Abstract
Objective
Acute visual impairment is the most feared complication of giant cell arteritis (GCA) but is challenging to predict. Magnetic resonance imaging (MRI) evaluates orbital pathology not visualized by an ophthalmologic examination. This study combined orbital and cranial vessel wall MRI to assess both orbital and cranial disease activity in patients with GCA, including patients without visual symptoms.
Methods
Patients with suspected active GCA who underwent orbital and cranial vessel wall MRI were included. In 14 patients, repeat imaging over 12 months assessed sensitivity to change. Clinical diagnosis of ocular or nonocular GCA was determined by a rheumatologist and/or ophthalmologist. A radiologist masked to clinical data scored MRI enhancement of structures.
Results
Sixty‐four patients with suspected GCA were included: 25 (39%) received a clinical diagnosis of GCA, including 12 (19%) with ocular GCA. Orbital MRI enhancement was observed in 83% of patients with ocular GCA, 38% of patients with nonocular GCA, and 5% of patients with non‐GCA. MRI had strong diagnostic performance for both any GCA and ocular GCA. Combining MRI with a funduscopic examination reached 100% sensitivity for ocular GCA. MRI enhancement significantly decreased after treatment (P < 0.01).
Conclusion
In GCA, MRI is a sensitive tool that comprehensively evaluates multiple cranial structures, including the orbits, which are the most concerning site of pathology. Orbital enhancement in patients without visual symptoms suggests that MRI may detect at‐risk subclinical ocular disease in GCA. MRI scores decreased following treatment, suggesting scores reflect inflammation. Future studies are needed to determine if MRI can identify patients at low risk for blindness who may receive less glucocorticoid therapy.
INTRODUCTION
Giant cell arteritis (GCA) is a large‐vessel vasculitis that frequently affects the cranial and orbital arteries. Acute visual impairment is the most feared complication of GCA and is most commonly caused by anterior ischemic optic neuropathy (AION). 1 Before the widespread use of glucocorticoids, the reported incidence of vision loss in patients with GCA ranged 2 , 3 from 35% to 60%. The current standard practice of initiating high‐dose glucocorticoids in all suspected cases of GCA has led to a decline in the rates of blindness but has also resulted in significant glucocorticoid‐related morbidity in this older population. 4 , 5 A reliable method to detect orbital disease and risk stratify for blindness from GCA is still lacking and could potentially revolutionize management of this highly morbid disease.
Advanced magnetic resonance imaging (MRI) is increasingly recognized as a promising tool for the diagnosis of GCA. As opposed to conventional techniques, such as computed tomography angiography or magnetic resonance angiography, that focus on luminal irregularities, vessel wall MRI requires high‐resolution scanners and specific sequences and parameters that are optimized to assess the vessel wall. 6 As a growing number of centers in the United States implement vessel wall MRI for other conditions (eg, stroke, aneurysm), there is an opportunity to use this technology in GCA. 7 In particular, vessel wall enhancement visualizes vasculitis of cranial arteries, and several prospective cohort studies demonstrated strong diagnostic performance of gadolinium‐enhanced MRI of the superficial temporal and occipital arteries when compared to clinical and histologic diagnosis of GCA. 8 , 9 Additionally, more than 30 case reports and several cohort studies also reported evidence of abnormal orbital MRI findings, such as optic nerve sheath and ophthalmic artery wall enhancement in patients with GCA‐related AION (ocular GCA). 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Intriguingly, authors of these studies hypothesized that MRI may detect early at‐risk subclinical ocular disease in GCA because of multiple reports of patients with unilateral vision loss presenting with bilateral orbital MRI enhancement. 10 , 12 , 18 However, little is known about the prevalence of orbital MRI enhancement in patients with GCA without vision loss (nonocular GCA) or the changes in orbital enhancement over time with treatment.
In this study, we combined dedicated orbital and cranial vessel wall MRI to assess orbital and cranial GCA activity in patients with suspected GCA with or without visual symptoms. Specifically, we aimed to (1) determine the prevalence of orbital and cranial pathology seen on MRI in patients with GCA, (2) evaluate the diagnostic performance of MRI for GCA and ocular GCA, and (3) examine changes in MRI enhancement with treatment, supporting the responsiveness and convergent validity of MRI for GCA.
PATIENTS AND METHODS
Study participants
Patients presenting with suspected new or relapsing GCA who underwent combined orbital and cranial vessel wall MRI were eligible for this study. A subset of patients who had positive MRI findings and a final diagnosis of GCA consented to participate in a longitudinal study in which combined orbital and cranial vessel wall MRI was repeated at months 1, 6, and 12. Patients without active GCA who underwent repeat MRI for other indications (eg, surveillance of an aneurysm) were also included in the longitudinal analysis. The study was conducted at the University of Pennsylvania Health System between 2019 and 2022 and was approved by the Institutional Review Board of the University of Pennsylvania. Written informed consent was obtained from participants who lacked a clinical indication and therefore obtained MRI for research purposes (eg, no visual symptoms, longitudinal repeat imaging).
MRI acquisition
All scans were obtained on a 3.0‐tesla magnetic resonance system (MAGNETOM Prisma; Siemens) with a 32‐channel head/neck coil. The imaging protocol was modified twice during the study period both to reduce scan time (for patient tolerability) and to optimize visualization. The final abbreviated protocol included the following: For angiographic imaging, a three‐dimensional time‐of‐flight magnetic resonance angiography of the head was performed (repetition time [TR]/echo time [TE], 21/3.7 milliseconds; flip angle [FA], 18°; field of view [FOV], 160 × 200; matrix, 586 × 768; slice thickness, 0.6 mm; time, 6:20 minutes), and two cranial vessel wall imaging sequences included a postcontrast cerebrospinal fluid–suppressed whole‐brain three‐dimensional variable FA turbo spin echo (TSE) sequence 19 , 20 (TR/TE, 900/16 milliseconds; FOV, 185 × 205; isotropic spatial resolution, 0.5 mm; time, 13 minutes) and a postcontrast axial‐oblique two‐dimensional T1‐weighted spin echo (TR/TE, 19/500 milliseconds; FOV, 210 × 210; spatial resolution, 0.2 × 0.2 × 3 mm; time, 10 minutes). For orbital imaging, coronal T2‐weighted TSE with fat suppression (TR/TE, 4,460/87 milliseconds; FOV, 179 × 179; slice thickness, 3 mm; time, 1:33 minutes) and postcontrast axial/coronal T1‐weighted TSE fat suppression (TR/TE, 500/10 milliseconds [axial]; TR/TE, 600/6.8 milliseconds [coronal]; FOV, 145–180; 15–26 slices; slice thickness, 3 mm; time, 3 min/plane) were used. Further details of the magnetic resonance protocol are in Supplementary Information 1.
MRI scoring
Anonymized MRIs were reviewed by a single radiologist (RR or JWS) with more than 10 years of experience in reading vessel wall MRIs. Readers were masked to all clinical data, including final clinical and histologic diagnosis. Based on existing data on the affected vasculature in GCA, 21 , 22 , 23 , 24 multiple cranial arteries were assessed using a previously reported semiquantitative scoring system 8 , 9 , 25 (Figure 1A and B).
Figure 1.
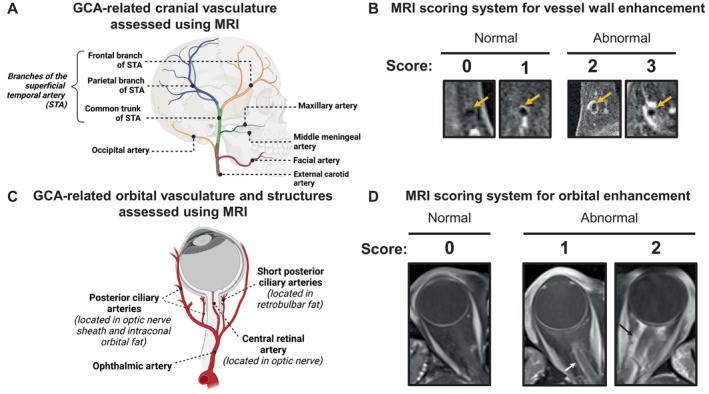
Scoring of cranial and orbital enhancement for GCA. (A) Illustration of vascular anatomy included in MRI scoring for GCA. Arteries included in analysis were selected based on prior knowledge of GCA, including data from postmortem studies. (B) Semiquantitative 4‐point scoring system was used to grade vessel wall contrast enhancement. Images demonstrate examples of each score, ranging from 0 to 3 (2–3 is abnormal). (C) Illustration of orbital vasculature and structures that were scored. Except for the ophthalmic artery, each artery is shown with its corresponding location in parentheses. Additional structures, such as the lacrimal gland and the optic chiasm, were also assessed but are not shown in the figure. (D) Semiquantitative 3‐point scoring system was used to grade contrast enhancement of orbital structures. Images demonstrate examples of each score using optic nerve sheath as an example. Scores range from 0 to 2 (1–2 is abnormal). Grading of the ophthalmic artery wall was according to the same 0 to 3 scoring system used for cranial arteries. GCA, giant cell arteritis; MRI, magnetic resonance imaging; STA, superficial temporal artery.
For orbital imaging, we included structures previously shown to have MRI enhancement in patients with ocular GCA: ophthalmic artery, 10 , 26 optic nerve sheath, 10 , 12 intraorbital fat, 27 , 28 retrobulbar fat at the posterior globe, 11 and optic nerve 27 (Figure 1C and D). In addition, enhancements of the temporalis muscles 29 , 30 and extraocular muscles 1 were also scored. Details of the MRI scoring are available in the Supplementary Information 2.
MRI criteria for diagnosis of GCA and ocular GCA
An MRI test positive for GCA was defined as abnormal vessel wall enhancement in at least one of the following eight arterial segments (right or left): frontal branch, parietal branch, or common trunk of the superficial temporal artery or occipital artery. This definition was selected based on prior studies in GCA. 8 , 9 Among patients with a positive MRI test (ie, abnormal cranial vessel wall enhancement), orbital imaging was read as consistent with ocular GCA if there was abnormal enhancement of at least one orbital structure or the ophthalmic artery wall based on prior reports. 10 , 12 , 26 , 27 , 28 , 31 Because enhancement of orbital structures is known to occur in other inflammatory conditions 32 (eg, malignancy, orbital pseudotumor, etc), orbital MRI was considered diagnostic for ocular GCA when concurrent cranial artery involvement was observed on imaging. When evaluating longitudinal changes in imaging across visits, readers were masked to the final clinical diagnosis, but to assess for change, they were not masked to visit order.
Clinical reference standards
A final clinical diagnosis of active GCA (new or relapsing) versus no or inactive GCA (non‐GCA) was determined by the treating clinician, which in most cases was a rheumatologist and/or neuro‐ophthalmologist. Diagnosis of GCA was determined by temporal artery biopsy, ophthalmologic examination, and other clinical features and was informed by classification criteria for GCA. Clinicians were not masked to MRI results. Among patients with a diagnosis of active GCA, patients were further classified as having GCA‐related ocular involvement (ocular GCA) versus no ocular involvement (nonocular GCA) according to the ophthalmologist's assessment and/or presence of suggestive visual symptoms (eg, transient vision loss, binocular diplopia). Patients with a final clinical diagnosis of non‐GCA underwent a rapid glucocorticoid taper, and all patient charts were reviewed at least six months after the baseline visit to ensure there was no change in disease classification.
Secondary clinical reference standards included histologic diagnosis based on temporal artery biopsy results and 2022 American College of Rheumatology (ACR)/EULAR classification criteria for GCA. 33 Temporal artery biopsies were performed either unilaterally or bilaterally depending on clinician preference and patient presentation. Surgeons were not masked to MRI results and, in some cases, had the option to use MRI to guide the site of biopsy. The histologic diagnosis was considered positive for GCA when the biopsy sample contained lymphocytic or other immune cell infiltrate in the intima, media, and/or adventitia with or without giant cells. The secondary clinical reference standard for ocular GCA included ophthalmologic examination, specifically the presence or absence of optic disc swelling on a funduscopic examination.
Statistical analysis
Group differences were assessed using the Wilcoxon rank sum test or Fisher's exact test. To test the diagnostic performance of MRI, sensitivity, specificity, positive predictive values, negative predictive values (NPVs), positive and negative likelihood ratios, and area under the receiver operating characteristic (ROC) curve were calculated. To compare different tests (eg, funduscopic examination vs MRI), McNemar's test and DeLong's test were used. Mixed‐effect ordinal regression models evaluated longitudinal changes in MRI enhancement score over time; the proportional odds or the parallel regression assumption was validated using the Brant–Wald test. Secondary analyses of baseline data compared MRI results by prednisone duration (≤5 vs >5 days 9 ) and dose (≤10 vs >10 mg) at the visit. A cutoff of 10 mg of prednisone was selected because some patients with pre‐existing polymyalgia rheumatica were on low‐dose prednisone at the time of the baseline MRI. Sensitivity analyses were performed by MRI protocol (original vs revisions) to determine if optimization steps altered diagnostic performance. A significance level of 0.05 was used for all tests of hypothesis. Statistical analyses were performed using Stata 17.0 (Stata Corp) and the R statistical environment (version 4.2.1).
RESULTS
Study population
All 64 patients presenting with clinically suspected GCA received orbital MRI. Cranial vessel wall imaging data were available in 59 (95%) patients but not in 5 patients because vessel wall imaging was either not performed or not interpretable; these 5 patients but were still included in orbital MRI analyses. Table 1 presents baseline characteristics of patients. Among the 64 patients, 25 patients received a final clinical diagnosis of active GCA. Among the 44 patients who underwent a temporal artery biopsy, 14 patients had a biopsy before MRI (6 in GCA group, 8 in non‐GCA group). Supplementary Table 1 lists the alternative diagnoses for patients in the non‐GCA group.
Table 1.
Baseline characteristics of study participants*
| Characteristic | All (N = 64) | Final clinical diagnosis | |
|---|---|---|---|
| Active GCA (n = 25) | No GCA or inactive (n = 39) | ||
| New diagnosis suspected, n (%) | 56 (88) | 20 (80) | 36 (92) |
| Relapse suspected, n (%) | 8 (12) | 5 (20) | 3 (8) |
| Temporal artery biopsy performed, n (%) | 44 (69) | 21 (84) | 23 (59) |
| Pathology consistent with GCA | 15 (34) | 15 (71) | 0 |
| Clinical manifestations, n (%) | |||
| Headache | 47 (73) | 18 (72) | 29 (74) |
| Jaw pain | 22 (34) | 13 (52) | 9 (23) |
| Scalp tenderness | 29 (45) | 12 (48) | 17 (44) |
| Temporal artery tenderness | 7 (11) | 5 (20) | 2 (5) |
| Polymyalgia rheumatica | 12 (19) | 8 (32) | 4 (10) |
| Any visual disturbance, n (%) | 47 (73) | 18 (72) | 29 (74) |
| Transient vision loss | 15 (23) | 10 (40) | 5 (13) |
| Blurry vision | 19 (30) | 6 (24) | 13 (33) |
| Diplopia | 11 (17) | 4 (16) | 7 (18) |
| Laterality of visual symptoms, n (%) | |||
| Right eye only | 10 (16) | 4 (16) | 6 (15) |
| Left eye only | 11 (17) | 2 (8) | 9 (23) |
| Both eyes | 24 (38) | 11 (44) | 13 (33) |
| Ophthalmologic examination performed, n (%) | 43 (67) | 15 (60) | 28 (72) |
| Ophthalmologic examination finding in at least one eye | |||
| Visual field defect | 17/43 | 8/15 | 9/28 |
| Relative afferent pupillary defect | 12/43 | 5/15 | 7/28 |
| Optic disc edema | 14/43 | 8/15 | 6/28 |
| Optic disc pallor | 12/43 | 7/15 | 5/28 |
| Peak ESR, median (IQR), mm/hr | 71 (30–97) | 80 (43–105) | 65 (23–86) |
| Peak CRP, median (IQR), mg/dL | 1.9 (0.5–5.2) | 3.4 (1.0–13.4) | 0.84 (0.4–2.9) |
| Days on prednisone at MRI visit, median (IQR) | 5 (0–54) | 13 (4–60) | 2 (0–35) |
| Dose of prednisone at MRI visit, median (IQR), mg | 40 (3–60) | 60 (40–60) | 15 (0–40) |
| At least one follow‐up MRI obtained | 14 | 11 | 3 |
| Month 1 | 12 | 11 | 1 |
| Month 6 | 8 | 6 | 2 |
| Month 12 | 6 | 6 | 0 |
For five patients with only orbital imaging data available, vessel wall imaging was either not performed or was uninterpretable because of motion degradation. CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; GCA, giant cell arteritis; IQR, interquartile range; MRI, magnetic resonance imaging.
Visual disturbances were reported in 73% of patients, and a majority of these patients were evaluated by an ophthalmologist. Among the 12 patients with a final clinical diagnosis of ocular GCA, transient vision loss was the most common symptom reported, and 8 patients showed signs of optic disc swelling on a funduscopic examination (Table 1). Additional ophthalmologic symptoms and signs are shown in Supplementary Table 2. Alternative clinical ocular diagnoses in the non‐GCA group are listed in Supplementary Table 1.
Description of orbital MRI findings in GCA
Patients with a final diagnosis of GCA (n = 25) were more likely to have orbital MRI abnormalities compared to patients without GCA (60% vs 5%; P < 0.01). Further, abnormal orbital MRIs were more likely to occur in ocular versus nonocular GCA (83% vs 38%). The most common orbital MRI findings in GCA were enhancement of the optic nerve sheath (14 [56%]) and ophthalmic artery wall enhancement (12 [48%]) (Figure 2). Orbital MRI changes were observed bilaterally in 14 of 15 patients. There was no significant difference in the proportion of patients with GCA with positive orbital MRIs grouped by prednisone duration (≤5 vs >5 days; P = 0.23) or dose of prednisone (≤10 vs >10 mg; P = 0.50). Results were similar when we compared across different versions of the MRI protocol used.
Figure 2.
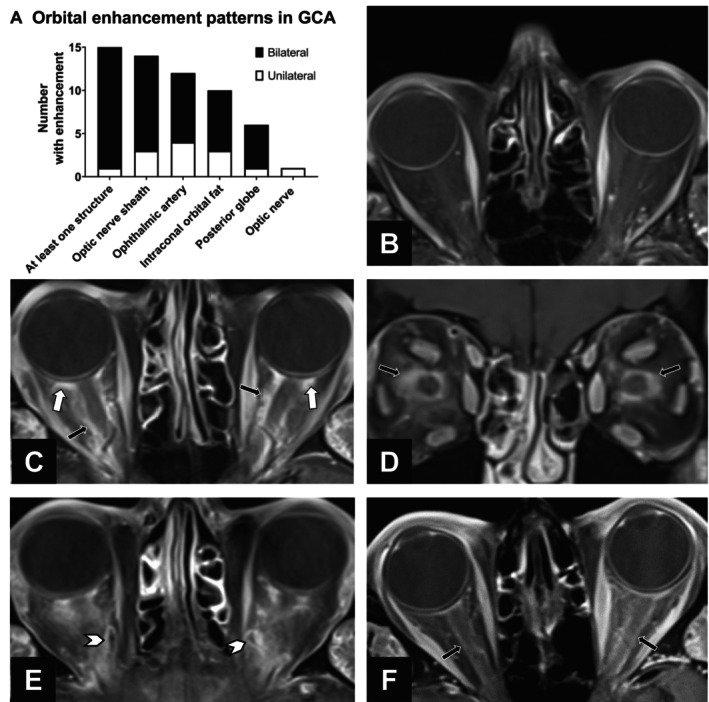
Orbital MRI enhancement in GCA. (A) Stacked bar graph of orbital structures with MRI enhancement among the 25 participants with a final clinical diagnosis of GCA. Enhancement was bilateral in a majority of cases. (B–E) Example MRIs of orbital enhancement using combined orbital and vessel wall MRI. All images are on an axial plane unless otherwise indicated. (B) Normal orbits in a patient without GCA and no visual symptoms. (C) Axial and (D) coronal images demonstrating bilateral enhancement of the optic nerve sheath and intraconal orbital fat (black arrows) and retrobulbar fat at the posterior globe (white arrows) in a patient diagnosed with GCA‐related arteritic ischemic optic neuropathy who had a positive temporal artery biopsy finding. (E) Ophthalmic vessel wall enhancement bilaterally (arrow heads) in a patient with GCA. (F) Bilateral MRI enhancement of optic nerve sheath (black arrows) in a patient with GCA. The patient presented with two weeks of diplopia, which was attributed to cranial nerve palsy, and had normal visual fields and funduscopic examination results. GCA, giant cell arteritis; MRI, magnetic resonance imaging.
Among the 39 patients with a final clinical diagnosis of non‐GCA, only 4 (10%) patients had a positive MRI result, including 2 with orbital enhancement. Nine patients with non‐GCA had orbital enhancement but because of the lack of concurrent cranial artery enhancement or alternative findings on imaging (eg, malignancy), these were interpreted by the reader as unrelated to GCA.
Asymptomatic orbital MRI enhancement in GCA
Our data revealed the presence of orbital enhancement not only in eyes with symptoms and signs of ocular GCA but also in eyes with no visual symptoms or normal or nondiagnostic ophthalmologic examination results. In the four patients with monocular vision loss, three had bilateral enhancement despite unilateral visual symptoms and signs (example MRI shown in Supplementary Figure 1). Most striking was the presence of orbital MRI enhancement among patients classified as having nonocular GCA. Among patients with nonocular GCA, 5 (38%) of 13 had imaging evidence of orbital involvement (Figure 3A and Supplementary Figure 1C), and in 4 (80%) of 5 of those patients, orbital enhancement was seen bilaterally. There was no significant difference in the types of orbital pathology seen on MRI between patients classified as having nonocular versus ocular GCA (all P > 0.05). In total, 13 (43%) of 30 asymptomatic eyes (eyes with no vision loss due to GCA) demonstrated abnormal orbital MRI enhancement.
Figure 3.
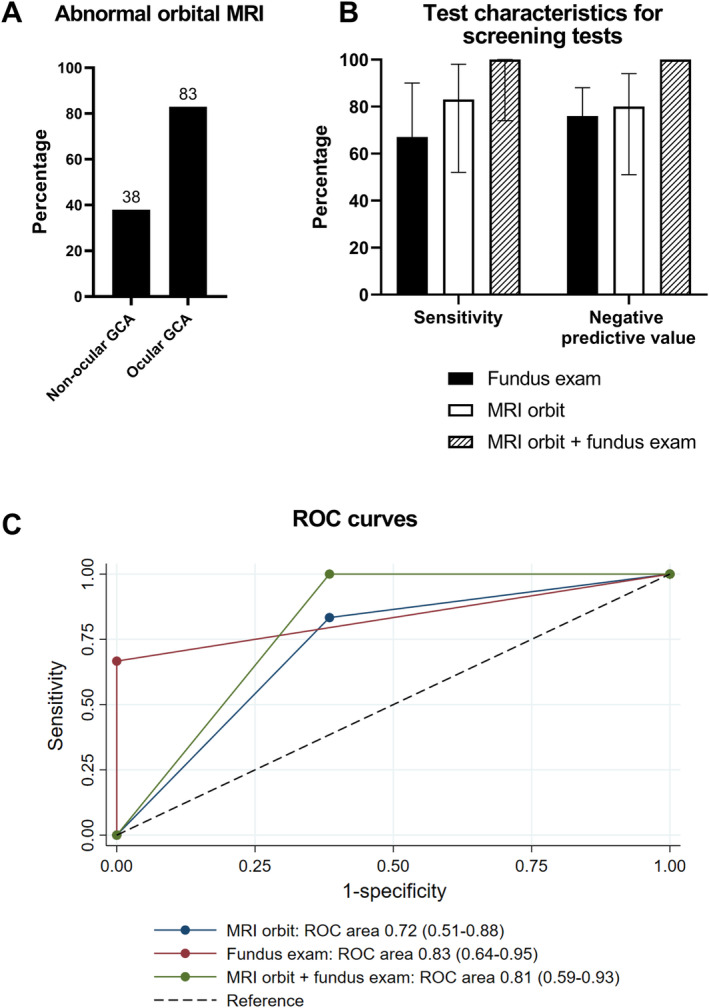
Diagnostic performance of orbital MRI compared to funduscopic examination for the diagnosis of ocular GCA. (A) Proportion of participants with abnormal orbital MRI enhancement among patients with ocular versus nonocular GCA (P = 0.04). The percentage of patients with abnormal MRI results is shown at the top of each bar. (B) Bar graph with 95% confidence intervals of sensitivity and the negative predictive value of optic disc edema on a funduscopic examination, positive orbital MRI enhancement, or both positive test results for diagnosing ocular involvement among patients with GCA. Combining funduscopic examination with MRI had a negative predictive value of 100%. (C) ROC curves for funduscopic examination, orbital MRI, and both combined in the diagnosis of ocular GCA. GCA, giant cell arteritis; MRI, magnetic resonance imaging; ROC, receiver operating characteristic.
Description of MRI findings in cranial arteries and structures in GCA
Beyond the superficial temporal arteries, MRI detected enhancement of other GCA‐related vasculature and structures (Figure 4). When we compared ocular versus nonocular GCA, there were generally more vascular and other structures demonstrating MRI enhancement in patients with ocular GCA; among the individual arterial segments, only the frontal branch of the superficial temporal artery had a significantly higher proportion with MRI enhancement in the ocular versus nonocular GCA group (91% vs 46%; P = 0.03). Data on facial and external carotid arteries were not analyzed because of inability to assess these structures in a large proportion of patients.
Figure 4.
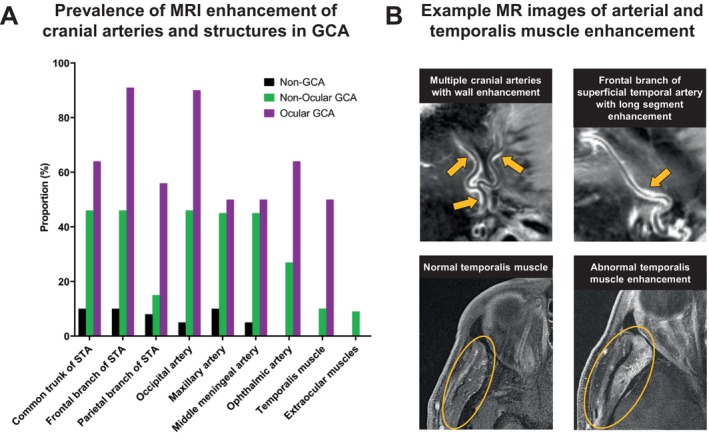
Multiple arteries and structures with MRI enhancement in participants with GCA. (A) Bar graph of the proportion of patients with MRI enhancement of each artery or muscle in patients without GCA (non‐GCA), with non‐ocular GCA, and with ocular GCA. For each individual arterial segment or structure, there was a higher proportion with MRI enhancement in the GCA versus non‐GCA groups (all P < 0.05). When comparing non‐ocular GCA to ocular GCA, the only structure with a significant difference between groups was the frontal branch of the STA (P = 0.03). (B) Example MRIs demonstrating long‐segment vessel wall enhancement of multiple cranial artery branches (arrows) as well as normal versus abnormal temporalis muscle enhancement (circle), which was observed in 32% of patients with GCA. GCA, giant cell arteritis; MRI, magnetic resonance imaging; STA, superficial temporal artery.
Diagnostic accuracy of MRI for GCA and ocular GCA
Among the 59 patients who had available vessel wall imaging of cranial arteries, vessel wall enhancement of at least one temporal or occipital artery was observed in 17 (74%) patients with a clinical diagnosis of GCA versus 4 (11%) patients with non‐GCA (P < 0.01). When compared against several reference standards, including clinical diagnosis, temporal artery biopsy, and ACR/EULAR classification criteria, cranial vessel wall enhancement had a good sensitivity and specificity (Supplementary Table 3 and Supplementary Figure 2). In particular, when we compared MRI to histologic diagnosis, absence of vessel wall enhancement on MRI perfectly predicted a negative temporal artery biopsy (NPV 100%). Among the six patients who underwent MRI after temporal artery biopsy, two patients with a clinical diagnosis of GCA but negative biopsy findings had positive MRI enhancement of the occipital arteries. Results were similar when we evaluated subgroups based on prednisone dose or duration as well as MRI protocol version.
We then evaluated whether adding orbital MRI to cranial vessel wall MRI improved diagnostic performance (Supplementary Table 4). We examined three definitions of a positive MRI result: (1) cranial artery enhancement alone, (2) either orbital or cranial artery enhancement (“or” definition), and (3) both orbital and cranial artery enhancement (“and” definition). For the diagnosis of GCA overall, the “or” definition had the highest sensitivity (83%), whereas the “and” definition produced the highest specificity (94%); none of the differences were statistically significant. For the diagnosis of ocular GCA, the “or” definition of a positive MRI result had the highest sensitivity (100%), whereas the “and” definition had the highest specificity (86%); compared to the “or” definition, the “and” definition did not significantly change sensitivity (P = 0.99) but significantly improved specificity (P < 0.01) for ocular GCA (Supplementary Table 4).
Among patients with GCA (n = 25), orbital MRI had a similar area under the ROC curve compared to presence of optic disc edema on a funduscopic examination, with numerically, but not significantly, higher sensitivity (Figure 3B and C, Supplementary Table 5). Specifically, compared against the reference standard of clinical diagnosis, MRI enhancement of at least one orbital structure or ophthalmic artery had a sensitivity of 83% and an NPV of 80%. When orbital MRI was combined with a funduscopic examination, the sensitivity and NPV both increased to 100%.
Responsiveness of MRI
To assess longitudinal changes in MRI with treatment, 14 patients underwent at least one follow‐up MRI, including 11 patients with a final clinical diagnosis of GCA and 3 patients with non‐GCA. Across visits, there was a significant decline in erythrocyte sedimentation rates and C‐reactive protein levels (all P < 0.05 compared to baseline), with normalization of median values by month 1. On MRI, cranial arteries with abnormal vessel wall enhancement at baseline had a significant decrease in enhancement scores over time, whereas arteries without enhancement at baseline remained unchanged (Figure 5A and C). Based on results of the ordinal regression model, for each one‐unit increase in visit (ie, one month), the odds of having a higher MRI score decreased by 28% after adjustment for baseline artery enhancement (odds ratio [OR] 0.72, 95% confidence interval [CI] 0.66–0.79). Similarly, enhancement scores for orbital structures also significantly decreased over each month after adjustment for baseline enhancement (OR 0.69, 95% CI 0.58–0.79) (Figure 5B and D). Interestingly, although scores significantly decreased by the month 1 visit (after at least one month of high‐dose glucocorticoid treatment), abnormally enhanced arteries after one month continued to have significantly higher scores compared to baseline normal arteries. Scores decreased to normal levels by the month 12 visit. One participant experienced a relapse of GCA and received a repeat MRI at the time of relapse, which was found to have an increase in vessel wall enhancement scores (the right occipital artery score increased from 0 to 2, and the left occipital artery score increased from 1 to 2 between the month 1 and month 6 visits).
Figure 5.
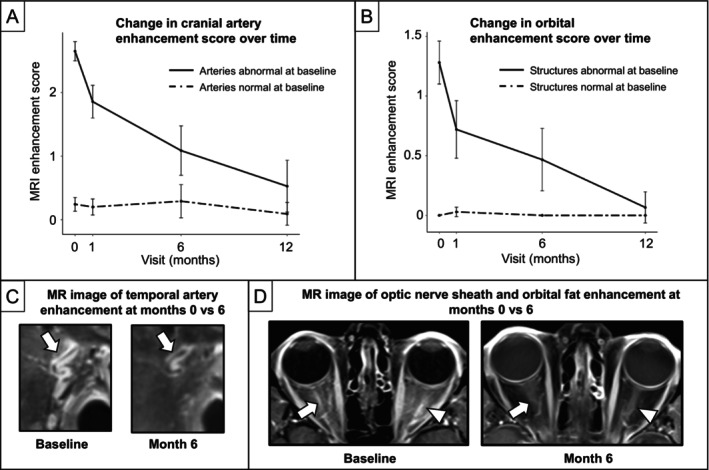
MRI enhancement score decreases over time with treatment of GCA. (A and B) Line graphs with 95% confidence intervals of mean MRI enhancement scores over time separated by baseline normal (dashed line) versus abnormal (solid line) enhancement. We conducted a multilevel mixed‐effect regression analysis for each cranial artery that did not undergo biopsy (branches of superficial temporal artery, occipital artery, and ophthalmic artery) and each orbital structure (optic nerve sheath, intraconal orbital fat, retrobulbar fat at posterior globe, and optic nerve) among 14 patients (11 with GCA, 3 with non‐GCA) who underwent at least one follow‐up MRI after baseline. Both (A) cranial arteries that did not undergo biopsy and (B) orbital structures demonstrated a significant decrease in score over time. Notably, MRI scores were still significantly elevated even after one month of treatment with glucocorticoids (P < 0.01). Example MRIs comparing MRI enhancement at month 0 versus 6 within the same individual for the (C) superficial temporal artery and (D) optic nerve sheath and intraconal fat. GCA, giant cell arteritis; MRI, magnetic resonance imaging.
DISCUSSION
In this study, we combined dedicated orbital and cranial vessel wall MRI to assess orbital and cranial GCA activity both at diagnosis and during follow‐up. We found that this comprehensive tool enabled visualization of multiple arteries and structures known to be affected by GCA all in a single MRI session. First, we found that MRI has strong diagnostic performance both for GCA overall and for ocular GCA. Second, we found evidence of orbital MRI enhancement not only in symptomatic eyes of patients with GCA‐related vision loss but also in asymptomatic eyes of patients with GCA, supporting the hypothesis that orbital MRI detects early subclinical ocular disease in GCA. Third, we identified MRI enhancement of additional cranial arteries and structures known to be affected in GCA, such as the maxillary and middle meningeal arteries and the temporalis muscle. Lastly, our longitudinal data demonstrate a decrease in MRI enhancement scores over one year with treatment of GCA, further suggesting that the enhancement observed on imaging reflects inflammatory activity. Overall, our findings suggest that MRI has the potential to significantly improve current management strategies in GCA by comprehensively and noninvasively assessing multiple cranial structures, including the orbits.
Our study found that the most prevalent enhancing orbital structures on MRI in patients with GCA are the optic nerve sheath and ophthalmic arteries. In most cases, enhancement is seen bilaterally. These enhancement patterns on orbital MRI in GCA are consistent with previous case series and small cohort studies of GCA, and previous studies comparing GCA‐AION to nonarteritic AION have found that orbital MRI can discriminate these two conditions. 10 , 12 The optic nerve sheath and orbital fat are the locations where the posterior ciliary arteries reside. 34 The posterior ciliary arteries branch off the ophthalmic artery and are the main blood supply for the optic nerves. Inflammation of the posterior ciliary and ophthalmic arteries is the most frequent cause of ischemic optic neuropathy in GCA. 35 In a postmortem study of 12 patients with GCA, 75% of posterior ciliary and ophthalmic arteries had pathologic evidence of vasculitis, and involvement was typically bilateral. 22 In these autopsies, it was noted that the most severe pathologic findings of the posterior ciliary arteries were in the areas around the optic nerve sheath, the same site where MRI contrast enhancement was observed in our study. In another case report, a patient with a diagnosis of ocular GCA and bilateral optic nerve sheath enhancement on MRI underwent biopsy of the optic nerve sheath, which showed an artery with transmural inflammation and multinucleated giant cells. 17 This case report provides histologic confirmation that optic nerve sheath enhancement on MRI in patients with GCA may represent GCA‐related disease activity. In summary, the orbital MRI enhancement pattern in patients with active GCA is characteristic of the vascular territory supplied by the ophthalmic artery and its branches and is anatomically concordant with histologic findings in previous literature of GCA.
Although common comorbidities frequently encountered in the GCA population, such as hypertension or glaucoma, are not expected to cause the orbital enhancement patterns observed this population, other conditions can cause similar patterns, including malignancy, infection, sarcoidosis, etc. 36 Therefore, it was not surprising that use of orbital imaging without concurrent cranial vessel wall imaging significant reduced specificity in this study. Although more studies are needed, this study suggests that interpretation of orbital MRI findings should be in conjunction other diagnostic findings of GCA (eg, cranial vessel wall enhancement, positive temporal artery biopsy finding, or positive temporal artery ultrasound result).
A striking finding from our study was the presence of orbital enhancement in asymptomatic eyes of patients with GCA. First, our study corroborated prior reports demonstrating bilateral orbital MRI changes in patients presenting with monocular vision loss due to GCA. 10 , 12 Given the high frequency of untreated monocular vision loss progressing to binocular involvement in GCA, MRI enhancement in the asymptomatic contralateral eye supports the hypothesis that MRI may detect at‐risk ocular disease before onset of visual symptoms. Second, in contrast to prior studies that focused on ocular GCA or included dedicated orbital imaging, our study reports orbital MRI changes even in patients without vision loss (nonocular GCA). Whether or not these patients would go on to develop vision loss could not be assessed because all patients are treated with high‐dose glucocorticoids. It is important to note that dedicated orbital imaging is optimized differently from routine brain MRIs to enable better visualization of the orbital structures. 37 Together, these findings suggest that MRI may be more sensitive than current diagnostic approaches to identifying ocular disease in GCA. Future studies are needed to determine if lower glucocorticoid doses could be used in patients with GCA who have a normal orbital MRI.
Furthermore, our study found that multiple cranial arterial territories beyond the superficial temporal arteries demonstrate MRI enhancement in GCA. For example, occipital artery enhancement was observed in 65% of patients with GCA and at times was observed in patients with negative temporal artery biopsy findings. We found that the temporalis muscle was frequently abnormal on MRI, particularly among patients with ocular GCA; inflammation or ischemia of the temporalis muscle may explain jaw claudication and scalp sensitivity in some patients with GCA. Future studies may determine if incorporating a larger scope of imaging data can enhance current methods of classification and prognostication in GCA.
Our study validates prior studies demonstrating strong diagnostic performance of MRI for GCA. 8 , 9 , 38 Interestingly, compared to temporal artery biopsy, MRI had an NPV of 100%, suggesting MRI may serve as useful screening test to rule out GCA and minimize unnecessary temporal artery biopsies. Furthermore, our study also demonstrated that MRI has very good sensitivity and specificity for ocular GCA. In particular, combining orbital MRI with a funduscopic examination reached an NPV of 100% for ocular GCA, again supporting this combination as a useful screening test. Whether patients with normal vision and negative orbital MRI results have a lower risk of developing blindness from GCA is unknown because all patients, regardless of visual symptoms, are treated empirically for ocular disease with high‐dose glucocorticoids. Future studies may evaluate the safety of lowering glucocorticoid therapy based on MRI results.
This prospective longitudinal study found that MRI enhancement significantly decreases over time with treatment of GCA, supporting the responsiveness and convergent validity of this imaging biomarker. A responsive objective measure that can noninvasively assess disease activity remains an unmet need in this relapsing disease. In particular, interleukin‐6 inhibitors, which are being increasingly used to treat GCA, suppress serum markers of inflammation, which further complicates the clinician's ability to assess disease activity. Interestingly, MRI scores continued to be elevated even after a month of immunosuppressive treatment and did not normalize until 12 months after diagnosis; this more gradual improvement in vascular inflammation on imaging is consistent with a prior study showing persistent inflammatory changes on repeat temporal artery biopsies 3 to 12 months later. 39 The persistent MRI changes at the one‐month visit demonstrates potential real‐world applicability of MRI in the management of GCA because many centers may not be able to perform MRIs within a few days. Whether persistent vasculitis on imaging is associated with future outcomes, such as relapse, remains unclear. Lastly, one patient who experienced a relapse during follow‐up also had a concurrent increase in MRI enhancement of cranial arteries at the time of relapse. The changes in MRI enhancement associated with disease activity supports the need for further studies investigating the role of MRI in longitudinal disease surveillance of GCA.
This study had a few limitations. First, misclassification of GCA may have occurred because there are no validated diagnostic criteria for GCA. We used multiple definitions of GCA and found similar results, which demonstrates the robustness of our findings. Furthermore, all patient charts were reviewed at least six months after diagnosis to ensure no change in classification and to ensure that patients without GCA received a rapid taper of prednisone. Second, ophthalmologic examination was not standardized, and some patients (eg, those without visual symptoms) did not receive an ophthalmologic examination. However, our data reflect real‐world care, and we expect that ophthalmologic misclassification would bias to the null. Third, the MRIs were scored by a single radiologist with many years of experience reading vessel wall and orbital MRIs. Therefore, we were not able to assess for interrater reliability, although previous studies demonstrated excellent agreement between readers. 9 , 10
Generalizability of our results may be limited because our cohort had a larger percentage of patients with visual disturbance (73%) compared to other cohorts of patients with GCA, likely because of targeted inclusion of this high‐risk subgroup for MRI examination. Radiologists were masked to clinical data, but clinicians were not masked to radiologic data, which may have introduced bias; however, the diagnostic performance of MRI was similar when comparing MRI to objective assessments (eg, temporal artery biopsy, funduscopic examination), mitigating this concern. The MRI protocol was optimized twice during the study, primarily to reduce scan time; secondary analyses stratifying by protocol did not demonstrate significant differences in results. The sample size of this study was small and further studies are needed to validate these results. Although the orbital MRI findings in this study raise interesting hypotheses, further studies are needed to validate the orbital imaging data and optimize this imaging biomarker before use in a clinical setting. Lastly, in terms of the clinical translation of this imaging tool, our MRI protocol uses existing US Food and Drug Administration–approved sequences, but further site‐specific refinement, based on vendor and software, may be needed especially for vessel wall imaging. Therefore, further external validation studies are needed before clinical implementation of this MRI technique.
In summary, by combining dedicated orbital and cranial vessel wall imaging, we can visualize abnormal MRI enhancement of multiple full‐length cranial arteries and structures affected by GCA, including orbital structures, which are the most concerning site of pathology. Intriguingly, bilateral orbital enhancement is observed in patients with unilateral vision loss and in patients without any vision loss, supporting the hypothesis that orbital MRI can identify patients at risk for blindness. The degree of MRI enhancement decreases over time with treatment of GCA, suggesting that MRI enhancement reflects inflammatory activity and is responsive to change. Together, our findings support the possibility that orbital and cranial vessel wall enhancement on MRI is a sensitive imaging biomarker both for the diagnosis of GCA and for risk of blindness and could be used longitudinally for disease surveillance. Future studies are needed to determine if MRI can identify patients at low risk for blindness due to GCA who may receive lower doses of glucocorticoids.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr Rhee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Rhee, Tamhankar, Song.
Acquisition of data
Rhee, Rebello, Tamhankar, Banerjee, Kurtz, Bhatt, Amudala, Chou, Liang, Sanchez, Burke, Desiderio, Fan, Loevner, Merkel, Song.
Analysis and interpretation of data
Rhee, Rebello, Tamhankar, Liu, Cao, Baker, Morris, Merkel, Song.
Supporting information
Disclosure form
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
We greatly appreciate the input of Dr Thorsten Bley for his input into our vessel wall imaging sequences and Dr Nader Khalidi for his input into study design.
Supported by the Rheumatology Research Foundation (Career Development Bridge Funding Award to Dr Rhee). Dr Rhee's work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K23‐AR‐071514). Dr Song's work was supported by the American Heart Association (grant 938082).
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/acr2.11649).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11649.
REFERENCES
- 1. Soriano A, Muratore F, Pipitone N, et al. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat Rev Rheumatol 2017;13(8):476–484. [DOI] [PubMed] [Google Scholar]
- 2. Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids: results in fifty‐five cases in which lesion was proved at biopsy. J Am Med Assoc 1957;163(10):821–827. [DOI] [PubMed] [Google Scholar]
- 3. Bruce GM. Temporal arteritis as a cause of blindness: review of literature and report of a case. Trans Am Ophthalmol Soc 1949;47:300–316. [PMC free article] [PubMed] [Google Scholar]
- 4. de Boysson H, Barakat C, Dumont A, et al. Tolerance of glucocorticoids in giant cell arteritis: a study of patient‐reported adverse events. Rheumatology (Oxford) 2022;61(9):3567–3575. [DOI] [PubMed] [Google Scholar]
- 5. Wilson JC, Sarsour K, Collinson N, et al. Incidence of outcomes potentially associated with corticosteroid therapy in patients with giant cell arteritis. Semin Arthritis Rheum 2017;46(5):650–656. [DOI] [PubMed] [Google Scholar]
- 6. Mandell DM, Mossa‐Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38(2):218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mossa‐Basha M, Zhu C, Yuan C, et al. Survey of the American Society of Neuroradiology membership on the use and value of intracranial vessel wall MRI. AJNR Am J Neuroradiol 2022;43(7):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhéaume M, Rebello R, Pagnoux C, et al. High‐resolution magnetic resonance imaging of scalp arteries for the diagnosis of giant cell arteritis: results of a prospective cohort study. Arthritis Rheumatol 2017;69(1):161–168. [DOI] [PubMed] [Google Scholar]
- 9. Klink T, Geiger J, Both M, et al. Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis: results from a multicenter trial. Radiology 2014;273(3):844–852. [DOI] [PubMed] [Google Scholar]
- 10. Mohammed‐Brahim N, Clavel G, Charbonneau F, et al. Three tesla 3D high‐resolution vessel wall MRI of the orbit may differentiate arteritic from nonarteritic anterior ischemic optic neuropathy. Invest Radiol 2019;54(11):712–718. [DOI] [PubMed] [Google Scholar]
- 11. Gospe SM III, Amrhein TJ, Malinzak MD, et al. Magnetic resonance imaging abnormalities of the optic nerve sheath and intracranial internal carotid artery in giant cell arteritis. J Neuroophthalmol 2021;41(1):54–59. [DOI] [PubMed] [Google Scholar]
- 12. Sommer NN, Treitl KM, Coppenrath E, et al. Three‐dimensional high‐resolution black‐blood magnetic resonance imaging for detection of arteritic anterior ischemic optic neuropathy in patients with giant cell arteritis. Invest Radiol 2018;53(11):698–704. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen KK, Al Othman BA, Kini AT, et al. Perineural optic nerve enhancement in giant cell arteritis: a case report and review of the literature. J Neuroophthalmol 2021;41(1):e42–e45. [DOI] [PubMed] [Google Scholar]
- 14. Liu TYA, Miller NR. Giant cell arteritis presenting as unilateral anterior ischemic optic neuropathy associated with bilateral optic nerve sheath enhancement on magnetic resonance imaging. J Neuroophthalmol 2015;35(4):360–363. [DOI] [PubMed] [Google Scholar]
- 15. Chen JJ, Kardon RH, Daley TJ, et al. Enhancement of the optic nerve sheath and temporal arteries from giant cell arteritis. Can J Ophthalmol 2015;50(5):e96–e97. [DOI] [PubMed] [Google Scholar]
- 16. Liu KC, Chesnutt DA. Perineural optic nerve enhancement on magnetic resonance imaging in giant cell arteritis. J Neuroophthalmol 2013;33(3):279–281. [DOI] [PubMed] [Google Scholar]
- 17. Morgenstern KE, Ellis BD, Schochet SS, et al. Bilateral optic nerve sheath enhancement from giant cell arteritis. J Rheumatol 2003;30(3):625–627. [PubMed] [Google Scholar]
- 18. Guggenberger KV, Vogt ML, Song JW, et al. Intraorbital findings in giant cell arteritis on black blood MRI. Eur Radiol 2023;33(4):2529–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Z, Yang Q, Deng Z, et al. Whole‐brain intracranial vessel wall imaging at 3 tesla using cerebrospinal fluid‐attenuated T1‐weighted 3D turbo spin echo. Magn Reson Med 2017;77(3):1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Q, Deng Z, Bi X, et al. Whole‐brain vessel wall MRI: a parameter tune‐up solution to improve the scan efficiency of three‐dimensional variable flip‐angle turbo spin‐echo. J Magn Reson Imaging 2017;46(3):751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooke WT, Cloake PCP. Temporal arteritis: a generalized vascular disease. Q J Med 1946;15:47–75. [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol 1972;27(5):378–391. [DOI] [PubMed] [Google Scholar]
- 23. Achkar AA, Lie JT, Gabriel SE, et al. Giant cell arteritis involving the facial artery. J Rheumatol 1995;22(2):360–362. [PubMed] [Google Scholar]
- 24. Bley TA, Geiger J, Jacobsen S, et al. High‐resolution MRI for assessment of middle meningeal artery involvement in giant cell arteritis. Ann Rheum Dis 2009;68(8):1369–1370. [DOI] [PubMed] [Google Scholar]
- 25. Bley TA, Uhl M, Carew J, et al. Diagnostic value of high‐resolution MR imaging in giant cell arteritis. AJNR Am J Neuroradiol 2007;28(9):1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geiger J, Ness T, Uhl M, et al. Involvement of the ophthalmic artery in giant cell arteritis visualized by 3T MRI. Rheumatology (Oxford) 2009;48(5):537–541. [DOI] [PubMed] [Google Scholar]
- 27. D'Souza NM, Morgan ML, Almarzouqi SJ, et al. Magnetic resonance imaging findings in giant cell arteritis. Eye (Lond) 2016;30(5):758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pappolla A, Silveira F, Norscini J, et al. Bilateral optic perineuritis as initial presentation of giant cell arteritis. Neurologist 2019;24(1):26–28. [DOI] [PubMed] [Google Scholar]
- 29. Veldhoen S, Klink T, Geiger J, et al. MRI displays involvement of the temporalis muscle and the deep temporal artery in patients with giant cell arteritis. Eur Radiol 2014;24(11):2971–2979. [DOI] [PubMed] [Google Scholar]
- 30. Joelson E, Ruthrauff B, Ali F, et al. Multifocal dural enhancement associated with temporal arteritis. Arch Neurol 2000;57(1):119–122. [DOI] [PubMed] [Google Scholar]
- 31. Remond P, Attyé A, Lecler A, et al. The central bright spot sign: a potential new MR imaging sign for the early diagnosis of anterior ischemic optic neuropathy due to giant cell arteritis. AJNR Am J Neuroradiol 2017;38(7):1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freddi TAL, Ottaiano AC. The optic nerve: anatomy and pathology. Semin Ultrasound CT MR 2022;43(5):378–388. [DOI] [PubMed] [Google Scholar]
- 33. Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR classification criteria for giant cell arteritis. Arthritis Rheumatol 2022;74(12):1881–1889. [DOI] [PubMed] [Google Scholar]
- 34. Hayreh SS. Orbital vascular anatomy. Eye (Lond) 2006;20(10):1130–1144. [DOI] [PubMed] [Google Scholar]
- 35. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol 1998;125(4):509–520. [DOI] [PubMed] [Google Scholar]
- 36. Gupta S, Sethi P, Duvesh R, et al. Optic perineuritis. BMJ Open Ophthalmol 2021;6(1):e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gokharman D, Aydin S. Magnetic resonance imaging in orbital pathologies: a pictorial review. J Belg Soc Radiol 2018;101(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta‐analysis informing the EULAR recommendations. RMD Open 2018;4(1):e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maleszewski JJ, Younge BR, Fritzlen JT, et al. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow‐up temporal artery biopsies in 40 treated patients. Mod Pathol 2017;30(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Information


