Abstract
Background
To explore the safety and effectiveness of personalized exercise intervention during chemotherapy for lung cancer patients who were relatively weak and with compromised cardiopulmonary function.
Methods
Thirty‐eight lung cancer patients treated with chemotherapy at Peking University Third Hospital were enrolled in this prospective study. The exercise group (N = 21) received individualized exercise guidance based on personal test results and exercised regularly, while the control group (N = 17) only received exercise education and planed exercise methods according to their own preferences. Both groups underwent three fitness tests and clinical indicator assessments at 0, 6, and 12 weeks after starting the exercise, and the differences in trends of various indicators between the two groups were compared.
Results
No exercise‐related adverse events occurred during the 12‐week exercise period. After 12 weeks of exercise training, in terms of fitness, the exercise group showed significant improvements in 6‐min walk test (6MWT) (p < 0.001), peak oxygen consumption (VO2peak) (p = 0.005), muscle content (p < 0.001), muscle percentage (p < 0.001), and grip strength (p = 0.008) compared to the control group. In terms of clinical indicators, the exercise group showed significant improvements in vital capacity (p = 0.018), D‐dimer (p = 0.031), and C‐reactive protein (CRP) (p = 0.01), uric acid (p = 0.003), triglycerides (p < 0.001), functional average score (p < 0.001), and main symptom average score (p = 0.004) compared to the control group in trends over time.
Conclusion
Rehabilitation exercises using individualized exercise prescriptions tailored by exercise prescription specialists during chemotherapy are safe for lung cancer patients. Adhering to exercise can achieve comprehensive improvements in physical fitness and quality of life at 12 weeks.
Keywords: efficacy, exercise prescription, lung cancer, personalized, safety
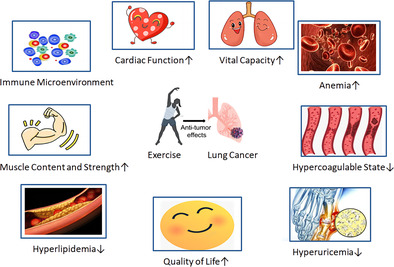
Compared to control group in lung cancer patients, the exercise group which followed individualized exercise prescriptions showed improvements not only in physical fitness indicators such as 6MWT, VO2peak, muscle content, grip strength, but also in clinical indicators such as lung capacity, D‐dimer, CRP, uric acid, triglycerides, hemoglobin, and quality of life after 12 weeks.
INTRODUCTION
Lung cancer has a high incidence and mortality rate and is the leading cause of cancer worldwide. With the advancement of chemotherapy, targeted therapy, and immunotherapy, the survival period of lung cancer patients has been significantly extended compared to the past. However, various complications related to cancer and its treatment, such as dyspnea, muscle atrophy, pain, fatigue, loss of appetite, and deterioration of physical fitness and lung function, may further impair the patient's condition. 1 Patients hope not only to live longer but also to have a better quality of life. The rehabilitation of cancer patients through exercise has been a focus since the 1980s, 2 and it has been found that exercise can help patients tolerate higher doses of chemotherapy. 3 In recent years, multiple studies have confirmed that exercise can bring benefits to the quality of life and survival of cancer patients and is safe. 4 , 5 The American Cancer Society released the third edition of the Nutrition and Physical Activity Guidelines for Cancer Survivors, recommending 150–300 min of moderate‐intensity activity or 75–150 min of vigorous activity per week, and ≥2 days of muscle‐strengthening activities per week during and after treatment. 6 Regular physical activity can bring many benefits to cancer patients: not only improving metabolic health, cardiopulmonary function, and quality of life but also extending survival time. 7 , 8 , 9 , 10 , 11 , 12
Researchers have proposed various exercise prescription recommendations based on the different side effects related to the treatment of patients. Most of these recommendations involve moderate to high‐intensity resistance and aerobic exercises, two to three times per week, continuing for 12 weeks. Researchers unanimously believe that there is no one‐size‐fits‐all prescription for all cancer patients, and due to the complexity of cancer and cancer treatment, personalization is crucial. It is necessary to formulate the most appropriate exercise prescription based on an individualized assessment. 13 , 14 To maximize the benefits of exercise therapy during the cancer survival period, patients should receive scientific exercise or rehabilitation plans as early as possible.
Although the importance of exercise for cancer patients is self‐evident, in reality, not many cancer patients actually engage in scientific and regular exercise. Most survivors do not participate in physical activities at the recommended level. 15 , 16 , 17 The main difficulty is that exercise prescriptions need to consider scientific validity, individualization, and safety, 14 , 18 as well as take into full account the patient's compliance, physical issues, psychological issues, 19 , 20 social support, 21 , 22 , 23 and so on. For lung cancer patients who have undergone lobectomy or are in a tumor‐bearing state, their cardiopulmonary function is weaker than before, and their willingness and physical strength to exercise have significantly decreased. 16 Therefore, it is more challenging to ensure patients can exercise regularly within a safe range. Hence, it is necessary to conduct pre‐exercise cardiopulmonary function and basic clinical indicator evaluations, exclude contraindications to exercise, and cautiously carry out exercise after assessing the patient's tolerable exercise intensity to avoid harm. 24 Regularly monitor the patient's fitness and clinical indicators after exercise, adjust continuously based on feedback, and achieve the most suitable amount of exercise for each patient. 25
Based on the project of the Ministry of Science and Technology of China on the development of precision exercise prescriptions for the Chinese population and the construction of an exercise prescription database, to better adhere to the principles of individualization and scientific nature of exercise for cancer patients, our center, in conjunction with Beijing Sport University, conducted related research on the exercise rehabilitation of cancer patients. Each patient needs a comprehensive assessment of physical fitness and clinical indicators before exercising. After exclusion of contraindications to exercise by clinical doctors and exercise experts, the most suitable exercise prescription for the patient is determined based on the assessment results. Exercise prescriptions are formulated by exercise prescription specialists from Beijing Sport University. Exercise evaluations and supervision are completed by graduate students majoring in sports human science at Beijing Sport University. Patients fully participate in 12 weeks of regular exercise under guidance and supervision. During the exercise period, three assessments of patient fitness and clinical indicators are performed, and the effectiveness and safety of the exercise prescription are continuously evaluated and fed back to ultimately determine the most suitable personalized exercise prescription for each patient.
METHODS
Research subjects
This study enrolled stage II–IV lung cancer patients who received chemotherapy in the Oncology Chemotherapy and Radiotherapy Department of Peking University Third Hospital from May 2019 to May 2022, with all clinical information coming from basic information upon admission. In the exercise group, 16 patients were undergoing first‐line chemotherapy, one was undergoing second‐line chemotherapy, and four were in the postoperative adjuvant chemotherapy period. Among the 21 patients, seven had previously undergone radical lung cancer surgery and re‐entered the advanced treatment stage after recurrence; one patient had received argon‐helium knife treatment before chemotherapy. The control group consisted of 17 patients who were all in the inoperable advanced or locally advanced stages at the time of enrollment, with 14 receiving first‐line chemotherapy and three in second‐line treatment. Among these 17 patients, two had previously received radiotherapy. The chemotherapy regimens for both groups were in accordance with NCCN guidelines, including the use of etoposide, pemetrexed, platinum agents, gemcitabine, taxanes, antiangiogenic drugs, and PD‐1 inhibitors. Patient baseline characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of the exercise and control groups.
| Variable | Type | Exercise group (n = 21) | Control group (n = 17) | p‐value |
|---|---|---|---|---|
| Gender | Male | 15 (71.43%) | 10 (58.82%) | 0.502 |
| Female | 6 (28.57%) | 7 (41.18%) | ||
| Age | ≤65 | 14 (66.67%) | 8 (47.06%) | 0.324 |
| >65 | 7 (33.33%) | 9 (52.94%) | ||
| Stage | II‐III | 11 (52.38%) | 7 (41.18%) | 0.532 |
| IV | 10 (47.62%) | 10 (58.82%) | ||
| Pathology | NSCLC | 17 (80.95%) | 11 (64.71%) | 0.293 |
| SCLC | 4 (19.05%) | 6 (35.29%) | ||
| Efficacy | CR/PR | 12 (57.14%) | 7 (41.18%) | 0.515 |
| SD/PD | 9 (42.86%) | 10 (58.82%) |
After signing the informed consent form, patients were divided into an exercise group (30 cases) and a control group (30 cases). Six patients in the exercise group, and seven patients in the control group were unable to come to the hospital for testing according to the schedule due to factors such as COVID‐19. In the exercise group, three patients were unable to continue and complete the 12‐week training due to deterioration in physical condition. In the control group, six patients were unwilling to further undergo testing due to worsening physical condition. Eventually, 21 lung cancer patients in the exercise group were able to regularly persist in 12 weeks of exercise training, and 17 patients in the control group were willing to regularly test their physical fitness. The plan has been certified by the Ethics Committee of Peking University Third Hospital. All patients signed an informed consent form. For inclusion and exclusion criteria, see Table 2.
TABLE 2.
Patient inclusion and exclusion criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
Research methods
The exercise group underwent exercise health education and 12 weeks of exercise guidance, following the international exercise guidelines for cancer patients. This meant combining 150 min per week of moderate‐intensity aerobic and resistance exercises, while also integrating evaluation results of the current status of the subjects to comprehensively develop exercise guidance plans. The exercise guidance method involved brisk walking for 30 min per day, 5 days a week, and two resistance training sessions per week, each lasting 20 min. Specific methods for resistance training were personalized based on individual assessment results. The control group was provided with general health education materials and was allowed to plan their own exercise methods according to their previous habits and preferences. Whether to join the exercise group or the control group is chosen by the patients themselves. Exercise prescriptions were formulated by exercise prescription specialists and exercise physiologist from the Sports Human Science major at Beijing Sport University. Exercise evaluations and supervisory guidance were completed by graduate students from the same major at Beijing Sport University. If adverse events such as falls, arrhythmias, falls from the bed, or severe diarrhea and vomiting occurred during the exercise guidance process, the exercise should be immediately stopped and medical assistance provided by clinical doctors to ensure the exercise guidance plan was scientifically safe and feasible.
Exercise prescription development
Based on the “American College of Sports Medicine (ACSM) Guidelines for Exercise Testing and Prescription (9th edition),” the recommended exercise intensity for cancer patients is to achieve a Rating of Perceived Exertion (RPE) of 13 or moderate exercise intensity. A weekly exercise time of 150 min, at a frequency of 5 days/week, is recommended, ideally including 2–3 resistance training sessions.
Exercise duration
The daily aerobic exercise time should be 30 min, meeting the requirement of 150 min of exercise per week. In addition to aerobic exercise, 2–3 resistance training sessions are tailored to actual conditions, each approximately lasting 20 min. For cancer patients with anemia, it is more appropriate to exercise multiple times a day. Initially, exercise can be performed for shorter durations, multiple times a day (in 5–10 min intervals), but each session should last at least 10 min, progressing to sustain exercise for more than 20 min.
Exercise frequency
The exercise frequency chosen for this study was >5 days a week without specifying fixed rest days. This is because participants might feel extremely fatigued on certain days or have unsatisfactory blood tests, so no rest days are scheduled, allowing participants to arrange rest days for other unexpected events. This maximizes the completion rate of the exercise guidance plan.
Exercise intensity
The prescribed exercise intensity is 50%–70% of the age‐predicted maximum heart rate. ACSM recommends that cancer patients exercise at a RPE of 13, which is equivalent to moderate intensity. Studies have found that the heart function of cancer patients can be affected by chemotherapy, leading to changes in heart rate. Therefore, using heart rate monitoring to gauge the exercise intensity of participants is no longer scientifically effective. Self‐assessment methods are more suitable for cancer patients to monitor their own exercise intensity. Since most of the study participants are older, using RPE is also more suitable for practical operation in this study. This study uses the RPE scale to monitor exercise intensity, which is consistent with the results of other current studies.
Exercise type
Aerobic exercises chosen involve walking with a cane, avoiding cycling or mountain climbing to prevent falls. The resistance exercises in this study comprehensively train the major muscle groups of the human body including the back, abdomen, buttocks, and lower limbs. However, due to most cancer patients having central venous catheters in place, shoulder, chest muscle groups, and full‐body movements are less involved. For patients who need to stay in bed for treatment, to alleviate muscle stiffness, low‐intensity exercises for activating back and buttock muscles, such as glute bridges and side‐lying leg lifts, are selected based on the principle of comprehensiveness. Ankle pump exercises and heel lifts are also given to promote overall blood circulation to prevent thrombosis. Due to the balance impairment often caused by chemotherapy, it is not appropriate to perform single‐leg or unstable exercises to avoid falls.
Exercise location
As cancer patients have reduced immune function during chemotherapy, it is not recommended for them to exercise in crowded or frequently used places during chemotherapy. The exercise plan in this study is designed for home‐based resistance training and aerobic exercises. There is no need to go to gyms or sports halls for resistance exercises, which reduces the likelihood of subjects refusing to exercise due to weather, venue, or equipment influences, and the less challenging walking exercises can be performed outdoors or on home treadmills. When walking outside, subjects should take protective measures to avoid contracting other diseases.
Effectiveness assessment
The effectiveness of the exercise guidance plan includes the assessment of clinical indicators, physical fitness level, and quality of life. Clinical indicators include monitoring the blood routine, biochemistry, coagulation function, CRP, lung capacity and the Response Evaluation Criteria in Solid Tumors (RECIST). Physical fitness indicators include 6‐min walk test, grip strength, sit‐and‐reach, one‐leg stand with eyes closed, and body composition. Quality of life assessment includes the International Physical Activity Questionnaires (IPAQ), cancer fatigue scale (CFS), and the third edition of the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ‐C30). As cancer patients typically undergo drug efficacy evaluation every two chemotherapy cycles (6 weeks), this study aligns with chemotherapy efficacy evaluations, conducting mid‐test and post‐test for subjects every two chemotherapy cycles. Each time patients undergo a physical fitness test, doctors are responsible for safety management and medical insurance, preparing emergency medications and equipment.
Statistical analysis
Statistical analysis was conducted using R 4.0.3 software. Continuous (quantitative) data were tested for normality using the Shapiro test. If the data followed a normal distribution, they were expressed as mean ± standard deviation, and an independent sample t‐test was used for comparison between groups; if not normally distributed, the median (25th percentile, 75th percentile) was used, and the Wilcoxon test was employed for between‐group comparisons. Categorical (qualitative) data are described using frequency (percentage) and were compared between groups using the chi‐square or Fisher's exact test. Repeated measures analysis of variance (ANOVA) was conducted using the stats package aov function, and survival curves were plotted using the survival and survminer packages. The log‐rank test was used to compare the survival rates between different groups, with a two‐sided p‐value <0.05 considered statistically significant.
RESULTS
At the three time points of exercise at 0, 6, and 12 weeks, comprehensive assessments of physical fitness indicators were conducted for both the exercise and control groups, including changes in 6MWT, VO2peak, muscle content, time standing on one leg with eyes closed, sit‐and‐reach, body fat percentage, and grip strength. The 6MWT test results within the exercise group after exercising showed significant improvement compared to the baseline (p = 0.009), with significant between‐group differences in trends post‐exercise (p < 0.001), and average values between groups also showing statistical differences (p = 0.006). VO2peak in the exercise group increased not significantly over time compared to pre‐exercise levels (p = 0.051), but with between‐group differences in trends over time (p = 0.005), and average values within the groups also showing significant statistical differences (p < 0.001). Muscle content and muscle percentage showed no significant differences before and after within the exercise and control groups, but there were significant between‐group differences in changes at 6 and 12 weeks post‐exercise (p < 0.001). Grip strength also showed between‐group differences in trends over time (p = 0.008). Details in Table 3.
TABLE 3.
The examination of within‐subject and between‐subject effects for physical fitness indicators.
| Outcome variables | Independent variable | F value | p‐value |
|---|---|---|---|
| 6MWT | Time | 6.397 | 0.009* |
| Time×group | 20.415 | 0.000* | |
| Group | 8.395 | 0.006* | |
| VO2peak | Time | 4.324 | 0.051 |
| Time×group | 5.664 | 0.005* | |
| Group | 18.475 | 0.000* | |
| Muscle content | Time | 2.615 | 0.240 |
| Time×group | 20.660 | 0.000* | |
| Group | 1.052 | 0.312 | |
| Muscle percentage | Time | 1.722 | 0.558 |
| Time×group | 11.467 | 0.000* | |
| Group | 3.829 | 0.058 | |
| Body fat percentage | Time | 2.630 | 0.237 |
| Time×group | 1.329 | 0.271 | |
| Group | 1.568 | 0.219 | |
| Body fat content | Time | 2.361 | 0.306 |
| Time×group | 0.166 | 0.848 | |
| Group | 0.945 | 0.338 | |
| Weight | Time | 1.755 | 0.540 |
| Time×group | 0.105 | 0.901 | |
| Group | 0.220 | 0.642 | |
| Time standing on one leg with eyes closed | Time | 2.854 | 0.273 |
| Time×group | 0.389 | 0.685 | |
| Group | 0.315 | 0.592 | |
| Grip strength | Time | 2.239 | 0.342 |
| Time×group | 5.154 | 0.008* | |
| Group | 0.907 | 0.347 | |
| Sit‐and‐reach | Time | 0.599 | 0.563 |
| Time×group | 0.056 | 0.946 | |
| Group | 0.466 | 0.517 |
At the 0, 6, and 12‐week marks, clinical indicators for both the exercise and control groups were evaluated. These included complete blood count, biochemistry, blood lipids, uric acid, and quality of life scores. The neutrophil‐to‐lymphocyte ratio (NLR) and the platelet‐to‐lymphocyte ratio (PLR) have some indicative value for the inflammatory state and prognosis of the tumor immune, therefore these ratios were calculated indirectly through the complete blood count and compared between groups.
After exercise, there was a statistically significant difference in the trends of lung capacity change post‐exercise between the exercise and control groups (p = 0.018). The uric acid levels showed a gradual decreasing trend in the exercise group, significantly differing from the control group's trend (p = 0.003), with a significant difference in the average values between the two groups (p = 0.009). Triglycerides in the exercise group showed a decreasing trend and differed significantly from the control group's trend (p < 0.001), with a significant difference in the average values between the groups (p = 0.030). Hemoglobin levels in the exercise group showed a rising trend post‐exercise compared to their baseline, which was statistically significant (p = 0.003). The functional average score improved gradually within the exercise group compared to their baseline (p = 0.003), showing a significant statistical difference in the trends of change between the two groups (p < 0.001), and there was a significant difference in the average scores between the groups (p = 0.042). The average score for primary symptoms showed a statistically significant difference in the trend of change between the exercise and control groups (p = 0.004). There was also a significant difference in the average level of overall health status between the two groups (p = 0.001), but there was no significant difference within each group's own pre‐ and post‐exercise comparison. No significant statistical differences were found in the intragroup comparisons before and after exercise and the intergroup comparisons for total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, eosinophil percentage, NLR, and PLR (Table 4).
TABLE 4.
The examination of within‐subject and between‐subject effects for clinical indicators.
| Outcome variables | Independent variable | F value | p‐value |
|---|---|---|---|
| Lung capacity | Time | 3.384 | 0.117 |
| Time×group | 4.239 | 0.018* | |
| Group | 3.795 | 0.059 | |
| NLR | Time | 1.750 | 0.543 |
| Time×group | 0.317 | 0.730 | |
| Group | 1.988 | 0.167 | |
| PLR | Time | 0.853 | 0.43 |
| Time×group | 0.204 | 0.816 | |
| Group | 0.187 | 0.668 | |
| Uric acid | Time | 0.820 | 0.445 |
| Time×group | 6.193 | 0.003* | |
| Group | 7.658 | 0.009* | |
| Triglycerides | Time | 1.725 | 0.558 |
| Time×group | 13.522 | 0.000* | |
| Group | 5.136 | 0.030* | |
|
Total cholesterol |
Time | 1.845 | 0.552 |
| Time×group | 2.179 | 0.139 | |
| Group | 0.492 | 0.499 | |
| High‐density lipoprotein cholesterol | Time | 0.075 | 0.928 |
| Time×group | 0.127 | 0.882 | |
| Group | 0.025 | 0.879 | |
| Low‐density lipoprotein cholesterol | Time | 0.632 | 0.544 |
| Time×group | 1.482 | 0.257 | |
| Group | 0.513 | 0.494 | |
| Hemoglobin | Time | 7.448 | 0.003* |
| Time×group | 1.242 | 0.295 | |
| Group | 1.258 | 0.269 | |
| Eosinophil percentage | Time | 0.406 | 0.668 |
| Time×group | 0.238 | 0.789 | |
| Group | 0.000 | 0.985 | |
| Functional status | Time | 7.186 | 0.003* |
| Time×group | 8.923 | 0.000* | |
| Group | 4.450 | 0.042* | |
| Primary symptoms | Time | 0.129 | 0.879 |
| Time×group | 6.122 | 0.004* | |
| Group | 0.836 | 0.367 | |
| Overall health status | Time | 1.751 | 0.181 |
| Time×group | 1.183 | 0.312 | |
| Group | 13.276 | 0.001* |
Since coagulation function and CRP were not tested every cycle, most patients only had two test results at the three time points. A paired‐test analysis comparing the last monitoring indicator post‐exercise with before exercise was conducted. The results revealed that the exercise group showed a significant downward trend in D‐dimer (p = 0.031) and CRP (p = 0.01), while the control group showed an upward trend in CRP (p = 0.042). The details are in Tables 5 and 6.
TABLE 5.
D‐dimer changes before and after exercise between the two groups.
| Variable | Group | Pre‐exercise | Post‐exercise | Diff | Statistic | p‐value |
|---|---|---|---|---|---|---|
| D‐dimer | Control (N = 13) | 0.66 ± 1.1 | 0.88 ± 1.25 | −0.22 ± 0.59 | −1.338 | 0.206 |
| Exercise (N = 21) | 0.69 ± 1.03 | 0.18 ± 0.04 | 0.52 ± 1.02 | 2.321 | 0.031* |
TABLE 6.
CRP changes before and after exercise between the two groups.
| Variable | Group | Pre‐exercise | Post‐exercise | Diff | Statistic | p‐value |
|---|---|---|---|---|---|---|
| CRP | Control (N = 15) | 32.13 ± 60.57 | 41.78 ± 74.82 | −9.65 ± 16.75 | −2.232 | 0.042* |
| Exercise (N = 12) | 15.87 ± 13.33 | 9.38 ± 9.98 | 6.49 ± 7.23 | 3.111 | 0.01* |
DISCUSSION
We found that after 12 weeks of training, the exercise group showed improvements in physical fitness indicators such as the 6MWT, VO2peak, muscle content, muscle percentage, and grip strength. At the same time, clinical indicators that improved included lung capacity, D‐dimer, CRP, uric acid, triglycerides, hemoglobin, functional average score, average score of primary symptoms, and overall health status. The control group, which received health education and kept their previous exercise routine, did not achieve such significant changes.
The cardiopulmonary function of patients in the exercise group improved significantly in our center. Studies specifically observing nonexercising lung cancer patients have shown that for 50 patients with non‐small cell lung cancer (NSCLC) (stages I–III) monitored over 6 months, the 6MWT significantly decreased from diagnosis to treatment period and continued to decrease after 6 months. 26 Cardiopulmonary health, which includes the respiratory system, cardiovascular system, vascular system, blood, and skeletal muscle system, can be reduced by many cancer‐related factors in lung malignancies. 27 The main reasons are: first, the presence of the tumor and related surgeries might decrease oxygen intake, thereby affecting the respiratory system; second, in advanced disease, the oxidative capacity of skeletal muscles can be impaired with reduced capillarization and mitochondrial density; third, chemotherapy drugs and radiation may damage the cardiac pump, blood cell groups, and vascular function. 27 Many cancer treatments have negative cardiovascular effects or cardiac toxicity. Exercise therapy has been found to improve the cardiopulmonary health of cancer patients and alleviate the cardiac toxicity of cancer treatments. 28
VO2peak and the 6MWT are the most applicable assessments of cardiopulmonary function for cancer patients. Similar to lung function, VO2peak provides clinically relevant diagnostic and prognostic information. It negatively correlated with peri‐ and postoperative complications and serves as an independent predictor of survival in lung cancer patients. To date, at least three studies have investigated the relationship between cardiopulmonary function and survival rates in lung cancer patients. 29 , 30 , 31 A retrospective study assessed lung cancer patients (stages IIB–IV) after 8–10 weeks of exercise rehabilitation, showing significant increases in forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1), with even larger increases in patients with existing respiratory impairments. 32 Another study on NSCLC patients (stages I–II) who had undergone surgical resection found that the exercise group had a significant increase in VO2peak compared to the control group. 33 Jones and colleagues prospectively found that every 50‐m improvement in the 6MWT reduced the risk of death for metastatic NSCLC patients by 13%. Additionally, compared to the group with the lowest 6MWT, there was a reduced risk of death and increased functional capacity (from 39% to 52%). 31 However, some results should be interpreted with caution due to the lack of a control group.
In other types of cancer, exercise exposure after cancer treatment has also been shown to improve cardiovascular outcomes for patients. During adjuvant chemotherapy for breast cancer, low‐intensity home exercises and supervised medium‐to‐high intensity resistance and aerobic exercises can mitigate the decline in cardiopulmonary function (−9% vs −17%). 34 Another study with a median follow‐up of 8.6 years found that the incidence of cardiovascular events in nonmetastatic breast cancer patients decreased as the level of physical activity increased, with a 23% reduction in cardiovascular events for those exercising ≥9 metabolic equivalent (MET)‐hours per week. 35 Breast cancer survivors who had undergone surgery, chemotherapy, and/or radiotherapy and engaged in aerobic exercise three times per week for 15 weeks experienced a 15% improvement in cardiopulmonary function and quality of life. 36 The OptiTrain study found that 16 weeks of aerobic exercise, combined with resistance training, had positive effects on the physical and mental health of breast cancer patients during chemotherapy. 37 Rogers and colleagues reported that nonmetastatic colorectal cancer patients who engaged in 12 weeks of moderate‐intensity aerobic exercise experienced a 20% improvement in cardiopulmonary function compared to those who received standard treatment. 38 Supervised 12‐week aerobic exercise improved the cardiopulmonary function of lymphoma patients undergoing chemotherapy by 17%, while those receiving standard treatment saw a 2% decline. 39 In adult survivors of childhood Hodgkin's lymphoma, a higher level of physical activity (≥9 MET‐h/week) was associated with a 7% absolute risk reduction in cardiovascular events (12.2% vs. 5.2%) compared to 0 MET‐h/week. 40 A meta‐analysis including 571 patients with lymphoma, prostate cancer, breast cancer, and colon cancer showed that compared to the control group, which had a significant decrease in VO2peak of 1.02 mL/kg/min, the exercise group had a significant increase of 2.90 mL/kg/min. 41 In a larger meta‐analysis of 48 randomized controlled trials (exercise group: 1900; control group: 1642), the exercise group had an increase in VO2peak of 2.80 mL/kg/min compared to the control group's 0.02 mL/kg/min. 42
We found that the exercise group showed a significant increase in muscle proportion and grip strength, consistent with literature reports. In a 2‐year follow‐up study of breast cancer, those who underwent a mix of aerobic and resistance training experienced lower cancer‐related fatigue and higher muscle strength. 43 Compared to low‐intensity exercise and control groups, breast cancer patients undergoing adjuvant chemotherapy who engaged in medium‐to‐high intensity exercise had better muscle strength, physical fitness, and cognitive function. 34 Exercise and nutritional interventions have shown great potential in reducing the risk of bone and muscle loss in cancer patients. 44 Exercise can mitigate chronic inflammatory responses by increasing muscle mass. Interleukin‐6 acts as an energy regulator within muscle tissue. 45 As muscle mass increases, insulin sensitivity (cell growth regulation) improves, along with mitochondrial function, oxidative capacity, and protein synthesis, all of which can synergistically inhibit tumor growth. 46 , 47
In our study, we found that the exercise group showed reductions in triglycerides, uric acid, and D‐dimer levels, and there was also an improvement in anemia. Exercise can induce beneficial changes in the tumor microenvironment by lowering blood lipid levels and modulating cytokine release, thereby reducing the protumorigenic effects of hyperlipidemia. 48 The sedentary lifestyle of most people leads to abnormalities in blood lipids, hypertension, and diabetes. Abnormal blood lipids are a fertile ground for the formation of atherosclerotic plaques. Aerobic and resistance exercises can significantly improve blood lipid levels and reduce mortality rates related to cardiovascular diseases. 49 Exercise can improve hyperuricemia, with moderate‐intensity exercise being more effective than low‐intensity exercise in this regard. 50 Exercise can improve anemia through multiple mechanisms, including bone marrow stimulation, improving the hematopoietic microenvironment, and controlling levels of inflammation and hormones. 51 , 52 Immobilization is recognized as a major risk factor in the Wells scoring criteria for deep vein thrombosis (DVT), which has become a consensus. 52 , 53 Studies have reported that NLR, PLR, and eosinophil percentage can reflect the immune and inflammatory status of tumor patients and have a certain predictive value for the prognosis of lung cancer 54 , 55 , 56 ; therefore, these indicators were included in this study, but no significant differences were found between the exercise and control groups in the end.
In this study, we found that the scores of the exercise group for main symptoms and functional assessments significantly improved. Cancer patients often experience a disconnection between body and mind, and participating in exercise can promote a reconnection. 57 The prevalence rates of anxiety, depression, and sleep disorders among cancer patients were 33%, 34%, and 45%–57%, respectively in previous studies 58 , 59 and patients can experience psychological distress due to cancer or the adverse effects of its treatment. Physical activity can improve anxiety and depression by regulating levels of monoamines and cortisol, 60 , 61 leading to adaptations in limbic structures. 62 Exercise has been shown to have a positive effect 63 , 64 , 65 , 66 or neutral effect 67 on improving anxiety and depression. Most cancer survivors who participate in exercise notice significant physical changes, which help them gain confidence to continue training, 68 and they derive enthusiasm for life from better physical condition. 69 , 70 Breast cancer survivors have said that exercise is a tool for rehabilitation; it heals the body as well as the mind. 71 Some cancer survivors have expressed that social support and physical activity can build confidence and hope in the fight against cancer. 21 Breast cancer survivors feel that participating in group training can also provide social support, helping them promote a sense of healing through discussion and sharing with other members. 57 , 72 All the women in the group got along very well, finding others to be “friendly”. 73 Exercise can also improve the sleep quality of cancer survivors. 74 A home‐based walking plan proposed by Chen et al. showed that compared to the control group, 12 weeks of moderate‐intensity exercise was effective in improving subjective and objective sleep quality in patients with lung cancer (stages I–IV). 75
Exercise promotes the recovery of immune function and alleviates chronic inflammatory responses. A prospective randomized study on postoperative patients with NSCLC found that exercise significantly promoted the percentage of peripheral blood mononuclear cells, circulating NK cells, and natural killer T cells. 76 Cancer patients who exercised using resistance bands during chemotherapy were able to maintain their white blood cell levels compared to the control group. 77 Exercise can inhibit tumor growth through adrenaline and IL‐6 dependent mobilization and redistribution of NK cells. 78 Serum from acute aerobic exercise conditions, through IL‐6 induced DNA damage, inhibited the proliferation of colon cancer cells. 79 In stage III lung cancer survivors, 24 weeks of moderate‐intensity aerobic exercise for 150 min per week resulted in a 35.4% and 29.6% reduction in hs‐CRP and IL‐6, respectively. 80 In a prospective cohort study of 1494 stage III colon cancer survivors, elevated hs‐CRP and IL‐6 were associated with a 65% and 52% higher relative risk of disease recurrence or death, respectively. 81
The formulation of an exercise program needs to be effective while ensuring safety. Throughout the duration of this study, no exercise‐related injuries or adverse events occurred in any patient. Safety standards that may affect the initiation or cessation of exercise have been summarized in position statements from Exercise and Sports Science Australia (ESSA) and guidance documents from the ACSM. 10 , 13 The reported rate of serious adverse events related to exercise during and after cancer treatment is <5%, and no life‐threatening adverse events or deaths related to exercise have been reported in any exercise and oncology clinical trial. 82 No serious adverse events occurred in 183 patients exercising in the community, at home, or in a tertiary cancer center before autologous stem cell transplant. 83 Under the premise of ensuring safety, too low an exercise intensity is not suitable for most tumor patients. Some exercise guidelines recommend that cancer patients engage in moderate‐level exercise training. 13 , 47 It has been reported that, during the entire treatment process for cancer patients, moderate‐intensity training has greater benefits than low‐intensity training. 84 Exercise training usually starts from an adaptation phase of moderate‐intensity aerobic exercise. Afterwards, the training enters a progressive phase, where high‐intensity exercise can be appropriately introduced. 85 However, high‐intensity exercise might be more suitable for application in early‐stage cancer patients. Studies have found that preoperative high‐intensity interval training has a positive impact on the exercise capacity of early‐stage lung cancer patients, with high rehabilitation rates (87%) and completion rates (92%). 86 A small trial for early‐stage colorectal cancer patients reported that both high‐ and moderate‐intensity interval training significantly improved the patients' cardiopulmonary function. 87 However, regarding moderate‐ and high‐intensity exercise programs, the Alberta Physical Activity and Breast Cancer Prevention Trial randomized 400 postmenopausal women to 52 weeks of 150 min per week or 300 min per week of aerobic exercise. Compared to 150 min per week, the group randomized to 300 min per week of exercise did not show significant changes in hs‐CRP, IL‐6, or TNFα. 88 Regarding the type of exercise, aerobic combined with resistance exercise is more effective than aerobic exercise alone. Engaging in resistance exercise twice or three times a week for 12 weeks, especially under supervision, can improve HRQoL during and after treatment, beyond the effects obtained from aerobic or resistance training alone. 13 In summary, to make exercise an effective treatment for improving fitness and strength, it is recommended to conduct moderate‐intensity aerobic combined with resistance training in cancer patients. There is still controversy regarding the use of high‐intensity exercise, the appropriate outcomes for measuring therapeutic effectiveness, and the mechanisms driving the therapeutic effects of exercise. 89
The implementation of exercise in cancer patients is challenging. Despite increasing evidence supporting the safety and effectiveness of exercise therapy for cancer, whether post‐surgery, during treatment, or post‐treatment, most patients are not active enough or are sedentary. A series of studies have reported low compliance and high dropout rates in exercise programs. 16 , 90 , 91 Among the reasons for dropout, side effects related to cancer and a lack of interest and motivation in most cases are prevalent. There are many barriers that limit the adherence of lung cancer patients to physical activity programs, such as interest, health status, and adverse reactions to treatment. Over time, environmental and personal exercise preferences, enjoyment, and social influences are important factors affecting the completion of physical activity programs. 92 There is a pressing need for ongoing collaboration between oncologists and cancer exercise specialists to tailor exercise programs to the needs, preferences, and physical and psychological conditions of patients. It is necessary to dynamically create exercise plans that fit the current conditions of patients, and more precise and flexible exercise prescriptions can improve patients' confidence and motivation to continue, leading to improvements in physical fitness and quality of life over time. Positive feedback can reinforce the confidence and motivation of patients to continue exercising.
This study was conducted during the COVID‐19 period, during which remote guidance and home exercise became the norm. Research has found that home interventions have improved overall quality of life, social function, and predicted VO2peak for breast cancer patients. 93 The use of telemedicine has provided opportunities to address health inequalities, improve the health status of all patients, and offer alternatives for patients with transportation difficulties. 94 Remote interventions and home exercises have shown advantages in efficiency, safety, and cost savings. 95 Studies show that patients prefer home interventions. 96 , 97 However, remote interventions have their drawbacks, such as the inability to accurately assess patient compliance, with reports indicating a wide range of adherence to rehabilitation plans, varying from 16% to 97%. 98 , 99 , 100 , 101 Digital or face‐to‐face exercise supervision remains the gold standard.
It is necessary to identify the drawbacks of remote guidance and find ways to overcome them. Following appropriate templates may increase clinical acceptance of effective exercise plans, facilitate research replication, reduce research waste, and improve patient outcomes. 102 Compliance can also be improved through behavior change, which, although complex, 103 can be used effectively during exercise interventions to improve health behaviors and maximize compliance. 104 Two rehabilitation trials have specified the use of behavior change techniques through mobile apps. 105 In addition, the enhancement of self‐efficacy is an important factor in improving compliance. 106 These strategies include participant education about the health impacts of exercise, setting achievable health‐related goals, determining perceptible effects, and strategies to overcome psychological barriers. Overall, the implementation of exercise can be stratified according to different patient situations, including supervised, unsupervised, community, or home‐based models. The flexibility offered by exercise therapy can maximize patient participation. High‐risk patients may benefit from supervised, hospital‐based, or community‐based exercise therapy programs, while low‐risk patients may participate in unsupervised, home‐based programs. For example, community‐based supervised exercise programs. 107
This study was a single‐center prospective trial, with professional sports personnel involved in the design, testing, and execution of the exercise. Throughout the 12‐week exercise period, individualized exercise prescriptions were dynamically and finely made for each patient. The study was completed under the multilayered review and supervision of clinicians, exercise prescribers, and sports science professionals, with a high quality of scientific design and strict quality control, providing a reference for future research on exercise rehabilitation in lung cancer patients. However, this study had some limitations, such as being disrupted by the pandemic, the use of online teaching methods for exercise, and a higher dropout rate early in the program. The sample size was small, and factors such as inconsistent treatment plans and lines of treatment affect the results. There is much room for exploration in the field of exercise rehabilitation for lung cancer. Given the characteristic reduction in cardiopulmonary function in lung cancer patients, inappropriate exercise may increase adverse events, and a customized multidisciplinary exercise prescription method should be adopted. This relies on strong collaboration between oncologists, cardiologists, and exercise physiologists.
CONCLUSION
Exercise during chemotherapy for lung cancer patients has been proven to be safe and effective at our center. It can increase patient muscle strength, improve cardiopulmonary function, enhance metabolic processes, alleviate inflammatory and hypercoagulable states, and lead to a comprehensive improvement in the quality of life through the enhancement of physical and emotional functions. The above results need to be validated with a larger sample size in the future.
AUTHOR CONTRIBUTIONS
Liwen Ma, Baoshan Cao, and Qian Li are responsible for the overall research design. Hong Ren and Yanchun Li are responsible for the design of exercise prescription and safety assessment, as well as the design of exercise research. Qian Li, Chen Guo, Fanjie Zhou, and Jiulong Wang Conducted physical fitness testing and exercise guidance for patients, patient safety management, health education, follow‐up, and collated data. Liwen Ma, Baoshan Cao, Qian Li, Mopei Wang, and Yane Liu were responsible for patient enrollment and clinical safety evaluation. Hua Zhang is responsible for the statistical design of the study. Qian Li is responsible for statistical analysis and article writing. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Li Q, Guo C, Cao B, Zhou F, Wang J, Ren H, et al. Safety and efficacy evaluation of personalized exercise prescription during chemotherapy for lung cancer patients. Thorac Cancer. 2024;15(11):906–918. 10.1111/1759-7714.15272
REFERENCES
- 1. Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non‐small cell lung cancer. Support Care Cancer. 2015;23(6):1699–1708. [DOI] [PubMed] [Google Scholar]
- 2. Winningham ML et al. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. 1989;16(5):683–689. [PubMed] [Google Scholar]
- 3. Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment‐related complications after high‐dose chemotherapy. Blood. 1997;90(9):3390–3394. [PubMed] [Google Scholar]
- 4. Voorn MJJ, Driessen EJM, Reinders RJEF, van Kampen‐van den Boogaart VEM, Bongers BC, Janssen‐Heijnen MLG. Effects of exercise prehabilitation and/or rehabilitation on health‐related quality of life and fatigue in patients with non‐small cell lung cancer undergoing surgery: a systematic review. Eur J Surg Oncol. 2023;49(10):106909. [DOI] [PubMed] [Google Scholar]
- 5. Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, et al. Physical activity and exercise in lung cancer care: will promises Be fulfilled? Oncologist. 2020;25(3):e555–e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):263–265. [DOI] [PubMed] [Google Scholar]
- 7. Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. [DOI] [PubMed] [Google Scholar]
- 8. Brown JC, Ma C, Shi Q, Fuchs CS, Meyer J, Niedzwiecki D, et al. Physical activity in stage III colon cancer: CALGB/SWOG 80702 (Alliance). J Clin Oncol. 2023;41(2):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingle L, Ruilova S, Cui Y, DeClercq V, Sweeney E, Yu ZM, et al. Substituting bouts of sedentary behavior with physical activity: adopting positive lifestyle choices in people with a history of cancer. Cancer Causes Control. 2022;33(8):1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes SC, Newton RU, Spence RR, Galvão DA. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–1199. [DOI] [PubMed] [Google Scholar]
- 11. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137(11):1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. Cardio‐oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139(21):e997–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell KL, Winters‐Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stout NL, Brown JC, Schwartz AL, Marshall TF, Campbell AM, Nekhlyudov L, et al. An exercise oncology clinical pathway: screening and referral for personalized interventions. Cancer. 2020;126(12):2750–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan A, Ports K, Neo P, Ramalingam MB, Lim AT, Tan B, et al. Barriers and facilitators to exercise among adult cancer survivors in Singapore. Support Care Cancer. 2022;30(6):4867–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips MJ, Sanders LH, et al. Exercise training for people following curative intent treatment for non‐small cell lung cancer: a randomized controlled trial. Braz J Phys Ther. 2017;21(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clifford BK, Mizrahi D, Sandler CX, Barry BK, Simar D, Wakefield CE, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer. 2018;26(3):685–700. [DOI] [PubMed] [Google Scholar]
- 18. Carter SJ, Baranauskas MN, Ballinger TJ, Rogers LQ, Miller KD, Nabhan DC. Exercise load monitoring: integrated approaches to advance the individualisation of exercise oncology. BMJ Open Sport Exerc Med. 2021;7(3):e001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbett T, Cheetham T, Müller AM, Slodkowska‐Barabasz J, Wilde L, Krusche A, et al. Exploring cancer survivors’ views of health behaviour change: “where do you start, where do you stop with everything?”. Psychooncology. 2018;27(7):1816–1824. [DOI] [PubMed] [Google Scholar]
- 20. Hyland KA, Jacobs JM, Lennes IT, Pirl WF, Park ER. Are cancer survivors following the national comprehensive cancer network health behavior guidelines? An assessment of patients attending a cancer survivorship clinic. J Psychosoc Oncol. 2018;36(1):64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonough MH, Beselt LJ, Kronlund LJ, Albinati NK, Daun JT, Trudeau MS, et al. Social support and physical activity for cancer survivors: a qualitative review and meta‐study. J Cancer Surviv. 2021;15(5):713–728. [DOI] [PubMed] [Google Scholar]
- 22. Monteiro‐Guerra F, Signorelli GR, Rivera‐Romero O, Dorronzoro‐Zubiete E, Caulfield B. Breast cancer Survivors’ perspectives on motivational and personalization strategies in Mobile app‐based physical activity coaching interventions: qualitative study. JMIR Mhealth Uhealth. 2020;8(9):e18867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Owusu C, Antognoli E, Nock N, Hergenroeder P, Austin K, Bennet E, et al. Perspective of older African‐American and non‐Hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: a qualitative study. J Geriatr Oncol. 2018;9(3):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eschke RK, Lampit A, Schenk A, Javelle F, Steindorf K, Diel P, et al. Impact of physical exercise on growth and progression of cancer in rodents‐a systematic review and meta‐analysis. Front Oncol. 2019;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grote M, Maihöfer C, Weigl M, Davies‐Knorr P, Belka C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial. Radiat Oncol. 2018;13(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Granger CL, McDonald CF, Irving L, Clark RA, Gough K, Murnane A, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83(2):292–299. [DOI] [PubMed] [Google Scholar]
- 27. Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9(5):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tong CKW, Lau B, Davis MK. Exercise training for cancer survivors. Curr Treat Options Oncol. 2020;21(7):53. [DOI] [PubMed] [Google Scholar]
- 29. Jones LW, Watson D, Herndon JE II, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long‐term all‐cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, et al. Prognostic value of the six‐minute walk in advanced non‐small cell lung cancer. J Thorac Oncol. 2009;4(5):602–607. [DOI] [PubMed] [Google Scholar]
- 31. Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non‐small cell lung cancer. Lung Cancer. 2012;76(2):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarumi S, Yokomise H, Gotoh M, Kasai Y, Matsuura N, Chang SS, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg. 2015;149(2):569–573. [DOI] [PubMed] [Google Scholar]
- 33. Messaggi‐Sartor M, Marco E, Martínez‐Téllez E, Rodriguez‐Fuster A, Palomares C, Chiarella S, et al. Combined aerobic exercise and high‐intensity respiratory muscle training in patients surgically treated for non‐small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med. 2019;55(1):113–122. [DOI] [PubMed] [Google Scholar]
- 34. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–1927. [DOI] [PubMed] [Google Scholar]
- 35. Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. [DOI] [PubMed] [Google Scholar]
- 37. Wengstrom Y, Bolam KA, Mijwel S, Sundberg CJ, Backman M, Browall M, et al. Optitrain: a randomised controlled exercise trial for women with breast cancer undergoing chemotherapy. BMC Cancer. 2017;17(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home‐based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. [DOI] [PubMed] [Google Scholar]
- 39. Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. [DOI] [PubMed] [Google Scholar]
- 40. Jones LW, Liu Q, Armstrong GT, Ness KK, Yasui Y, Devine K, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta‐analysis. Oncologist. 2011;16(1):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta‐analysis. J Clin Oncol. 2018;36(22):2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bolam KA, Mijwel S, Rundqvist H, Wengström Y. Two‐year follow‐up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiss N, Baguley BJ, Dalla Via J, Fraser SF, Bolam KA, Daly RM. Exercise and nutritional approaches to combat cancer‐related bone and muscle loss. Curr Osteoporos Rep. 2020;18(3):291–300. [DOI] [PubMed] [Google Scholar]
- 45. Kistner TM, Pedersen BK, Lieberman DE. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. 2022;4(2):170–179. [DOI] [PubMed] [Google Scholar]
- 46. Thomas RJ, Kenfield SA, Jimenez A. Exercise‐induced biochemical changes and their potential influence on cancer: a scientific review. Br J Sports Med. 2017;51(8):640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mavropalias G, Sim M, Taaffe DR, Galvão DA, Spry N, Kraemer WJ, et al. Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects. J Cancer Res Clin Oncol. 2022;148(6):1389–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buss LA, Dachs GU. The role of exercise and Hyperlipidaemia in breast cancer progression. Exerc Immunol Rev. 2018;24:10–25. [PubMed] [Google Scholar]
- 49. Sulague RM, Suan NNM, Mendoza MF, Lavie CJ. The associations between exercise and lipid biomarkers. Prog Cardiovasc Dis. 2022;75:59–68. [DOI] [PubMed] [Google Scholar]
- 50. Hou Y, Ma R, Gao S, Kaudimba KK, Yan H, Liu T, et al. The effect of low and moderate exercise on hyperuricemia: protocol for a randomized controlled study. Front Endocrinol (Lausanne). 2021;12:716802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avancini A, Belluomini L, Tregnago D, Trestini I, Milella M, Lanza M, et al. Exercise and anemia in cancer patients: could it make the difference? Expert Rev Hematol. 2021;14(11):979–985. [DOI] [PubMed] [Google Scholar]
- 52. Bockenstedt P. D‐dimer in venous thromboembolism. N Engl J Med. 2003;349(13):1203–1204. [DOI] [PubMed] [Google Scholar]
- 53. Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 54. Mandaliya H, Jones M, Oldmeadow C, Nordman IIC. Prognostic biomarkers in stage IV non‐small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ozturk AE, Komurcuoglu B, Karakurt GK, Ozturk O. Prognostic value of diffuse cancer inflammation index (ALI), serum neutrophil/lymphocyte (NLR) and platelet/lymphocyte (PLR) in advanced‐stage lung cancer. J Cancer Res Ther. 2023;1–5. [DOI] [PubMed] [Google Scholar]
- 56. Sibille A, Corhay JL, Louis R, Ninane V, Jerusalem G, Duysinx B. Eosinophils and lung cancer: from bench to bedside. Int J Mol Sci. 2022;23(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nock NL, Owusu C, Flocke S, Krejci SA, Kullman EL, Austin K, et al. A community‐based exercise and support group program improves quality of life in African‐American breast cancer survivors: a quantitative and qualitative analysis. Int J Sports Exerc Med. 2015;1(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality‐of‐life data. J Clin Oncol. 2000;18(4):893–903. [DOI] [PubMed] [Google Scholar]
- 59. Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008;62(3):391–400. [DOI] [PubMed] [Google Scholar]
- 60. O'Neil PM, Aroda VR, Astrup A, Kushner R, Lau DCW, Wadden TA, et al. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta‐meta‐analysis of the effect of physical activity on depression and anxiety in non‐clinical adult populations. Health Psychol Rev. 2015;9(3):366–378. [DOI] [PubMed] [Google Scholar]
- 62. Wegner M, Helmich I, Machado S, Nardi A, Arias‐Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta‐ analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13(6):1002–1014. [DOI] [PubMed] [Google Scholar]
- 63. Law CYJ, Yu THJ, Chen T. Effectiveness of aerobic and resistance exercise in cancer survivors with depression: a systematic review and meta‐analysis of randomized controlled trials. J Psychosom Res. 2023;173:111470. [DOI] [PubMed] [Google Scholar]
- 64. Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home‐based walking exercise on anxiety, depression and cancer‐related symptoms in patients with lung cancer. Br J Cancer. 2015;112(3):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced‐stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14(4):341–349. [DOI] [PubMed] [Google Scholar]
- 66. Sommer MS, Trier K, Vibe‐Petersen J, Christensen KB, Missel M, Christensen M, et al. Changes in health‐related quality of life during rehabilitation in patients with operable lung cancer: a feasibility study (PROLUCA). Integr Cancer Ther. 2018;17(2):388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise in patients with non‐small cell lung cancer. Med Sci Sports Exerc. 2014;46(4):656–663. [DOI] [PubMed] [Google Scholar]
- 68. Brunet J, Saunders S, Gifford W, Thomas R, Hamilton R. An exploratory qualitative study of the meaning and value of a running/walking program for women after a diagnosis of breast cancer. Disabil Rehabil. 2018;40(9):1041–1048. [DOI] [PubMed] [Google Scholar]
- 69. Osypiuk K, Kilgore K, Ligibel J, Vergara‐Diaz G, Bonato P, Wayne PM. “making peace with our bodies”: a qualitative analysis of breast cancer Survivors’ experiences with qigong mind‐body exercise. J Altern Complement Med. 2020;26(9):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wurz A, St‐Aubin A, Brunet J. Breast cancer survivors’ barriers and motives for participating in a group‐based physical activity program offered in the community. Support Care Cancer. 2015;23(8):2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cantwell M, Walsh D, Furlong B, Loughney L, McCaffrey N, Moyna N, et al. Physical activity across the cancer journey: experiences and recommendations from people living with and beyond cancer. Phys Ther. 2020;100(3):575–585. [DOI] [PubMed] [Google Scholar]
- 72. Ray HA, Verhoef MJ. Dragon boat racing and health‐related quality of life of breast cancer survivors: a mixed methods evaluation. BMC Complement Altern Med. 2013;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sabiston CM, McDonough MH, Crocker PR. Psychosocial experiences of breast cancer survivors involved in a dragon boat program: exploring links to positive psychological growth. J Sport Exerc Psychol. 2007;29(4):419–438. [DOI] [PubMed] [Google Scholar]
- 74. Salhi B, Huysse W, van Maele G, Surmont VF, Derom E, van Meerbeeck JP. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I‐III lung cancer patients. Lung Cancer. 2014;84(1):56–61. [DOI] [PubMed] [Google Scholar]
- 75. Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. Br J Cancer. 2016;115(11):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu J, Chen P, Wang R, Yuan Y, Wang X, Li C. Effect of tai chi on mononuclear cell functions in patients with non‐small cell lung cancer. BMC Complement Altern Med. 2015;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Karvinen KH, Esposito D, Raedeke TD, Vick J, Walker PR. Effect of an exercise training intervention with resistance bands on blood cell counts during chemotherapy for lung cancer: a pilot randomized controlled trial. Springerplus. 2014;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–562. [DOI] [PubMed] [Google Scholar]
- 79. Orange ST, Jordan AR, Odell A, Kavanagh O, Hicks KM, Eaglen T, et al. Acute aerobic exercise‐conditioned serum reduces colon cancer cell proliferation in vitro through interleukin‐6‐induced regulation of DNA damage. Int J Cancer. 2022;151(2):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brown JC, Compton SLE, Meyerhardt JA, Spielmann G, Yang S. The dose‐response effect of aerobic exercise on inflammation in colon cancer survivors. Front Oncol. 2023;13:1257767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cheng E, Shi Q, Shields AF, Nixon AB, Shergill AP, Ma C, et al. Association of Inflammatory Biomarkers with Survival among Patients with Stage III colon cancer. JAMA Oncol. 2023;9(3):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta‐analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–2636. [DOI] [PubMed] [Google Scholar]
- 83. Crowe J, Francis JJ, Edbrooke L, Loeliger J, Joyce T, Prickett C, et al. Impact of an allied health prehabilitation service for haematologic patients receiving high‐dose chemotherapy in a large cancer centre. Support Care Cancer. 2022;30(2):1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate‐intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta‐regression. J Physiother. 2016;62(2):68–82. [DOI] [PubMed] [Google Scholar]
- 85. Klijn P, van Keimpema A, Legemaat M, Gosselink R, van Stel H. Nonlinear exercise training in advanced chronic obstructive pulmonary disease is superior to traditional exercise training. A randomized trial. Am J Respir Crit Care Med. 2013;188(2):193–200. [DOI] [PubMed] [Google Scholar]
- 86. Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C, et al. Short‐term preoperative high‐intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323–333. [DOI] [PubMed] [Google Scholar]
- 87. Minnella EM, Ferreira V, Awasthi R, Charlebois P, Stein B, Liberman AS, et al. Effect of two different pre‐operative exercise training regimens before colorectal surgery on functional capacity: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(11):969–978. [DOI] [PubMed] [Google Scholar]
- 88. Friedenreich CM, O'Reilly R, Shaw E, Stanczyk FZ, Yasui Y, Brenner DR, et al. Inflammatory marker changes in postmenopausal women after a year‐long exercise intervention comparing high versus moderate volumes. Cancer Prev Res (Phila). 2016;9(2):196–203. [DOI] [PubMed] [Google Scholar]
- 89. Denehy L, Edbrooke L. The role of exercise before cancer treatment. Semin Oncol Nurs. 2022;38(5):151330. [DOI] [PubMed] [Google Scholar]
- 90. Leach HJ, Devonish JA, Bebb DG, Krenz KA, Culos‐Reed SN. Exercise preferences, levels and quality of life in lung cancer survivors. Support Care Cancer. 2015;23(11):3239–3247. [DOI] [PubMed] [Google Scholar]
- 91. Missel M, Pedersen JH, Hendriksen C, Tewes M, Adamsen L. Exercise intervention for patients diagnosed with operable non‐small cell lung cancer: a qualitative longitudinal feasibility study. Support Care Cancer. 2015;23(8):2311–2318. [DOI] [PubMed] [Google Scholar]
- 92. Granger CL, Connolly B, Denehy L, Hart N, Antippa P, Lin KY, et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer. 2017;25(3):983–999. [DOI] [PubMed] [Google Scholar]
- 93. Farajivafa V, Khosravi N, Rezaee N, Koosha M, Haghighat S. Effectiveness of home‐based exercise in breast cancer survivors: a randomized clinical trial. BMC Sports Sci Med Rehabil. 2023;15(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cannon C. Telehealth, Mobile applications, and wearable devices are expanding cancer care beyond walls. Semin Oncol Nurs. 2018;34(2):118–125. [DOI] [PubMed] [Google Scholar]
- 95. Murray E, Hekler EB, Andersson G, Collins LM, Doherty A, Hollis C, et al. Evaluating digital health interventions: key questions and approaches. Am J Prev Med. 2016;51(5):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Waterland JL, Ismail H, Amin B, Granger CL, Denehy L, Riedel B. Patient acceptance of prehabilitation for major surgery: an exploratory survey. Support Care Cancer. 2021;29(2):779–785. [DOI] [PubMed] [Google Scholar]
- 97. Waterland JL, Chahal R, Ismail H, Sinton C, Riedel B, Francis JJ, et al. Implementing a telehealth prehabilitation education session for patients preparing for major cancer surgery. BMC Health Serv Res. 2021;21(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bennell KL, Lawford BJ, Metcalf B, Mackenzie D, Russell T, van den Berg M, et al. Physiotherapists and patients report positive experiences overall with telehealth during the COVID‐19 pandemic: a mixed‐methods study. J Physiother. 2021;67(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McDermott MM, Spring B, Berger JS, Treat‐Jacobson D, Conte MS, Creager MA, et al. Effect of a home‐based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. Jama. 2018;319(16):1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97(8):1187–1197. [DOI] [PubMed] [Google Scholar]
- 101. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–1082. [DOI] [PubMed] [Google Scholar]
- 102. Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med. 2016;50(23):1428–1437. [DOI] [PubMed] [Google Scholar]
- 103. Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 104. Grimmett C, Bradbury K, Dalton SO, Fecher‐Jones I, Hoedjes M, Varkonyi‐Sepp J, et al. The role of behavioral science in personalized multimodal Prehabilitation in cancer. Front Psychol. 2021;12:634223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barberan‐Garcia A, Navarro‐Ripoll R, Sánchez‐Lorente D, Moisés‐Lafuente J, Boada M, Messaggi‐Sartor M, et al. Cost‐effectiveness of a technology‐supported multimodal prehabilitation program in moderate‐to‐high risk patients undergoing lung cancer resection: randomized controlled trial protocol. BMC Health Serv Res. 2020;20(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van Stralen MM, Lechner L, Mudde AN, de Vries H, Bolman C. Determinants of awareness, initiation and maintenance of physical activity among the over‐fifties: a Delphi study. Health Educ Res. 2010;25(2):233–247. [DOI] [PubMed] [Google Scholar]
- 107. Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123(7):1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]


