Abstract
The protein encoded by the US28 gene of human cytomegalovirus (HCMV) has homology to G protein-coupled receptors (GCR). Previous studies demonstrated that recombinant US28 protein can bind the β class of chemokines (K. Neote, D. DiGregorio, J. Y. Mak, R. Horuk, and T. J. Schall, Cell 72:415–425, 1993) and induce a rise in intracellular calcium after the binding of chemokines (J. L. Gao and P. M. Murphy, J. Biol. Chem. 269:28539–28542, 1994). In order to investigate the function of the US28 protein in virus-infected cells, a recombinant HCMV (HV5.8) was constructed, with the US28 open reading frame disrupted by the insertion of the Escherichia coli gpt gene and the gene for the green fluorescent protein. The US28 gene is not required for growth in human fibroblasts (HF). HF infected with wild-type HCMV bound RANTES at 24 h postinfection and demonstrated an intracellular calcium flux induced by RANTES. In cells infected with HV5.8, RANTES did not bind or induce a calcium flux, demonstrating that US28 is responsible for the β-chemokine binding and induced calcium signaling in HCMV-infected cells. The ability of the US28 gene to bind chemokines was shown to cause a significant reduction in the concentration of RANTES in the medium of infected cells. Northern analysis of RNA from infected cells showed that US28 is an early gene, while US27 (another GCR) is a late gene.
Open reading frames (ORF) with homology to cellular seven transmembrane spanning receptors have been identified in the genomes of both beta and gamma herpesviruses (15, 37). Many cellular seven transmembrane spanning receptors have been shown to be G protein-coupled receptors (GCR) and comprise a superfamily of genes encoding the receptors for a variety of biological compounds, including neurotransmitters, hormones, odorants, and chemotactic agents. GCR link the binding of an extracellular ligand to processes within the cell by their activation of associated G proteins. G proteins can activate serine/threonine kinases, phosphatidylinositol 3-kinase, phospholipases, or Ras (9). These proteins, in turn, can stimulate mitogen-activated protein kinase or generate second messenger molecules, such as diacylglycerol and inositol triphosphate, resulting in the activation of protein kinase C and increases in intracellular Ca2+ levels (9). Ultimately, these processes result in the amplification of the initial signal transduced by the ligand-GCR interaction into complex cellular processes such as chemotaxis.
GCR are receptors for chemokines, derived from chemotactic cytokine, a multigene family of 70- to 90-amino-acid soluble proteins that are excreted from a variety of cell types and play important roles in leukocyte trafficking and immune regulation (7). Two classes of these structurally similar proteins are defined by the first two of four conserved cysteines. In the α class (e.g., interleukin-8 [IL-8], MGSA, and GCP-2) the first two cysteines are separated by an intervening residue (C-X-C), while in the β class (e.g., RANTES, MIP-1α, MIP-1β, and MCP-1) they are adjacent (C-C). In general, the α-chemokines attract primarily neutrophils, while β-chemokines can have activity on monocytes, lymphocytes, eosinophils, and basophils (46).
The human cytomegalovirus (HCMV) US28 ORF shows approximately 33% homology to the cellular β-chemokine receptor CCR-1 (35). Conserved features of viral and cellular proteins include the putative seven-membrane spanning regions and cysteines implicated in disulfide bond formation. The sequence homology between US28 and cellular GCR led to the identification of β-chemokines as the ligand for the viral receptor. Recombinant HCMV US28 protein expressed in 293 cells was shown to bind β-chemokines (35), and as with the binding of chemokines by their cellular receptors, the binding of ligand by recombinant US28 expressed in K562 cells led to an increase in intracellular calcium (21).
During an acute infection, HCMV can be found in the blood as well as in numerous tissues, with the lungs, kidneys, salivary gland, and liver being commonly involved. HCMV has been identified in a wide variety of cells both in culture and in patients’ tissue, including epithelial cells, endothelial cells, fibroblasts, monocytes/macrophages, and lymphocytes (33, 47, 52). Because HCMV can infect cell types that respond to chemokines and cell types that produce chemokines, the viral GCR may mimic the functions of cellular GCR, but the role of the expression of a viral GCR in viral biology and the cell type in which it is important are not known.
While researchers examined US28 function with recombinant protein in previous studies (21, 36), we have investigated the functions of the US28 gene expressed from the viral genome. We have constructed a recombinant HCMV with the US28 ORF disrupted by the genes for the green fluorescent protein (GFP) and guanine phosphoribosyl transferase (GPT) and have demonstrated that US28 is responsible for the functions of a GCR in HCMV-infected cells.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts (HF) were grown in Dulbecco’s modified Eagle’s medium (Gibco Laboratories) supplemented with 10% Nu serum (Collaborative Research, Inc.), 0.1 mg of streptomycin, 100 U of penicillin, and 2 mM l-glutamine in a humidified 5% CO2 37°C incubator. HCMV (Towne) was used for all experiments.
Construction of recombinant virus.
The 6-kb BamHI Q fragment (216298 to 222296 of the published sequence [14]) of AD169 was cloned into the BamHI site of pOK7 to generate pQ62. A deletion was then generated between the BspHI (219198) and the StuI (219627) sites contained within the BamHI Q fragment to create pQ63. This construct, pQ63, was digested with BclI and a 2.5-kb BamHI fragment consisting of the Escherichia coli gpt gene expressed by the herpes simplex virus (HSV) tk promoter and the GFP gene under the control of the rat β-actin promoter was inserted into the BclI site (position 219666) to create pQ64. For the transfection of HF, pQ64 was digested with BamHI, extracted with phenol-CHCl3 and CHCl3, ethanol precipitated, and dried. The DNA was resuspended in STE (5 mM NaCl, 5 mM Tris-HCl [pH 7.5], 1 mM EDTA) at a concentration of 1 to 2 mg/ml, and 20 μg was used per electroporation. For electroporation, HF were trypsinized, mixed with an equal volume of medium, pelleted at 500 × g for 5 min, and resuspended in electroporation buffer (a 1:3 mixture of OptiMEM I [Gibco] and cytomix [54] [120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4-KH2PO4 [pH 7.6], 5 mM MgCl2]). In 0.4 ml of electroporation buffer 2 × 106 to 5 × 106 HF cells plus DNA were electroporated with a BTX ECM 600 instrument set at 285 V and 1,075 μF in a 4-mm cuvette at room temperature. Cells were plated following electroporation and infected with HCMV at a multiplicity of infection (MOI) of 2 to 5 at 18 to 24 h postelectroporation. Progeny virus from these cultures, harvested 5 days postinfection (dpi), was used to infect fresh HF and cultured in medium containing 25 μg of xanthine per ml and 15 μg of mycophenolic acid per ml. Virus was harvested 2 days post-100% cytopathic effect, and selection with mycophenolic acid was repeated. Progeny virus from the second mycophenolic acid selection was then used to plaque purify recombinant virus by twofold-limiting dilution in 96-well plates. Recombinant virus was identified as green fluorescent plaque under 450- to 490-nm UV illumination.
Two additional viruses, HV5.6 and HV5.7, that have the same US28 deletion and use the same insertion site as HV5.8 have been constructed. HV5.6 was constructed with pQ63 with the insertion of the BamHI LacZ-GPT cassette of pON855 (55) at the BclI site. This virus was generated and selected for with mycophenolic acid and xanthine as described above for HV5.8. Recombinant virus was plaque purified with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overlay as described previously (49). HV5.7, which has only the insertion of the 240-bp simian virus 40 (SV40) polyadenylation signal (position 2533 to 2770) at the BclI site, was generated from HV5.6 with back selection against the gpt gene with the drug 6-thioguanine by growing HV5.6 in human Lesch-Nyhan (hypoxanthine-GPT deficient) skin fibroblasts (25). The generation of HV5.7 is possible because the LacZ-GPT cassette of pON855 is flanked by direct repeats of the SV40 polyadenylation signals and recombination can occur between these two repeated sequences, deleting the LacZ and GPT genes, making the virus resistant to 6-thioguanine, and leaving a single SV40 polyadenylation signal as the insert (41, 56). Genome structure of these recombinant HCMV was confirmed by viral DNA analysis as described previously for HV5.8 (data not shown).
Radioligand binding and displacement.
Displaceable binding of 125I-labeled chemokine was performed on HF at various times after infection. Cells (2 × 106 cells per ml) were incubated with 0.5 nM radiolabeled ligands and varying concentrations of unlabeled ligands at 4°C for 2 h. The incubation was terminated by removing aliquots from the cell suspension and separating cells from buffer by centrifugation through a silicon-paraffin oil mixture (43). Nonspecific binding was determined in the presence of 1 mM unlabeled ligand. Individual assay determinations, representative of at least three separate experiments, are plotted. Iodine-labeled chemokines were obtained from Dupont/NEN (Boston, Mass.), and unlabeled chemokines were obtained from R&D Systems (Minneapolis, Minn.). Both homologous and heterologous chemokine displacements were measured. Experiments were carried out with two independently isolated recombinant viruses.
Cytoplasmic calcium measurements.
HF were loaded with 2 mM indo-1/AM (Molecular Probes, Inc., Eugene, Oreg.) in complete growth medium at 20°C for 45 min. Cells were then washed, resuspended in Na-Hanks balanced salt solution (2 mM CaCl2, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM glucose, 20 mM HEPES, pH 7.3) containing 1% bovine serum albumin and maintained at 20°C for up to 2 h. Approximately 5 × 105 cells were then suspended in 2 ml of Na-Hanks balanced salt solution and maintained at 37°C in a constantly stirred acrylic cuvette. Fluorescence measurements to determine the increase in cytosolic-free Ca2+ concentration ([Ca2+]i) were done with a Photon Technologies Inc. spectrofluorimeter with an excitation wavelength of 350 nm (4-nm bandwidth) and dual simultaneous monitoring of emission at 405 and 485 nm (10-nm bandwidth). The ratio of emission at 405 and 485 nm was measured at a rate of 2 Hz. Experiments were carried out with two independently isolated recombinant viruses.
RNA analysis.
HF in 60-mm-diameter dishes were infected at an MOI of 5 PFU/cell. When used, ganciclovir (30 μm) was added to the culture medium 1 h before infection. At various times postinfection, whole-cell RNA was harvested (16) and analyzed by formaldehyde agarose gel electrophoresis and Northern hybridization (22) with a 32P-labeled probe. The US27 probe included the sequence from 217761 to 219008 of the AD169 sequence, and the US28 probe was composed of a PCR-generated clone of the US28 ORF (36).
Determination of RANTES concentration in culture supernatants.
HF seeded in 15.5-mm wells were infected at an MOI of 3, with Towne, HV5.8, UV-inactivated Towne, or heat-inactivated Towne (60°C, 20 min) or left uninfected. At the end of 1 h of absorption, the inoculum was removed and the cells washed with phosphate-buffered saline, and 0.3 ml of medium was added to each well. For each experimental interval, the medium was removed from the culture and 0.3 ml of fresh medium was added. The collected medium was centrifuged for 5 min at 1,500 × g, and the supernatant was removed to a fresh tube and stored at −70°C until analyzed. The RANTES concentration was determined with an enzyme-linked immunosorbent assay kit for RANTES (R&D Systems) following the manufacturer’s instructions.
RESULTS
Construction and analysis of recombinant HCMV.
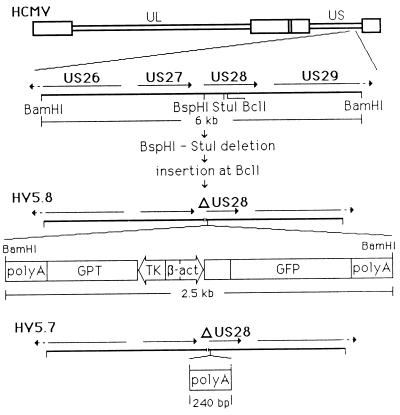
The steps for the construction of HV5.8, a recombinant HCMV (Towne) with the disruption of the US28 ORF by a deletion and the insertion of a GPT-GFP cassette, are diagrammed in Fig. 1. The construct used to construct HV5.8, pQ64, consisted of the BamHI Q fragment (position 216298 to 222296 of the Ad169 sequence) with a deletion of the first 40% of the US28 ORF between positions 219200 (BspHI) and 219629 (StuI). A GPT-GFP cassette was inserted at position 219666 (BclI) within the US28 ORF. The GPT-GFP construct (Fig. 1) contains the E. coli gpt gene expressed by the HSV thymidine kinase promoter (55) and the GFP gene (13) expressed from the rat β-actin promoter (38). The transcripts of both genes are terminated by early polyadenylation signals of the bidirectional SV40 polyadenylation signals (12), which can provide polyadenylation functions for viral genes upstream of the insertion site (55). HV5.8 was generated by pQ64-transfected HF, which were subsequently infected with HCMV (Towne). Progeny virus was grown under selection for the gpt gene for two cycles, and recombinant virus containing the GFP was plaque purified by the identification of green fluorescent plaques under 450- to 490-nm illumination.
FIG. 1.
Structure of HV5.8, HV5.7, and the GPT-GFP insert. Schematic diagram of the HCMV genome with the expansion of the 6-kb BamHI fragment containing the US28 gene used to construct recombinant virus. The BspHI-to-StuI deletion removed 430 bp of the US28 ORF. The BclI site is the insertion point for the GPT-GFP cassette in HV5.8 or the SV40 polyadenylation segment (nucleotides 2533 to 2774 [20]) in HV5.7. The components of the GPT-GFP cassette are (starting at the left) SV40 sequence [poly(A)], nucleotides 2533 to 2774 (20); the E. coli guanine-xanthine phosphoribosyl transferase gene (GPT), nucleotides 667 to 121 (40); the HSV thymidine kinase promoter (TK), nucleotides 47925 to 48108 (31); SV40 sequence, nucleotides 128 to 37 (20); the rat β-actin promoter (β-act), nucleotides 124 to 262 (38); the human T-cell leukemia virus R region, nucleotides 96 to 270 (42); the A. victoria GFP gene, nucleotides 26 to 742 (39); SV40 sequence [poly(A)], nucleotides 2774 to 2533 (20).
Two additional viruses, with the same deletion of US28 as HV5.8, but with different inserts at the BclI site (219666), have also been constructed. HV5.6 has the LacZ-GPT insert from pON855 (55) at the BclI site, and HV5.7 (Fig. 1) has only the 240-bp SV40 polyadenylation sequence inserted and therefore does not express any heterologous proteins. HV5.7 was generated from HV5.6 by back selection against the gpt gene with the drug 6-thioguanine (25).
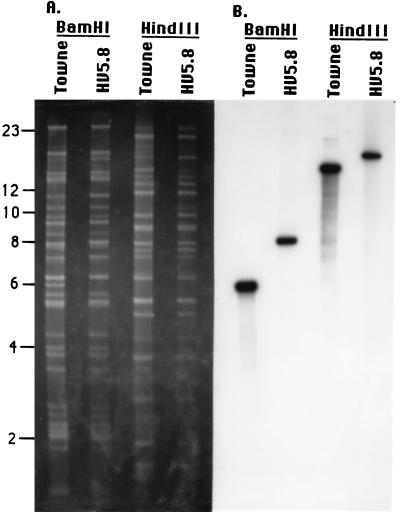
Viral DNA from HV5.8 was isolated to examine the structure of the viral genome and to confirm the absence of wild-type HCMV. Viral DNAs of HV5.8 and HCMV (Towne) were digested with BamHI or HindIII and separated by agarose gel electrophoresis. The DNA was analyzed by ethidium bromide staining (Fig. 2A) and by hybridization with a 32P-labeled DNA probe containing US27 and US28 sequences (Fig. 2B). The comparison of HCMV and HV5.8 DNAs in the ethidium bromide gel showed no alterations outside of the US28-containing fragments. The hybridization analysis showed that the 6-kb BamHI and the 14-kb HindIII fragments (which contain US28) detected by the probe in HCMV (Towne) were absent from HV5.8 and that fragments 2 kb larger than the HCMV (Towne) fragments were present, as expected.
FIG. 2.
Analysis of the genomic structure of HV5.8. Viral DNA was prepared from HCMV (Towne) and HV5.8, digested with BamHI or HindIII, and electrophoresed on a 0.6% agarose gel. (A) Ethidium-bromide-stained viral DNA. Molecular size standards in kilobase pairs are indicated on the left. (B) Autoradiograph resulting from the hybridization analysis of the viral DNAs with the US27-US28 32P-labeled probe depicted in Fig. 7.
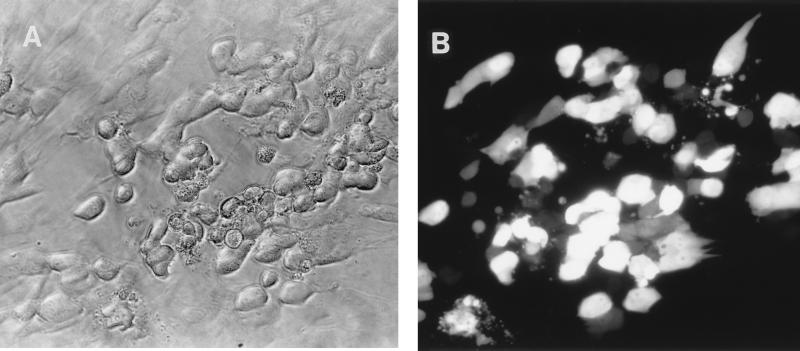
The recombinant virus HV5.8 carries the gene for the GFP from the jellyfish Aequorea victoria (39) expressed from the rat β-actin promoter. The use of the GFP as a marker for recombinant virus allows the visualization of infected cells by fluorescent microscopy (Fig. 3). In addition, the presence of the GFP makes possible the sorting of infected cells by fluorescence-activated cell sorting, sorted cells remained viable, and recombinant virus could be recovered (data not shown).
FIG. 3.
Visualization of HCMV plaque on HF. (A) Photomicrogragh of viral plaque under normal illumination. (B) HV5.8 plaque on HF photographed under 450- to 490-nm UV illumination with a Nikon microscope at 200× magnification.
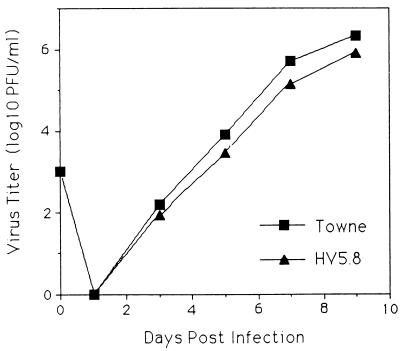
The growth kinetics of HV5.8 and HCMV (Towne) were compared (Fig. 4). A four- to fivefold reduction in peak titers was seen in HV5.8. This result was not likely due to secondary mutations outside of the US28 region, because three independently derived recombinant viruses showed the same phenotype (data not shown). Whether this phenotype is due to the loss of the US28 ORF, the loss of an ORF of 290 codons that overlaps approximately 75% of the US28 ORF and is also disrupted in HV5.8, or an effect of the insert has not been resolved.
FIG. 4.
Growth kinetics of HCMV (Towne) and HV5.8. HF were infected at an MOI of 0.05 with either wild-type HMCV or HV5.8, harvested at the time indicated, and frozen at −70°C. Samples were thawed and sonicated at the time of titering. Initial inocula took place on day 0, and the detection limit was 10 PFU/ml.
Chemokine binding.
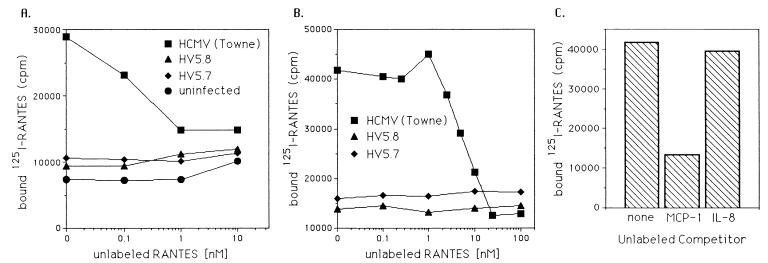
To determine if HCMV-infected cells would show specific chemokine binding, experiments with labeled chemokines as ligands were performed. HF infected with HCMV 24 and 48 h postinfection were incubated with 125I-RANTES and increasing amounts of unlabeled RANTES. The amount of labeled RANTES bound to the cells was determined, showing that 125I-RANTES bound to HCMV-infected cells, and this binding was prevented by competition with unlabeled RANTES (Fig. 5A and B). HF and HF infected with HV5.8 or HV5.7 at 24 or 48 h postinfection did not demonstrate 125I-RANTES binding (Fig. 5A and B). This binding was specific for β-chemokines in that MCP-1, like unlabeled RANTES, could compete with labeled RANTES binding, but IL-8 did not (Fig. 5C). In addition, cells 4 h postinfection with wild-type HCMV or HV5.8 did not show increased RANTES binding compared to uninfected cells (data not shown). The lack of chemokine binding by cells infected by both HV5.8 and HV5.7 supports the finding that US28 is the protein responsible for β-chemokine binding by infected cells.
FIG. 5.
Binding of 125I-labeled RANTES by infected cells. (A) Uninfected HF and HF infected for 24 h with wild-type HCMV, HV5.7, or HV5.8 were incubated with 125I-labeled RANTES in the presence of increasing amounts of unlabeled RANTES, and the amount of labeled RANTES was determined. (B) The displacement of 125I-labeled RANTES by unlabeled RANTES on cells infected with wild-type HCMV, HV5.7, or HV5.8 at 48 h postinfection. (C) The displacement of 125I-labeled RANTES from HCMV-infected cells 48 h postinfection by either MCP-1 or IL-8, at a concentration of 100 nM. cpm, counts per minute.
Calcium flux.
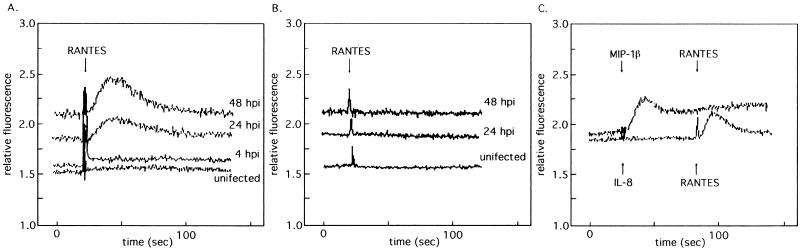
To test if the US28 ORF, expressed from the viral genome, was responsible for the induction of a calcium flux, the intracellular calcium level in HF infected with HCMV (Towne) or HV5.8 was monitored upon the addition of various chemokines. Changes in the intracellular calcium levels were monitored by loading infected cells with the fluorescent calcium indicator indo-1/AM and measuring the change in fluorescence, with an increase in relative fluorescence illustrating an increase in intracellular calcium. No change in intracellular calcium levels was seen upon the addition of RANTES in uninfected HF or in cells infected for 4 h with HCMV, while HF infected for 24 or 48 h demonstrated an increase in calcium with the addition of RANTES (Fig. 6A). In experiments with HV5.8, no calcium flux was detected with the addition of RANTES at any time point (Fig. 6B).
FIG. 6.
Intracellular calcium flux in HF infected by HCMV and HV5.8. At the times indicated after infection, cells were loaded with the calcium indicator, indo-1/AM, and monitored spectrofluorometrically during the addition of the indicated chemokine. (A) HCMV-infected cells at 4, 24, and 48 h postinfection and uninfected HF, treated with 1 μM RANTES. (B) HF and HF infected with HV5.8 at 24 and 48 h postinfection with the addition of 1 μM RANTES. (C) HF infected with HCMV at 24 h postinfection. The top trace shows cells treated with 1 μM MIP-1α followed by 1 μM RANTES, and the bottom trace shows cells treated with 1 μM IL-8 and then 1 μM RANTES.
It has been demonstrated that a recombinant US28 gene expressed in cell lines can elicit a calcium response to β-chemokines other than RANTES but not to α-chemokines (21). HF infected with HCMV were tested for the generation of a calcium flux in response to MIP-1β (a β-chemokine) and IL-8 (an α-chemokine) (Fig. 6C). A response to MIP-1β was detected, and a subsequent addition of RANTES did not give a second calcium flux. The addition of IL-8 to HCMV-infected cells did not elicit a calcium flux, but the subsequent addition of RANTES did generate a response. In addition, an increase in intracellular calcium was detected in response to the β-chemokines, MCP-1 and MIP-1α, but to a lesser degree than to RANTES or MIP-1β (data not shown).
Northern analysis of US27 and US28.
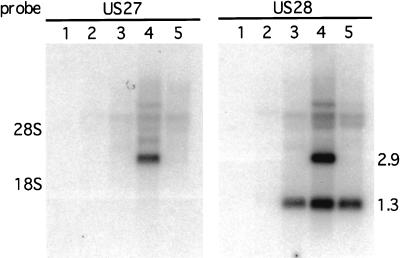
The binding and calcium flux data indicated that the US28 gene was expressed by 24 h postinfection. To confirm that this was true, the temporal expression of US28 and that of US27 were determined by Northern analysis of total RNA isolated from HF at 4, 24 and 48 h postinfection with HCMV (Towne) and at 48 h postinfection in the presence of ganciclovir. Figure 7 shows the Northern analysis of these samples with US27, or US28, derived probes. The US27 and US28 genes are transcribed in the same direction and terminate at a common polyadenylation signal at the end of US28, which results in a 2.9-kb US27 transcript and a 1.3-kb US28 transcript (57). No signal was detected in uninfected HF or at 4 h postinfection. A 1.3-kb transcript, corresponding to US28, was detected at 24 and 48 h postinfection, and a 2.9-kb transcript, from US27, was detected only at 48 h postinfection. In the presence of ganciclovir at 48 h postinfection only the 1.3-kb RNA was detected, identifying US28 as an early gene and US27 as a late gene.
FIG. 7.
Northern blot analysis of the US27 and US28 transcripts. Whole-cell RNA was isolated from mock-infected HF, HF infected at 4, 24, and 48 h postinfection, and HF infected at 48 h postinfection in the presence of ganciclovir. RNA was electrophoresed on a 1% agarose–1.2 M formaldehyde gel and blotted onto nitrocellulose. The autoradiograph resulting from the hybridization analysis of the RNA with either a US27-derived or a US28-derived 32P-labeled probe is shown. Lane 1, mock infected; lane 2, 4 h postinfection; lane 3, 24 h postinfection; lane 4, 48 h postinfection; lane 5, 48 h postinfection with ganciclovir. The positions of the 1.3 US28 and the 2.9 US27 transcripts as well as the positions of 28S and 18S rRNA are indicated.
US28 moderation of RANTES concentration in CMV-infected cultures.
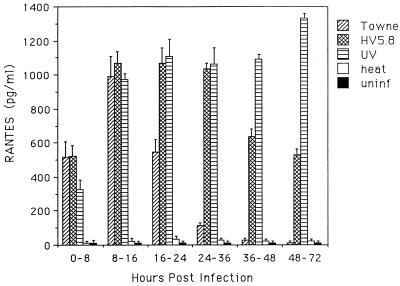
Human fibroblasts can be induced to produce RANTES by inoculation with tumor necrosis factor alpha (34) or CMV (32). The effect of US28 on the level of RANTES present in medium of infected cultures was determined by assaying the RANTES concentration in culture supernatants from HF, and HF infected with Towne, HV5.8, UV-inactivated virus, or heat-inactivated virus, at the time intervals indicated in Fig. 8. Uninfected HF and those infected with heat-inactivated virus produced minimally detectable levels of RANTES at all time points, while HF infected with Towne, HV5.8, and UV-inactivated virus showed a significant increase in RANTES by 8 h postinfection, with increased amounts at the 8- to 16-h interval. The level of RANTES in cultures infected with Towne rapidly declined after 16 h postinfection until 36 h postinfection, when levels were similar to those for uninfected cells. HV5.8-infected cells maintained a high level of RANTES through 36 h postinfection, but a reduction did occur 2 and 3 dpi. HF infected with UV-inactivated virus maintained elevated levels of RANTES expression at all time intervals.
FIG. 8.
RANTES concentration in culture supernatants collected at the time intervals indicated from cultures of HF infected with Towne, HV5.8, Towne inactivated with UV (UV), Towne inactivated with heat (heat), and uninfected HF (uninf). Results plotted are the averages of four experiments.
DISCUSSION
It was previously demonstrated that cell lines expressing recombinant US28 could bind β-chemokines and show a calcium flux in the presence of chemokine ligands (21, 35). In this study, we demonstrate that human fibroblasts infected with HCMV gain the functions consistent with those of a GCR that recognizes β-chemokines as a ligand. HCMV-infected cells bind RANTES, a β-chemokine, and respond to the addition of β-chemokines with the induction of an intracellular calcium flux. Cells infected with a virus lacking US28 did not show these functions, demonstrating that the functions of a GCR for β-chemokines seen in infected cells are due to US28 and not to other viral genes or to an induced cellular gene.
It was found that US28 is an early gene, while US27 is a late gene. The expression of the US28 transcript temporally corresponded to the functions of a GCR for β-chemokines identified in HCMV-infected cells. The expression of the US28 mRNA at 24 h postinfection contrasts with earlier work with AD169 indicating that both US28 and US27 were late genes (57). Possible reasons for the different results could be viral strain differences, the use of ganciclovir instead of phosphonoformic acid (PFA), or that in the previous work RNA was examined at only a single time point, 7 dpi, and the extended PFA treatment may have had nonspecific effects. A recent study in which PCR was used for the detection of RNA also suggested that the US28 gene is an early gene (32), but their methods could not distinguish between US27 or US28 as an early gene. It has been postulated that the HCMV GCR may be constituents of the virion and may account for the changes in intracellular calcium levels seen in cells shortly after infection (57). While the UL33 protein was shown to be a component of the virion (30), we did not detect the activity of the US28 protein in the first few hours of infection, indicating that the US28 protein is not a virion protein or that it is present at too low a level to be detected by our methods.
Human fibroblasts are capable of producing RANTES (34), and inoculation of HF with CMV can induce RANTES expression (32). We found that the US28 gene has a profound effect on the level of RANTES present in the medium of infected cells. There was a rapid increase in RANTES present in culture supernatants from cells inoculated with Towne, HV5.8, or UV-inactivated virus, whereas a steep decline in RANTES was seen in Towne-infected cultures starting at the 16- to 24-h postinfection interval; the level in HV5.8 infected cultures remained elevated. However, a reduction of RANTES in supernatants of cultures infected with HV5.8 was seen at 2 and 3 dpi, compared to cultures infected with UV-inactivated virus, suggesting that factors other than US28 can contribute to lowered chemokine levels. While this result would support a role for US28 in reducing the immune response to sites of infection, it remains to be determined whether this is indicative of a true immune evasion function for US28 or simply an in vitro effect of the expression of a functional chemokine receptor by the virus.
Genes encoding GCR are a common theme in the genomes of gamma and beta herpesviruses. The UL33, UL78, US27, and US28 ORFs of HCMV (15, 24), the U12 and U51 ORFs of human herpesvirus 6 (HHV-6) (24), ORF 74 of HHV-8 (45), and ECRF3 of herpesvirus saimiri (1) all have homology to cellular GCR. Epstein-Barr virus, a gamma herpesvirus without a viral GCR, induces the expression of two cellular GCR, EBI-1 and EBI-2 (8). EBI-1 is also induced by HHV-6 and -7 (26). The ubiquitous presence of GCR with beta and gamma herpesviruses suggests an important role for these proteins in viral pathogenesis, but this role has not yet been elucidated. Three possible functions have been suggested for viral GCR: (i) immune evasion, (ii) cell activation, and (iii) promotion of cell migration and virus dissemination. Our data showing that HCMV-infected cells at early times after infection gain the function of a GCR for β-chemokines can lend support to any of these roles postulated for viral GCR.
Viral genes involved in immune evasion that mimic cellular functions have been identified previously. Poxviruses express soluble proteins that bind IL-1β and gamma interferon (3, 51, 53), and the use of recombinant virus in animal models has shown that these proteins can influence the host response (2, 3). It is possible that the US28 protein sequesters chemokines, thereby reducing the host immune response to sites of infection. The potential importance of chemokines to the immune response is illustrated in transgenic mice lacking the β-chemokine MIP-1α, in which a delayed clearance of influenza virus was found (18). In support of its functioning to adsorb chemokines, US28 was shown to have greater binding affinity than CCR-1, one of the cellular receptors, for a spectrum of β-chemokines (35, 45a). Our data demonstrating that the US28 gene can reduce the level of RANTES released by an infected cell in vitro also support a possible role in immune evasion. Although the US28 gene may function in immune evasion by binding chemokines, the fact that the US28 protein transmits an intracellular signal suggests that it also has other functions, since signal transduction should not be required for sequestering chemokines alone.
The early kinetics of expression of US28 and the induction of processes involved in cell activation shown by the US28 protein are consistent with its having a role in activating a cell to allow or enhance viral replication. While CMV has been shown to replicate in a number of cell types that may express a chemokine receptor (10, 11, 19, 47), the superior binding of chemokines by the US28 GCR may be important for the activation of an infected cell at a level of ligand below that which activates uninfected cells. The US28 protein may also lead to the activation of cells by chemokines that do not naturally express a chemokine receptor.
Chemokines function as cell chemotactic agents in the induction of cell adhesion to endothelium and the enhancement of cell migration across the endothelium (6, 44). Thus, the US28 gene may function to influence the migration of infected cells. The β-chemokines can act as chemotactic agents for monocytes, T cells, and NK cells (4, 6, 29, 44), some of which can be infected by HCMV (10, 17, 19, 23, 27, 47, 48). The expression of the US28 protein would add to infected cells a GCR with high affinity for a number of β-chemokines (36). This receptor may therefore give infected cells an improved or novel migratory ability and thereby contribute to virus dissemination.
In the construction of the HCMV US28 mutant, the GFP was used as a marker that allowed the identification of recombinant plaques under UV illumination as well as the fluorescence-activated cell sorting of infected cells and the recovery of virus. Since the recombinant GFP gene, isolated from the jellyfish A. victoria (39), was first used as a genetic marker in Caenorhabditis elegans (13), it has been used in a variety of organisms, including yeast (5), mammalian cells (50), and transgenic mice (28). GFP is a valuable biological marker which allows a nonevasive detection that requires only illumination by UV light to yield green fluorescence. While the GFP is useful for the identification of recombinant virus, the greatest utility of GFP-tagged viruses may be for the noninvasive identification of infected cells. Future investigations on the functions of viral GCR will likely involve the study of monocytes and other cell populations in which only a subset of cells may be experimentally infected by CMV. In such cell populations, the GFP can allow the specific observation of infected cells or the isolation of infected cells for use in subsequent experiments regarding the study of viral GCR.
ACKNOWLEDGMENT
This study was supported in part by NIH grant AI26672 (awarded to A.P.G.).
REFERENCES
- 1.Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 2.Alcam’i A, Smith G L. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci USA. 1996;93:11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcam’i A, Smith G L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Bianchi G, Zhou D, van Damme J, J’Ilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 5.Atkins D, Izant J G. Expression and analysis of the green fluorescent protein gene in the fission yeast Schizosaccharomyces pombe. Curr Genet. 1995;28:585–588. doi: 10.1007/BF00518173. [DOI] [PubMed] [Google Scholar]
- 6.Audran R, Lesimple T, Delamaire M, Picot C, Van Damme J, Toujas L. Adhesion molecule expression and response to chemotactic agents of human monocyte-derived macrophages. Clin Exp Immunol. 1996;103:155–160. doi: 10.1046/j.1365-2249.1996.d01-4.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baggiolini M B, Dewald B, Moser B. IL-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–149. [PubMed] [Google Scholar]
- 8.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokoch G M. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 10.Braun R W, Reiser H C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986;60:29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brytting M, Mousavi Jazi M, Bostrom L, Larsson M, Lunderberg J, Ljungman P, Ringd’en O, Sundqvist V A. Cytomegalovirus DNA in peripheral blood leukocytes and plasma from bone marrow transplant recipients. Transplantation. 1995;60:961–965. [PubMed] [Google Scholar]
- 12.Carswell S, Alwine J C. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989;9:4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 14.Chee M S, Bankier A T, Beck S, Bohini R, Brown C M, Cerny T, Horsnell T, Hutchinson III C A, Kouzarides T, Martignetti E, Preddie E, Satchwell S C, Weston P K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:123–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 15.Chee M S, Satchwell S C, Preddie E, Weston P K M, Barrell B G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- 16.Chomezynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Cinatl J, Jr, Vogel J U, Cinatl J, Weber B, Rabenau H, Novak M, Kornhuber B, Doerr H W. Long-term productive human cytomegalovirus infection of a human neuroblastoma cell line. Int J Cancer. 1996;65:90–96. doi: 10.1002/(SICI)1097-0215(19960103)65:1<90::AID-IJC16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 19.Dankner W M, McCutchan J A, Richman D D, Hirata K, Spector S A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990;161:31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, Van Herreweghe J, Volckaert G, Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978;273:113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- 21.Gao J L, Murphy P M. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- 22.Geballe A P, Leach F S, Mocarski E S. Regulation of cytomegalovirus late gene expression: γ genes are controlled by posttranscriptional events. J Virol. 1986;57:864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerna G, Zipeto D, Percivalle E, Parea M, Revello M G, Maccario R, Peri G, Milanesi G. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J Infect Dis. 1992;166:1236–1244. doi: 10.1093/infdis/166.6.1236. [DOI] [PubMed] [Google Scholar]
- 24.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 25.Greaves R F, Brown J M, Vieira J, Mocarski E S. Selectable insertion and deletion mutagenesis of the human cytomegalovirus genome using the Escherichia coli guanosine phosphoribosyl transferase (gpt) gene. J Gen Virol. 1995;76:2151–2160. doi: 10.1099/0022-1317-76-9-2151. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa H, Utsunomiya Y, Yasukawa M, Yanagisawa K, Fujita S. Induction of G protein-coupled peptide receptor EBI 1 by human herpersvirus 6 and 7 infection in CD4+ T cells. J Virol. 1994;68:5326–5329. doi: 10.1128/jvi.68.8.5326-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibanez C E, Schrier R, Ghazal P, Wiley C, Nelson J A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikawa M, Kominami K, Yoshimura Y, Tanaka K, Nishimune Y, Okabe M. A rapid and non-invasive selection of transgenic embryos before implantation using green fluorescent protein (GFP) FEBS Lett. 1995;375:125–128. doi: 10.1016/0014-5793(95)01162-8. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher P, Seitz M, Clark Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- 30.Margulies B J, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of the herpes somplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 32.Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos F, Virelizier J-L, Landini M P. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J Virol. 1997;71:6495–6500. doi: 10.1128/jvi.71.9.6495-6500.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myerson D, Hackman R C, Nelson J A, Ward D C, McDougal J K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984;15:430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 34.Nelson P J, Kim H T, Manning W C, Goralski T J, Krensky A M. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- 35.Neote K, Darbonne W, Ogez J, Horuk R, Schall T J. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993;268:12247–12249. [PubMed] [Google Scholar]
- 36.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 37.Nicholas J, Cameron K R, Honess R W. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 38.Nudel U, Zakut R, Neuman S, Levy Z, Yaffe D. The nucleotide sequence of the rat cytoplasmic β-actin gene. Nucleic Acids Res. 1983;11:1759–1768. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasher D C, Eckenrode C K, Ward W W, Prendergast F G, Cormier M J. Primary structure of the Aequorea victoria green fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 40.Pratt D, Subramani S. Nucleotide sequence of the Escherichia coli xanthine-guanine phosphoribosyl transferase gene. Nucleic Acids Res. 1983;11:8817–8823. doi: 10.1093/nar/11.24.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard, M., and E. S. Mocarski. Personal communication.
- 42.Retzel E, Evanelista A, Maroushek S, Minnigan H, Larson A, Haase A T, Gonzalez-Dunia D, McFarlin D, Mingiolo E, Jacabson S, Osame M, Sonoda S. Nucleotide sequence analysis of a provirus derived from an individual with tropical spastic paraparesis. Microb Pathog. 1990;8:259–278. doi: 10.1016/0882-4010(90)90052-r. [DOI] [PubMed] [Google Scholar]
- 43.Robb R J, Greene W C, Rusk C M. Low and high affinity receptors for interleukin 2. J Exp Med. 1984;160:1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth S J, Carr M W, Springer T A. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon-gamma inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995;25:3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- 45.Russo J J, Bohensky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Yuan C, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Schall, T. Unpublished observations.
- 46.Schall T J, Bacon K B. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 47.Schrier R D, Nelson J A, Oldstone M B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985;230:1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- 48.Soderberg C, Larsson S, Bergstedt Lindqvist S, Moller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993;67:3166–3175. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spate R R, Mocarski E S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci USA. 1987;84:7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stauber R, Gaitanaris G A, Pavlakis G N. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology. 1995;213:439–449. doi: 10.1006/viro.1995.0016. [DOI] [PubMed] [Google Scholar]
- 51.Symons J A, Alcam’i A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 52.Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 53.Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 54.van der Hoff M J B, Moorman A F M, Lamers W H. Electorporation in ‘intracellular’ buffer increases cell survival. Nucleic Acids Res. 1992;20:2902. doi: 10.1093/nar/20.11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieira J, Farrell H E, Rawlinson W D, Mocarski E S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells: insertion mutagenesis with a lacZ/gpt cassette. J Virol. 1994;68:4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieira, J. Unpublished data.
- 57.Welch A R, McGregor L M, Gibson W. Cytomegalovirus homologs of cellular G protein-coupled receptor genes are transcribed. J Virol. 1991;65:3915–3918. doi: 10.1128/jvi.65.7.3915-3918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]