Abstract
Increasing epidemiological evidence supports the view that dietary flavonoids have protective roles in oral diseases, including cancer. However, the dietary forms of flavonoids, the flavonoid glycosides, must first be hydrolyzed to the aglycones, which is thought to occur mainly in the intestine. In the present study we tested whether this hydrolytic activity occurs in the oral cavity. Saliva was collected from human subjects, incubated with flavonoid glycosides, and analyzed for aglycone formation by HPLC. When quercetin 4′-glucoside or genistein 7-glucoside was incubated with human saliva, hydrolysis to quercetin and genistein, respectively, was detected within minutes. Studies of additional flavonoid glycosides demonstrated that glucose conjugates were rapidly hydrolyzed, but not conjugates with other sugars, i.e., rutin, quercitrin, and naringin. In a limited study of 17 subjects, the interindividual variability in the hydrolysis of genistein 7-glucoside was >20-fold. This supports the contention that salivary hydrolysis of certain flavonoid glucosides may be important in some individuals but not in others. Support for a bacterial contribution to this hydrolysis was obtained from the inhibitory effect of antibacterials in vivo and in vitro and from experiments with subcultured oral bacterial colonies. However, cytosol isolated from oral epithelial cells was also capable of effective hydrolysis. Dietary flavonoid glucosides may thus be hydrolyzed in the oral cavity by both bacteria and shedded epithelial cells to deliver the biologically active aglycones at the surface of the epithelial cells. The aglycones quercetin and genistein both potently inhibited proliferation of oral cancer cells. The large interindividual variability in this hydrolytic activity may be a factor that should be taken into consideration in future studies.
Keywords: flavonoid glycosides, salivary hydrolysis, β-glucosidase, oral cancer
Epidemiological studies have clearly demonstrated a protective role of fruits and vegetables in oral cancers, presumably mediated by their content of polyphenols, particularly flavonoids (1–3). Numerous mechanisms for these effects have been suggested, mainly based on in vitro and cellular studies (4–6). The dietary sources of flavonoids, except for the tea flavonoids (7), are flavonoid glycosides, which in most cases first must undergo hydrolysis to their aglycones to be able to produce effects. Still, serious questions remain regarding how these dietary components gain access to proposed cellular sites of action in the human body. For the tea flavonoids, which are gallic acid esters rather than glycosides, the access to oral epithelial cells may be less complex, as very recently noted (7).
In the past, it was strongly believed that flavonoid glycosides could not be absorbed per se but only after hydrolysis by the bacterial flora in the lower part of the intestine (8,9). In 1995 researchers proposed that flavonoid glycosides can be absorbed intact, presumably via the sodium-dependent glucose transporter SGLT1 (10). Although this was later confirmed (11), it was also shown that many glycosides are not absorbed due to efficient efflux transport by multidrug resistance-associated protein-2 (12). Other studies suggested that hydrolysis of flavonoid glycosides can occur in the small intestine (13), maybe by the broad-specific enterocyte β-glucosidase (14) and/or the lactase phloridzin hydrolase (15). Once this hydrolysis occurs, the aglycones formed are efficiently absorbed, although the bioavailability may be extremely low due to extensive presystemic metabolism (16). Thus, the potential protective effects of dietary flavonoids against cancers of the oral cavity are not understood.
Saliva has been suggested to be able to hydrolyze flavonoid glycosides (17–19), but it has never been considered an important factor. In the present study we attempted to establish the importance of salivary hydrolysis, using as substrates 4 different glycosides of quercetin, i.e., quercitrin (the 3-rhamnoside), rutin (the 3-rhamnoglucoside), isoquercitrin (the 3-glucoside), and spiraeoside (the 4′-glucoside), which previously have been shown to be hydrolyzed by β-glucosidases of human (14,15) and/or bacterial origin (9). We also tested genistin (genistein 7-glucoside), naringin (naringenin 7-rhamnoglucoside), and phloridzin (phloretin 2′-glucoside) as substrates, all commonly present in the human diet.
MATERIALS AND METHODS
Materials.
Genistin, genistein, naringin, phloridzin, quercitrin (quercetin 3-rhamnoside), rutin (quercetin 3-rhamnoglucoside), quercetin, and 3-[4,5-dimethylthiozol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)4 were obtained from Sigma; spiraeoside (quercetin 4′-glucoside) and isoquercitrin (quercetin 3-glucoside) were purchased from Indofine Chemicals. Colgate manufactured Chlorhexidine Gluconate Oral Rinse (PerioGard), and Listerine® was distributed by Warner-Lambert Consumer Health Care. Dimethyl sulfoxide (DMSO), glacial acetic acid, and methanol were purchased from Fisher Scientific; trifluoroacetic acid was obtained from Aldrich Chemical Company. Fetal bovine serum was produced by Atlanta Biologicals, and other cell culture medium components were obtained from Cellgro Mediatech, Fisher Scientific.
Collection of human saliva.
The study was approved by the Institutional Review Board for Human Research. The limited population study was conducted using 17 volunteers recruited from 1 high school science class (ages 15–17 y). Other saliva samples were from adult subjects (23–64 y). Unstimulated saliva (2 mL) was collected in the morning with the subject abstaining from toothbrushing since the previous evening. In some adult subjects saliva was collected before and after brushing (no toothpaste) or before and after rinsing with an antibacterial mouthrinse (chlorhexidine or Listerine) for 30 s. In the latter experiments, saliva was collected at 6 min and 1, 2, 6, and 24 h after the mouthrinse.
Flavonoid hydrolysis by saliva.
The saliva (2 mL) was diluted 1:1 with distilled water and shaken vigorously to reduce viscosity. In some experiments, diluted saliva was centrifuged at 10,000 × g and filtered with 1- and 0.2-μm filters to remove oral cells and bacteria, respectively. Other saliva samples (1 mL) were incubated on a shaking water bath at 37°C for 24 h with an equal volume of DMEM (containing 10% fetal bovine serum) with or without 200,000 U/L penicillin and 0.2 g/L streptomycin prior to incubation with flavonoid glycoside.
Flavonoid glycosides dissolved in DMSO (final concentration < 0.1%) were added to two 1-mL aliquots of the saliva mixture to a final flavonoid concentration of 25 or 50 μmol/L. After vigorous shaking and vortexing, argon was added to the samples before incubation for specified times at 37°C. The pH of the saliva samples was 6.7 ± 0.2 (mean ± SEM) before and 6.4 ± 0.1 after incubation. An equal volume of methanol was added to the samples following incubation. The samples were centrifuged at 16,000 × g for 2 min and the supernatant was analyzed by HPLC with flavonoid-specific UV detection.
Flavonoid hydrolysis by cultured oral bacteria.
A cotton swab was held in the mouth of 1 volunteer whose saliva had been shown previously to have a high level of flavonoid hydrolysis. The swab was streaked on blood agar and chocolate agar plates and incubated overnight. Eight different-looking common bacterial colonies were subcultured twice to ensure a homogeneous population. One plate for each colony was used for heavy inoculation of fresh plates. Twenty-four hours later, the bacterial colonies were removed with a cotton swab to tubes of sterile broth, vortexed, and rocked at room temperature for 1 h. Aliquots (0.5 mL) of the bacterial suspensions as well as broth (negative control) were incubated with 50 μmol/L genistin for 1 h at 37°C on a shaking water bath. The samples were analyzed as above. The experiment was done twice with separate inoculates.
Flavonoid hydrolysis by SCC-9 oral squamous carcinoma cell cytosol.
Oral squamous carcinoma SCC-9 cells obtained from the American Type Culture Collection were cultured in F-12/DMEM containing glutamine and HEPES, fetal calf serum (10%), 100,000 U/L penicillin, 0.1 g/L streptomycin, and hydrocortisone (0.2 g/L). SCC-9 cytosol was prepared by harvesting the cells at confluency. The cell pellet was resuspended in buffer containing protease inhibitors and was then sonified and centrifuged at 100,000 × g for 15 min at 7°C. The cytosol (supernatant) was stored at −80°C. Aliquots of cytosol were mixed with 0.1 mol/L acetate buffer (pH 6) to mimic the pH of the oral environment. Quercetin 4′-glucoside was added to a final concentration of 25 μmol/L, and the samples were incubated at 37°C for 1 h. After the addition of an equal volume of methanol and centrifugation, the samples were subjected to HPLC analysis as described below.
HPLC.
All flavonoid glycosides and their respective aglycones were detected by reverse phase HPLC of 200-μL samples on a Millennium HPLC system with a Symmetry C18 column (3.9 × 150 mm) and a Model 996 photodiode array detector, using slight modifications of previous studies (20,21). The flow rate was 0.9 mL/min. A mobile phase consisting of 35% methanol and 5% acetic acid with UV detection at 370 nm was used for the quercetin glycosides and at 260 nm for genistin and naringin. Phloridzin hydrolysis was analyzed using a mobile phase of 45% methanol and 0.3% trifluoroacetic acid with detection at 283 nm. Quantitation was done by peak area measurements in comparison with standard curves for each of the flavonoid glycosides and aglycones. The detection limits were adequate for all experiments. The recoveries were estimated to exceed 90% for all compounds tested, as previously noted (20,21).
Cell proliferation assay.
SCC-9 cells were seeded in 96 wells at a density of 5000 cells/well and cultured as described above. On d 2–4 after plating, fresh medium including 0.1–200 μmol/L flavonoid or DMSO (0.25%, v:v) was added. On d 5 the medium was aspirated and the cells were incubated with MTT in buffer (0.5 g/L) for 3 h before the addition of 0.1 mol/L HCl and 10% Triton X-100 in 2-propanol (Sigma protocol) to lyse the cells and dissolve the formazan crystals. The absorbance was read with a plate reader at 570 nm with 690-nm background subtraction. This assay measures the conversion of MTT to blue formazan by mitochondrial dehydrogenases in living cells.
Statistics.
Data are means ± SEM. The statistical significance of differences between 2 treatments was evaluated using a 2-tailed unpaired Student t test with a significant level of P < 0.05. In experiments with multiple treatments, ANOVA with Dunnett’s posttest (InStat) or 2-way ANOVA with Bonferroni’s posttest (Prism) was used.
RESULTS
The hydrolysis of the flavonoid glycosides to their respective aglycones by saliva was examined using HPLC separation with flavonoid-specific UV detection. This was shown for quercetin 4′-glucoside (spiraeoside), one of the most important flavonoid glycosides in the human diet, present in large amounts in onions, for example (22). Both the glucoside and its aglycone quercetin had excellent chromatographic properties, and there was little interference from other salivary components at the 370-nm wavelength used. The hydrolytic reaction was linear with time for at least 3 h. There was no evidence of other products formed under these conditions. The reactions were carried out under argon to avoid degradation of any of the aglycones formed. For a given saliva sample, the reproducibility of the reaction was acceptable with a CV of about 15%.
The hydrolysis of all 7 of the flavonoid glycosides studied was examined using saliva from 1 subject in replicate analyses (Table 1). For estimation of the rates of hydrolysis, the percentage conversion to the aglycones, taking into account the molar extinction coefficients, was determined after 2-h incubations at 37°C. Four of the glycosides were hydrolyzed efficiently, spiraeoside, phloridzin, genistin, and isoquercitrin, with spiraeoside being by far the best substrate. On the other hand, rutin was hydrolyzed very little, and quercitrin and naringin were not hydrolyzed at all. The same pattern was seen with saliva from 3 other subjects, although the interindividual variability in the hydrolysis rate was large (see below).
TABLE 1.
| Flavonoid glycoside | Aglycone formation |
|---|---|
| % of added glycoside | |
| Spiraeoside (quercetin 4′-glucoside) | 85.6 ± 1.3 |
| Phloridzin (phloretin 2′-glucoside) | 67.8 ± 2.9 |
| Genistin (genistein 7-glucoside) | 44.3 ± 4.3 |
| Isoquercetin (quercetin 3-glucoside) | 40.5 ± 6.6 |
| Rutin (quercetin 3-rhamnoglucoside) | 9.0 ± 0.6 |
| Quercitrin (quercetin 3-rhamnoside) | 0 |
| Naringin (naringenin 7-rhamnoglucoside) | 0 |
Values are means ± SEM, n = 3–4.
Saliva from 1 volunteer was incubated for 2 h at 37°C with 25 μmol/L flavonoid glycosides and analyzed by HPLC for the aglycone products and the parent flavonoid glycosides.
We next examined the hydrolysis of one flavonoid glucoside in 17 healthy student volunteers. For this purpose we used genistin, i.e., the 7-glucoside of genistein, because of its superior stability under the trial conditions used. The purpose of this trial was to determine the interindividual variability in hydrolysis rate. Variability was extremely large (Fig. 1). One subject (No. 7) showed no hydrolysis at all [minimum detectable amount 1 μmol/(L saliva h)] and 4 subjects (Nos. 1, 3, 5, and 8) had barely detectable levels of hydrolysis. On the other hand, 3 subjects (Nos. 12, 14, and 17) had very high hydrolysis rates, consuming virtually all of the genistin substrate. The only known phenotypes among the subjects that were examined, i.e., age, race, sex, and regular use of mouthwash, were not correlated with hydrolysis rate in this limited number of subjects.
FIGURE 1.
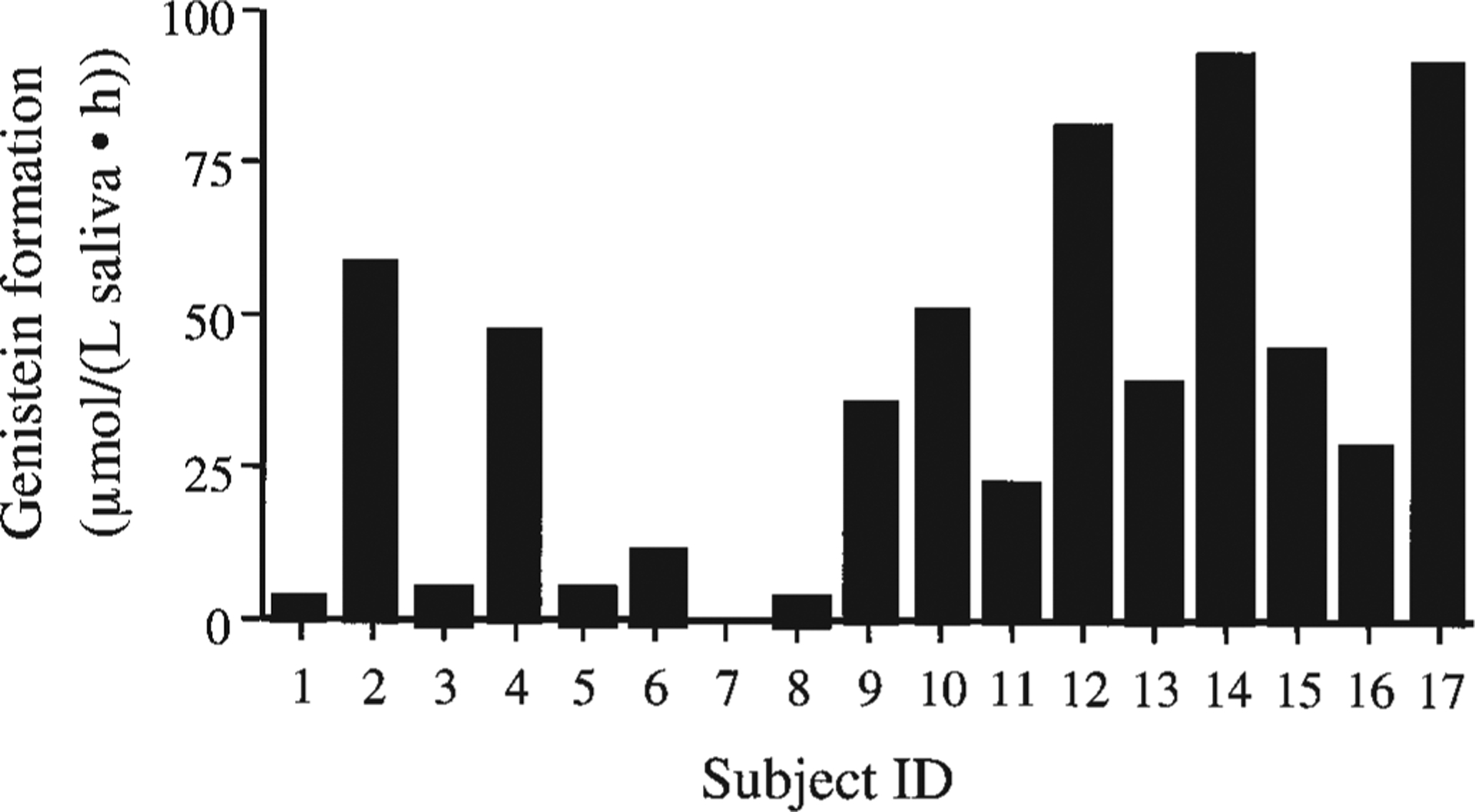
Genistin hydrolysis to genistein in the saliva from a group of young, healthy human subjects. Saliva was incubated for 1 h with 25 μmol/L genistin.
To examine the effect of antibacterial mouthwashes, we performed time course studies in several adults after they rinsed with chlorhexidine and Listerine (Fig. 2). Chlorhexidine efficiently inhibited the hydrolysis of genistin and this inhibition persisted for at least 6 h. Listerine had a modest effect, persisting for only 2 h. These effects were consistent with the antibacterial potencies of these agents (23). The findings with the antibacterial agents strongly indicated that the hydrolysis was due to the oral bacterial flora. Centrifugation (10,000 × g) and filtration (1- and 0.2-μm filters) of the saliva also removed >90% of the enzymatic activity, indicating that the activity was related to a cellular source rather than to soluble enzymes. To further determine the importance of the bacterial flora, we compared the hydrolysis of genistin to genistein by raw saliva and raw saliva preincubated with cell culture medium (without antibiotics) for 24 h. There was a dramatic increase consistent with the anticipated bacterial growth in cultured saliva (Fig. 3). In the presence of penicillin and streptomycin, this increase was abolished (Fig. 3). To further confirm the importance of bacteria in the hydrolysis of flavonoid glycosides, blood agar and chocolate agar plates were inoculated with saliva from 1 volunteer who had previously been shown to have high salivary hydrolytic activity. Eight different oral bacterial colonies subcultured to homogeneity were tested for their ability to hydrolyze genistin. Two of the cultures showed a high level of activity, about 25% hydrolysis after a 1-h incubation with 25 μmol/L genistin, whereas the others were totally inactive. The experiment was repeated with the same results. The 2 active strains were classified as gram-positive cocci in tetrads and gram-negative diplococci, both common nonpathogenic oral bacteria.
FIGURE 2.
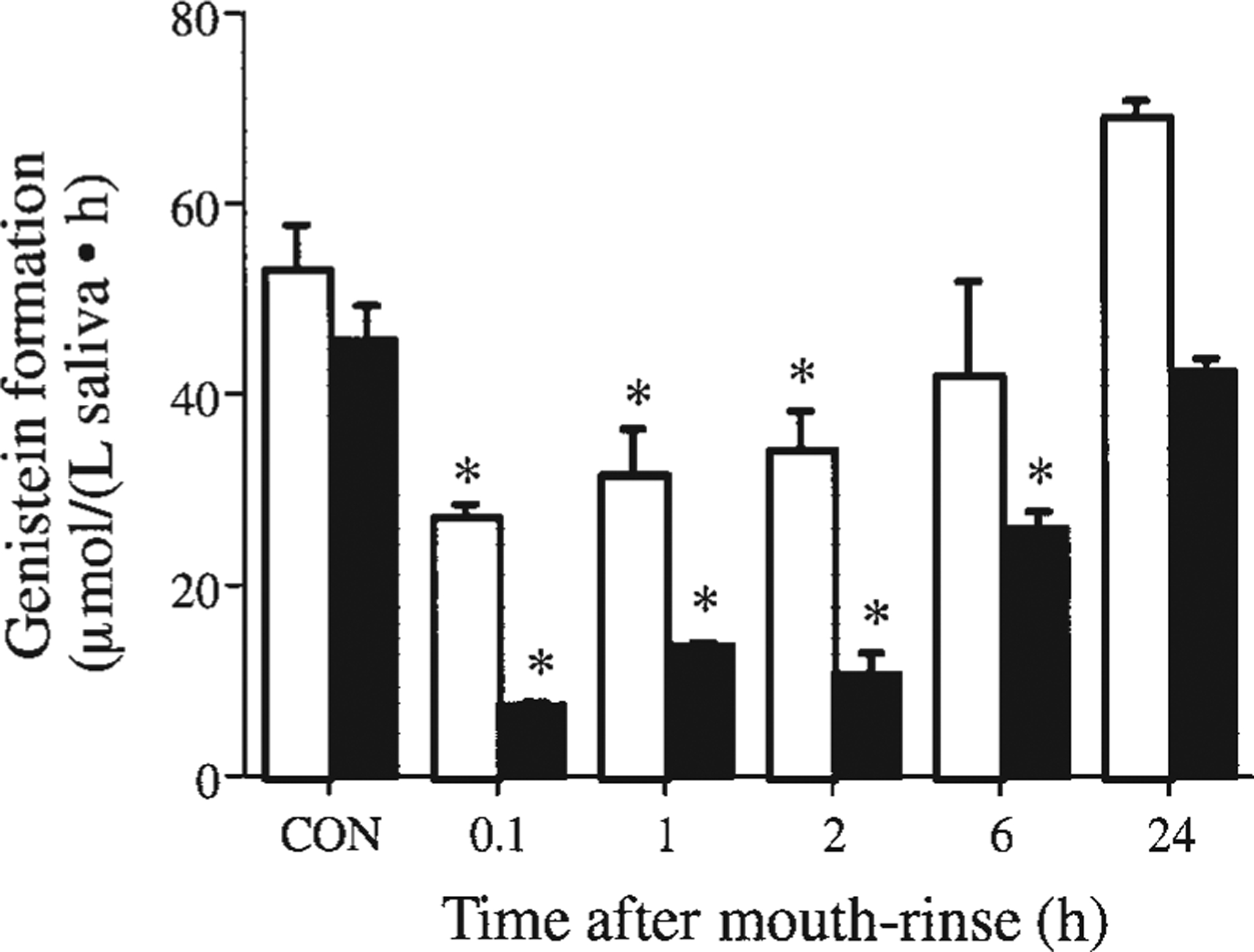
Inhibitory effect of chlorhexidine (■) and Listerine (□) on the salivary hydrolysis of genistin to genistein after a 30-s mouthrinse followed by a brief rinse with tap water. Human saliva samples were collected before (CON) and at various times after the mouthrinses and incubated with 25 μmol/L genistin for 1 h. Values are means ± SEM, n = 3–6. *Different from CON, P < 0.05.
FIGURE 3.
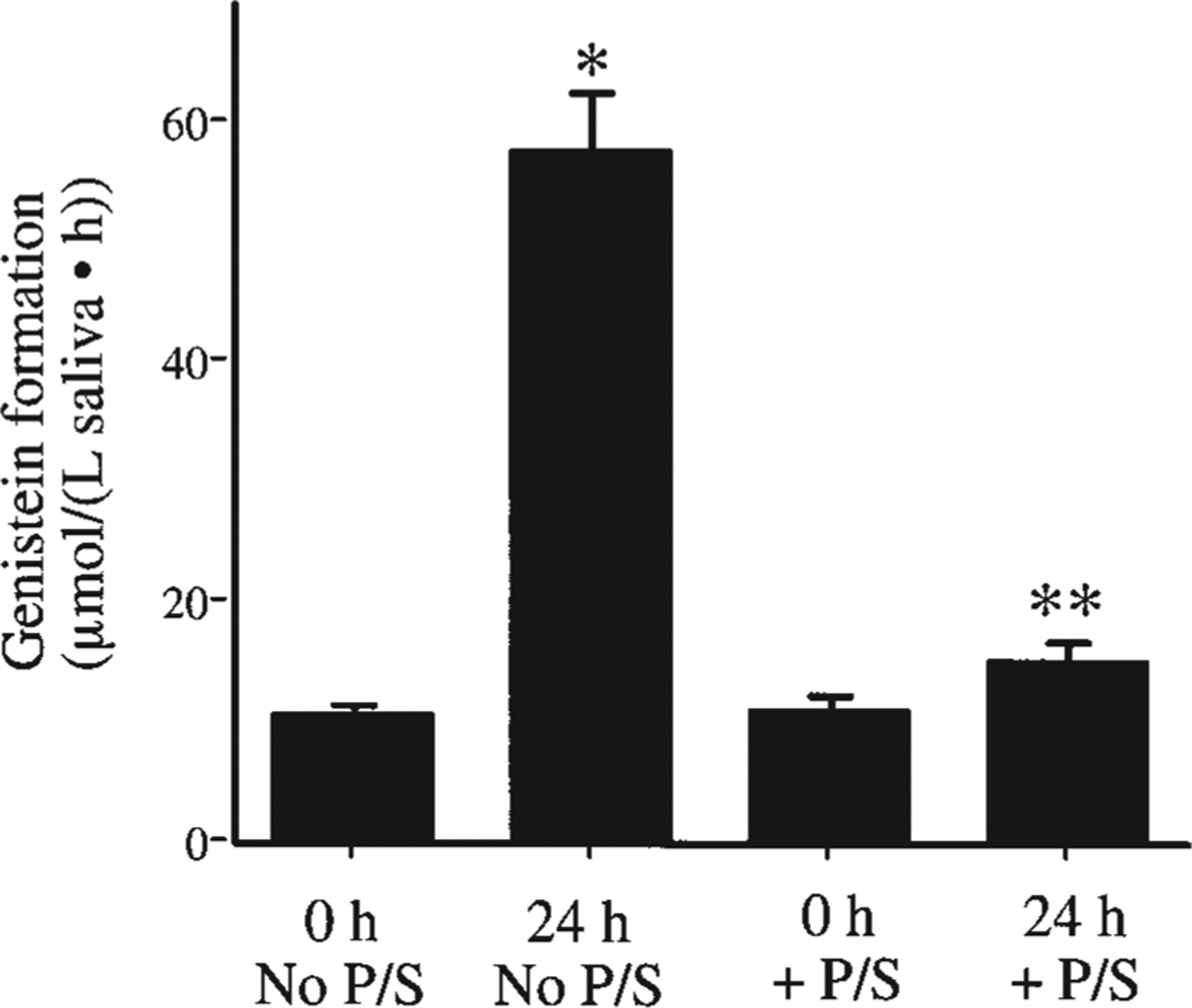
Effect of preincubating saliva from 1 human volunteer with cell culture medium for 0 or 24 h in the absence and presence of penicillin and streptomycin (P/S) on the hydrolysis rate of 50 μmol/L genistin (1-h incubation). Values are means ± SEM, n = 6. *Different from 0 h, no P/S, P < 0.001; **Different from 24 h, no P/S, P < 0.001.
These observations, although highly supportive of a bacterial source for the β-glucosidase activity, did not rule out a contribution from shedded oral epithelial cells. When saliva samples were examined by microscopy, they all contained variable numbers of scaly oral epithelial cells, about half of which appeared to be intact. To examine the effect of these cells on hydrolysis, 3 subjects brushed their cheeks, gums, and tongue with a toothbrush before providing saliva samples, an established procedure to collect loosely attached epithelial cells. Microscopy confirmed a substantial increase in cell numbers. The quercetin 4′-glucoside to quercetin hydrolysis in these samples increased substantially compared to samples obtained before brushing (P < 0.05; n = 3), consistent with a contribution by shed epithelial cells. The aglycone/glucoside ratios after a 30-min incubation were 0.12 ± 0.03 before brushing vs. 0.55 ± 0.17 after brushing and after a 60-min incubation were 0.33 ± 0.08 vs. 4.42 ± 0.34. To further test the hydrolytic activity of oral epithelial cells without the confounding presence of oral bacteria we used cytosols prepared from oral squamous carcinoma SCC-9 cells (24). Hydrolysis of quercetin 4′-glucoside to quercetin by this cytosol was efficient (Fig. 4). The reaction was clearly saturable over the 5–100 μmol/L quercetin 4′-glucoside concentration range. Nonlinear regression analysis of the data yielded an apparent Km of 34 μmol/L and a Vmax of 64 nmol/(h mg protein).
FIGURE 4.
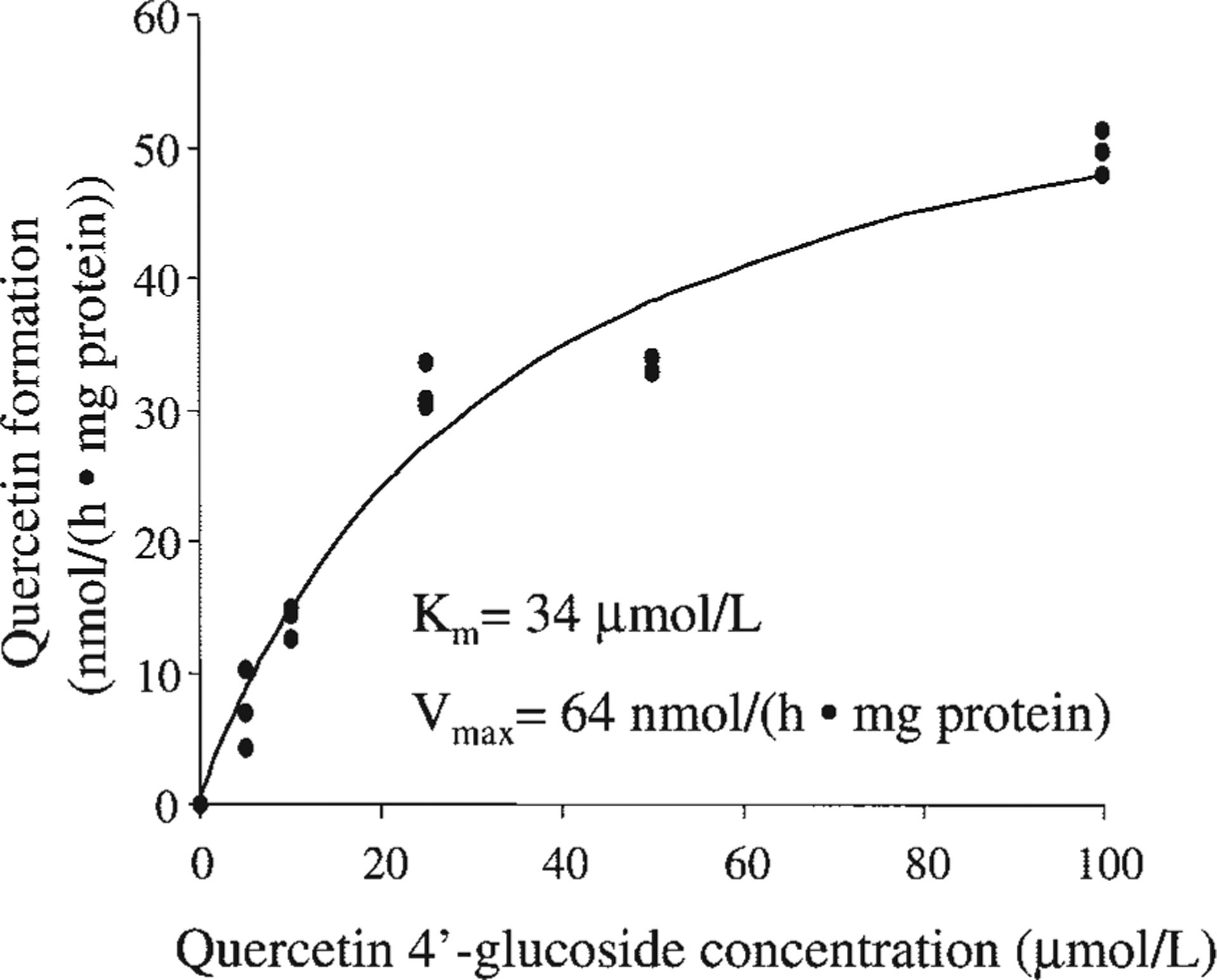
Michaelis-Menten kinetics of the hydrolysis of quercetin 4′ -glucoside to quercetin by cytosol from human oral epithelial SCC-9 cells. The incubation time was 1 h.
To obtain a quantitative estimate of the effectiveness of the oral hydrolysis of flavonoid glycosides irrespective of mechanisms, we conducted experiments in situ. Thus, 15 mL of a 10 μmol/L solution of quercetin-4′-glucoside was held in the mouth of 1 volunteer for 5 min, and glucoside and aglycone quercetin content were assayed by HPLC. In 3 experiments, a consistent 57–63% of the glucoside disappeared during this short time period, taking into account volume changes. Also, 17–24% of the glucoside was hydrolyzed to quercetin.
To determine the potential biological importance of the collective findings, we examined the antiproliferative effects of quercetin and genistein, the 2 flavonoids demonstrating efficient salivary formation from their precursor glucosides, on the oral squamous carcinoma SCC-9 cells using the MTT assay and a wide range of flavonoid concentrations (0.1–200 μmol/L) (Fig. 5). Both flavonoids produced potent inhibition with a minimum effective concentration (MEC) of 5 and 10 μmol/L for quercetin and genistein, respectively. Thus, the aglycones formed in the oral cavity may have anticancer and antibacterial (3) effects or more generally scavenge hydrogen peroxide and other reactive oxygen species (19).
FIGURE 5.
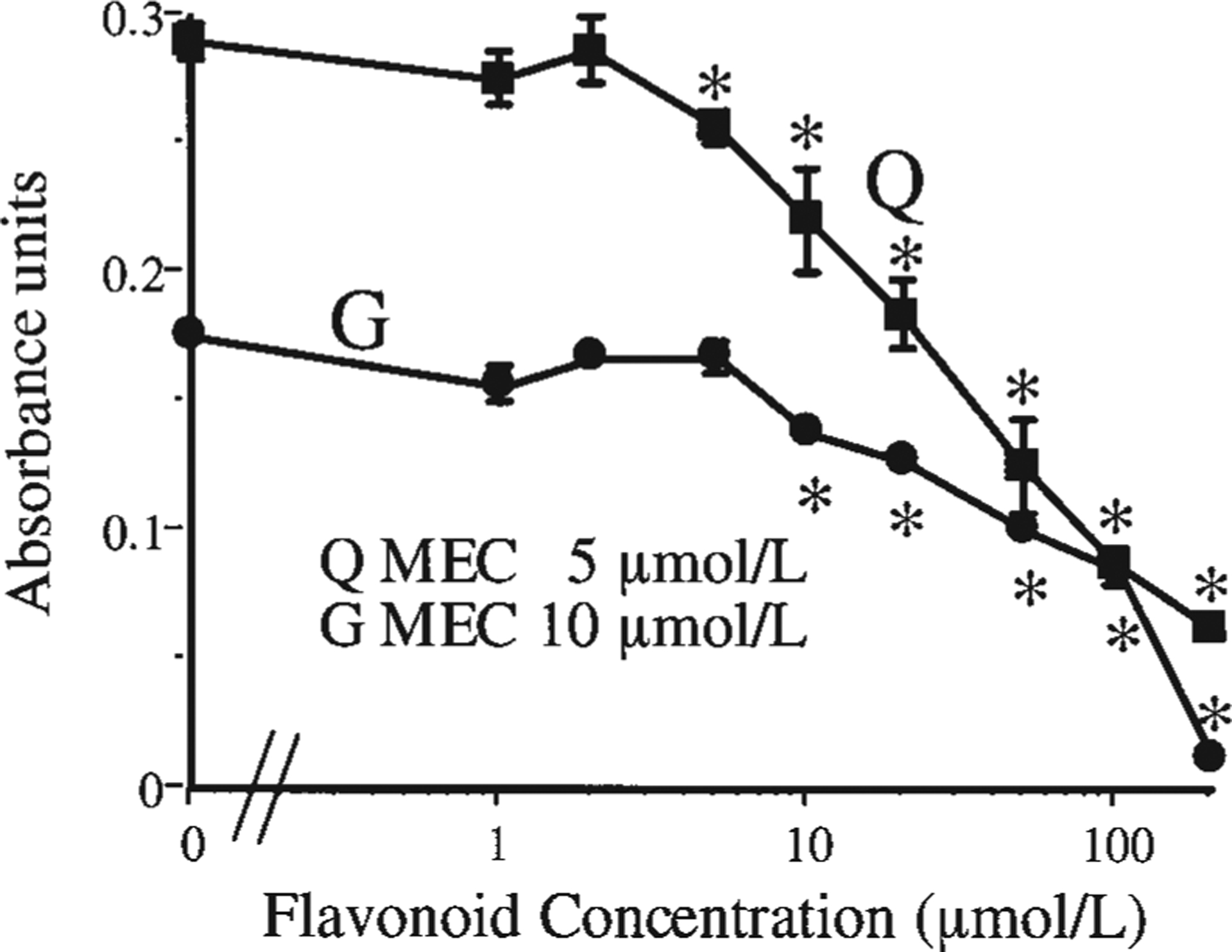
Antiproliferative effects of quercetin (Q) and genistein (G) in human oral squamous carcinoma SCC-9 cells. MEC, minimum effective concentration. Values are means ± SEM, n = 12–18. *Different from the flavonoid-free (0 μmol/L) DMSO control; P < 0.05.
DISCUSSION
Although several previous studies have demonstrated that saliva can hydrolyze certain flavonoid glycosides (17–19), those studies were limited in scope. From the present study it appears that this hydrolysis is limited to glucose conjugates, because other glycosides, such as the rhamnosides (quercitrin) and rhamnoglucosides (rutin and naringin), either were hydrolyzed very slowly or were resistant to salivary hydrolysis. Interestingly, these latter types of glycosides are easily hydrolyzed by the human fecal flora (9). Quercetin 4′-glucoside in particular, but also genistin, i.e., genistein 7-glucoside, can be rapidly hydrolyzed by saliva. These are 2 of the more abundant flavonoids in the human diet (22,25). Thus, the formation of quercetin and genistein from their dietary sources may be rapid enough in certain individuals to be important also in vivo. Holding a solution of quercetin 4′-glucoside in the mouth for 5 min resulted in a 60% loss of glucoside. About 20% of that loss could be accounted for as quercetin in the saliva, with the remaining 40% being absorbed by the oral epithelial cells, presumably as quercetin.
Still, whether this oral hydrolysis will be of importance for local effects on the oral epithelium, considering the relatively short residence time of most foods in the oral cavity, is difficult to assess. Our study of 17 high school students demonstrated a remarkable interindividual variability in hydrolysis rate. These observations suggest that oral hydrolysis may be quantitatively important in some individuals but not in others. Such variability may have a genetic origin, but could also involve environmental factors.
Hydrolysis of flavonoid glucosides by β-glucosidases in the oral cavity is also likely to be influenced by the food matrix in which they are contained. Thus, if contained in a liquid form, they will obviously have greater access to the enzymes than if contained in a solid form requiring extensive chewing. Even so, it should be clear that the hydrolytic activity will start in the oral cavity and continue through the passage of the food throughout the aerodigestive tract.
The mechanism of hydrolysis was presumably through β-glucosidases, but the source of these enzymes was difficult to pinpoint. Bacterial flora in the oral cavity play a role. This can be deduced from the effectiveness of the antibacterial agents chlorhexidine and Listerine in inhibiting the salivary hydrolysis and is also strongly supported by the experiments using cell culture techniques, either for the whole saliva or for subcultured oral bacterial colonies. However, cytosols from cultured oral epithelial cells also displayed a high rate of hydrolysis and the saliva from all subjects, in particular after brushing the cheeks, gums, and tongue, contained high numbers of epithelial cells. It is interesting to note that the cytosolic hydrolysis of quercetin 4′-glucoside to quercetin proceeded with an apparent Km of 34 μmol/L and a Vmax of 64 nmol/(h mg protein), very similar to the hydrolysis by cytosol from human liver and small intestine (14), implying the involvement of the same β-glucosidase enzyme. Clearly, more studies will be needed to better determine the contribution of bacterial and human epithelial β-glucosidases.
Finally, it was of great interest to note that both quercetin and genistein were able to inhibit proliferation of the oral squamous carcinoma SCC-9 cells. This occurred with an MEC of 5–10 μmol/L for these flavonoids, which should be achievable in the oral cavity after consumption of a diet containing these flavonoids. The mechanisms of these effects are likely different. Quercetin inhibits PI3-kinase (26), whereas genistein inhibits tyrosine kinases (26). A mixture of flavonoids with different mechanistic properties may be an advantage in cancer prevention.
In conclusion, flavonoid glucosides were hydrolyzed to their aglycones in the oral cavity. The β-glucosidase enzymes responsible were derived both from bacteria and shedded oral epithelial cells. Two of the flavonoid aglycones studied, quercetin and genistein, showed potent inhibition of oral cancer cell proliferation. The ability of some individuals but not others to hydrolyze these protective dietary flavonoids in the oral cavity should be an important consideration in future studies.
ACKNOWLEDGMENTS
Several students, including Sarah London, Kristen French, Cheryl Widejko, and Teresita Alston, contributed to various aspects of this study.
Supported by the Department of Defense/Hollings Cancer Center Grant N6311602MD200 and the National Institutes of Health Grants GM55561 and RR01070.
Abbreviations used:
- DMSO
dimethyl sulfoxide
- MEC
minimum effective concentration
- MTT
3-[4,5-dimethylthiozol-2-yl]-2,5-diphenyl tetrazolium bromide
Footnotes
Presented in part at the Experimental Biology 2004 meeting in Washington, DC, April 17–21, 2004 [Walle, U. K., Browning, A. M., Steed, L. L., Reed, S. G. & Walle, T. (2004) Hydrolysis of flavonoid glycosides in the oral cavity—contribution by both bacteria and shedded epithelial cells. FASEB J. 18: A890 (abs.)].
LITERATURE CITED
- 1.Block G, Patterson B & Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer 18: 1–29. [DOI] [PubMed] [Google Scholar]
- 2.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S & Monnier P (1998) Food groups and risk of oral and pharyngeal cancer. Int. J. Cancer 77: 705–709. [DOI] [PubMed] [Google Scholar]
- 3.Sakagami H, Oi T & Satoh K (1999) Prevention of oral diseases by polyphenols (review). In Vivo 13: 155–172. [PubMed] [Google Scholar]
- 4.Middleton EJ, Kandaswami C & Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev 52: 673–751. [PubMed] [Google Scholar]
- 5.Rice-Evans C (2001) Flavonoid antioxidants. Curr. Med. Chem 8: 797–807. [DOI] [PubMed] [Google Scholar]
- 6.Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol. Ther 96: 67–202. [DOI] [PubMed] [Google Scholar]
- 7.Lee M-J, Lambert JD, Prabhu S, Meng X, Lu H, Maliakal P, Ho C-T & Yang CS (2004) Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epidemiol. Biomark. Prev 13: 132–137. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths LA & Barrow A (1972) Metabolism of flavonoid compounds in germ-free rats. Biochem. J 130: 1161–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokkenheuser VD, Shackleton CHL & Winter J (1987) Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J 248: 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollman PCH, de Vries JHM, van Leeuwen SD, Mengelers MJB & Katan MB (1995) Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr 62: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 11.Walle T & Walle UK (2003) The β-D-glucoside and sodium-dependent glucose transporter 1 (SGLT1)-inhibitor phloridzin is transported by both SGLT1 and multidrug resistance-associated proteins 1/2. Drug Metab. Dispos 31: 1288–1291. [DOI] [PubMed] [Google Scholar]
- 12.Walgren RA, Karnaky KJ Jr., Lindenmayer GE & Walle T (2000) Efflux of dietary flavonoid quercetin 4′-β-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J. Pharmacol. Expt. Ther 294: 830–836. [PubMed] [Google Scholar]
- 13.Walle T, Otake Y, Walle UK & Wilson FA (2000) Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J. Nutr 130: 2658–2661. [DOI] [PubMed] [Google Scholar]
- 14.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJC, Morgan MRA & Williamson G (1998) Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 436: 71–75. [DOI] [PubMed] [Google Scholar]
- 15.Day AJ, Cañada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MRA & Williamson G (2000) Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 468: 166–170. [DOI] [PubMed] [Google Scholar]
- 16.Walle T, Walle UK & Halushka PV (2001) Carbon dioxide is the major metabolite of quercetin in humans. J. Nutr 131: 2648–2652. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald IA, Mader JA & Bussard RG (1983) The role of rutin and quercitrin in stimulating flavonol glycosidase activity by cultured cell-free microbial preparations of human feces and saliva. Mutat. Res 122: 95–102. [DOI] [PubMed] [Google Scholar]
- 18.Laires A, Pacheco P & Rueff J (1989) Mutagenicity of rutin and the glycosidic activity of cultured cell-free microbial preparations of human faeces and saliva. Food Chem. Toxicol 27: 437–443. [DOI] [PubMed] [Google Scholar]
- 19.Hirota S, Nishioka T, Shimoda T, Miura K, Ansai T & Takahama U (2001) Quercetin glucosides are hydrolyzed to quercetin in human oral cavity to participate in peroxidase-dependent scavenging of hydrogen peroxide. Food Sci. Technol. Res 7: 239–245. [Google Scholar]
- 20.Walgren RA, Walle UK & Walle T (1998) Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem. Pharmacol 55: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 21.Walle UK, French KL, Walgren RA & Walle T (1999) Transport of genistein-7-glucoside by human intestinal Caco-2 cells: potential role for MRP2. Res. Comm. Mol. Pathol. Pharmacol 103: 45–56. [PubMed] [Google Scholar]
- 22.Kiviranta J, Huovinen K & Hiltunen R (1988) Variation of phenolic substances in onion. Acta. Pharm. Fenn 97: 67–72. [Google Scholar]
- 23.Rosin M, Welk A, Kocher T, Majic-Todt A, Kramer A & Pitten FA (2002) The effect of a polyhexamethylene biguanide mouthrinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque regrowth. J. Clin. Periodont 29: 392–399. [DOI] [PubMed] [Google Scholar]
- 24.Rheinwald JG & Beckett MA (1981) Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 41: 1657–1663. [PubMed] [Google Scholar]
- 25.Barnes S, Kirk M & Coward L (1994) Isoflavones and their conjugates in soy foods: extraction conditions and analysis by HPLC-mass spectrometry. J. Agric. Food Chem 42: 2466–2474. [Google Scholar]
- 26.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Ré mést C, Chap H & Payrastre B (1997) Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem. Pharmacol 53: 1649–1657. [DOI] [PubMed] [Google Scholar]


