Abstract
Background
The relationship between the NHHR and kidney stone risk remains unknown. The purpose of this study was to evaluate the association between adult NHHR and kidney stone occurrence in USA.
Methods
This study used a variety of statistical techniques such as threshold effects, subgroup analysis, smooth curve fitting, multivariate logistic regression, and data from the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2014. We aimed to clarify the relationship between the NHHR and kidney stone risk.
Results
The average age of the 21,058 individuals in this research was 49.70 ± 17.64 years. The mean NHHR was 3.00 ± 1.47, and the overall prevalence of kidney stone occurrence was 9.05%. The prevalence within the quartile ranges (Q1–Q4) was 7.01%, 8.71%, 9.98%, and 10.49%, respectively. The overall average recurrence rate of kidney stones was 3.05%, demonstrating a significant increase with increasing NHHR (Q1: 1.92%, Q2: 2.92%, Q3: 3.35%, Q4: 4.00%, P < 0.01). The occurrence of kidney stones increased by 4% (95% CI: 1.00-1.08, P = 0.0373) and the chance of recurrence increased by 9% (95% CI: 1.03–1.14, P < 0.01) with each unit increase in NHHR. The interaction analysis results demonstrated that the relationship between the NHHR and the risk of kidney stones was not significantly impacted by the following factors: sex, body mass index, poverty income ratio, diabetes, or hypertension. Curve fitting and threshold effect analysis also demonstrated a non-linear association, with a breakpoint found at 3.17, between the NHHR and the risk of kidney stones.
Conclusions
In adults in the USA, there is a substantial correlation between elevated NHHR levels and a higher probability of kidney stones developing and recurring. Timely intervention and management of NHHR may effectively mitigate the occurrence and recurrence of kidney stones.
Keywords: NHHR, Lipid ratio, Kidney stones, NHANES, Cross-sectional study
Introduction
Nephrolithiasis is a prevalent malady of the urogenital system and is attributed to excessive mineral saturation in urine, giving rise to crystalline formations that subsequently precipitate within the renal pelvis and calyces [1]. According to epidemiological statistics, there are 114–720 cases per 100,000 people in Italy, Japan, Germany, Scotland, Spain, Sweden, and the USA, with prevalence rates ranging from 1.7 to 14.8% [2]. Moreover, its incidence rate has increased sharply over the past three decades [3]. Furthermore, the recurrence rate of nephrolithiasis is notably elevated, with an estimated annual recurrence rate ranging from 10 to 23%, escalating to 50% within 5–10 years and reaching 75% within two decades [4, 5].
Nephrolithiasis can cause renal colic, haematuria, obstructive hydronephrosis, and impaired renal function. In severe cases, complications, including infections, may occur with potentially life-threatening implications [6–8]. Obesity [9], diabetes [10, 11], hypertension [12], and metabolic syndrome [13]are acknowledged as pivotal risk factors. The current study underscores the correlation between dyslipidaemia and nephrolithiasis. A nationwide survey conducted by Kohjimoto et al. established an association between dyslipidaemia and an increased prevalence of stone recurrence or multiplicity. Dyslipidaemia may exacerbate the risk of nephrolithiasis through mechanisms such as insulin resistance, inflammatory responses, and oxidative stress [14]. Additionally, studies have suggested a significant elevation in uric acid levels and decrease in urine pH among individuals with metabolic syndrome, which correlates with an increased occurrence of uric acid stones. Within this demographic, reduced levels of high density lipoprotein (HDL) and increased levels of triglycerides have been linked to an increased incidence of uric acid stones [15]. A newly identified risk indicator for atherosclerosis is the ratio of non-high density lipoprotein cholesterol to high density lipoprotein cholesterol (NHHR) [16]. Recent investigations have indicated that the NHHR can independently determine the risk of metabolic syndrome, chronic kidney disease, and nonalcoholic fatty liver disease (NAFLD) [17–20]. However, the relationship between the NHHR and nephrolithiasis has not yet been explored. Thus, by leveraging NHANES data from 2007 to 2014, this study sought to elucidate the correlation between NHHR and the likelihood of developing nephrolithiasis. This study hypothesised that high NHHR increases the likelihood of nephritis, and exploring the association between lipid metabolism and kidney stones is expected to fill a knowledge gap in the research field. Simultaneously, we opened up a new area of research to explore the potential application of the NHHR in predicting the outcome of kidney stones.
Methods
Data source
National Health and Nutrition Examination Survey (NHANES) is a comprehensive survey addressing various ethnic groups and health-related issues in the USA. It endeavours to amass information pertaining to the health, nutritional, and sociological aspects of the American population. The program ensures that all participants have provided their given permission by conducting health and nutrition evaluations every two years, which are examined and authorized by the National Centre for Health Statistics Research Ethics Review Board. The database contains structured questionnaires, physical examinations, and laboratory tests. Requisite data were procured from the official NHANES website.
Study population
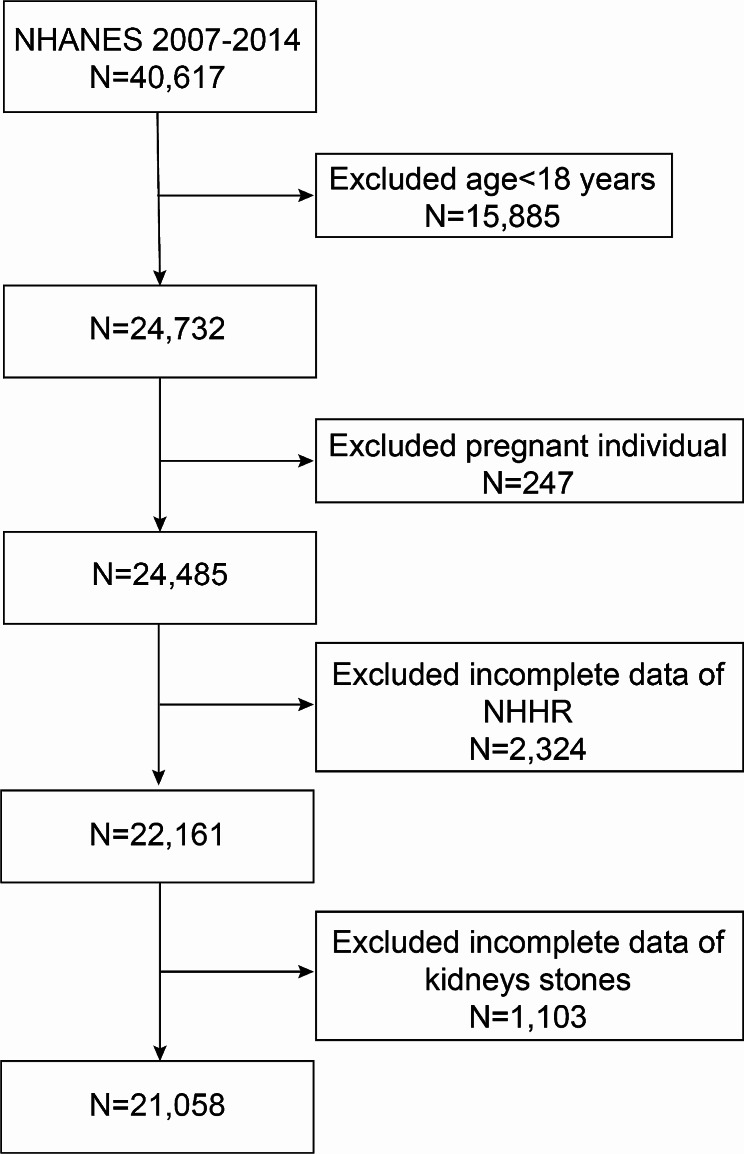
This study meticulously curated data from four NHANES survey cycles from 2007 to 2014. Initially, the cohort comprised of 40,617 individuals. Through a methodological screening process, certain demographic characteristics were excluded as follows: individuals under the age of 18 years (15,885 individuals), pregnant women (247 individuals), those lacking NHHR data (2,324 individuals), and those who did not provide information on kidney stones (1,103 individuals). Following this rigorous selection process, 21,058 individuals met the inclusion criteria (Fig. 1).
Fig. 1.
The flowchart depicting sample selection for the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2014
Exposure definition
The focal exposure variable in this study was NHHR, which denotes the ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol [21]. The computation of non-high-density lipoprotein cholesterol involves subtracting high-density lipoprotein cholesterol from total cholesterol (TC). Stratifying participants based on their NHHR values, this study categorised them into four tiers for analytical convenience.
Outcome definition
Nephrolithiasis occurrence and recurrence rates were regarded as outcome measures in this study. These metrics were ascertained through a health status questionnaire in the NHANES, relying on the participants’ self-reports during personal interviews regarding two specific renal health enquiries (KIQ026 and KID028). These queries respectively enquire, ‘Have you ever suffered from nephrolithiasis?’ and ‘How many instances of nephrolithiasis did you experience?’ Participants were required to choose ‘yes’ or ‘no’ as their responses. An affirmative response was defined as a history of nephrolithiasis. If there were two or more documented instances of affliction, it was classified as a nephrolithiasis recurrence. Prior research has substantiated the heightened accuracy of nephrolithiasis information obtained using self-reporting methods [22].
Covariates
Drawing on prior research, this study extracted a myriad of covariates from the NHANES database, encompassing dimensions such as demographics, dietary habits, examinations, laboratory assays, and questionnaire surveys. Numerous categorical factors were included in these covariates, such as body mass index (BMI), age, sex, ethnicity, marital status, educational level, poverty income ratio (PIR), vigorous and moderate physical activities, daily alcohol consumption (defined as a minimum intake of four alcoholic beverages per day), smoking habits (defined as at least 100 cigarettes smoked throughout an individual’s lifetime), and the presence of hypertension and diabetes. Notably, the PIR is stratified into three tiers: ‘1’, ‘1–3’, and ‘3’ or above. Similarly, the BMI is divided into ranges of less than 25, 25–29.9, and more than 30 kg/m², which represent normal weight, overweight, and obesity, respectively. For continuous variables, our considerations were extended to the levels of TC and high-density lipoprotein cholesterol (HDL-C).
Statistical analysis
In accordance with the guidelines issued by the Centres for Disease Control and Prevention (CDC) (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx), this study conducted refined statistical analyses. The specific procedures were as follows: initially, quartile division of the NHHR was performed, with the lowest quartile (Q1) serving as the reference group. Frequencies and percentages are used to portray categorical data, and the standard deviation, or mean, is used to describe continuous variables. The association between the NHHR and the occurrence and recurrence rates of kidney stones was investigated using a multivariate logistic regression model. Model 2 contained sex, age, and ethnicity adjustments, and Model 3 added more adjustments for BMI, education level, marital status, PIR, alcohol and smoking habits, diabetes, hypertension, and vigorous and moderate physical activity in addition to the adjustments for sex, age, and ethnicity. Step three involved testing the threshold effect of the NHHR on the occurrence rate of kidney stones using a segmented linear regression model and examining the nonlinear connection between the NHHR and the occurrence and recurrence rates of kidney stones using a smooth curve-fitting approach. Finally, an in-depth exploration of the potential differences among different populations was conducted through subgroup analysis and interaction testing. EmpowerStats (version 2.0) and R software (version 4.1.3) were utilized for each of the statistical evaluations, with a significance level of P below 0.05.
Results
Baseline characteristics of participants
Table 1 delineates the baseline characteristics of the participants selected from the NHANES between 2007 and 2014, stratified by NHHR quartiles. The study included 21,058 participants, with a sex distribution of 49.18% males and 50.82% females. The mean age was 49.70 years, with a standard deviation of 17.64 years. The quartile ranges for NHHR were 0.45–1.98, 1.98–2.72, 2.72–3.71, and 3.71–24.3. The overall occurrence rate of kidney stones averaged 9.05%, with the rates for each quartile (Q1 to Q4) being 7.01%, 8.71%, 9.98%, and 10.49%. The overall average recurrence rate of kidney stones was 3.05%, exhibiting a significant increase with increasing NHHR (Q1:1.92%; Q2:2.92%; Q3:3.35%; Q4:4.00%; P < 0.01).
Table 1.
Based on the baseline characteristics of the study population ascertained by NHANES from 2007 to 2014
| Characteristic | Total | Q1 (0.45–1.98) | Q2 (1.98–2.72) | Q3 (2.72–3.71) | Q4 (3.71–24.3) | P-value |
|---|---|---|---|---|---|---|
| N | 21,058 | 5261 | 5268 | 5258 | 5271 | |
| Age (years) | 49.70 ± 17.64 | 49.48 ± 19.31 | 50.50 ± 18.28 | 49.86 ± 17.08 | 48.95 ± 15.63 | < 0.001 |
| Sex (%) | < 0.001 | |||||
| Male | 10,357 (49.18%) | 1928 (36.65%) | 2248 (42.67%) | 2841 (54.03%) | 3340 (63.37%) | |
| Female | 10,701 (50.82%) | 3333 (63.35%) | 3020 (57.33%) | 2417 (45.97%) | 1931 (36.63%) | |
| Race (%) | < 0.001 | |||||
| Mexican American | 3156 (14.99%) | 561 (10.66%) | 705 (13.38%) | 887 (16.87%) | 1003 (19.03%) | |
| Other Hispanic | 2137 (10.15%) | 410 (7.79%) | 517 (9.81%) | 576 (10.95%) | 634 (12.03%) | |
| Non-Hispanic White | 9426 (44.76%) | 2341 (44.50%) | 2353 (44.67%) | 2316 (44.05%) | 2416 (45.84%) | |
| Non-Hispanic Black | 4237 (20.12%) | 1382 (26.27%) | 1142 (21.68%) | 975 (18.54%) | 738 (14.00%) | |
| Other Races | 2102 (9.98%) | 567 (10.78%) | 551 (10.46%) | 504 (9.59%) | 480 (9.11%) | |
| Marital status (%) | < 0.001 | |||||
| Married | 10,891 (51.72%) | 2435 (46.28%) | 2642 (50.15%) | 2868 (54.55%) | 2946 (55.89%) | |
| Single | 8607 (40.87%) | 2479 (47.12%) | 2226 (42.26%) | 2021 (38.44%) | 1881 (35.69%) | |
| with partner | 1560 (7.41%) | 347 (6.60%) | 400 (7.59%) | 369 (7.02%) | 444 (8.42%) | |
| Education level (%) | < 0.001 | |||||
| Below high school | 2317 (11.00%) | 424 (8.06%) | 541 (10.27%) | 617 (11.73%) | 735 (13.94%) | |
| High school | 3195 (15.17%) | 738 (14.03%) | 729 (13.84%) | 809 (15.39%) | 919 (17.44%) | |
| Above high school | 15,546 (73.82%) | 4099 (77.91%) | 3998 (75.89%) | 3832 (72.88%) | 3617 (68.62%) | |
| PIR (%) | < 0.001 | |||||
| < 1 | 4299 (22.31%) | 1031 (21.36%) | 1017 (21.13%) | 1045 (21.76%) | 1206 (24.99%) | |
| 1–3 | 7979 (41.41%) | 1865 (38.64%) | 1919 (39.87%) | 2097 (43.66%) | 2098 (43.48%) | |
| > 3 | 6989 (36.27%) | 1930 (39.99%) | 1877 (39.00%) | 1661 (34.58%) | 1521 (31.52%) | |
| BMI category (%) | < 0.001 | |||||
| Normal weight | 6147 (29.56%) | 2623 (50.37%) | 1679 (32.31%) | 1135 (21.85%) | 710 (13.66%) | |
| Overweight | 7001 (33.66%) | 1503 (28.86%) | 1739 (33.46%) | 1849 (35.60%) | 1910 (36.74%) | |
| Obese | 7649 (36.78%) | 1081 (20.76%) | 1779 (34.23%) | 2210 (42.55%) | 2579 (49.61%) | |
| Vigorous activity (%) | < 0.001 | |||||
| Yes | 3799 (18.04%) | 787 (14.96%) | 895 (16.99%) | 988 (18.79%) | 1129 (21.42%) | |
| No | 17,259 (81.96%) | 4474 (85.04%) | 4373 (83.01%) | 4270 (81.21%) | 4142 (78.58%) | |
| Moderate activity (%) | < 0.001 | |||||
| Yes | 7279 (34.57%) | 1708 (32.47%) | 1755 (33.31%) | 1890 (35.95%) | 1926 (36.54%) | |
| No | 13,779 (65.43%) | 3553 (67.53%) | 3513 (66.69%) | 3368 (64.05%) | 3345 (63.46%) | |
| Alcohol habit (%) | < 0.001 | |||||
| Yes | 2865 (17.42%) | 599 (14.70%) | 642 (15.77%) | 729 (17.82%) | 895 (21.26%) | |
| No | 13,582 (82.58%) | 3476 (85.30%) | 3428 (84.23%) | 3363 (82.18%) | 3315 (78.74%) | |
| Smoking habit (%) | < 0.001 | |||||
| Yes | 9541 (45.31%) | 2191 (41.65%) | 2281 (43.30%) | 2369 (45.06%) | 2700 (51.22%) | |
| No | 11,517 (54.69%) | 3070 (58.35%) | 2987 (56.70%) | 2889 (54.94%) | 2571 (48.78%) | |
| Hypertension (%) | < 0.001 | |||||
| Yes | 7596 (36.07%) | 1786 (33.95%) | 1877 (35.63%) | 1963 (37.33%) | 1970 (37.37%) | |
| No | 13,462 (63.93%) | 3475 (66.05%) | 3391 (64.37%) | 3295 (62.67%) | 3301 (62.63%) | |
| Diabetes (%) | 0.138 | |||||
| Yes | 2611 (12.40%) | 619 (11.77%) | 637 (12.09%) | 660 (12.55%) | 695 (13.19%) | |
| No | 18,447 (87.60%) | 4642 (88.23%) | 4631 (87.91%) | 4598 (87.45%) | 4576 (86.81%) | |
| TC, mmol/L | 5.01 ± 1.08 | 4.42 ± 0.89 | 4.76 ± 0.90 | 5.08 ± 0.89 | 5.77 ± 1.12 | < 0.001 |
| HDL-C, mmol/L | 1.35 ± 0.41 | 1.77 ± 0.42 | 1.43 ± 0.28 | 1.22 ± 0.22 | 0.99 ± 0.20 | < 0.001 |
| NHHR | 3.00 ± 1.47 | 1.53 ± 0.32 | 2.34 ± 0.21 | 3.18 ± 0.28 | 4.95 ± 1.41 | < 0.001 |
| Nephrolithiasis (%) | < 0.001 | |||||
| Yes | 1906 (9.05%) | 369 (7.01%) | 459 (8.71%) | 525 (9.98%) | 553 (10.49%) | |
| No | 19,152 (90.95%) | 4892 (92.99%) | 4809 (91.29%) | 4733 (90.02%) | 4718 (89.51%) | |
| Nephrolithiasis recurrence (%) | 0.009 | |||||
| Yes | 642 (3.05%) | 101 (1.92%) | 154 (2.92%) | 176 (3.35%) | 211 (4.00%) | |
| No | 20,416 (96.95%) | 5160 (98.08%) | 5114 (97.08%) | 5082 (96.65%) | 5060 (96.00%) |
Associations between the NHHR and kidney stones
Regarding the incidence of kidney stones, this study demonstrated a positive link between the likelihood of kidney stone occurrence and an increase in NHHR. After making all necessary modifications, the chance of developing kidney stones rose by 4% (95% CI: 1.00-1.08, P = 0.0373) for every incremental unit rise in NHHR. In addition, using the NHHR as a stratified variable (quartiles) for further analysis, individuals in the highest quartile (Q4) had a 1.28-fold higher risk of kidney stone incidence in a fully corrected model (95% CI: 1.07–1.52, P = 0.0058) than those in the lowest quartile (Q1). This strengthens the favorable link that has been seen throughout time between higher NHHR and kidney stone risk.
The study also shows a correlation between elevated NHHR and a higher chance of kidney stone recurrence (Model 1: OR = 1.14, 95% CI: 1.10–1.19, P < 0.01; Model 2: OR = 1.12, 95% CI: 1.07–1.17, P < 0.01; Model 3: OR = 1.09, 95% CI: 1.03–1.14, P < 0.01). A consistent positive connection was found between the risk of kidney stone recurrence and NHHR rise in the fully calibrated Model 3, with data showing that for every unit increase in NHHR, the likelihood of recurrence rose by 9% (Table 2).
Table 2.
Association of NHHR with kidney stone and a recurrence of passing kidney stones
| Exposure | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR(95%CI), P-value | OR(95%CI), P-value | OR(95%CI), P-value | |
| Nephrolithiasis | |||
| NHHR | 1.08 (1.05, 1.12) < 0.0001 | 1.07 (1.04, 1.11) < 0.0001 | 1.04 (1.00, 1.08) 0.0373 |
| Categories | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.27 (1.10, 1.46) 0.0012 | 1.22 (1.05, 1.41) 0.0074 | 1.15 (0.97, 1.37) 0.1132 |
| Q3 | 1.47 (1.28, 1.69) < 0.0001 | 1.40 (1.22, 1.62) < 0.0001 | 1.25 (1.05, 1.49) 0.0104 |
| Q4 | 1.55 (1.35, 1.78) < 0.0001 | 1.47 (1.27, 1.69) < 0.0001 | 1.28 (1.07, 1.52) 0.0058 |
| P for trend | 1.14 (1.10, 1.19) < 0.0001 | 1.13 (1.08, 1.18) < 0.0001 | 1.08 (1.02, 1.14) 0.0086 |
| Nephrolithiasis recurrence | |||
| NHHR | 1.14 (1.10, 1.19) < 0.01 | 1.12 (1.07, 1.17) < 0.01 | 1.09 (1.03, 1.14) < 0.01 |
| Categories | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.54 (1.19, 1.98) 0.0009 | 1.46 (1.13, 1.89) 0.0037 | 1.26 (0.93, 1.70) 0.1380 |
| Q3 | 1.77 (1.38, 2.27) < 0.0001 | 1.63 (1.27, 2.09) 0.0001 | 1.34 (1.00, 1.81) 0.0527 |
| Q4 | 2.13 (1.68, 2.71) < 0.0001 | 1.90 (1.49, 2.44) < 0.0001 | 1.58 (1.18, 2.12) 0.0024 |
| P for trend | 1.24 (1.16, 1.33) < 0.0001 | 1.20 (1.12, 1.29) < 0.0001 | 1.15 (1.05, 1.25) 0.0026 |
In the analysis, NHHR was transformed from a continuous variable into a categorical variable using quartiles
OR, odds ratio; 95% CI, 95% confidence interval
Model 1 did not incorporate any variable adjustments
Model 2 adjusted for sex, age, and race
Model 3 extended these adjustments to include a more comprehensive set of variables: sex, age, race, educational level, marital status, Poverty Income Ratio (PIR), Body Mass Index (BMI), vigorous activity, moderate activity, hypertension, diabetes, alcohol and smoking habits
Nonlinear association between the NHHR and Kidney Stones
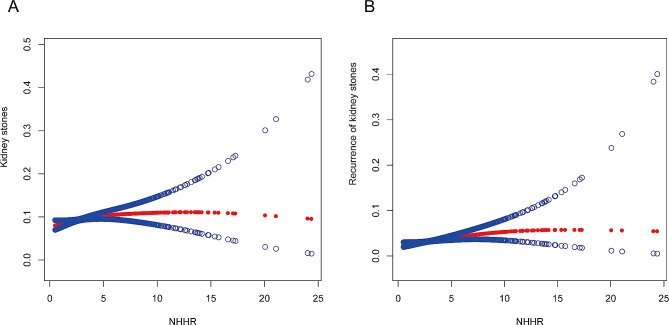
This study investigated the nonlinear relationship between the NHHR and the risk of kidney stones, as seen in Fig. 2. Through meticulous analysis of smooth curves, this study revealed a nonlinear association between the occurrence of kidney stones and the NHHR (Part A). In the correlation analysis of kidney stone recurrence, a similar nonlinear pattern is observed (Part B). On contrasting standard linear models with biphasic linear models, the research findings indicated that in the likelihood ratio test, the P-value for the association between NHHR and kidney stone recurrence was 0.07, whereas the P-value for NHHR and the risk of kidney stone occurrence was significantly < 0.05. By employing biphasic linear models and recursive algorithms, the study identifies an inflection point at an NHHR value 3.17 (refer to Table 3). When NHHR is below 3.17, the chance of developing kidney stones increases by 15% (OR: 1.15, 95% CI: 1.04–1.27) for every additional unit of NHHR. Conversely, when the NHHR exceeds 3.17, no significant change in the relative risk of kidney stones was observed.
Fig. 2.
(A) The correlation between NHHR and kidney stones. (B) The association between NHHR and the recurrence of kidney stones. The red solid line signifies the smooth curve fit between variables. The blue band represents the 95% confidence interval derived from the fit
Table 3.
Utilize the two-segment piecewise linear regression model for the analysis of threshold effects between NHHR and nephrolithiasis
| Adjusted | ||
|---|---|---|
| OR (95% CI) | P value | |
| Nephrolithiasis | ||
| Fitting by standard linear model | 1.04 (1.00, 1.08) | 0.037 |
| Fitting by two-piecewise linear model | ||
| <3.17 | 1.15 (1.04, 1.27) | 0.0076 |
| >3.17 | 1.00 (0.95, 1.06) | 0.9566 |
| Log-likelihood ratio | 0.039 | |
| Nephrolithiasis recurrence | ||
| Fitting by standard linear model | 1.09 (1.03, 1.14) | 0.0025 |
| Fitting by two-piecewise linear | ||
| <5.43 | 1.15 (1.06, 1.25) | 0.0009 |
| >5.43 | 0.99 (0.87, 1.13) | 0.8860 |
| Log-likelihood ratio | 0.070 | |
Adjusted for sex, age, race, educational level, marital status, Poverty Income Ratio (PIR), Body Mass Index (BMI), vigorous activity, moderate activity, hypertension, diabetes, alcohol and smoking habits
Subgroup analysis
This study investigated whether kidney stones and NHHR status are consistently associated in the general population using subgroup analyses and interaction testing. It seeks to identify possible differences in particular demographic scenarios according to BMI, PIR, sex, diabetes, and hypertension, among others. Table 4 presents the research findings, which indicate a positive association consistently across sex, BMI, PIR, diabetes, and hypertension subgroups with kidney stone occurrence. This finding suggests that the correlation applies to a wide variety of demographic situations.
Table 4.
Subgroup analysis
| NHHR | Nephrolithiasis | Nephrolithiasis recurrence | ||
|---|---|---|---|---|
| OR(95%CI), P-value | P for interaction | OR(95%CI), P-value | P for interaction | |
| Sex | 0.4571 | 0.4464 | ||
| Male | 1.04 (0.99, 1.09) 0.1221 | 1.08 (1.01, 1.16) 0.0277 | ||
| Female | 1.07 (1.01, 1.14) 0.0325 | 1.13 (1.03, 1.23) 0.0063 | ||
| BMI | 0.5341 | 0.6919 | ||
| Normal weight | 1.03 (0.92, 1.14) 0.6090 | 1.06 (0.89, 1.27) 0.4856 | ||
| Overweight | 1.01 (0.95, 1.08) 0.6755 | 1.05 (0.95, 1.16) 0.3062 | ||
| Obese | 1.06 (1.01, 1.11) 0.0223 | 1.10 (1.03, 1.18) 0.0037 | ||
| PIR | 0.2518 | 0.5233 | ||
| < 1 | 1.00 (0.93, 1.08) 0.9755 | 1.11 (1.02, 1.22) 0.0185 | ||
| 1–3 | 1.03 (0.98, 1.09) 0.2798 | 1.05 (0.96, 1.14) 0.2803 | ||
| > 3 | 1.09 (1.02, 1.16) 0.0152 | 1.12 (1.00, 1.24) 0.0458 | ||
| Diabetes | 0.7834 | 0.5237 | ||
| Yes | 1.05 (0.97, 1.13) 0.2285 | 1.05 (0.94, 1.18) 0.3913 | ||
| No | 1.04 (0.99, 1.08) 0.1053 | 1.09 (1.03, 1.16) 0.0031 | ||
| Hypertension | 0.8378 | 0.3268 | ||
| Yes | 1.03 (0.97, 1.08) 0.3320 | 1.05 (0.97, 1.13) 0.2459 | ||
| No | 1.03 (0.98, 1.09) 0.1947 | 1.10 (1.03, 1.18) 0.0060 | ||
Discussion
The purpose of this study was to investigate the possible relationship between kidney stones and NHHR. The analysis of 21,058 participants revealed a connection between an elevated NHHR and an increased risk of both the initial occurrence and recurrence of kidney stones. This correlation remained consistent when the subgroups were stratified based on sex, BMI, PIR, diabetes, and hypertension. Further smoothing curve fitting and threshold effect analysis indicated a nonlinear relationship between the NHHR and kidney stones, with a turning point identified at 3.17. Before this inflection point, an increase in the NHHR correlated positively with the risk of kidney stone occurrence, whereas beyond this point, the correlation was not statistically significant.
This study provides a preliminary investigation into the association between the NHHR and kidney stones. The mounting evidence acknowledges NHHR as a precise indicator of lipid-related disease risks [18, 23]. Despite the lack of direct research exploring the link between kidney stones and lipid metabolism, extensive studies have revealed associations between kidney stones and various lipid-related factors. An 8-year prospective study found a significant increase in kidney stone risk with elevated triglyceride levels [24]. Comparative studies have indicated higher serum triglyceride and lower HDL-C levels in patients with kidney stones [25–30]. A retrospective analysis of 2,442 kidney stone patients showed an association between lipid levels and changes in urine composition [31]. Furthermore, dyslipidemia is linked to an increased prevalence of stone recurrence or multiplicity [14]. In individuals with metabolic syndrome, elevated triglyceride and low HDL levels are closely associated with an increased incidence of uric acid stones [15]. Fundamentally, kidney stones consist of crystals and organic matrices, with the matrix containing various lipids that facilitate crystal nucleation and drive stone formation [32–36].
NHHR, which is recognized as a novel lipid indicator of atherosclerosis [37], serves as an independent risk factor for atherosclerotic plaques and is a crucial lipid marker for preventing plaque formation [21]. NHHR has demonstrated diagnostic value beyond traditional lipid markers in predicting metabolic syndrome, insulin resistance [19, 38], and NAFLD [17]. The close association of the NHHR with various diseases validates its effectiveness as a lipid management tool. This study also identified a potential positive correlation between the NHHR and the occurrence and recurrence of kidney stones. Therefore, the NHHR is a useful tool for determining how lipid metabolism affects kidney stone occurrence and probable recurrence.
Study strengths and limitations
There were several significant research strengths in this study. It started with a nationwide poll of individuals in the United States. Second, meticulously adjusting for confounding variables ensured the credibility and generalizability of the research outcomes. Finally, employing smoothing curve fitting and bilinear regression models, it explores the nonlinear relationship between NHHR and kidney stones.
However, this study had some limitations. First, the diagnosis of kidney stones relied on self-reporting by participants, introducing subjectivity and inevitable recall bias. Second, despite adjusting for numerous confounding factors based on prior research, the potential impact of unmeasured or unknown confounding factors on the study results cannot be completely eliminated. The cross-sectional design of this investigation precluded the establishment of a causal link between kidney stones and the NHANES. Finally, due to the data design of the NHANES database, this study implemented some exclusion criteria that may have been subject to selection bias.
Conclusion
The findings of this study showed that the NHHR and the risk of kidney stones and their recurrence were positively correlated. The potential prognostic value of the NHHR for kidney stone outcomes was another new area investigated in this study. Controlling NHHR and mitigating the risk of kidney stones have significant clinical implications. However, further prospective clinical trials are required to confirm the potential role of lipids in kidney disease.
Acknowledgements
We are grateful to the National Health and Nutrition Examination Survey for the data provided and to all participants for their selfless dedication.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- NHHR
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- BMI
Body mass index
- PIR
Poverty income ratio
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
Author contributions
HHJ: Principal manuscript writer, designed the study and wrote the manuscript. HYJ and GZQ: contributed to the data analysis and data interpretation. QYL and FJL: contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Shenyang Science and Technology Program for Public Health Research and Development (22-321-33-52) and the Wu Jieping Medical Foundation.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The portions of this study involving human participants, human materials, or human data were conducted in accordance with the Declaration of Helsinki and were approved by the NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang B, Lu X, Li Y, Li Y, Yu D, Zhang W, Duan C, Taguchi K, Yasui T, Kohri K, Gao B. A Proteomic Network Approach across the kidney Stone Disease reveals endoplasmic reticulum stress and Crystal-Cell Interaction in the kidney. Oxid Med Cell Longev. 2019;2019:9307256. doi: 10.1155/2019/9307256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- 3.Mayans L, Nephrolithiasis Prim Care. 2019;46:203–12. doi: 10.1016/j.pop.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–44. doi: 10.1016/s0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 5.Siener R. Nutrition and kidney Stone Disease. Nutrients. 2021;13. 10.3390/nu13061917 [DOI] [PMC free article] [PubMed]
- 6.Gottlieb M, Long B, Koyfman A. The evaluation and management of urolithiasis in the ED: a review of the literature. Am J Emerg Med. 2018;36:699–706. doi: 10.1016/j.ajem.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684–93. doi: 10.1056/NEJMcp030813. [DOI] [PubMed] [Google Scholar]
- 8.Pfau A, Knauf F. Update on Nephrolithiasis: Core Curriculum 2016. Am J Kidney Dis. 2016;68:973–85. doi: 10.1053/j.ajkd.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 10.Daudon M, Jungers P. Diabetes and nephrolithiasis. Curr Diab Rep. 2007;7:443–8. doi: 10.1007/s11892-007-0075-6. [DOI] [PubMed] [Google Scholar]
- 11.Lieske JC, de la Vega LS, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ, Leibson CL. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48:897–904. doi: 10.1053/j.ajkd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257–64. doi: 10.1038/ajh.2007.62. [DOI] [PubMed] [Google Scholar]
- 13.Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116:c159–171. doi: 10.1159/000317196. [DOI] [PubMed] [Google Scholar]
- 14.Kohjimoto Y, Sasaki Y, Iguchi M, Matsumura N, Inagaki T, Hara I. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61:923–9. doi: 10.1053/j.ajkd.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Cho ST, Jung SI, Myung SC, Kim TH. Correlation of metabolic syndrome with urinary stone composition. Int J Urol. 2013;20:208–13. doi: 10.1111/j.1442-2042.2012.03131.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, Lu Z, Zhu L, Ouyang X, Yang Y, He W, Feng Y, Yi F, Song Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. 2015;73:931–8. doi: 10.5603/KP.a2015.0086. [DOI] [PubMed] [Google Scholar]
- 17.Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): is it more than just an association? Hepatology. 2013;58:1166–74. doi: 10.1002/hep.26390. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Zhong J, Ye M, Miao L, Lu G, Xu C, Xue Z, Zhou X. Association between the non-HDL-cholesterol to HDL-cholesterol ratio and non-alcoholic fatty liver disease in Chinese children and adolescents: a large single-center cross-sectional study. Lipids Health Dis. 2020;19:242. doi: 10.1186/s12944-020-01421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC, Kim SW, Chung JH, Min YK, Lee MS, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168:2678–83. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Zuo PY, Chen XL, Liu YW, Zhang R, He XX, Liu CY. Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease. Nutr Metab Cardiovasc Dis. 2015;25:582–7. doi: 10.1016/j.numecd.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang A, Li Y, Zhou L, Liu K, Li S, Zong C, Song B, Gao Y, Li Y, Tian C, et al. Non-HDL-C/HDL-C ratio is associated with carotid plaque stability in general population: a cross-sectional study. Front Neurol. 2022;13:875134. doi: 10.3389/fneur.2022.875134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–8. doi: 10.1056/nejm199303253281203. [DOI] [PubMed] [Google Scholar]
- 23.Iannuzzi A, Giallauria F, Gentile M, Rubba P, Covetti G, Bresciani A, Aliberti E, Cuomo G, Panico C, Tripaldella M, et al. Association between Non-HDL-C/HDL-C ratio and carotid intima-media thickness in Post-menopausal Women. J Clin Med. 2021;11. 10.3390/jcm11010078 [DOI] [PMC free article] [PubMed]
- 24.Kang HW, Lee SK, Kim WT, Kim YJ, Yun SJ, Lee SC, Kim WJ. Hypertriglyceridemia and low high-density lipoprotein cholesterolemia are associated with increased hazard for urolithiasis. J Endourol. 2014;28:1001–5. doi: 10.1089/end.2014.0135. [DOI] [PubMed] [Google Scholar]
- 25.Ding Q, Ouyang J, Fan B, Cao C, Fan Z, Ding L, Li F, Tu W, Jin X, Wang J, Shi Y. Association between Dyslipidemia and Nephrolithiasis Risk in a Chinese Population. Urol Int. 2019;103:156–65. doi: 10.1159/000496208. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Kim CH, Sung EJ, Kim SR, Shin HC, Jung WJ. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism. 2013;62:808–13. doi: 10.1016/j.metabol.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AJ, Adamsky MA, Nottingham CU, Pruitt J, Lapin B, Wang CH, Park S. Impact of statin intake on kidney stone formation. Urology. 2019;124:57–61. doi: 10.1016/j.urology.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 29.Masterson JH, Woo JR, Chang DC, Chi T, L’Esperance JO, Stoller ML, Sur RL. Dyslipidemia is associated with an increased risk of nephrolithiasis. Urolithiasis. 2015;43:49–53. doi: 10.1007/s00240-014-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung JA, Li CH, Geng JH, Wu DW, Chen SC. Dyslipidemia increases the risk of incident kidney Stone Disease in a large Taiwanese Population Follow-Up study. Nutrients. 2022;14. 10.3390/nu14071339 [DOI] [PMC free article] [PubMed]
- 31.Torricelli FC, De SK, Gebreselassie S, Li I, Sarkissian C, Monga M. Dyslipidemia and kidney stone risk. J Urol. 2014;191:667–72. doi: 10.1016/j.juro.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Khan SR, Atmani F, Glenton P, Hou Z, Talham DR, Khurshid M. Lipids and membranes in the organic matrix of urinary calcific crystals and stones. Calcif Tissue Int. 1996;59:357–65. doi: 10.1007/s002239900140. [DOI] [PubMed] [Google Scholar]
- 33.Khan SR, Glenton PA. Increased urinary excretion of lipids by patients with kidney stones. Br J Urol. 1996;77:506–11. doi: 10.1046/j.1464-410x.1996.09324.x. [DOI] [PubMed] [Google Scholar]
- 34.Khan SR, Glenton PA, Backov R, Talham DR. Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int. 2002;62:2062–72. doi: 10.1046/j.1523-1755.2002.00676.x. [DOI] [PubMed] [Google Scholar]
- 35.Khan SR, Shevock PN, Hackett RL. In vitro precipitation of calcium oxalate in the presence of whole matrix or lipid components of the urinary stones. J Urol. 1988;139:418–22. doi: 10.1016/s0022-5347(17)42447-5. [DOI] [PubMed] [Google Scholar]
- 36.Khan SR, Shevock PN, Hackett RL. Membrane-associated crystallization of calcium oxalate in vitro. Calcif Tissue Int. 1990;46:116–20. doi: 10.1007/bf02556095. [DOI] [PubMed] [Google Scholar]
- 37.Sheng G, Liu D, Kuang M, Zhong Y, Zhang S, Zou Y. Utility of Non-high-density Lipoprotein Cholesterol to high-density lipoprotein cholesterol ratio in evaluating Incident Diabetes Risk. Diabetes Metab Syndr Obes. 2022;15:1677–86. doi: 10.2147/dmso.S355980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin D, Qi Y, Huang C, Wu M, Wang C, Li F, Yang C, Yan L, Ren M, Sun K. Associations of lipid parameters with insulin resistance and diabetes: a population-based study. Clin Nutr. 2018;37:1423–9. doi: 10.1016/j.clnu.2017.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.