Abstract
This study evaluated the toxicity of the pyrazino isoquinoline anthelmintic praziquantel (PZQ) to the Danio rerio zebrafish and Daphnia magna water flea. The estimated 24 h and 96 h LC50 of PZQ to the zebrafish was 39.9 mg/l and 30.4 mg/l, respectively. The highest 24 h and 96 h non-lethal concentration (LC0) was 21.7 mg/l and 21.2 mg/l, respectively. The mobility inhibition test of the juvenile Daphnia magna revealed a 48 h EC50 of 42.7 mg/l.
Keywords: 96 h LC50, antiparasitic, bath treatment, water flea, zebrafish
Praziquantel (PZQ) is an anthelmintic drug developed during the 1970s to treat platyhelminth infections in humans and animals. Its mode of action is the impairment of the parasite neuromuscular system, inhibiting the attachment and affecting the permeability of their integument, leading to an osmotic and nutritional imbalance (Treves-Brown 2000). In aquaculture, PZQ can be administered by bath or orally. Bath treatments are effective against monogeneans (Schmahl and Mehlhorn 1985; Morales-Serna et al. 2018; Maciel and Affonso 2021), digeneans (Szekely and Molnar 1991; Zuskova et al. 2018), and cestodes (Mitchell 2004; Mitchell and Darwish 2009). Oral treatments via intubation or in the feed are effective against monogeneans (Kim and Cho 2000; Forwood et al. 2016), cestodes (Sudova et al. 2010), blood flukes (Shirakashi et al. 2012), and acanthocephalans (Zuskova et al. 2018).
The pharmacokinetics of PZQ in fish depends on the mode of administration (Bjorklund and Bylund 1987; Kim et al. 2001; Xie et al. 2015), the dose (Kim et al. 2003) and the size of the fish (Hirazawa et al. 2013) as well as the environmental conditions, such as the temperature (Bjorklund and Bylund 1987) or salinity (Xie et al. 2015), e.g., a single in feed administration of PZQ at 15 mg/kg body weight led to the PZQ concentration in the tissues peaking between 0.5 and 1.5 h following administration, where PZQ was undetectable after 24 h (Ishimaru et al. 2013). Ensuring chemical residue levels are below accepted thresholds is vital to ensuring consumer safety (Norbury et al. 2022).
Unlike in some non-EU states, there is no maximum residue limit for PZQ in fish for consumption in the EU, although it is normally used only in ornamental and non-food fish, with applications in other situations controlled by EU legislation within the cascade rules under Article 10 of Directive 2001/82/EC as amended by Directive 2004/28/EC for veterinarians (Zuskova et al. 2018). Although PZQ has its limitations compared with compounds, such as benzimidazole, mebendazole, and febantel, it is the most widely used chemical for controlling parasites in fish (Ogawa 2015).
The aim of this study was to determine the lethal concentration of PZQ to Danio rerio zebrafish, and, using the Daphnia magna water flea as model, to determine its effect on aquatic invertebrates that represent a crucial part of the freshwater ecosystem as feed for vertebrate organisms.
MATERIAL AND METHODS
Chemicals
The praziquantel (PZQ) powder was obtained from the Ecological Laboratories Inc. (Cape Coral, USA). The ethanol (96%) was purchased from Merck KGaA (Darmstadt, Germany).
Toxicity tests
Acute toxicity tests were carried out on two-month-old aquarium zebrafish, Danio rerio, with a length 27 ± 4 mm and weight 0.3 ± 0.1 g obtained from the breeding facility of the Faculty of Fisheries and Protection of Waters in Vodňany, Czech Republic.
The method followed the Organisation for Economic Co-operation and Development 203 Guide-lines for the Testing of Chemicals (Fish, Acute Toxicity Test) (OECD 1992) under a semi-static condition with a solution replacement after 24 h to ensure a stable PZQ concentration in test solutions. Prior to the test, the fish were acclimatised in a 200-l tank for a minimum of seven days at 20 ± 1 °C and with a 12 : 12 light/dark cycle and fed commercial fish pellets. Feeding was suspended 24 h prior to the trial.
The fish were divided into two control and six experimental groups of 10 fish in 20 l aquaria. Each group was run in triplicate (three 20 l glass aquaria with 10 fish, i.e., 30 fish per group in total). A stock solution of PZQ was prepared by adding the required concentration of PZQ dissolved in 96% ethanol (1 ml/l) due to the low solubility of PZQ in water. In a 96-h preliminary test, the mortality was 0% and 100% at 20 and 70 mg/l PZQ, respectively. The PZQ was dissolved in ethanol to concentrations equivalent to 20, 30, 40, 50, 60, and 70 mg/l. Two control groups were used: C1 in ethanol/dechlorinated tap water at the PZQ concentrations, and C2 exposed to dechlorinated tap water only. The water quality parameters were the total ammonia 0.03 mg/l; NO3– 4.6 mg/l; PO43– < 0.02 mg/l; chemical oxygen demand – CODMn 1.4 mg/l; acid neutralisation capacity – ANC4.5 1.15 mmol/l; Σ Ca2+ + Mg2+ 0.95 mmol/l; Cl– 11 mg/l. The water temperature, oxygen saturation and pH were measured daily and ranged from 20.0 °C to 21.5 °C, 85–94%, and 7.2–7.6, respectively. The PZQ concentrations were measured before and after each water replacement by ultrahigh–performance liquid chromatography (UHPLC) using the method of Zrncic et al. (2014), confirming the presence of PZQ at > 87% of the nominal concentrations.
The mobility inhibition testing was carried out on < 24 h old Daphnia magna, conducted according to the OECD 202 Guideline for the Testing of Chemicals (Daphnia sp. Acute Immobilisation Test) (OECD 2004) obtained from the breeding facility of the Faculty of Fisheries and Protection of Waters in Vodňany. Daphnia were exposed to praziquantel at 5, 10, 15, 20, 50, and 100 mg/l along with two controls (C1 exposed to ethanol at the PZQ dilutions and C2 to the dechlorinated tap water only) at 20 ± 2 °C and a 16 : 8 light : dark cycle. Each group consisted of 10 individuals and the test was undertaken in duplicate. The water quality parameters were the total ammonia 0.03 mg/l; NO3– 4.6 mg/l; PO43– < 0.02 mg/l; chemical oxygen demand – CODMn 1.4 mg/l; acid neutralisation capacity – ANC4.5 1.15 mmol/l; Σ Ca2+ + Mg2+ 0.95 mmol/l; Cl– 11 mg/l. The water temperature, oxygen saturation and pH were measured daily and ranged from 20.0 °C to 20.5 °C, 88–97%, and 7.2–7.4, respectively. After 48 h, the numbers of immobilised specimens were counted, and a probit analysis was used to calculate the 48 h EC50 concentration.
Ethics
All the procedures complied with the relevant legislative regulations of the Czech Republic (166/1996 and 246/1992). The testing of acute toxicity to fish was approved by the Ministry of Education, Youth, and Sports of the Czech Republic (Permission No. 3126/2021-3-MSMT). The study did not involve endangered or protected species.
Statistical analysis
The number of dead/non-motile specimens at the test concentrations was subjected to a probit analysis using the EKO-TOX v5.2 program to determine the LC50 and EC50 values of PZQ.
RESULTS AND DISCUSSION
The determination of the PZQ acute toxicity is important not only to establish safe levels in therapeutic applications, but also to address the potential contamination of the environment. Recommended concentrations of PZQ therapeutic baths range from 0.25 mg/l to 50 mg/l depending on the bath duration and parasite species (Bader et al. 2019). Bath treatments typically involve a concentration up to 10 mg/l of PZQ for an extended period of time. In contrast, a dip utilises tens of mg/l of PZQ for a shorter period. Both methods provide uniform treatment to each fish (Samuelsen and Lunestad 1996). In our study, the estimated 24 h and 96 h LC50 were 39.9 mg/l and 30.4 mg/l, respectively (Figure 1). The highest 24 h and 96 h non-lethal concentrations (LC0) were 21.7 mg/l and 21.2 mg/l, respectively. Although the values are within the therapeutic concentration range for baths, the exposure time of the observed LC50 was several times longer that of recommended dip treatment at the same PZQ concentrations. In our study, the zebrafish in the control groups and at 20 mg/l PZQ showed typical behaviour and no mortality throughout the test period. The fish from the other exposed groups showed respiratory stress and worsening uncoordinated movement after 1 h of exposure, indicating an incipient toxic effect of PZQ, at which point it is advisable to terminate the exposure. No differences were found among the controls. A number of toxicity tests have been performed to optimise the therapeutic concentrations for individual fish species and age categories under the relevant conditions. A PZQ 96 h LC50 of 28.6 mg/l was reported for juvenile barbels (Zuskova et al. 2018) and 29.22 mg/l for juvenile goldfish (Zhang et al. 2014), while a higher 96 h LC50 value of 53.52 mg/l was found for juvenile Clarias gariepinus (Nwani et al. 2014). Mitchell and Hobbs (2007) reported PZQ 24 h LC50 estimates for Ctenopharyngodon idella and Notemigonus crysoleucas juveniles of 55.1 and 63.4 mg/l, respectively. On the contrary, a lower 24 h LC50 value of 13.4 mg/l was found for the fry of Clarias gariepinus (Obiekezie and Okafor 1995). These indicate varying levels of toxicity depending on the species and age of the exposed fish, where younger individuals are more sensitive to praziquantel exposure (Thoney and Hargis 1991; Obiekezie and Okafor 1995). Another factor affecting the absorption, metabolism and, consequently, also the toxicity of praziquantel is the temperature.
Figure 1. Mean cumulative mortality of Danio rerio zebrafish at the tested concentrations in the acute toxicity tests.
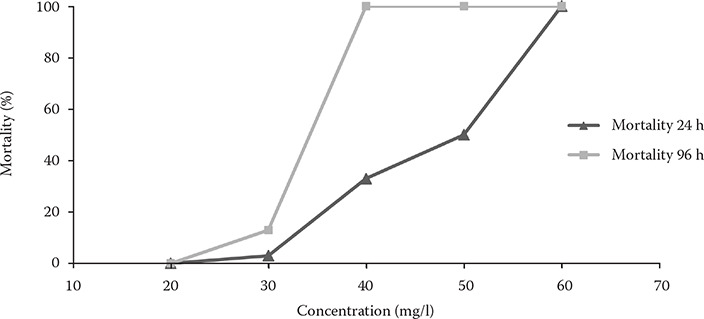
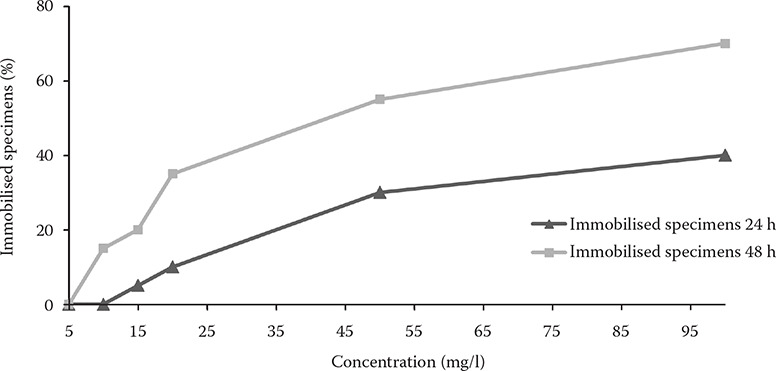
Figure 2. Mean immobilised specimens of Daphnia magna at the tested concentrations in the mobility inhibition tests.
Bjorklund and Bylund (1987) confirmed the more rapid absorption of PZQ from the gastrointestinal tract at 18 °C than at 12 °C, indicating the faster onset of effects in warmer water. Summarised, a range of factors can influence PZQ pharmacokinetics and toxicity in fish; this variability means the fate of PZQ should be contemplated in regard to each species under the relevant conditions (Norbury et al. 2022).
A limited number of studies have been performed to observe the effects of PZQ on arthropods. Hoai and Van (2014) state that 48 h exposure of PZQ concentrations exceeding 2.5 mg/l reduced the infection intensity of Lernaea sp. in common carp. It indicates an influence of PZQ on arthropod organisms already at low concentrations. The effect of PZQ on other non-target organisms, such as parasites of fish, was reviewed by Norbury et al. (2022). Praziquantel released into water for a bath or dip treatment or excreted by fish after an oral or a parenteral application could impact other resident organisms (Morley 2009). In our mobility inhibition test on Daphnia magna, the calculated 48 h EC50 was 42.7 mg/l. This value is greater than that what we found toxic to fish and, therefore, lower therapeutic concentrations or sufficiently diluted wastewater containing PZQ should not affect the viability of these planktonic organisms. On the other hand, there is a concern that PZQ present in small quantities in the environment may lead to resistance and indirectly complicate the treatment of human parasitic disease (Cupit and Cunningham 2015). Therefore, PZQ as a therapeutic tool should only be applied to fish in justified cases under the supervision of a veterinarian.
Acknowledgement
We thank to Lucidus Consultancy for English proofreading.
Funding Statement
Supported by the Ministry of Agriculture of the Czech Republic (Project No. QK21010113) and by the Ministry of Education, Youth and Sports of the Czech Republic, Project: Sustainable Production of Healthy Fish in Various Aquaculture Systems, PROFISH (No. CZ.02.1.01/0.0/0.0/16_019/0000869).
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Bader C, Starling DE, Jones DE, Brewer MT. Use of praziquantel to control platyhelminth parasites of fish. J Vet Pharmacol Ther. 2019 Mar;42(2):139-53. [DOI] [PubMed] [Google Scholar]
- Bjorklund H, Bylund G. Absorption, distribution and excretion of the anthelmintic praziquantel (Droncit) in rainbow trout (Salmo gairdneri R.). Parasitol Res. 1987;73(3):240-4. [DOI] [PubMed] [Google Scholar]
- Cupit PM, Cunningham C. What is the mechanism of action of praziquantel and how might resistance strike? Future Med Chem. 2015;7(6):701-5. [DOI] [PubMed] [Google Scholar]
- Forwood JM, Bubner EJ, Landos M, D’Antignana T, Deveney MR. Praziquantel treatment for yellowtail kingfish (Seriola lalandi): Dose and duration safety study. Fish Physiol Biochem. 2016 Feb;42(1):103-9. [DOI] [PubMed] [Google Scholar]
- Hirazawa N, Akiyama K, Umeda N. Differences in sensitivity to the anthelmintic praziquantel by the skin-parasitic monogeneans Benedenia seriolae and Neobenedenia girellae. Aquaculture. 2013 Aug;404-405:59-64. [Google Scholar]
- Hoai T, Van K. Efficacy of praziquantel against external parasites infecting freshwater fish. J Sci Dev. 2014;12(5):711-9. [Google Scholar]
- Ishimaru K, Mine R, Shirakashi S, Kaneko E, Kubono K, Okada T, Sawada Y, Ogawa K. Praziquantel treatment against Cardicola blood flukes: Determination of the minimal effective dose and pharmacokinetics in juvenile Pacific bluefin tuna. Aquaculture. 2013 Jul;402-403:24-7. [Google Scholar]
- Kim KH, Kim CS, Kim JW. Depletion of praziquantel in plasma and muscle tissue of cultured rockfish Sebastes schlegeli after oral and bath treatment. Dis Aquat Organ. 2001 Aug 2;45(3):203-7. [DOI] [PubMed] [Google Scholar]
- Kim CS, Cho JB, Ahn KJ, Lee JI, Kim KH. Depletion of praziquantel in muscle tissue and skin of cultured rockfish (Sebastes schlegeli) under the commercial culture conditions. Aquaculture. 2003 Apr;219(1-4):1-7. [Google Scholar]
- Kim KH, Cho JB. Treatment of Microcotyle sebastis (Monogenea: Polyopisthocotylea) infestation with praziquantel in an experimental cage simulating commercial rockfish Sebastes schlegeli culture conditions. Dis Aquat Organ. 2000 Apr 20;40(3):229-31. [DOI] [PubMed] [Google Scholar]
- Maciel PO, Affonso EG. Praziquantel against monogeneans of tambaqui (Colossoma macropomum). Aquacult Int. 2021 Oct;29(5):2369-86. [Google Scholar]
- Mitchell A, Darwish A. Efficacy of 6-, 12-, and 24-h praziquantel bath treatments against Asian tapeworms Bothriocephalus acheilognathi in grass carp. N Am J Aquac. 2009 Jan;71(1):30-4. [Google Scholar]
- Mitchell AJ, Hobbs MS. The acute toxicity of praziquantel to grass carp and golden shiners. N Am J Aquac. 2007 Jan;69(3):203-6. [Google Scholar]
- Mitchell AJ. Effectiveness of praziquantel bath treatments against Bothriocephalus acheilognathi in grass carp. J Aquat Anim Health. 2004 Jan;16(3):130-6. [Google Scholar]
- Morales-Serna FN, Chapa-Lopez M, Martinez-Brown JM, Ibarra-Castro L, Medina-Guerrero RM, Fajer-Avila EJ. Efficacy of praziquantel and a combination anthelmintic (Adecto®) in bath treatments against Tagia ecuadori and Neobenedenia melleni (Monogenea), parasites of bullseye puffer fish. Aquaculture. 2018 Jun;492:361-8. [Google Scholar]
- Morley NJ. Environmental risk and toxicology of human and veterinary waste pharmaceutical exposure to wild aquatic host-parasite relationships. Environ Toxicol Pharmacol. 2009 Mar;27(2):161-75. [DOI] [PubMed] [Google Scholar]
- Norbury LJ, Shirakashi S, Power C, Nowak BF, Bott NJ. Praziquantel use in aquaculture – Current status and emerging issues. Int J Parasitol Drugs Drug Resist. 2022 Apr;18:87-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwani CD, Nnaji MC, Oluah SN, Echi PC, Nwamba HO, Ikwuagwu OE, Ajima MN. Mutagenic and physiological responses in the juveniles of African catfish, Clarias gariepinus (Burchell 1822) following short term exposure to praziquantel. Tissue Cell. 2014 Aug;46(4):264-73. [DOI] [PubMed] [Google Scholar]
- Obiekezie A, Okafor N. Toxicity of four commonly used chemotherapeutic compounds to fry of the African catfish, Clarias gariepinus (Burchell). Aquacult Res. 1995 Jun;26(6):441-5. [Google Scholar]
- OECD – Organisation for Economic Cooperation and Development. Guideline for testing of chemicals 203. Fish, acute toxicity test. Paris: OECD; 1992. 9 p. [Google Scholar]
- OECD – Organisation for Economic Cooperation and Development. Guideline for testing of chemicals 202. Daphnia sp. acute immobilisation test. Paris: OECD; 2004. 12 p. [Google Scholar]
- Ogawa K. Diseases of cultured marine fishes caused by Platyhelminthes (Monogenea, Digenea, Cestoda). Parasitology. 2015 Jan;142(1):178-95. [DOI] [PubMed] [Google Scholar]
- Samuelsen OB, Lunestad BT. Bath treatment, an alternative method for the administration of the quinolones flumequine and oxolinic acid to halibut Hippoglossus hippoglossus, and in vitro antibacterial activity of the drugs against some Vibrio sp. Dis Aquat Org. 1996 Oct;27(1):13-8. [Google Scholar]
- Schmahl G, Mehlhorn H. Treatment of fish parasites. 1. Praziquantel effective against Monogenea (Dactylogyrus vastator, Dactylogyrus extensus, Diplozoon paradoxum). Z Parasitenkd. 1985;71(6):727-37. [DOI] [PubMed] [Google Scholar]
- Shirakashi S, Andrews M, Kishimoto Y, Ishimary K, Okada T, Swasa Y, Ogawa K. Oral treatment of praziquantel as an effective control measure against blood fluke infection in Pacific bluefin tuna (Thunnus orientalis). Aquaculture. 2012 Jan;326-329:15-9. [Google Scholar]
- Sudova E, Piackova V, Velisek J, Pijacek M, Svobodova Z. Efficacy testing of orally administered praziquantel to common carp naturally infected by caryophyllidean tapeworms (Platyhelminthes: Eucestoda). Acta Vet Brno. 2010;79(9):73-8. [Google Scholar]
- Szekely C, Molnar K. Praziquantel (Droncit) is effective against diplostomosis of grass carp Ctenopharyngodon idella and silver carp Hypophthalmichthys molitrix. Dis Aquat Org. 1991 Aug;11(2):147-50. [Google Scholar]
- Thoney DA, Hargis Jr WJ. Monogenea (Platyhelminthes) as hazards for fish in confinement. Annu Rev Fish Dis. 1991;1:133-53. [Google Scholar]
- Treves-Brown KM. Anthelmintics. In: Poxton MG, editor. Applied fish pharmacology. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2000. p. 200-5. [Google Scholar]
- Xie X, Zhao Y, Yang X, Hu K. Comparison of praziquantel pharmacokinetics and tissue distribution in fresh and brackish water cultured grass carp (Ctenopharyngodon idellus) after oral administration of single bolus. BMC Vet Res. 2015 Apr 1;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XP, Li WX, Ai TS, Zou H, Wu SG, Wang GT. The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea). Aquaculture. 2014 Jan;420-421:302-7. [Google Scholar]
- Zrncic M, Gros M, Babic S, Kastelan-Macan M, Barcelo D, Petrovic M. Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. Chemosphere. 2014 Mar;99:224-32. [DOI] [PubMed] [Google Scholar]
- Zuskova E, Piackova V, Machova J, Chupani L, Steinbach C, Stara A, Velisek J. Efficacy and toxicity of praziquantel in helminth-infected barbel (Barbus barbus L.). J Fish Dis. 2018 Apr;41(4):643-9. [DOI] [PubMed] [Google Scholar]


