Figure 4. Glycosylation patterns observed for the MST1 PPII helix.
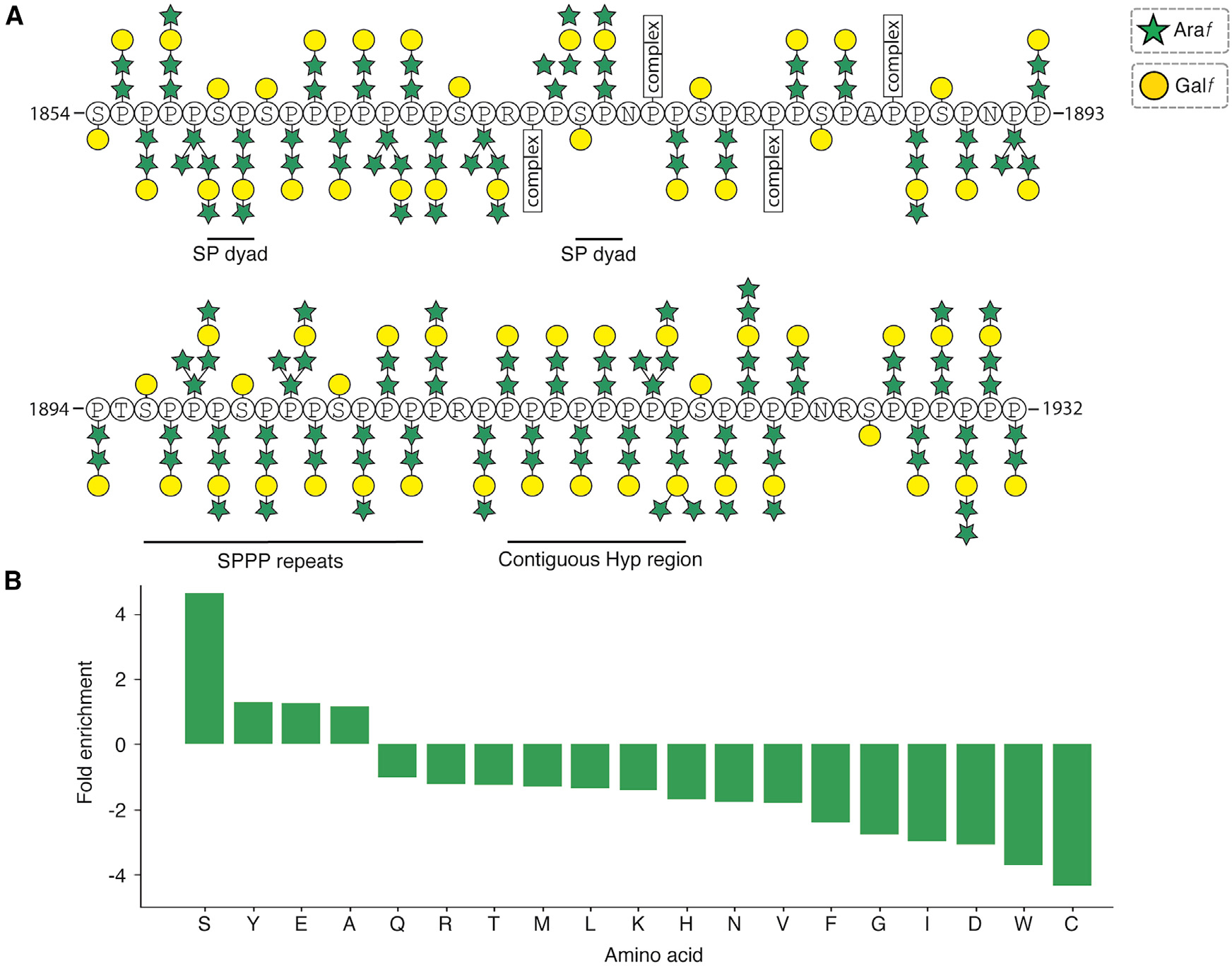
(A) Schematic showing the glycan structures associated with each hydroxyproline and serine residue of the poly(proline)-rich region of MST1 (residues 1,854–1,932). Saccharides are represented following the Symbol Nomenclature for Glycans (SNFG). Note that these assignments are only tentative, as the cryo-EM density does not allow individual sugar types to be distinguished. The number of saccharide units represents either the exact number or the minimum that can be confidently identified from the cryo-EM density. Large branched glycans are labeled as “complex.” Non-proline/serine residues are not modified.
(B) Histogram showing the fold enrichment of each non-proline amino acid in poly(proline)-rich regions of the C. reinhardtii proteome. Poly(proline)-rich regions were defined as 20-residue nonoverlapping regions containing 10 or more proline residues. Only serine (S), tyrosine (Y), glutamate (E), and alanine (A) are enriched in poly(proline)-rich regions compared with their general frequency in the proteome.
