Abstract
Herpes simplex virus (HSV) glycoproteins gE and gI form an immunoglobulin G (IgG) Fc receptor (FcγR) that binds the Fc domain of human anti-HSV IgG and inhibits Fc-mediated immune functions in vitro. gE or gI deletion mutant viruses are avirulent, probably because gE and gI are also involved in cell-to-cell spread. In an effort to modify FcγR activity without affecting other gE functions, we constructed a mutant virus, NS-gE339, that has four amino acids inserted into gE within the domain homologous to mammalian IgG FcγRs. NS-gE339 expresses gE and gI, is FcγR−, and does not participate in antibody bipolar bridging since it does not block activities mediated by the Fc domain of anti-HSV IgG. In vivo studies were performed with mice because the HSV-1 FcγR does not bind murine IgG; therefore, the absence of an FcγR should not affect virulence in mice. NS-gE339 causes disease at the skin inoculation site comparably to wild-type and rescued viruses, indicating that the FcγR− mutant virus is pathogenic in animals. Mice were passively immunized with human anti-HSV IgG and then infected with mutant or wild-type virus. We postulated that the HSV-1 FcγR should protect wild-type virus from antibody attack. Human anti-HSV IgG greatly reduced viral titers and disease severity in NS-gE339-infected animals while having little effect on wild-type or rescued virus. We conclude that the HSV-1 FcγR enables the virus to evade antibody attack in vivo, which likely explains why antibodies are relatively ineffective against HSV infection.
Herpes simplex virus (HSV) establishes latency within sensory ganglia and periodically reactivates to produce recurrent infections. Latency is one mechanism used by HSV to evade immune attack, since during latency few if any viral proteins are produced and the virus remains hidden from the host. But how does the virus evade host immunity during recurrent infection? Virus can generally be recovered from lesions for several days after reactivation despite an already primed immune system.
HSV encodes at least 11 glycoproteins (48), several of which are essential for virus replication since they mediate virus entry or egress (30, 40, 53). Others are nonessential for replication in vitro yet are conserved in nature, suggesting an important role in vivo. Glycoproteins gE and gI are among the nonessential HSV glycoproteins. gE and gI form a hetero-oligomer complex that functions as a receptor for the Fc domain of immunoglobulin G (IgG) (5, 32, 33, 41). gE alone acts as a lower affinity IgG Fc receptor (FcγR), binding IgG aggregates but not IgG monomers, while the gE-gI complex acts as a higher-affinity FcγR, binding both IgG monomers and aggregates (6, 12).
IgG FcγRs are fairly widely distributed among human pathogens. Cells infected by HSV type 2 (HSV-2) (42), varicella-zoster virus (36), and cytomegalovirus (37) express virus-encoded IgG FcγRs. Certain protozoa (schistosomes and trypanosomes) (15, 50) and bacteria (for example, staphylococci [protein A] and streptococci [protein G]) (7, 47) also express IgG Fc binding proteins. Therefore, understanding the role of the HSV-1 FcγR in immune evasion may have broad implications for understanding microbial pathogenesis.
Initial studies of the HSV FcγR focused on its role in binding nonimmune IgG (1, 8, 11); however, the FcγR preferentially binds anti-HSV IgG by a process called antibody bipolar bridging (16, 51). This occurs when an HSV antibody molecule binds to its antigenic target by its Fab end and the Fc domain of the same molecule binds to the HSV-1 FcγR. In vitro studies indicate that the HSV FcγR inhibits complement-enhanced antibody neutralization (16), antibody-dependent cellular cytotoxicity (13), and attachment of granulocytes to the Fc domain of antibodies on HSV-infected cells (51). These results support a possible role for the FcγR in immune evasion and form the basis for studying the biologic relevance of the HSV-1 FcγR in vivo.
gE and gI play an important role in virus spread from cell to cell (2, 9, 10). This has created an obstacle to investigate the role of the HSV-1 FcγR in pathogenesis, since HSV-1 gE or gI null viruses are practically avirulent (2, 10, 43), probably because of their inability to spread. Therefore, to study the role of the FcγR in virulence it was necessary to develop HSV-1 mutant viruses that are deficient in Fc binding while retaining other gE and gI functions. Using this rationale, an HSV-1 mutant virus that has a four-amino-acid insert within the gE IgG Fc binding domain was generated (3, 14). This FcγR− virus remained intact for virus spread at the skin inoculation site in mice and caused disease comparable in severity to that caused by wild-type and marker-rescued viruses. In the presence of anti-HSV IgG, the FcγR− virus was significantly more susceptible to antibody attack than FcγR+ strains, indicating that the HSV FcγR promotes immune evasion in vivo.
MATERIALS AND METHODS
Cells and antibodies.
African green monkey kidney cells (Vero) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 20 μg of gentamicin per ml, and 20 mM HEPES (pH 7.3). Anti-gE monoclonal antibody (MAb) 1BA10 (17) and anti-gI MAb Fd69 (39) were previously described. Pooled human IgG (165 mg/ml) was purchased from the Michigan Department of Public Health, Lansing. This reagent is prepared by pooling serum from thousands of normal donors. Characteristics of the pooled human anti-HSV IgG are as follows: anti-HSV-1 enzyme-linked immunosorbent assay (ELISA) titer, 1:150,000; anti-gE ELISA titer, 1:30,000, anti-gI ELISA titer, 1:10,000; neutralizing antibody titer against wild-type or FcγR− mutant virus in the absence of complement, 100 μg/ml. In addition, by Western blot analysis, human anti-HSV IgG recognizes purified HSV-1 glycoproteins gC, gD, gE, and gH, and in an immunoprecipitation assay the IgG reacts comparably with wild-type gE and a mutant form of gE that has four amino acids inserted at position 339. The human anti-HSV IgG nonimmune serum was obtained from HSV-1- and HSV-2-seronegative donors (18) and purified on a protein G affinity column (Sigma Chemical Co., St. Louis, Mo.). Murine anti-HSV serum was pooled from mice 2 to 4 weeks after recovering from wild-type HSV-1 flank infection. IgG was purified from serum on a protein A affinity column (Bio-Rad Laboratories, Hercules, Calif.). The neutralizing titer of murine anti-HSV IgG in the absence of complement was 50 μg/ml against wild-type and FcγR− mutant virus.
Viruses.
Wild-type HSV-1 strain NS is a low-passage-number clinical isolate and was used for the generation of mutant viruses (18). To construct a gE null virus (NS-gEnull), the entire gE coding sequence was excised from pCMV3gE-1 with XbaI and cloned into pSPT18 (14). A 1.1-kb HpaI-BglII fragment from amino acids 124 to 508 (Fig. 1B) was excised, and the HpaI site was changed to a BglII site. A 4.3-kb fragment derived from pD6P (22) containing the Escherichia coli β-galactosidase gene under the control of the HSV ICP6 promoter was cloned into the BglII site. The resultant vector contains 374 bp of NS DNA sequences 5′ and 225 bp 3′ of the ICP6::lacZ cassette and was used to construct the gE null virus. The XbaI fragment containing the flanking sequence vector was isolated, and 750 ng was cotransfected into Vero cells with 1.0 μg of NS DNA by the calcium phosphate transfection method. Four hours later, the DNA-calcium phosphate mixture was removed and cells were shocked with 15% glycerol. Cells were harvested when cytopathic effects were noted in 30 to 40% of cells, and cells were sonicated to prepare a virus pool. Recombinant gE null virus expressing β-galactosidase was selected by infecting Vero cells and overlaying with 0.5% agarose, 5.0% fetal bovine serum, and 300 μg of 5-bromo-d-galactopyranoside (X-Gal). Blue plaques were picked and purified twice in X-Gal agarose overlay and once by limiting dilution. Virus was purified from supernatant fluids of infected Vero cells on a 5 to 70% sucrose gradient.
FIG. 1.
(A) Southern blot of wild-type, gE mutant, and rescued viruses. NS, NS-gEnull, rNS-gEnull, NS-gE339, rNS-gE339, and NS-gE406 were digested with NruI alone (lanes 1 to 6) or with NruI and XhoI to detect XhoI linkers in NS-gE339 and NS-gE406 (lanes 7 to 12). The blot was probed with a 1.1-kb HpaI-BglII gE fragment. The position of the 2.4-kb gE band is shown on the right, and positions (in kilobases) of DNA size markers are shown on the left. (B) Model of HSV-1 gE, a 550-amino-acid glycoprotein. Sig, predicted signal sequence, amino acids 1 to 23; # # #, the domain on gE, amino acids 235 to 264, that interacts with gI to form a hetero-oligomer complex (3); ∗∗∗, the region of gE, amino acids 235 to 380, which comprises the IgG Fc binding domain (3, 14); •••, the gE domain of homology with mammalian FcγRs. gE amino acids 322 to 359 have 46% identity and 66% similarity with domain 2 of human FcγRII (14). TM refers to the predicted transmembrane domain of gE, amino acids 420 to 444. Arrows at amino acids 339 and 406 indicate the positions of four amino acids (ARAA) inserted within and outside, respectively, the IgG Fc binding domain. HpaI and BglII sites were used to delete gE amino acids 124 to 508. The ICP6::lacZ cassette was cloned into this site to generate the gE null virus. The shaded balloons indicate potential N-linked glycosylation sites at gE amino acids 124 and 243. C’s mark the cysteine positions in the extracellular domain of gE at amino acids 63, 88, 271, 280, 289, 297, 314, 323, and 359.
Rescued virus, rNS-gEnull, was prepared by cotransfection of Vero cells with 1.0 μg of NS-gEnull DNA and 1.5 μg of wild-type gE fragment purified from pCMV3gE-1 (14). Progeny viruses were examined by immunoperoxidase staining using anti-gE MAb 1BA10 to confirm expression of gE on the surface of infected cells (29). Plaques were purified by limiting dilution, and virus pools were prepared.
gE linker insertion plasmids H339 and H406 (14) were used to generate recombinant viruses NS-gE339 and NS-gE406, respectively. Plasmid H339 has a four-amino-acid XhoI linker inserted after gE amino acid 339, which is within the IgG Fc binding domain (3, 14). Plasmid H406 contains the same four amino acids inserted after gE amino acid 406, which lies outside the IgG Fc binding domain (Fig. 1B) (3, 14). To construct recombinant viruses, 1.0 μg of NS-gEnull DNA was cotransfected with 1.5 μg of plasmid H339 or H406, and recombinants were selected by immunoperoxidase staining using anti-gE MAb 1BA10 and purified by limiting dilution.
Marker rescue of NS-gE339 virus was accomplished by cotransfecting Vero cells with virus DNA from NS-gE339 and wild-type gE DNA purified from pCMV3gE-1. Virus was harvested and used to infect cells in a 48-well plate at a concentration of 0.5 to 1 PFU/well. Virus was expanded from wells containing single plaques that were screened for rescue of FcγR activity by using biotin-labeled nonimmune monomeric IgG, which was incubated with infected cells for 30 min at 4°C and then reacted with strepavidin-phycoerythrin (4). Viruses positive for immunofluorescence were purified three times by limiting dilution, and one clone, designated rNS-gE339, was chosen for further studies.
Southern blots were performed to confirm proper construction of mutant and rescued viruses. When infected Vero cells reached 100% cytopathic effects, DNA was extracted and 1.0 μg of DNA was digested with NruI alone or NruI and XhoI to detect XhoI linkers in NS-gE339 or NS-gE406. The blot was probed using a 1.1-kb HpaI-BglII fragment (Fig. 1B) deleted from gE in NS-gEnull virus.
Fluorescence-activated cell sorting analysis.
Double-label staining was performed to detect gE expression and IgG Fc binding. Vero cells were infected at a multiplicity of infection of 5 for 16 h and harvested by using cell dissociation buffer (Gibco BRL, Grand Island, N.Y.). Cells were incubated for 60 min at 4°C with anti-gE MAb 1BA10 and biotin-labeled nonimmune monomeric human IgG (50 μg/ml). Cells were washed and then incubated with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse IgG and strepavidin-phycoerythrin for 60 min at 4°C, fixed in 1% paraformaldehyde, and analyzed by dual-channel immunofluorescence (FACScan; Becton Dickinson). To detect gI expression, infected Vero cells were incubated with anti-gI MAb Fd69 (39) and FITC-conjugated goat F(ab′)2 anti-mouse IgG.
Complement-enhanced antibody neutralization.
Assays were performed by incubating 104 to 105 PFU of wild-type or mutant viruses with anti-HSV pooled human IgG at a concentration (100 μg/ml) that neutralized 50% of the virus in the absence of complement (16). Ten percent human serum obtained from an HSV-1- and HSV-2-seronegative donor, or heat-inactivated serum as a control, was added as source of complement for 60 min at 37°C, and titers were determined by plaque assay on Vero cells. Complement-enhanced antibody neutralization results are expressed as titer (log10) when virus is incubated with antibody plus heat-inactivated complement minus titer (log10) when virus is incubated with antibody plus active complement.
Murine flank model.
The shaved right flanks of 5- to 6-week-old female BALB/c mice were denuded by using a depilatory cream. Twenty-four hours later, virus (5 × 103 to 5 × 105 PFU) in 10 μl of sterile Dulbecco modified Eagle medium was applied to the denuded flank several millimeters from the spinal column and scratched gently 30 times with a 27-gauge needle in an approximate 3- by 3-mm area (45, 46). Disease scores were recorded daily and expressed as the sum of the scores from days 3 to 8 postinfection. Disease at the inoculation site was scored as follows: 0 points for no disease; 0.5 for swelling without vesicles; 1.0 point for each vesicle or scab to a maximum daily score of 5 points. If vesicles or scabs became confluent, points were assigned based on the size of the confluent lesion. Swelling and lesions at locations separate from the site of inoculation were considered dermatomal or zosteriform lesions. Scoring of these lesions was the same as at the inoculation site except that a maximum daily score of 10 was used since a larger number of lesions could be counted over the greater skin area involved.
Mice were passively immunized intraperitoneally (44, 46) with pooled human anti-HSV IgG, murine anti-HSV IgG, or nonimmune human IgG 16 h prior to flank infection. Animals were scored for disease at the inoculation and zosteriform spread sites. Passive transfer experiments were performed with 200, 500, or 2,000 μg of IgG/mouse, concentrations which were selected based on previous reports using human antibodies for passive protection (24).
Viral titers of skin lesions.
Mice were euthanized, and a 0.5-cm2 area of skin was excised from the inoculation site 1, 2, 3, or 5 days postinfection and stored at −70°C. Skin samples were thawed and Dounce homogenized, and virus titers were determined on Vero cells.
RESULTS
Characterization of HSV-1 gE mutant viruses.
We previously reported that gE mutant viruses NS-gEnull and NS-gE339 are FcγR−, and that NS-gE339 has a cell-to-cell spread phenotype similar to that of wild-type virus in epidermal keratinocyte (HaCaT) cells (52). We now report results that confirm proper construction of gE null virus NS-gEnull, gE mutant viruses NS-gE339 and NS-gE406, and gE rescued viruses rNS-gEnull and rNS-gE339, and we define the importance of the HSV-1 FcγR in immune evasion by examining in vitro and in vivo characteristics of NS-gE339.
A Southern blot was prepared from viral DNA digested with NruI or NruI plus XhoI to demonstrate XhoI linkers inserted in gE (Fig. 1A). The blot was probed with a 1.1-kb HpaI-BglII gE fragment deleted from NS-gEnull (Fig. 1B). Following NruI digestion, a 2.4-kb fragment containing the entire gE protein coding sequence was detected in all viruses except NS-gEnull (Fig. 1A, lanes 1 to 6). Digestion with NruI and XhoI revealed two DNA gE-1 fragments in NS-gE339 and NS-gE406, indicating the presence of XhoI linkers at the expected positions in these viruses (Fig. 1A, lanes 10 and 12). The absence of XhoI linkers in rNS-gE339 indicates rescue of this mutant (Fig. 1A, lane 11). To confirm that NS-gEnull contains the ICP6::lacZ gene, DNA was digested with NruI and analyzed by Southern blotting using lacZ as a probe. The expected 6.4-kb fragment was detected (result not shown). When the blot was probed with the 374-bp 5′ gE-1 flanking sequence, the lacZ insert was demonstrated to be within gE (result not shown).
NS-gE339 is a gE+ gI+ FcγR− mutant virus.
A number of FcγR− viruses have been studied previously (2, 9, 10); however, mutant viruses were gE null, which complicates efforts to separate FcγR activity from other functions mediated by gE. Double-label flow cytometry was performed to measure both FcγR activity and gE expression at the surface of cells infected with wild-type, gE mutant, or rescued viruses (Fig. 2). NS expresses gE and binds IgG (Fig. 2A). NS-gEnull does not express gE or bind IgG (Fig. 2B). NS-gE339 expresses gE but does not bind IgG (Fig. 2C), indicating that this mutant virus is gE+ FcγR−. This was anticipated since the mutation was created within the gE Fc binding domain (Fig. 1B). NS-gE406 expresses gE and binds IgG (Fig. 2D), indicating that this mutant virus is gE+ FcγR+, which was expected since the mutation was outside the gE Fc binding domain (Fig. 1B). rNS-gEnull expresses gE and binds IgG (Fig. 2E), indicating rescue of gE null. rNS-gE339 expresses gE and binds IgG (Fig. 2F), indicating rescue of the FcγR phenotype. We conclude that NS and our panel of mutant and rescued viruses have the expected gE and Fc binding phenotypes. Our previous studies demonstrated that both gE and gI are required to bind nonimmune monomeric IgG (12). Therefore, NS-gE339 gI expression was measured at the surface of infected cells by flow cytometry and was found similar to expression of wild-type and rescued virus (not shown), which indicates that the phenotype of mutant virus NS-gE339 is gE+ gI+ FcγR−.
FIG. 2.
Double-label flow cytometry for gE expression and monomeric IgG binding. Cells were infected with wild-type, gE mutant, and rescued viruses and analyzed for gE expression by using MAb anti-gE 1BA10 and FITC F(ab′)2 anti-mouse IgG (x axis) or for FcγR activity by using biotin-labeled monomeric nonimmune IgG and strepavidin-phycoerythrin (y axis). Fluorescence in the upper right quadrant indicates both gE expression and IgG binding, fluorescence in the lower left quadrant indicates neither gE expression nor IgG binding, while fluorescence in the lower right quadrant indicates gE expression but no IgG binding. (A) NS; (B) NS-gEnull; (C) NS-gE339; (D) NS-gE406; (E) rNS-gEnull; (F) rNS-gE339.
NS-gE339 is defective in antibody bipolar bridging.
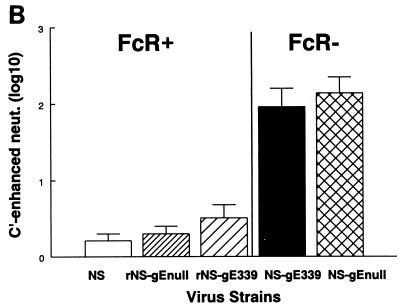
We previously showed that an FcγR− mutant virus is not capable of antibody bipolar bridging (16). Thus, FcγR− virus or virus-infected cells are more susceptible to activities mediated by the IgG Fc domain, including complement activation and antibody-dependent cellular cytotoxicity (13, 16). We tested whether FcγR− virus NS-gE339 is capable of antibody bipolar bridging by measuring its susceptibility to complement in an assay that detects complement-enhanced antibody neutralization (Fig. 3A). To this end, 104 to 105 PFU was incubated with human anti-HSV IgG and active complement, or heat-inactivated complement as a control, and the neutralization mediated by of the addition of complement was measured. Human anti-HSV IgG was used at a concentration of 100 μg/ml, which was the titer that neutralized 50% of each virus strain. FcγR+ viruses NS, rNS-gE339, and rNS-gEnull showed only 0.2- to 0.5-log10 (2- to 3-fold) additional neutralization when complement was added to human HSV antibodies, while FcγR− viruses NS-gE339 and NS-gEnull demonstrated 2- to 2.1-log10 (100- to 126-fold) additional neutralization in the presence of complement (P < 0.01, FcγR− viruses compared with the FcγR+ strains) (Fig. 3B). As controls, viruses were incubated with antibody or complement but not both. Differences in neutralization among the viruses emerged only when viruses were incubated with antibody plus complement. As an additional control, viruses were incubated with murine anti-HSV IgG. The HSV-1 FcγR binds the Fc domain of human, but not murine, IgG (31); therefore, the viral FcγR should not protect against complement-enhanced antibody neutralization using murine IgG as the source of antibody (16). When the panel of viruses was incubated with murine antibody plus complement, no differences were detected among the viruses (result not shown). Therefore, we conclude that the four-amino-acid insert in NS-gE339 renders the virus incapable of antibody bipolar bridging and that the NS-gE339 mutant virus is as susceptible to complement-enhanced antibody neutralization as is NS-gEnull virus.
FIG. 3.
(A) Model showing the HSV-1 FcγR blocking complement-enhanced antibody neutralization. On the left, an antibody molecule (red) binds to its target antigen (shown in green as HSV-1 glycoprotein gD) by its Fab domain. The absence of an FcγR enables C1q (brown) to bind to the antibody Fc domain, leading to activation of complement and complement-enhanced antibody neutralization. On the right is shown an example of antibody bipolar bridging in which an antibody molecule (red) binds to its target antigen (green) by the Fab domain while the Fc domain of the same antibody molecule binds to the HSV-1 FcR (blue), which blocks the interaction of C1q (brown) with the IgG Fc domain. (B) Complement-enhanced antibody neutralization of FcγR+ viruses NS, rNS-gE339, and rNS-gEnull and FcγR− viruses NS-gE339 and NS-gEnull. Each virus was incubated with pooled human IgG at 100 μg/ml, which resulted in 50% neutralization in the absence of complement. Then 10% nonimmune human serum or heat-inactivated serum was added, and complement-enhanced neutralization was calculated by determining the additional neutralization mediated by including complement in the reaction. Results are the mean (± SEM) of three experiments.
NS-gE339 causes disease at the inoculation site in the mouse flank model.
Previous studies showed that gE null viruses are virtually avirulent (43), likely because gE is required for virus spread (2, 10). Our intent was to develop an FcγR− mutant virus that remained intact for cell-to-cell spread so that we could separate the multiple functions mediated by gE. We hypothesized that experiments using mice may enable us to separate FcγR and cell spread functions, since murine IgG Fc does not bind to the HSV FcγR (31). Therefore, the absence of an FcγR should have no impact on disease in mice.
FcγR− virus NS-gE339 was scratched onto the flanks of mice, and the skin lesions at the inoculation site were counted. At an inoculum of 5 × 105 PFU, NS-gE339 produced disease scores comparable to those of NS, NS-gE406, and rNS-gE339, while NS-gEnull produced few lesions (each virus compared with NS-gEnull, P < 0.001) (Fig. 4A). Additional experiments were performed with wild-type, NS-gE339, and rNS-gE339 viruses to determine if NS-gE339 produced comparable disease scores over a range of inocula. Figure 4B demonstrates that disease scores were similar for the three viruses when inoculated at 10- and 100-fold-lower doses. We conclude that FcγR− mutant virus NS-gE339 differs from previously described gE mutants since it produces disease similar to those produced by wild-type and rescued viruses at the inoculation site.
FIG. 4.
(A) Disease scores at the inoculation site in the mouse flank after infection by wild-type, NS, FcγR+ mutant, NS-gE406, FcγR− mutants, NS-gE339 and NS-gEnull, and rescued virus, rNS-gE339. An inoculum of 5 × 105 PFU was scratched onto the denuded flank of 10 BALB/c mice per group. The average (± SEM) cumulative disease scores from days 3 to 8 are shown at the inoculation site. (B) An inoculum of 5 × 104 or 5 × 103 PFU was scratched onto the denuded flank of each of five mice per group, and the mean (± SEM) cumulative disease scores from days 3 to 8 were calculated.
In the flank model, HSV-1 travels from the skin to the dorsal root ganglia and then returns to the skin by axonal transport to produce lesions in a zosteriform distribution (45, 46). Animals infected with NS, rNS-gE339, NS-gE339, or NS-gEnull were scored for zosteriform spread disease. NS-gEnull caused no disease, most likely because of defective spread. FcγR− mutant NS-gE339 produced zosteriform lesions (at 5 × 105 PFU, NS-gE339 lesion scores were 21.5 ± 3.5 compared with NS-gEnull score of 0; P < 0.001); however, NS-gE339 caused less disease than NS, NS-gE406, or rNS-gE339 (NS scores were 41.5 ± 1.8, NS-gE406 scores were 37.3 ± 1.6, and rNS-gE339 scores were 38 ± 1.9; P < 0.01 compared with NS-gE339). Thus, NS-gE339 is intact for cell spread at the skin inoculation site; however, the virus may be partially defective in neuronal spread. In support of a possible neuronal spread defect is the observation that zosteriform lesions developed on average 1.3 to 1.4 days later in animals infected with NS-gE339 than rNS-gE339 or NS. We conclude that NS-gE339 is a gE+ gI+ FcγR− mutant virus that is defective in antibody bipolar bridging but capable of causing disease scores similar to those of wild-type and rescued viruses at the inoculation site. By focusing on inoculation site disease rather than on zosteriform spread disease, we are now able to evaluate the role of the HSV-1 FcγR in immune evasion without confounding interpretation of results because of defective cell spread.
Passive transfer of IgG.
A second set of murine experiments was performed to evaluate the hypothesis that the HSV FcγR is critical in pathogenesis because it mediates immune evasion. The experiments involved passive transfer of human anti-HSV IgG into mice and then infecting the mice with FcγR− or FcγR+ virus. These studies take advantage of the fact that human anti-HSV IgG is capable of binding to the HSV FcγR by antibody bipolar bridging (16). We postulated that FcγR− virus should be more readily inhibited by human anti-HSV IgG because the Fc domain of the antibody molecule would be available to mediate activities such as complement-enhanced antibody neutralization, antibody-dependent cellular cytotoxicity, and complement-dependent cellular cytotoxicity.
Pooled human IgG was used as source of HSV antibodies. Passive immunization experiments were performed by intraperitoneal injection with 200 μg of IgG, 2,000 μg of IgG, or saline as a control; 16 h later, mice were infected in the flank with 5 × 105 PFU of FcγR− virus NS-gE339 or FcγR+ virus NS or rNS-gE339. Prior to infection, mouse serum was tested for neutralizing antibody titers. An intraperitoneal inoculation of 2,000 μg of anti-HSV IgG resulted in antibody neutralizing titers of 1:16 in the absence of complement, while an inoculum of 200 μg produced titers of <1:8. Passive transfer of antibody at each IgG concentration had little effect on FcγR+ viruses; however, antibody significantly reduced disease scores in animals infected with FcγR− virus and passively immunized with 200 μg of human anti-HSV IgG (P < 0.0001, NS-gE339 compared with NS or rNS-gE339) or 2,000 μg of human anti-HSV IgG (P < 0.001, NS-gE339 compared with NS or rNS-gE339) (Fig. 5A). We conclude that HSV antibody is significantly more effective in reducing disease scores of FcγR− than FcγR+ viruses.
FIG. 5.
(A) Mice were passively immunized with 200 or 2,000 μg of human anti-HSV IgG, or saline (0 μg IgG) as a control, and then infected 16 h later with FcγR+ virus NS or rNS-gE339 or FcγR− virus NS-gE339. Ten mice were evaluated at each data point. Disease scores in saline controls were set as 100%, and as shown in Fig. 4A, these scores were similar for all three viruses. Percent disease scores at 200 or 2,000 μg of human anti-HSV IgG were calculated as [mean (± SEM) disease score in animals receiving human anti-HSV IgG/mean disease score in saline-treated animals] × 100. (B) Mice were passively immunized with 200 or 2,000 μg of nonimmune human IgG, or saline (0 μg of IgG) as a control. Five mice were included at each data point. Percent disease scores were calculated as [mean (± SEM) disease score in animals receiving nonimmune IgG/mean disease score in saline-treated animals] × 100. (C) Mice were passively immunized with 200 or 2,000 μg of murine anti-HSV IgG, or saline (0 μg of IgG), as a control. Five mice were included at each data point. Percent disease scores were calculated as [mean (± SEM) disease score in animals receiving murine anti-HSV IgG/mean disease score in saline-treated animals] × 100.
Mice were passively immunized with 200 or 2,000 μg of nonimmune human IgG to determine if antibodies must be capable of antibody bipolar bridging to have a greater effect on FcγR− than FcγR+ viruses. The IgG Fc domain of nonimmune IgG can bind to the HSV-1 FcγR; however, the Fab domain does not bind to HSV antigens, and therefore nonimmune IgG is not capable of bipolar bridging. Nonimmune IgG had little effect on disease (Fig. 5B). Since nonimmune human IgG did not modify HSV-1 disease, we conclude that a more important function of the HSV-1 FcγR is to block activities of the Fc domain of anti-HSV IgG.
As an additional control, mice were passively immunized with murine anti-HSV-1 IgG inoculated at 200 or 2,000 μg/mouse. This antibody is also not capable of antibody bipolar bridging because the Fc domain of murine IgG does not bind to the HSV-1 FcγR (31). We postulated that murine anti-HSV IgG would have equal effects on FcγR− and FcγR+ viruses. Results shown in Fig. 5C support our hypothesis. We conclude that the role of the HSV-1 FcγR is to protect the virus or virus-infected cell against human anti-HSV IgG.
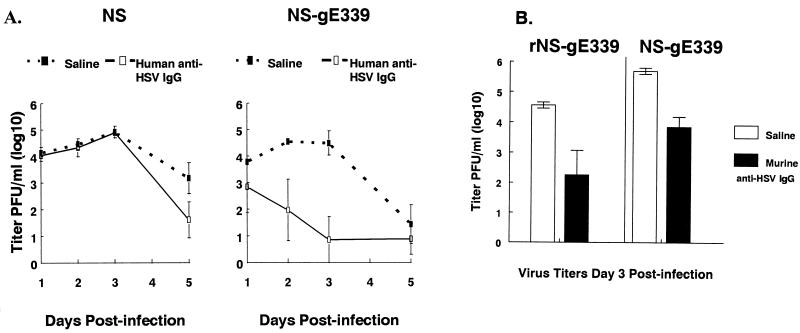
To further demonstrate a role for the HSV-1 FcγR in immune evasion, viral titers were performed on skin excised from the inoculation site. Animals were passively immunized with human anti-HSV IgG at 500 μg/animal, or saline as control, and then infected with wild-type or NS-gE339 virus (Fig. 6A). NS and NS-gE339 viruses grew to similar titers in saline control animals, which indicates that there is no defect in NS-gE339 replication in skin. Anti-HSV IgG had little effect on NS titers but had a dramatic effect on NS-gE339 titers. By day 1 postinfection, NS-gE339 titers were approximately 10-fold lower in antibody-treated animals than in saline controls, and by day 3, differences in titers reached approximately 10,000-fold (P < 0.01). Virus titers at day 3 were also measured in animals infected with rescued strain, rNS-gE339. Virus titers in saline-treated controls were 5.2 log10 ± 0.1 (1.6 × 105 PFU/ml), compared with 4.6 log10 ± 0.1 (4 × 104 PFU/ml) in animals treated with human anti-HSV IgG (mean ± standard error of the mean [SEM] of four animals each). Thus, the rescued strain showed fourfold differences in titers between antibody-treated and untreated animals. This contrasts sharply with the FcγR− mutant strain, which showed 10,000-fold differences. Additional studies comparing the effects of murine anti-HSV IgG on virus titers in animals infected with FcγR− mutant or rescued virus were performed (Fig. 6B). At 3 days postinfection, murine anti-HSV IgG had similar effects on the two virus strains, showing an approximate 2-log10 decrease in titers. We conclude that the FcγR provides marked protection to the virus or infected cell against human, but not murine, anti-HSV IgG.
FIG. 6.
(A) Viral titers in skin excised from the inoculation site 1, 2, 3, or 5 days after infection with FcγR+ virus NS or FcγR− virus NS-gE339. Animals were passively immunized with 500 μg of human anti-HSV IgG or saline as a control and infected 16 h later. Data represent the mean (± SEM) of four (days 1 and 2), five (day 3), and eight (day 5) mice per group. (B) Viral titers in skin excised from the inoculation site 3 days postinfection with rescued FcγR− virus rNS-gE339 or FcγR− virus NS-gE339. Animals were passively immunized with 500 μg of murine anti-HSV IgG or saline as a control and infected 16 h later. Results are the mean (± SEM) of four mice except rNS-gE339 saline controls, which represent three mice.
DISCUSSION
Studies to define the biological relevance of the HSV-1 FcγR have been hampered by the fact that gE null viruses are markedly attenuated in vivo, probably because of defects in cell spread. The approach in the present study was to alter only a small region within the gE IgG Fc binding domain so that we could isolate an FcγR− mutant virus that retains other functions mediated by gE. We constructed gE mutant virus NS-gE339, which has the following phenotype: gE+, gI+, negative for binding nonimmune monomeric IgG, negative for antibody bipolar bridging, and intact for producing lesions at the skin inoculation site. Therefore, FcγR− mutant virus NS-gE339 has the phenotype required for in vivo studies to define the role of the HSV-1 FcγR in pathogenesis.
In prior experiments using cells transfected with gE linker insertion plasmids, we found that plasmid H339 did not form an FcγR, whereas cells transfected with plasmid H406 expressed an FcγR (3, 14). When these mutant genes were introduced into the HSV genome, the resulting HSV-1 virus, NS-gE339, failed to demonstrate FcγR activity, while NS-gE406 had FcγR activity similar to wild-type activity. These results indicate that the FcγR phenotypes of gE linker insertion plasmids H339 and H406 were maintained when recombined into virus.
The mutation at gE amino acid 339 causes loss of FcγR activity. This mutation lies within a cysteine-rich region of the molecule (Fig. 1B), which could raise concerns about whether the gE protein was grossly malformed. However, we believe that the mutation disrupts the FcγR domain, as intended, for the following reasons. (i) The mutation at position 339 is within the gE domain homologous to the site on mammalian FcγRII that binds IgG (14, 28), suggesting that this region is involved in IgG Fc binding. (ii) Prior studies using gD-gE fusion proteins demonstrated that a gE domain from amino acid 183 to 402 binds IgG (14). This includes the cysteine-rich domain of gE, which suggests that amino acids in this region form the FcγR. Therefore, the mutation at position 339 is likely to be within the Fc binding domain. (iii) In NS-gE339, gE is expressed on the virus and at the infected cell surface. If the protein were grossly malfolded, we would not expect gE transport to remain intact. (iv) A four-amino-acid mutation at position 380, which is outside the cysteine-rich region, has been recombined into virus, and this mutant strain is also FcγR− (18a). This suggests that gross alterations in structure are not required to disrupt FcγR activity. (v) NS-gE339 causes disease similar to that caused by wild-type virus in skin, while NS-gEnull does not. If the mutation at 339 had grossly altered gE conformation, we would not expect virulence to remain intact in skin (2). Therefore, we conclude that the mutation at position 339 alters FcγR activity because it disrupts the Fc binding domain.
Studies were performed with mice by using passive transfer of human anti-HSV IgG to determine if the HSV-1 FcγR protects against antibody attack. Based on in vitro results that demonstrated that FcγR+ viruses are capable of binding to and inhibiting activity of the IgG Fc domain, we postulated that passively transferred antibodies would have a greater effect on FcγR− than FcγR+ virus in vivo. Our results showed highly significant reduction in disease caused by FcγR− virus NS-gE339 compared with FcγR+ viruses NS and rNS-gE339. Results of passive transfer experiments using murine anti-HSV IgG or nonimmune human IgG further supported the hypothesis that the FcγR protects by blocking Fc-mediated activities. Neither murine anti-HSV IgG nor human nonimmune IgG is capable of bipolar bridging. The former binds only by its Fab domain to HSV antigens, while the latter binds only by its Fc domain to the HSV FcγR. Murine anti-HSV IgG inhibited disease scores of FcγR− and FcγR+ viruses in vivo to comparable extents, which supports the interpretation that antibody bipolar bridging accounts for the greater effects of human anti-HSV IgG on FcγR− virus. Nonimmune human IgG had no effect on FcγR− virus NS-gE339. This was expected, since this virus cannot bind IgG; however, the lack of effect on FcγR+ virus indicates that binding of nonimmune human IgG to the HSV-1 FcγR has no apparent impact on virulence.
Virus titers were measured in skin samples to support the conclusion that the HSV-1 FcγR promotes immune evasion. We noted marked differences in virus titers in skin of mice passively immunized with human anti-HSV IgG and infected with FcγR− virus. By day 3, NS-gE339 titers were 10,000-fold lower in animals immunized with human anti-HSV IgG compared with saline controls. In contrast, anti-HSV IgG had little effect on wild-type virus titers, since only small differences were detected between antibody-treated and saline controls and these differences were not detected until day 5 postinfection. Titers of the two viruses were similar in saline-treated controls, which indicates that the differences between NS and NS-gE339 cannot be explained by defective NS-gE339 cell-to-cell spread in skin. As controls, virus titers were also measured at the inoculation site 3 days after infection of animals passively immunized with murine anti-HSV IgG. As expected, murine antibody had similar effects on FcγR+ and FcγR− viruses. Therefore, differences in animals treated with human anti-HSV IgG are attributable to the effects of the HSV-1 FcγR in modifying IgG Fc-mediated activity.
A role for gE in epidermal spread is supported by the findings that gE null virus produces little disease at the inoculation site and small plaques in epidermal cells (52). In contrast, NS-gE339 is intact for epidermal spread, since virus skin titers, inoculation site disease scores, and epidermal cell plaque size (52) are comparable to those for wild-type virus. However, NS-gE339 causes less zosteriform spread disease than wild-type virus, suggesting that the mutant virus may be defective in epidermal-neuronal spread, neuronal transport, or transneuronal spread. Therefore, the gE domain interrupted by the mutation at position 339 disrupts spread in some cell types without affecting others, which suggests that different gE domains may mediate epidermal and neuronal spread.
Additional immune evasion strategies have been described for HSV-1. Glycoprotein gC binds complement components C3 and its enzymatic cleavage products, C3b, iC3b, and C3c (17, 34). gC prevents the interaction of properdin with C3b (29), and blocks C5 interaction with C3b (19, 34). These activities inhibit activation of the complement cascade, thereby protecting HSV-1 from complement-mediated neutralization (18, 21, 25, 38) or HSV-infected cells from complement-mediated injury (23). ICP47 is an immediate-early HSV-1 protein that interferes with the TAP (transporter associated with antigen processing) system, preventing HSV peptides from being expressed within the context of major histocompatibility complex class I antigens (20, 26, 49, 54). ICP47 inhibits peptide expression in human but not mouse cells (49, 54), while gC, gE, and gI also show species specificity for human immune proteins (18, 27, 31). Proof that immune evasion is important in vivo has been hampered by the species specificity of the interactions between viral proteins and the immune system. To circumvent this, the approach taken in this study was to passively transfer human IgG into mice, which enabled an assessment of the importance of human IgG-FcγR interactions in pathogenesis.
Results of this study help explain why antibodies are relatively ineffective in modifying HSV infection; however, the experiments do not address which aspects of IgG Fc-mediated immunity, such as complement activation, antibody-dependent cellular cytotoxicity, or complement-dependent cellular cytotoxicity, are inhibited by the HSV-1 FcγR. Our results suggest that virus neutralization in the absence of complement does not account for the effects of antibodies, since serum obtained from mice passively immunized with human anti-HSV IgG at 200 μg per mouse had a neutralizing titer of <1:8; nevertheless, this concentration had a marked effect on disease scores and viral titers of NS-gE339 virus.
The passive transfer murine model mimics conditions that exist following HSV vaccination, in that antibodies are present prior to infection. A modification of the model can be used to more closely simulate conditions during reactivation infection by delaying passive immunization with anti-HSV IgG until virus reaches the ganglion. Rabbit corneal infection can also be used to define the role of the FcγR in reactivation disease, since virus reactivates spontaneously in this model (35), and passive transfer of IgG is not necessary because rabbit IgG Fc binds to the HSV-1 FcγR (31). However, studies of reactivation disease will require an FcγR− mutant virus that is intact for spread from ganglion to skin so that the role of gE in FcγR activity and cell spread can be clearly distinguished. The experiments performed in this study define a role for the HSV-1 FcγR in antibody evasion but do not address whether the FcγR is most important during primary or reactivation infection.
The fact that the HSV-1 FcγR blocks the effectiveness of antibodies administered prior to infection raises important questions regarding vaccine strategies to prevent HSV disease. Will the HSV-1 FcγR reduce the effectiveness of vaccine-induced antibodies? If so, attempts to block HSV-1 immune evasion may require that gE and/or gI be included in a subunit vaccine. Vaccine strategies to block HSV FcγR activity may be difficult to develop, since despite the high titers of antibodies to gE and gI in the pooled human IgG used for passive transfer studies, the antibodies did not effectively block FcγR activity of wild-type virus. This was apparent in complement-enhanced antibody neutralization experiments that used pooled human IgG and in antibody passive transfer studies. In these experiments pooled human IgG did not block FcγR activity since FcγR+ virus did not escape antibody attack. Critical epitopes involved in forming the HSV FcγR may be inaccessible to the immune system, or perhaps the epitopes are immunologically privileged because of sequence homology with mammalian FcγRs.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by NIH grant AI 33063.
We thank Stuart Isaacs and Ronald Collman for thoughtful comments on the manuscript and Cindy Friedman for help with the artwork.
REFERENCES
- 1.Adler R, Glorioso J C, Cossman J, Levin M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978;21:442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Dubin G, Basu M, Nguyen V, Friedman H M. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J Immunol. 1995;154:260–267. [PubMed] [Google Scholar]
- 4.Basu S, Dubin G, Nagashunmugam T, Basu M, Goldstein L T, Wang L, Weeks B, Friedman H M. Mapping regions of herpes simplex virus type 1 glycoprotein I required for formation of the viral Fc receptor for monomeric IgG. J Immunol. 1997;158:209–215. [PubMed] [Google Scholar]
- 5.Baucke R B, Spear P G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979;32:779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell S, Cranage M, Borysiewicz L, Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoprotein E and I of herpes simplex virus type 1. J Virol. 1990;64:2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;133:969–974. [PubMed] [Google Scholar]
- 8.Costa J, Rabson A S, Yee C, Tralka T S. Immunoglobulin binding to herpes simplex virus-induced Fc receptors inhibits virus growth. Nature. 1977;269:251–252. doi: 10.1038/269251a0. [DOI] [PubMed] [Google Scholar]
- 9.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell to cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell K S, Doering L C, Johnson D C. Glycoprotein E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowler K W, Veltri R W. In vitro neutralization of HSV-2: inhibition by binding normal IgG and purified Fc to virion Fc receptor (FcR) J Med Virol. 1984;13:251–259. doi: 10.1002/jmv.1890130307. [DOI] [PubMed] [Google Scholar]
- 12.Dubin G, Frank I, Friedman H M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990;64:2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubin G, Socolof E, Frank I, Friedman H M. The herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991;650:7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubin G, Basu S, Mallory D L P, Basu M, Tal-Singer R, Friedman H M. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J Virol. 1994;68:2478–2486. doi: 10.1128/jvi.68.4.2478-2485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira de Miranda-Santos I K, Campos-Neto A. Receptor for immunoglobulin Fc on pathogenic but not on nonpathogenic protozoa of the Trypanosomatidae. J Exp Med. 1981;154:1732–1742. doi: 10.1084/jem.154.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank I, Friedman H M. A novel function of the herpes simplex virus type 1 FcR: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989;63:4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman H M, Cohen G H, Eisenberg R J, Seidel C A, Cines D B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 18.Friedman H M, Wang L, Fishman N O, Lambris J D, Eisenberg R J, Cohen G H, Lubinski J. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70:4253–4260. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Friedman, H. M., et al. Unpublished data.
- 19.Fries L F, Friedman H M, Cohen G H, Eisenberg R J, Hammer C H, Frank M M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986;137:1636–1641. [PubMed] [Google Scholar]
- 20.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 21.Gerber S I, Belval B J, Herold B C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology. 1995;214:29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein D J, Weller S K. An ICP6::lacZ insertion mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris S L, Frank I, Yee A, Cohen G H, Eisenberg R J, Friedman H M. Glycoprotein C of herpes simplex virus 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990;162:331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 24.Hayashida I, Nagafuchi S, Hayashi Y, Kino Y, Mori R, Oda H, Ohtomo N, Tashiro A. Mechanism of antibody-mediated protection against herpes simplex virus infection in athymic nude mice: requirement of Fc portion of antibody. Microbiol Immunol. 1982;26:497–509. doi: 10.1111/j.1348-0421.1982.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 25.Hidaka Y, Sakai Y, Toh Y, Mori R. gC of HSV-1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of infected cells. J Gen Virol. 1991;72:915–921. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- 26.Hill A, Jugovic P, York I, Russ G, Bennick J, Yewdell J, Ploegh H, Johnson D C. Herpes simplex virus turns of the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 27.Huemer H P, Larcher C, van Drunen Littel-van den Hurk S, Babiuk L A. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch Virol. 1993;130:353–364. doi: 10.1007/BF01309666. [DOI] [PubMed] [Google Scholar]
- 28.Hulett M D, Wifurt E, Brinkworth R I, McKenzie I F C, Hogarth P M. Identification of the IgG binding site of the low affinity receptor for IgG Fc gamma RII: enhancement and ablation of binding by site-directed mutagenesis. J Biol Chem. 1994;269:15287–15293. [PubMed] [Google Scholar]
- 29.Hung S-L, Peng C, Kostavasili I, Friedman H M, Lambris J D, Eisenberg R J, Cohen G H. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology. 1994;203:299–312. doi: 10.1006/viro.1994.1488. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson L, Johnson D C. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson P J H, Myhre E B, Blomberg J. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J Virol. 1985;56:489–494. doi: 10.1128/jvi.56.2.489-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N G. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostavasili I, Sahu A, Friedman H M, Eisenberg R J, Cohen G H, Lambris J D. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J Immunol. 1997;158:1763–1771. [PubMed] [Google Scholar]
- 35.Kwon B S, Gangarosa L P, Green K, Hill J M. Kinetics of ocular herpes simplex virus shedding induced by epinephrine iontophoresis. Invest Ophthalmol Visual Sci. 1982;22:818–821. [PubMed] [Google Scholar]
- 36.Litwin V, Jackson W, Grose C. Receptor properties of two varicella-zoster virus glycoproteins gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacCormac L P, Grundy J E. Human cytomegalovirus induces an Fc γ receptor in endothelial cells and fibroblasts that is distinct from the human cellular FcγRs. J Infect Dis. 1996;174:1151–1161. doi: 10.1093/infdis/174.6.1151. [DOI] [PubMed] [Google Scholar]
- 38.McNearney T A, Odell C, Holers V M, Spear P G, Atkinson J P. HSV gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987;166:1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metcalf J F, Chaterjee S, Coga J, Whitley R J. Protection against herpetic ocular disease by immunotherapy with monoclonal antibodies to herpes simplex virus glycoprotein. Intervirology. 1988;29:39–49. doi: 10.1159/000150027. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 41.Para M F, Bauke R B, Spear P G. Immunoglobulin G (Fc)-binding receptor on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980;34:512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Para M F, Goldstein L, Spear P G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982;41:137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajcani J, Hergert U, Kaermer H C. Spread of herpes simplex virus strains SC16, ANG, ANGpath, and its gC and gE mutants in DBA/2 mice. Acta Virol. 1990;34:305–320. [PubMed] [Google Scholar]
- 44.Sanna P P, De Logu A, Williamson R A, Hom Y-L, Straus S E, Bloom F E, Burton D R. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215:101–106. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
- 45.Simmons A, Nash A A. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons A, Nash A A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985;53:944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjodahl J. Repetitive sequences in protein A from Staphylococcus aureus—arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur J Biochem. 1977;73:343–351. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 48.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 49.Tomazin R, van Schoot N E G, Goldsmith K, Jugovic P, Sempe P, Fruh K, Johnson D C. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol. 1998;72:2560–2563. doi: 10.1128/jvi.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torpier G, Capron A, Ouissi M A. Receptor for IgG (Fc) and human β 2-microglobulin on S. mansoni schistosomula. Nature. 1979;278:447–449. doi: 10.1038/278447a0. [DOI] [PubMed] [Google Scholar]
- 51.Van Vliet K E, De Graaf-Miltenburg L A M, Verhof J, Van Strijp J A G. Direct evidence of antibody bipolar bridging on herpes simplex virus-infected cells. Immunology. 1992;77:109–115. [PMC free article] [PubMed] [Google Scholar]
- 52.Weeks B S, Sundaresan P, Nagashunmugam T, Kang E, Friedman H M. The herpes simplex virus-1 glycoprotein E (gE) mediates IgG binding and cell-to-cell spread through distinct gE domains. Biochem Biophys Res Commun. 1997;235:31–35. doi: 10.1006/bbrc.1997.6720. [DOI] [PubMed] [Google Scholar]
- 53.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce-de-Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]