Abstract
IMPORTANCE
Recent clinical and imaging studies underscore that major adverse cardiac events (MACE) outcomes are associated not solely with severe coronary obstructions (ischemia hypothesis or stenosis hypothesis), but with the plaque burden along the entire coronary tree. New research clarifies the pathobiologic mechanisms responsible for plaque development/progression/destabilization leading to MACE (plaque hypothesis), but the translation of these insights to clinical management strategies has lagged. This narrative review elaborates the plaque hypothesis and explicates the current understanding of underlying pathobiologic mechanisms, the provocative destabilizing influences, the diagnostic and therapeutic implications, and their actionable clinical management approaches to optimize the management of patients with chronic coronary disease.
OBSERVATIONS
Clinical trials of management strategies for patients with chronic coronary artery disease demonstrate that while MACE rate increases progressively with the anatomic extent of coronary disease, revascularization of the ischemia-producing obstruction does not forestall MACE. Most severely obstructive coronary lesions often remain quiescent and seldom destabilize to cause a MACE. Coronary lesions that later provoke acute myocardial infarction often do not narrow the lumen critically. Invasive and noninvasive imaging can identify the plaque anatomic characteristics (plaque burden, plaque topography, lipid content) and local hemodynamic/biomechanical characteristics (endothelial shear stress, plaque structural stress, axial plaque stress) that can indicate the propensity of individual plaques to provoke a MACE.
CONCLUSIONS AND RELEVANCE
The pathobiologic construct concerning the culprit region of a plaque most likely to cause a MACE (plaque hypothesis), which incorporates multiple convergent plaque features, informs the evolution of a new management strategy capable of identifying the high-risk portion of plaque wherever it is located along the course of the coronary artery. Ongoing investigations of high-risk plaque features, coupled with technical advances to enable prognostic characterization in real time and at the point of care, will soon enable evaluation of the entire length of the atheromatous coronary artery and broaden the target(s) of our therapeutic intervention to include all regions of the plaque (both flow limiting and nonflow limiting).
Accumulating evidence reinforces the concept that many major adverse cardiac events (MACE) in patients with chronic ischemic heart disease are related less to the flow-limiting coronary artery luminal lesions, but rather to the overall atherosclerotic burden, be it obstructive or nonobstructive (what we term the plaque hypothesis).1–5 Recent work has shed important new light into the basic pathobiologic mechanisms that operate along the length of individual nonobstructive portions of plaque responsible for these MACE outcomes. Yet, the translation of these pathobiologic insights into clinical diagnostic and management strategies has lagged. This review explores new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support the plaque hypothesis. It provides an update on pathobiologic mechanisms, the provocative destabilizing triggers, and the diagnostic and therapeutic implications that inform actionable clinical management approaches to optimize the management of patients with chronic ischemic heart disease.
The Ischemia Hypothesis or Stenosis Hypothesis of the Natural History and Management of Coronary Artery Disease
Classic pathogenetic concepts of coronary artery disease (CAD) complications emerged from observations that inducible myocardial ischemia from a severe coronary luminal obstruction caused angina. A reasonable extrapolation from these findings posited that obstructive lesions also provoked MACE (what we term the ischemia hypothesis or stenosis hypothesis). Accordingly, risk stratification aimed to identify those patients with the most ischemic myocardium at risk since they were considered most likely to benefit from revascularization strategies to reduce ischemia and thereby prevent MACE.
However, recent natural history follow-up studies of individual coronary plaques using intravascular ultrasonography (IVUS) or optical coherence tomography (OCT) invasive imaging consistently demonstrated that the majority of severely obstructive coronary lesions, even those with putative high-risk pathobiologic anatomic features and causing severe ischemia, often remain quiescent and do not destabilize to cause a MACE, even over several years of follow-up.6–11 Large-scale noninvasive imaging investigations, using coronary computed tomography angiography (CCTA), which can evaluate the full length of the coronary artery and coronary plaques, also underscore that coronary arterial lesions that later provoke acute myocardial infarction (MI) often do not narrow the lumen critically.12–14Most importantly, such studies indicate that the risk of CAD events is associated more with the extent of the plaque burden throughout the coronary tree than the severity of individual luminal obstructions.2,3,5These more recent studies affirmed the inferences from earlier studies that used angiography, a modality that images the lumen rather than the lesions themselves.15–18
Pharmacologic management of obstructive CAD now includes more biologically directed therapeutic interventions and disease-modifying noninvasive therapies than in the past. In addition to pharmacologic measures directed mainly at improving the balance between oxygen supply and demand distal to flow-limiting stenoses, we currently possess agents that alter plaques themselves or the risk factors or thrombotic milieu (eg, statins, ezetimibe, PCSK9 inhibitors, icosapent ethyl, late-generation antiplatelet agents, and now even agents developed to reduce glycemia such as sodium-glucose cotransporter-2 inhibitors and glucagonlike peptide-1 receptor agonists) or the inflammatory milieu (eg, interleukin 1β inhibitors, colchicine). These newer therapies appear to reduce MACE not so much by luminal expansion, but by biological modification of atherosclerotic involvement along the full-length of coronary arteries, not just the obstructive lesions.5
The results of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial19 and later Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI2D) trial20 most clearly challenged the ischemia hypothesis by using a management strategy of intensive medical therapy plus percutaneous coronary intervention (PCI) and/or coronary artery bypass grafting (CABG) (BARI2D trial20) vs intensive medical therapy alone. These studies demonstrated equivalent cumulative incidence of events by the disease-modifying medical therapies or by invasive revascularization. PCI did not reduce death or MI compared with medical therapy alone over the period of observation, even in patients with extensive 3-vessel CAD, or proximal left anterior descending artery stenosis of 90% or more. Limitations to these studies included the very low rate of drug-eluting stent use, the absence of a predefined threshold for the extent and severity of baseline ischemia at entry, and the determination of eligibility only after coronary angiography.
A study that guided revascularization based on fractional flow reserve (FFR) (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 [FAME 2] trial8) reinforced for many the concept that a flow-limiting obstruction caused the composite primary end point of death, MI, or urgent revascularization. Yet, the sole driver of the favorable composite outcome with FFR-guided PCI was the unblinded component of urgent revascularization, while the objective outcomes of death or MI did not improve.
The more recent International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial21 aimed to avoid the limitations of the prior large-scale investigations of the ischemia hypothesis. As in most other trials, the rate of the primary outcome (composite of cardiovascular death, MI or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest) progressively and significantly increased as the anatomic extent and severity of angiographically defined atherosclerotic coronary obstructions increased from single-(8.2%) to double-(11.9%) and to triple-vessel (23.9%) disease over a median 3.2-year follow-up. In contrast, adverse outcomes were not associated with the extent and severity of myocardial ischemia.21,22 Moreover, and consistent with the evolving understanding of the culprit lesion(s) responsible for MACE, mechanical revascularization of the flow-limiting obstruction(s) with either PCI or CABG did not reduce those MACE outcomes.21 In a subgroup analysis, the patients who underwent invasive treatment in the ISCHEMIA trial manifested fewer spontaneous MIs than the patients who underwent conservative treatment,23 but the relationship between the revascularization procedure and the MI reduction is unclear since the reduction in spontaneous MIs was observed even if the patient had no PCI performed or had no obstructive CAD. Invasively managed patients also may have had fewer spontaneous MIs due to the ongoing use of dual antiplatelet therapy or to ascertainment bias.23
The recent prospective, double-blind COMBINE OCT-FFR11 natural history study of FFR-guided PCI and identification of FFR-negative thin cap fibroatheroma (TCFA) character lesions in 550 patients with diabetes with either chronic CAD or an acute coronary syndrome (ACS) demonstrated convincingly that many culprit plaques responsible for future MACE had TCFA characteristics but unimpaired FFR. The patients who had evidence of such TCFA (25% of the cohort) had a 5-fold higher rate of MACE over an 18-month follow-up (>80% of future MACE) compared with patients without a TCFA character lesion, despite the absence of ischemia. These MACE outcomes were mainly spontaneous MIs and also target lesion revascularization related to worsening angina due to plaque progression and minimal lumen area (MLA) reduction. The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) 3 trial,24 which randomized patients with 3-vessel CAD to revascularization with FFR-guided PCI or to CABG, also demonstrated that FFR-guided PCI was not noninferior to CABG at 1-year follow-up. Since all lesions with abnormal FFR in the FFR-guided PCI group underwent PCI, the results suggested that the lesions responsible for MACE in that group during follow-up were lesions that were not flow limiting at baseline, and, per protocol, did not undergo PCI. In contrast, CABG bypassed both the flow-limiting and many nonflow-limiting lesions, and these patients experienced a significantly lower incidence of the composite primary end point.
The Plaque Hypothesis: Fundamental Concepts Linking the Pathobiology of Coronary Atherosclerosis to MACE
The ensemble of recent large-scale clinical trial results and earlier angiographic findings affirm that severely obstructive focal plaque regions traditionally targeted for revascularization do not necessarily cause the abrupt plaque complications that generally provoke MACE. Hence, epicardial coronary artery stenosis relief by PCI or CABG did not improve prognosis. These more recent studies reinforce the concept that while PCI may ameliorate regional ischemia, and therefore reduce symptoms of angina,25 the most consequential complications of atherosclerosis, ie, nonfatal MI and cardiac death, often originate from plaques or portions of plaque that do not produce the most severe obstructions.
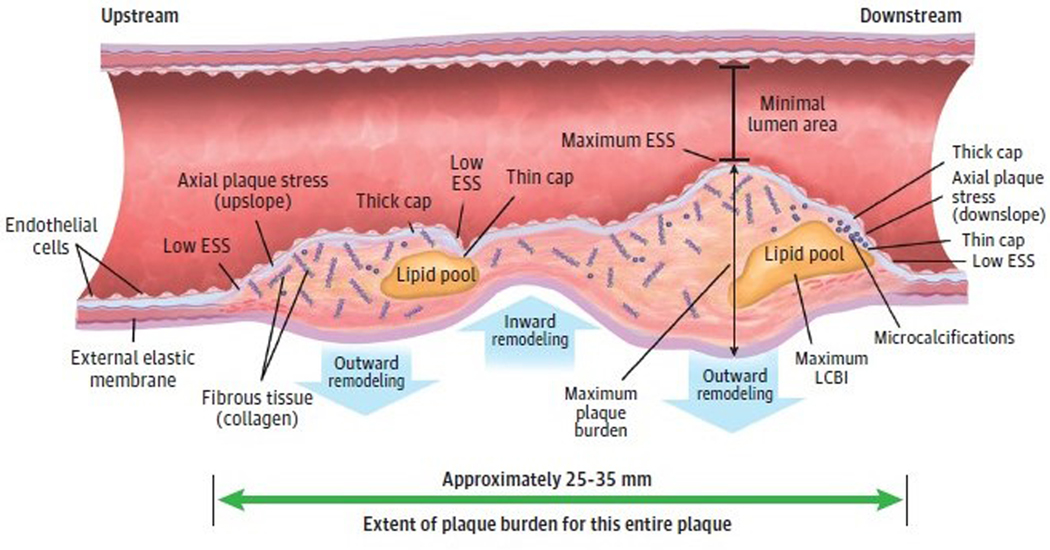
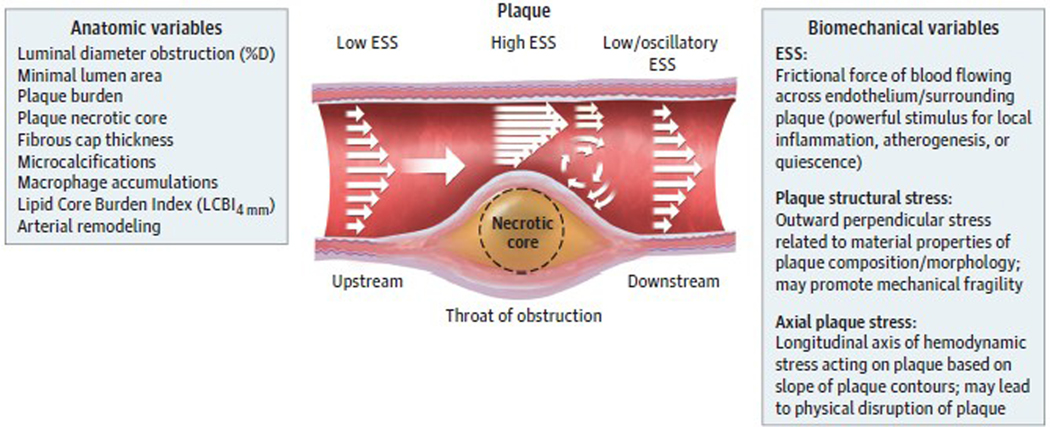
These observations provide further support for the view that obstructive plaques serve principally as a marker for atherosclerotic burden, including complex and heterogeneous plaques that may be nonobstructive or obstructive or that may contain regions of both flow-limiting obstruction and nonflow-limiting disease (Figure 1). Despite this accumulating evidence, our prevailing diagnostic and therapeutic management strategies and guidelines have lagged and still largely reflect the ischemia hypothesis that posits that alleviation of stenosis as the key to effective treatment. Accordingly, we need to broaden our management approach for chronic CAD to focus on identifying and altering pathobiological aspects of plaques along the course of atheromatous arteries, not merely those lesion segments that provoke ischemia.
Figure 1. Coronary Atherosclerotic Plaque as a Complex, Lengthy, and Heterogeneous Pathobiologic Lesion.
Many different constituents, morphologies, and resultant pathobiologic and biomechanical environments localize spatially distant from the minimal lumen area. ESS indicates endothelial shear stress; LCBI, lipid core burden index.
Vascular Pathobiology of Coronary Atherosclerosis and the Role of Biomechanics in Plaque Destabilization
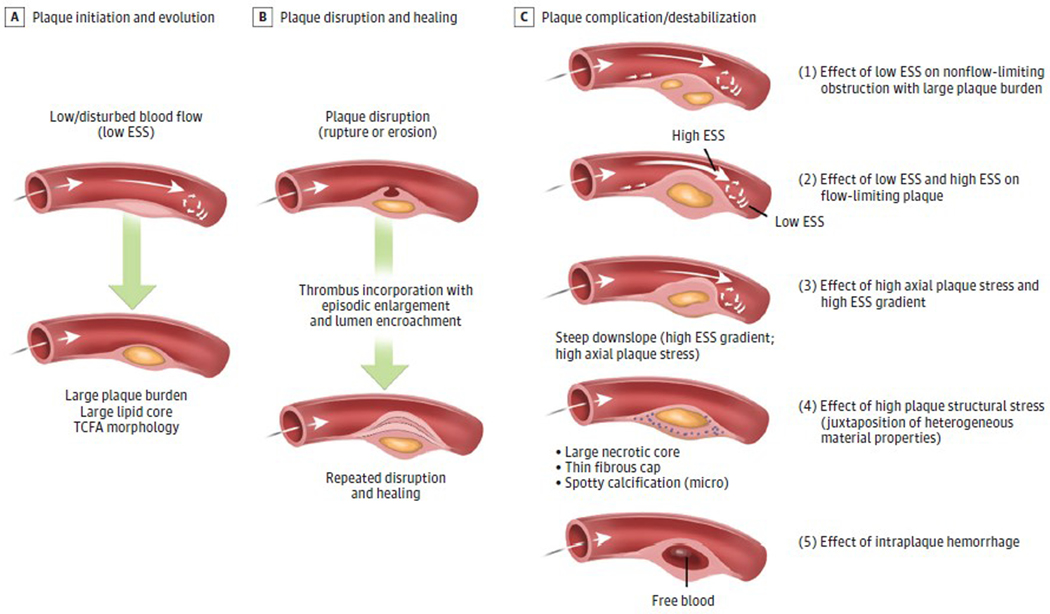
Our evolving understanding of the natural history of CAD derives from early observations of the vascular biology and clinical manifestations of atherosclerosis. More than 30 years ago, Glagov et al,26 Clarkson et al,27 and others emphasized the expansive outward remodeling that accommodates an enlarging plaque during much of a lesion’s progression. Such compensatory enlargement of arteries can prevent luminal encroachment by even very large lesions and preserve myocardial blood flow distal to that plaque. Such culprit lesions are not necessarily small but do not cause critical stenosis due to expansive remodeling that accommodates plaque growth abluminally, preserving the luminal caliber. Indeed, positive remodeling determined by CCTA characterizes plaques with elevated risk of provoking an ACS.28 Plaque disruption in patients with acute MI and nonobstructive CAD, defined as coronary luminal obstruction less than 50%,29 also supports the concept that culprit plaques causing ACS need not obstruct the lumen. Recent invasive and noninvasive studies investigating the size, shape, constituents, and hemodynamic environment surrounding coronary plaque provide essential new pathobiologic understanding concerning the detailed plaque regions that are prone to destabilize and likely give rise to future adverse clinical events (Figure 2).31
Figure 2. Pathobiologic Mechanisms of Plaque Progression and Disruption.
Shown are the coronary plaque and arterial features that may lead to plaque progression and destabilization culminating in major adverse cardiac events in a variety of plaque scenarios involving a constellation of pathobiologic and biomechanical mechanisms, which may operate alone or in concert with other pathologic mechanisms. A, Plaque initiation and development begin in zones of low and disturbed blood flow (ie, low endothelial shear stress [ESS]), regions that typically occur on the inner aspect of an artery curve, outer waists of a bifurcation, and upstream and downstream from a luminal obstruction. Local low ESS leads endothelial cells to switch from expressing a palette of atheroprotective properties to adopt proinflammatory, pro-atherogenic, and prothrombotic functions. Ongoing exposure to low ESS leads to progressive plaque burden, lipid accumulation, and thin cap fibroatheroma (TCFA) formation. B, Plaques can progress in a stepwise manner to destabilization (rupture, superficial erosion, or calcium nodule eruption, events that can provoke thrombosis), followed by plaque healing.30 Repeated destabilization and the healing response to disruption including thrombus resorption can lead to progressive plaque fibrosis, constrictive remodeling, and encroachment into the lumen. C, Prominent pathobiologic mechanisms contribute to plaque destabilization and complications. (1) Regions along the course of a plaque may encounter ongoing pro-atherogenic low ESS (Figure 1) and continue to develop local progressive inflammation, lipid accumulation, and elaboration of matrix-degrading metalloproteinases that promote fragility and instability of the fibrous cap and internal plaque structures, thereby fostering plaque rupture. These events may occur in a nonobstructive plaque or in plaque portions upstream or downstream from a luminal obstruction. (2) Portions of the plaque that encroach into the lumen create local high ESS at the throat of the obstruction that may cause endothelial cell elaboration of matrix-degrading metalloproteinases, endothelial death or desquamation, and platelet activation, rendering plaques more prone to provoke thrombosis. Plaque regions immediately adjacent to the high ESS typically also exhibit sites of low and oscillatory ESS, with its attendant pro-atherogenic and proinflammatory consequences31 as described in scenario 1. (3) High ESS gradients, which represent abrupt large differences in the magnitude of ESS in immediately adjacent endothelial cells, or steep plaque upslope/downslope, with or without associated high ESS, will increase axial plaque stress and promote plaque disruption. This adverse biomechanical stress operates independently of stenosis severity, drop in perfusion pressure, or local ESS. (4) The composition and spatial proximity of internal plaque constituents of different material properties can create inhomogeneities that affect cellular function and modify the structural integrity of the plaque and foster disruption (plaque structural stress or tensile stress).32 Computation of plaque structural stress requires accurate depiction of both atherosclerotic plaque composition and architecture. (5) Intraplaque hemorrhage may develop either as a result of microruptures of the plaque cap or leaking from immature and leaky vasa vasorum within an enlarging plaque, leading to an abrupt conformational change due to the atherogenic properties of lipids from degraded red blood cell membranes and released free hemoglobin and heme.33–35 Iron derived from heme can drive local oxidative stress, further promoting lesion complication.35
The first efforts to identify high-risk plaques at risk to trigger an ACS (so-called vulnerable plaques) emerged from morphological characterization alone (large plaque burden, TCFA morphology, narrow MLA, and lipid accumulation).6,10,36–38 While these anatomic plaque characteristics were associated with increased MACE outcomes, the positive predictive value was less than 20%. This recognition spurred the investigation of the biomechanical stresses that may influence whether an individual plaque will progress, destabilize, or remain quiescent.32 Earlier studies demonstrated that low endothelial shear stress (ESS), the frictional force of blood acting on the endothelial cells of the arterial wall, disrupted the homeostatic atheroprotective properties of the normal endothelium. Low ESS elicits proinflammatory, pro-atherogenic, and prothrombotic properties of the intimal lining and impairs basal vasodilatory and other atheroprotective endothelial functions.31,39,40 Serial invasive studies confirmed that low local ESS tracked with plaque initiation and progression9,41–43 and that local low- or high-ESS environments predicted future MACE when added to plaque anatomic assessment.9,44,45
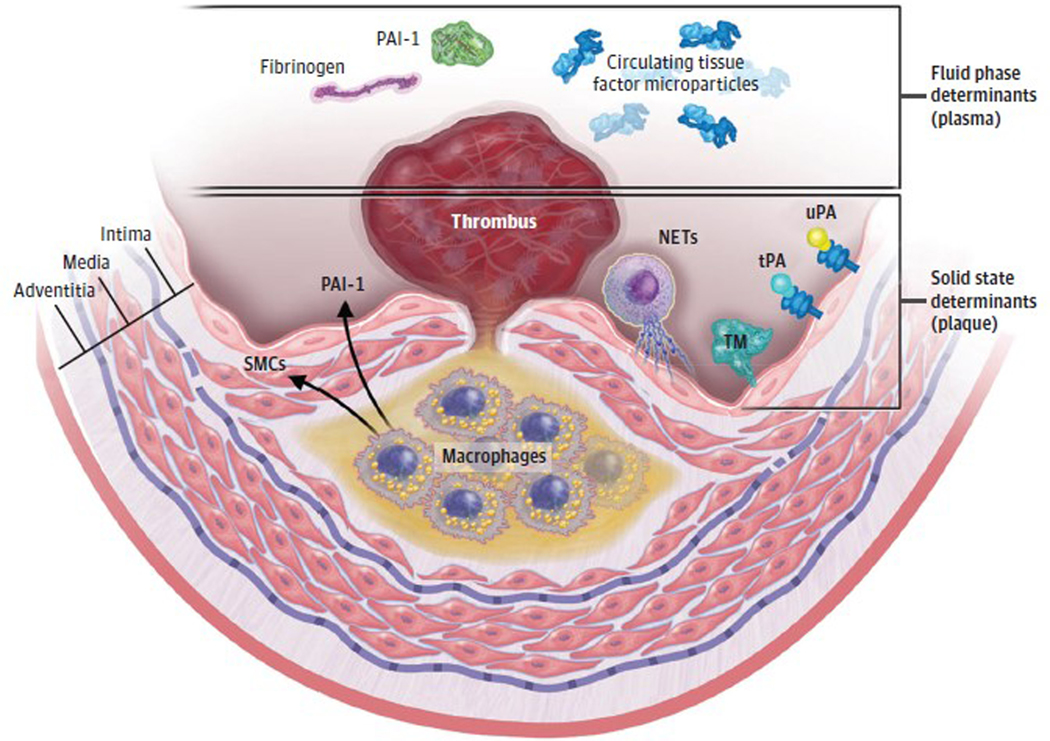
These and other current data obtained with intravascular or CCTA imaging indicate a much more heterogenous and dynamic nature of plaque morphology and behavior than traditionally conceived, and the appreciation that plaque destabilization and MACE may require a perfect storm of a constellation of a number of highrisk plaque features (the solid state), as well as an unfavorable thrombotic/fibrinolytic balance in blood (the fluid phase) (Figure 2 and Figure 3).46–49
Figure 3. A 2-State Concept of Atherothrombosis.
The classic high-risk atheroma has a thin fibrous cap overlying a large lipid core that contains tissue factor–bearing macrophages. When the fibrous cap fractures, coagulation proteins in the fluid phase of blood gain access to tissue factor–associated macrophages and tissue factor–bearing microparticles derived from apoptotic cells in the solid state of the plaque, these events trigger thrombus formation on the ruptured plaque. The clinical consequences depend on the amount of tissue factor and apoptosis in the plaque’s core and on the levels of fibrinogen and plasminogen activator inhibitor 1 (PAI-1) in the fluid phase of blood. The interaction of the fluid phase with the solid state determines whether a given plaque disruption provokes a partial or transient coronary artery occlusion (that can be clinically silent or causes an episode of unstable angina) or a persistent and occlusive thrombus that can precipitate an acute myocardial infraction. Neutrophil extracellular traps (NETs) can localize at the interface of the solid state of the intima with the fluid phase of blood. Their externalized strands of extruded nuclear DNA are decorated with mediators including tissue factor and can propagate and amplify local inflammation and thrombosis around this critical interface. SMC indicates smooth muscle cells; tPA, tissue plasminogen activator; TM, thrombomodulin; uPA, urokinase-type plasminogen activator.
For example, local areas of proinflammatory low ESS may lead to destabilization of a portion of the plaque in the absence of flow limitation due to local elaboration of interstitial collagenases and elastolytic proteases that degrade internal plaque structures.42,44,50,51 Plaque topography, especially the upslope and downslope that surround a luminal obstruction (axial plaque stress), may substantially impact the proclivity, location, and nature of focal plaque disruption, which may also explain why even nonobstructive plaques may destabilize if their topographical slope is adverse.52,53 Recent OCT studies similarly demonstrate that focal areas of high ESS and, in particular, high ESS gradient (the difference in ESS values of immediately adjacent endothelial areas), which also correlates with plaque slope, contribute to the plaque destabilization process of erosion or rupture.53–55
The location of plaque constituents and their material properties often vary markedly along the course of an individual plaque, leading to very heterogeneous patterns of plaque structural stress, which can influence subsequent plaque destabilization and the occurrence of MACE.56 Invasive or noninvasive imaging can identify these constituents, which may include necrotic core, fibrofatty tissue, fibrous tissue, and calcium. Plaques that heal following disruption may manifest plates of calcification, which provide mechanical stability to the plaque,30 while spotty calcification, which may represent an earlier form of calcification development, is associated with plaque instability.57
Intraplaque hemorrhage may result from leaky vasa vasorum, or from microruptures of a thin plaque cap, regardless of plaque size or lumen encroachment. The presence of free blood within the plaque may lead to a structural change due to the atherogenic properties of lipids from degraded red blood cell membranes and released free hemoglobin and heme.33–35 Marked worsening of angina, a frequent component of MACE,6,9,11 may also result from this plaque change in shape without a thrombotic component. Ferrous iron derived from heme may drive local oxidative stress regionally within plaques as well via the Fenton reaction.35,58
These various pathobiologic features may occur in a variety of locations along the course of the plaque, regardless of the magnitude of plaque luminal encroachment, and thus a therapeutic mechanical intervention such as PCI targeted to the ischemia-producing stenotic segment alone would leave untreated adjacent proinflammatory and prothrombotic plaque regions upstream or downstream from the site of greatest stenosis. Indeed, a 2017 IVUS study observed that plaque rupture occurred at the site of the MLA in only 16% of culprit lesions, while the site of plaque rupture localized either substantially upstream or downstream from the MLA in more than 80% of cases.52
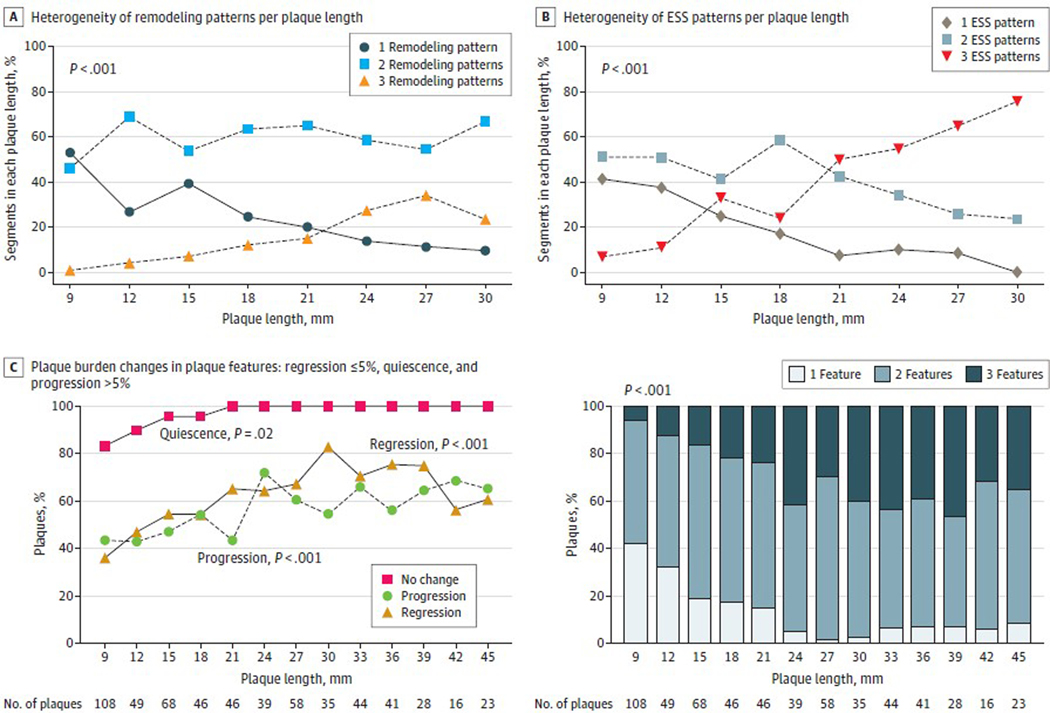
The PREDICTION study confirmed the highly heterogeneous nature of evolution of focal plaque anatomic features. This prospective, invasive, serial imaging study of patients after having ACS investigated the effect of baseline ESS patterns of individual plaques on subsequent characteristics of 3-mm plaque subsegments within that plaque 6 to 10 months later. The baseline mean (SD) plaque length in 661 plaques from 302 patients was 26 (14) mm, and plaques of greater length had significantly increased numbers of distinct regions with different arterial remodeling and focal shear stress patterns within each plaque, which, in turn, led to highly varied focal 3-mm areas of plaque progression, regression, and quiescence at follow-up (Figure 4).59,60 Serial invasive studies of plaque characteristics highlight that lesions typically change substantially over time as plaques heal after an episode of destabilization30 or as inflammatory and vascular remodeling characteristics evolve reflecting changing local vascular conditions.61,62 In contrast to the limited benefits of PCI to prevent MACE, CABG surgery bypasses more extensive areas of both flow-limiting and nonflow-limiting arterial plaques than PCI and thus may more likely reduce the risk of subsequent spontaneous MI.20,24,63,64
Figure 4. Heterogeneity of Local Arterial Remodeling and Endothelial Shear Stress (ESS) Within Plaques and Resultant Changes in Plaque Burden.
Local patterns of arterial remodeling and ESS in 3-mm segments within individual plaques are heterogeneous (A and B) and lead to heterogeneous natural history changes of local 3-mm plaque burden along the course of the individual plaque (C) over 6 to 10 months’ follow-up. Vascular and plaque heterogeneity becomes more complex as plaques become longer. Modified from Antoniadis et al59 and Wentzel et al60 with permission.
Focal vs Systemic Therapeutic Approaches to Treating Culprit Plaques
These considerations argue for diagnostic and therapeutic strategies that focus on the entire length of an atheromatous coronary artery to reduce cardiac events. Systemic vasculoprotective strategies of pharmacologic and lifestyle interventions can reduce inflammation and lipid accumulation throughout the length of the coronary artery, but we must acknowledge that despite intense adherence to systemic vasculoprotective interventions, a substantial number of adverse events nevertheless occur.11 For example, despite the dramatic lipid-lowering potential of PCSK9 inhibitors to reduce low-density lipoprotein cholesterol to even below 10 mg/dL (to convert to millimoles per liter, multiply by 0.0259) and direct anti-inflammatory strategies with interleukin 1β inhibition or colchicine, which significantly reduced MACE by 15% to 25% compared with standard care,65–67 75% to 85% of MACE still occurred during the follow-up period in patients who underwent more intensive treatment. Addressing this residual risk despite systemic pharmacologic therapy remains a major clinical challenge today.
In patients with ACS, because the culprit plaque has already destabilized, early mechanical revascularization of the culprits of ST-segment elevation myocardial infarction and many non–ST-segment elevation acute coronary syndrome do confer clinical benefit in these acute settings.
Evolving Diagnostic Strategies and Methods to Improve Characterization of High-Risk Plaques
Understanding the propensity of distinct regions within individual coronary plaques to cause MACE will require assessment and incorporation of multiple plaque features, including anatomic, biochemical, and biomechanical characteristics that can contribute to thrombotic complications (Figure 5).9,14,44,45,56 Characterization of individual plaques has generally used invasive assessment with OCT or IVUS imaging, but the ability of noninvasive imaging, particularly CCTA, to characterize plaques and their hemodynamic features, has evolved very rapidly despite providing less spatial resolution than the invasive intravascular modalities.68–70 High-risk plaque features based on CCTA, such as low-attenuation plaque, positive remodeling, spotty calcification, and napkin-ring sign, especially when combined with adverse biomechanical characteristics (ESS, FFR, axial plaque stress), show great promise to predict which patients and plaques may produce future MACE.14
Figure 5. Multimodality Variables to Predict Plaque Development, Progression, Destabilization, or Quiescence.
Anatomic and biomechanical pathobiologic features can be routinely characterized by invasive coronary imaging (optical coherence tomography, intravascular ultrasonography, and near-infrared spectroscopy) and noninvasive imaging (computed tomography angiography). These variables report on characteristics that foster plaque formation, progression or quiescence. Modified from Stone47 with permission. ESS indicates endothelial shear stress; LCBI, lipid core burden index.
A major impediment to adoption of existing risk-stratification methods that interrogate the entire length of individual regions of atheroma is the current requirement for offline analyses of plaque anatomic/hemodynamic/biomechanical characteristics that are time consuming and require substantial technical and computational resources. Nevertheless, intense efforts underway to enhance imaging and postprocessing systems and the application of artificial intelligence and machine learning should eventually permit more rapid and detailed assessment of these high-risk characteristics at the point of care within a very few minutes of image acquisition. Such advances would enable real-time assessment and subsequent deployment and monitoring of highly selective appropriate therapeutic interventions, such as PCI or local intracoronary balloon drug delivery, regardless of the location of a high-risk region along the course of the atheromatous artery. Such pathobiologic diagnostic and management considerations pertain largely to patients with chronic CAD.
Compelling Need for a Systematic and Fundamental Shift in Our Management Approach for Chronic CAD
Our current approach of identifying and treating mainly flow-limiting epicardial coronary obstructions in chronic CAD fails to prevent many MACE. Results from recent strategy clinical trials, such as ISCHEMIA,21 FAME 3,24 and COMBINE OCT-FFR,11 underscore that strategies to identify the severity of an epicardial coronary obstruction or to quantify the magnitude and extent of ischemia provide little value. Patients who fit the entry criteria of the ISCHEMIA trial21 who have little or no angina and an acceptable quality of life, who likely comprise a majority of patients with chronic CAD (approximately 80% of patients in the ISCHEMIA trial21), are appropriately managed with an intensive medical therapy approach rather than an initial invasive diagnostic or revascularization approach.71 The invasive strategy offers evidence-based value for those patients with frequent or angina that limits quality of life despite intensive medical therapy.21,25,71
Indeed, current data compel us to adopt a broader view (eBoxes1 and 2 in the Supplement). The continued refinement of the invasive and noninvasive imaging and computational methods will enable rapid examination of the full length of the coronary artery wall and identify those plaque features that constitute the highest risk of destabilization and MACE. CCTA currently provides an initial, noninvasive diagnostic assessment of the extent of plaque burden, and the nature and localization of certain high-risk plaque features, including the local coronary hemodynamic and plaque biomechanical environment. Rapidly evolving methods of computational fluid dynamics will enable this risk assessment to be completed and reported in real time at the point of care. This noninvasive strategy would likely be appropriate for patients with known CAD and those at highest risk of CAD, which could include those with high risk but asymptomatic clinical features such as marked hyperlipidemia and elevation of other coronary risk factors. This strategy could also be repeated periodically as the underlying plaque risk may change over time.61,62 CCTA also can guide clinicians on the detailed localization of CAD, such as the presence of left main CAD, which may dictate CABG surgery.
Invasive risk assessment of the full array of adverse plaque features will be appropriate for patients who undergo coronary angiography for routine clinical indications, as well as those patients identified to be at high risk from noninvasive CCTA screening. Invasive assessment with IVUS or OCT not only enables more detailed and precise assessment of plaque risk, due to their higher resolution than CCTA, but also could inform possible preemptive PCI since real-time reporting of high-risk plaque features will soon be available while the patient is in the catheterization laboratory. This strategy would require rigorous validation of clinical efficacy when added to current and evolving highly effective noninterventional therapies. Future research will be necessary to determine if high-risk features identified by CCTA that persist despite such intensive medical therapy are appropriate considerations for preemptive PCI, even in the absence of symptoms.
Such advances could also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy. In some cases, the high-risk portion of a potential culprit plaque may be suitable for preemptive invasive local intervention, whether by PCI or by local administration of pharmacologic agents, regardless of the magnitude of that plaque’s encroachment into the coronary lumen. Such proactive strategies might modulate the adverse features of the high-risk portion of plaque in a controlled manner and reduce its ability to destabilize and provoke a new MACE. The full palette of biologically directed and disease-modifying current medical treatments should serve as comparators in further trials of revascularization vs medical therapy.
Future Directions and Conclusions
The ISCHEMIA,21 FAME 3,24 and COMBINE OCT-FFR11 trials results emphasize that application of the ischemia hypothesis and the treatment of obstructive epicardial flow-limiting stenoses alone do not suffice to reduce MACE in high-risk patients with chronic CAD or ACS. Thus, in addition to systemic therapies directed at reducing residual dyslipidemic, thrombotic, metabolic, and inflammatory cardiovascular risk, we need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery (the plaque hypothesis) and broaden the target(s) of our therapeutic intervention to include all regions of the plaque (both flow-limiting and nonflow-limiting), even those that are distant from the presumed ischemia-producing obstruction.
Supplementary Material
Funding/Support:
This work was supported in part by the National Heart, Lung, and Blood Institute (grants R01 HL146144–01 and RO1 HL140498 to Dr Stone and grants 1R01HL134892 and 1R01HL163099–01 to Dr Libby), the American Heart Association (grant 18CSA34080399 to Dr Libby), the RRM Charitable Fund (Dr Libby), and the Simard Fund (Dr Libby). We gratefully acknowledge the generous support of the Schaubert Family.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Additional Contributions: We acknowledge Rob Flewell, BFA (Anatomy By Design, Inc), for illustrating Figures 1, 2, 3, and 5; he received compensation for this work.
Conflict of Interest Disclosures: Dr Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Eulicid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech, Inc; Dr Libby’s laboratory has received research funding in the last 2 years from Novartis; is on the board of directors of XBiotech, Inc; has a financial interest in Xbiotech, a company developing therapeutic human antibodies, in TenSixteen Bio, a company targeting somatic mosaicism and clonal hematopoiesis of indeterminate potential (CHIP) to discover and develop novel therapeutics to treat age-related diseases, and in Soley Therapeutics, a biotechnology company that is combining artificial intelligence with molecular and cellular response detection for discovering and developing new drugs, currently focusing on cancer therapeutics. Dr Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies. No other disclosures were reported.
REFERENCES
- 1.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91(11):2844–2850. doi: 10.1161/01.CIR.91.11.2844 [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. 2016;68(22):2467–2478. doi: 10.1016/j.jacc.2016.08.069 [DOI] [PubMed] [Google Scholar]
- 3.Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. 2020;76(24): 2803–2813. doi: 10.1016/j.jacc.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 4.Villines TC, Rodriguez Lozano P. Transitioning from stenosis to plaque burden in the cardiac CT era: the changing risk paradigm. J Am Coll Cardiol. 2020;76(24):2814–2816. doi: 10.1016/j.jacc.2020.10.030 [DOI] [PubMed] [Google Scholar]
- 5.Ferraro R, Latina JM, Alfaddagh A, et al. Evaluation and management of patients with stable angina: beyond the ischemia paradigm: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76 (19):2252–2266. doi: 10.1016/j.jacc.2020.08.078 [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 7.Oemrawsingh RM, Cheng JM, García-García HM, et al. ; ATHEROREMO-NIRS Investigators. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64(23):2510–2518. doi: 10.1016/j.jacc.2014.07.998 [DOI] [PubMed] [Google Scholar]
- 8.De Bruyne B, Pijls NH, Kalesan B, et al. ; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 9.Stone PH, Saito S, Takahashi S, et al. ; PREDICTION Investigators. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126(2):172–181. doi: 10.1161/CIRCULATIONAHA.112.096438 [DOI] [PubMed] [Google Scholar]
- 10.Waksman R, Di Mario C, Torguson R, et al. ; LRP Investigators. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. 2019; 394(10209):1629–1637. doi: 10.1016/S0140-6736(19)31794-5 [DOI] [PubMed] [Google Scholar]
- 11.Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42(45):4671–4679. doi: 10.1093/eurheartj/ehab433 [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi A, Leipsic J, Blankstein R, et al. Do plaques rapidly progress prior to myocardial infarction? the interplay between plaque vulnerability and progression. Circ Res. 2015;117(1): 99–104. doi: 10.1161/CIRCRESAHA.117.305637 [DOI] [PubMed] [Google Scholar]
- 13.Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511–2522. doi: 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Choi G, Koo BK, et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc Imaging. 2019;12(6):1032–1043. doi: 10.1016/j.jcmg.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 15.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56–62. doi: 10.1016/0735-1097(88)90356-7 [DOI] [PubMed] [Google Scholar]
- 16.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69(8):729–732. doi: 10.1016/0002-9149(92)90495-K [DOI] [PubMed] [Google Scholar]
- 17.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157–1166. doi: 10.1161/01.CIR.78.5.1157 [DOI] [PubMed] [Google Scholar]
- 18.Nobuyoshi M, Tanaka M, Nosaka H, et al. Progression of coronary atherosclerosis: is coronary spasm related to progression? J Am Coll Cardiol. 1991;18(4):904–910. doi: 10.1016/0735-1097(91)90745-U [DOI] [PubMed] [Google Scholar]
- 19.Boden WE, O’Rourke RA, Teo KK, et al. ; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 20.Frye RL, August P, Brooks MM, et al. ; BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron DJ, Hochman JS, Reynolds HR, et al. ; ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395–1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds HR, Shaw LJ, Min JK, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation. 2021;144 (13):1024–1038. doi: 10.1161/CIRCULATIONAHA.120.049755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaitman BR, Alexander KP, Cyr DD, et al. ; ISCHEMIA Research Group. Myocardial infarction in the ISCHEMIA trial: impact of different definitions on incidence, prognosis, and treatment comparisons. Circulation. 2021;143(8):790–804. doi: 10.1161/CIRCULATIONAHA.120.047987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fearon WF, Zimmermann FM, De Bruyne B, et al. Fractional flow reserve-guided PCI as compared with coronary bypass surgery. N Engl J Med. 2021;386(2):128–137. doi: 10.1056/NEJMoa2112299 [DOI] [PubMed] [Google Scholar]
- 25.Spertus JA, Jones PG, Maron DJ, et al. ; ISCHEMIA Research Group. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15): 1408–1419. doi: 10.1056/NEJMoa1916370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204 [DOI] [PubMed] [Google Scholar]
- 27.Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of coronary arteries in human and nonhuman primates. JAMA. 1994;271 (4):289–294.”https://www.ncbi.nlm.nih.gov/pubmed/8295288“ doi: 10.1001/jama.1994.03510280051032 [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337–346. doi: 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 29.Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143(7):624–640. doi: 10.1161/CIRCULATIONAHA.120.052008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med. 2020;383(9):846–857. doi: 10.1056/NEJMra2000317 [DOI] [PubMed] [Google Scholar]
- 31.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49(25):2379–2393. doi: 10.1016/j.jacc.2007.02.059 [DOI] [PubMed] [Google Scholar]
- 32.Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol. 2016;13(4):210–220. doi: 10.1038/nrcardio.2015.203 [DOI] [PubMed] [Google Scholar]
- 33.Jeney V, Balla G, Balla J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front Physiol. 2014;5:379. doi: 10.3389/fphys.2014.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornelissen A, Guo L, Sakamoto A, Virmani R, Finn AV. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine. 2019;47:598–606. doi: 10.1016/j.ebiom.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel JB, Libby P, Franck G. Internal bleeding: is intraplaque hemorrhage a decoration or a driver? JACC Basic Transl Sci. 2018;3(4):481–484. doi: 10.1016/j.jacbts.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvert PA, Obaid DR, O’Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. JACC Cardiovasc Imaging. 2011;4(8):894–901. doi: 10.1016/j.jcmg.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35 (10):639–647. doi: 10.1093/eurheartj/eht484 [DOI] [PubMed] [Google Scholar]
- 38.Erlinge D, Maehara A, Ben-Yehuda O, et al. ; PROSPECT II Investigators. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet. 2021;397(10278):985–995. doi: 10.1016/S0140-6736(21)00249-X [DOI] [PubMed] [Google Scholar]
- 39.Gimbrone MA Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;99 (8):1809–1813. doi: 10.1172/JCI119346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108(4):438–444. doi: 10.1161/01.CIR.0000080882.35274.AD [DOI] [PubMed] [Google Scholar]
- 42.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117(8):993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254 [DOI] [PubMed] [Google Scholar]
- 43.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124(7): 779–788. doi: 10.1161/CIRCULATIONAHA.111.021824 [DOI] [PubMed] [Google Scholar]
- 44.Stone PH, Maehara A, Coskun AU, et al. Role of low endothelial shear stress and plaque characteristics in the prediction of nonculprit major adverse cardiac events: the PROSPECT study. JACC Cardiovasc Imaging. 2018;11(3):462–471. doi: 10.1016/j.jcmg.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Thompson EW, Lefieux A, et al. High Coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J Am Coll Cardiol. 2018;72(16):1926–1935. doi: 10.1016/j.jacc.2018.07.075 [DOI] [PubMed] [Google Scholar]
- 46.Stone PH. The hazardous longitudinal heterogeneity of plaques: a complex mountain range, not a single volcano. JACC Cardiovasc Imaging. 2020;13(5):1219–1220. doi: 10.1016/j.jcmg.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 47.Stone PH. Evaluation of individual plaque risk based on plaque anatomic and biomechanical characteristics: methodologies and clinical applications are approaching an inflection point. Eur Heart J. 2019;40(18):1423–1425. doi: 10.1093/eurheartj/ehz208 [DOI] [PubMed] [Google Scholar]
- 48.Varshney AS, Coskun AU, Siasos G, et al. Spatial relationships among hemodynamic, anatomic, and biochemical plaque characteristics in patients with coronary artery disease. Atherosclerosis. 2021;320: 98–104. doi: 10.1016/j.atherosclerosis.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25): 3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878 [DOI] [PubMed] [Google Scholar]
- 50.Chatzizisis YS, Baker AB, Sukhova GK, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011;123(6):621–630. doi: 10.1161/CIRCULATIONAHA.110.970038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koskinas KC, Sukhova GK, Baker AB, et al. Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler Thromb Vasc Biol. 2013;33(7): 1494–1504. doi: 10.1161/ATVBAHA.112.300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JM, Choi G, Hwang D, et al. Impact of longitudinal lesion geometry on location of plaque rupture and clinical presentations. JACC Cardiovasc Imaging. 2017;10(6):677–688. doi: 10.1016/j.jcmg.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 53.Hakim D, Coskun AU, Maynard C, et al. Abstract 11768: role of endothelial shear stress and endothelial shear stress gradient in plaques associated with acute erosion vs. stable control plaques and relationship between plaque slope and localization of plaque erosion. Circulation. 2021;144: A11768. doi: 10.1161/circ.144.suppl_1.11768 [DOI] [Google Scholar]
- 54.Thondapu V, Mamon C, Poon EKW, et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res. 2021; 117(8):1974–1985. doi: 10.1093/cvr/cvaa251 [DOI] [PubMed] [Google Scholar]
- 55.Adriaenssens T, Allard-Ratick MP, Thondapu V, et al. Optical coherence tomography of coronary plaque progression and destabilization: JACC focus seminar part 3/3. J Am Coll Cardiol. 2021;78(12): 1275–1287. doi: 10.1016/j.jacc.2021.07.032 [DOI] [PubMed] [Google Scholar]
- 56.Costopoulos C, Maehara A, Huang Y, et al. Heterogeneity of plaque structural stress is increased in plaques leading to MACE: insights from the PROSPECT study. JACC Cardiovasc Imaging. 2020;13(5):1206–1218. doi: 10.1016/j.jcmg.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127–142. doi: 10.1016/j.jcmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 58.Matic LP, Jesus Iglesias M, Vesterlund M, et al. Novel multiomics profiling of human carotid atherosclerotic plaques and plasma reveals biliverdin reductase B as a marker of intraplaque hemorrhage. JACC Basic Transl Sci. 2018;3(4):464–480. doi: 10.1016/j.jacbts.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antoniadis AP, Papafaklis MI, Takahashi S, et al. Arterial remodeling and endothelial shear stress exhibit significant longitudinal heterogeneity along the length of coronary plaques. JACC Cardiovasc Imaging. 2016;9(8):1007–1009. doi: 10.1016/j.jcmg.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wentzel JJ, Papafaklis MI, Antoniadis A, et al. Abstract 15270: coronary plaque natural history displays marked longitudinal heterogeneity along the length of individual coronary plaques. Circulation. 2020;142:A15270 doi: 10.1161/circ.142.suppl_3.15270 [DOI] [Google Scholar]
- 61.Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55(15):1590–1597. doi: 10.1016/j.jacc.2009.07.078 [DOI] [PubMed] [Google Scholar]
- 62.Koskinas KC, Feldman CL, Chatzizisis YS, et al. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation. 2010;121 (19):2092–2101. doi: 10.1161/CIRCULATIONAHA.109.901678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doenst T, Haverich A, Serruys P, et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2019;73(8):964–976. doi: 10.1016/j.jacc.2018.11.053 [DOI] [PubMed] [Google Scholar]
- 64.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 65.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18): 1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 66.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 67.Nidorf SM, Fiolet ATL, Mosterd A, et al. ; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020; 383(19):1838–1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 68.Coskun A, Siasos G, Cefalo N, et al. Abstract 13006: non-invasive calculation of local coronary endothelial shear stress: head-to-head comparison of CCTA-derived vs IVUS-derived intracoronary imaging using computational fluid dynamics (CFD). Circulation. 2019;140:A13006. [Google Scholar]
- 69.Eslami P, Hartman EMJ, Albaghadai M, et al. Validation of wall shear stress assessment in non-invasive coronary CTA versus invasive imaging: a patient-specific computational study. Ann Biomed Eng. 2021;49(4):1151–1168. doi: 10.1007/s10439-020-02631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hakim D, Coskun A, Maynard C, et al. Abstract 13620: comparison of endothelial shear stress (ESS) computation utilizing non-invasive coronary computed tomography angiography (CCTA) vs invasive intravascular ultrasound (IVUS) imaging. Circulation. 2021;144:A13620. doi: 10.1161/circ.144.suppl_1.13620 [DOI] [Google Scholar]
- 71.Boden WE, Stone PH. To stent or not to stent? treating angina after ISCHEMIA-why a conservative approach with optimal medical therapy is the preferred initial management strategy for chronic coronary syndromes: insights from the ISCHEMIA trial. Eur Heart J. 2021;42(14):1394–1400. doi: 10.1093/eurheartj/ehab069.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.