Abstract
Background
The neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) are inflammatory biomarkers. Until now, it is unknown the impact of opioid dosage on perioperative immunity in glioma patients. The aim of this study was to explore the effect of intraoperative opioid dosage on perioperative immune perturbations using NLR and LMR as inflammatory biomarkers and evaluate the correlation between inflammatory biomarkers and pathological grade of glioma.
Methods
The study included 208 patients with primary glioma who underwent glioma resection from February 2012 to November 2019 at Harbin Medical University Cancer Hospital. Complete blood count (CBC) was collected at 3 time points: one week before surgery, and 24 hours and one week after surgery. Patients were divided into high-dose and low-dose groups, based on the median value of intraoperative opioid dose. The relationships between perioperative NLR, LMR and intraoperative opioid dosage were analyzed using repeated measurement analysis of variance (ANOVA). Correlations between preoperative various factors and pathological grade were analyzed by Spearman analysis. Receiver operating characteristic (ROC) curves were performed to assess the predictive performance of the NLR and LMR for pathological grade.
Results
The NLR (P=0.020) and lower LMR (P=0.037) were statistically significant different between high-dose and low-dose groups one week after surgery. The area under the curve (AUC) of the NLR to identify poor diagnosis was 0.685, which was superior to the LMR (AUC: 0.607) and indicated a correlation between the NLR with pathological grade. The preoperative NLR (P=0.000), LMR (P=0.009), age (P=0.000) and tumor size (P=0.001) exhibited a significant correlation with the pathological grade of glioma.
Conclusion
Intraoperative opioids in the high-dose group were associated with higher NLR and lower LMR in postoperative glioma patients. The preoperative NLR and LMR demonstrated predictive value for distinguishing between high-grade and low-grade gliomas.
Keywords: glioma, intraoperative opioid dosage, neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, pathological grade
Introduction
Glioma is the most common primary malignant tumor of the brain.1 The prognosis of high-grade glioma is poor despite combination therapy of surgery, chemotherapy and radiotherapy. Perioperative factors such as intraoperative blood transfusion and methods of anesthesia may have impact on the prognosis of patients. Studies have found that higher doses of intraoperative opioids are associated with lower overall survival in patients with stage I non-small cell lung cancer2 and kidney cancer.3
Opioids may suppress immunity, forming a microenvironment that promotes tumor progression. Opioids exert an impact on various components of the immune system, encompassing NK cell function, macrophage proliferation, phagocytic activity and lymphocyte function.4 The pivotal role of NK cells in innate immunity is widely acknowledged, as they orchestrate early defense mechanisms through their innate cytotoxicity against both microbial-infected and malignant-transformed cells.5,6 The cytotoxicity of human NK cells was found to be inhibited upon exposure to various opioids commonly used in clinical settings.7 Studies have demonstrated that long-term opioids impair the macrophages’ ability to defend against invading pathogens both in vitro and in vivo.8 The impact on lymphocytes primarily manifests as the suppression of both T and B lymphocyte function and quantity.
Inflammation plays an important role in the occurrence of tumor and the progression of cancer.9,10 The pathogenesis of cancer shares many similarities with the processes of inflammation and wound healing.6 Inflammation represents an intrinsic defensive response triggered by detrimental stimuli and neoplastic cells, serving as a pivotal mechanism to eradicate the initial cause of cellular injury, eliminate necrotic cells and damaged tissues, and initiate tissue regeneration. While acute inflammation confers advantageous protection against infections or injuries and preneoplastic cells, it is imperative for this immune reaction to terminate upon completion of its purpose in order to prevent chronic inflammation, which could potentially facilitate tumor initiation and metastasis.11,12 The presence of numerous triggers for chronic inflammation significantly heightens the susceptibility to cancer development. For example, chronic inflammation resulting from microbial infections has been associated with the development of specific malignancies, including gastric, hepatic and cervical cancers.13–15
Inflammatory markers have great predictive ability in various cancers. Relevant studies have demonstrated that the neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) possess prognostic value in rectal cancer, stage IV non-small cell lung cancer (NSCLC) and Hilar Cholangiocarcinoma.16–19 Studies have found that the MRI-based radiomics signature can predict survival of high-low grade gliomas, which is more complex than inflammatory markers.20,21 Studies have shown that neutrophil-to-lymphocyte ratios are correlated with the pathological grade22,23 and prognosis of glioma.24,25 The NLR and LMR are useful predictors for inflammation and surgery-induced immunosuppression, combining the strengths of both systems to enhance accuracy.26 The aim of this study was to investigate the perioperative alterations in NLR and LMR induced by intraoperative opioid-mediated immune perturbation in patients with glioma.
Methods
Study Design
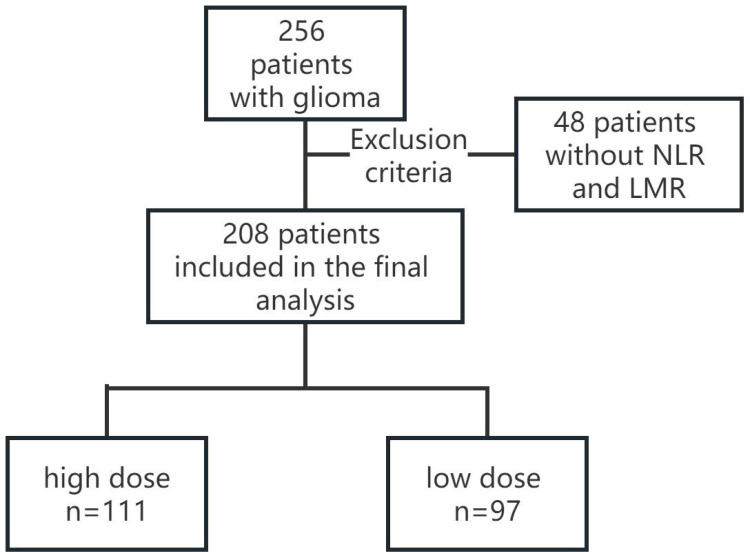
In this study, data for 256 patients with primary glioma who received tumor resection at Harbin Medical University Cancer Hospital from February 2012 to November 2019 were retrospectively collected. Inclusion criteria were that patients were confirmed by histopathological examination, first resection of glioma, general anesthesia and non-immune-related diseases. Patients with incomplete demographic data, surgical or anesthetic records or loss of follow-up were excluded. A total of 208 patients were included finally after selection, as shown in Figure 1.
Figure 1.
Flow diagram.
Outcomes
Demographic information such as age, sex, body mass index (BMI), medical history, American Society of Anesthesiologists physical status (ASA), World Health Organization (WHO) pathological grade, tumor size, operation duration, tumor site, intraoperative opioid dose and type, NLR and LMR one week before surgery (T1), 24 hours after surgery (T2) and one week after surgery (T3) were collected. All patients were divided into high-dose and low-dose groups based on the median value of intraoperative opioid dose. The data were retrieved from the electronic medical record data of the Harbin Medical University Cancer Hospital.
Anesthetic Management
Anesthesia induction was achieved by intravenous infusion of sufentanil 0.4 μg/kg or fentanyl 4 μg/kg, propofol 2 mg/kg, and cisatracurium 0.15 mg/kg. Remifentanil 0.3 μg/(kg·min), propofol 4 μg/(kg·h), and rocuronium 0.3 mg/kg or cisatracurium 0.6 mg/kg were used for anesthesia maintenance. Opioids were converted into fentanyl dosage: 1 μg fentanyl=0.1 μg sufentanil=1 μg remifentanil.
Statistical Analysis
Categorical variables were reported as percentages and the χ2 test was used for comparison between groups. Normally distributed variables were expressed as means±standard deviations (SDs) and analyzed by the Student’s t-test. Nonnormally distributed variables are expressed as the median and interquartile range (IQR) and analyzed by the Mann–Whitney U-test. Inflammatory markers of patients in different groups were contrasted T1, T2 and T3 by repeated-measures ANOVA. Spearman analysis was used to examine the correlation between factor indicators and pathological grade of patients, and univariate analysis of variance was used in different grades under the control of covariates. All were analyzed using SPSS 26.0 statistical software.
Results
Comparison of Clinical Characteristics
We collected information on 208 glioma patients who were divided into two groups with the median of intraoperative opioid dose (2.000 mg). There was a significant difference in duration of operation between the high-dose and low-dose groups (P=0.000). There were no differences in age, gender, BMI, history of previous diseases, ASA, WHO and tumor size between two groups, as shown in Table 1.
Table 1.
Data Assessed by Chi-Square Test, Independent-Sample t-Test and Mann–Whitney U-Test Statistically Significant at P<0.05
| Various | Low-Dose Group | High-Dose Group | Z/t/x2 | P |
|---|---|---|---|---|
| Age | 52.60±14.06 | 52.7±14.61 | −0.089 | 0.929 |
| Female | 48 (46.2%) | 56 (53.8%) | 0.016 | 0.898 |
| Male | 49 (47.1%) | 55 (52.9%) | ||
| BMI | 23.94 (14.88–33.79) | 23.99 (17.93–41.32) | −0.502 | 0.615 |
| High blood pressure or diabetes | 0.048 | 0.827 | ||
| Yes | 92 (46.5%) | 106 (53.5%) | ||
| No | 5 (50.0%) | 5 (50.0%) | ||
| ASA I | 2 (25.0%) | 6 (75.0%) | 4.215 | 0.202 |
| II | 92 (48.4%) | 98 (51.6%) | ||
| III | 2 (22.2%) | 7 (77.8%) | ||
| IV | 1 (100.0%) | 0 (0.0%) | ||
| WHO I | 6 (37.5%) | 10 (62.5%) | 3.703 | 0.295 |
| II | 45 (51.1%) | 43 (48.9%) | ||
| III | 21 (37.5%) | 35 (62.5%) | ||
| IV | 25 (52.1%) | 23 (47.9%) | ||
| Tumor size | 3.41±1.94 | 3.61±2.13 | −0.458 | 0.647 |
| Duration of operation (h) | 3.83 (3.06–4.87) | 4.5 (3.67–5.5) | −4.179 | 0.000 |
Abbreviations: ASA, American Society of Anesthesiologists physical status; BMI, body mass index; WHO, World Health Organization.
Statistically significant at P<0.05.
Changes of NLR and LMR in Different Time Points Between High-Dose and Low-Dose Groups
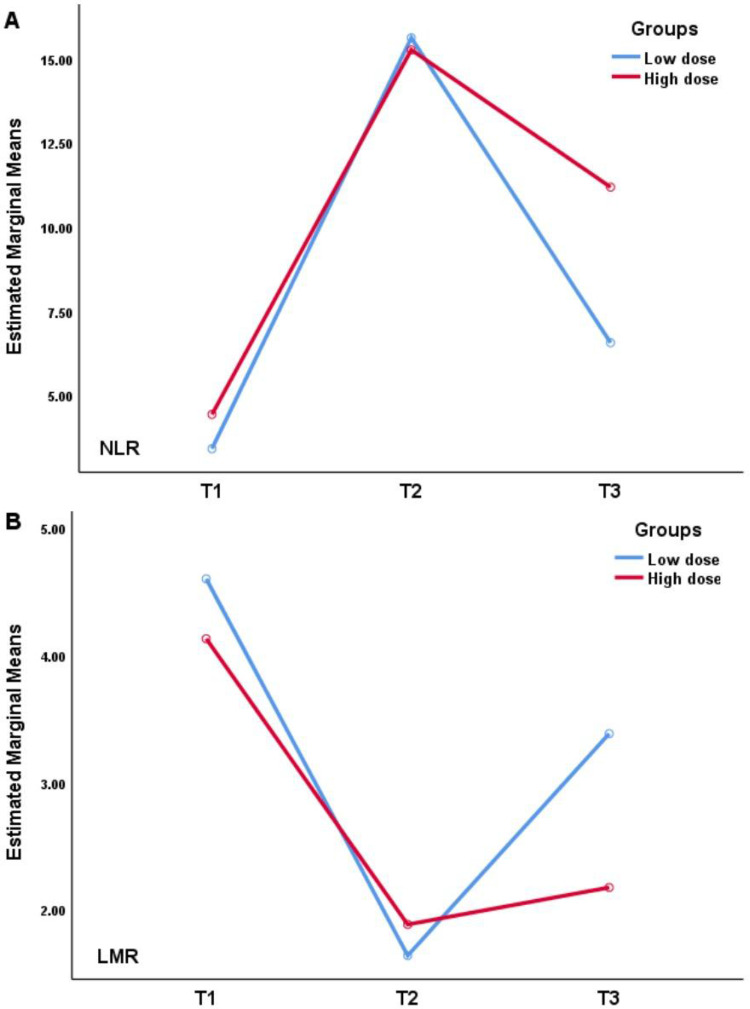
We collected NLR and LMR one week before surgery, and 24 hours and one week after surgery, which were expressed as T1, T2 and T3. Table 2 and Figure 2 present the significant differences in NLR and LMR between the high-dose and low-dose groups one week after surgery, with no differences in the other times.
Table 2.
Data Assessed by Repeated-Measures ANOVA
| Variable | Low-Dose Group | High-Dose Group | P |
|---|---|---|---|
| NLR | |||
| T1 | 2.40 (1.65–3.23) | 2.49 (1.62–5.1) | 0.131 |
| T2 | 11.17 (5.95–24.26) | 12.24 (6.64–18.62) | 0.842 |
| T3 | 4.66 (2.41–8.55) | 6.72 (3.19–12.35) | 0.020 |
| LMR | |||
| T1 | 4.29 (3.44–5.42) | 4.16 (2.79–5.53) | 0.137 |
| T2 | 1.23 (0.73–2.00) | 1.34 (0.86–2.04) | 0.291 |
| T3 | 2.23 (1.25–3.39) | 1.57 (1.12–2.86) | 0.037 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
Statistically significant at P<0.05. One week before surgery, 24 hours after surgery and one week after surgery are defined as T1, T2 and T3.
Figure 2.
Repeated-measures analysis of variance of perioperative NLR (A) and LMR (B).
Predictive Value of Inflammatory Biomarkers Before Surgery
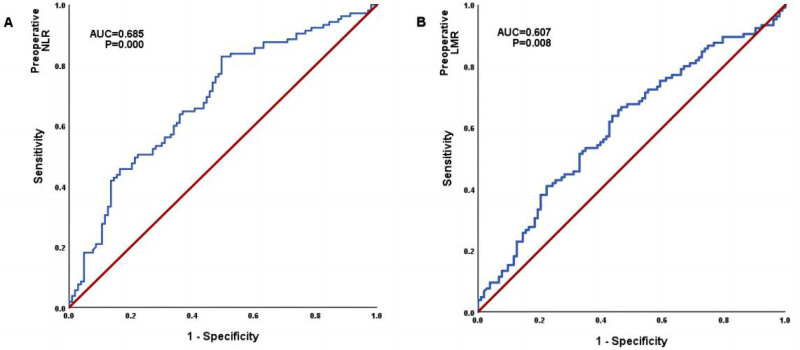
According to the WHO classification, WHO grade I and II are classified as low-grade gliomas, and WHO grade III and IV are defined as high-grade gliomas. The predictive value of NLR and LMR for pathology classification is indicated by the ROC curve. As shown in Figure 3, the NLR showed an area under the curve (AUC) of 0.685 [95% confidence interval (CI), 0.613–0.757, P=0.000]. The LMR showed an area under the curve (AUC) of 0.607 [95% confidence interval (CI), 0.530–0.684, P=0.008].
Figure 3.
The ROC of neutrophil-to-lymphocyte ratio (A) and lymphocyte-to-monocyte ratio (B) to predictive capability for high and low grade of gliomas; area under the ROC curve appears in cartouche with 95% confidence interval.
Correlation Analysis of Preoperative NLR and LMR with Pathological Grade of Glioma
The preoperative NLR (P=0.000), LMR (P=0.009), age (P=0.000) and tumor size (P=0.001) were significantly correlated with the pathological grade of glioma, and the pathological grade was positively correlated with NLR, and glioma with higher WHO grade had higher NLR one week before surgery, as shown in Table 3.
Table 3.
Data Assessed by Univariate Analysis of Variance
| NLR | P | LMR | P | |
|---|---|---|---|---|
| WHO I | 2.28±1.99 | 0.021 | 5.58±2.43 | 0.039 |
| WHO II | 3.06±3.36 | 4.42±1.83 | ||
| WHO III | 4.92±6.53 | 3.79±1.93 | ||
| WHO IV | 5.07±5.16 | 4.46±3.08 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; WHO, World Health Organization.
Statistically significant at P<0.05.
Discussion
In this study, we investigated the correlation of intraoperative opioid dose on perioperative immune function in glioma patients. It has been considered that the NLR and LMR are useful inflammatory biomarkers of systemic inflammation. This study indicated that intraoperative high dosage of opioids is associated with postoperative immune function as indicated by NLR and LMR values.
The use of opioids as essential anesthetic agents in surgical procedures has prompted extensive clinical research on their impact on the immune system of patients.27 Some studies have shown that the regulation of the immune system by opioids occurs through their binding to mu opioid receptors (MOP receptors).28–30 Opioid receptors are present on various cells, and regulate the proliferation and apoptosis of various cells, including cancer cells.4,31 There are some studies that have found a significant reduction in the number of lymphocytes in patients treated with opioids compared with controls and that opioids are able to inhibit the growth of lymphocytes.32–34 NK cells are crucial for innate immunity, as they defend against microbial infections and cancerous cells through innate cytotoxicity. However, opioids impair NK cell function through opioid receptors and Toll-like receptor 4 (TLR4).29 The immunosuppressive effects through many mechanisms induced by opioids have detrimental implications in cancer development, as it has been found that morphine or fentanyl in combination with tumor cells appears to facilitate metastatic dissemination.35 This effect on the immune system appears to be related to the dosage of opioids.36 In our study, we observed the effects of different doses of opioids on postoperative immunity in patients with glioma as expressed by NLR and LMR values.
Inflammation and the immune system have been widely implicated in the development of various cancers, such as infection with Helicobacter pylori is associated with gastric cancer and Hepatitis virus is associated with liver cancer. Cancer-related inflammation involves inflammatory cells and mediators in tumor tissues, leading to tissue remodeling, repair and angiogenesis similar to chronic inflammatory responses. A proinflammatory environment increases the risk of excessive cell proliferation, which can lead to cancer.37 The inflammatory already exists before the occurrence of cancers and accompanies tumor progression. The neutrophil-to-lymphocyte ratio (NLR) and the lymphocyte-to-monocyte ratio (LMR) can not only reflect the inflammatory state, but also predict the malignant degree of glioma.38,39 Inflammatory cells are an important part of the tumor microenvironment, in which neutrophils account for 55–70% of the total number of white blood cells and participate in natural immunity. Neutrophils secrete cytokines and chemokines to promote the recruitment and activation of inflammatory cells, thereby promoting neovascularization and facilitating the transendothelial migration of cancer cells to promote tumor metastasis.40,41 It has been reported that activated neutrophils release mutagenic reactive oxygen species and proteolytic enzymes to damage normal cells. Neutrophils, as direct effector cells, enable tumor cells to evade immune recognition, providing advantages for tumor cell growth.42 As the core component of immune system, lymphocytes can reflect the immune function and balance the immune strength of the body. Lymphocytes are directly involved in anticancer reactions. CD4+, CD8+ lymphocytes and T regulatory cells play an indispensable role in immune surveillance of tumor growth.43 CD8+ cytotoxic lymphocytes constitute the effector of adaptive immunity, which can slow down the growth rate of tumors.44 Under the action of the tumor microenvironment, monocytes can promote immunosuppression, accelerate angiogenesis and promote disease progression.45 NLR and LMR are useful markers for predicting opioid-induced immunosuppression, linking neutrophils, lymphocytes and monocytes, and are more valuable than a single system, which can be effectively, simply and rapidly to get in patients. In addition, NLR values have a good ability to predict the degree of malignancy. Many studies also have shown that the higher NLR values are, the more malignant the glioma is,46,47 which is consistent with the data analysis obtained in our experiment.
There has been no study on the relationship between intraoperative opioid dose and perioperative immune response with NLR and LMR values in glioma. In our study, T1, T2 and T3 correspond to 1 week before surgery, and 24 hours after surgery and 1 week after surgery, respectively. The patient’s immune response was at the baseline level one week before surgery, but within 24 hours after surgery the patient was under acute stress due to surgical factors, anesthesia factors and other factors, and the immune response fluctuated sharply. However, one week after surgery, the patient’s immune stress basically returned to the baseline level. There were significant differences in NLR and LMR between the high-dose and low-dose groups one week after surgery in our study. Our results suggest that opioid doses are associated with the immune response of patients with glioma, and whether this correlation can shorten or prolong the survival of patients needs to be further explored. As an indispensable intraoperative anesthetic drug, opioids need to be explored for the appropriate dosage, even if it affects the immune system but does not shorten survival. Multi-modal analgesia is the recommended approach for pain management, combining regional anesthesia techniques and combination drug therapy to reduce opioid usage. The combination of drugs with distinct mechanisms of action allows for effective pain relief. Studies show that NSAIDs combined with opioids can decrease immunosuppression caused by opioid use.48
Our study has some limitations. Firstly, it is single-center research, which restricts the generalizability of our findings. To enhance the robustness of our results, we recommend expanding the sample size and collecting data from multiple hospitals. It is important to note that we did not compare the intraoperative opioid dose with alternative analgesic strategies in this study, which may limit our comprehensive understanding of their effects on inflammatory markers.
Therefore, we propose designing a prospective clinical study to investigate perioperative changes in inflammatory markers among cancer patients undergoing multi-mode analgesia regimens and opioid monotherapy for pain management. Additionally, we aim to conduct survival analysis by following up with patients at 1, 3 and 5 years post surgery to assess overall survival and relapse-free survival rates. The study will not be limited to a specific type of cancer but aims to include multiple cancer types for comprehensive observation.
Conclusion
There were significant differences in the NLR and LMR between the different dose groups in that the high-dose group of patients have higher NLR and lower LMR one week after surgery, and no differences in one week before surgery and 24 hours after surgery in our study. The preoperative NLR and LMR demonstrated predictive value for distinguishing between high-grade and low-grade gliomas.
Acknowledgment
We thank members in the Department of Pain Medicine at Harbin Medical University Cancer Hospital who helped the present study.
Funding Statement
The study was supported by the Haiyan Foundation of Harbin Medical University Cancer Hospital (JJZD2023-12). The funding agencies did not participate in the experimental design and data analysis.
Data Sharing Statement
Data and materials are available from the corresponding author on reasonable request.
Ethical Approval
This study met the ethical criteria described in the Declaration of Helsinki. This study was approved by the Institutional Review Board of Harbin Medical University Cancer Hospital (2022-126R). Informed consent from the patients was waived by the IRB because the nature of this retrospective study was reanalyzing existing data which does not involve any potential risks and benefits to the patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no competing financial interests.
References
- 1.Guoping J, Xin L, Yuqian S, Tao S, Shan W. Expression of Glypican-1 in brain glioma and its relationship with clinicopathological features and prognosis. Chin J Mod Med. 2020;30:19–23. [Google Scholar]
- 2.Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non‐small cell lung cancer resection. Cancer Med. 2014;3:900–908. doi: 10.1002/cam4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silagy AW, Hannum ML, Mano R, et al. Impact of intraoperative opioid and adjunct analgesic use on renal cell carcinoma recurrence: role for onco-anaesthesia. Br J Anaesth. 2020;125:e402–e404. doi: 10.1016/j.bja.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zajączkowska R, Leppert W, Mika J, et al. Perioperative immunosuppression and risk of cancer progression: the impact of opioids on pain management. Pain Res Manag. 2018;2018:1–8. doi: 10.1155/2018/9293704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björkström NK, Strunz B, Ljunggren H-G. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112–123. doi: 10.1038/s41577-021-00558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang F, Xie S, Chen M, et al. Advances in NK cell production. Cell Mol Immunol. 2022;19:460–481. doi: 10.1038/s41423-021-00808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher DP, Walia D, Heller NM. Suppression of human natural killer cells by different classes of opioids. Anesth Analg. 2019;128:1013–1021. doi: 10.1213/ANE.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plein LM, Rittner HL. Opioids and the immune system – friend or foe. Br J Pharmacol. 2018;175:2717–2725. doi: 10.1111/bph.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985 [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 11.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-López L, Adrados I, Ferres-Marco D, Dominguez M. A blueprint for cancer-related inflammation and host innate immunity. Cells. 2021;10:3211. doi: 10.3390/cells10113211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 14.Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B virus-associated hepatocellular carcinoma. Viruses. 2022;14:986. doi: 10.3390/v14050986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyouni AAA. Human papillomavirus in cancer: infection, disease transmission, and progress in vaccines. J Infect Public Health. 2023;16:626–631. doi: 10.1016/j.jiph.2023.02.014 [DOI] [PubMed] [Google Scholar]
- 16.Mandaliya H, Jones M, Oldmeadow C, Nordman IIC. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8:886–894. doi: 10.21037/tlcr.2019.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portale G, Bartolotta P, Azzolina D, Gregori D, Fiscon V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis. Langenbecks Arch Surg. 2023;408:85. doi: 10.1007/s00423-023-02786-8 [DOI] [PubMed] [Google Scholar]
- 18.Hamid HKS, Davis GN, Trejo-Avila M, Igwe PO, Garcia-Marín A. Prognostic and predictive value of neutrophil-to-lymphocyte ratio after curative rectal cancer resection: a systematic review and meta-analysis. Surg Oncol. 2021;37:101556. doi: 10.1016/j.suronc.2021.101556 [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Ma C, Cao W, Ning Z, Tan G. Prognostic significance of NLR, PLR, LMR and tumor infiltrating T lymphocytes in patients undergoing surgical resection for hilar cholangiocarcinoma. Front Oncol. 2022;12:908907. doi: 10.3389/fonc.2022.908907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le VH, Minh TNT, Kha QH, Le NQK. A transfer learning approach on MRI-based radiomics signature for overall survival prediction of low-grade and high-grade gliomas. Med Biol Eng Amp Comput. 2023;61:2699–2712. doi: 10.1007/s11517-023-02875-2 [DOI] [PubMed] [Google Scholar]
- 21.Kha Q-H, Le V-H, Hung TNK, Le NQK. Development and validation of an efficient MRI radiomics signature for improving the predictive performance of 1p/19q co-deletion in lower-grade gliomas. Cancers. 2021;13:5398. doi: 10.3390/cancers13215398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng W, Chen X, Gong S, Guo L, Zhang X. Preoperative neutrophil–lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol Res. 2018;40:917–922. doi: 10.1080/01616412.2018.1497271 [DOI] [PubMed] [Google Scholar]
- 23.Gomes Dos Santos A, de Carvalho RF, de Morais ANLR, et al. Role of neutrophil-lymphocyte ratio as a predictive factor of glioma tumor grade: a systematic review. Crit Rev Oncol Hematol. 2021;163:103372. doi: 10.1016/j.critrevonc.2021.103372 [DOI] [PubMed] [Google Scholar]
- 24.Lei Y, Li Y, Hu Q, Wang J, Sui A. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:152. doi: 10.1186/s12957-019-1686-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Kang K, Lin Q, Hai J. Prognostic significance of preoperative systemic cellular inflammatory markers in gliomas: a systematic review and meta‐analysis. Clin Transl Sci. 2020;13:179–188. doi: 10.1111/cts.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J-H, Zhai E-T, Yuan Y-J, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261. doi: 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14–18. doi: 10.1097/SPC.0b013e3282f5272e [DOI] [PubMed] [Google Scholar]
- 28.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid Therapy and Immunosuppression. Am J Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85 [DOI] [PubMed] [Google Scholar]
- 29.Malafoglia V, Ilari S, Gioia C, et al. An observational study on chronic pain biomarkers in fibromyalgia and osteoarthritis patients: which role for mu opioid receptor’s expression on NK cells? Biomedicines. 2023;11:931. doi: 10.3390/biomedicines11030931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afsharimani B, Cabot P, Parat M-O. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–238. doi: 10.1007/s10555-011-9285-0 [DOI] [PubMed] [Google Scholar]
- 32.Wang ZY, Wang CQ, Yang JJ, et al. Which has the least immunity depression during postoperative analgesia—morphine, tramadol, or tramadol with lornoxicam? Clin Chim Acta. 2006;369:40–45. doi: 10.1016/j.cca.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 33.Fuggetta MP, Di Francesco P, Falchetti R, et al. Effect of morphine on cell-mediated immune responses of human lymphocytes against allogeneic malignant cells. J Exp Clin Cancer Res CR. 2005;24:255–263. [PubMed] [Google Scholar]
- 34.Haroutounian S. Postoperative opioids, endocrine changes, and immunosuppression. PAIN Rep. 2018;3:e640. doi: 10.1097/PR9.0000000000000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus–pituitary–adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun. 2007;21:767–774. doi: 10.1016/j.bbi.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Wodehouse T, Demopoulos M, Petty R, et al. A randomized pilot study to investigate the effect of opioids on immunomarkers using gene expression profiling during surgery. Pain. 2019;160:2691–2698. doi: 10.1097/j.pain.0000000000001677 [DOI] [PubMed] [Google Scholar]
- 37.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan P, Li J-W, Mo L-G, Huang Q-R. A nomogram combining inflammatory markers and clinical factors predicts survival in patients with diffuse glioma. Medicine. 2021;100:e27972. doi: 10.1097/MD.0000000000027972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P-F, Song H-W, Cai H-Q, et al. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget. 2017;8:50117–50123. doi: 10.18632/oncotarget.15235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory AD, McGarry Houghton A. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583 [DOI] [PubMed] [Google Scholar]
- 41.Hofman PM. Pathobiology of the neutrophil-intestinal epithelial cell interaction: role in carcinogenesis. World J Gastroenterol. 2010;16:5790. doi: 10.3748/wjg.v16.i46.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b‐positive neutrophil‐to‐CD8‐positive T‐cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456 [DOI] [PubMed] [Google Scholar]
- 43.Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–372. doi: 10.1016/j.athoracsur.2008.10.067 [DOI] [PubMed] [Google Scholar]
- 44.Mony JT, Schuchert MJ. Prognostic implications of heterogeneity in intra-tumoral immune composition for recurrence in early stage lung cancer. Front Immunol. 2018;9:2298. doi: 10.3389/fimmu.2018.02298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanmei X, Tianxiang C, Xiaojing Z, Dezhi L, Jianguo S. Correlation analysis between peripheral blood monocyte number and near-and long-term efficacy of radiotherapy for lung cancer. Chin J Lung Dis. 2014;7:159–164. [Google Scholar]
- 46.Mou L, Zou W, Zhang L, Du D. The value of preoperative blood inflammatory response markers for the diagnosis of different grades of glioma. Tumor Prevent Treat. 2022;35:996–1000. [Google Scholar]
- 47.Bao Y, Yang M, Jin C, et al. Preoperative hematologic inflammatory markers as prognostic factors in patients with glioma. World Neurosurg. 2018;119:e710–e716. doi: 10.1016/j.wneu.2018.07.252 [DOI] [PubMed] [Google Scholar]
- 48.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available from the corresponding author on reasonable request.