Abstract
A monoclonal antibody (MAb) that blocks most echoviruses (EVs) from infecting rhabdomyosarcoma (RD) cells has been isolated. By using the CELICS cloning method (T. Ward, P. A. Pipkin, N. A. Clarkson, D. M. Stone, P. D. Minor, and J. W. Almond, EMBO J. 13:5070–5074, 1994), the ligand for this antibody has been identified as β2-microglobulin (β2m), the 12-kDa protein that associates with class I heavy chains to form class I HLA complexes. A commercial MAb (MAb 1350) against β2m was also found to block EV7 infection without affecting binding to its receptor, DAF, or replication of EV7 viral RNA inside cells. Entry of EV7 into cells was reduced by only 30% by antibody and cytochalasin D, an inhibitor of endocytosis mediated by caveolae and clathrin-coated pits, but was not significantly reduced by sodium azide. The block to virus entry by cytochalasin D was additive to the block induced by antibody. We suggest that EV7 rapidly enters into a multicomponent receptor complex prior to entry into cells and that this initial entry event requires β2m or class I HLA for infection to proceed.
Echoviruses (EVs) are members of the Enterovirus genus of the family Picornaviridae and are important human pathogens. They are associated with a wide spectrum of clinical syndromes, including rashes, diarrhea, aseptic meningitis, respiratory disease, and possibly conditions such as chronic fatigue syndrome. This range of clinical manifestations is probably a reflection of virus tissue tropisms, which seem to be mediated, at least in part, by utilization of a range of cellular receptors.
Anti-cell surface monoclonal antibodies (MAbs) that block EV infection have been isolated previously and have been used to determine the identity of some of these receptors. In 1992 Bergelson et al. demonstrated that EV serotypes 1 and 8 use the collagen receptor VLA-2 (6) by attaching to the α2 subunit (7). Previously, we and others have shown that a regulator of complement activity, decay-accelerating factor (DAF), is the receptor for a range of hemagglutinating EVs (3, 37). Other EVs appear to use neither of these, but the identity of their receptor(s) is unknown. Mbida et al. have isolated a MAb (MAb 143) that blocks most EV serotypes from infecting a range of cell types. MAb 143 was also found to block coxsackievirus A9 but not poliovirus or coxsackievirus serotypes B1 to B6 (21). The ligand for MAb 143 was found by affinity purification to be an unknown 44-kDa glycoprotein (22). It was therefore suggested that the 44-kDa protein was part of a multicomponent receptor complex used by most EVs to infect cells. A direct role for the 44-kDa protein in virus attachment seems unlikely, since MAb 143 blocks infection by the viruses that have been shown to use other proteins, such as DAF (3, 37) and VLA-2 (6), as their primary receptors.
Here, we report the isolation of a MAb similar to that described by Mbida et al. (21, 22) and describe the cloning and identification of its ligand. The ligand is β2-microglobulin (β2m), a 12-kDa protein that associates with the class I HLA heavy chains (44 kDa) and presents antigenic peptides (20). We show that MAbs to β2m block EV infection partly by reducing the entry of virus into cells, although other postbinding effects cannot be ruled out.
MATERIALS AND METHODS
Cell culture and virus propagation.
Rhabdomyosarcoma (RD) cells, Ohio HeLa cells, and COS cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) unless otherwise stated. All EV serotypes (prototype strains) were obtained from Colindale (Public Health Laboratory Service, Colindale, United Kingdom) and were propagated in RD cells in serum-free DMEM. Poliovirus (type 3, Leon) was propagated in Ohio HeLa cells. The titers of stock virus preparations were determined as the 50% tissue culture infective dose (TCID50) in RD cells.
Antibodies and chemicals.
MAbs 854 and 918 were isolated after immunizing mice with Ohio HeLa cell membrane extracts, as described previously (23). MAb 854 is reactive against DAF (37). The goat anti-mouse immunoglobulin–β-galactosidase conjugate used in the immunofocal assays (11) was from Harlan Seralab, Loughborough, United Kingdom, and was used at a dilution of 1/400. The anti-β2m antibody, MAb 1350, was obtained as ascitic fluid from Chemicon International Inc., Temecula, Calif. The anti-enterovirus MAb 5-D8/1 was from Dako A/S, High Wycombe, United Kingdom, and was used at a dilution of 1/200. In the immunofocal assays, antibodies were used after dilution in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (Fraction V; Sigma). Unless otherwise stated, all other chemicals were purchased from Sigma. Soluble DAF (sDAF) was expressed in the yeast Pichea pastoris and purified to >95% purity (26).
Virus-blocking assays.
Ninety-six-well plates of RD cells at 80% confluency were washed with serum-free DMEM and incubated with 50-μl volumes of MAb 1350 for 1 h at 37°C before the addition of 50-μl volumes of EV. The plates were incubated at 37°C in a 5% CO2 atmosphere for 24 or 48 h, and the cells were then fixed and stained with Giemsa crystal violet.
Cloning of the MAb 918 ligand.
The previously described (37) cloning technique CELICS was used. Briefly, 108 COS cells were transfected by electroporation (10) with 100 μg of cDNA library from human umbilical vein endothelial cells (HUVEC) in the high-expression vector pCDM8 (29) (a kind gift from Dave Simmons, Imperial Cancer Research Fund, Oxford, United Kingdom). After 48 h of incubation in tissue culture, transformants were screened by an immunofocal assay (11) for MAb 918-reactive cells. MAb 918-reactive cells stained blue and were isolated by using a fine capillary tube. Plasmid DNA was then extracted from the isolated blue cells and transformed into Escherichia coli (MC1063/P3) by electroporation (15). The resultant E. coli colonies were then pooled, and their plasmid DNA was extracted (8) and transfected into COS cells for a further round of CELICS. The E. coli clones from the second round of CELICS were grown individually in Luria-Bertani broth overnight, and small-scale preparations of their plasmid DNA were transfected into COS cells and screened for MAb 918 reactivity. Positive cDNA clones were thus isolated and sequenced.
Virus neutralization assay.
EV7 (1,000 TCID50) in 40-μl volumes was incubated with purified β2m (0 to 20 μg in PBS; Scripps Laboratories, San Diego, Calif.) for 1 h at 37°C. Virus was then added to RD cells at 80% confluency in 96-well plates. The RD cells were then incubated at 37°C (5% CO2) for 1 or 2 days before being fixed and stained with Giemsa crystal violet.
EV7 replication in RD cells.
RD cells (80% confluent) in 25-cm2 flasks were incubated in the presence or absence of MAb 1350 (5 ml, diluted 1/250 in DMEM containing 10% FCS) for 1 h at 37°C. The antibody was then removed, and 1 ml of EV7 at 1 TCID50/cell was added to the cells and allowed to adsorb for 1 h at 37°C. The cells were then washed four times with 5-ml volumes of DMEM. Antibody was added back to the cells, which were then incubated for 16 h under normal growth conditions. An equal amount of antibody was added to the control flask. The anti-β2m MAbs were then sequestered by addition of 20 μg of purified β2m to both flasks. Virus yields from the flasks were titered on RD cells.
EV7 vRNA replication in RD cells.
Cells (5 × 106) were incubated in the presence or absence of MAb 1350 (5 ml, diluted 1/250 in DMEM with 10% FCS) for 1 h at 37°C. The cells were then transfected with 6 μg of purified EV7 viral RNA (vRNA) or poliovirus vRNA (27) by electroporation at 300 V and 250 μF (10). The cells were seeded in 6-well dishes and incubated in DMEM containing 10% FCS and HEPES at 10 mM for 6 h. The cells were then washed four times with 3-ml volumes of PBS, fixed with acetone-methanol (1:1) for 3 min, washed four times with PBS, and stained for EV7-infected cells by using the anti-enterovirus MAb 5-D8/1 in an immunofocal assay (11).
Anti-β2m time-dependent block to entry of EV7.
RD cells (2 × 107) were harvested from fully confluent 75-cm2 flasks by washing with PBS alone and then resuspended in 1 ml of DMEM (10% FCS) at 4°C. MAb 1350 (1/50) was added to the cells, which were then aliquoted into ice-cold 1.5-ml plastic tubes at 2 × 106/tube. The tubes were transferred to a 37°C water bath and incubated for various periods up to 1 h before being placed back on ice. Radiolabelled EV7 (104 cpm) was then added to the cells and allowed to adsorb for 1 h on ice. Nonadsorbed virus was removed by three washes with 1-ml volumes of ice-cold DMEM, each followed by centrifugation at 2,000 rpm in a Microfuge (MSE; Micro Centaur). The cells were incubated at 37°C for 5 min to allow adsorbed virus to enter. The cells were then pelleted by centrifugation, and the amount of eluted radiolabelled virus in the supernatants was determined by scintillation counting. The cells were then resuspended in 100 μl of sDAF (100 μg/ml in PBS) for 1 h on ice; sDAF was added in order to remove from the cell surface by competition any virus that had not yet entered the cells or become irreversibly bound into virus-cell complexes (26). The cells were then pelleted, and the 35S counts in both the supernatant and the cells were determined by scintillation counting.
Time course of EV7 entry into RD cells.
RD cells were harvested from fully confluent 75-cm2 flasks by washing with PBS alone and were resuspended in 1 ml of DMEM (10% FCS) at 4°C. MAb 1350 (1/50) was then added to the cells, which were placed in 1.5-ml plastic tubes at 2 × 107/tube. The tubes were then transferred to a warm (37°C) room and were rotated for 1 h (six revolutions/min) to keep the cells in suspension. The cells were then chilled on ice for 5 min before the addition of 105 cpm of radiolabelled EV7, which was allowed to adsorb to the cells for 1 h on ice. Nonadsorbed virus was then removed by three washes with 1-ml volumes of ice-cold DMEM, each followed by centrifugation at 2,000 rpm in a Microfuge (MSE; Micro Centaur) in a cold (4°C) room. The cell pellets were then resuspended in 1 ml of DMEM with or without antibody, and the cells were aliquoted into fresh 1.5-ml tubes at 2 × 106/tube. The cells were incubated at 37°C from 0 to 60 min to allow adsorbed virus to enter them. The cells were then pelleted by centrifugation, and eluted radiolabelled virus in the supernatant was determined by scintillation counting. The cells were resuspended in 100 μl of sDAF (100 μg/ml in PBS) for 1 h on ice; sDAF was added in order to remove from the cell surface by competition any virus that had not yet entered the cells (26). The cells were then pelleted by centrifugation, and the 35S counts in both the supernatant and the cells were determined by scintillation counting.
RESULTS
MAb 918 blocks EV infection.
We have previously described the isolation of MAbs against the cell surface that block infection by various enteroviruses (23, 37). As described previously, we observed that one antibody, MAb 918, blocked infection of RD cells by a range of EVs, including serotypes 1 to 6, 9, 12 to 19, 21, 24, 26, 27, and 29 to 33, and enterovirus 70. This includes viruses that have previously been shown to utilize DAF (e.g., EV6, -13, -21, -29, and -33) and VLA-2 (EV1) as their primary receptors (3, 6, 37). We first determined that MAb 918 was not (and did not contain) an antibody against DAF; this was done by transfecting a DAF cDNA clone into mouse WOP cells and assessing antibody binding. Such cells did not bind MAb 918, whereas they strongly bound the anti-DAF antibody MAb 854 (data not shown). Moreover, we were able to conclude that the MAb 918 blocking was a specific rather than a general property of ascites, since other MAbs isolated at the same time had different enterovirus-blocking properties; most MAbs did not block EV infection, and one blocked poliovirus infection (MAb 280 [23]).
We then determined whether MAb 918 was directed against a cell surface protein involved in attachment by assessing its ability to block the binding of radiolabelled virus as described previously (37). We included EV6 and -7 as well those viruses that do not utilize DAF (or VLA-2), namely, EV4, -14, -18, and -31, in our assay. In no case was blocking observed (data not shown). We therefore concluded that the ligand for MAb 918 was not a primary receptor and therefore was probably a secondary factor required for EV infection of RD cells after the virus has attached to its receptor.
Identification of the MAb 918 ligand, using CELICS.
To identify the putative secondary factor involved in EV infection, we used CELICS to clone the MAb 918 ligand (37). The CELICS method was developed for the cloning of cell surface proteins and is based on a sensitive immunofocal screen exploiting anti-species antibodies conjugated to β-galactosidase (11). Approximately 108 COS cells were transfected with a HUVEC cDNA library in pCDM8. Rare MAb 918-reactive cDNA-transformed cells were identified by being stained blue after sequential incubations with MAb 918, goat anti-mouse immunoglobulin–β-galactosidase-conjugated antibody, and the β-galactosidase chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). Just four blue cells were found and isolated in the first round of screening. No blue cells were found among control COS cells. Plasmid DNA extracted from these four blue cells gave about 104 E. coli (MC1063/P3) colonies after transformation by electroporation (15). The E. coli colonies were pooled, and a small-scale preparation of their plasmid DNA was transfected into 107 fresh COS cells. In this second round, over 103 MAb 918-reactive blue cells were observed, and 40 were picked. Plasmid DNA was then extracted from the isolated blue cells and transformed into E. coli. Many colonies were obtained, and to determine which clones were MAb 918 reactive, small-scale preparations of the cloned plasmid DNA were transfected into COS cells. Of the 10 cDNA clones screened, 2 were MAb 918 positive, as indicated by their ability to cause the majority of cells to turn blue in a CELICS assay. These clones contained inserts of 1 kb, the sequences of which were 100% concordant with the cDNA sequence of human β2m in the EMBL database (accession no. X07621).
β2m was confirmed as the MAb 918 ligand by enzyme-linked immunosorbent assay on purified commercial protein. Moreover, fluorescence-activated cell sorter analysis of known β2m-negative and -positive cells was consistent with this conclusion (data not shown).
Other anti-β2m MAbs but not anti-major histocompatibility complex antibodies block EV infection of RD cells.
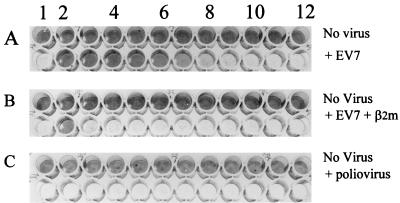
To confirm a role for β2m in EV infection of RD cells, we repeated the blocking assays with a commercially available anti-β2m MAb, MAb 1350. This antibody was higher titer than MAb 918 and, in addition, blocked infection by EV7 (Fig. 1A), -8, -11, -25, and -28 and CA9. To further check that these blocking results were anti-β2m specific, we also performed the blocking assay in the presence of excess purified β2m. The results of this experiment (Fig. 1B) show that free β2m protein inhibits the blocking action of MAb 1350. This indicates that the blocking of EV infection is anti-β2m specific. MAb 1350 was also found to have no affect on poliovirus infection of RD cells (Fig. 1C).
FIG. 1.
Anti-β2m MAb 1350 blocks EV7 infection of RD cells. RD cells in 96-well plates were incubated with MAb 1350 for 1 h in tissue culture in the absence (A) or presence (B) of β2m (200 ng/well) and were then infected with 10 TCID50/cell of EV7. MAb 1350-treated RD cells were also infected with the same titer of poliovirus (C). After infection, the cells were incubated in tissue culture for 24 h and then fixed and stained with Giemsa crystal violet. Lane 1, no MAb; lanes 2 to 12, MAb 1350 at dilutions of 1/125 to 1/128,000.
β2m molecules are coexpressed on the cell surface with class I HLA heavy chains. We therefore investigated the possible involvement of the class I heavy chain in EV infection. The panspecific anti-class I MAb W6/32 (2) was used in virus infection-blocking assays. This MAb did not inhibit EV infection, suggesting that class I antigens recognized by W6/32 are not involved.
EV7 binding and neutralization.
We chose to study the involvement of β2m in EV7 infection of RD cells. EV7 binding to these cells is well characterized and can be blocked by an anti-DAF MAb, MAb 854 (12, 37). Neither MAb 1350 nor purified β2m protein at concentrations of up to 20 μM inhibited EV7 binding to RD cells, whereas the anti-DAF MAb blocked over 95% of binding (12, 37). Furthermore, in virus neutralization assays micromolar concentrations of β2m had no effect on EV7 or EV11 virus titer. These results indicate that EV7 does not interact directly with free β2m in solution. However, they do not rule out the possibility that cell-bound β2m directly interacts with viruses at a postattachment stage.
β2m is not involved in EV7 infection of all cell types.
We next investigated whether semipermissive Ohio HeLa cells and permissive MRC5 cells were also protected from infection by anti-β2m antibody. Like RD cells, MRC5 cells were protected by MAb 1350. Surprisingly, however, neither MAb 1350 nor MAb 918 blocked infection of HeLa cells even though these cells were shown by fluorescence-activated cell sorter analysis to express levels of β2m similar to those of RD cells (data not shown). In experiments performed to show whether β2m was required for infection, Daudi cells, a B-cell line that does not express β2m, were inoculated with EV7 and the infection of cells was measured by immunofocal assay and compared with that of inoculated Raji cells, a β2m-positive cell line. Both cell lines supported virus infection. These results suggest a role for β2m in EV infection of some but not all cell types.
Effect of anti-β2m MAb 1350 on EV7 replication in RD cells.
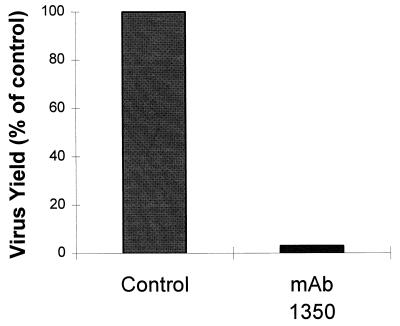
The infection of RD cells by EV7 could be quantified easily by the immunofocal staining method with a commercial MAb against EV coat protein (MAb 5-D/8; Dako). Pretreatment of cells for 1 h with a 1/250 dilution of the anti-β2 antibody (MAb 1350) resulted in a 95% decrease in the number of cells that stained blue with the anti-coat protein MAb at 6 h postinfection. Moreover, the same concentration of MAb 1350 was found to cause a 95% reduction in EV7 yield at 16 h postinfection, as measured by TCID50 assay (Fig. 2). This latter assay was made possible by the addition of purified β2m to the virus preparations to sequester excess antibody so that replication was not blocked on recipient cell monolayers, as described in Materials and Methods. The absence of detectable virus coat protein in the majority of cells by 6 h postinfection suggests that the block to infection occurs at an early stage, possibly at penetration and/or uncoating. This conclusion was reinforced by transfecting EV7 and poliovirus vRNAs into MAb 1350-treated cells. No significant inhibition was observed by the immunofocal assay (data not shown).
FIG. 2.
EV7 infection is blocked by MAb 1350. RD cells (80% confluent) in 25-cm2 flasks were incubated in the presence or absence of MAb 1350 as described in the text. The antibody was then removed, and 1 ml of EV7 at 1 TCID50/cell was added to the cells and allowed to adsorb for 1 h at 37°C. The cells were washed, and the antibody was added back to the cells, which were then incubated for 16 h under normal growth conditions. Antibody was then added to the control flask, followed by the addition of 20 μg of purified β2m to both flasks. The virus yields were then measured (see text).
MAb 1350 causes only a partial inhibition of EV7 entry into RD cells.
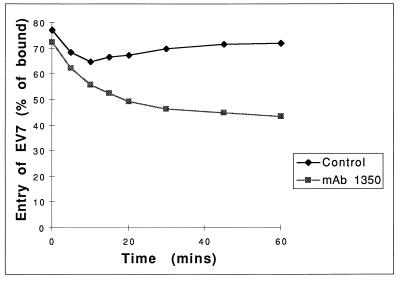
Having established that MAb 1350 blocks neither virus binding nor translation and replication of transfected vRNA, we investigated the postbinding entry of virus into RD cells in the presence of the antibody. We have shown previously that EV7 bound to cells at 4°C can be displaced by the addition of excess recombinants DAF (26). To determine the dynamics of the MAb 1350-induced block to EV7 entry, RD cells were preincubated with MAb 1350 at 37°C from 0 to 60 min before the addition of virus, which was allowed to attach at 4°C. The cells were then moved to 37°C for 5 min and resuspended in PBS containing sDAF at a level known to displace ∼90% of bound virus from the cell surfaces. Noneluted virus was measured and compared to the levels in control cells, which had not been pretreated with MAb 1350. Antibody treatment was found to induce a partial block to entry of EV7 within minutes and reached a maximum block of 30% after 60 min (Fig. 3). However, the extent of this inhibition was significantly less than the 95% block to virus infection observed under similar conditions. This result suggests that although the anti-β2m MAb has some effect on virus entry, its inhibition of virus infection may involve other mechanisms.
FIG. 3.
Anti-β2m time-dependent block to virus entry. RD cells were incubated with MAb 1350 for 0 to 60 min at 37°C and then chilled on ice (see text). The cells were then incubated with 35S-radiolabelled virus on ice for 1 h before being washed to remove nonadsorbed virus. The entry of virus into the cells was then stimulated by incubation at 37°C for 5 min, the cells were placed back on ice, and supernatants were removed. Nonentered virus was then competed off the cell surfaces by the addition of sDAF (100 μg/ml), the cells were pelleted by centrifugation, and the 35S counts in the supernatant and the cell pellet were determined by scintillation counting. The results shown are representative of four independent experiments.
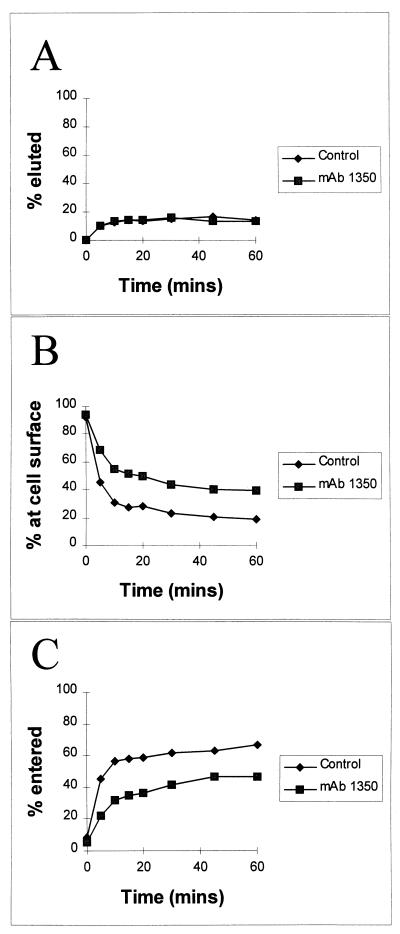
In similar experiments, the rate of virus entry into cells was measured after RD cells were pretreated with antibody for 1 h, after which entry was allowed at 37°C for 0 to 60 min. Unlike poliovirus (19), EV7 does not spontaneously elute from cells in substantial quantities (26). MAb 1350 did not alter the rate of spontaneous elution; most virus (∼90%) remained associated with the cells at 1 h (Fig. 4A). Virus entry (as measured by virus becoming resistant to displacement by sDAF) was rapid, reaching a plateau by approximately 10 min (Fig. 4B and C). Similar results were obtained for virus entry as measured by virus becoming resistant to displacement by proteinase K (data not shown). As discussed above, MAb 1350 inhibited entry by about 30%, and this was apparent at all time points analyzed. By contrast, neither MAb 1350 that had been pretreated with purified β2m nor the anti-class I MAb W6/32 had any effect on virus entry (data not shown). Similar experiments measured EV7 entry into HeLa cells pretreated with MAb 1350. The antibody had no measurable effect.
FIG. 4.
Time course of EV7 entry into RD cells. RD cells were incubated with MAb 1350 for 1 h at 37°C and placed on ice. 35S-radiolabelled virus was then added to the cells, and 1 h later nonadsorbed virus was washed away (see text). The entry of virus into cells was stimulated by incubation at 37°C for 0 to 60 min, and the eluted counts in the supernatants were determined (A). Nonentered virus was then competed off the cell surfaces by the addition of sDAF (100 μg/ml) and counted (B). Counts remaining in the cell pellet were also determined (C). The results are expressed as percent total virus bound and are representative of four independent experiments.
The use of RD cells in suspension in these experiments is unlikely to have influenced their permissiveness for virus compared to that of cells in monolayers. This is because RD cells kept in suspension for up to 6 h postinfection showed productive infection at a level comparable to that of those in monolayers (data not shown).
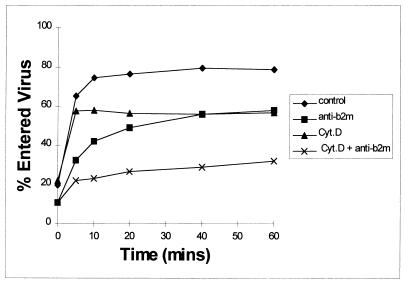
These results indicate that the anti-β2m antibodies partially inhibit entry of EV7. However, the extent of this inhibition of entry is not sufficient to account for the full extent of the block on infection. We therefore considered whether virus might enter cells via more than one mechanism, only one of which would be productive and subject to inhibition by anti-β2m antibodies. To determine whether caveolae or non-clathrin- or clathrin-coated pits have a role in EV7 infection, these routes of endocytosis were inhibited by preincubating cells with cytochalasin D, a potent inhibitor of actin-myosin polymerization and thus of endocytosis via pits and caveolae (25). We found that cytochalasin D treatment also caused a partial block to EV7 entry into RD cells, although it did not block infection as determined by cytopathic effect and immunofocal assay. However, the time course of entry of the noninhibited fraction was consistently different from that in antibody-treated cells (Fig. 5). Moreover, the block to entry found in cells treated with both cytochalasin D and antibody was double that of the cells which received single treatments. In addition, a 1 h preincubation of cells in 0.1% sodium azide had little effect on the entry of virus (data not shown). Since both of these chemical treatments inhibit endocytosis via caveolae and pits, the most likely explanation for these results is that our assay not only measures penetration of virus into cells but also entry of virus into proteinase K-resistant receptor complexes at the cell surface. Formation of such receptor complexes would prevent elution of EV7 by recombinant sDAF. Our recently published results on EV7-DAF interaction also suggest that EV7 escapes neutralization by recombinant sDAF by entering into a receptor complex (26). Therefore, the results showing that the blocks to entry for cytochalasin D and antibody are additive indicate that recruitment of virus into a receptor complex may be inhibited by MAbs against β2m.
FIG. 5.
Effect of cytochalasin D on EV7 entry into RD cells. RD cells were incubated with cytochalasin D (cyt. D; 2 μg/ml) and/or MAb 1350 (anti-β2m) for 1 h at 37°C and placed on ice. 35S-radiolabelled virus was then added to the cells, and 1 h later nonadsorbed virus was washed away (see text). The entry of virus into the cells was stimulated by incubation at 37°C for 0 to 60 min. Nonentered virus was then competed off the cell surfaces by the addition of sDAF (100 μg/ml). Counts remaining in the cell pellet were determined. The results are expressed as percent total virus bound and are representative of four independent experiments.
Treatment of RD cells with specific inhibitors of caveolae uptake, i.e., the phorbol ester phorbol myristate acetate (10 μM) (1, 33) or nystatin (25 μg/ml) (1), 1 h before the addition of virus failed to block EV7 infection as measured by immunofocal staining at 6 h postinfection (data not shown). These results suggest that caveolae are not involved in the EV7 infectious pathway.
DISCUSSION
A MAb directed against RD cells, MAb 918, was found to block EV infection. We therefore cloned the cDNA encoding the ligand of MAb 918 by the CELICS cloning method. The ligand was identified as β2m. Use of a commercial anti-β2m antibody, MAb 1350, confirmed that antibodies to β2m block EV infection of RD and MRC5 cells.
An antibody with properties similar to those described here has been reported previously by Mbida et al. (21, 22). Their antibody, MAb 143, blocks infection by most EVs in a variety of human and primate cell lines, including KB, P2002, Wish, Vero, and BGM cells, although its action on RD and MRC5 cells was not reported. Since MAb 143 was found to affinity purify a 44-kDa protein, it is possible that MAb 143 is directed against either β2m or class I HLA heavy chains. These proteins would be expected to copurify as a complex under the conditions used (22). Mbida et al. reported that their purified protein neutralized EV11 in vitro (22). However, we found that neither EV11 nor EV7 is neutralized by purified β2m, and we also found that this protein does not induce A-particle formation in the presence of the purified soluble receptor, DAF (unpublished data). Also, we were unable to show blocking of virus infection by an anti-class I HLA antibody. However, given that β2m exists primarily on the cell surface as a complex with many haplotypes of class I HLA, we cannot rule out the possibility that other anti-class I HLA antibodies may block infection. An observation that argues against a direct role for class I HLA is that Daudi cells, which do not express HLAs, can be infected by EVs. However, Daudi cells may express other cell surface proteins that can substitute for β2m in infection. The inability of the anti-β2m MAbs to block EV infection of HeLa cells may also be due to the possible expression of other cell surface proteins that can substitute for β2m and play a role in infection. Some picornaviruses have been reported to have the capacity to use more than one primary receptor (4, 5, 24, 30, 31). It is possible that EVs can also use more than one secondary factor for infection.
It is not clear from our results at precisely what stage antibodies to β2m block EV infection. Naked vRNA transfected into RD cells was not inhibited by the anti-β2m antibodies. This suggests that the block may occur at a stage in replication up to, and including, uncoating. We measured virus uptake on the basis of labelled virus becoming attached to the cell and progressing into a state in which it could not be eluted with excess soluble receptor (DAF) or by protease digestion. Although the anti-β2m antibodies caused a partial block to this virus uptake, the level of the block may be insufficient to account for the 95% block to virus infection. However, cytochalasin D or other inhibitors of pit and/or caveoli entry did not inhibit EV7 infection. This, together with the observation that the block to entry caused by cytochalasin D was additive to that caused by the anti-β2m antibodies, suggests that entry via clathrin-coated pits and caveolae may constitute dead-end entry mechanisms. Moreover, since uptake of EV7 is azide resistant, we suggest that virus may enter into receptor complexes prior to penetration. We also speculate that anti-β2m antibodies block EV7 infection by affecting virus-receptor complex formation.
The best-characterized role of β2m is its association with the 44-kDa class I heavy chains forming the HLA complex at the cell surface. This complex presents foreign antigens to T cells. There is, however, strong evidence that the HLA and/or MHC complex has other roles in the biology of the cell. Contact inhibition (14), platelet aggregation (13), and many hormone receptor signal transduction systems are influenced by class I expression. For example, it has been shown that class I antigen heavy chains interact with the luteinizing hormone receptor after binding its ligand, and it is thought that this interaction is essential for luteinizing hormone receptor micro-aggregation prior to signal transduction (34, 35). Also, the insulin receptor contains the class I heavy chain, and its activity can be blocked by antibodies to class I antigen (16). Indeed, it has been suggested that class I antigen may influence many receptors by facilitating their aggregation, which is required for efficient signal transduction (16, 28, 34, 35). If antibodies to β2m block aggregation and thereby influence signal transduction events, they may induce a general down regulation of a variety of cellular activities, some of which may be necessary for productive entry of EVs.
This is the first reported observation that MAbs against β2m block EV infection. However, it has previously been shown that MAbs to class I antigen and β2m can block infection by other pathogens, e.g., simian virus 40 (9, 36), adenovirus 2 and 5 (18), and Theileria parva (32). These pathogens are thought to use class I antigen as a receptor. Salmonella typhimurium may also require class I antigen for invasion of cells (17). On binding to the epidermal growth factor receptor, EGFR, the signalling of which is regulated by class I antigen (28), Salmonella induces a selective microaggregation of class I antigen, β2m, CD44, and the fibronectin receptor, α5β1. It is therefore possible that some invasive pathogens may share with EVs entry pathways or receptor complexes that are regulated by HLA and/or MHC antigens.
In conclusion, our results show that β2m, possibly as part of the HLA class I complex, is required for EV infection of certain, but not all, cell types. The block to infection is postattachment but prior to RNA translation and replication. The precise mechanism by which β2m plays a role in infection, including its possible role in signal transduction mechanisms, is under further investigation.
ACKNOWLEDGMENTS
We thank Moy Robson for excellent technical support.
This work was funded by the Medical Research Council Programme Grant G006199.
REFERENCES
- 1.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnstable C J, Bodmer W F, Brown G, Galfre G, Milstein C, Williams A F, Zigler A. Production of monoclonal antibodies to group A erythrocytes HLA and other human cell surface antigens. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Chan M, Solomon K R, St. John N F, Lin H, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Mohanty J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson J M, Shepley M P, Chan B M, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1525. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breau W C, Atwood W J, Norkin L C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992;66:2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham P R, McKnight A, Weiss R A. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531–3537. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson N A, Kaufman R, Lublin D M, Ward T, Pipkin P A, Minor P D, Evans D J, Almond J W. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J Virol. 1995;69:5497–5501. doi: 10.1128/jvi.69.9.5497-5501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry R A, Messner R P, Johnson G J. Inhibition of platelet aggregation by monoclonal antibody reactive with beta-2 microglobulin chain of HLA complex. Science. 1984;224:509–511. doi: 10.1126/science.6324346. [DOI] [PubMed] [Google Scholar]
- 14.Curtis A S G, Rooney P. H-2 restriction of contact inhibition of epithelial cells. Nature. 1979;281:222–223. doi: 10.1038/281222a0. [DOI] [PubMed] [Google Scholar]
- 15.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Due C, Simonsen M, Olsson L. The major histocompatibility complex class-I heavy chain as a structural subunit of the human cell membrane insulin receptor: implications for the range of biological functions of histocompatibility antigens. Proc Natl Acad Sci USA. 1986;83:6007–6011. doi: 10.1073/pnas.83.16.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-del Portillo F, Pucciarelli M G, Jefferies W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I alpha 2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joklik W K, Darnell J E. The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- 20.Kvist S, Levy F. Early events in the assembly of MHC class I antigens. Semin Immunol. 1993;5:105–116. doi: 10.1006/smim.1993.1014. [DOI] [PubMed] [Google Scholar]
- 21.Mbida A D, Gaudin O G, Sabido O, Pozzetto B, Lebihan J C. Monoclonal antibody specific for the cellular receptor of echoviruses. Intervirology. 1992;33:17–22. doi: 10.1159/000150226. [DOI] [PubMed] [Google Scholar]
- 22.Mbida A D, Pozzetto B, Gaudin O G, Grattard F, Lebihan J C, Akono Y, Ros A. A 44,000-glycoprotein is involved in the attachment of echovirus-11 onto susceptible cells. Virology. 1992;189:350–353. doi: 10.1016/0042-6822(92)90714-z. [DOI] [PubMed] [Google Scholar]
- 23.Minor P D, Pipkin P A, Hockley D, Schild G C, Almond J W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1:203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- 24.Modlin J F, Bergelson J M, Wielandalter W, Finberg R W. Group-B coxsackieviruses (CBV) use both decay-accelerating factor and a 50 kd protein as receptors. Pediatr Res. 1996;39:1064. [Google Scholar]
- 25.Parton R G, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell R M, Ward T, Evans D J, Almond J W. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J Virol. 1997;71:9306–9312. doi: 10.1128/jvi.71.12.9306-9312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rico Hesse R, Pallansch M A, Nottay B K, Kew O M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987;160:311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A B, Schlessinger J, Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984;98:725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw M K, Tilney L G, Musoke A J, Teale A J. MHC class-I molecules are an essential cell surface component involved in Theileria parva sporozoite binding to bovine lymphocytes. J Cell Sci. 1995;108:1587–1596. doi: 10.1242/jcs.108.4.1587. [DOI] [PubMed] [Google Scholar]
- 33.Smart E J, Foster D C, Ying Y S, Kamen B A, Anderson R G W. Protein kinase-C activators inhibit receptor mediated potocytosis by preventing internalization of caveolae. J Cell Biol. 1994;124:307–313. doi: 10.1083/jcb.124.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solano A R, Cremaschi G, Sanchez M L, Borda E, Sterinborda L, Podesta E J. Molecular and biological interaction between major histocompatibility complex class I antigens and luteinizing hormone receptors or beta-adrenergic receptors triggers cellular response in mice. Proc Natl Acad Sci USA. 1988;85:5087–5091. doi: 10.1073/pnas.85.14.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solano A R, Sanchez M L, Sardanons M L, Dada L, Podesta E J. Luteinizing hormone triggers a molecular association between its receptor and the major histocompatibility complex class I antigen to produce cell activation. Endocrinology. 1988;122:2080–2083. doi: 10.1210/endo-122-5-2080. [DOI] [PubMed] [Google Scholar]
- 36.Stang E, Kartenbeck J, Parton R G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward T, Pipkin P A, Clarkson N A, Stone D M, Minor P D, Almond J W. Decay accelerating factor (CD55) identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 1994;13:5070–5074. doi: 10.1002/j.1460-2075.1994.tb06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]