Summary
Background
GST-HG171 is a potent, broad-spectrum, orally bioavailable small-molecule 3C like protease inhibitor that has demonstrated greater potency and efficacy compared to Nirmatrelvir in pre-clinical studies. We aimed to evaluate the efficacy and safety of orally administered GST-HG171 plus Ritonavir in patients with coronavirus disease 2019 (COVID-19) infected with emerging XBB and non-XBB variants.
Methods
This randomised, double-blind, placebo-controlled phase 2/3 trial was conducted in 47 sites in China among adult patients with mild-to-moderate COVID-19 with symptoms onset ≤72 h. Eligible patients were randomised 1:1 to receive GST-HG171 (150 mg) plus Ritonavir (100 mg) or corresponding placebo tablets twice daily for 5 days, with stratification factors including the risk level of disease progression and vaccination status. The primary efficacy endpoint was time to sustained recovery of clinical symptoms within 28 days, defined as a score of 0 for 11 COVID-19-related target symptoms for 2 consecutive days, assessed in the modified intention-to-treat (mITT) population. This trial was registered at ClinicalTrials.gov (NCT05656443) and Chinese Clinical Trial Registry (ChiCTR2200067088).
Findings
Between Dec 19, 2022, and May 4, 2023, 1525 patients were screened. Among 1246 patients who underwent randomisation, most completed basic (21.2%) or booster (74.9%) COVID-19 immunization, and most had a low risk of disease progression at baseline. 610 of 617 who received GST-HG171 plus Ritonavir and 603 of 610 who received placebo were included in the mITT population. Patients who received GST-HG171 plus Ritonavir showed shortened median time to sustained recovery of clinical symptoms compared to the placebo group (13.0 days [95.45% confidence interval 12.0–15.0] vs. 15.0 days [14.0–15.0], P = 0.031). Consistent results were observed in both SARS-CoV-2 XBB (45.7%, 481/1053 of mITT population) and non-XBB variants (54.3%, 572/1053 of mITT population) subgroups. Incidence of adverse events was similar in the GST-HG171 plus Ritonavir (320/617, 51.9%) and placebo group (298/610, 48.9%). The most common adverse events in both placebo and treatment groups were hypertriglyceridaemia (10.0% vs. 14.7%). No deaths occurred.
Interpretation
Treatment with GST-HG171 plus Ritonavir has demonstrated benefits in symptom recovery and viral clearance among low-risk vaccinated adult patients with COVID-19, without apparent safety concerns. As most patients were treated within 2 days after symptom onset in our study, confirming the potential benefits of symptom recovery for patients with a longer duration between symptom onset and treatment initiation will require real-world studies.
Funding
Fujian Akeylink Biotechnology Co., Ltd.
Keywords: Anti SARS-CoV-2 drug, COVID-19, RCT, 3CL protease, XBB variants
Research in context.
Evidence before this study
We searched PubMed with the terms (“3C-like protease” OR “main protease”) AND (COVID-19 OR SARS-CoV-2) AND (phase 3) and filters of (clinical trial OR randomised controlled trial) for articles published in any language up to Mar 1, 2024. Our search identified 4 results, including the phase 2/3 trial indicating the efficacy of Nirmatrelvir in high-risk, non-hospitalized patients with coronavirus disease 2019 (COVID-19) for preventing disease progression, and the phase 2/3 trial indicating the efficacy of Ensitrelvir in mild-to-moderate COVID-19 patients regarding the recovery of 5 specific fever and respiratory symptoms. Also, this study was designed based on the evidence obtained from the discovery and early clinical development of GST-HG171. GST-HG171 showed more potent and effective antiviral activity than Nirmatrelvir in pre-clinical studies, and a favorable pharmacokinetic characteristics and excellent safety profile in phase 1 clinical trial.
Added value of this study
GST-HG171 plus Ritonavir significantly reduced the time to achieve overall recovery from 11 COVID-19 target symptoms and to clear the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in adult patients with mild-to-moderate COVID-19, whether or not they had risk factors for disease progression. Importantly, this study was conducted during the emergence of recent Omicron variants, particularly XBB. Consistent effectiveness of GST-HG171 plus Ritonavir in both XBB- and non-XBB-infected sub-populations was demonstrated in this trial.
Implications of all the available evidence
GST-HG171 plus Ritonavir demonstrated significant efficacy compared with a placebo regimen in overall symptom recovery for COVID-19 caused by emerging Omicron variants, including the most recent XBB subtypes. While the short time period between symptom onset and treatment initiation in our study may not fully represent all clinical scenarios in the real world, GST-HG171, as an orally administered, broad-spectrum anti-COVID-19 drug, provides substantial value in combatting the pandemic, especially with continuously evolving SARS-CoV-2 variants.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to evolve during the coronavirus disease 2019 (COVID-19) pandemic, leading to a compromised efficacy of vaccines and neutralizing monoclonal antibodies.1,2 As of August 2023, most of the new COVID-19 cases worldwide were caused by SARS-CoV-2 Omicron variants,3, 4, 5, 6 particularly by the Omicron XBB variants in China as reported by Chinese Center for Disease Control and Prevention.7 Although symptoms associated with Omicron infections are generally less severe than the preceding strains,8,9 the risk of disease progressing to hospitalization and deaths remains, especially in immune-deficient or compromised, or elderly population with chronic diseases. In addition, the risk of long-term consequence (i.e., long Covid) caused by continuously emerging variants is also unpredictable. Therefore, broad-spectrum, and more effective and safer antiviral small-molecule drugs targeting the intrinsic and more conserved viral replication cycle are still in urgent need to combat the outbreak of the emerging Omicron variants and to treat patients with COVID-19 across all risk levels.10, 11, 12, 13
Several drugs have undergone evaluation and shown significant clinical benefits in patients with COVID-19.14 Most previous trials used hospitalization or death as the primary endpoint, such as the MOVE-OUT trial for Molnupiravir, the EPIC-HR trial for Nirmatrelvir plus Ritonavir, and the TOGETHER trial for fluvoxamine.10,11,15 As the mortality rate has significantly decreased with the emergence of recent Omicron variants, however, there has been a shift in focus towards outcomes related to clinical symptoms. Among drugs evaluated for symptoms related outcomes, Nirmatrelvir plus Ritonavir (in the EPIC-SR trial) did not show benefits.16 The PANORAMIC trial and the PRINCIPLE trial exhibited benefits in symptom recovery for Molnupiravir and budesonide, respectively,17,18 yet both were evaluated in open-label trials. The SCORPIO-SR trial demonstrated efficacy for a 3C-like (3CL) protease inhibitor Ensitrelvir with the time to resolution of 5 characteristic symptoms shortened.19 VV116, an RNA-dependent RNA polymerase (RdRp) inhibitor approved in China, was evaluated among adults with mild-to-moderate COVID-19, establishing non-inferior efficacy to Nirmatrelvir plus Ritonavir and demonstrating superiority to placebo with respect to the time to sustained clinical recovery.12,20 Simnotrelvir and Leritrelvir are 3CL protease inhibitors recently approved in China, both of which shortened the time to sustained resolution of COVID-19 symptoms compared to placebo in their phase III trials.21,22 However, the trials mentioned above were all conducted before the 2023 Omicron XBB wave in China.20, 21, 22 To date, evidence of efficacy on symptom recovery for orally administrated antiviral drugs in mild-to-moderate COVID-19 remains limited, and no evidence of efficacy has been reported in patients infected with emerging XBB variants.
GST-HG171 (known as Atilotrelvir) is a novel, potent, broad-spectrum, orally administered small-molecule inhibitor targeting SARS-CoV-2 3CL protease. It demonstrated superior antiviral activities compared to Nirmatrelvir in pre-clinical studies both in vitro and in vivo.20 Additionally, GST-HG171 exhibits favorable pharmacokinetic characteristics and has demonstrated an excellent safety profile in both pre-clinical studies and phase 1 clinical trials with healthy subjects (ClinicalTrials.gov number, NCT05668897).23,24 Ritonavir, a known cytochrome P450 family 3 subfamily A member 4 (CYP3A4) inhibitor, was found to increase plasma exposure of GST-HG171 significantly in healthy subjects when taken together with GST-HG171.24 In this pivotal phase 2/3 study, we aimed to evaluate the efficacy and safety of GST-HG171 plus Ritonavir in patients with mild-to-moderate COVID-19 infected with emerging Omicron XBB and non-XBB variants. Based on the results of this study, GST-HG171 plus Ritonavir has been conditionally approved by the Chinese National Medical Products Administration (NMPA) for treating adult patients with mild to moderate COVID-19.
Methods
Study design and participants
This multicenter, randomised, double-blind, placebo-controlled phase 2/3 trial evaluating the efficacy and safety of orally administered GST-HG171 plus Ritonavir in adult patients with mild-to-moderate COVID-19 was done at 47 sites in China.
The trial was designed and monitored by the principal investigators and the sponsor, Fujian Akeylink Biotechnology Co., Ltd. Data were collected by the investigators and site personnel, analyzed by statisticians employed by the sponsor, and interpreted by the authors. Safety oversight was performed by the sponsor, the IRB/EC at each site, and an independent Data and Safety Monitoring Board (DSMB). All the data were available to all the authors, who vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, which is available online.
Eligible patients were required to be adults aged ≥18 years with mild-to-moderate COVID-19, regardless of previous SARS-CoV-2 infection and risk factors for progression to severe diseases25; with a positive result of SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) test detected within 5 days and at least 2 of the 11 COVID-19-related target symptoms (Appendix p 10) onset within 72 h before randomisation. Key exclusion criteria were diagnosed as severe or critical COVID-19, prior (within 28 days before randomisation) or planned receipt of COVID-19 vaccine, prior COVID-19 treatment with antiviral drugs within 14 days before randomisation, monoclonal antibodies, human immunoglobulin, or convalescent plasma, and use of drugs with possible drug interaction risks. Full eligibility criteria and representative drugs with possible drug interaction risks are provided in the Appendix (pp 4–8). Sex data were collected from self-report.
Important changes of eligibility criteria across protocol amendment included:
-
1)
Time from onset of symptoms to randomisation (within 48 h in protocol Version 1.3 was changed to within 72 h in protocol Version 1.4/1.5), and time from first positive SARS-CoV-2 result to randomisation (within 4 days in protocol Version 1.3 was changed to within 5 days in protocol Version 1.4/1.5). These eligibility criteria were modified to better adapt to current clinical reality of COVID-19 infection for facilitating recruitment;
-
2)
Time of excluding prior vaccinations (excluding vaccinations within 3 months prior to randomization in protocol Version 1.3/1.4 was changed to excluding vaccinations within 28 days prior to randomization). The reason for the change is to expand the eligible population of the study to include the patients who received the vaccinations for over 28 days, but still got infected by SARS-CoV2. The patients who receive vaccinations, but still get infections may also benefit from the treatment of GST-HG171.
A full discussion on changes to eligibility criteria during protocol amendment is provided in the Appendix (pp 5–6), and the complete record of protocol revision is provided in the protocol (pp 7–17).
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki, the Good Clinical Practice guidelines, and other applicable regulations and guidelines. The study was reviewed and approved by the Chinese NMPA (reference number: CXHL2200684, CXHL2200685), and the institutional review board (IRB) or ethics committee (EC) at each participating site listed in the Appendix (p 2) before the start of recruitment. Written informed consent was obtained from all patients or their legally acceptable representatives.
Randomisation and masking
Eligible patients were randomly assigned in a 1:1 ratio to either the GST-HG171 plus Ritonavir group or the placebo group. Randomisation was stratified according to the presence of a high-risk factor of progression to severe disease (yes vs. no) and COVID-19 vaccination status (incomplete basic immunization, completed basic immunization, or completed booster immunization). The randomisation list was prepared by a randomisation statistician not involved in the trial using the PLAN procedure of SAS version 9.4, and the random number of the patient was generated by the randomisation statistician using the stratified block randomisation method. Investigators were responsible for participant enrollment and intervention assignment according to the random number. The Interactive Web Response System (IWRS) with patient random allocation table imported by the randomisation statistician was used to patient assignment. Patients, investigators, sponsor, and other involved personnel remained blinded to the treatment assignments until the end of the study, except for the independent DSMB established to assess the efficacy and safety data of study treatment.
Procedures
Patients received either GST-HG171 (150 mg/dose, Fujian Cosunter Pharmaceutical Co., Ltd.) plus Ritonavir (100 mg/dose, Jiangsu Sinotherapeutics Co., Ltd.) or placebo for GST-HG171 (Fujian Cosunter Pharmaceutical Co., Ltd.) plus Ritonavir placebo (Ascletis Pharmaceutical Co., Ltd.) tablets, administered orally twice daily for 5 days (10 doses in total). Tablets in the two intervention groups have identical appearance. Clinical assessments were performed mainly by daily COVID-19-related symptom scores (Appendix p 10, including 11 target symptoms and 3 other symptoms, as reported by patients) until day 28. Virology assessments were performed using both qualitative and quantitative SARS-CoV-2 RT-PCR assay by Teddy Clinical Research Laboratory (Wuxi) (Jiangsu, China) with nasopharyngeal swabs collected at baseline and on pre-specified days (due to protocol amendment, compulsory nasopharyngeal swabs were collected at day 3, day 4 and day 5 per protocol Version 1.3, and day 4 per protocol Version 1.4 and 1.5) following the treatment. Patients were followed up for safety throughout the 28-day assessment. More details of assessments were provided in the Appendix pp 62–65. The study design schematic was provided in the Appendix (p 25).
Outcomes
The primary efficacy endpoint was time to sustained recovery of clinical symptoms within 28 days after treatment. Key secondary efficacy endpoints included changes in viral load from baseline to day 4, time to sustained recovery of fever and respiratory symptoms (i.e., cough, congestion or runny nose, sore throat or dry throat, shortness of breath or difficulty breathing) within 28 days, and time to negative conversion of SARS-CoV-2 nucleic acid within 28 days. Other secondary efficacy endpoints included time to sustained relief of clinical symptoms within 28 days, area under the viral load-time curve (AUC) within 14 days, clinical symptom score-time AUC within 14 days, percentage of COVID-19 progression (defined as progression to severe/critical COVID-19 or all-cause mortality) and sustained recovery of clinical symptoms by visits, and changes in the scores of COVID-19 symptoms and chest CT scan from baseline. Sustained recovery or relief of clinical symptoms was defined as the score of 0 or 1 for all COVID-19-related target symptoms for 2 consecutive days, with the event date defined as the first day of the 2 consecutive days. Safety endpoints included the incidence of adverse events and serious adverse events, as well as any clinically significant abnormality of vital signs, physical examination, laboratory tests, and electrocardiograms during the study.
To streamline the PCR test sample collection during the initial 7 days of the study, ensuring better patient compliance, and also considering the observed peak effect of viral load reduction around day 4, as reported by Ensitrelvir treatment,13 we modified the sampling time points. In Protocol Version 1.3, sampling occurred at day 3, 4, 5, and 7, which was revised to day 4 and 7 in Protocol Version 1.4/1.5 during the first week of the study. Accordingly, the key secondary endpoint evaluating changes in viral load from baseline to day 5 in Protocol Version 1.3 was adjusted to day 4 in Protocol Version 1.4/1.5. Moreover, to better assess the effect on viral clearance, the time to negative conversion of SARS-CoV-2 nucleic acid within 28 days was highlighted as an additional key secondary endpoint in Protocol Version 1.4/1.5.
Statistics
Approximately 1200 patients (1:1 ratio, 600 in each group) were planned to be enrolled in this study, of which 960 patients and 856 events were expected to be analyzed to ensure a power of 90% under the significance criterion with the one-sided of 0.0238 (two interim analyses require partial alpha spent) to detect the assumed difference in the median time to sustained recovery of clinical symptoms between GST-HG171 and placebo (8 days vs. 10 days).
Two interim analyses and a final analysis were planned to be performed for this study. The first interim analysis was the unblinded safety review and preliminary efficacy observation by DSMB for the first 100 patients (sentinel cohort). The second interim analysis took place when about 70% of the expected events (i.e., sustained recovery of clinical symptoms) occurred to provide support info for recommendation made by DSMB, which was to continue the study with original sample size. Here we report the final analysis performed after the last patient had completed the last assessment. The boundary value generated by the Lan-DeMets spending function method to approximate O'Brien-Fleming26,27 was used to control class I errors (α < 0.05 for a two-sided test).
All efficacy analysis were performed in the modified intention-to-treat (mITT) population that were confirmed to be positive for SARS-CoV-2 nucleic acid by RT-PCR at baseline, non-positive for influenza virus, and had at least 1 visit from post-baseline to day 28 (see Appendix p 12 for definitions of all analysis populations). The primary analysis compared the median time to sustained recovery of clinical symptoms in the two groups using the Kaplan–Meier method and the log-rank test or Peto–Peto test (only if the proportional hazard assumption was not met) adjusted for randomisation factors (using the treatment group as a group variable, tests are stratified on the 6 levels stratum based on 2 stratification factors, and the patients' data were presented separately as 2 Kaplan–Meier curves under each stratum. After adjusting for the effects of the two stratification factors, a log-rank/Peto–Peto test of homogeneity of the 2 curves was performed), with the hazard ratio (HR, investigational group/placebo group) calculated by the Cox regression model and corrected by randomisation factors. Same method was used in all time-to-event analyses for secondary endpoints. Several supplementary analyses and sensitivity analyses were also performed to assess the robustness of the primary analysis results (Appendix p 9). Changes in viral load from baseline were estimated using the analysis of covariance (ANCOVA) with the log10-transformed viral load as the dependent variable, the treatment group as the independent variable, and the randomisation factors and baseline viral load as the covariates. Viral rebound was analyzed in the mITT population and defined as a re-positive status after negative conversion of SARS-CoV-2 nucleic acid. Subgroups analysis was planned based on sex, age category, severity, COVID-19 vaccination status (yes [complete basic immunization vs. complete booster immunization] vs. no) and presence of high-risk factor of progression to severe illness. In addition to pre-specified subgroup analyses, we conducted post hoc subgroup analysis according to SARS-CoV-2 variants (XBB vs. non-XBB) for the primary endpoint and two key secondary endpoints (i.e., change in viral load and time to SARS-CoV-2 negative conversion). Safety analyses were done in all patients who had received at least one dose of drug or placebo (safety population). Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 26.0, from the time of consent through 28-day follow-up or early withdrawal.
Statistical analyses were done using SAS version 9.4. Full statistical analysis plan (SAP) was provided online. This study is registered with ClinicalTrials.gov, NCT05656443, and Chinese Clinical Trial Registry, ChiCTR2200067088.
Role of the funding source
The study sponsor (funding source) participated in study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication.
Results
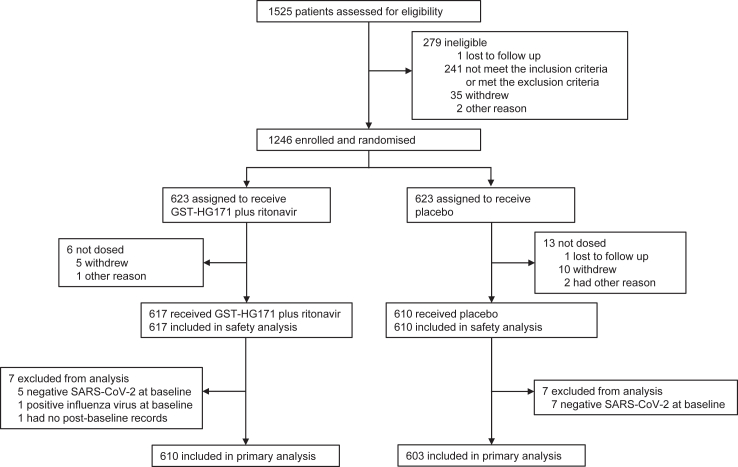
Between Dec 19, 2022 and May 4, 2023, a total of 1525 patients were screened at 47 sites in China (Fig. 1). The pre-specified sample size was reached before the study ended. 1246 eligible patients were enrolled and underwent randomisation (623 assigned to each group), of whom 19 (1.5%) were not dosed (mostly due to voluntary withdrawal), 617 received GST-HG171 plus Ritonavir, and 610 received placebo; 1209 (97.0%) completed the 28-day assessment and 37 (3.0%) discontinued the trial (Appendix p 13). All patients received GST-HG171 plus Ritonavir or placebo were included in safety analysis population; 610 received GST-HG171 plus Ritonavir and 603 received placebo were included in the mITT population and assessed for the primary endpoint. Among 14 patients who were excluded from mITT population, 12 patients had negative results of SARS-CoV-2 RT-PCR test at baseline, one patient had no post-baseline records, and one patient had positive result of influenza virus test at baseline.
Fig. 1.
CONSORT Flow chart of participants.
Patient characteristics at baseline were balanced between the two groups among 1227 patients in the full analysis population (Table 1). The mean age was 34.5 years (standard deviation [SD] 11.04); 538 (43.8%) patients were male, and 1223 (99.7%) patients were Asian. Most patients completed basic (260 of 1227, 21.2%) or booster (919 of 1227, 74.9%) COVID-19 immunization and had mild COVID-19 (1132 of 1227, 92.3%), with a mean total score for all COVID-19-related target symptoms of 9.4 points (SD 4.15) at baseline. 1090 (88.8%) patients were free from high-risk factors of disease progression at baseline. Therefore, most of the patients in this study was low-risk, vaccinated population. All patients with SARS-CoV-2 viral sequence data were infected by the SARS-CoV-2 Omicron strain, including 484 (39.4%) infected by the XBB strains and 572 (46.6%) infected by the Non-XBB strains. Among 1053 patients with SARS-CoV-2 viral sequence data in the mITT population, 481 (45.7%) were infected by the XBB strains and 572 (54.3%) were infected by the Non-XBB strains (more information on viral strains provided in Appendix p 14). Patients received the first dose at an average of 2.1 days (SD 0.87) after COVID-19 symptom onset, with similar medication adherence in the two groups.
Table 1.
Demographic and clinical characteristics of the full analysis population.a
| Characteristic | GST-HG171 plus Ritonavir (N = 617) | Placebo (N = 610) |
|---|---|---|
| Age at randomisation, years | 34.3 (10.76) | 34.7 (11.31) |
| Sex | ||
| Male | 282 (45.7%) | 256 (42.0%) |
| Female | 335 (54.3%) | 354 (58.0%) |
| Raceb | ||
| Asian | 615 (99. 7%) | 608 (99.7%) |
| Other | 2 (0.3%) | 2 (0.3%) |
| COVID-19 vaccination status | ||
| Incomplete basic immunization | 23 (3.7%) | 25 (4.1%) |
| Completed basic immunization | 132 (21.4%) | 128 (21.0%) |
| Completed booster immunization | 462 (74.9%) | 457 (74.9%) |
| COVID-19 severity | ||
| Mild | 574 (93.0%) | 558 (91.5%) |
| Moderate | 43 (7.0%) | 52 (8.5%) |
| COVID-19-related symptoms | ||
| Time from first symptom to first dose, days | 2.1 (0.87) | 2.1 (0.87) |
| Total score for all COVID-19–related target symptoms, points | 9.4 (4.20) | 9.5 (4.10) |
| High-risk factor of progression to severe disease | ||
| No risk factor | 548 (88.8%) | 542 (88.9%) |
| At least one risk factor | 69 (11.2%) | 68 (11.1%) |
| Elderly people aged >60 yr | 20 (3.2%) | 21 (3.4%) |
| Underlying diseases | 33 (5.3%) | 29 (4.8%) |
| Obesityc | 24 (3.9%) | 29 (4.8%) |
| Heavy smokers | 3 (0.5%) | 7 (1.1%) |
| Immune deficiency | 1 (0.2%) | 0 |
| Virology | ||
| Baseline SARS-CoV-2 nucleic acid by RT-PCR | ||
| Positive | 612 (99.2%) | 603 (98.9%) |
| Negative | 5 (0.8%) | 7 (1.1%) |
| SARS-CoV-2 virus straind | ||
| Non-XBB | 303 (49.1%) | 269 (44.1%) |
| XBB | 233 (37.8%) | 251 (41.1%) |
COVID-19, coronavirus disease 2019; SD, standard deviation; RT-PCR, reverse-transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Shown are data for all patients who underwent randomisation and received at least one dose of GST-HG171 plus Ritonavir or Placebo. Patients were grouped according to treatment assignment. Data are mean (SD) or n (%).
Race was reported by the patients.
Obesity was defined by a body-mass index of 30 or higher.
“Non-XBB” includes Omicron BA.5.2, BF.7, BQ.1 and other variants. “XBB” includes several lineages of Omicron XBB strain. See Appendix p14 for details.
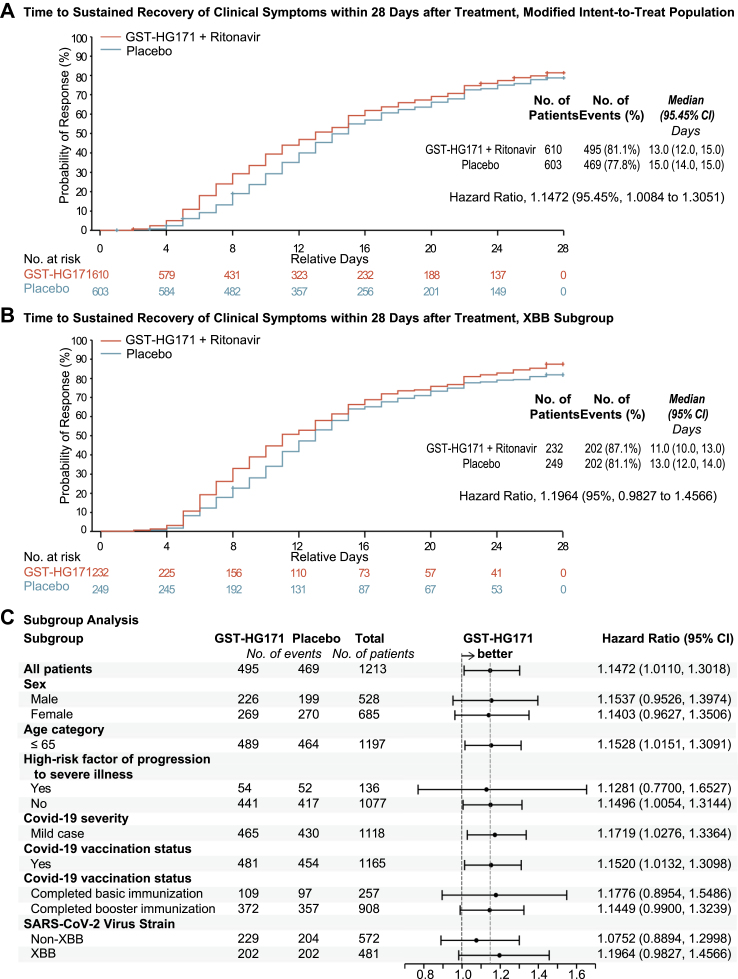
In the primary efficacy analysis based on the mITT population, sustained recovery of clinical symptoms occurred in 495 (81.1%) of 610 patients in the GST-HG171 plus Ritonavir group and 469 (77.8%) of 603 patients in the placebo group, with the estimated median time to be 13.0 days (95.45% confidence interval [CI] 12.0–15.0) and 15.0 days (14.0–15.0), respectively, showing a significant 2-day decrease vs. placebo under the treatment (P = 0.031; HR 1.15 [95.45% CI 1.01–1.31]) (Fig. 2A and Appendix pp 15–17). Consistent results were also observed in sensitivity analyses, supplementary analyses (Appendix pp 15–17), and all the subgroups analyses regardless of sex, age, high-risk factors of severe disease, COVID-19 severity, vaccination status, and SARS-CoV-2 strains at baseline (Fig. 2C). Based on post hoc analysis, in the subgroup of patients infected by SARS-CoV-2 XBB variants, the median time to sustained recovery of clinical symptoms decreased by 2 days (11.0 days [95% CI 10.0–13.0] vs. 13.0 days [12.0–14.0], HR 1.20 [95% CI 0.98–1.46]) (Fig. 2B and Appendix pp 18–20). As for sustained relief of clinical symptoms (scores ≤1), the estimated median time was 5.0 days (95% CI 5.0–6.0) and 6.0 days (5.0–6.0) in the GST-HG171 plus Ritonavir and placebo group, respectively (HR 1.13 [95% CI 1.00–1.29]) (Appendix pp 18–20, 26).
Fig. 2.
Time to Sustained Recovery of Clinical Symptoms. Shown are the time to sustained recovery of clinical symptoms within 28 days after treatment in the mITT population (1213 patients, Panel A) and the subgroup of population that infected with XBB variants (481 patients, Panel B), as estimated with the use of the Kaplan–Meier method and compared with the use of the log-rank test adjusted for randomisation factors, and the hazard ratio (HR, investigational group/placebo group) calculated by the Cox regression model and corrected by randomisation factors. Sustained recovery of clinical symptoms is defined as the score of 0 for all COVID-19-related target symptoms for 2 consecutive days, with the event date defined as the first day of the 2 consecutive days. + indicates censored values. Data for the patients who used prohibited medications or therapies that may affect the efficacy endpoints (as identified at the data review meeting), progressed to severe/critical COVID-19 before recovery, or were assessed by investigators as having poor efficacy and were withdrawn from treatment early, and dead before recovery were censored on Day 28. Panel C shows subgroup analysis of the time to sustained recovery of clinical symptoms within 28 days after treatment in the mITT population, analyzed using the same statistical methods as the primary analysis shown in Panel A. CI, confidence interval; GST-HG171 as a shorthand for GST-HG171 plus Ritonavir.
Besides the overall symptom recovery, fever and respiratory symptoms, as the most representative symptoms of Omicron infection, also recovered faster in the GST-HG171 plus Ritonavir group with the estimated median time to sustained recovery to be 13.0 days (95.45% CI 11.0–14.0) compared to 14.0 days (13.0–15.0) in the placebo group (P = 0.0021; HR 1.13 [95.45% CI 0.99–1.29]) (Appendix pp 18–20, 27). Moreover, time to sustained recovery of each single respiratory symptom including cough, congestion or runny nose, and sore throat or dry throat, was significantly shortened in the GST-HG171 plus Ritonavir group compared to placebo (Appendix pp 28–29). By the time of the final analysis, no patients in this trial died or experienced progression to severe/critical COVID-19, and no significant inter-group difference in the change of chest CT scan were detected due to limited sample numbers (Appendix pp 18–20).
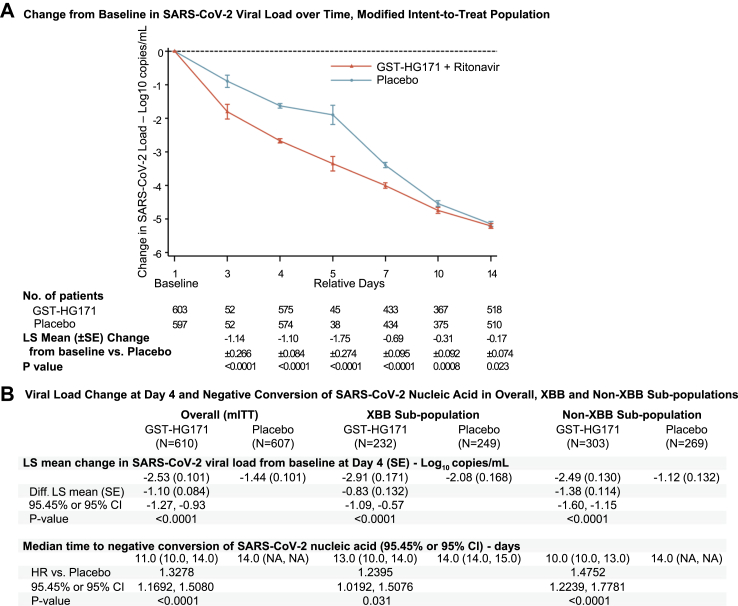
Although the SARS-CoV-2 viral load decreased with time in both groups, the change from baseline was significantly greater in the GST-HG171 plus Ritonavir group through day 14 (Fig. 3A). GST-HG171 plus Ritonavir reduced the viral load by an additional least-squares (LS) mean of 1.14 log10 copies/mL (standard error [SE] 0.266; 95.45% CI 0.60–1.67; P < 0.0001) at day 3, 1.10 log10 copies/mL (0.084; 0.93–1.27; P < 0.0001) at day 4, and 1.75 log10 copies/mL (0.274; 1.19–2.30; P < 0.0001) at day 5, compared to placebo (Fig. 3A and Appendix pp 18–21). The significant difference in LS mean change in SARS-CoV-2 viral load from baseline to day 4 (the pre-specified key secondary endpoint) was also observed in the XBB and non-XBB subgroups, showing an additional 0.83 log10 copies/mL (SE 0.132; 95% CI 0.57–1.09; P < 0.0001) and 1.38 log10 copies/mL (0.114; 1.60–1.15; P < 0.0001) reduction in the GST-HG171 plus Ritonavir group compared to placebo, respectively (Fig. 3B and Appendix pp 18–20). GST-HG171 plus Ritonavir treatment significantly decreased the estimated median time to negative conversion of SARS-CoV-2 nucleic acid compared to placebo by 3 days, 1 day, and 4 days in mITT overall population (11.0 days [95.45% CI 10.0–14.0] vs. 14.0 days [95.45% CI NA], P < 0.0001, Appendix p 30), XBB subgroup (13.0 days [95% CI 10.0–14.0] vs. 14.0 days [14.0, 15.0], P = 0.030), and non-XBB subgroup (10.0 days [95% CI 10.0–13.0] vs. 14.0 days [NA], P < 0.0001), respectively (Fig. 3B and Appendix pp 18–20). Viral rebound occurred in 11 of the 610 patients (1.8%) in the GST-HG171 plus Ritonavir group and 10 of the 603 (1.7%) in the placebo group. There was no adjustment for multiple comparisons.
Fig. 3.
Antiviral Efficacy of GST-HG171 plus Ritonavir. Panel A shows the Log10-transformed mean change in SARS-CoV-2 viral load from baseline among all the patients in the mITT population with available viral load data. Panel B shows virological efficacy results among the overall mITT population, and subgroup of populations infected with SARS-CoV-2 XBB or non-XBB variants. Lower limit of quantification of viral RNA is 2.3 log10 copies/mL. Data regarding SARS-CoV-2 viral load are presented as LS means ± SEs; LS mean, difference in LS mean change versus placebo, SE, CI, and P-value are based on the analysis of covariance (ANCOVA) method with treatment group as independent variable and baseline viral load and presence of a high-risk factor of progression to severe disease, COVID-19 vaccination status as covariates. Analysis in time to negative conversion of SARS-CoV-2 nucleic acid only includes the patients with baseline SARS-CoV-2 nucleic acid positive; hazard ratio and CI is based on COX model test stratified by presence of a high-risk factor of progression to severe illness, COVID-19 vaccination status; P-value is based on Log-rank test stratified by presence of a high-risk factor of progression to severe illness, COVID-19 vaccination status. Data with 95.45% CI and 95% CI were calculated in the overall population and subgroup populations, respectively. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LS, least-squares; SE, standard error; CI, confidence interval; Diff., difference; HR, hazard ratio; GST-HG171 as a shorthand for GST-HG171 plus Ritonavir.
The incidence of adverse events was similar in the GST-HG171 plus Ritonavir (320/617, 51.9%) and placebo (298/610, 48.9%) group, with all these collected adverse events emerging during or after the treatment period. Most adverse events were non-serious and of grade 1/2, and the most common adverse events in both placebo and treatment groups were hypertriglyceridaemia (10.0% vs 14.7%). Patients received GST-HG171 plus Ritonavir reported fewer adverse events leading to any drug withdrawal (4/617, 0.6% vs. 7/617, 1.1%) or interruption (0 vs. 1/617, 0.2%) than placebo; none of the adverse events led to death (Table 2). One serious adverse event (arteriosclerosis coronary artery) was reported in 1 (0.2%) patient in the GST-HG171 plus Ritonavir group; two serious adverse events (pneumonia and cervical dysplasia) were reported in 2 (0.3%) patients in the placebo group. None of serious adverse events were drug-related (Table 2).
Table 2.
Adverse events in the safety population.a
| Adverse event | GST-HG171 plus ritonavir (N = 617) | Placebo (N = 610) |
|---|---|---|
| No. of adverse events | 822 | 720 |
| Patients with adverse events | ||
| Any adverse event | 320 (51.9%) | 298 (48.9%) |
| Treatment-emergent adverse eventb | 320 (51.9%) | 298 (48.9%) |
| Any Drug-related AEc | 222 (36.0%) | 182 (29.8%) |
| Serious AE | 1 (0.2%) | 2 (0.3%) |
| Any Drug-related Serious AEc | 0 | 0 |
| AE leading to any drug withdrawal | 4 (0.6%) | 7 (1.1%) |
| AE leading to any drug interruption | 0 | 1 (0.2%) |
| AE leading to death | 0 | 0 |
| AE by NCI-CTCAEd | ||
| Grade 1 | 291 (47.2%) | 269 (44.1%) |
| Grade 2 | 77 (12.5%) | 66 (10.8%) |
| Grade 3 | 16 (2.6%) | 12 (2.0%) |
| Grade 4 | 1 (0.2%) | 1 (0.2%) |
| Grade 5 | 0 (0%) | 0 (0%) |
Data are n (%). AE, adverse event; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Shown are results for all the adverse events (AEs) as coded according to the Medical Dictionary for Regulatory Activities, version 26.0, from the time of consent through 28-day follow-up or early withdrawal. Patients were those who received at least one dose of GST-HG171 plus Ritonavir or Placebo as grouped according to the actual treatment received.
Treatment-Emergent Adverse Event (TEAE) is defined as any adverse event occurring or worsening relative to baseline from the first dose of the study drug through 28-day follow-up or early withdrawal.
Drug-related AEs include all AEs with relationships that are “Definitely related”, “Probably related”, and “Possibly related” as determined by the investigators.
The same patient can be included in different grades at the same time because of the grades of adverse events that have occurred.
Drug-related adverse events were reported for 222 (36.0%) of 617 patients in the GST-HG171 plus Ritonavir group and 182 (29.8%) of 610 patients in the placebo group (Table 2), with all of which to be non-serious, most of grade 1/2, and more than half resolved by the end of the study. The ≥ grade 3 drug-related adverse events reported in the GST-HG171 plus Ritonavir (8/617, 1.3%) or placebo (6/610, 1.0%) group were hypertriglyceridaemia (4 [0.6%] with GST-HG171 plus Ritonavir and 4 [0.7%] with placebo), neutropenia (2 [0.3%] and 1 [0.2%], respectively), hyperlipidaemia (1 [0.2%] with GST-HG171 plus Ritonavir), hypokalaemia (1 [0.2%] with GST-HG171 plus Ritonavir), and decrease in neutrophil count (1 [0.2%] with placebo) (Appendix p 22).
Discussion
Results from this pivotal phase 2/3 study in adult patients with mild-to-moderate COVID-19 demonstrated the efficacy and safety of oral administration of GST-HG171 (150 mg) with Ritonavir (100 mg) twice daily for 5 days. GST-HG171 plus Ritonavir treatment within 72 h after COVID-19 symptoms onset shortened the median time to sustained recovery of clinical symptoms by 2 days compared to placebo. Similar trends favoring GST-HG171 were observed in subgroup analyses regardless of sex, age, high-risk factors for severe disease, COVID-19 severity, vaccination status, and SARS-CoV-2 variants (XBB or non-XBB) at baseline. GST-HG171 plus Ritonavir also showed significant efficacy in terms of the time to sustained recovery of fever and respiratory symptoms, sustained relief of clinical symptoms, and negative conversion of SARS-CoV-2 nucleic acid. Treatment with GST-HG171 plus Ritonavir significantly reduced SARS-CoV-2 viral load from baseline versus placebo through 14 days of the treatment, especially at day 3, 4, and 5. GST-HG171 is generally safe with a similar adverse events incidence to placebo in this study.
An outstanding contribution of this study is providing positive results for GST-HG171 in patients infected with the latest SARS-CoV-2 XBB variants. To our knowledge, this is the first demonstration of the clinical benefits of an oral 3CL protease inhibitor in a randomised, double-blind, placebo-controlled clinical study with XBB variant-infected patients. This study was conducted from late 2022 to mid-2023 during the SARS-CoV-2 Omicron pandemic in China, with the rapid circulation of XBB and its sub-lineages beginning approximately in April 2023.8 All of the patients included in the efficacy analysis of this study were infected by SARS-CoV-2 Omicron strain, among whom 45.7% were infected by the XBB variants and 54.3% were infected by the non-XBB variants, demonstrating the clinical benefits of GST-HG171 plus Ritonavir against different variants of SARS-CoV-2 Omicron strain including XBB and non-XBB. These clinical efficacy results are consistent with the potent and broad-spectrum antiviral activity of GST-HG171 observed in pre-clinical studies in vitro and in vivo.23 In pre-clinical studies, GST-HG171 has shown potent antiviral activities against various SAR-CoV-2 strains including the original strain, beta, delta, and omicron, with 7-10-fold higher potency than Nirmatrelvir in the cytopathic effect (CPE) assays in vitro. It also potently inhibits several variants of omicron lineages including BA.4, BA.5, BQ.1.1, XBB.1, XBB.1.5, with 5-10-fold higher potency than Nirmatrelvir in the CPE assay. Further, GST-HG171 has >4-fold higher lung exposure and lung/plasma exposure ratio than Nirmatrelvir in animals, and demonstrated >20-fold higher efficacy in vivo than Nirmatrelvir in reducing viral load in lung tissue in mice infected with SARS-CoV-2 virus.23
Although several antiviral drugs have been reported to be effective in the treatment of COVID-19, GST-HG171 has shown several specific strengths. As an orally administrated drug, GST-HG171 is more convenient than Remdesivir that is currently used intravenously to treat severe COVID-19 disease.28 Paxlovid (Nirmatrelvir plus Ritonavir) and Molnupiravir were approved for non-hospitalized patients with COVID-19 but only indicated for those at high-risk for progression to severe disease.10,11 In patients with mild-to-moderate COVID 19 at standard risk, however, no statistically significant effect on symptom resolution was demonstrated by Nirmatrelvir plus Ritonavir,16 whereas GST-HG171 is able to target a wider patient population regardless of their risk-levels for overall symptom recovery. The superior potency, efficacy, and PK characteristics of GST-HG171 over Nirmatrelvir in preclinical studies in vitro and in vivo may contribute to the efficacy observed in patients across all risk levels in the current clinical study.23 Among published studies assessing efficacy of COVID-19 drugs, Ensitrelvir, approved in Japan only, was evaluated in similar population compared to this study, but efficacy of GST-HG171 on recovery of 11 COVID-19 symptoms was not observed by Ensitrelvir treatment.19 The significant difference in the change of subtotal of 5 fever and respiratory symptoms versus placebo achieved with treatment of Ensitrelvir was also observed with GST-HG171 in the current study. In the trial comparing VV116 (an RdRp inhibitor) and placebo, the median time to sustained clinical symptom resolution for 2 consecutive days was 10.9 days for the VV116 group and 12.9 days for the placebo group, indicating a two-day difference over placebo,20 which aligns closely with our study's observations. It is, however, noteworthy that the dominant subvariants in VV116 trial were mostly Omicron BA.5.2.48 (58.7%) and BF.7.14 (30.7%).20 Simnotrelvir and Leritrelvir, two 3CL protease inhibitors recently approved in China, were both evaluated in the pivotal trials with the same primary endpoints as our study. Faster resolution of the 11 COVID-19 symptoms was observed in both trials, with a difference of 35.8 h (Simnotrelvir plus Ritonavir) and 20.31 h (Leritrelvir) compared to placebo, respectively,21,22 which were smaller than that (48 h) observed in our study. Moreover, both trials were conducted before the 2023 Omicron XBB wave in China, and XBB variant was only detected in 1 out of 140 patients evaluated for viral strains in the pivotal trial of Simnotrelvir, with viral strains of patients not reported in the pivotal trial of Leritrelvir.21,22 Consequently, GST-HG171 stands out as the only drug showing benefits in patients infected with XBB variants. In addition, a greater reduction of viral load was observed with GST-HG171 plus Ritonavir (a reduction of 1.10 log10copies/mL compared to placebo) than Leritrelvir (a reduction of 0.82 log10copies/mL compared to placebo) on day 4. Similarly, a greater reduction of viral load was observed with GST-HG171 plus Ritonavir (a reduction of 1.75 log10copies/mL compared to placebo) than Simnotrelvir plus Ritonavir (a reduction of 1.51 log10copies/mL compared to placebo) on day 5. Considering the viral load of SARS-CoV-2 is associated with disease severity, potential mortality, and risk of transmission,29,30 GST-HG171 may bring more clinical benefits than currently approved drugs targeting 3CL. In addition, the recommended dose of GST-HG171 (150 mg, twice daily) is the lowest among the approved 3CL protease inhibitors,10,19,21,22 potentially leading to better medication compliance.
The study has, however, several limitations. First, most (92.3%) patients had mild COVID-19 and most (88.8%) had a low risk of disease progression, reflecting the current reality of population immunity and the SARS-CoV-2 Omicron pandemic, with limited data in patients at high risk; and further, only a limited number of elderly patients (4.0%, aged older than 60 years) and patients other than Asians (0.3%) were included in this study. Secondly, the faster clearance of the SARS-CoV-2 virus and shortened clinical recovery of symptoms under the treatment with GST-HG171 plus Ritonavir may bring potential benefits in minimizing disease transmission and burden, whereas the efficacy of GST-HG171 in preventing disease progression or death could not be evaluated as no such events occurred in this study. Thirdly, as the target population of this study was patients with mild-to-moderate COVID-19, it remains unknown whether GST-HG171 would also be beneficial for patients with severe-to-critical COVID-19, which warrants further investigations. Fourthly, the use of Ritonavir together with GST-HG171 may affect the concentrations of certain drugs, and thus a careful evaluation should be performed before use of the concomitant medication, and monitoring and treatment of the potential adverse events should be educated, whereas, in patients who use low-dose Ritonavir as a PK enhancer for a short duration, risk is expected to be lower than that in routine HIV treatment at higher doses. Finally, the short time period between symptom onset and treatment initiation in our study may not fully represent all clinical scenarios in the real world, and benefits for patients treated after 2 days following symptom onset are to be confirmed in real-world studies.
In conclusion, oral treatment with GST-HG171 plus Ritonavir has demonstrated benefits in clinical symptom recovery and viral clearance in low-risk, vaccinated, young patients with mild-to-moderate COVID-19, regardless of whether infection is caused by newly emerging SARS-CoV-2 XBB or non-XBB variants. There were no apparent safety concerns associated with the treatment. As most patients were treated within 2 days after symptom onset in our study, confirming the potential benefits of symptom recovery for patients with a longer duration between symptom onset and treatment initiation will require real-world studies.
Contributors
NZ, HongzL, GZ, JM, YZ, XiaC, ZY, XinC, GW, and XiaoC conceived and designed the study. NZ and HongzL are co-principal investigators, and FL, LL, FZ, YL, YZ, KZ, WenfY, RS, LH, PH, YL, XZ, and FW are investigators. YT, TZ, WenhY, XY, HT, LM, FH, ZH, and ZY executed the study, with supervision from GZ, JM, YZ, ZY, ZheL, HongmL, and GL. ZW, ZhuL, and HF contributed to statistical analysis. GZ, XiaC, and XT drafted the manuscript, with critical revision by NZ, HongzL, JM, YZ, TZ, XY, ZhuL, GW, XiaoC, XinC, and FW. NZ, HongzL, and GZ have directly accessed and verified the underlying data reported in the manuscript. All authors had full access to all the data in the study, and approved of this manuscript to be submitted for publication.
Data sharing statement
After approval from the Human Genetic Resources Administration of China, this trial data can be shared with qualifying researchers who submit a proposal with a valuable research question. A contract should be signed.
Declaration of interests
GZ, JM, TZ, YT, WenhY, XY, and HT are employees and GZ, JM, TZ, YT, HT, FH, HongmL, and GL are shareholders of Fujian Akeylink Biotechnology, Co., Ltd. FH, ZH, HongmL, and GL are employees and shareholders of Fujian Cosunter Pharmaceutical Co., Ltd. ZW, XiaC, ML, XT, and ZhuL from Tigermed and HF from Zenith Medical Research provided paid expert service and received consulting fees from Fujian Akeylink Biotechnology. All other authors declare no competing interests.
Acknowledgements
This study was supported by Fujian Akeylink Biotechnology Co., Ltd. We thank all investigators who performed the study (as listed in the appendix p 2), and all patients who volunteered for this trial, as well as their families, and the study site personnel for their contributions. We also thank the members of the independent data and safety monitoring board for their dedication and their diligent review of the data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102582.
Contributor Information
Hongzhou Lu, Email: luhongzhou@fudan.edu.cn.
George Zhang, Email: george.zhang@akeylink.cn.
John Mao, Email: jmao@jwmpharma.com.
Xiaochun Chen, Email: chenxc998@fjmu.edu.cn.
Yangqing Zhan, Email: zhan071119@163.com.
Ling Lin, Email: linl6@163.com.
Tianxiang Zhang, Email: zhangtianxiang@akeylink.cn.
Yanan Tang, Email: tangyanan@cosunter.com.
Feng Lin, Email: lin_fenghn@126.com.
Feiyue Zhu, Email: hnldzfy@126.com.
Yuanlong Lin, Email: 553039465@qq.com.
Yiming Zeng, Email: zeng_yi_ming@126.com.
Kaiyu Zhang, Email: kaiyu@jlu.edu.cn.
Wenfang Yuan, Email: 413699442@qq.com.
Zhenyu Liang, Email: 490458234@qq.com.
Ruilin Sun, Email: sunruilin213@126.com.
Liya Huo, Email: hly0311@sina.com.
Peng Hu, Email: hp_cq@163.com.
Yihua Lin, Email: yhlin_xm@163.com.
Xibin Zhuang, Email: zxbqz@163.com.
Zhaohui Wei, Email: zhaohui.wei@tigermedgrp.com.
Xia Chen, Email: connie.chen@tigermedgrp.com.
Wenhao Yan, Email: yanwenhao@akeylink.cn.
Xiuping Yan, Email: yanxiuping@akeylink.cn.
Lisa Mu, Email: lisha.mu@tigermedgrp.com.
Zhuhua Lin, Email: Ella.Lin@tigermedgrp.com.
Xinyu Tu, Email: tuxinyu1997@gmail.com.
Hongshan Tan, Email: tanhongshan@akeylink.cn.
Fuhu Huang, Email: hfh@cosunter.com.
Zhiqiang Hu, Email: huzhiqiang@cosunter.com.
Hongming Li, Email: lihongming@cosunter.com.
Guoping Li, Email: Lgp@cosunter.com.
Haijun Fu, Email: hadrian.fu@zenithcro.com.
Zifeng Yang, Email: jeffyah@163.com.
Xinwen Chen, Email: chen_xinwen@gzlab.ac.cn.
Fu-Sheng Wang, Email: fswang302@163.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Appendix A. Supplementary data
References
- 1.Hachmann N.P., Miller J., Collier A.Y., et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Guo Y., Iketani S., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song S., Ma L., Zou D., et al. The global landscape of SARS-CoV-2 genomes, variants, and haplotypes in 2019nCoVR. Dev Reprod Biol. 2020;18(6):749–759. doi: 10.1016/j.gpb.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao W.M., Song S.H., Chen M.L., et al. The 2019 novel coronavirus resource. Yi Chuan. 2020;42(2):212–221. doi: 10.16288/j.yczz.20-030. [DOI] [PubMed] [Google Scholar]
- 5.Gong Z., Zhu J.W., Li C.P., et al. An online coronavirus analysis platform from the National Genomics Data Center. Zool Res. 2020;41(6):705–708. doi: 10.24272/j.issn.2095-8137.2020.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu D., Yang X., Tang B., et al. Coronavirus GenBrowser for monitoring the transmission and evolution of SARS-CoV-2. Brief Bioinform. 2022;23(2):bbab583. doi: 10.1093/bib/bbab583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Center for Disease Control and Prevention . 2023. Report on epidemic of SARS-CoV-2 in China.https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202309/t20230906_269361.html [Google Scholar]
- 8.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Z., Gao W., Bao H., et al. VV116 versus nirmatrelvir-ritonavir for oral treatment of covid-19. N Engl J Med. 2023;388(5):406–417. doi: 10.1056/NEJMoa2208822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukae H., Yotsuyanagi H., Ohmagari N., et al. Efficacy and safety of Ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76(8):1403–1411. doi: 10.1093/cid/ciac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maimeri N., Marmiere M., Losiggio R., et al. Interventions reducing mortality in COVID-19 patients: a systematic review of randomized evidence. Minerva Med. 2023;115:61. doi: 10.23736/S0026-4806.23.08590-7. [DOI] [PubMed] [Google Scholar]
- 15.Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial [published correction appears in Lancet Glob Health. 2022;10(4):e481] [published correction appears in Lancet Glob Health. 2022 Sep;10(9):e1246] Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfizer reports additional data on Paxlovid supporting upcoming new drug application submission to U.S. FDA. 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting
- 17.Butler C.C., Hobbs F.D.R., Gbinigie O.A., et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–293. doi: 10.1016/S0140-6736(22)02597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L.M., Bafadhel M., Dorward J., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial [published correction appears in Lancet. 2021 Aug 18] Lancet. 2021;398(10303):843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yotsuyanagi H., Ohmagari N., Doi Y., et al. Efficacy and safety of 5-day oral Ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial. JAMA Netw Open. 2024;7(2) doi: 10.1001/jamanetworkopen.2023.54991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan X., Dai X., Ling Y., et al. Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study. Lancet Infect Dis. 2023;24:129. doi: 10.1016/S1473-3099(23)00577-7. [DOI] [PubMed] [Google Scholar]
- 21.Cao B., Wang Y., Lu H., et al. Oral Simnotrelvir for adult patients with mild-to-moderate covid-19. N Engl J Med. 2024;390(3):230–241. doi: 10.1056/NEJMoa2301425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y., Lin Z., Liang J., et al. Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial. eClinicalMedicine. 2023;67 doi: 10.1016/j.eclinm.2023.102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G., Mao J., He H., et al. Discovery of GST-HG171, A potent and selective oral 3CL protease inhibitor for the treatment of COVID-19. SM J Infect Dis. 2023;6:9. [Google Scholar]
- 24.Zhang H., Zhou J., Chen H., et al. Phase I study, and dosing regimen selection for a pivotal COVID-19 trial of GST-HG171. Antimicrob Agents Chemother. 2023;68:e0111523. doi: 10.1128/aac.01115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diagnosis and treatment protocol for COVID-19 (trial version 10) issued by the national health commission of the People's Republic of China. 2023. [Google Scholar]
- 26.Lan K.K.G., DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 27.O'Brien P.C., Fleming T.R. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Gottlieb R.L., Vaca C.E., Paredes R., et al. Early Remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fajnzylber J., Regan J., Coxen K., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown E.R., O'Brien M.P., Snow B., et al. A prospective study of key correlates for household transmission of severe acute respiratory syndrome coronavirus 2. Open Forum Infect Dis. 2023;10(7) doi: 10.1093/ofid/ofad271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.