Abstract
Objective
To investigate the prevalence of sarcopenia and its impact on clinical outcomes in patients with esophageal, gastric, or colorectal cancer (EC, GC, and CRC) receiving neoadjuvant therapy through Meta-analysis.
Methods
We searched the PubMed, Embase databases, and Cochrane Library for the prevalence of sarcopenia and its impact on clinical outcomes in EC, GC, or CRC patients treated with neoadjuvant therapy (NAT) from inception to November 2022. The primary endpoints were the prevalence of sarcopenia and overall survival in patients with EC, GC, or CRC treated with NAT. Secondary outcomes included recurrence-free survival, total postoperative complications, grade 3–4 chemotherapy toxicity, and 30-day mortality after surgery.
Results
Thirty-one retrospective studies with 3651 subjects were included. In a fixed-effects model, the prevalence of muscle loss was higher in patients with EC, GC, or CRC at 50% (95% CI = 42% to 58%). The results of the multivariate analysis showed that preoperative patients with sarcopenia had a 1.91 times shorter overall survival (95% CI = 1.61–2.27) and a 1.77 times shorter recurrence-free survival time (95% CI = 1.33–2.35) than patients without sarcopenia, and that patients with sarcopenia had a higher risk of total postoperative complications than patients without sarcopenia OR = 1.27 (95% CI = 1.03–1.57). However, the two groups had no statistical difference in grade 3–4 chemotherapy toxicity (P = 0.84) or 30-d postoperative mortality (P = 0.88).
Conclusions
The prevalence of sarcopenia in patients with EC, GC, or CRC during NAT is high, and it is associated with poorer clinical outcomes. Clinicians should closely monitor the changes in patients’ body composition and guide patients to carry out a reasonable diet and appropriate exercise to improve their poor prognosis and quality of life.
Systematic review registration
CRD42023387817.
Keywords: Esophageal cancer, Gastric cancer, Colorectal cancer, Sarcopenia, Neoadjuvant therapy, Clinical outcomes
Introduction
Gastrointestinal cancer is one of the most essential malignant diseases threatening people's health. According to related reports, the global incidence of esophageal, gastric, and colorectal cancer (EC, GC, and CRC) ranks seventh, third, and fifth, respectively.1 The case fatality rate ranked sixth, second, and fourth, respectively. Neoadjuvant therapy (NAT) is an essential part of the comprehensive treatment of gastrointestinal cancer. It has many advantages, such as reducing tumor size, improving tumor staging, improving the success rate of surgical resection, improving the overall survival rate, and so on. In the course of NAT for patients with EC, GC, and CRC, a series of related adverse reactions such as nausea, vomiting, and loss of appetite can lead to reduced food intake, weight loss, and even cancer cachexia, resulting in muscle being unable to absorb enough energy, resulting in sarcopenia.2 The prevalence rate of sarcopenia in patients with gastrointestinal cancer is 12%–78%.3 The European Working Group on Sarcopenia 2019 updated the definition of sarcopenia, which is a syndrome characterized by low muscle strength, decreased skeletal muscle mass and quantity, and decreased physical mobility, leading to adverse outcomes such as disability, poor quality of life, and death.4 Sarcopenia hurts the quality of life, reduces the tolerance to anticancer therapy, and increases the risk of chemotherapy toxicity.5,6 Pedrosa et al.7 found that chemotherapy affects the regulation of multiple molecular pathways in skeletal muscle. Muscle atrophy and growth result from the balance of these pathways during and after chemotherapy. The catabolic pathway overcomes the anabolic pathway, aggravating the muscular dystrophy that often occurs in cancer patients. Studies by Tantai et al.8 have shown that the severity and duration of muscular dystrophy in patients with liver cirrhosis are significantly correlated with increased mortality, seriously affecting the cumulative survival time of patients.
The diagnosis and intervention of sarcopenia are often carried out after surgery.9, 10, 11 However, some studies have shown that the effect of diet and exercise interventions on sarcopenia diagnosed before the surgery is better than that diagnosed after the surgery.12,13 Most studies explore the effect of postoperative diagnosis of sarcopenia on the prognosis of patients with gastrointestinal cancer.14, 15, 16 There are few studies on the prognosis of gastrointestinal cancer patients with NAT who were diagnosed with sarcopenia before the surgery. Some studies have shown that improving the patient's ability to cope with stress, such as with chemotherapy and surgery with pre-rehabilitation, can reduce chemotherapy and postoperative complications.17 Given the limitations of the above study, the purpose of this study is to conduct a meta-analysis. To investigate the prevalence of sarcopenia and its effect on clinical outcomes in patients with EC, GC, and CRC, who received NAT before surgery.
Methods
The protocol for this meta-analysis is registered on PROSPERO (submitted for registration) with ID number CRD42023387817 and adheres to the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (checklists can be found in Supplementary file 1).
Inclusion criteria
(1) A Cohort study or a retrospective study; (2) patients aged 18 years or older with EC, GC, or CRC receiving NAT (neoadjuvant chemotherapy or radiotherapy); (3) skeletal muscle assessment before and after NAT, and a second assessment must be completed before additional treatment such as surgery or postoperative chemotherapy; (4) appropriate changes in skeletal muscle data were reported (specific values or rates of change before and after NAT were provided), as well as clinical outcomes (e.g., survival, postoperative complications, adverse effects of chemotherapy, mortality); (5) published in English only.
Exclusion criteria
(1) The study design was an animal study; (2) reviews, case reports, conference abstracts, unpublished data, and duplicate publications.
Literature search
The search strategies were based on keywords and the medical subject headings (Mesh) according to the PICO framework. The keywords for the literature search were as follows: P: “colorectal neoplasms” [Mesh] OR “esophageal neoplasms” [Mesh] OR “stomach neoplasms” [Mesh]; I: “drug therapy” [Mesh Terms] OR “therapy drug” [Title/Abstract] OR “drug therapies” [Title/Abstract] OR “therapies drug” [Title/Abstract] OR “chemotherapy” [Title/Abstract] OR “chemotherapies” [Title/Abstract] OR “pharmacotherapy” [Title/Abstract] OR “pharmacotherapies” [Title/Abstract] OR “radiotherapy” [Title/Abstract]; O: “sarcopenia” [MeSH Terms] OR “sarcopenias” [Title/Abstract] OR “muscle mass” [Title/Abstract] OR “muscle loss” [Title/Abstract] OR “muscle strength” [Title/Abstract] OR “muscle wasting” [Title/Abstract] OR “muscular atrophy” [Mesh Terms]. Details are provided in Supplementary file 2.
Study selection and data extraction
Literature screening and data extraction were performed independently by two researchers who had received systematic evidence-based training, and a third researcher judged whether there was disagreement. EndNote X9 was used to deduplicate and preliminary screen the acquired literature. The full text of the literature that met the inclusion criteria was carefully read to determine the included literature. A unified table was used to extract data, including the name of the first author, publication year, country, tumor type, clinical stage, neoadjuvant chemotherapy regimen, mean age, treatment method, measurement tool, cutoff value for diagnosis of sarcopenia, prevalence of sarcopenia during NAT, and clinical outcomes (over survival, postoperative total complications, grade 3 to 4 chemotherapy toxicity, 30-day mortality).
Quality assessment
The quality evaluation was conducted by two researchers independently, and the evaluation results were checked. If there were different opinions, the third researcher was involved. The cohort study was scored according to the Newcastle Ottawa Scale (NOS), and the evaluation content included the selection of the study population (4 items in total, with a full score of 4 points); inter-group comparability (1 item, full score of 2 points); results/measurement of exposure factors (3 items in total, full score of 3 points). The total score is 9 points; a score of 5 or less is considered low quality; a score of 6 or 7 is considered medium quality; and a score of 8 or 9 is considered high quality.
Statistical analysis
Continuous data were expressed as mean ± standard deviation, and the mean prevalence of sarcopenia, which refers to the proportion of people diagnosed with sarcopenia during NAT in the total sample, was analyzed using a random effects model. Multivariate Cox proportional hazards regression analysis was used to study the effect of sarcopenia on overall survival in the original literature. The hazard ratio (HR) and 95% confidence interval (95% Cl) of OS and recurrence-free survival (RFS) were extracted from the literature to pool effect sizes. Odds ratio (OR) and 95% confidence intervals for total postoperative complications, grade 3–4 chemotherapy toxicity, and 30-day mortality were extracted for effect size pooling. Heterogeneity was assessed using I2, such as I2 < 50% and P > 0.1 using the fixed effects model, and vice versa using the random effects model.18 After confirming the correctness of the extracted data, subgroup analysis was performed according to the characteristics of the included studies to reduce heterogeneity, and sensitivity analysis was performed to test the stability of the results. Egger's test was used to detect publication bias; if Egger's test P < 0.05, further clipping and fill analysis were performed. STATA 17 and RevMan 5.4 software were used for analysis.
Results
Search results
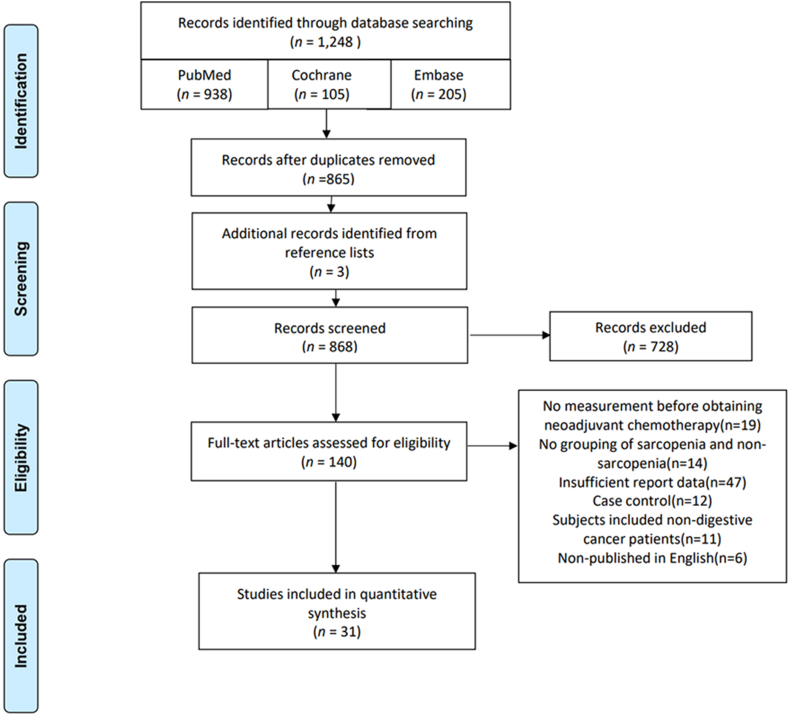
The initial search yielded 1248 articles, and after removing duplicates, reading titles and abstracts, and the unavailability of the full text, 137 articles remained, excluding nine articles that were not measured before neoadjuvant therapy, 14 articles that were not grouped for sarcopenia versus non-sarcopenia, 47 articles that reported insufficient data, 12 case–control, 11 studies that included patients with other types of cancer, 6 articles that were not published in English, 28 articles that remained, and three articles that were manually searched, resulting in a total of 31 articles19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 included (Fig. 1).
Fig. 1.
PRISMA search flow diagram. PRISMA, Reporting Items for Systematic reviews and Meta-Analyses.
Study characteristic and quality assessment
A total of 31 studies involving 3651 subjects were included. All of them were retrospective studies, and 15 studies19,20,23,24,29, 30, 31,33, 34, 35, 36,39,40,42,48 were from the Asian population. Thirteen articles were from the European population,21,22,25,27,28,37,38,41,43, 44, 45, 46,49 two articles26,32 were from South America, and one47 was from Oceania. The average age of the included population in 30 articles19, 20, 21, 22, 23, 24, 25,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 was more than 60 years old, and the average age of only one article26 was 56.2 years old. Among them, 24 articles were on esophageal cancer,19, 20, 21,23, 24, 25,29, 30, 31,33, 34, 35, 36,38, 39, 40, 41, 42, 43, 44, 45, 46, 47,49 two articles.27,37 were gastroesophageal junction cancer, two articles22,32 were gastric cancer, three articles were colorectal cancer,26,28,48 and one48 was rectal cancer. Neoadjuvant chemotherapy was used in 20 articles,19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33,35,37,43,44,47 neoadjuvant radiotherapy in three articles,38, 39, 40 neoadjuvant chemotherapy combined with radiotherapy in one article45 and neoadjuvant chemotherapy or radiotherapy in seven articles.34,36,41,42,46,48,49 In addition, computed tomography (CT) was the most commonly used tool to measure muscle mass, and only one study35 used bioelectrical impedance analysis (BIA), 11 studies19, 20, 21, 22,24,29,33,34,36,39,42 used psoas muscle index as the diagnostic criterion, 19 studies25, 26, 27, 28,30, 31, 32,35,37,38,40,41,43, 44, 45, 46, 47, 48, 49 used skeletal muscle index as the diagnostic criterion, and only one study23 used skeletal muscle mass, the characteristics of the included studies are detailed in Table 1, Table 2.
Table 1.
Characteristics of included studies.
| Author, year, country | Cancer type, stage | Neoadjuvant chemotherapy | Age (year) | Therapy method | Muscle assessment | Cut offs for sarcopenia (cm2/m2) |
|---|---|---|---|---|---|---|
| Ishida, 2021, Japan |
EC I-IV |
DCF or ACF | 71 | NAC: 100% | CT L3 PMI | M < 6.36 F < 3.92 |
| Kamitani, 2019, Japan |
EC IB-III |
DCF or FP | 68 | NAC: 100% | CT L3 PMI | M < 52.4 F < 38.5 |
| Tan, 2014, England |
EC I-III |
FP or ECX | 68.6 | NAC: 100% | CT L3 PMI | M < 52.4 F < 38.5 |
| Palmela, 2017, Portugal |
GC II-III |
NR | 69.3 | NAC: 100% | CT L3 PMI | M < 43 (BMI < 25 kg/m2); 53 (BMI ≥ 25 kg/m2) F < 41 |
| Miyata, 2017, Japan |
EC I-IV |
ACF or DCF | 64.2 | NAC: 100% | BIA skeletal muscle mas |
< 90% of standard |
| Onishi, 2022, Japan |
EC II-III |
FP/DCF | 66.2 | NAC: 100% | CT L3 PMI | M < 6.36 F < 3.92 |
| Yip, 2014, England |
EC I-IV |
5-FU or platinum or EXC | 63 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Okuno, 2018, America |
CRC NR |
NR | 56.2 | NAC: 100% | CT L3 SMI | M < 43 (BMI < 25 kg/m2); 53 (BMI ≥ 25 kg/m2) F < 41 |
| Boer, 2020, England |
EC I-III |
NR | 66.1 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Eriksson, 2016, Sweden |
CRC NR |
NR | 67.3 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Ishida, 2019, Japan |
EC I-IV |
DCF or ACF | 66.7 | NAC: 100% | CT L3 PMI | M < 6.36 F < 3.92 |
| Mayanagi, 2017, Japan |
EC II-III |
Platinum + fluorouracil | 63.3 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Harada, 2022, Japan |
EC III-VI |
FP or DCF | 71.1 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Mirkin, 2017, America |
GC NR |
DCF or ECX or ECF or other | 64.5 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Ishibashi, 2019, Japan |
EC II-III |
FP | 68.3 | NAC: 100% | CT L3 PMI | M < 6.36 F < 3.92 |
| Ozawa, 2019, Japan |
EC T1-3N0-3 |
Cisplatinum + 5-FU | 63.5 | NAC: 46% NCRT: 54% |
CT L3 PMI | M < 6.36 F < 3.92 |
| Kita, 2021, Japan |
EC T1-4N0-3 |
PAF | 62.8 | NAC: 100% | CT L3 SMI | 25th cut off |
| Nakayama, 2021, Japan |
EC II-III |
FP or DCF | 66.3 | NAC: 84% NCRT: 16% |
CT L3 PMI | M < 6.36 F < 3.92 |
| Awad, 2011, England |
EC T1-4N0-3 |
ECF or capecitabine/cisplatin or epirubicin/oxaliplatin | 63 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Hagens, 2019, Netherlands |
EC T1-4N0-3 |
Carboplatin + paclitaxel | 63.7 | NCRT: 100% | CT L3 SMI | M < 43 (BMI < 25 kg/m2); 53 (BMI ≥ 25 kg/m2) F < 41 |
| Kawakita, 2019, Japan |
EC T1-4N0-3 |
Cisplatin/nedaplatin + 5-FU | 64 | NCRT: 100% | CT L3 PMI | M < 3.85 F < 2.42 |
| Yoon, 2020, Korea |
EC T1-4N0-3 |
Cisplatin + 5-FU | 63.5 | NCRT: 100% | CT L3SMI | M < 52.4 F < 38.5 |
| Järvinen, 2018, Finland |
EC I-III |
EOX | 63 | NAC: 76% NCRT: 24% |
CT L3SMI | M < 52.4 F < 38.5 |
| Liu, 2016, Japan |
EC T1-4N0-3 |
5-FU + cisplatin/nedaplatin | 62.2 | NAC: 76% NCRT: 24% |
CT L3 PMI | NR |
| Reisinger, 2015, Netherlands |
EC I-IV |
CF or ECC or PC | 63 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Grün, 2020, Germany |
EC T1-4N0-3 |
NR | 67.4 | NAC: 100% | CT L3 SMI | M < 52.4 F < 38.5 |
| Panje, 2019, Netherlands |
EC II-IV |
Docetaxe + cisplatin | 61 | NAC + NCRT | CT L3 SMI | M < 43 (BMI < 25 kg/m2); 53 (BMI ≥ 25 kg/m2) F < 41 |
| Elliot, 2017, Ireland |
EC I-III |
Cisplatin + 5-FU or carboplatin + paclitaxel | 61.6 | NAC: 32% NCRT: 68% |
CT L3 SMI | M < 52.4 F < 38.5 |
| Paireder, 2016, Australia |
EC I-III |
NR | 61.4 | NAC: 100% | CT L3 SMI | M < 55.0 F < 39.0 |
| Fukuoka, 2019, Japan |
RC I-III |
mFOLFOX6 or XELOX or XELOX + Cetuximab | 66 | NAC: 43% NCRT: 57% |
CT L3 SMI | M < 6.36 F < 3.92 |
| Yassaie, 2019, Netherlands |
EC NR |
MAGIC or carboplatin/paclitaxel | 65.8 | NAC: 89% NCRT: 11% |
CT L3 SMI | M < 6.36 F < 3.92 |
5-FU, 5-fluorouracil; ACF, adriamycin + cisplatin + 5-FU; CF, cisplatin + 5-FU; CRC, colorectal cancer; DCF, docetaxel + cisplatin + 5-FU; EC, esophagus cancer; ECC, epirubicin + cisplatin + capecitabine; ECF, epirubicin + cyclophosphamide + 5-FU; ECX, cisplatin + 5-FU + capecitabine; EOX, epirubicin + oxaliplatin + capecitabine; FP, cisplatin + 5-FU; GC, gastric carcinoma; MAGIC, epirubicin + cisplatin + capecitabine; PAF, cisplatin + adriamycin + 5-FU; PC, paclitaxel + carboplatin; PMI, psoas major muscle index; RC, rectal cancer; SMI, skeletal muscle index.
Table 2.
Main clinical outcomes included in meta-analysis.
| Author, year | Prevalence |
Clinical outcome |
|||||
|---|---|---|---|---|---|---|---|
| Sample (n) | Sarcopenia (n) | OS | RFS | Postoperative total complications |
Grade 3 to 4 chemotherapy toxicity |
30-day mortality |
|
| (Sarcopenia/Non-Sarcopenia) | |||||||
| Ishida, 2021 | 333 | 37 | 1.68 (1.07–2.66) | 25/114 | |||
| Kamitani, 2019 | 90 | 72 | 2.49 (1.12–5.53) | 48/6 | 29/3 | ||
| Tan, 2014 | 89 | 44 | 24/13 | ||||
| Palmela, 2017 | 47 | 11 | |||||
| Miyata, 2017 | 94 | 44 | 6/5 | ||||
| Onishi, 2022 | 175 | 139 | 2.92 (0.86–9.96) | 44/13 | |||
| Yip, 2014 | 35 | 9 | |||||
| Okuno, 2018 | 169 | 22 | 1.82 (1.07–3.10) | 1.82 (1.07–3.10) | 17/94 | ||
| Boer, 2020 | 199 | 91 | 1/1 | ||||
| Eriksson, 2016 | 97 | 50 | 5.99 (2.43–14.79) | 18/8 | |||
| Ishida, 2019 | 165 | 43 | 29/38 | ||||
| Mayanagi, 2017 | 66 | 55 | |||||
| Harada, 2022 | 150 | 23 | 2.49 (1.12–5.53) | 13/55 | |||
| Mirkin, 2017 | 36 | 12 | 6/6 | ||||
| Ishibashi, 2019 | 85 | 54 | |||||
| Ozawa, 2019 | 82 | 21 | |||||
| Kita, 2021 | 87 | 65 | 10/10 | ||||
| Nakayama, 2021 | 93 | 47 | 10/4 | ||||
| Awad, 2011 | 47 | 27 | |||||
| Hagens, 2019 | 322 | 125 | 1.81 (1.30–2.52) | 103/63 | |||
| Kawakita, 2019 | 113 | 27 | 5.45 (2.48–11.99) | 2.36 (1.23–4.53) | 13/28 | 13/43 | |
| Yoon, 2020 | 248 | 156 | 2.30 (1.42–3.73) | 1.57 (1.07–2.32) | |||
| Järvinen, 2018 | 115 | 92 | 62/17 | 3/0 | |||
| Liu, 2016 | 84 | 42 | 2.44 (0.93–6.36) | 20/15 | 1/1 | ||
| Reisinger, 2015 | 123 | 16 | 6/5 | ||||
| Grün, 2020 | 52 | 31 | |||||
| Panje, 2019 | 61 | 31 | 15/22 | ||||
| Elliot, 2017 | 207 | 49 | 39/98 | ||||
| Paireder, 2016 | 130 | 80 | 1.72 (1.05–2.83) | ||||
| Fukuoka, 2019 | 47 | 15 | 15/32 | ||||
| Yassaie, 2019 | 53 | 33 | 8/0 | ||||
OS, overall survival; RFS, recurrence-free survival.
The Newcastle–Ottawa Scale demonstrated high quality for seven studies,19,20,32,35,45,47,48 and moderate quality for 23 studies21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31,33,34,36, 37, 38, 39, 40, 41, 42, 43, 44,46 (Table 3).
Table 3.
Quality assessment for included studies based on the Newcastle–Ottawa Scale.
| Author, year | Select | Comparability | Result | Total |
|---|---|---|---|---|
| Ishida, 2021 | ★★★★ | ★★ | ★★ | 8 |
| Kamitani, 2019 | ★★★★ | ★★ | ★★ | 8 |
| Tan, 2014 | ★★★★ | ★ | ★★ | 7 |
| Palmela, 2017 | ★★★★ | ★ | ★★ | 7 |
| Miyata, 2017 | ★★★★ | ☆ | ★★ | 6 |
| Onishi, 2022 | ★★★★ | ★ | ★★ | 7 |
| Yip, 2014 | ★★★ | ★ | ★★ | 6 |
| Okuno, 2018 | ★★★★ | ★ | ★★ | 7 |
| Boer, 2020 | ★★★★ | ★ | ★ | 6 |
| Eriksson, 2016 | ★★★★ | ★ | ★ | 6 |
| Ishida, 2019 | ★★★★ | ★ | ★★ | 7 |
| Mayanagi, 2014 | ★★★★ | ★ | ★ | 6 |
| Harada, 2022 | ★★★★ | ★ | ★★ | 7 |
| Mirkin, 2017 | ★★★★ | ★ | ★★★ | 8 |
| Ishibashi, 2019 | ★★★★ | ★ | ★ | 6 |
| Ozawa, 2019 | ★★★★ | ☆ | ★★ | 6 |
| Kita, 2021 | ★★★★ | ★ | ★★★ | 8 |
| Nakayama, 2021 | ★★★★ | ★ | ★★ | 7 |
| Awad, 2011 | ★★★★ | ★ | ★★ | 7 |
| Hagens, 2019 | ★★★★ | ★ | ★ | 6 |
| Kawakita, 2019 | ★★★★ | ★ | ★★★ | 8 |
| Yoon, 2020 | ★★★★ | ★ | ★★ | 7 |
| Järvinen, 2018 | ★★★★ | ★ | ★ | 6 |
| Liu, 2016 | ★★★★ | ★ | ★ | 6 |
| Reisinger, 2015 | ★★★★ | ★ | ★ | 6 |
| Grün, 2020 | ★★★★ | ★ | ★ | 6 |
| Panje, 2019 | ★★★★ | ★ | ★★★ | 8 |
| Elliot, 2017 | ★★★★ | ★ | ★★ | 7 |
| Paireder, 2016 | ★★★★ | ★★ | ★★★ | 9 |
| Fukuoka, 2019 | ★★★★ | ★★ | ★★★ | 9 |
| Yassaie, 2019 | ★★★★ | ★ | ★★ | 7 |
The prevalence of sarcopenia during neoadjuvant therapy in patients with esophageal, gastric, or colorectal cancer
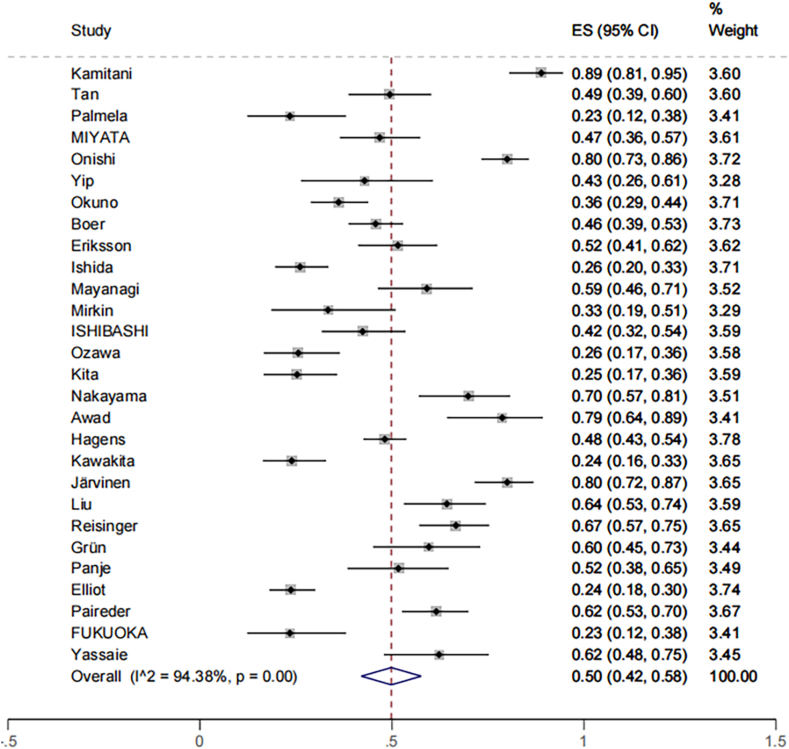
Meta-analysis of the prevalence data of sarcopenia in 3651 participants from 28 studies showed that the average prevalence of sarcopenia during NAT in patients with EC, GC, or CRC was 50% (95% CI = 42% to 58%), and there was significant heterogeneity among the studies (I2 = 94.38%; P < 0.001) (Fig. 2). The results showed that the prevalence of sarcopenia was 49.7% (95% CI = 41.5% to 68.2%) in patients with EC, GC, or CRC who were older than 65 years of age. It was higher than the average prevalence of patients under 65 years old, 46.7% (95% CI = 37.1% to 56.3%), but there was no statistical significance (P > 0.05). The results of subgroup analysis by region showed that the average prevalence of people from Asia 47% (95% CI = 34.3% to 59.8%) was slightly lower than that of people from other regions 51% (95% CI = 42.1% to 59.9%). However, the difference was not statistically significant (P > 0.05). The prevalence of sarcopenia in male patients with EC, GC, or CRC was 39.3% (95% CI = 30.0% to 48.5%) and 35.3% (95% CI = 22.4% to 48.2%) in female patients, and the difference was not statistically significant (P > 0.05). The average prevalence estimated by the method of measuring the skeletal muscle index (SMI) at the L3 level and the psoas major muscle index (PMI) at the L3 level was 50.7% (95% CI = 41.2% to 60.1%) and 47.4% (95% CI = 27.6% to 67.2%), respectively, with no significant difference (Table 4).
Fig. 2.
The prevalence of sarcopenia preoperatively during NAT in patients with EC, GC, or CRC. CRC, colorectal cancer; EC, esophageal; GC, gastric; NAT, neoadjuvant therapy.
Table 4.
Subgroup analysis of the mean prevalence of sarcopenia in patients with gastrointestinal cancer.
| Subgroup | Study (n) | Prevalence rate (%) | 95% CI | Heterogeneity across the studies |
Heterogeneity between groups (P-value) | |
|---|---|---|---|---|---|---|
| I2 | P | |||||
| Age (year) | ||||||
| < 65 | 17 | 46.7 | 37.1–56.3 | 94.68% | < 0.001 | 0.376 |
| > 65 | 11 | 49.7 | 41.5–68.2 | 95.89% | < 0.001 | |
| Studying regional | ||||||
| Asia | 15 | 47.0 | 34.3–59.8 | 97.50% | < 0.001 | 0.619 |
| Other | 16 | 51.0 | 42.1–59.9 | 96.23% | < 0.001 | |
| Gender | ||||||
| Male | 23 | 39.3 | 30.0–48.5 | 96.68% | < 0.001 | 0.628 |
| Female | 23 | 35.3 | 22.4–48.2 | 95.64% | < 0.001 | |
| Skeletal muscle index | ||||||
| SMI | 20 | 50.7 | 41.2–60.1 | 96.05% | < 0.001 | 0.769 |
| PMI | 7 | 47.4 | 27.6–67.2 | 96.04% | < 0.001 | |
PMI, psoas major muscle index; SMI, skeletal muscle index.
Relationship between sarcopenia during neoadjuvant therapy and clinical outcomes
The relationship between sarcopenia during neoadjuvant therapy and overall survival
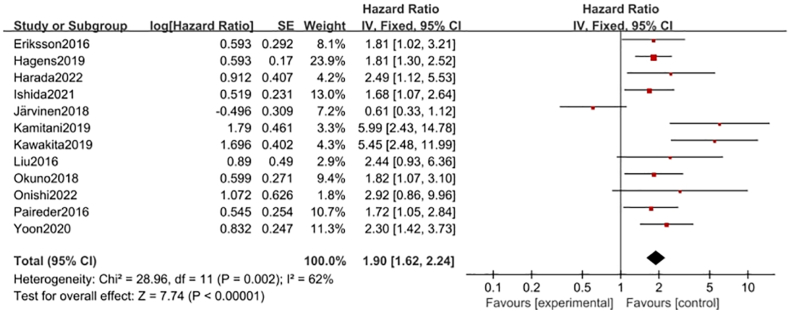
Twelve studies19,20,24,26,28,31,38, 39, 40, 41, 42,47 showed the relationship between preoperative NAT and OS in patients with EC, GC, or CRC. Multivariate Cox proportional regression survival analyses of the association between sarcopenia and OS in these studies were pooled. The results showed that the risk of shortened overall survival in patients with sarcopenia was 1.91 times that in patients without sarcopenia (HR = 1.91, 95% CI = 1.61–2.27, Z = 7.42, P < 0.001; heterogeneity test I2 = 65%, P = 0.002; Fig. 3). Further sensitivity analysis found that the heterogeneity of Jarvinen41 was high, and the deletion of this article had no effect on the results of the study (HR = 2.11, 95% CI = 1.77–2.52, Z = 8.21, P < 0.001; heterogeneity test I2 = 30%, P = 0.16). The metaninf command was used to conduct sensitivity analysis to explore the robustness of the meta-analysis results. The results showed that no study significantly affected the stability of the combined effect size (Fig. S1), and the funnel plot showed no high publication bias in each study (Egger's test, P = 0.200 > 0.05, Fig. S2).
Fig. 3.
Forest plots of the relationship between sarcopenia preoperative during NAT and overall survival (multivariate analysis). NAT, neoadjuvant therapy.
The relationship between sarcopenia during neoadjuvant therapy and recurrence-free survival
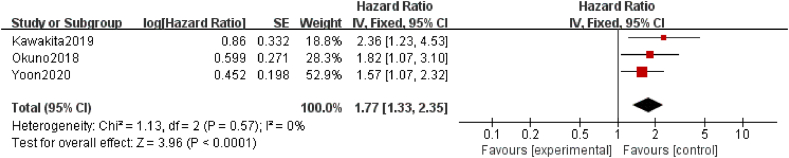
The effect of sarcopenia on RFS was reported in three studies26,39,40 included in this meta-analysis. In patients with EC, GC, or CRC, the meta-analysis revealed a significant reduction in RFS among patients with sarcopenia compared with those without sarcopenia (HR = 1.77; 95% CI = 1.33–2.35, P < 0.001; heterogeneity test I2 = 0%, P = 0.57; Fig. 4).
Fig. 4.
Forest plots of the relationship between sarcopenia preoperative during NAT and recurrence-free survival (multivariate analysis). NAT, neoadjuvant therapy.
Relationship between sarcopenia during neoadjuvant therapy and postoperative outcomes
The relationship between sarcopenia during neoadjuvant therapy and postoperative total complications
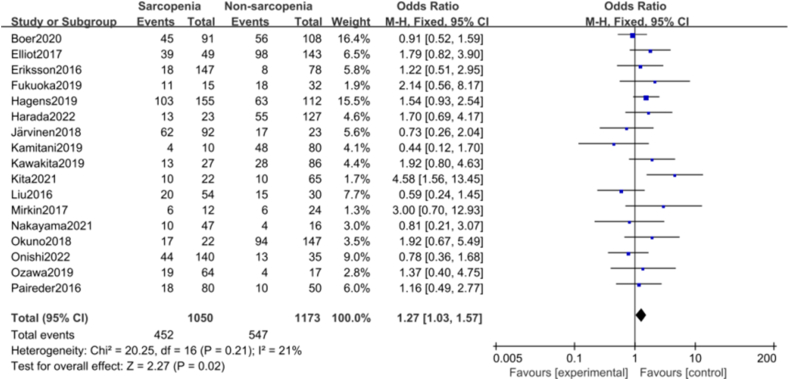
There were 17 studies on the effect of intraoperative muscle loss on total postoperative complications of NAT.20,24,26, 27, 28,31,32,34, 35, 36,38,39,41,42,46, 47, 48 The pooled results of the fixed effect model showed that sarcopenia was significantly associated with total postoperative complications (OR = 1.27; 95% CI = 1.03–1.57, Z = 2.27, P = 0.02; heterogeneity test I2 = 21%, P = 0.21 > 0.1; Fig. 5). No studies that significantly affected the stability of the combined effect size were found (Fig. S3). The funnel plot showed no high publication bias in each study (Egger's test, P = 0.710 > 0.05, Fig. S4).
Fig. 5.
Forest plots of the relationship between sarcopenia preoperative during NAT and postoperative total complications. NAT, neoadjuvant therapy.
The relationship between sarcopenia during neoadjuvant therapy and grade three to four chemotherapy toxicity
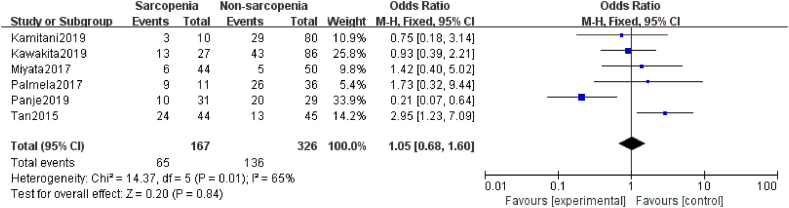
A total of six studies20, 21, 22, 23,39,45 elucidated the relationship between sarcopenia and grade 3–4 chemotherapy toxicity. One21 study supported that sarcopenia during neoadjuvant therapy may be a predictor of increased incidence of grade 3–4 chemotherapy toxicity in patients with EC, GC, or CRC. In contrast, the remaining five studies20,22,23,39,45 did not support this finding. However, the results of the meta-analysis showed that the effect of sarcopenia on grade 3–4 chemotherapy toxicity was not statistically significant (OR = 1.05, 95% CI = 0.68–1.60, Z = 0.20, P = 0.84; heterogeneity test I2 = 65%, P = 0.01; Fig. 6).
Fig. 6.
Forest plots of the relationship between sarcopenia preoperative during NAT and grade 3/4 chemotherapy toxicity. NAT, neoadjuvant therapy.
The relationship between sarcopenia during neoadjuvant therapy and postoperative 30-day mortality
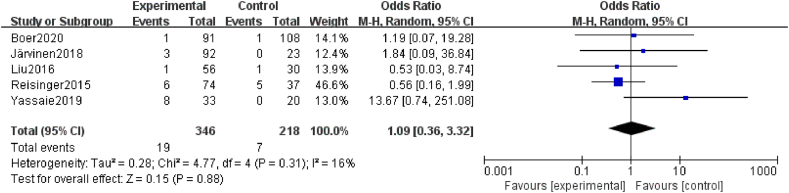
Five studies27,41, 42, 43,49 mentioned postoperative 30-day mortality, and these results all demonstrated that there were no significant differences between the sarcopenia and non-sarcopenia groups (OR = 1.09, 95% CI = 0.36–3.32, Z = 0.63, P = 0.88 > 0.05, heterogeneity test I2 = 16%, P = 0.31; Fig. 7).
Fig. 7.
Forest plots of the relationship between sarcopenia preoperative during NAT and 30-day mortality. NAT, neoadjuvant therapy.
Discussion
Clinicians increasingly consider skeletal muscle loss a new imaging biomarker, especially in diseases characterized by systemic depletion, such as cancer. Skeletal muscle loss can cause systemic metabolic damage, manifested as weakened antioxidant capacity of the body, inhibition of anabolic metabolism, insulin resistance, etc. It can ultimately lead to metabolic syndrome, malaise, dyslipidemia, etc. and poor patient prognoses.50
According to previous reports, the European Working Group on Sarcopenia (EWGSOP)51 in 2010 counted the prevalence of sarcopenia as 6%–12% globally, and the Asian Working Group on Sarcopenia (AWGS) 2019 reported that the prevalence of sarcopenia in the elderly Asian population was 5.5%–25.7%.52 Among solid tumors, the prevalence of sarcopenia was 42% in head and neck tumors,53 43% in nonsmall cell lung cancer, and about 25% in breast cancer.54,55 The results of this study show that the prevalence of sarcopenia in patients with EC, GC, or CRC is 50% (95% CI = 42% to 58%), which shows that the prevalence of sarcopenia is already high in patients with EC, GC, or CRC who received NAT before surgery. This high probability may be because EC, GC, or CRC are malignant, wasting diseases with a high prevalence incidence of obstruction or hemorrhage and that tumor progression is often associated with increased levels of systemic inflammation, decreased diet, appetite, anorexia, pain, and an increased incidence of malnutrition, all of which are associated with sarcopenia prevalence.
Research has demonstrated that changes in skeletal muscle from pretreatment to posttreatment may be a more significant prognostic factor than the pretreatment status of skeletal sarcopenia.30 Additionally, the American College of Surgeons' guidelines emphasize the importance of preoperative assessment of sarcopenia in elderly patients with gastric cancer who are undergoing surgical intervention.56 However, most studies explore the effect of postoperative diagnosis of sarcopenia on the prognosis of patients with gastrointestinal cancer. The study found a significant association between sarcopenia and impaired overall survival, shorter recurrence-free survival, and a high incidence of postoperative complications. The combined multifactorial analyses revealed that patients with preoperative combined sarcopenia had a 1.91 times shorter overall survival, a 1.77 times shorter recurrence-free survival, and a 1.27 times higher risk of postoperative total complications compared to those who were not sarcopenic. Many of the included studies reported increased muscle loss during NAT, and a significant number of patients developed sarcopenia, indicating a continuous state of change in body composition and nutritional status. During neoadjuvant therapy, tumor patients may be susceptible to complications of sarcopenia due to various reasons, such as inflammatory response, mitochondrial dysfunction, nutritional metabolism disorders, chemotherapeutic response, and changes in hormone levels.57, 58, 59 It is important to note that these reasons are objective and supported by evidence. The simultaneous presence of sarcopenia may result in delayed healing of surgical incisions, an increased risk of surgical complications, shortened overall and recurrence-free survival of patients, and an increased risk of mortality.
Moreover, sarcopenia is associated with an increased risk of falls, osteoporosis, and fractures. A cross-sectional study investigating the relationship between sarcopenia and osteoporotic vertebral compression fractures (OVCF)60 discovered that the prevalence of sarcopenia was 12.0%. Furthermore, 66.7% of patients with sarcopenia developed OVCF, indicating that individuals with sarcopenia are more vulnerable to OVCF than the general population. Sarcopenia affects not only the physical health of patients but also their self-care abilities and quality of life. Furthermore, it may be linked to an increased risk of cognitive impairment. According to a study,61 the risk of mild cognitive impairment in the sarcopenia population with a normal body mass index (BMI) was 1.84 times higher than that in the sarcopenia population with a normal BMI. It is important to note that there is a longitudinal association between sarcopenia and mental health problems. According to a cross-sectional analysis, individuals with sarcopenia were more likely to experience depressive symptoms than those without sarcopenia.62
The results of this study indicate that patients with a preoperative diagnosis of sarcopenia have a poor prognosis after undergoing neoadjuvant therapy. Therefore, we speculate that early sarcopenia prevention or treatment may improve patients' prognosis and quality of life. Currently, sarcopenia interventions mainly include nutritional management, exercise guidance, etc. Early implementation of an exercise intervention or an intervention combining exercise and nutrition is an effective strategy to avoid muscle mass loss during treatment and support cancer care. The Clinical Oncology Society of Australia (COSA)63 also proposed that exercise can help alleviate the adverse effects of cancer and its treatment; it should be part of standard practice in cancer care. Some studies64,65 have shown that exercise increases nerve conduction velocity and reduces loss of muscle strength and volume. Among them, resistance exercise, as recommended by the American College of Sports Medicine (ACSM) exercise guidelines for cancer survivors66 and the Chinese Expert consensus on Nutrition and Exercise Intervention for Sarcopenia,67 can effectively enhance muscle strength and improve physical function by increasing muscle protein synthesis, reducing inflammation, and reducing oxidative stress.68 In the nutritional management of sarcopenia, in recent years, the intake of some substances has also been emphasized, such as whey protein, branched-chain amino acids, glutathione, l-carnitine, vitamin D, etc.69 Many studies have shown that nutritional management combined with exercise can more effectively improve the limb function, activities of daily living, and nutritional status of sarcopenia patients.70, 71, 72 We believe that future research on whether early prevention or treatment of sarcopenia will improve the prognosis of patients is significant and promising. At the same time, the population receiving neoadjuvant chemotherapy is far beyond these three types of cancer patients, such as breast cancer, liver cancer, etc. and whether the occurrence of sarcopenia will also affect the prognosis of these patients remains to be confirmed.
There are some limitations in this study. First, all the documents included in this study are published in English, and most of the studies are of medium quality, which may increase the risk of bias. Secondly, there is some heterogeneity in this research literature, which may affect the results of this study. In addition, this study discussed the prevalence of sarcopenia in patients with EC, GC, or CRC who received NAT before surgery and its influence on the clinical outcome of patients. It did not analyze other influencing factors, such as the neoadjuvant chemotherapy scheme and pathological reactions.
Conclusions
This meta-analysis showed a higher prevalence of sarcopenia in EC, GC, or CRC patients during NAT and was associated with worse clinical outcomes. Monitoring changes in body composition, reasonable diet structure, and appropriate exercise are beneficial to reduce the occurrence of sarcopenia. Furthermore, for patients with sarcopenia, these measures may improve their prognosis. Therefore, the results of this study also call for clinicians to pay more attention to the possibility of sarcopenia and effective nursing measures in patients with EC, GC, or CRC during NAT.
Ethics statement
Not required.
Funding
This study received no external funding.
CRediT Authorship Contribution Statement
Lin Luo: Methodology, Writing – original draft preparation. Yidan Fan: Methodology, Formal analysis, Data curation. Yanan Wang: Formal analysis, Data curation. Zhen Wang: Visualization. Jian Zhou: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Declaration of Generative AI and AI-assisted technologies in the writing process
No AI tools/services were used during the preparation of this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.apjon.2024.100436.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xu X.Y., Jiang X.M., Xu Q., et al. Skeletal muscle change during neoadjuvant therapy and its impact on prognosis in patients with gastrointestinal cancers: a systematic review and meta-analysis. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.892935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen C., de Heer P., Bjerre E.D., et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 2018;268(1):58–69. doi: 10.1097/sla.0000000000002679. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020;21(1):7. doi: 10.1007/s11864-019-0691-9. [DOI] [PubMed] [Google Scholar]
- 6.Ryan A.M., Prado C.M., Sullivan E.S., et al. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. 2019;67–68 doi: 10.1016/j.nut.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa M.B., Barbosa S., Vitorino R., et al. Chemotherapy-induced molecular changes in skeletal muscle. Biomedicines. 2023;11(3):905. doi: 10.3390/biomedicines11030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantai X., Liu Y., Yeo Y.H., et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76(3):588–599. doi: 10.1016/j.jhep.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Min J., An K.Y., Park H., et al. Postoperative inpatient exercise facilitates recovery after laparoscopic surgery in colorectal cancer patients: a randomized controlled trial. BMC Gastroenterol. 2023;23(1):127. doi: 10.1186/s12876-023-02755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S., Meng Q., Jiang Y., et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clin Nutr. 2021;40(1):47–53. doi: 10.1016/j.clnu.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner A., Olpe T., Griot S., et al. Association of CT-based diagnosis of sarcopenia with prognosis and treatment response in patients at risk of malnutrition – a secondary analysis of the Effect of early nutritional support on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial (EFFORT) trial. Clin Nutr. 2023;42(2):199–207. doi: 10.1016/j.clnu.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Alavi D.T., Henriksen H.B., Lauritzen P.M., et al. Effect of a one-year personalized intensive dietary intervention on body composition in colorectal cancer patients: results from a randomized controlled trial. Clin Nutr ESPEN. 2023;57:414–422. doi: 10.1016/j.clnesp.2023.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Anandavadivelan P., Malberg K., Vikstrom K., et al. Home-based physical activity after treatment for esophageal cancer-A randomized controlled trial. Cancer Med. 2023;12(3):3477–3487. doi: 10.1002/cam4.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y., Hao Q., Zhou J., et al. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):188. doi: 10.1186/s12877-017-0569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura E., Matsuda S., Kawakubo H., et al. The impact of thoracic duct resection on the long-term body composition of patients who underwent esophagectomy for esophageal cancer and survived without recurrence. Dis Esophagus. 2023;36(9):doad002. doi: 10.1093/dote/doad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki M., Fukuoka T., Shibutani M., et al. Usefulness of the skeletal muscle index in postoperative ileus of colorectal cancer patients: a retrospective cohort study. BMC Surg. 2022;22(1):448. doi: 10.1186/s12893-022-01887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallois C., Bourillon C., Auclin E., et al. Skeletal muscle loss during chemotherapy and its association with survival and systemic treatment toxicity in metastatic colorectal cancer: an AGEO prospective multicenter study. Clin Res Hepatol Gastroenterol. 2021;45(6) doi: 10.1016/j.clinre.2020.101603. [DOI] [PubMed] [Google Scholar]
- 18.Jin S.B., Tian Z.B., Ding X.L., et al. The impact of preoperative sarcopenia on survival prognosis in patients receiving neoadjuvant therapy for esophageal cancer: a systematic review and meta-analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.619592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida T., Makino T., Yamasaki M., et al. Quantity and quality of skeletal muscle as an important predictor of clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28(12):7185–7195. doi: 10.1245/s10434-021-10025-x. [DOI] [PubMed] [Google Scholar]
- 20.Kamitani N., Migita K., Matsumoto S., et al. Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today. 2019;49(12):1022–1028. doi: 10.1007/s00595-019-01846-1. [DOI] [PubMed] [Google Scholar]
- 21.Tan B.H., Brammer K., Randhawa N., et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–338. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Palmela C., Velho S., Agostinho L., et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J Gastric Cancer. 2017;17(1):74–87. doi: 10.5230/jgc.2017.17.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyata H., Sugimura K., Motoori M., et al. Clinical assessment of sarcopenia and changes in body composition during neoadjuvant chemotherapy for esophageal cancer. Anticancer Res. 2017;37(6):3053–3059. doi: 10.21873/anticanres.11660. [DOI] [PubMed] [Google Scholar]
- 24.Onishi S., Tajika M., Tanaka T., et al. Effect of body composition change during neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Clin Med. 2022;11(3):508. doi: 10.3390/jcm11030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yip C., Goh V., Davies A., et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24(5):998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 26.Okuno M., Goumard C., Kopetz S., et al. Loss of muscle mass during preoperative chemotherapy as a prognosticator for poor survival in patients with colorectal liver metastases. Surgery. 2019;165(2):329–336. doi: 10.1016/j.surg.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 27.den Boer R.B., Jones K.I., Ash S., et al. Impact on postoperative complications of changes in skeletal muscle mass during neoadjuvant chemotherapy for gastro-oesophageal cancer. BJS Open. 2020;4(5):847–854. doi: 10.1002/bjs5.50331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson S., Nilsson J.H., Strandberg Holka P., et al. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford) 2017;19(4):331–337. doi: 10.1016/j.hpb.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T., Makino T., Yamasaki M., et al. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166(6):1041–1047. doi: 10.1016/j.surg.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Mayanagi S., Tsubosa Y., Omae K., et al. Negative impact of skeletal muscle wasting after neoadjuvant chemotherapy followed by surgery on survival for patients with thoracic esophageal cancer. Ann Surg Oncol. 2017;24(12):3741–3747. doi: 10.1245/s10434-017-6020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada T., Tsuji T., Ueno J., et al. Prognostic impact of the loss of skeletal muscle mass during neoadjuvant chemotherapy on older patients with esophageal cancer. Ann Surg Oncol. 2022;29(13):8131–8139. doi: 10.1245/s.10434-022-12379-2. [DOI] [PubMed] [Google Scholar]
- 32.Mirkin K.A., Luke F.E., Gangi A., et al. Sarcopenia related to neoadjuvant chemotherapy and perioperative outcomes in resected gastric cancer: a multi-institutional analysis. J Gastrointest Oncol. 2017;8(3):589–595. doi: 10.21037/jgo.2017.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishibashi Y., Tsujimoto H., Hiraki S., et al. Predictive value of immuno-inflammatory and nutritional measures modulated by neoadjuvant chemotherapy on the response of neoadjuvant chemotherapy and long-term outcomes in patients with esophageal cancer. Oncol Lett. 2020;19(1):487–497. doi: 10.3892/ol.2019.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozawa Y., Nakano T., Taniyama Y., et al. Evaluation of the impact of psoas muscle index, a parameter of sarcopenia, in patients with esophageal squamous cell carcinoma receiving neoadjuvant therapy. Esophagus. 2019;16(4):345–351. doi: 10.1007/s10388-019-00670-3. [DOI] [PubMed] [Google Scholar]
- 35.Kita R., Miyata H., Sugimura K., et al. Clinical effect of enteral nutrition support during neoadjuvant chemotherapy on the preservation of skeletal muscle mass in patients with esophageal cancer. Clin Nutr. 2021;40(6):4380–4385. doi: 10.1016/j.clnu.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama T., Furuya S., Kawaguchi Y., et al. Prognostic value of preoperative psoas muscle index as a measure of nutritional status in patients with esophageal cancer receiving neoadjuvant therapy. Nutrition. Oct 2021;90 doi: 10.1016/j.nut.2021.111232. [DOI] [PubMed] [Google Scholar]
- 37.Awad S., Tan B.H., Cui H., et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31(1):74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Hagens E.R.C., Feenstra M.L., van Egmond M.A., et al. Influence of body composition and muscle strength on outcomes after multimodal oesophageal cancer treatment. J Cachexia Sarcopenia Muscle. 2020;11(3):756–767. doi: 10.1002/jcsm.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakita Y., Motoyama S., Sato Y., et al. Decreases in the psoas muscle index correlate more strongly with survival than other prognostic markers in esophageal cancer after neoadjuvant chemoradiotherapy plus esophagectomy. World J Surg. 2020;44(5):1559–1568. doi: 10.1007/s00268-019-05344-w. [DOI] [PubMed] [Google Scholar]
- 40.Yoon H.G., Oh D., Ahn Y.C., et al. Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers. 2020;12(4):925. doi: 10.3390/cancers12040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Järvinen T., Ilonen I., Kauppi J., et al. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16(1):27. doi: 10.1186/s12957-018-1327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J., Motoyama S., Sato Y., et al. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Anticancer Res. 2016;36(12):6677–6685. doi: 10.21873/anticanres.11278. [DOI] [PubMed] [Google Scholar]
- 43.Reisinger K.W., Bosmans J.W., Uittenbogaart M., et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. 2015;22(13):4445–4452. doi: 10.1245/s10434-015-4558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grün J., Elfinger L., Le H., et al. The influence of pretherapeutic and preoperative sarcopenia on short-term outcome after esophagectomy. Cancers. 2020;12(11):3409. doi: 10.3390/cancers12113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panje C.M., Höng L., Hayoz S., et al. Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: a SAKK 75/08 substudy. Radiat Oncol. 2019;14(1):166. doi: 10.1186/s13014-019-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott J.A., Doyle S.L., Murphy C.F., et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg. 2017;266(5):822–830. doi: 10.1097/sla.0000000000002398. [DOI] [PubMed] [Google Scholar]
- 47.Paireder M., Asari R., Kristo I., et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–484. doi: 10.1016/j.ejso.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Fukuoka T., Maeda K., Nagahara H., et al. Change in PMI during neoadjuvant therapy is a predictive prognostic marker in rectal cancer. Anticancer Res. 2019;39(9):5157–5163. doi: 10.21873/anticanres.13711. [DOI] [PubMed] [Google Scholar]
- 49.Yassaie S.S., Keane C., French S.J.H., et al. Decreased total psoas muscle area after neoadjuvant therapy is a predictor of increased mortality in patients undergoing oesophageal cancer resection. ANZ J Surg. 2019;89(5):515–519. doi: 10.1111/ans.15106. [DOI] [PubMed] [Google Scholar]
- 50.Biolo G., Cederholm T., Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33(5):737–748. doi: 10.1016/j.clnu.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L.K., Woo J., Assantachai P., et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Surov A., Wienke A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A meta-analysis. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211008844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia L., Zhao R., Wan Q., et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;9(21):7964–7978. doi: 10.1002/cam4.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang M., Shen Y., Tan L., Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. 2019;156(1):101–111. doi: 10.1016/j.chest.2019.04.115. [DOI] [PubMed] [Google Scholar]
- 56.Chow W.B., Rosenthal R.A., Merkow R.P., et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons national surgical quality improvement program and the American geriatrics society. J Am Coll Surg. 2012;215(4):453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Batsis J.A., Villareal D.T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steffl M., Bohannon R.W., Sontakova L., et al. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi: 10.2147/cia.S132940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan L., Xie W., Fu X., et al. Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp Gerontol. 2021;154 doi: 10.1016/j.exger.2021.111544. [DOI] [PubMed] [Google Scholar]
- 60.de Almeida L.L., Ilha T., de Carvalho J.A.M., et al. Sarcopenia and its association with vertebral fractures in people living with HIV. Calcif Tissue Int. 2020;107(3):249–256. doi: 10.1007/s00223-020-00718-y. [DOI] [PubMed] [Google Scholar]
- 61.O'Donovan G., Sarmiento O.L., Hessel P., et al. Associations of body mass index and sarcopenia with screen-detected mild cognitive impairment in older adults in Colombia. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1011967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao K., Ma W.Z., Huck S., et al. Association between sarcopenia and depressive symptoms in Chinese older adults: evidence from the China health and retirement longitudinal study. Front Med. 2021;8 doi: 10.3389/fmed.2021.755705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cormie P., Atkinson M., Bucci L., et al. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184–187. doi: 10.5694/mja18.00199. [DOI] [PubMed] [Google Scholar]
- 64.Tezze C., Sandri M., Tessari P. Anabolic resistance in the pathogenesis of sarcopenia in the elderly: role of nutrition and exercise in young and old people. Nutrients. 2023;15(18) doi: 10.3390/nu15184073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S.H., Lee H. Effectiveness of combined exercise and nutrition interventions in preventing and improving sarcopenia in frail or healthy older adults: a systematic review. Res Gerontol Nurs. 2023:1–9. doi: 10.3928/19404921-20230817-03. [DOI] [PubMed] [Google Scholar]
- 66.Rock C.L., Thomson C.A., Sullivan K.R., et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230–262. doi: 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 67.Jianqin S., Jian Z., Cuiqing C., et al. Chinese expert consensus on nutritional and exercise interventions for muscle weakening syndrome. Acta Nutr Sin. 2015;37(4):320–324. doi: 10.13325/j.cnki.acta.nutr.sin.2015.04.006. [DOI] [Google Scholar]
- 68.Wiskemann J., Clauss D., Tjaden C., et al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: a randomized controlled trial. Pancreas. 2019;48(2):257–266. doi: 10.1097/mpa.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 69.Ceglia L., Niramitmahapanya S., da Silva Morais M., et al. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927–E1935. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupont J., Dedeyne L., Dalle S., et al. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31(6):825–836. doi: 10.1007/s40520-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jang M.K., Park C., Tussing-Humphreys L., et al. The effectiveness of sarcopenia interventions for cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancer Nurs. 2023;46(2):E81–E90. doi: 10.1097/ncc.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodacki C.L., Rodacki A.L., Pereira G., et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012;95(2):428–436. doi: 10.3945/ajcn.111.021915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.