Abstract
The spaceflight environment, including microgravity and radiation, may have considerable effects on the health and performance of astronauts, especially for long-duration and Martian missions. Conventional on-ground and in-space experimental approaches have been employed to investigate the comprehensive biological effects of the spaceflight environment. As a class of recently emerging bioengineered in vitro models, tissue chips are characterized by a small footprint, potential automation, and the recapitulation of tissue-level physiology, thus promising to help provide molecular and cellular insights into space medicine. Here, we briefly review the technical advantages of tissue chips and discuss specific on-chip physiological recapitulations. Several tissue chips have been launched into space, and more are poised to come through multi-agency collaborations, implying an increasingly important role of tissue chips in space medicine.
Keywords: microfluidics, spaceflight, microgravity, radiation, drug development
1. Introduction
Space exploration and travel are both exciting themselves and are of great scientific values and implications for human beings (Schwartz, 2020; Board and Council, 2012). In the past half-century, space travel and exploration have progressed substantially through the efforts of the National Aeronautics and Space Administration (NASA) and other governmental agencies and private companies, as exemplified by the human landing on Moon in 1969, Perseverance rover landing on Mars in 2021, and the International Space Station (ISS). The ISS has orbited the Earth at an approximate altitude of 400 km and a speed of 28,000 km per hour for the past two decades. Recently, due to the advent of reusable rockets such as SpaceX Falcon 9, the cost of launching a spacecraft has been reduced by approximately 50% and continues to decline, which makes personal space travel possible and perhaps popular in the near future. The field and market of space travel and exploration seem to burgeon in the next decade.
However, the biological effects of the spaceflight environment may overshadow space exploration, especially for long-duration and Martian missions (Afshinnekoo et al., 2020). The spaceflight environment, different from that on Earth, is primarily characterized by microgravity and high-energy radiation (Fig. 1a), which broadly affects biological systems and may lead to pathological conditions (Nicogossian, 2003). Microgravity can result in alterations of multiple organs/tissues, affecting the immune and musculoskeletal systems as well as renal and cardiac functions (Nicogossian, 2003). Some physiological alternations undermine the health and performance of astronauts, including the losses of muscle and bone masses (Lee et al., 2020). It is because that microgravity is unable to provide necessary mechanical stimuli to maintain tissue homeostasis and regeneration (Grimm et al., 2016; Juhl et al., 2021; Comfort et al., 2021). Also, microgravity leads to changes in the blood flow, such as flow turbulence and local density, which is associated with a rare venous thrombosis in an astronaut after working in the ISS for 2 months (Auñón-Chancellor et al., 2020). Microgravity was suggested to alter stem cell differentiation and gene regulation (Nagaraja and Risin, 2013; Zhang et al., 2018). For example, human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), onboard the ISS under microgravity for approximately 6 weeks, altered the expressions of 2,635 genes (Wnorowski et al., 2019).
Fig 1.
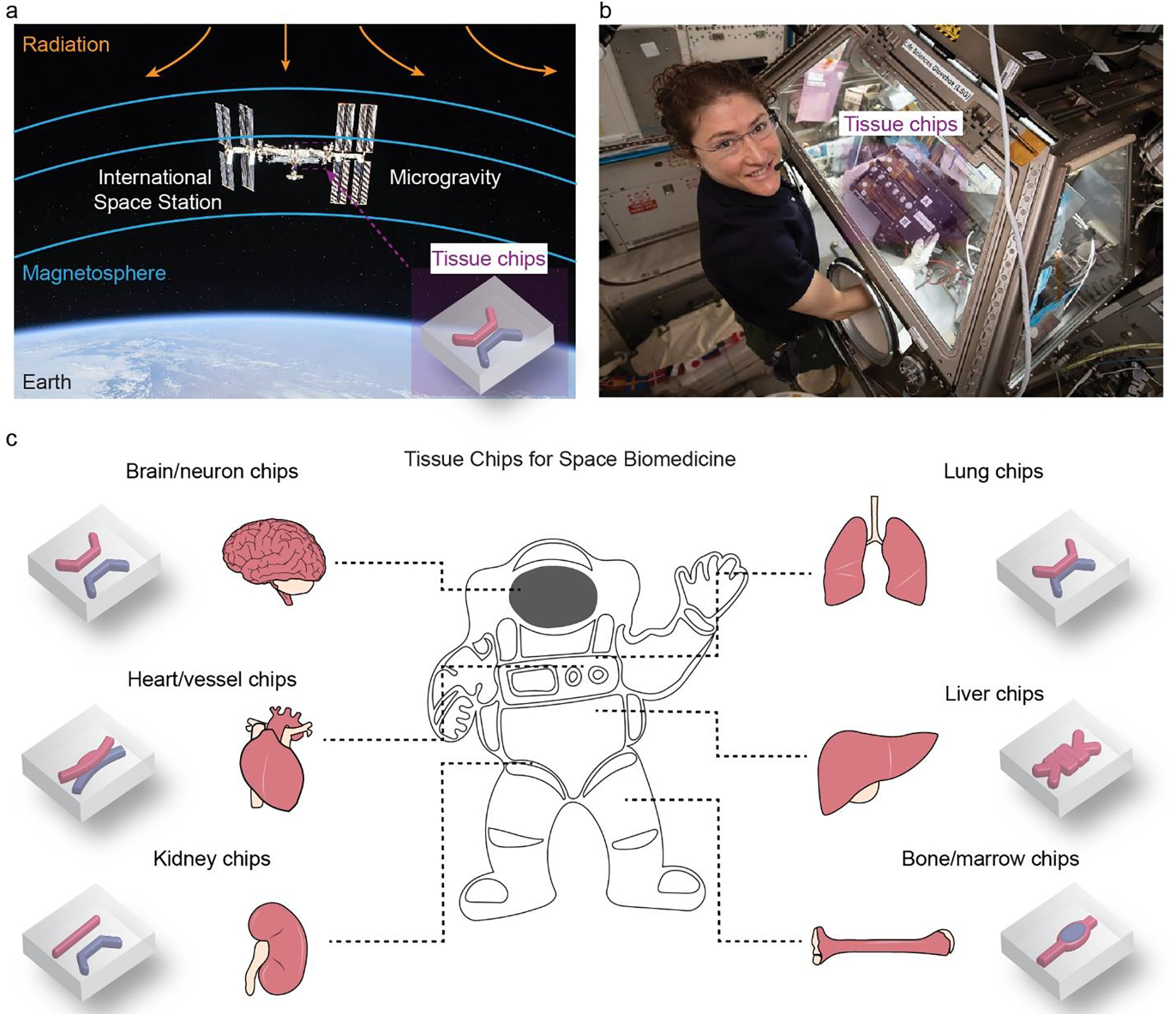
a. Tissue chips are a promising tool for understanding the biological effects of spaceflight environments, including microgravity and radiation. Photograph credit (iss056e201352): NASA/Roscosmos. b. Astronaut Christina Koch operated kidney chips in the International Space Station (ISS). The reduced footprint and improved automated manipulation of liquids make tissue chips compelling. Reproduced with permission (Yeung et al., 2020). Copyright 2020, John Wiley and Sons. c. Overview of tissue chips for recapitulating multiple tissue-level physiological functions. Microchannels and in-chip microstructures are labeled in red and blue. Multiple tissues can be incorporated into one chip.
Space radiation is another important feature of the spaceflight environment and a conundrum in space medicine (Chancellor et al., 2014). Space radiation has been associated with DNA damage (Dubrova et al., 2000), cancer (Sridharan et al., 2016), and other diseases (Wakayama et al., 2021; Mishra and Luderer, 2019). Away from the earth, space radiation may include energetic protons, alpha particles, and high-energy, and high-charge (HZE) nuclei, which may affect the welfare of astronauts during deep-space travels. In low-Earth orbit (LEO, with an altitude of 2,000 km or less), the space radiation is partially shielded by the Earth’s magnetic field.
It remains challenging to investigate the complicated space-related biological effects and develop space medicine. One obstacle is to recapitulate the microgravity and space radiation on Earth. Many on-ground approaches have been developed to resemble microgravity, including clinostat, random positioning machine, diamagnetic levitation, and drop towers (Ferranti et al., 2020), as well as to simulate galactic cosmic rays (GCR). In particular, the NASA Space Radiation Laboratory at Brookhaven National Laboratory is the only operating facility in the US for GCR simulation (Slaba et al., 2021; Norbury et al., 2019). A range of studies on GCR-induced biological effects has been carried out in this facility, including behavioral effects (Kiffer et al., 2019), omics datasets (Beheshti et al., 2018), and plants and plant propagules (Zhang et al., 2022). However, some on-ground approaches are limited to either short time scales or incomplete simulation. Furthermore, the on-ground approaches and datasets may be challenging to investigate the combined effects of space radiation and microgravity (Wakayama et al., 2021; Furukawa et al., 2020).
In addition to the on-ground approaches, the ISS and other in-space facilities have been used to investigate the limits of life in space and the habitability of Mars (De Vera et al., 2019; Poghosyan and Golkar, 2017). Of note, space radiation on the ISS is mitigated due to the shielding effect of the Earth’s magnetosphere (Fig. 1a). Due to the orbiting motion, the ISS is subject to microgravity around 1×10−6 m s−2, which is much lower than that on Earth, around 9.81 m s−2, thus suitable for microgravity studies. The ISS has hosted approximately 3,000 experiments from a diverse range of disciplines, including biology, technology, and physics, to benefit space exploration and fundamental biomedical research (Witze, 2020; Thirsk et al., 2009). However, the ISS and other in-space facilities are restricted by the accommodating/loading capacity, limiting the weight and footprint of research instruments. Also, the hands-on operation of astronauts is often limited. These experimental limitations in spaceflight probably lead to reduced complexity, oversimplification, and the narrowed scope of experiments, representing a major challenge in exploiting space-induced biological effects and space medicine.
Tissue chips or microphysiological systems (MPS) are promising toolsets for space biomedicine (Fig. 1b), primarily due to their relatively small footprints, the capability to recapitulate tissue-level physiology, and the possible automated operations (Low and Giulianotti, 2020; Low et al., 2021; Zhang et al., 2017; Mu et al., 2013a; Zhang et al., 2009). In the microchannels of tissue chips, surface tension and viscous forces dominate, benefiting fluid-handling and transportation (Nijhuis et al., 2022). Yet gravity is still playing a role in the microchannel (Huh et al., 2007; Giorello et al., 2020; Sun et al., 2019). In particular, continuous mass-dependent separation of particles has been carried out in microfluidic chips (Huh et al., 2007). Other gravity-induced phenomena have been reported, including the flow-focusing of liquids with unmatched densities (Giorello et al., 2020) and the participation of bacteria (Sun et al., 2019). Thus, it is still promising to deploy tissue chips in space for exploiting microgravity-related biological effects. Also, tissue chips will be a valuable alternative to animal models for in-space and on-ground studies.
NASA and the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) have collaborated with the ISS-National Laboratory (ISS-NL) along with other agencies to launch an array of tissue chips into the ISS and perform a range of biomedical experiments in space (Table 1). These tissue chips in the ISS promote the understanding of the molecular and cellular mechanism of spaceflight environment-related biological effects (Fig. 1c).
Table 1.
Tissue chips for space exploration in the ISS (Tagle, 2022).
| # | Targeted tissues & cells | Physiological pathways and diseases | Launch dates | Funding agencies |
|---|---|---|---|---|
|
| ||||
| 1 | Brain, Liver, and Gut | Brain-liver-gut axis, aging | 2022–2025 | NASA NIH BARDA FDA |
| 2 | Brain | Neurotoxic stress | ||
| 3 | Neurovasculature | Chronic inflammation and neurodegeneration | ||
| 4 | Vessel | Atherosclerosis | ||
| 5 | Multiple tissues | Human tissues to stressors | ||
| 6 | Multiple tissues | Repair after hypoxia | ||
| 7 | Heart, vascular tissues | Response to radiation exposure | ||
| 8 | Kidney | Acute and chronic exposure to drugs and toxins | ||
|
|
||||
| 9 | Myocytes | Muscle wasting | Dec 2020 | NCATS |
| 10 | Heart | Cardiomyopathy | Dec 2020 | |
| 11 | Intestine | Bacterial infection | Mar 2020 | |
| 12 | Cartilage-bone-synovium | Musculoskeletal disease | May 2019; Dec 2020 | |
| 13 | Lung | Lung host defense | May 2019 | |
| 14 | Blood-brian barrier | Microgravity effects | May 2019; Dec 2021 | |
| 15 | Stem cells | Immunological senescence | Dec 2018 | |
| 16 | Kidney | Proximal and distal tubule functions | May 2019; Jun 2021 | |
|
|
||||
| 17 | Human iPSC, heart | Cardiac dysfunction | Mar 2020 | NCATS & NIBIB |
NASA, National Aeronautics and Space Administration; NIH, National Institutes of Health; BARDA: Biomedical Advanced Research and Development Authority; FDA, Food and Drug Administration; NCATS, National Center for Advancing Translational Sciences; NIBIB, National Institute for Biomedical Imaging and Bioengineering.
In this review, we describe the technical fundamentals of tissue chips and their promising utility in space medicine. In particular, tissue chips are characterized by small footprints, tissue-level anatomy, and long-term maintenance of the tissue functions, thus highly promising for studying long-term space travel-related biological effects. We also review promising examples of tissue chips for space medicine, including drug development and fundamental biomedicine research, such as cancer biology.
2. Tissue chip techniques
Tissue chips are bioengineered microdevices that originate from microfluidic chips (Bhatia and Ingber, 2014; Mitchell, 2001) and can recapitulate essential physiological features at the tissue level, such as the breathing lung alveoli (Huang et al., 2021; Huh et al., 2012a; Huh et al., 2010) and glomerular filtration (Valverde et al., 2022; Ashammakhi et al., 2018; Mu et al., 2013b). The on-chip recapitulation of tissue-level physiological features are achieved by the combination of human cells, mammalian extracellular matrix (ECM) (Moses et al., 2021), advanced microfabrication, and well-controlled environmental cues (Viravaidya et al., 2004; Thompson et al., 2020). Also, tissue chips can integrate multiple cell types and tissue/organ-analogs for investigating the metabolic pathway of drugs and the toxicity of metabolites, superior to single-tissue/cell models (Miller and Shuler, 2016; Zhang et al., 2017; Skardal et al., 2017; Nahmias et al., 2007; Trapecar et al., 2021; Herland et al., 2020; Novak et al., 2020). This feature of tissue chips also allows the construction of quantitative pharmacokinetic-pharmacodynamic (PK-PD) models (Sung and Shuler, 2009; Esch et al., 2011; Lee et al., 2017).
Tissue chips are expected to offer a more realistic and accessible physiological environment and predict human responses better than two-dimensional (2D) Petri dish-based cell culture and some animal models. It has been increasingly recognized that animal models exhibit physiological and pathological conditions distinct from human beings, thus leading to a high rate of failure in clinical trials (Akhtar, 2015; Horejs, 2021). Also, compared with animal models, tissue chips are characterized by well-controlled and decoupled experimental conditions and convenient real-time imaging. Depending on the substrate materials and fabrication techniques, tissue chips can be high in cost and thus largely used for low- to moderate-throughput screening (Leung et al., 2022). However, the cost of tissue chips can be lowered by adopting affordable fabrication techniques (Winkler et al., 2020). Tissue chips have been recognized as a potential lower-cost alternative to animal models (Huh et al., 2013) and hold great promise for facilitating clinical trials and speeding up drug development (Caplin et al., 2015; Ma et al., 2021). Tissue chips may further enable the integration of various types of biosensors for in-situ, continually, and automatically monitoring of on-chip cellular responses (Zhang et al., 2017; Aleman et al., 2021; Lima et al., 2014; Miller et al., 2021). Due to all these technical benefits, tissue chips are one of the most promising technical platforms for exploiting space medicine. To deploy tissue chips in space is worth taking all necessary costs. Below, we discuss the space medicine-related technical benefits of tissue chips, including relatively small footprints, long-term preservation of tissue functions, and heterogeneous cellular composition, as well as other promising biomedical utility of tissue chips in space, such as cancer biology and drug development.
2.1. Relatively small footprint
Compared with conventional approaches, tissue chip-based systems, including tissue chips and peripherals, may be of relatively small footprint and thus be suitable for loading into the space-limited ISS and other spacecrafts. Tissue chips are usually not larger than the size of a palm, a huge reduction of footprint compared to conventional bioreactors. The relatively small footprint of tissue chips is due to advanced microfabrication approaches, including lithography (McDonald et al., 2000; Whitesides et al., 2001) and recently, three-dimensional (3D) printing (Ching et al., 2019; Au et al., 2016; Rahmani Dabbagh et al., 2022). The advanced fabrication approaches allow the construction of precise microstructures in the length scales of a few to a few hundreds of micrometers, such as networks of microchannels and micropillar arrays.
Notably, tissue chips also require external power sources, tubing, and regulating systems, which are oftentimes much larger than the chips themselves yet still contribute to a small footprint compared with conventional approaches. For example, a kidney chip system required four syringe pumps, two incubators and tubing over 30 meters, accounting for a total volume of approximately 1,350 L (Yeung et al., 2020). Through optimizing the external perfusion and environmental control systems, the size of a tissue chip-based device can be reduced from approximately 1,350 L to 45 L, which fits well the launch capsule and the limited experimental space in the ISS (Yeung et al., 2020). The footprint of the tissue chip-based systems may be further reduced through continued engineering innovations, for example, capillary phenomena-based sequential liquid-manipulation (Yafia et al., 2022).
The tissue chips with microchannels and micrometer-sized structures may provide other size-related benefits. These microstructures are comparable to the size of cells, thus beneficial for manipulating cells, such as patterning and trapping (Mu et al., 2013a; Ma et al., 2018). Also, the mass/heat-transport is usually faster on a small length scale than on a large one (Dittrich and Manz, 2006). Flow phenomena under small scales, such as multistream laminar flow and multi-phase droplets, provide flow-based approaches for manipulating cells, which reduces the system complexity (Mu et al., 2009; Teh et al., 2008; Mu et al., 2014).
2.2. Long-term culture
Tissue chips enable the long-term cell culture and the preservation of tissue-level physiology, resulting from the bioengineered on-chip environment that is physiologically relevant. The long-term on-chip cell culture benefits the investigations of non-acute biological effects of the spaceflight environment (Vernetti et al., 2016; Qiu et al., 2018; Sieber et al., 2018), and allows the investigations of non-acute cellular responses, which is important for the pathogenesis of chronic diseases. In addition, the launch of a spacecraft may experience unexpected changes and delays (Yeung et al., 2020), which can be mitigated by the long-term self-contained culture capacity of tissue chips. Most of current tissue chips enable cell culture for 3–4 weeks, but the more recent multi-agency collaborative effort is to extend the culture period for at least 6 months, which supports eight tissue chip-based projects that are supposed to function in an automated manner and are projected to be onboard the ISS (Tagle, 2022). Below we discuss two exemplary tissue chips that can support cell culture and tissue-level functions for around 1 month.
One liver chip consists of human hepatocytes, non-parenchymal cell lines, and collagen hydrogels, maintaining metabolic functions for at least 28 days (Vernetti et al., 2016). The liver chip reveals the acute toxicity of 180-μM troglitazone and 210-μM nimesulide within 2–4 days, characterized by sharply decreased albumin and urea, increased reactive oxygen species (ROS), and cellular apoptosis. More importantly, the 28-μM troglitazone did not lead to significant cellular apoptosis until 14 days. This result underscores the importance of long-term on-chip culture for uncovering non-acute and chronic drug toxicity.
Another vessel chip maintained endothelial barrier functions over 1 month; the long-term on-chip culture is attributed to the physiologically relevant microenvironment, including substrate stiffness and laminar flow (Fig. 2a–c) (Qiu et al., 2018). In particular, the vessel chip employed an agarose-alginate interpenetrating-polymer-network (IPN) hydrogel with Young’s modulus of roughly 20 kPa, mimicking that of the tissues surrounding the vessels (~35 kPa) (Huynh et al., 2011). In contrast, a stiffer material of ~50 kPa, silicone rubber polydimethylsiloxane (PDMS), led to significantly increased permeability; a softer one of ~1 kPa was not feasible to form an endothelial barrier. The long-term preservation of vascular barrier function enables investigations of the interactions between pathological red blood cells and endothelial cells. The vessel chips demonstrated that pathological Plasmodium falciparum-infected blood cells alone led to increased vascular permeability, which does not require the participation of other types of cells, such as immune cells. This result thus provides insights into a long-standing controversy regarding the cellular mechanism of malaria (Frevert and Nacer, 2014).
Fig. 2.
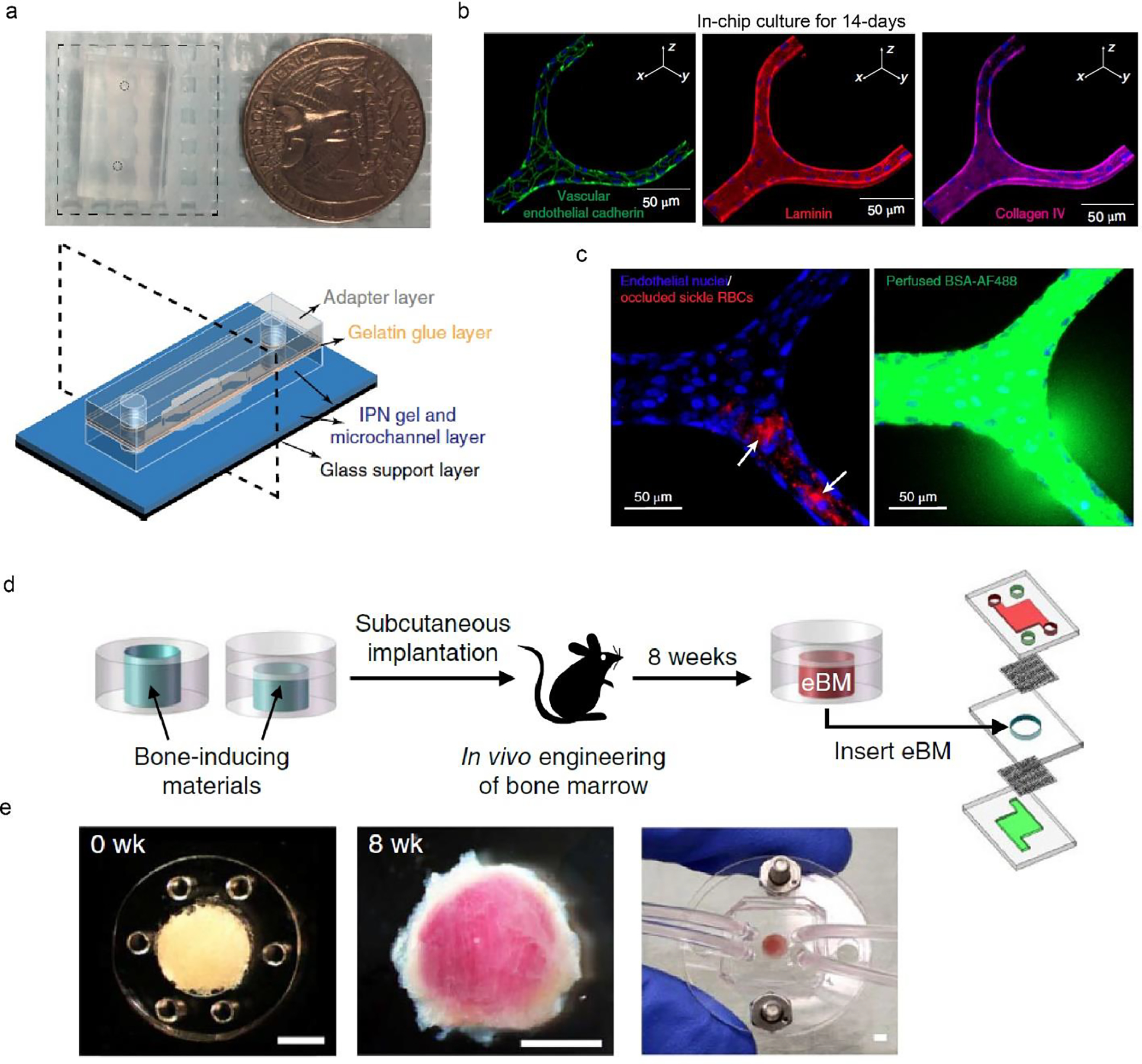
Two tissue chips for recapitulating the blood vessel and the bone marrow. a. Macroscopic view of a blood vessel chip, the size of which is compared with a quarter. Schematic of the tissue chip design, including glass support layer and several hydrogel layers. b. Immunostaining of endothelial cells after 14-day in-chip culture. c. Blockage of sickle RBCs in the engineered vessel leads to local leakage. a-c, Reproduced with permission (Qiu et al., 2018). Copyright 2018, Springer Nature. d. Schematic of a bone marrow chip using subcutaneous implantation for producing engineered bone marrow (eBM). e. Bone marrow tissues before (0 week) and after 8 weeks of implantation. The chip enables media perfusion and the delivery of biochemical cues. Scale bars, 2 mm. d-e, Reproduced with permission (Torisawa et al., 2014). Copyright 2014, Springer Nature.
2.3. Heterogenous cellular composition
Tissues are composed of heterogonous cells. The complex and dynamic interactions between these cells are key to tissue homeostasis and disease pathogenesis. For example, the respiratory system, including the alveoli and large and small airways, contains more than 40 types of cells; the lung alveolus alone contains two types of epithelial cells, macrophages, endothelial cells, and fibroblasts, among others (Franks et al., 2008). Tissue chips employ microstructures and microchannels to incorporate and pattern multiple cells to resemble the complex anatomical and physiological characteristics, thus promising to decipher the role of cellular interactions at the tissue level (Mertz et al., 2018; Huh et al., 2012b). In particular, epithelial and endothelial cells were cultured in individual yet interconnected channels, recapitulating the tissue interface in the lung alveoli (Huh et al., 2012b) and the nephron (Mu et al., 2013b).
One bone marrow chip reconstitutes a complex population of bone marrow cells to recapitulate the hematopoietic niche and to model radiation toxicity and potential countermeasures of drugs (Fig. 2d, e) (Torisawa et al., 2014). This bone marrow chip exploited an in vivo approach, i.e., 8-week subcutaneous implantation, for forming trabecular bone-like tissues with a central region of blood-filled marrow. The reconstituted bone marrow resembled the morphological, biochemical, and cellular aspects of natural bone marrow. The cell population residing in the bone marrow included hematopoietic stem cells (HSCs), hematopoietic progenitor cells (HPCs), and all other differentiated blood cells, such as erythrocytes, lymphocytes, and myeloid cells. The complete set of bone marrow cells was suggested to work collectively to maintain hematopoietic functions and mimic the response to radiation.
The reconstituted bone marrow was incorporated into a microfluidic chip, where on-chip perfusion provided physiological flow stress and delivered nutrients and water-soluble chemicals for keeping cells’ self-renewal and differentiation for more than 1 week. After exposing to 1- and 4-Gy doses of γ-radiation, the bone marrow chip exhibited a dose-dependent decrease in the proportion of HSCs, HPCs, lymphoid cells, and myeloid cells. This on-chip result is nearly identical to that of live mice yet not observed with a static stroma-supported model. Also, colony-stimulating factor (G-CSF), a therapeutic agent for radiation toxicity (Hérodin and Drouet, 2005), can increase the number of hematopoietic stem cells and progenitor cells in the bone marrow chip. These results imply the potential of the bone marrow chip, as a useful alternative to animal models, for mimicking radiation responses and screening anti-radiation drugs.
In addition to using mouse cells, some bone marrow chips can be composed of all human cells (Aleman et al., 2019; Chou et al., 2020), thus minimizing potentially biased results due to species differences. One study used multiple bone marrow-derived cells, including sinusoidal endothelial cells, arterial endothelial cells, mesenchymal stromal cells (MSCs), and MSCs-differentiated osteoblasts, to construct 4 distinct bone marrow niches within an integrated, recirculating perfusion system (Aleman et al., 2019). Another study employed two microfluidic channels to recapitulate hematopoietic and vascular morphologies, respectively (Chou et al., 2020). The on-chip results, such as ionizing radiation-induced decrease of bone marrow cells, quantitatively matched human radiation sensitivity.
2.4. Cancer biology
Tissue chips can be deployed in space to empower other fundamental biomedical research in space, such as cancer biology (Krüger et al., 2019). It is primarily because of the space environment that impacts cancer cell behaviors and perhaps carcinogenesis and is often challenging to replicate on ground. Tissue chips have been exploited to reconstruct key tumor features and investigate the pathogenesis, metastasis, and potential treatments for several cancers (Mu and Zhang, 2022; Sontheimer-Phelps et al., 2019), paving the way for in-space investigation.
Microgravity has been shown to affect the behavior of cancerous breast cells (MCF-7), such as invasion, adhesion, migration, vinculin expression, and apoptosis, compared to normal breast cells (MCF10A) (Monti et al., 2021). Some regulating genes and signaling pathways have been suggested to involve microgravity-induced cellular alternations (Ma et al., 2014; Arun et al., 2017). Moreover, cancer cells in space tend to form 3D tissue-like spheroids that may capture some tissue-level features and facilitate testing anti-cancer drugs (Grimm et al., 2018; Aleshcheva et al., 2016).
An all-glass tissue chip has been used to investigate skin melanoma cell line A375 under short-term (2 hr) simulated microgravity using a 3D clinostat (Przystupski et al., 2021). The simulated microgravity was found to increased caspase activity and reduce proliferation of cancer cells, which may be linked to apoptosis and are consistent with previous results (Arun et al., 2017; Kossmehl et al., 2003). Although these results are interesting, more efforts are most likely needed to extend the culture and simulation period.
2.5. Drug development
The biological effects of the space environment are also important to medication and drug development (Braddock, 2020; Giulianotti and Low, 2020; Eyal and Derendorf, 2020). The bioavailability, metabolic pathway, and pharmacokinetics of drugs in space may differ from those on Earth (Eyal and Derendorf, 2019). Also, the drug-drug interactions (DDIs) may lead to the compromised performance of astronauts. For example, the combination of sleep aids and anti-emetics involves cumulative central nervous system (CNS) depression, and such sedation may be problematic for extravehicular activity (spacewalk) (Berman and Eyal, 2019). Furthermore, most DDIs potentially encountered in space have not been fully investigated. Thus, it is desirable to avoid non-established drug combinations in space and to develop a space-related clinical decision support (CDS) system (Berman and Eyal, 2019). Tissue chips in space are promising to verify the efficacy of drugs and the potential crosstalk. In particular, some vessel chips with on-chip mechanical stimuli and sensors may be useful for screening drugs in space for vascular diseases (Ribas et al., 2017; Sadlowski et al., 2018; Giulianotti and Low, 2020).
The spaceflight environment leads to rapid physiological alternations that resemble the effects of expedited aging, including changes in telomere length, cardiovascular dysregulation, neurodegeneration, and a mosaic of somatic mutations (Afshinnekoo et al., 2020). These accelerated physiological alternations could shorten the required experimental time of tissue chips for observing disease development and cellular responses to drug candidates, thus promising to expedite drug discovery (Giulianotti and Low, 2020). Of note, it remains unclear if in-space aging and disease development’s mechanisms are the same as the terrestrial ones. Nevertheless, tissue chips enable further exploration to compare the mechanisms on-Earth and in-space.
Some PDMS-constituted tissue chips may suffer from the bulk absorption of small hydrophobic molecules due to the porosity and hydrophobicity of the polymer network of PDMS (Toepke and Beebe, 2006). The bulk absorption may bias the concentration of drug candidates within the microchannels, thus leading to challenges in predicting cellular responses. There are several approaches to ameliorate this issue, including surface modification (Ren et al., 2010), a simulation-facilitated experimental approach (Grant et al., 2021), and the adoption of other polymeric materials for fabricating tissue chips, such as Teflon and thermoplastic polypropylene (Ren et al., 2011; Sun et al., 2019; Pourmand et al., 2018; Shaegh et al., 2018).
3. Conclusion and perspective
In summary, the tissue chip techniques as versatile bioengineered small-footprint physiological models have exhibited particular advantages in understanding biological effects in space for developing space medicine. The huge potential of tissue chips in space is in part reflected in the ongoing projects supported by the multi-agency collaboration. As tissue chips are naturally multi-disciplinary, the collaboration between scientists and engineers with diverse backgrounds and between different regulating agencies would be key to future success. Tissue chips are still in their infancy, yet we envision them playing an increasingly important role in space exploration and space medicine.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health (R21EB025270, R00CA201603, UG3TR003274) and the Brigham Research Institute.
References
- Afshinnekoo E, Scott RT, MacKay MJ, Pariset E, Cekanaviciute E, Barker R, Gilroy S, Hassane D, Smith SM, Zwart SR (2020) Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell 183 (5):1162–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A (2015) The flaws and human harms of animal experimentation. Cambridge Quarterly of Healthcare Ethics 24 (4):407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman J, George SK, Herberg S, Devarasetty M, Porada CD, Skardal A, Almeida-Porada G (2019) Deconstructed Microfluidic Bone Marrow On-A-Chip to Study Normal and Malignant Hemopoietic Cell–Niche Interactions. Small 15 (43):1902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman J, Kilic T, Mille LS, Shin SR, Zhang YS (2021) Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat Protoc 16 (5):2564–2593. doi: 10.1038/s41596-021-00511-7 [DOI] [PubMed] [Google Scholar]
- Aleshcheva G, Bauer J, Hemmersbach R, Slumstrup L, Wehland M, Infanger M, Grimm D (2016) Scaffold-free tissue formation under real and simulated microgravity conditions. Basic & clinical pharmacology & toxicology 119:26–33 [DOI] [PubMed] [Google Scholar]
- Arun RP, Sivanesan D, Vidyasekar P, Verma RS (2017) PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of Colorectal Cancer Cells under Simulated Microgravity. Sci Rep 7 (1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N, Wesseling-Perry K, Hasan A, Elkhammas E, Zhang YS (2018) Kidney-on-a-chip: untapped opportunities. Kidney international 94 (6):1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au AK, Huynh W, Horowitz LF, Folch A (2016) 3D-printed microfluidics. Angew Chem Int Ed 55 (12):3862–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auñón-Chancellor SM, Pattarini JM, Moll S, Sargsyan A (2020) Venous thrombosis during spaceflight. N Engl J Med 382 (1):89–90 [DOI] [PubMed] [Google Scholar]
- Beheshti A, Miller J, Kidane Y, Berrios D, Gebre SG, Costes SV (2018) NASA GeneLab project: bridging space radiation omics with ground studies. Radiat Res 189 (6):553–559 [DOI] [PubMed] [Google Scholar]
- Berman E, Eyal S (2019) Drug interactions in space: a cause for concern? Pharm Res 36 (8):1–6 [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32 (8):760–772. doi: 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- Board SS, Council NR (2012) Recapturing a future for space exploration: life and physical sciences research for a new era. National Academies Press, [Google Scholar]
- Braddock M (2020) From target identification to drug development in space: using the microgravity assist. Current Drug Discovery Technologies 17 (1):45–56 [DOI] [PubMed] [Google Scholar]
- Caplin JD, Granados NG, James MR, Montazami R, Hashemi N (2015) Microfluidic organ-on-a-chip technology for advancement of drug development and toxicology. Adv Healthcare Mater 4 (10):1426–1450 [DOI] [PubMed] [Google Scholar]
- Chancellor JC, Scott GB, Sutton JP (2014) Space radiation: the number one risk to astronaut health beyond low earth orbit. Life 4 (3):491–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T, Li Y, Karyappa R, Ohno A, Toh Y-C, Hashimoto M (2019) Fabrication of integrated microfluidic devices by direct ink writing (DIW) 3D printing. Sensors and Actuators B: Chemical 297:126609. doi: 10.1016/j.snb.2019.05.086 [DOI] [Google Scholar]
- Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bölükbaşı Ö, Joyce CE, Moreira Teixeira LS (2020) On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng 4 (4):394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort P, McMahon J, Jones P, Cuthbert M, Kendall K, Lake J, Haff GG (2021) Effects of spaceflight on musculoskeletal health: a systematic review and meta-analysis, considerations for interplanetary travel. Sports Medicine 51 (10):2097–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vera J-P, Alawi M, Backhaus T, Baqué M, Billi D, Böttger U, Berger T, Bohmeier M, Cockell C, Demets R (2019) Limits of life and the habitability of Mars: the ESA space experiment BIOMEX on the ISS. Astrobiology 19 (2):145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich PS, Manz A (2006) Lab-on-a-chip: microfluidics in drug discovery. Nature Reviews Drug Discovery 5 (3):210–218. doi: 10.1038/nrd1985 [DOI] [PubMed] [Google Scholar]
- Dubrova YE, Plumb M, Gutierrez B, Boulton E, Jeffreys AJ (2000) Transgenerational mutation by radiation. Nature 405 (6782):37–37 [DOI] [PubMed] [Google Scholar]
- Esch MB, King TL, Shuler ML (2011) The Role of Body-on-a-Chip Devices in Drug and Toxicity Studies. In: Yarmush ML, Duncan JS, Gray ML(eds) Annual Review of Biomedical Engineering, Vol 13, vol 13. Annual Review of Biomedical Engineering. Annual Reviews, Palo Alto, pp 55–72. doi: 10.1146/annurev-bioeng-071910-124629 [DOI] [PubMed] [Google Scholar]
- Eyal S, Derendorf H (2019) Medications in space: in search of a pharmacologist’s guide to the galaxy. Pharm Res 36 (10):1–13 [DOI] [PubMed] [Google Scholar]
- Eyal S, Derendorf H (2020) One Giant Leap for Pharmacology. vol 37. Springer, [DOI] [PubMed] [Google Scholar]
- Ferranti F, Del Bianco M, Pacelli C (2020) Advantages and limitations of current microgravity platforms for space biology research. Applied Sciences 11 (1):68 [Google Scholar]
- Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, Cardoso WV, Crystal RG, Drake CJ, Engelhardt J (2008) Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proceedings of the American Thoracic Society 5 (7):763–766 [DOI] [PubMed] [Google Scholar]
- Frevert U, Nacer A (2014) Fatal cerebral malaria: a venous efflux problem. Frontiers in cellular and infection microbiology 4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S, Katsube T, Wang B, Tsuruoka C, Shirai T, Nakamura AJ (2020) Space radiation biology for “Living in Space”. BioMed Research International 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorello A, Minetti F, Nicastro A, Berli CL (2020) The effect of gravity on microfluidic flow focusing. Sensors and Actuators B: Chemical 307:127595 [Google Scholar]
- Giulianotti MA, Low LA (2020) Pharmaceutical research enabled through microgravity: perspectives on the use of the International Space Station US National Laboratory. Pharm Res 37 (1):1–4 [DOI] [PubMed] [Google Scholar]
- Grant J, Özkan A, Oh C, Mahajan G, Prantil-Baun R, Ingber DE (2021) Simulating drug concentrations in PDMS microfluidic organ chips. Lab Chip 21 (18):3509–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Egli M, Krüger M, Riwaldt S, Corydon TJ, Kopp S, Wehland M, Wise P, Infanger M, Mann V (2018) Tissue engineering under microgravity conditions–use of stem cells and specialized cells. Stem Cells and Development 27 (12):787–804 [DOI] [PubMed] [Google Scholar]
- Grimm D, Grosse J, Wehland M, Mann V, Reseland JE, Sundaresan A, Corydon TJ (2016) The impact of microgravity on bone in humans. Bone 87:44–56 [DOI] [PubMed] [Google Scholar]
- Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, Chalkiadaki A, Benson Chou D, Marquez S, Delahanty A, Jalili-Firoozinezhad S, Milton Y, Sontheimer-Phelps A, Swenor B, Levy O, Parker KK, Przekwas A, Ingber DE (2020) Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng 4 (4):421–436. doi: 10.1038/s41551-019-0498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérodin F, Drouet M (2005) Cytokine-based treatment of accidentally irradiated victims and new approaches. Experimental hematology 33 (10):1071–1080 [DOI] [PubMed] [Google Scholar]
- Horejs C (2021) Organ chips, organoids and the animal testing conundrum. Nat Rev Mater 6 (5):372–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Liu T, Liao J, Maharjan S, Xie X, Pérez M, Anaya I, Wang S, Mayer AT, Kang Z (2021) Reversed-engineered human alveolar lung-on-a-chip model. Proc Natl Acad Sci 118 (19): e2016146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Bahng JH, Ling Y, Wei H-H, Kripfgans OD, Fowlkes JB, Grotberg JB, Takayama S (2007) Gravity-driven microfluidic particle sorting device with hydrodynamic separation amplification. Analytical chemistry 79 (4):1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE (2013) Microfabrication of human organs-on-chips. Nat Protoc 8 (11):2135–2157 [DOI] [PubMed] [Google Scholar]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE (2012a) A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4 (159):159ra147–159ra147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting Organ-Level Lung Functions on a Chip. Science 328 (5986):1662–1668. doi: 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Torisawa Y-s, Hamilton GA, Kim HJ, Ingber DE (2012b) Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12 (12):2156–2164 [DOI] [PubMed] [Google Scholar]
- Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3 (112):112ra122–112ra122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl OJ, Buettmann EG, Friedman MA, DeNapoli RC, Hoppock GA, Donahue HJ (2021) Update on the effects of microgravity on the musculoskeletal system. npj Microgravity 7 (1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffer F, Boerma M, Allen A (2019) Behavioral effects of space radiation: a comprehensive review of animal studies. Life sciences in space research 21:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmehl P, Shakibaei M, Cogoli A, Infanger M, Curcio F, nberger J, Eilles C, Bauer J, Pickenhahn H, Schulze-Tanzil G (2003) Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology 144 (9):4172–4179 [DOI] [PubMed] [Google Scholar]
- Krüger M, Melnik D, Kopp S, Buken C, Sahana J, Bauer J, Wehland M, Hemmersbach R, Corydon TJ, Infanger M (2019) Fighting thyroid cancer with microgravity research. Int J Mol Sci 20 (10):2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DS, Ha SK, Choi I, Lee JM, Sung JH (2017) A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic–pharmacodynamic (PK–PD) model. Biotechnology and bioengineering 114 (2):432–443 [DOI] [PubMed] [Google Scholar]
- Lee S-J, Lehar A, Meir JU, Koch C, Morgan A, Warren LE, Rydzik R, Youngstrom DW, Chandok H, George J (2020) Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc Natl Acad Sci 117 (38):23942–23951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CM, De Haan P, Ronaldson-Bouchard K, Kim G-A, Ko J, Rho HS, Chen Z, Habibovic P, Jeon NL, Takayama S (2022) A guide to the organ-on-a-chip. Nature Reviews Methods Primers 2 (1):1–29 [Google Scholar]
- Lima EA, Snider RM, Reiserer RS, McKenzie JR, Kimmel DW, Eklund SE, Wikswo JP, Cliffel DE (2014) Multichamber multipotentiostat system for cellular microphysiometry. Sensors and Actuators B: Chemical 204:536–543. doi: 10.1016/j.snb.2014.07.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LA, Giulianotti MA (2020) Tissue chips in space: modeling human diseases in microgravity. Pharm Res 37 (1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA (2021) Organs-on-chips: into the next decade. Nature Reviews Drug Discovery 20 (5):345–361. doi: 10.1038/s41573-020-0079-3 [DOI] [PubMed] [Google Scholar]
- Ma C, Peng Y, Li H, Chen W (2021) Organ-on-a-Chip: A new paradigm for drug development. Trends in pharmacological sciences 42 (2):119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L-D, Wang Y-T, Wang J-R, Wu J-L, Meng X-S, Hu P, Mu X, Liang Q-L, Luo G-A (2018) Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 18 (17):2547–2562 [DOI] [PubMed] [Google Scholar]
- Ma X, Pietsch J, Wehland M, Schulz H, Saar K, Hübner N, Bauer J, Braun M, Schwarzwälder A, Segerer J (2014) Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. The FASEB Journal 28 (2):813–835 [DOI] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu HK, Schueller OJA, Whitesides GM (2000) Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21 (1):27–40 [DOI] [PubMed] [Google Scholar]
- Mertz DR, Ahmed T, Takayama S (2018) Engineering cell heterogeneity into organs-on-a-chip. Lab Chip 18 (16):2378–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Schaffer DK, Neely MD, McClain ES, Travis AR, Block FE, McKenzie JR, Werner EM, Armstrong L, Markov DA, Bowman AB, Ess KC, Cliffel DE, Wikswo JP (2021) A bistable, multiport valve enables microformulators creating microclinical analyzers that reveal aberrant glutamate metabolism in astrocytes derived from a tuberous sclerosis patient. Sensors and Actuators B: Chemical 341:129972. doi: 10.1016/j.snb.2021.129972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PG, Shuler ML (2016) Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnology and bioengineering 113 (10):2213–2227 [DOI] [PubMed] [Google Scholar]
- Mishra B, Luderer U (2019) Reproductive hazards of space travel in women and men. Nature Reviews Endocrinology 15 (12):713–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P (2001) Microfluidics—downsizing large-scale biology. Nat Biotechnol 19 (8):717–721 [DOI] [PubMed] [Google Scholar]
- Monti N, Masiello MG, Proietti S, Catizone A, Ricci G, Harrath AH, Alwasel SH, Cucina A, Bizzarri M (2021) Survival pathways are differently affected by microgravity in normal and cancerous breast cells. Int J Mol Sci 22 (2):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SR, Adorno JJ, Palmer AF, Song JW (2021) Vessel-on-a-chip models for studying microvascular physiology, transport, and function in vitro. American Journal of Physiology-Cell Physiology 320 (1):C92–C105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Liang Q, Zhou J, Ren K, Hu P, Wang Y, Zheng Z, Luo G (2014) Oil–water biphasic parallel flow for the precise patterning of metals and cells. Biomedical Microdevices 16:245–253. doi: 10.1007/s10544-013-9828-y [DOI] [PubMed] [Google Scholar]
- Mu X, Liang QL, Hu P, Ren KN, Wang YM, Luo GA (2009) Laminar flow used as “liquid etch mask” in wet chemical etching to generate glass microstructures with an improved aspect ratio. Lab Chip 9 (14):1994–1996. doi: 10.1039/b904769g [DOI] [PubMed] [Google Scholar]
- Mu X, Zhang YS (2022) Tumor-on-a-chip devices for cancer immunotherapy. In: Engineering Technologies and Clinical Translation. Elsevier, pp 155–195 [Google Scholar]
- Mu X, Zheng W, Sun J, Zhang W, Jiang X (2013a) Microfluidics for Manipulating Cells. Small 9 (1):9–21. doi: 10.1002/smll.201200996 [DOI] [PubMed] [Google Scholar]
- Mu X, Zheng W, Xiao L, Zhang W, Jiang X (2013b) Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip 13 (8):1612–1618 [DOI] [PubMed] [Google Scholar]
- Nagaraja MP, Risin D (2013) The current state of bone loss research: data from spaceflight and microgravity simulators. J Cell Biochem 114 (5):1001–1008 [DOI] [PubMed] [Google Scholar]
- Nahmias Y, Berthiaume F, Yarmush ML (2007) Integration of technologies for hepatic tissue engineering. In: Lee K, Kaplan D (eds) Tissue Engineering Ii: Basics of Tissue Engineering and Tissue Applications, vol 103. Advances in Biochemical Engineering-Biotechnology. pp 309–329. doi: 10.1007/10_029 [DOI] [PubMed] [Google Scholar]
- Nicogossian A (2003) Medicine and space exploration. The Lancet 362:s8–s9 [DOI] [PubMed] [Google Scholar]
- Nijhuis J, Schmidt S, Tran NN, Hessel V (2022) Microfluidics and Macrofluidics in Space: ISS-Proven Fluidic Transport and Handling Concepts. Frontiers in Space Technologies 2:779696 [Google Scholar]
- Norbury JW, Slaba TC, Aghara S, Badavi FF, Blattnig SR, Clowdsley MS, Heilbronn LH, Lee K, Maung KM, Mertens CJ (2019) Advances in space radiation physics and transport at NASA. Life Sciences in Space Research 22:98–124 [DOI] [PubMed] [Google Scholar]
- Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, Maoz BM, Jeanty SSF, Somayaji MR, Burt M, Calamari E, Chalkiadaki A, Cho A, Choe Y, Chou DB, Cronce M, Dauth S, Divic T, Fernandez-Alcon J, Ferrante T, Ferrier J, FitzGerald EA, Fleming R, Jalili-Firoozinezhad S, Grevesse T, Goss JA, Hamkins-Indik T, Henry O, Hinojosa C, Huffstater T, Jang K-J, Kujala V, Leng L, Mannix R, Milton Y, Nawroth J, Nestor BA, Ng CF, O’Connor B, Park T-E, Sanchez H, Sliz J, Sontheimer-Phelps A, Swenor B, Thompson G, Touloumes GJ, Tranchemontagne Z, Wen N, Yadid M, Bahinski A, Hamilton GA, Levner D, Levy O, Przekwas A, Prantil-Baun R, Parker KK, Ingber DE (2020) Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng 4 (4):407–420. doi: 10.1038/s41551-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poghosyan A, Golkar A (2017) CubeSat evolution: Analyzing CubeSat capabilities for conducting science missions. Progress in Aerospace Sciences 88:59–83 [Google Scholar]
- Pourmand A, Shaegh SAM, Ghavifekr HB, Aghdam EN, Dokmeci MR, Khademhosseini A, Zhang YS (2018) Fabrication of whole-thermoplastic normally closed microvalve, micro check valve, and micropump. Sensors and Actuators B: Chemical 262:625–636 [Google Scholar]
- Przystupski D, Górska A, Michel O, Podwin A, Śniadek P, Łapczyński R, Saczko J, Kulbacka J (2021) Testing Lab-on-a-Chip technology for culturing human melanoma cells under simulated microgravity. Cancers 13 (3):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH, Ofori-Acquah SF, Lam WA (2018) Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng 2 (6):453–463. doi: 10.1038/s41551-018-0224-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Dabbagh S, Rezapour Sarabi M, Birtek MT, Mustafaoglu N, Zhang YS, Tasoglu S (2022) 3D bioprinted organ-on-chips. Aggregate:doi.org/ 10.1002/agt1002.1197 [DOI] [Google Scholar]
- Ren K, Zhao Y, Su J, Ryan D, Wu H (2010) Convenient method for modifying poly (dimethylsiloxane) to be airtight and resistive against absorption of small molecules. Analytical chemistry 82 (14):5965–5971 [DOI] [PubMed] [Google Scholar]
- Ren KN, Dai W, Zhou JH, Su J, Wu HK (2011) Whole-Teflon microfluidic chips. Proc Natl Acad Sci U S A 108 (20):8162–8166. doi: 10.1073/pnas.1100356108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J, Zhang YS, Pitrez PR, Leijten J, Miscuglio M, Rouwkema J, Dokmeci MR, Nissan X, Ferreira L, Khademhosseini A (2017) Biomechanical strain exacerbates inflammation on a progeria-on-a-Chip model. Small 13 (15):1603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlowski C, Balderston S, Sandhu M, Hajian R, Liu C, Tran TP, Conboy MJ, Paredes J, Murthy N, Conboy IM (2018) Graphene-based biosensor for on-chip detection of bio-orthogonally labeled proteins to identify the circulating biomarkers of aging during heterochronic parabiosis. Lab Chip 18 (21):3230–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JS (2020) The value of science in space exploration. Oxford University Press, [Google Scholar]
- Shaegh SAM, Pourmand A, Nabavinia M, Avci H, Tamayol A, Mostafalu P, Ghavifekr HB, Aghdam EN, Dokmeci MR, Khademhosseini A (2018) Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sensors and Actuators B: Chemical 255:100–109 [Google Scholar]
- Sieber S, Wirth L, Cavak N, Koenigsmark M, Marx U, Lauster R, Rosowski M (2018) Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J Tissue Eng Regener Med 12 (2):479–489 [DOI] [PubMed] [Google Scholar]
- Skardal A, Murphy SV, Devarasetty M, Mead I, Kang H-W, Seol Y-J, Shrike Zhang Y, Shin S-R, Zhao L, Aleman J, Hall AR, Shupe TD, Kleensang A, Dokmeci MR, Jin Lee S, Jackson JD, Yoo JJ, Hartung T, Khademhosseini A, Soker S, Bishop CE, Atala A (2017) Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7 (1):8837. doi: 10.1038/s41598-017-08879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaba T, Rusek A, Simonsen L, Guida P (2021) An overview of the GCR simulator at the NASA Space Radiation Laboratory. 43rd COSPAR Scientific Assembly Held 28 January-4 February 43:1851 [Google Scholar]
- Sontheimer-Phelps A, Hassell BA, Ingber DE (2019) Modelling cancer in microfluidic human organs-on-chips. Nature Reviews Cancer 19 (2):65–81 [DOI] [PubMed] [Google Scholar]
- Sridharan DM, Asaithamby A, Blattnig SR, Costes SV, Doetsch PW, Dynan WS, Hahnfeldt P, Hlatky L, Kidane Y, Kronenberg A (2016) Evaluating biomarkers to model cancer risk post cosmic ray exposure. Life sciences in space research 9:19–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Chan C-W, Wang Y, Yao X, Mu X, Lu X, Zhou J, Cai Z, Ren K (2019) Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid, low-cost antimicrobial susceptibility test. Lab Chip 19 (17):2915–2924 [DOI] [PubMed] [Google Scholar]
- Sung JH, Shuler ML (2009) A micro cell culture analog (mu CCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 9 (10):1385–1394. doi: 10.1039/b901377f [DOI] [PubMed] [Google Scholar]
- Tagle D (2022) Tissue Chips in Space. NCATS. https://ncats.nih.gov/tissuechip/projects/space. Accessed March 28 2022 [Google Scholar]
- Teh SY, Lin R, Hung LH, Lee AP (2008) Droplet microfluidics. Lab Chip 8 (2):198–220 [DOI] [PubMed] [Google Scholar]
- Thirsk R, Kuipers A, Mukai C, Williams D (2009) The space-flight environment: the International Space Station and beyond. Cmaj 180 (12):1216–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Fu S, Heywood HK, Knight MM, Thorpe SD (2020) Mechanical stimulation: a crucial element of organ-on-chip models. Frontiers in Bioengineering and Biotechnology:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepke MW, Beebe DJ (2006) PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 6 (12):1484–1486 [DOI] [PubMed] [Google Scholar]
- Torisawa Y-s, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE (2014) Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat Methods 11 (6):663–669 [DOI] [PubMed] [Google Scholar]
- Trapecar M, Wogram E, Svoboda D, Communal C, Omer A, Lungjangwa T, Sphabmixay P, Velazquez J, Schneider K, Wright CW (2021) Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases. Sci Adv 7 (5):eabd1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde MG, Mille LS, Figler KP, Cervantes E, Li VY, Bonventre JV, Masereeuw R, Zhang YS (2022) Biomimetic models of the glomerulus. Nature Reviews Nephrology:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Ying Shun T, Gough A, Taylor DL (2016) A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Experimental biology and medicine 241 (1):101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viravaidya K, Sin A, Shuler ML (2004) Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol Progr 20 (1):316–323. doi: 10.1021/bp0341996 [DOI] [PubMed] [Google Scholar]
- Wakayama S, Ito D, Kamada Y, Shimazu T, Suzuki T, Nagamatsu A, Araki R, Ishikawa T, Kamimura S, Hirose N (2021) Evaluating the long-term effect of space radiation on the reproductive normality of mammalian sperm preserved on the International Space Station. Sci Adv 7 (24):eabg5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE (2001) Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373 [DOI] [PubMed] [Google Scholar]
- Winkler TE, Feil M, Stronkman EF, Matthiesen I, Herland A (2020) Low-cost microphysiological systems: feasibility study of a tape-based barrier-on-chip for small intestine modeling. Lab Chip 20 (7):1212–1226 [DOI] [PubMed] [Google Scholar]
- Witze A (2020) Astronauts have conducted nearly 3,000 science experiments aboard the ISS. Nature [DOI] [PubMed] [Google Scholar]
- Wnorowski A, Sharma A, Chen H, Wu H, Shao N-Y, Sayed N, Liu C, Countryman S, Stodieck LS, Rubins KH (2019) Effects of spaceflight on human induced pluripotent stem cell-derived cardiomyocyte structure and function. Stem cell reports 13 (6):960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafia M, Ymbern O, Olanrewaju AO, Parandakh A, Sohrabi Kashani A, Renault J, Jin Z, Kim G, Ng A, Juncker D (2022) Microfluidic chain reaction of structurally programmed capillary flow events. Nature 605 (7910):464–469 [DOI] [PubMed] [Google Scholar]
- Yeung CK, Koenig P, Countryman S, Thummel KE, Himmelfarb J, Kelly EJ (2020) Tissue chips in space—challenges and opportunities. Clinical and Translational Science 13 (1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li L, Jiang Y, Wang C, Geng B, Wang Y, Chen J, Liu F, Qiu P, Zhai G (2018) Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. The FASEB Journal 32 (8):4444–4458 [DOI] [PubMed] [Google Scholar]
- Zhang K, Liang QL, Ma S, Mu XA, Hu P, Wang YM, Luo GA (2009) On-chip manipulation of continuous picoliter-volume superparamagnetic droplets using a magnetic force. Lab Chip 9 (20):2992–2999. doi: 10.1039/b906229g [DOI] [PubMed] [Google Scholar]
- Zhang Y, Richards JT, Feiveson AH, Richards SE, Neelam S, Dreschel TW, Plante I, Hada M, Wu H, Massa GD (2022) Response of Arabidopsis thaliana and Mizuna Mustard Seeds to Simulated Space Radiation Exposures. Life 12 (2):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Shaegh SAM, Massa S, Riahi R, Chae S, Hu N (2017) Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci 114 (12):E2293–E2302 [DOI] [PMC free article] [PubMed] [Google Scholar]


