Abstract
The cytolytic T-lymphocyte (CTL) response to respiratory infection with equine herpesvirus 1 (EHV-1) in CBA (H-2k) mice was investigated. Intranasal (i.n.) inoculation of mice with the attenuated EHV-1 strain KyA resulted in the generation of a primary virus-specific CTL response in the draining mediastinal lymph nodes 5 days following infection. EHV-1-specific CTL could be restimulated from the spleen up to 26 weeks after the resolution of infection, indicating that a long-lived memory CTL population was generated. Depletion of CD8+ T cells by treatment with antibody and complement prior to assay eliminated CTL activity from both primary and memory populations, indicating that cytolytic activity in this model was mediated by class I major histocompatibility complex-restricted, CD8+ T cells. A single i.n. inoculation with KyA induced protective immunity against infection with the pathogenic EHV-1 strain, RacL11. The adoptive transfer of splenocytes from KyA-immune donors into sublethally irradiated recipients resulted in a greater than 250-fold reduction in RacL11 in the lung. The elimination of both CD4+ and CD8+ T cells from the transferred cells abrogated clearance of RacL11, while the selective depletion of either subpopulation alone had little effect. These results suggested that both lymphocyte subpopulations contribute to viral clearance, with either subpopulation alone being sufficient.
Equine herpesvirus 1 (EHV-1) is a prevalent respiratory pathogen of horses worldwide (8, 17, 38). Infection of horses with EHV-1 results in fever, respiratory distress, abortagenic disease, and severe neurological sequelae (3, 13, 27, 35, 36). The highly contagious respiratory transmission of EHV-1 has resulted in disastrous outbreaks of disease in domestic horse populations and has had a significant economic impact on the equine industry. EHV-1 infection of the horse results in the generation of a short-lived humoral response but does not confer long-term protection, as disease often occurs following natural infection (10, 22). Although both live and inactivated vaccines are currently available for EHV-1, only relatively short-lived protection has been observed (11, 12, 24). Furthermore, it is not clear which immune functions are responsible for conferring the short-lived protection following vaccination. In addition to specific antibody responses, peripheral blood leukocytes from vaccinated horses produce gamma interferon in culture (19). EHV-1-specific, CD8+ class I major histocompatibility complex (MHC)-restricted cytotoxic T lymphocytes (CTL) have been identified in peripheral blood mononuclear cells, reaching maximal levels 2 to 3 weeks postinfection (p.i.) (4, 20). However, the effectiveness of the current vaccines in stimulating EHV-1-specific CTL and their role in protective immunity in vivo are currently not known.
Horses inoculated with the attenuated EHV-1 strain Kentucky A (KyA) exhibited a reduction in clinical signs following challenge with a pathogenic EHV-1 strain (33, 34). Although the attenuated strain induced a protective response, in terms of a reduced duration of viral shedding and viremia, the ability of this strain to induce an EHV-1-specific antibody response was weaker than that of the virulent strain. This finding suggested that immune functions other than the generation of specific antibodies might be critical in the resolution of infection. To generate a more effective EHV-1 vaccine, a better understanding of the precise immune functions associated with protection and resolution from EHV-1 infection is essential.
A murine model of respiratory EHV-1 infection which closely mimicked EHV-1 infection in the natural host was established in various strains of mice (5). Common features included replication in the respiratory mucosae, the development of pneumonitis, cell-associated viremia, and abortion (5, 6). Specific immune responses are important for modulating infection. In the mouse, the passive transfer of hyperimmune polyclonal rabbit EHV-1-specific antibodies into infected mice significantly reduced the viremia following challenge with live EHV-1 (5). Subsequent studies demonstrated that various EHV-1 glycoproteins, including gB, gC, gD and gH, were capable of inducing the generation of neutralizing antibodies (9, 23, 40, 49, 52, 56). Furthermore, inoculation of mice with gB, gC, and/or gD elicited a protective response against subsequent challenge with pathogenic EHV-1 (39, 49, 50, 52, 56). However, each of these EHV-1 gene products is capable of eliciting both B- and T-cell responses; thus, the role of distinct immune functions conferring protection is not clearly defined.
In the most extensively studied BALB/c mouse model of EHV-1 infection, adoptive transfer experiments demonstrated that immune spleen cells isolated from mice primed with live, but not heat-killed, EHV-1 reduced viral levels in both the lungs and nasal turbinates of infected recipient mice (5). Although the specific cell population responsible for protection was not determined, the data suggested that cell-mediated immune functions may be critical in the resolution of EHV-1 infection. The adoptive transfer of defined T-cell subpopulations demonstrated that both CD4+ and CD8+ T cells play a role in controlling EHV-1 respiratory infection. The CD8+ T-cell subpopulation appeared to play a more dominant role, although the functions by which protection was mediated were not defined (7). If the immune response was elicited by immunization with recombinant baculovirus-derived EHV-1 glycoproteins, the response was altered so that CD4+ T cells were predominantly associated with protection (52). Thus, either T-cell subpopulation is likely to play an important role in the optimal response to infection.
While adoptive transfer studies have implicated an important role for CD8+ T cells in the control of EHV-1 infection in the lungs of BALB/c mice (7), there has been no direct assessment of CD8+ T-cell effector functions in this model. This is due predominantly to the lack of suitable class I MHC-compatible, H-2d-expressing murine cells that are susceptible to infection with EHV-1. However, the attenuated KyA strain of EHV-1 has been propagated in our laboratory in suspension cultures of murine L-M fibroblasts that express the H-2k haplotype. Therefore, an alternative mouse model, using mice expressing the H-2k haplotype, was adopted principally to assess the activation of EHV-1-specific CTL responses. Initial studies by Awan et al. (5) demonstrated that CBA (H-2k) mice were susceptible to infection with EHV-1 strain Ab4. In the present study, CBA mice were susceptible to respiratory tract infection by both the nonpathogenic KyA and the pathogenic RacL11 strains of EHV-1. Furthermore, infection of CBA mice with the attenuated KyA strain generated a vigorous CD8+, class I MHC-restricted, EHV-1-specific primary CTL response in the draining mediastinal lymph nodes (MLN) and a long-term memory CTL response in the spleen. These studies provide the basis for a model system to analyze the potential importance of class I MHC-restricted CTL activity in controlling EHV-1 infection in vivo.
MATERIALS AND METHODS
Virus and cell culture.
The Kentucky A KyA strain was propagated in suspension cultures of L-M mouse fibroblasts as previously described (41). EHV-1 strain RacL11, a kind gift of Anton Mayr, Institute for Medical Microbiology, Infectious and Epidemic Diseases, Ludwig-Maximilian-University, Munich, Germany, was grown in NBL6 equine dermal cells (American Type Culture Collection). Titers of both viruses were determined on rabbit kidney cells (ATCC RK-13). L-M, NBL6, and RK cell lines were maintained at 37°C in Eagle’s minimal essential medium supplemented with penicillin (100 U per ml), streptomycin (100 μg per ml), nonessential amino acids, and 5% fetal calf serum (FCS). For suspension cultures, L-M fibroblasts were maintained at 37°C in Eagle’s minimal essential medium supplemented with yeast extract-lactalbumin hydrolysate-peptone, antibiotics, and 5% FCS (41).
Mice.
Female CBA (H-2k) mice, 3 to 6 weeks of age, were obtained from Harlan Sprague Dawley, Indianapolis, Ind., or Jackson Laboratory Bar Harbor, Maine. Mice were maintained in the Animal Resource Facility of the Louisiana State University Medical Center, Shreveport, in cages equipped with filter tops. All mice were rested for a minimum of 1 week prior to use.
Infection and assessment of CTL activity.
Mice were anesthetized with Halothane (Sigma Chemical Co., St. Louis, Mo.) and inoculated intranasally (i.n.) with 2 × 106 PFU of EHV-1 KyA in a volume of 50 μl. To assess primary CTL responses, lymphocytes were isolated from the MLN or superficial cervical lymph nodes (CLN) 5 days postinoculation, and a single-cell suspension was obtained by pressing the lymphoid tissues through a 60-gauge wire mesh screen. The lymphocytes were washed and cultured (107 cells per well) for 3 days at 37°C and 5% CO2 in 12-well flat-bottom plates (Corning Inc., Corning, N.Y.) in complete RPMI 1640 (Sigma) containing 5% FCS, 20 μM β-mercaptoethanol, 20 mM HEPES, 2 mM l-glutamine, and antibiotics. To assess memory CTL responses, mice were infected as described above and maintained for 2 to 26 weeks. Spleen cell suspensions were generated as described above except for a brief exposure at 37°C to Tris-buffered 0.83% NH4Cl to lyse erythrocytes. The resulting lymphocytes were cultured (107 cells per well) in complete RPMI 1640 at 37°C and 5% CO2, for 5 days in 12-well flat-bottom plates in the presence of 3 × 105 mitomycin C-treated, EHV-1 KyA-infected L-M cells (28). Stimulator cells were infected (EHV-1 KyA, multiplicity of infection of 10 for 18 h) to allow the expression of late viral gene products (38). Cytolytic activity was assessed in a standard 4-h 51Cr release assay in 96-well V-bottom plates (Nunc, Denmark) to allow analysis of a range of effector-to-target ratios against 104 51Cr-labeled, infected or uninfected target cells. The percentage of specific lysis was determined by using the formula [(A − B)/(C − B)] × 100, where A is 51Cr released by target cells incubated with effector cells (experimental release), B is 51Cr released from targets incubated in medium alone (spontaneous release), and C is 51Cr released from targets incubated in 3% acetic acid (maximum release). Each effector-to-target ratio was assayed in triplicate. The spontaneous release never exceeded 20%, and the variability between the values of specific lysis of triplicate cultures did not exceed 5%.
Adoptive transfer of lymphocytes.
Donor immune lymphocytes were obtained from the spleens of mice infected with EHV-1 KyA 2 weeks previously. The lymphocytes were processed, restimulated in vitro for 5 days, and resuspended in RPMI 1640 without FCS at a concentration of 8 × 107 cells/ml. Recipient mice were given a sublethal dose of 500 cGy of total-body γ irradiation and infected i.n. 1 h later with 1.5 × 106 PFU of EHV-1 RacL11. One to two hours following infection, recipients received 2 × 107 immune lymphocytes via tail vein injection. Control animals received 2 × 107 nonimmune splenocytes from uninfected mice. Four days following transfer, the lungs were removed, and the titer of infectious EHV-1 RacL11 was determined.
Selective depletion of T-lymphocyte subpopulations.
To identify the functional T-lymphocyte subpopulation(s) in the in vitro cytolytic assays and the in vivo protection assays, cultured lymphocytes were first subjected to two rounds of antibody-dependent, complemented-mediated depletion (21). CD4+ T cells were depleted with monoclonal antibody (MAb) RL-172 (rat immunoglobulin M [IgM] [15]) and rabbit complement (Low-Tox M; Accurate Scientific, Westbury, N.Y.). CD8+ T cells were depleted with the MAb 3.155 (rat IgM [45]) and complement. Depleted populations were resuspended in the original volume to avoid increasing the concentration of the remaining T cells. The efficacy of depletion was determined by subsequent staining with fluorescein isothiocyanate-conjugated anti-CD4 (GK1.5, rat IgG2b [55]) or phycoerythrin-conjugated anti-CD8 (CT-CD8b, rat IgG2a; Caltag Laboratories, Burlingame, Calif.). Flow cytometric analysis was performed with a Profile II analyzer (Coulter, Hialeah, Fla.).
Statistical analysis.
The significance of differences in virus recovery from immunized mice or from recipients of immune donor cells and the appropriate control groups was determined by Student’s two-tailed t test (Mann-Whitney analysis of variance).
RESULTS
Infection of CBA mice with EHV-1.
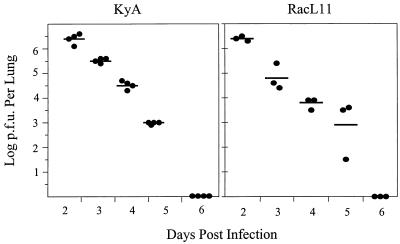
To assess the susceptibility of CBA mice to infection with EHV-1, groups of animals were infected in the respiratory tract with 2 × 106 PFU of KyA or 1.5 × 106 RacL11 by i.n. inoculation, and the titers of infectious virus were determined at different times p.i. The results (Fig. 1) demonstrated that the levels of both EHV-1 strains were highest at 2 days p.i. and steadily declined thereafter to undetectable levels by day 6. Histological examination of infected lungs revealed that inflammatory infiltration was evident by day 3 p.i., with substantial consolidation involving up to 30% of the lungs by day 5 of infection for KyA and greater than 50% of the lungs for RacL11 (data not shown). In some experiments, mice infected with RacL11 died 6 to 7 days p.i., while no animals died following KyA infection. Lung consolidation was most severe when virus titers were at their lowest, suggesting that severe pneumonia was independent of virus levels in the lung.
FIG. 1.
Kinetics of EHV-1 and RacL11 growth in the lungs of CBA mice. CBA mice were infected i.n. with 2 × 106 PFU of KyA or 1.5 × 106 PFU of RacL11 and on days 2 through 6; the lungs were removed and homogenized in a 3-ml tissue grinder. The amount of infectious EHV-1 was determined by standard plaque titration on RK monolayers as described in Materials and Methods. Each dot represents the log PFU per lung determined from a single mouse. The horizontal bars represent the mean log PFU per experimental group.
Assessment of EHV-1-specific cytolytic activity during primary EHV-1 infection.
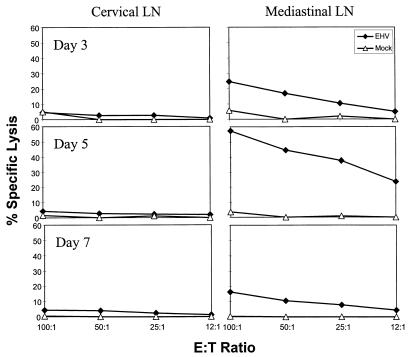
CBA mice were infected i.n. with 2 × 106 PFU of KyA, and on day 5 p.i. the CLN draining the nasal turbinates and the MLN draining the lungs were removed and cultured for 3 days to allow cytolytic activity to develop, as demonstrated previously for other herpesviruses (14, 28, 42, 47). The results (Fig. 2) demonstrated that EHV-1-specific CTL were present in the MLN by day 3 p.i., peaked by day 5, and diminished by day 7. In contrast, little or no cytolytic activity was detected in the CLN at any time during infection. This finding suggested that EHV-1-specific CTL activity, elicited by the avirulent KyA strain, was compartmentalized primarily to the MLN draining the lung, even though there was an increase in cellularity in the CLN draining the nasal turbinates, the initial site of virus replication (5) (data not shown).
FIG. 2.
Cytolytic activity in the MLN and CLN. CBA mice were infected intranasally with 2 × 106 PFU of KyA. On days 3, 5, and 7 p.i., the MLN and CLN were removed, a single-cell suspension was obtained, and the resulting LN cells were cultured for 3 days as described in Materials and Methods. Cytolytic activity was measured in a standard 4-h 51Cr release assay using mock-infected (▵) or KyA-infected (⧫) L-M fibroblasts as targets. Each effector-to-target (E:T) ratio was assayed in triplicate. The spontaneous release never exceeded 20%, and the variability between the values of specific lysis of triplicate cultures did not exceed 5%.
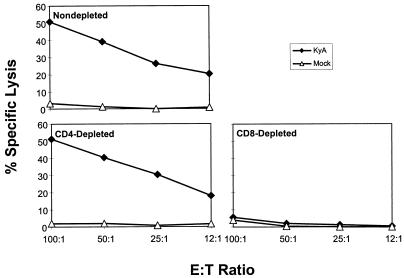
EHV-1-specific CTL activity is mediated by CD8+ T cells.
The phenotype of the MLN-resident T-cell subpopulation mediating cytolytic activity was assessed by selective depletion prior to assay. MLN lymphocytes were obtained 5 days p.i. with KyA, cultured for 3 days in vitro, and depleted by treatment with MAb specific for murine CD4 or CD8α in the presence of complement. Depletion of CD4+ T cells had no effect on the cytolytic activity observed, while the elimination of CD8+ lymphocytes from this population abrogated EHV-1-specific CTL (Fig. 3). These results indicated that the cytolytic activity is mediated exclusively by CD8+, class I MHC-restricted T cells, although the formal demonstration of class I MHC restriction was not possible due to the lack of allogeneic murine cell lines susceptible to infection with EHV-1.
FIG. 3.
Abrogation of primary CTL activity by depletion of CD8+ T lymphocytes in vitro. MLN were removed 5 days after i.n. infection with 2 × 106 PFU of KyA, and a single-cell suspension was obtained and cultured as described in Materials and Methods. The resulting lymphocytes were either nondepleted or depleted of CD4+ or CD8+ T lymphocytes with specific antibody plus complement immediately prior to assay as described in Materials and Methods. Flow cytometric analyses of depleted cell populations revealed that the efficacy of CD4 and CD8 depletion was greater than 99%. Cytolytic activity was measured in a standard 4-h 51Cr release assay using mock-infected (▵) or KyA-infected (⧫) L-M fibroblasts as targets. Each effector-to-target (E:T) ratio was assayed in triplicate. The spontaneous release never exceeded 20%, and the variability between the values of specific lysis of triplicate cultures did not exceed 5%.
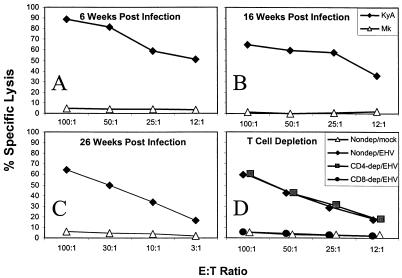
Determination of EHV-1-specific memory CTL activity.
To determine whether EHV-1-specific memory CTL activity was established following infection, splenocytes from mice infected with strain KyA at 6, 16, and 26 weeks previously were restimulated for 5 days in vitro with mitomycin C-treated, EHV-1-infected L-M cells. The results demonstrated that EHV-1 infection generated a virus-specific memory CTL response that was detected at all time points (Fig. 4). Importantly, a vigorous CTL response was recalled as late as 26 weeks following a single i.n. inoculation of the candidate vaccine strain, KyA (Fig. 4). As with the primary CTL response, depletion of the CD8+ T-cell subpopulation eliminated the cytolytic activity, while depletion of CD4+ T cells was without effect (Fig. 4), indicating that the cytolytic activity recalled by antigenic stimulation in vitro was also mediated by class I MHC-restricted, CD8+ T cells. These results demonstrated that in addition to the generation of long-lived antibody (16) and CD4+ T-cell (56) responses, the attenuated KyA strain also generated a long-lived CD8+ T-cell response. Therefore, the candidate vaccine strain has the ability to elicit all components of the acquired immune response that are likely to be involved in controlling viral infections.
FIG. 4.
Memory CD8+ CTL isolated from the spleen following KyA infection. CBA mice were infected i.n. with 2 × 106 PFU of KyA and at 6 weeks (A), 16 weeks (B), and 26 weeks (C) p.i., the spleens were removed and a single-cell suspension was obtained as described in Materials and Methods; 107 splenocytes/well were restimulated for 5 days in vitro in the presence of 3 × 105 KyA-infected, mitomycin C-treated L-M fibroblasts as stimulators. Following in vitro restimulation, cytolytic activity was measured in a standard 4-h 51Cr release assay using mock-infected (▵) or KyA-infected (⧫) L-M fibroblasts as targets. Splenocytes isolated from CBA mice infected 4 weeks prior (D) were either nondepleted and tested against mock-infected (▵) or KyA-infected (⧫) targets or depleted of CD4+ (▪) or CD8+ (•) T cells by specific antibody and complement treatment immediately prior to cytolytic assay against KyA-infected targets. Lysis of mock-infected targets by depleted populations never exceeded that observed by nondepleted splenocytes (data not shown). In all panels, each effector-to-target (E:T) ratio was assayed in triplicate. The spontaneous release never exceeded 20%, and the variability between the values of specific lysis of triplicate cultures did not exceed 5%.
Adoptive transfer of protection by EHV-1-specific, restimulated memory lymphocytes expressing cytolytic function.
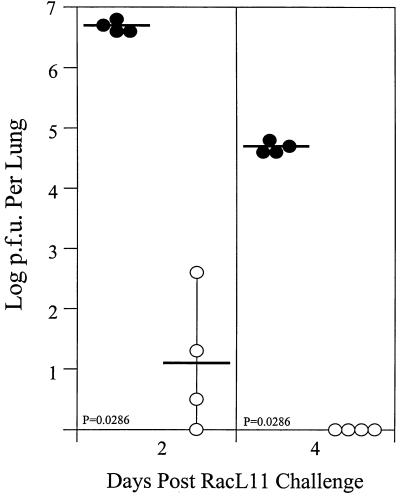
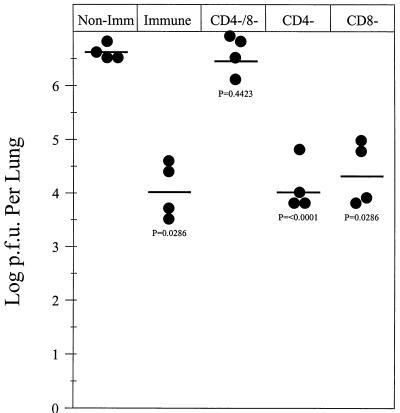
Infection of BALB/c mice with the nonpathogenic EHV-1 strain KyA results in long-term protection against challenge with pathogenic EHV-1 strain RacL11 (16). The present study confirmed that a similar protective response was elicited in the CBA mouse model. Mice infected with strain KyA were protected against infection with strain RacL11, compared to age-matched control mice, as demonstrated by reduced viral titers and faster clearance from the lungs (Fig. 5). Since viral clearance has been associated with the presence of EHV-1-specific antibody and T-cell responses, the contribution of the EHV-1-specific CD8+ T cells was determined. To achieve this, donor mice were immunized by the i.n. route with 2 × 106 PFU of KyA. Two weeks following immunization, the spleens were removed and the splenocytes were restimulated in vitro for 5 days. The donor cells, expressing high levels of EHV-1-specific CTL activity, were selectively depleted of CD4+ and/or CD8+ T cells immediately prior to transfer into irradiated recipient mice infected 1 h previously with 1.5 × 106 PFU of pathogenic RacL11. It was found that 2 × 107 immune donor cells, treated with complement alone, reduced markedly (>250-fold) the levels of RacL11 recovered from the lungs 4 days posttransfer compared to recipients of nonimmune donor cells (Fig. 6). In contrast, depletion of both CD4+ and CD8+ T cells abrogated completely the protective capabilities of the transferred cells (Fig. 6). Surprisingly, depletion of either CD4+ or CD8+ T cells alone had no effect on the levels of conferred protection (Fig. 6), suggesting that either each subpopulation alone was protective or mechanisms independent of CD4+ and CD8+ T cells were also present in the cultures and were responsible for viral clearance. These results indicated that while T cells activated in vitro are capable of reducing EHV-1 RacL11 in the lungs of challenged mice, either T-cell subpopulation, independently, was sufficient for the transfer of protection.
FIG. 5.
Inoculation i.n. with the attenuated KyA strain is protective against subsequent challenge with the pathogenic EHV-1 strain RacL11. Groups of CBA mice were either uninfected (•) or infected i.n. with 2 × 106 PFU of KyA (○). Two weeks following KyA infection, both groups of mice were infected i.n. with 1.5 × 106 PFU of RacL11. On days 2 and 4 postchallenge, the lungs were removed and homogenized, and the amount of infectious EHV-1 was determined by standard plaque titration as previously described. Each dot represents the log PFU per lung determined from a single mouse. The horizontal bars represent the mean log PFU per experimental group.
FIG. 6.
Adoptive transfer of immune lymphocytes into RacL11-infected, irradiated recipient CBA mice. Immune lymphocytes for transfer were isolated from the spleens of donor mice inoculated 2 weeks previously with 2 × 106 PFU of KyA. Donor lymphocytes were restimulated in vitro for 5 days in the presence of 3 × 105 KyA-infected, mitomycin C-treated L-M fibroblast stimulators. On the day of transfer, recipient mice were given 500 cGy of total-body γ irradiation and were infected i.n. with 1.5 × 106 PFU of RacL11. Immune donor lymphocytes were either left untreated, depleted in vitro of CD4+ or CD8+ T cells, or depleted of both CD4+ and CD8+ T cells immediately prior to transfer. Following in vitro depletion, the remaining cells were transferred in a volume of 0.25 ml to recipient mice via tail vein injection. Control mice received 2 × 107 nonimmune (Non-Imm) splenocytes. The amount of infectious EHV-1 was determined by standard plaque titration on RK monolayers as described in Materials and Methods. Each dot represents the log PFU per lung determined from a single mouse. The horizontal bars represent the mean log PFU per experimental group.
DISCUSSION
It has long been recognized that class I MHC-restricted, CD8+ T cells play an important role in controlling acute and chronic viral infections (46, 57, 58). Their ability to recognize viral peptides presented in association with class I MHC gives them a central role in surveillance for infected cells. In experimental murine models of infection with the prototypical alphaherpesvirus, herpes simplex virus type 1, a role for both class I MHC-restricted, CD8+ T cells and class II MHC-restricted, CD4+ T cells has been demonstrated (reviewed in reference 46). The relative importance of either subpopulation is highly dependent on the site of infection, but both subpopulations appear to be necessary for the optimal response to infection (46). Studies of a variety of viral infections of the respiratory tract have suggested a crucial role for CD8+ CTL in recovery from acute infection and protection against reinfection (1, 18, 29, 31, 44, 51). It is likely that this T-cell subpopulation plays a role in the immune response to EHV-1 infection in the natural equine host, because EHV-1-specific, class I MHC-restricted, CD8+ T cells have been isolated from the peripheral blood of infected animals (4) and infiltrate in high numbers into the infected lung (30). In the mouse model of EHV-1 infection, a role for CD8+ T cells in controlling infection in the lung was demonstrated by adoptive transfer studies (7). However, no functional studies of this T-cell subpopulation in the mouse have been reported.
In the present study, the primary and memory CD8+ CTL responses to EHV-1 respiratory tract infection in a susceptible mouse strain were evaluated. It was demonstrated that upper respiratory tract infection by the i.n. inoculation of CBA mice with the attenuated EHV-1 KyA strain resulted in the generation of cytolytic effectors in the draining MLN during the acute phase of infection. Kinetic analysis of the response revealed that CTL activity in the draining MLN peaked at 5 days p.i. and declined thereafter. This pattern is similar to the response to other acute viral infections (28, 32, 48) and corresponded well with the kinetics of viral clearance from the lungs. Moreover, EHV-1 infection in the lung resulted in long-lived memory EHV-1-specific CTL activity in the spleen, which could be detected in infected mice at 26 weeks postimmunization by stimulating resting memory CTL precursors with EHV-1-infected stimulator cells in culture. In conjunction with the findings of previous studies of the immune response to EHV-1 in mice (2, 7, 16, 37, 49, 52, 56), the demonstration of class I MHC-restricted, EHV-1-specific CTL indicated that all of the components of the acquired immune response identified during the natural infection in equines have their counterpart in the mouse model.
While EHV-1-specific CTL activity was readily detected in the MLN, relatively little CTL activity was detected in the CLN at any time during infection. This was surprising, since the CLN drains the nasal turbinate region, where EHV-1 is known to replicate (5). Therefore, the CTL response to EHV-1 was compartmentalized to the LN draining the lung, which is the major site of infection. Compartmentalization of CTL effector function associated with respiratory viral infections has been described elsewhere (25, 26). Although EHV-1 replicates to slightly lower levels in the nasal turbinates than in the lungs, the kinetics of EHV-1 growth and clearance from the tissue is almost identical to that observed in the lungs (5). Therefore, lack of CTL activity due to a variation in the duration and extent of antigenic stimulation is an unlikely explanation for the CTL compartmentalization. Furthermore, flow cytometric analyses of lymphoid cell populations isolated at 5 days p.i. revealed little difference in the percentage of CD8+ cells in the CLN and MLN. Routinely, the percentage of CD8+ T cells in both lymphoid sites ranged from 22 to 25%.
It is possible that EHV-1-specific CTL are present in the CLN but at frequencies too low to be detected in the assay used. An important component of this assay is the target cell. Subsequent studies have shown that the L-M cell line used in these assays has reduced expression of H-2Dk (unpublished observation). Because this class I MHC molecule may be an important restriction element for EHV-1-specific CTL in CBA mice, the lower levels of its expression would hinder the detection of that component of the response restricted to H-2Dk. However, our recent studies using an L2 mouse fibroblast that expresses both the H-2Dk and the H-2Kk molecules support the observation that significant differences in the CTL response occur in the MLN versus the CLN in EHV-1-infected CBA mice.
The demonstration of EHV-1-specific CTL activity in mice identifies a potential weapon in the arsenal of responses directed against infection but does not directly implicate this subpopulation in controlling infection. While recent studies of influenza virus infection of mice have clearly implicated cytolytic mechanisms as essential for eradication of virus from the lung (53), direct evidence of cytolytic activity as the principal mechanism against other respiratory pathogens is lacking. Adoptive transfer of restimulated spleen cell cultures expressing high levels of cytolytic function resulted in an approximate 250-fold reduction of infectious RacL11 in the lungs of irradiated, infected recipient mice. The depletion of both CD4+ and CD8+ T cells abrogated totally the ability of the transferred cells to mediate viral clearance. Interestingly, the selective depletion of either T-cell subpopulation alone had little effect. These results suggested that either CD4+ or CD8+ T cells alone, or other, as yet undefined components remaining in the activated spleen cell population, were able to mediate the functions responsible for clearance of RacL11 from the lung. These results also clearly indicated that EHV-1-specific CD4+ T cells were also restimulated in culture, presumably by the processing and presentation of virion components or infected-cell components by antigen-presenting cells present in the spleen. The ability of CD4+ T cells to mediate protection against viral pathogens in the absence of CD8+ T cells has been observed previously (26, 43, 48). Although the functions employed by CD4+ T cells in this model have not yet been evaluated, the production of cytokine such as gamma interferon (48) is a likely mechanism. It is clear, however, that CD8+ T cells mediate their function through direct interaction with infected cells in the lung (25), while CD4+ T cells may be effective by more indirect interactions (54). Future studies will address which effector functions, whether cytolytic or cytokine mediated, are important for optimal viral clearance.
Although the adoptive transfer experiments do not conclusively establish a role for the CD8+ T-cell subpopulation in controlling EHV-1 infection in the mouse, other evidence does support this contention. First, CD8+ T cells isolated from the spleens of EHV-1-infected BALB/c mice and adoptively transferred into recipient animals without further antigenic stimulation are more effective than CD4+ T cells in controlling respiratory infection (7). Second, analysis of the infiltrating cells isolated from the lungs of EHV-1-infected CBA mice showed a high proportion of CD8+ T cells in the infiltrate relative to other lymphocyte subpopulations. These cells, when isolated directly from the lung, express cytolytic function against EHV-1-infected target cells (47a), suggesting they express direct cytolytic function in situ.
The results described here and elsewhere (16, 56) indicate that the attenuated EHV-1 strain KyA is able to induce a long-term protective response against challenge with pathogenic EHV-1 RacL11 in the murine model. This protection was shown to be associated with elevated levels of EHV-1-specific antibody and CD4+ T-cell responses, and the present study has demonstrated for the first time that a component of the response is cytolytic activity mediated by EHV-1-specific CD8+ T lymphocytes. The ability to generate and measure cytolytic activity and to transfer immune protection to naive recipients should provide a valuable model to determine the precise functions associated with protection and recovery from respiratory EHV-1 infection. In addition, this model will be invaluable in determining if specific EHV-1 proteins are capable of eliciting a protective response without the inflammatory immunopathology commonly associated with viral infection of the lung. Although the identification of viral components that generate EHV-1-specific CTL in CBA mice will not be directly applicable to the equine infection, basic information of this type may be important for the design and engineering of a recombinant KyA vaccine. In this regard, initial experiments indicate that the sole immediate-early protein and the novel IR6 protein that is made in large quantities throughout infection are EHV-1 proteins that serve as CTL targets and thus should be considered in the design of candidate vaccine viruses.
ACKNOWLEDGMENTS
We thank Suzanne Zavecz for excellent technical assistance.
This study was supported in part by research grants AI 22001 (D.J.O.) and NS 32464 (S.R.J.) from the National Institutes of Health and by funds made available through Boehringer Ingleheim Vetmedica GmbH, Ingleheim, Germany.
REFERENCES
- 1.Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Alber D G, Greensill J, Killington R A, Stokes A. Role of T-cells, virus neutralising antibodies and complement-mediated antibody lysis in the immune response against equine herpesvirus type-1 (EHV-1) infection of C3H (H-2k) and BALB/c (H-2d) mice. Res Vet Sci. 1995;59:205–213. doi: 10.1016/0034-5288(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 3.Allen G P, Bryans J T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- 4.Allen G P, Yeargan M, Costa L R R, Cross R. Major histocompatibility complex class I-restricted cytotoxic T-lymphocyte response in horses infected with equine herpesvirus 1. J Virol. 1995;69:606–612. doi: 10.1128/jvi.69.1.606-612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awan A R, Chong Y-C, Field H J. The pathogenesis of equine herpesvirus type 1 in the mouse: a new model for studying host responses to the infection. J Gen Virol. 1990;71:1131–1140. doi: 10.1099/0022-1317-71-5-1131. [DOI] [PubMed] [Google Scholar]
- 6.Awan A R, Gibson J S, Field H J. A murine model for studying EHV-1 induced abortion. Res Vet Sci. 1991;51:94–99. doi: 10.1016/0034-5288(91)90038-p. [DOI] [PubMed] [Google Scholar]
- 7.Azmi M, Field H J. Cell-mediated antiviral response to equine herpesvirus type 1 demonstrated in a murine infection model by means of adoptive transfer of immune cells. J Gen Virol. 1993;74:275–280. doi: 10.1099/0022-1317-74-2-275. [DOI] [PubMed] [Google Scholar]
- 8.Bagust T J. The equine herpesviruses. Vet Bull. 1971;41:79–91. [Google Scholar]
- 9.Bell C W, Boyle D B, Whalley J M. Transcript analysis of the equine herpesvirus glycoprotein B gene homologue and its expression by a recombinant vaccinia virus. J Gen Virol. 1990;71:1119–1129. doi: 10.1099/0022-1317-71-5-1119. [DOI] [PubMed] [Google Scholar]
- 10.Bryans J T, Allen G P. Equine viral rhinopneumonitis. Rev Sci Tech Off Int Epizoot. 1986;5:837–847. doi: 10.20506/rst.5.4.273. [DOI] [PubMed] [Google Scholar]
- 11.Burki F, Rofsmanith W, Nowotny N, Pallan C, Mostl, K. K, Lussy H. Viremia and abortions are not prevented by two commercial equine herpesvirus-1 vaccines after experimental challenge of horses. Vet Q. 1990;12:80–86. doi: 10.1080/01652176.1990.9694249. [DOI] [PubMed] [Google Scholar]
- 12.Burrows R, Goodridge D, Denyer M S. Trials of an inactivated equid herpesvirus-1 vaccine: challenge with a subtype-1 virus. Vet Res. 1984;114:369–374. doi: 10.1136/vr.114.15.369. [DOI] [PubMed] [Google Scholar]
- 13.Carrol C L, Westbury H A. Isolation of equine herpesvirus type 1 from the brain of a horse affected with paresis. Aust Vet J. 1985;62:345–346. doi: 10.1111/j.1751-0813.1985.tb07660.x. [DOI] [PubMed] [Google Scholar]
- 14.Carter V C, Schaffer P A, Tevethia S S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981;126:1655–1660. [PubMed] [Google Scholar]
- 15.Ceredig R, Lowenthal J W, Nabholz M, MacDonald H R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 16.Colle C F, III, Tarbet E B, Grafton W D, Jennings S R, O’Callaghan D J. Equine herpesvirus-1 strain KyA, a candidate vaccine strain, reduces viral titers in mice challenged with a pathogenic strain RacL. Virus Res. 1996;43:111–124. doi: 10.1016/0168-1702(96)01324-x. [DOI] [PubMed] [Google Scholar]
- 17.Crabb B S, Studdert M J. Equine herpesvirus 4 (equine rhinopneumonitis virus) and 1 (equine abortion virus) Adv Virus Res. 1995;45:153–190. doi: 10.1016/s0065-3527(08)60060-3. [DOI] [PubMed] [Google Scholar]
- 18.de Waal L P, Kast W M, Melvoid R W, Melief C J M. Regulation of the cytotoxic T lymphocyte response against Sendai virus analyzed with H-2 mutants. J Immunol. 1983;130:1090–1096. [PubMed] [Google Scholar]
- 19.Ellis J A, Bogdan J R, Kanara E W, Morley P S, Haines D M. Cellular and antibody responses to equine herpesviruses 1 and 4 following vaccination of horses with modified-live and inactivated viruses. J Am Vet Med Assoc. 1995;206:823–832. [PubMed] [Google Scholar]
- 20.Ellis J A, Steeves E, Wright A K, Bogdan J R, Davis W C, Kanara E W, Haines D M. Cell-mediated cytolysis of equine herpesvirus-infected cells by leukocytes from young vaccinated horses. Vet Immunol Immunopathol. 1997;57:201–214. doi: 10.1016/s0165-2427(96)05749-2. [DOI] [PubMed] [Google Scholar]
- 21.Flyer D C, Anderson R W, Tevethia S S. Lyt phenotype of H-2b CTL effectors and precursors specific for the SV40 transplantation rejection antigen. J Immunol. 1982;129:2368–2371. [PubMed] [Google Scholar]
- 22.Grandell R A, Mock R E, Lock T F. Vaccination of pregnant ponies against equine rhinopneumonitis. Am J Vet Res. 1980;41:994–996. [PubMed] [Google Scholar]
- 23.Guo P I, Goebel S, Perkus M E, Taylor J, Norton E, Allen G, Languet B, Desmettre P, Paoletti E. Coexpression by vaccinia virus recombinants of equine herpesvirus 1 glycoproteins gp13 and gp14 results in potentiated immunity. J Virol. 1990;64:2399–2406. doi: 10.1128/jvi.64.5.2399-2406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannant D, Jessett D M, O’Neill T, Dolby C A, Cook R F, Mumford J A. Responses of ponies to equid herpesvirus-1 ISCOM vaccination and challenge with virus of the homologous strain. Res Vet Sci. 1993;54:299–305. doi: 10.1016/0034-5288(93)90126-z. [DOI] [PubMed] [Google Scholar]
- 25.Hou S, Doherty P C. Clearance of Sendai virus by CD8+ T cells requires direct targeting to virus-infected epithelium. Eur J Immunol. 1995;25:111–116. doi: 10.1002/eji.1830250120. [DOI] [PubMed] [Google Scholar]
- 26.Hou S, Doherty P C, Zijlstra M, Jaenisch R, Katz J M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 27.Jackson T A, Kendrick J W. Paralysis of horses associated with equine herpesvirus 1 infection. J Am Vet Med Assoc. 1971;158:1351–1357. [PubMed] [Google Scholar]
- 28.Jennings S R, Bonneau R H, Smith P M, Wolcott R M, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 29.Kast W M, Bronkhorst A M, de Waal L P, Melief C J M. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated, a model for MHC-disease associations. J Exp Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kydd J H, Hannant D, Mumford J A. Residence and recruitment of leucocytes to the equine lung after EHV-1 infection. Vet Immunol Immunopathol. 1996;52:15–26. doi: 10.1016/0165-2427(95)05533-9. [DOI] [PubMed] [Google Scholar]
- 31.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific T lymphocytes is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch F, Doherty P C, Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989;142:3592–3598. [PubMed] [Google Scholar]
- 33.Matsumura T, Kondo T, Sugita S, Damiani A M, O’Callaghan D J, Imagawa H. An equine herpesvirus type 1 recombinant with a deletion in the gE and gI genes is avirulent in young horses. Virology. 1998;242:68–79. doi: 10.1006/viro.1997.8984. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura T, O’Callaghan D J, Kondo T, Kamada M. Lack of virulence of the murine fibroblast adapted strain, Kentucky A (KyA), of equine herpesvirus type 1 (EHV-1) in young horses. Vet Microbiol. 1996;48:353–365. doi: 10.1016/0378-1135(09)59999-3. [DOI] [PubMed] [Google Scholar]
- 35.Meyer H, Thein P, Hubert P. Characterization of two equine herpesvirus (EHV) isolates associated with neurological disorders in horses. Zentbl Veterinaermed Reihe B. 1987;34:545–548. doi: 10.1111/j.1439-0450.1987.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 36.Mumford J A, Hannant D A, Jessett D M, O’Neill T, Smith K C, Ostlund E N. Abortigenic and neurological disease caused by infection with equid herpesvirus-1. In: Nakajima H, Plowright W, editors. Proceedings of the 7th International Conference of Equine Infectious Diseases. Newmarket, United Kingdom: R&W Publisher; 1995. pp. 261–275. [Google Scholar]
- 37.Neubauer A, Beer M, Brandmuller C, Kaaden O-R, Osterrieder N. Equine herpesvirus 1 mutants devoid of glycoprotein B or M are apathogenic for mice but induce protection against challenge infection. Virology. 1997;239:36–45. doi: 10.1006/viro.1997.8857. [DOI] [PubMed] [Google Scholar]
- 38.O’Callaghan D J, Osterrieder N. Equine herpesviruses. In: Webster R G, Granoff A, editors. Encyclopedia of virology. 2nd ed., in press. San Diego, Calif: Academic Press, Harcourt Brace & Company, Publishers; 1998. [Google Scholar]
- 39.Osterrieder N, Wagner R, Brandmuller C, Schmidt P, Wolf, H. H, Kaaden O-R. Protection against EHV-1 challenge infection in the murine model after vaccination with various formulations of recombinant glycoprotein gp14 (gB) Virology. 1995;208:500–510. doi: 10.1006/viro.1995.1181. [DOI] [PubMed] [Google Scholar]
- 40.Osterrieder N, Wagner R, Pfeffer M, Kaaden O-R. Expression of equine herpesvirus type 1 glycoprotein gp14 in Escherichia coli and in insect cells: a comparative study on protein processing and humoral immune responses. J Gen Virol. 1994;75:2041–2046. doi: 10.1099/0022-1317-75-8-2041. [DOI] [PubMed] [Google Scholar]
- 41.Perdue M L, Kemp M C, Randall C C, O’Callaghan D J. Studies of the molecular anatomy of the L-M strain of equine herpesvirus type 1: protein of the nucleocapsid and intact virion. Virology. 1974;59:201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- 42.Pfizenmaier K, Jung H, Starzinski-Powitz A, Rollinghof M, Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977;119:939–944. [PubMed] [Google Scholar]
- 43.Polic B, Jonjic S, Pavic I, Crnkovic I, Zorica I, Hengel H, Lucin P, Koszinowski U H. Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J Gen Virol. 1996;77:217–225. doi: 10.1099/0022-1317-77-2-217. [DOI] [PubMed] [Google Scholar]
- 44.Reddehase M J, Mutter W, Munch K, Buhring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarmiento M, Glasebrook A L, Fitch F W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 46.Schmid D S, Rouse B T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 47.Sinickas V G, Ashman R B, Blanden R V. The cytotoxic response to murine cytomegalovirus. II. In vitro requirements for generation of cytotoxic T cells. J Gen Virol. 1985;66:757–765. doi: 10.1099/0022-1317-66-4-757. [DOI] [PubMed] [Google Scholar]
- 47a.Smith, P. M. Unpublished data.
- 48.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon Interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 49.Stokes A, Alber D G, Cameron R S, Marshall R N, Allen G P, Killington R A. The production of a truncated form of baculovirus expressed EHV-1 glycoprotein C and its role in protection of C3H (H-2Kk) mice against virus challenge. Virus Res. 1996;44:97–109. doi: 10.1016/0168-1702(96)01339-1. [DOI] [PubMed] [Google Scholar]
- 50.Stokes A, Cameron R S, Marshall R N, Killington R A. High level expression of equine herpesvirus 1 glycoproteins D and H and their role in protection against virus challenge in the C3H (H-2Kk) murine model. Virus Res. 1997;50:159–173. doi: 10.1016/s0168-1702(97)00067-1. [DOI] [PubMed] [Google Scholar]
- 51.Taylor P M, Askonas B A. Influenza nucleoprotein-specific cytotoxic T cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 52.Tewari D, Whalley J M, Love D N, Field H J. Characterization of immune responses to baculovirus-expressed equine herpesvirus type 1 glycoproteins D and H in a murine model. J Gen Virol. 1994;75:1735–1741. doi: 10.1099/0022-1317-75-7-1735. [DOI] [PubMed] [Google Scholar]
- 53.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 54.Topham D J, Tripp R A, Sarawar S R, Sangster M Y, Doherty P C. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II −/− respiratory epithelium. J Virol. 1996;70:1288–1291. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilde D B, Marrack P, Kappler J, Dialynas D P, Fitch F W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983;131:2178–2183. [PubMed] [Google Scholar]
- 56.Zhang, Y., P. M. Smith, E. B. Tarbet, N. Osterrieder, S. R. Jennings, and D. J. O’Callaghan. Protective immunity against equine herpesvirus type 1 (EHV-1) infection in mice induced by recombinant EHV-1 gD. Virus Res., in press. [DOI] [PubMed]
- 57.Zinkernagel R M. Immunity to viruses. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1993. pp. 1211–1250. [Google Scholar]
- 58.Zinkernagel R M, Doherty P C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]