Abstract
Protein tyrosine kinase (PTK) phosphorylation is involved in cellular proliferation and differentiation processes that are key factors for human immunodeficiency virus type 1 (HIV-1) regulation in infected monocytic cells. Short-term exposure of the chronically infected promyelocytic OM10 cell line with the PTK inhibitor genistein induced a dose-dependent increase in p24 antigen production in culture supernatants. This induction persisted in the presence of the reverse transcriptase inhibitor, zidovudine, and was associated with an increased transcription of HIV-1 multiply spliced and unspliced RNAs, suggesting a transcriptional mechanism targeting the integrated provirus. Genistein induced cell differentiation, apoptosis, and a G2 arrest in the OM10 cells. Cell differentiation and apoptosis were not directly involved in the observed increase in HIV-1 replication that was closely linked to genistein-induced G2 arrest. Alleviation of the G2 arrest by pentoxyfylline resulted in a concomitant reduction of HIV-1 to baseline replication. Additionally, by flow cytometry, a significant increase in the number of p24 antigen-expressing cells was observed in cells arrested in G2 compared to those located in G1 or S. Tyrosine kinase inhibition was found not to be essential for enhanced viral replication, which seemed to be related to two other properties of genistein, inhibition of topoisomerase II activity and inhibition of phosphotidylinositol turnover. These findings are consistent with the recent observation that HIV-1 Vpr induces viral replication through preventing proliferation of cells by arresting them in G2 of the cell cycle and strongly suggest that manipulation of the cell cycle plays an important role in HIV-1 pathogenesis.
Cells of monocyte/macrophage lineage represent a major reservoir of human immunodeficiency virus type 1 (HIV-1) in vivo. Despite the usual absence of virus-induced cytopathic effect, these cells produce high levels of virus, even at the later stages of HIV-1 infection when CD+ T cells are declining (30). Thus, the regulation of HIV-1 replication in monocytes/macrophages plays an important role in the pathogenesis of AIDS and is particularly critical for HIV-1 persistence and dissemination in infected individuals.
Among the many factors able to influence the levels of HIV-1 replication in macrophages, proinflammatory cytokine production (for a review, see reference 33), as well as the state of cell activation and differentiation, seems to play an important role. For this latter aspect, most studies have found that cell maturation enhances HIV-1 replication, via either an increased susceptibility of the cell to HIV-1 infection (22, 36, 40) or an increase in viral transcription of a quiescent provirus (7, 26). However, some reports have recently demonstrated a dissociation between cell differentiation and HIV-1 expression (15). The modulation of HIV-1 transcription after integration into host cell DNA is determined to a great extent by the activity of the viral protein Tat on its RNA-responsive element located in the long terminal repeat (LTR) and by transcription factors acting on binding sites also located in the LTR (for a review, see reference 14). The second messenger systems, acting upstream of the transcriptional control of the integrated provirus, are not fully characterized and probably involve a complex network of protein phosphorylation and dephosphorylation. Protein tyrosine kinase (PTK) phosphorylation plays a crucial role in cell proliferation and differentiation and therefore may also regulate some aspect of HIV-1 latency-reactivation in infected cells. HIV-1 is known to increase the level of tyrosine phosphorylation of several proteins within the infected cells (4, 9, 31), involving the enhancement of the Src family PTK activity. On the other hand, PTK is required for the transduction of signals initiated by the action of lipopolysaccharide on monocytes/macrophages (3, 42, 46, 47), leading to the increase of several cytokines known to induce HIV-1 (3, 42, 46). However, the consequences of tyrosine kinase activation or inhibition on HIV-1 expression itself have not been investigated.
While studying the effects of the tyrosine kinase inhibitor genistein on HIV-1 expression in chronically infected promyelocytic cells, we demonstrated a strong and dose-dependent increase of HIV-1 expression. In the research described herein, we have characterized this upregulation of HIV-1 replication in the promyelocytic cell line OM10. The enhancement of HIV-1 appears to be transcriptional, since both p24 antigen production and transcription of viral RNAs are induced and persist in the presence of zidovudine. We also present evidence that arrest of cells in G2 is critical for the increase in HIV-1 expression. Finally, the ability of genistein to inhibit topoisomerase II activity and phosphotidylinositol turnover seems to be important in the upregulation of HIV-1 rather than its inhibition of PTK.
MATERIALS AND METHODS
Cells and chemicals. (i) Cell lines.
Two cell lines were used for these experiments: the OM10 cell line, which is a chronically infected promyelocytic clone (LAI strain) harboring a single proviral DNA integrated in the chromosome, a low basal HIV-1 expression, and a persistent surface expression of CD4 until HIV-1 activation; and the U1 cell line, which is a more differentiated promonocytic clone, containing two integrated copies of provirus and also expressing low levels of virus until HIV-1 activation. Both cell lines were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program and maintained in RPMI 1640 complemented with 10% fetal calf serum and antibiotics. Cells were resuspended at 5 × 105 cells/ml prior to stimulation.
(ii) Reagents.
Genistein, herbimycin A, psi-tectorigenin, and etoposide were purchased from Calbiochem (La Jolla, Calif.) and diluted in dimethyl sulfoxide (DMSO) prior to use. The final concentration of DMSO in the cultures never exceeded 0.2% (vol/vol). Pentoxyfylline (Sigma, Carpinteria, Calif.) was diluted in water as a 500× stock solution (500 mM). A stock solution of dihydroxyvitamin D3 (Sigma) was dissolved in ethanol and preserved at −80°C until final dilution (10−7 M) in RPMI. Nonspecific esterase (NSE) activity was determined on a cytospin preparation of the cells, by a staining procedure, according to the manufacturer’s protocol (α-naphthyl acetate esterase staining kit; Sigma).
(iii) Cytokine quantification.
Tumor necrosis factor alpha (TNF-α) present in the supernatants of the cells was quantified by enzyme-linked immunosorbent assay (ELISA) (Biosource, Camarillo, Calif.) according to the manufacturer’s protocol.
HIV-1 expression. (i) p24 antigen production.
The production of HIV-1 p24 antigen in cell culture supernatants was determined by an ELISA (Abbott, Chicago, Ill.) according to the manufacturer’s protocol. All determinations were performed in duplicate.
(ii) RT-PCR.
A semiquantitative reverse transcription-PCR (RT-PCR) was used to evaluate the transcription of both unspliced and multiply spliced viral RNAs in OM10 cells. Briefly, total RNA was extracted from 2 × 106 cells with the RNAzolB procedure (Tel-Test, Inc., Friendswood, Tex.), resuspended in diethylpyrocarbonate-treated water, and quantified with a spectrophotometer. The same amount of RNA (1 μg) was reverse transcribed for 1 h at 37°C, with Moloney murine leukemia virus reverse transcriptase (Gibco BRL, Grand Island, N.Y.) and random hexanucleotides. Serial dilutions of cDNA were then amplified by PCR, in the presence of 32P-radiolabeled dCTP and primers specific for either HIV-1 unspliced RNAs (SK38 and SK39) (17) or multispliced RNAs (MS1 and MS2) (38). After 30 cycles of amplification, aliquots of amplified products were separated in a 6% polyacrylamide gel and subsequently exposed to autoradiography.
(iii) Intracytoplasmic detection of HIV-1 antigen.
Flow cytometry was used to detect the expression of HIV-1 p24 antigen in the cytoplasm of treated cells or controls. Cells were washed in phosphate-buffered saline (PBS) and fixed and permeabilized in 250 μl of PermeaFix (Gibco BRL) for 40 min. Cells were washed and labeled for 30 min at 4°C in 100 μl of PBS containing 0.2 μl of monoclonal antibody (MAb) against HIV-1 p24 antigen (clone 314219 [American Type Culture Collection]) or its appropriate control. After a second wash, a fluorescein isothiocyanate (FITC)-conjugated secondary anti-mouse antibody (Dako, Carpinteria, Calif.) was added for 30 min, at 4°C. After the last wash, cells were resuspended in 0.5% paraformaldehyde in PBS and analyzed on a Coulter Elite flow cytometer (Coulter, Miami, Fla.), in parallel with the DNA content of the nuclei.
Flow cytometry analysis of cell phenotype. (i) Cell cycle.
The quantitative measure of cell cycle was performed by flow cytometry analysis of nuclear DNA contents, after propidium iodide staining. Briefly, cells were washed in PBS and fixed in 50% ethanol. After washing, the nuclear DNA was treated with propidium iodide (50 μg/ml) and RNase (10 mg/ml) for 20 min at room temperature. Cell cycle determination was performed with a Coulter Elite flow cytometer and analyzed with the help of Multicycleav software (Phoenix Flow, San Diego, Calif.). At least 104 cells were assayed for each determination.
(ii) Detection of apoptosis.
Annexin V is a Ca2+-dependent, phospholipid-binding protein with high affinity for phosphotidylserine. Normally, phosphotidylserine is found only on the inner side of cell membranes, but during early phases of apoptosis, cells lose membrane phospholipid asymmetry and expose phosphotidylserine to the outer membrane. This process can be monitored by using annexin V-FITC. 7-AAD is a nucleic acid stain that labels dead cells with a compromised membrane. Used together, these two markers allow for the discrimination among vital cells (7-AAD negative–annexin negative), live cells early in apoptosis (7-AAD negative–annexin positive), dead cells late in apoptosis (7-AAD positive–annexin positive), and necrotic cells (7-AAD positive–annexin negative). Cell samples (5 × 105) were washed with PBS and resuspended in 0.5 ml of 1× binding buffer (BB) containing 10 μl of FITC-conjugated annexin V (apoptosis detection kit; R&D Systems, Minneapolis, Minn.) and 7-AAD (20 μg/ml) (Molecular Probes, Eugene, Oreg.). Cells were incubated for 10 min at room temperature, washed once with 1× BB, and fixed in 0.5 ml of 1× BB–1% alcohol-free paraformaldehyde. These cells were analyzed on a Coulter Elite flow cytometer. Apoptosis was also evaluated by the proportion of cells harboring less than 1 N DNA in the nucleus, after staining with propidium iodide.
(iii) Surface expression of differentiation markers.
Cell surface expression of CD11b and CD14 was evaluated by cytofluorometric analysis, with MAbs directly tagged with R-phycoerythrin and FITC, respectively. Cells were washed in PBS, incubated at 4°C for 30 min with 5 μg of the specific MAbs per ml or the appropriate controls (Dako), washed again, and fixed in 1% paraformaldehyde prior to their analysis on a Coulter Elite flow cytometer.
Phosphoprotein analysis. (i) Immunoblotting.
Protein (30 μg) obtained from a total cell lysate was resolved on 10% polyacrylamide gels and electrotransferred to a nylon membrane (Protan 0.1 μm pore size; Schleicher and Schuell). The membrane was blocked for 1 h at 22°C, probed overnight at 4°C with 0.5 μg of 4G-10 murine antiphosphotyrosine MAb (Upstate Biotechnology Inc., Lake Placid, N.Y.) per ml, washed, and visualized with an ECL chemiluminescence system (Amersham, Arlington Heights, Ill.).
(ii) Immunofluorescence.
The total tyrosine-phosphorylated protein content was also evaluated by flow cytometry as described previously with slight modifications (13). Briefly, 106 cells were washed in PBS, fixed and permeabilized with PermeaFix (Ortho Diagnostics), washed again, and labeled for 30 min at 4°C with 1 μg of FITC-conjugated antiphosphotyrosine antibody (Sigma) or its appropriate control. After a second wash, cells were resuspended in 0.5% paraformaldehyde in PBS and analyzed by flow cytometry.
RESULTS
Effect of genistein on HIV-1 expression in OM10 and U1 cells.
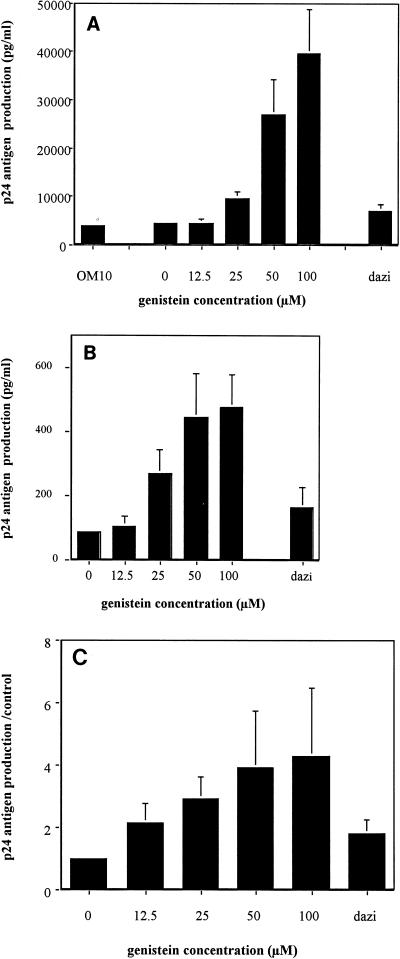
In initial experiments, OM10 cells exposed to genistein for 4 h demonstrated an increase in HIV-1 replication as demonstrated by an increase in p24 antigen production at day 3 postexposure. The increase in HIV-1 expression was found to follow a dose response, with increasing doses of genistein from 25 to 100 μM resulting in an increase in HIV-1 p24 antigen detected in culture supernatant (Fig. 1A). To determine if this observation was restricted to the OM10 cell line, we examined the impact of genistein on HIV-1 replication on a more differentiated promonocytic cell line, U1. As previously observed with OM10 cells, genistein enhanced HIV-1 production in U1 cells (Fig. 1B). In contrast, the inactive analog of genistein, dazidzein, did not increase HIV-1 p24 antigen production, indicating that the observed effect is related to a specific biochemical activity of genistein.
FIG. 1.
Genistein increases HIV-1 expression in OM10 and U1 cells. (A and B) OM10 cells (A) and U1 cells (B) were treated with increasing doses of genistein for 4 h at 37°C, washed in PBS, and resuspended (5 × 105 cells/ml) in RPMI plus 10% fetal calf serum. Supernatants were collected at day 3. HIV-1 p24 antigen production was determined in duplicate by an ELISA. Data are the means ± standard errors of the means of at least five separate experiments. (Dazi represents cells treated with dazidzein [100 μM]). (C) OM10 cells were cultured in the presence of zidovudine (10 μg/ml) after the treatment with genistein. Data are the means ± standard errors of the means of four experiments expressed as ratios of p24 antigen production in genistein-treated cells to that in controls.
Because OM10 cells remain CD4 positive despite being chronically infected with HIV-1 (5), we next determined if the genistein-induced increase in HIV-1 replication was due predominantly to the induction of proviral DNA already integrated in the cell line or to reinfection of cells. OM10 cells were treated with zidovudine in addition to genistein, and culture supernatants were tested for the quantity of HIV-1 p24 antigen produced. The continued finding of increased production of p24 antigen in the zidovudine-treated cultures (Fig. 1C) indicates that genistein effectively increases the ability of the already infected cell to support replication of its own integrated provirus and that reinfection of cells is unlikely to play an important role in the enhanced HIV-1 replication.
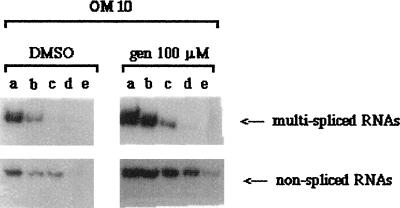
A semiquantitative RT-PCR analysis of HIV-1 RNA showed increased levels of the multiply spliced RNA, as well as of unspliced species in genistein-treated cells (Fig. 2), suggesting that the increase of HIV-1 replication in genistein-induced OM10 cells is predominantly transcriptional, which is consistent with the transcriptional control in HIV-1 expression in this cell line (6).
FIG. 2.
Autoradiograph of semiquantitative RT-PCR analysis of multiply spliced and unspliced HIV-1 RNAs in OM10 cells treated with DMSO (control) or 100 μM genistein (gen) (induced). Lanes a, b, c, d, and e represent 10-fold serial dilutions of 1 μg of total RNA submitted to RT-PCR (as described in Materials and Methods).
Effect of genistein on growth of OM10 cells.
Several studies have demonstrated that genistein, in addition to inhibiting tyrosine kinase, also may reduce cell proliferation, modify the cell cycle, and induce cell differentiation (11, 19, 25, 41, 45). Because each of these cellular modifications may impact on HIV-1 expression in chronically infected cells, the effects of genistein on OM10 cells were examined. For cell growth experiments, OM10 cells were treated for 4 h with genistein at increasing concentrations, and the proportion of viable cells was determined at days 1, 3, and 5. A dose-dependent inhibition of cell growth was observed for genistein concentrations between 12.5 and 100 μM, with a 50% inhibitory concentration at 38 μM at day 3. At 50 μM, most cell proliferation was inhibited; however, more than 80% of cells were still alive. Cell toxicity appeared at 100 μM, with 35% of the cells being dead by 3 days (data not shown).
Effect of genistein on cell cycle progression.
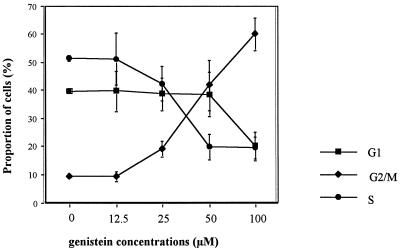
To evaluate the effect of genistein on the cell cycle, the DNA content of OM10 cells was analyzed by flow cytometry at 24 h after exposure to genistein. As shown in Fig. 3, genistein induced a strong G2/M arrest of the cells at the concentrations shown to induce HIV-1. The increase in the proportion of cells in G2/M was first observed at a genistein concentration of 25 μM but was more pronounced at 50 and 100 μM. Time course analysis of the DNA content shows that this arrest appears at 8 h, peaks between 16 and 24 h, and persists for at least 48 h postexposure (data not shown). The mitotic index (mean ± standard deviation, evaluated by counting the proportion of cells in mitosis) was (2.3 ± 0.9)% (n = 3; counted on 103 cells) in genistein (100 μM)-treated cells compared to (1.8 ± 0.5)% (n = 3; counted on 103 cells) in the control cells, indicating that cells are arrested in G2 phase rather than in M.
FIG. 3.
Genistein induces a G2/M arrest in OM10 cells. At day 1 after a 4-h induction with different concentrations of genistein, the DNA content of nuclei of OM10 cells was determined by flow cytometry. The percentages of cells in G1, S, and G2/M are shown as means of five experiments (± standard errors of the means).
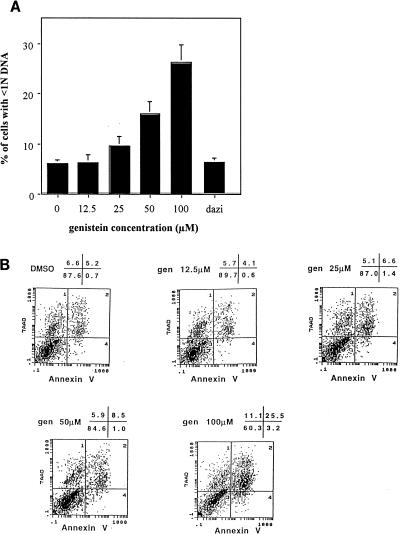
A significant increase in cells with less than 1N DNA present within the nucleus was detected in the cells treated with the highest concentrations of genistein (Fig. 4A), suggesting that genistein is able to induce apoptosis in OM10 cells. This induction of apoptosis was confirmed by flow cytometry analysis of cells stained with annexin V and 7-AAD (Fig. 4B). In the cells treated with 100 μM genistein, 26% were positive for both annexin V and 7-AAD.
FIG. 4.
Genistein induces apoptosis in OM10 cells. (A) After OM10 cells were washed and fixed in 50% ethanol, the nuclear DNA was treated with propidium iodide and RNase for 20 min at room temperature. Cell cycle determination was performed by flow cytometry. The figure shows the percentages of cells with a DNA content in the nucleus of less than 1N, 24 h after treatment of OM10 cells with increasing concentrations of genistein. The numbers indicated are means of five experiments (± standard errors of the means). (Dazi represents cells treated with dazidzein [100 μM]). (B) A total of 10 × 105 cells were washed in PBS and, after resuspension in BB containing 10 μl of FITC-conjugated annexin V and 7-AAD (20 μg/ml), were incubated, washed, and analyzed by flow cytometry. The figure shows the dual staining of control and treated cells with 7-AAD and annexin V. The percentages of cells present in each quadrant are shown in the upper right corner. These data are from one experiment representative of two separate experiments. gen, genistein. The x and y axes denote log fluorescence of FITC and 7-AAD, respectively.
Effect of genistein on cell differentiation.
In order to determine the differentiation state of genistein-treated cells, OM10 cells were evaluated at 5 days post-genistein exposure by morphologic analysis, by quantitation of surface expression of CD14 and CD11b, and for induction of an NSE activity. Genistein was found to induce profound changes in the morphology of OM10 cells indicative of cell differentiation, including enlargement of cells, induction of polymorphic and irregular shape, indentation of the nuclei, less-apparent nucleoli, and increased coarseness of chromatin (data not shown). Additionally, at the highest concentrations (50 and 100 μM), NSE activity was induced within the cells (Table 1), confirming the differentiation effect of genistein. Flow cytometry analysis of surface expression of CD14 and CD11b also indicated a significant increase after genistein induction, at the highest concentration tested (Table 1).
TABLE 1.
Genistein induces differentiation of OM10 cellsa
| Marker | Value for cell groupb
|
||||||
|---|---|---|---|---|---|---|---|
| OM10 | DMSO | Genistein (12.5 μM) | Genistein (25 μM) | Genistein (50 μM) | Genistein (100 μM) | Dihydroxyvitamin D3 | |
| CD14 | 5.1 | 4.8 | 4.8 | 7.7 | 14 | 37 | 32.7 |
| CD11b | 0.6 | 0.4 | 0.3 | 0.8 | 10 | 30.5 | 87.5 |
| NSE | 2 | 3 | ND | ND | 22 | 38.3 | 21.7 |
| HIV increasec | 1 | 1 | 2.8 | 6.3 | 6.4 | 1.9 | |
OM10 cells were treated for 4 h with increasing doses of genistein, cultured for 5 days, and analyzed for the presence of differentiation markers as described in Materials and Methods. Controls included untreated cells, cells treated with DMSO only, and cells induced to differentiation with vitamin D3 (10−7 M). The HIV-1 increase was evaluated by the HIV-1 p24 antigen production (ratio of induced cells to untreated controls). Results are expressed as means of three experiments. ND, not done.
Percentage of positive cells.
Ratio of p24 antigen production in treated versus OM10 control cells.
Dissociation between cell differentiation induced by genistein and HIV-1 replication.
In cells of the monocyte/macrophage lineage, a differentiation process is usually associated with an increased susceptibility to HIV-1 infection (22, 36, 40) or an increase in viral transcription of a quiescent provirus (7, 26). However, differentiation has not always been shown to enhance HIV-1 replication (14). We found a similar dissociation in the OM10 cells. With dihydroxyvitamin D3, which is a strong inducer of OM10 cell differentiation, there was no increase in HIV-1 replication (Table 1). Other indirect evidence for this dissociation is the ability of genistein to increase HIV-1 replication in the more differentiated promonocytic cell line, U1 (Fig. 1C), and the finding that the concentrations of genistein able to induce the differentiation phenotype (>50 μM) are greater than those required to significantly induce HIV-1 (25 μM).
Genistein-induced G2 arrest is associated with enhanced HIV-1 replication.
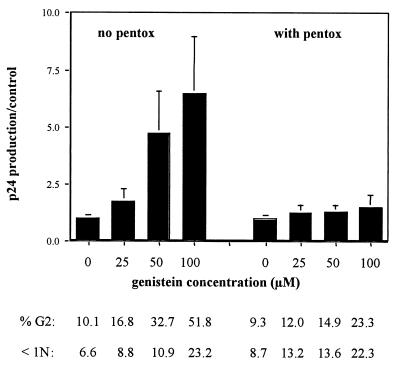
Two approaches were used to further investigate the precise role of the genistein-induced cell cycle arrest in enhancing HIV-1 replication in OM10 cells. First, HIV-1 replication was examined following treatment with methylxanthine pentoxyfylline, which is known to reverse G2 arrest induced by several DNA-damaging agents. In three separate experiments, pentoxyfylline was able to alleviate the genistein-induced G2 arrest as well as the genistein-induced enhanced HIV-1 replication (Fig. 5). Thus, the increased production of HIV-1 appears to be related to the cell cycle arrest in G2. In contrast, the proportion of cells with less than 1N DNA was not reduced by methylxanthine pentoxyfylline, indicating that the induction of apoptosis by genistein was not directly responsible for the increase in HIV-1 replication. Similarly, the genistein-induced increase of surface expression of CD14 and CD11b persisted in the presence of pentoxyfylline (data not shown), confirming that the induction of HIV-1 was not linked to the differentiation process. Since pentoxyfylline is also known as an inhibitor of TNF-α synthesis (12), we measured the amount of TNF-α in the supernatants of genistein-treated and control cultures. At days 1 and 3 post-genistein exposure, TNF-α levels remained very low (below 15 pg) and did not differ between genistein-treated cells and controls.
FIG. 5.
Treatment with pentoxyfylline alleviates the G2 arrest as well as the increase of HIV induced by genistein. OM10 cells were treated with genistein or DMSO alone, washed, and resuspended in RPMI with (right side) or without (left side) pentoxyfylline (pentox) (1 mM). For DNA content analysis by flow cytometry, cells were stained after 24 h with propidium iodide as described in Materials and Methods. Below the concentrations of genistein are the respective proportions of cells arrested in G2 and with less than 1N DNA (data are the means of four experiments). At day 3, supernatants were collected for HIV-1 p24 antigen production. Values are expressed as ratios of genistein-induced cells to controls (means of four experiments ± standard errors of the means).
We next examined by flow cytometry, at 2 days postexposure, the effect of genistein on intracytoplasmic HIV-1 p24 antigen expression within cells located in G1 compared to those blocked in G2 (Table 2). In the control cells, the mean proportion of p24 antigen-expressing cells did not significantly differ within the G1 and G2 phases (23 and 28%, respectively). In contrast, within the cells treated with 100 μM genistein, the mean proportion of HIV-1-positive cells was significantly higher in G2 than in G1 (35 and 25%, respectively; P < 0.02). This result confirms that the G2 arrest mediated by genistein promotes a higher HIV-1 expression in chronically infected cells.
TABLE 2.
HIV expression is enhanced in cells arrested in G2 by genisteina
| Treatment | Proportion of p24 antigen-positive cells (%) according to stage in cell cycleb
|
Pc | |
|---|---|---|---|
| G1 | G2 | ||
| DMSO | 22.7 (13.0–32.4) | 27.5 (13.3–43.7) | NS |
| Genistein | 25.0 (19.1–30.9) | 35.4 (27.7–43.1) | 0.016 |
OM10 cells were treated with 100 μM genistein for 4 h, cultured for 2 days, and analyzed by flow cytometry for intracytoplasmic HIV-1 p24 antigen expression in parallel with nuclear DNA content as described in Materials and Methods.
Means of five experiments with 95% confidence intervals in parentheses.
Wilcoxon rank test. NS, not significant.
The effects of genistein are related to the inhibition of topoisomerase II activity and phosphatidylinositol turnover rather than to tyrosine kinase inhibition.
To investigate whether the induction of HIV-1 was due to the decrease of tyrosine phosphorylation of a specific protein in the OM10 cells, we analyzed the phosphotyrosine protein contents of treated cells. The phosphotyrosine proteins were first analyzed in untreated and genistein-induced cells, with immunoblotting with antiphosphotyrosine. The basal level of phosphotyrosine was high in OM10 cells, but surprisingly, no clear difference was observed in genistein-treated cells in comparison to controls (data not shown). This result suggests that the increase of HIV-1 may not be a direct consequence of tyrosine kinase inhibition. Analysis by flow cytometry of the total phosphotyrosine protein content of the cells treated with genistein at concentrations up to 100 μM demonstrated little reduction from controls (data not shown). Additionally, another tyrosine kinase inhibitor, herbimycin A, evaluated in a range of concentrations known to have significant tyrosine kinase inhibition (0.1 to 10 μM), failed to induce any increase of HIV-1 expression in the same OM10 cells (data not shown).
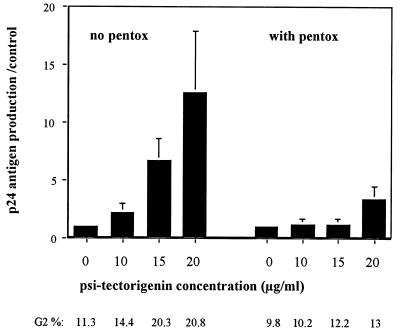
In addition to inhibiting tyrosine kinase, genistein has also been shown to inhibit topoisomerase II activity (29) and phosphatidylinositol turnover (20). To investigate whether either of these properties of genistein was involved in the induction of HIV-1 replication, cells were treated with (i) another inhibitor of topoisomerase II activity, etoposide; or (ii) a strong inhibitor of phosphatidylinositol turnover, psi-tectorigenin. In two independent experiments, OM10 cells treated for 4 h with 1 to 4 μM etoposide produced a moderate (2.1- to 2.8-fold) but reproducible increase of p24 antigen production, as well as a clear arrest of the cells in G2/M (34 to 51% of cells arrested at 24 h). Similarly, a strong and dose-dependent increase of HIV-1 expression in OM10 cells treated with psi-tectorigenin at 10 to 20 μg/ml was observed, in conjunction with a concomitant arrest at G2/M. Of interest, pentoxyfylline was also able to inhibit both the enhanced HIV-1 replication and the G2 arrest induced by psi-tectorigenin (Fig. 6).
FIG. 6.
Induction of HIV-1 by psi-tectorigenin is also linked to a G2/M arrest. OM10 cells were treated for 4 h with increasing concentrations of psi-tectorigenin or DMSO alone, washed, and resuspended in RPMI with (right side) or without (left side) pentoxyfylline (pentox) (1 mM). At day 3, supernatants were collected for HIV-1 p24 antigen production. Values are expressed as ratios of genistein-induced cells to controls (means of three experiments ± standard errors of the means). Below each concentration of psi-tectorigenin is the respective proportion of cells arrested in G2/M (flow cytometric analysis of nuclear DNA performed at day 1).
DISCUSSION
In this research paper, we describe the ability of the isoflavone genistein to enhance the expression of HIV-1 in two chronically infected monocytic cell lines, OM10 and U1. The dose-dependent increase of HIV-1 p24 antigen production in the supernatant of treated OM10 cells persisted in the presence of zidovudine and was associated with an increase of both multiply spliced and unspliced RNAs. These findings suggest an increase in transcription of the integrated HIV-1 proviral DNA in genistein-treated chronically infected cells. Our data suggest that this effect appears to be independent of the inhibitory activity of genistein on tyrosine kinase because there was no qualitative or quantitative difference between the proteins phosphorylated on tyrosine residues of treated cells and those on untreated cells, and another tyrosine kinase inhibitor, herbimycin A, failed to increase HIV-1 replication. An alternative explanation is that an undetected inhibition of the tyrosine phosphorylation of a minor protein could cause these findings. However, two other properties of genistein, its inhibition of phosphatidylinositol turnover and its inhibition of topoisomerase II activity, seem to be involved with induction of HIV-1 replication. Supporting findings for these properties of genistein inducing HIV-1 replication include data demonstrating that psi-tectorigenin (an inhibitor of phosphatidylinositol turnover) and etoposide (an inhibitor of topoisomerase II activity) also upregulate HIV-1 replication.
Although we have not identified the molecular pathways that regulate the transcriptional activation of HIV-1 by genistein, we have established a link between enhanced HIV-1 replication and specific changes in cell phenotype. Of interest, we failed to identify a specific association between cell differentiation and HIV-1 replication in the monocytic cell lines studied. These findings are consistent with recent observations in U1 cells that also found a lack of association between cell differentiation and HIV-1 replication (15) and may be explained by the opposite effect of cell differentiation on viral transcription and viral release described in differentiated THP1 cells and monocyte-derived macrophages (39).
Our findings support an association between the G2 arrest induced by genistein in OM10 cells and enhanced HIV-1 replication. The ability of pentoxyfylline to prevent G2 arrest in genistein-treated cells with a concomitant reduction in HIV-1 p24 antigen production to baseline supports the importance of arresting the cells in G2 for upregulating HIV-1 replication. Additionally, we observed by flow cytometry a significant increase of HIV-1 expression in cells arrested in G2 compared to cells located in S or G1. The in vivo significance of this result is unclear. However, it is reasonable to hypothesize that a virally induced modification of the cell cycle would be important for HIV-1 to maximally produce viral progeny prior to killing the infected cell. In support of this hypothesis, HIV-1 Vpr has recently been shown to induce G2 arrest (2, 16, 21, 35, 37) by maintaining the cyclin-dependent kinase Cdc2 in an inactive, phosphorylated form (16, 34, 35). Vpr is highly conserved among different HIV-1 isolates and is also present in HIV-2 and simian immunodeficiency virus (18). The ability of Vpr to induce a G2 arrest is also observed among other species of lentiviruses (32), indicating that this property may be an important strategy of the virus for continued survival.
In further studies, recombinant purified Vpr protein has been shown to increase HIV-1 replication after infection of promonocytic cell lines or peripheral blood mononuclear cells and to reactivate virus in latently infected cells (24). Additionally, an increase in HIV-1 LTR activity has been associated with some domains of the Vpr protein (10, 28, 44). Our findings correlating a G2 arrest with an increase in HIV-1 transcription suggest that the cell cycle modifications associated with Vpr increase viral transcription and that, by arresting the cell in G2, provide a cellular environment conducive to enhanced HIV-1 replication (1, 44). The recent report (8) of an increase of HIV-1 LTR activity and virus expression during the G2 arrest caused by Vpr strengthens this hypothesis. Moreover, the in vivo selection for the wild-type Vpr gene during human or chimpanzee infection over a defective Vpr clone provides further supporting evidence (8).
In total, our findings suggest that modification of the cell cycle may represent an important strategy for HIV-1 to promote its own replication that may be critical for the virus to maximally propagate itself while infecting cells that are further programmed by the virus for cell death (27). Increasing evidence suggests that apoptosis follows the arrest of the cell in G2 induced either by Vpr (43) or by cellular interactions between the HIV-1 Env glycoprotein and CD4-expressing cells (23). Further understanding of the interactions between HIV-1 and alterations of the cell cycle will provide new insights into HIV-1 pathogenesis and could provide novel strategies for inhibiting HIV-1 replication through manipulation of the cell cycle of HIV-1-infected cells.
ACKNOWLEDGMENTS
This research was supported by grants AI39004 and AI27563, the UCSD Center for AIDS Research (AI36214), and a fellowship from NATO for J. Gozlan.
We acknowledge Karen Hsia for providing reagents and procedures used for RT-PCR analysis, J. Nordberg and M. Lutz (Veterans Affairs Hospital, Medical Center Laboratory, San Diego, Calif.) for flow cytometry analysis, and J. Corbeil (Department of Medicine, University of California, San Diego) for providing the MAb 314219.
REFERENCES
- 1.Agostini I, Navarro J M, Rey F, Bouhamdan M, Spire R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 2.Bartz S, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaty C D, Franklin T L, Uehara Y, Wilson C B. Lipopolysaccharide-induced cytokine production in human monocyte: role of tyrosine phosphorylation in transmembrane signal transduction. Eur J Immunol. 1994;24:1278–1284. doi: 10.1002/eji.1830240606. [DOI] [PubMed] [Google Scholar]
- 4.Briand G, Barbeau B, Tremblay M. Binding of HIV-1 to its receptor induces tyrosine phosphorylation of several CD4-associated proteins, including the phosphatidylinositol 3-kinase. Virology. 1997;228:171–179. doi: 10.1006/viro.1996.8399. [DOI] [PubMed] [Google Scholar]
- 5.Butera S T, Perez V L, Wu B-Y, Nabel G J, Folks T M. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991;65:4645–4653. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butera S T, Roberts B D, Lam L, Hodge T, Folks T M. Human immunodeficiency virus type 1 RNA expression by four chronically infected cell lines indicates multiple mechanisms of latency. J Virol. 1994;68:2726–2730. doi: 10.1128/jvi.68.4.2726-2730.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon P M, Tenen D G, Feinberg M B, Shin H S, Kim S. Human immunodeficiency virus-1 infection of the human promyelocytic cell line HL-60: high frequency of low level infection and effect of subsequent cell differentiation. Blood. 1993;81:437–445. [PubMed] [Google Scholar]
- 8.Chun Goh W, Rogel M E, Kinsey M, Michael S F, Fultz P N, Nowak M A, Hanh B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D I, Tani Y, Tian H, Boone E, Samelson L E, Lane H C. Participation of tyrosine phosphorylation in the cytopathic effect of human immunodeficiency virus-1. Science. 1992;256:542–545. doi: 10.1126/science.1570514. [DOI] [PubMed] [Google Scholar]
- 10.Cohen E A, Terwilliger E F, Jalinosa Y, Proulx J, Sodroski J G, Haseline W A. Identification of HIV-1 vpr products and function. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 11.Constantinou A, Kiguchi K, Huberman E. Induction of differentiation and DNA strand breakage in human HL-60 and K562 leukemia cells by genistein. Cancer Res. 1990;50:2618–2624. [PubMed] [Google Scholar]
- 12.Dezube B J. Pentoxifylline for the treatment of infection with human immunodeficiency virus. Clin Infect Dis. 1994;18:285–287. doi: 10.1093/clinids/18.3.285. [DOI] [PubMed] [Google Scholar]
- 13.Far D F, Peyron J-F, Imbert V, Rossi B. Immunofluorescent quantification of tyrosine phosphorylation of cellular proteins in whole cells by flow cytometry. Cytometry. 1994;15:327–334. doi: 10.1002/cyto.990150408. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Goletti D, Kinter A L, Biswas P, Bende S M, Poli G, Fauci A S. Effect of cellular differentiation on cytokine-induced expression of human immunodeficiency virus in chronically infected promonocytic cells: dissociation of cellular differentiation and viral expression. J Virol. 1995;69:2540–2546. doi: 10.1128/jvi.69.4.2540-2546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Choe S, Walker R, DiMarzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsia K, Spector D H, Spector S A. Molecular analysis of the differential restriction of human immunodeficiency virus type 1 replication in neuronal cell lines. J Gen Virol. 1997;78:3255–3264. doi: 10.1099/0022-1317-78-12-3255. [DOI] [PubMed] [Google Scholar]
- 18.Huang L M, Jeang K T. HIV vpr: roles in viral replication and cellular metabolism. In: Meyers G, et al., editors. Human retrovirus and AIDS. N.Mex: Theoretical Biology and Biophysics, Los Alamos; 1995. pp. 3–9. [Google Scholar]
- 19.Hunàkovà L, Sedlàk J, Klobusickà M, Durai J, Chorvàth B. Tyrosine kinase inhibitor-induced differentiation of K-562 cells: alterations of cell cycle cycle and cell surface phenotype. Cancer Lett. 1994;81:81–87. doi: 10.1016/0304-3835(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 20.Imoto M, Yamashita T, Sawa T, Kurasawa S, Naganawa H, Takeuchi T, Bao-quan Z, Umeawa K. Inhibition of cellular phosphatidylinositol furnover by psi-tectorigenin. FEBS Lett. 1988;230:230–243. doi: 10.1016/0014-5793(88)80638-0. [DOI] [PubMed] [Google Scholar]
- 21.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitano K, Baldwin G C, Raines M A, Golde D W. Differentiating agents facilitate infection of myeloid leukemia cell lines by monocytotrophic HIV-1 strains. Blood. 1990;76:1980–1988. [PubMed] [Google Scholar]
- 23.Kolesnitchenko V, Wahl L M, Tian H, Sunila I, Yoshihiko T, Hartmann D-P, Cossman J, Raffeld M, Orenstein J, Samelson E, Cohen D J. Human immunodeficiency virus 1 envelope-initiated G2-phase programmed cell death. Proc Natl Acad Sci USA. 1995;142:1880–1893. doi: 10.1073/pnas.92.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D N, Refaeli Y, Weiner D B. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makishima M, Honma Y, Hozumi M, Sampi K, Hattori M, Umezawa K, Moyoyoshi K. Effects of inhibitors of protein tyrosine kinase activity and/or phosphatidylinositol turnover on differentiation of some human myelomonocytic leukemia cells. Leuk Res. 1991;15:701–708. doi: 10.1016/0145-2126(91)90072-2. [DOI] [PubMed] [Google Scholar]
- 26.Moses A V, Ibanez C, Gaynor R, Ghazal P, Nelson J A. Differential role of long terminal repeat control elements for the regulation of basal and Tat-mediated transcription of the human immunodeficiency virus in stimulated and unstimulated primary human macrophages. J Virol. 1994;68:298–307. doi: 10.1128/jvi.68.1.298-307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noraz N, Gozlan J, Corbeil J, Brunner T, Spector S A. HIV-induced apoptosis of activated primary CD4+ T lymphocytes is not mediated by Fas/Fas ligand. AIDS. 1997;11:1671–1680. doi: 10.1097/00002030-199714000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- 30.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 31.Phipps D J, Read S E, Piovesan J P, Mills G B, Branch D R. HIV infection in vitro enhances the activity of src-family protein tyrosine kinase. AIDS. 1996;10:1191–1198. doi: 10.1097/00002030-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Planelles V, Jowett J B, Li O, Xie Y, Hahn B, Chen S Y. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon B, Jowett B M, Stewart S A, Armstrong R W, Rishton G M, Chen I S Y. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich E A, Chen I S Y, Zack J A, Leonard M L, O’Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV 1) J Clin Investig. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogel M, Wu L, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saskela K, Muchmore E, Girard M, Fultz P, Baltimore D. High viral load in lymph nodes and latent human immunodeficiency virus (HIV) in peripheral blood cells of HIV-1-infected chimpanzees. J Virol. 1993;67:7423–7427. doi: 10.1128/jvi.67.12.7423-7427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shattock R J, Friedland J S, Griffin G E. Release of human immunodeficiency virus by THP-1 and human macrophages is regulated by cellular adherence and activation. J Virol. 1993;67:3569–3575. doi: 10.1128/jvi.67.6.3569-3575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinozzi F, Pagliacci C, Migliorati G, Moraca R, Grignani F, Riccardi C, Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in jurkat T-leukemia cells. Leuk Res. 1994;18:431–439. doi: 10.1016/0145-2126(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 42.Stefanova I, Corcoran M L, Horak E M, Wahk L M, Bolen J B, Horak I D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 43.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activator of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe T, Kondo K, Oishi M. Induction of differentiation of mouse erythroleukemia cells by genistein, an inhibitor of tyrosine protein kinases. Cancer Res. 1991;51:764–768. [PubMed] [Google Scholar]
- 46.Weinstein S L, June C H, DeFranco A L. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol. 1993;151:3829–3838. [PubMed] [Google Scholar]
- 47.Weinstein S L, Sanghera J S, Lemke K, DeFranco A L, Pelech S L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]