Abstract
Diets with high carbohydrate (HC) was reported to have influence on appetite and intermediary metabolism in fish. To illustrate whether betaine could improve appetite and glucose-lipid metabolism in aquatic animals, mandarin fish (Siniperca chuatsi) were fed with the HC diets with or without betaine for 8 weeks. The results suggested that betaine enhanced feed intake by regulating the hypothalamic appetite genes. The HC diet-induced downregulation of AMPK and appetite genes was also positively correlated with the decreased autophagy genes, suggesting a possible mechanism that AMPK/mTOR signaling might regulate appetite through autophagy. The HC diet remarkably elevated transcriptional levels of genes related to lipogenesis, while betaine alleviated the HC-induced hepatic lipid deposition. Additionally, betaine supplementation tended to store the energy storage as hepatic glycogen. Our findings proposed the possible mechanism for appetite regulation through autophagy via AMPK/mTOR, and demonstrated the feasibility of betaine as an aquafeed additive to regulate appetite and intermediary metabolism in fish.
Keywords: Mandarin fish, Betaine, AMPK/mTOR, Appetite regulation, Glucose-lipid metabolism
1. Introduction
Carbohydrates are the major energy-providing nutrients in commercial fish feed formulations due to its economic indispensability [1]. However, fish's capacity to use carbohydrates are highly variable both between and within species, which is depended on the feeding habits [2]. Compared to omnivorous and herbivorous species, carnivorous fish have a high intolerance for carbohydrates in diets [2]. Consequently, high carbohydrate (HC) diets invariably induce growth retardation and atypical regulation of intermediary metabolism, including the metabolic syndrome such as persistent postprandial hyperglycaemia and hepatic steatosis in aquatic animals [3,4]. Additionally, several reports have reported a reduction on food intake in carnivorous fish after being exposed to diet rich in carbohydrate [5,6]. Feed intake regulation is of great importance in maintaining energy balance, thus further exploration is required to reveal the mechanism underlying the appetite regulation in HC diet-induced reduction in feed intake.
In fish, food intake regulation is a multifaceted process that involves diverse pathways in central nervous system (CNS), in which the hypothalamus acts as the primary regulatory center [7]. Similarly to mammals, the regulation of energy expenditure and food intake in fish relies on the detection of changes in nutrient levels by various sensing mechanisms in the hypothalamus [8,9]. Additionally, the food intake regulation is governed by two principal parts of neurons in the hypothalamus. One population is the orexigenic neuropeptides neuropeptide Y (NPY) and agouti-related neuropeptide (AGRP), which promote appetite. The other parts express the anorexigenic neuropeptides CART prepropeptide (CART) and pro-opiomelanocortin (POMC), which suppress food ingestion [7,8]. Research have shown that hyperglycemia may result in reduced food intake in fish and mammals fed a HC diet [5,6,10]. Nevertheless, the regulatory mechanisms in the hypothalamus of fish underlying the effect of HC diet on appetite regulation remain rarely understood.
The hypothalamic AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway serves as a sensor of energy and controller of cellular metabolism [11,12]. In mammals, the control of food intake by hypothalamic AMPK/mTOR signaling has gained significant attention as it acts as a connection between signals from peripheral and the regulation of central feeding behavior [13]. It has been reported that under conditions of nutrient deprivation, AMPK can be activated, leading to elevated orexigenic genes NPY/AgRP mRNA levels, and reduced anorexigenic genes CART/POMC mRNA levels [13,14]. Consequently, activation of hypothalamic AMPK and mTOR showed opposite roles in regulating food intake, as AMPK activation inhibit mTOR activity through direct phosphorylation at Thr2446 [11,12]. Emerging evidence has revealed that autophagy, a process that cells use to maintain their internal environment, is closely linked to the hypothalamus' control of food intake [8,15,16]. In conditions of nutrient sufficiency, AMPK directly activates Ulk1, which is a homologue of yeast ATG1, to promote autophagy [17]. Studies showed that deleting autophagy related 7 (Atg7) in POMC neurons of mice demonstrated significant effects on food intake [18]. Therefore, it is plausible that regulating autophagy is a mechanism by which AMPK/mTOR signaling controls neuropeptide expression and feeding behavior in mammals. However, whether this mechanism applies to aquatic animals remains unclear.
To mitigate negative impact on feed intake and metabolic disturbances caused by the HC diet, several studies have investigated different strategies. One potential approach involves the use of betaine, which has been employed as a feed additive in the poultry industry to enhance growth, boost antioxidant defenses, and improve immune function in animals [19]. Notably, betaine has been shown to function as a feed attractant, promoting feed intake in aquatic animals, including the mandarin fish [20]. Betaine has also shown promise as a supplement for the treating or preventing type 2 diabetes in mice, as it improves glucose homeostasis and reduces lipid accumulation in the liver [21]. In black seabream (Acanthopagrus schlegelii), dietary betaine has been found to alleviate the high fat diet induced liver steatosis and inflammation [22]. In addition, betaine could regulate the fatty acids synthesis via mTOR pathway in zebrafish [23]. Therefore, we hypothesized that dietary betaine could alleviate the adverse effects on food intake and metabolic disorders, such as HC diet-induced hepatic steatosis in aquatic animals. Moreover, whether this regulation is via the AMPK/mTOR signaling pathway remains to be investigated.
Mandarin fish (Siniperca chuatsi) is a kind of carnivorous freshwater fish with significant economic potential in Chinese aquaculture. However, mandarin fish was reported to exhibit anorexia and hepatic steatosis after subjected to a diet rich in carbohydrates [6,24]. Previous study has reported that including dietary betaine can reduce could mitigate the oxidative status and apoptosis induced by diet rich in high carbohydrate in mandarin fish [25]. Hence, this study aims to explore if dietary betaine usage could improve the HC diet-induced food intake reduction and metabolic disturbances in glucose and lipid metabolism. The findings might offer new insights for the mechanisms that regulate appetite regulation and metabolic homeostasis in aquatic animals fed a HC diet.
2. Materials and methods
2.1. Diets
Diets containing fishmeal as the protein source and fish oil as the lipid source were formulated to have identical nitrogen and lipid content. The diets were divided into four groups based on their carbohydrate and betaine content, which were termed the NC, BET, HC, and HC + BET group. The addition level of betaine and HC diet in the diets were 1% and 20%, respectively. The betaine used in the study had a minimum purity of 98.0%. Table 1 presents the formulation of the experimental diets. All of the ingredients were subjected to a thorough grinding process and pelletized using a pelletizing machine. The formulated diets were then dried and stored at a temperature of −20 °C until further use.
Table 1.
Diet formulation.
| Ingredients (%) | Experimental diet |
|||
|---|---|---|---|---|
| NC | BET | HC | HC + BET | |
| Fish meal | 70 | 70 | 70 | 70 |
| Corn starch | 0 | 0 | 20 | 19 |
| Fish oil | 3 | 3 | 3 | 3 |
| Vitamin mixa | 2 | 2 | 2 | 2 |
| Mineral mixb | 2 | 2 | 2 | 2 |
| Microcrystalline cellulose | 20 | 19 | 0 | 0 |
| Carboxymethylcellulose sodium | 3 | 3 | 3 | 3 |
| Betainec | 0 | 1 | 0 | 1 |
| Proximate composition | ||||
| Moisture (%) | 6.30 | 6.88 | 5.30 | 8.29 |
| Crude protein (%) | 45.08 | 45.98 | 45.48 | 45.69 |
| Crude lipid (%) | 8.02 | 7.84 | 8.10 | 8.05 |
| Ash (%) | 16.11 | 15.83 | 16.09 | 15.59 |
| Energy (kJ/g) | 15.66 | 15.60 | 18.96 | 18.78 |
Vitamin mix: produced by Guangdong Nutriera Group, (Guangzhou, China): Inositol 100; niacinamide 50; vitamin A 10; vitamin B1 6; vitamin B2 5; vitamin B6 7.5; vitamin B12 (1%) 4; vitamin D3 5; vitamin K3 10; vitamin E (50%) 200; biotin (2%) 2.5; calcium pantothenate 20; ascorbyl calcium phosphate (35%) 500; folic acid 5; corn protein powder 75.
Mineral mix: produced by Guangdong Nutriera Group (Guangzhou, China): Zeolite 638; FeSO4·H2O 300; ZnSO4·H2O 200; CoCl2·6H2O (10% Co) 5; NaCl 100; MnSO4·H2O 100; Na2SeO3 (10% Se) 67; KIO3 (10%) 80; CuSO4·5H2O 10.
Betaine: IB0150, Beijing Solarbio Science & Technology Co., Ltd (Beijing, China).
2.2. Fish and feeding trail
The research was performed at the Pearl River Fisheries Research Institute Chinese Academy of Fishery Sciences (Guangzhou, China), using an indoor rearing system. A detailed description of the experimental procedure is given in previous publications by Li et al. (2023). In brief, juvenile mandarin fish (Siniperca chuatsi), already adapted to consuming artificial diets, were acclimated to the rearing environments for two weeks. Following a 24-h fast, 300 healthy mandarin fish (initial weight, 23.73 ± 0.05 g) were assigned to four groups at random. The fish experiment involved groups of three tanks, each with 25 fish, in triplicate. Then, fish were fed to apparent satiation twice daily, using the four experimental diets at 08:30 and 16:00. Unconsumed food was collected with a siphon 20 min after feeding, then oven-dried to correct for ingested amounts. During the eight-week rearing period, regular monitoring was conducted on water quality parameters, including temperature (maintained at 27.0–28.5 °C), total ammonia nitrogen (<0.1 mg/L), dissolved oxygen (4.6–5.5 mg/L), and pH (7.0–8.5).
2.3. Sampling procedure
After an 8-week feeding trial, MS-222 (60 mg/L, Sigma, USA) was used to the anesthetize the fish. The number and weight of fish were recorded before sampling. Blood was obtained from three randomly selected fish from each tank using 0.02% heparin sodium (Aladdin, China)-rinsed sterile syringe. The supernatant was carefully collected after being centrifuged in sterile centrifuge tubes (4000 rpm for 10 min). One portion of the liver was fixed by 4% formaldehyde for histological analysis, while the other part of liver tissue was immediately removed on ice and stored at a temperature of −80 °C for future use.
2.4. Biochemical and enzymatic analysis
The levels of plasma triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were detected with commercial-available kits following the manufacturer's instructions (A110-1-1, A112-1, and A113-1, Nanjing Jiancheng Bioengineering Institute, China). The liver enzyme activities including the acid phosphatase (ACP) and alkaline phosphatase (AKP) were detected using commercially available kits (A060-2-2, A059-2-2, Nanjing Jiancheng Bioengineering Institute, China). The TG and cholesterol (T-CHO) levels were measured in the plasma and liver of mandarin fish using commercial kits (A111-1-1, Nanjing Jiancheng Bioengineering Institute, China).
2.5. Oil-red O staining analysis
In order to observe the fat droplets accumulation in the liver, sections of liver were made using neutral Oil Red O (Wako Pure Chemicals, Japan) staining, and the protocal was the same as in previous studies [26]. Briefly, fixed livers were sectioned and embedded in TissueTek OCT compound (Sakura Finetek, Japan). Then, the samples were snap-frozen with liquid nitrogen-cooled isopentane and cut into 5 mm sections using a cryostat. After undergoing staining and rinsing steps, observations were performed using an optical microscope (Olympus BX41, Olympus Corporation).
2.6. RT-PCR analysis
The TRizol reagent (Invitrogen, Carlsbad, USA) was utilized for liver tissue RNA extraction in accordance with the manufacturer's instructions. The RNA concentration was measured using Implen NanoPhotometer (Implen Inc, Germany). The integrity of the RNA was detected by 1% agarose electrophoresis. Subsequently, cDNA was synthesized using a Takara reverse transcription kit (Takara, China). Table 2 presents the primer sequences that used in this experiment. β-actin and rpl13α were applied as the reference genes. Quantitative real-time PCR was carried out in triplicate on a Light Cycler®96-Time PCR instrument (Roche, Switzerland). The 20 μL reaction systems comprised of diluted first-strand cDNA product (2 μL), 2 × SYBR Premix Ex TaqII (10 μL), forward/reverse primer (0.8 μL of each), and sterilized ddH2O (6.4 μL). The qRT-PCR cycling procedure were set as follows: 95 °C for 5 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. Pfaffl's mathematical model was used to calculate the relative gene expression [27].
Table 2.
Primers sequences selected for analysis.
| Genes | Sequence from 5′-3′ | Accession number |
|---|---|---|
| beta-actin (β-actin) | F: TGCGTGACATCAAGGAGAAGC | XM_044169301.1 |
| R: GAGGAAGGAAGGCTGGAAGAG | ||
| ribosomal protein L13a (rpl13a) | F: CACCCTATGACAAGAGGAAGC | MK770673 |
| R: TGTGCCAGACGCCCAAG | ||
| amp-activated protein kinase (AMPK) | F: GGGATGCAAACCAAGATG | XM_044175461.1 |
| R: ACAGACCCAGAGCGGAGA | ||
| mammalian target of rapamycin (mTOR) | F: GCATCAACGAGAGCACCA | XM_044211707.1 |
| R: CGCTTCAAAATTCATAACCG | ||
| unc-51 like autophagy activating kinase 1 (ulk1) | F: GTGCCTGCCCAGTTTCCC | XM_044195569.1 |
| R: GCAGGTTCTGTTCCATACGCT | ||
| beclin 1 (becn1) | F: AGGAGGTGAAGAGCGATAAGG | XM_044167873.1 |
| R: CCAGGCGACGGTTGTGA | ||
| microtubule-associated protein 1 light chain 3b (lc3b) | F: AGAGCAGCACCCCAGCAA | XM_044194970.1 |
| R: CGTTGACCAGCAGGAAGAAA | ||
| autophagy related 4c (atg4c) | F: TCAGCACCAGCGATTTCCC | XM_044200084.1 |
| R: GCGGGGTATTTCTCCTTCG | ||
| neuropeptide y (npy) | F: GTTGAAGGAAAGCACAGACA | EF554594 |
| R: GCTCATAGAGGTAAAAGGGG | ||
| agouti gene-related protein 2 (agrp2) | F: GAGCCAAGCGAAGACCAGA | MK770670 |
| R: GCAGCACGGCAAATGAGAG | ||
| pro-opiomelanocortin a (pomca) | F: CTGTCAGGAGCTCAACTCTG | MN818827 |
| R: AGGAAGGGAGGATGAAGGAG | ||
| cocaine and amphetamine regulated transcript (cart) | F: CGAACCTAACCAGTGAGAAG | MN818823 |
| R: GGGACAGTCGCACATCTT | ||
| glucokinase (gk) | F: CTGCGTTTAGAGACCCACGA | MW140068.1 |
| R: TTCACCAGCATCACACGGAA | ||
| 6-phosphofructokinase (pfk) | F: GCTACCATCAGCAACAACG | XM_044218098.1 |
| R: GCCACAGAATCCACCCAT | ||
| pyruvate kinase (pk) | F: CGCCCTCGCTGTCCTATTA | XM_044207998.1 |
| R: TGCCGAAGTTGACCCTGTTG | ||
| glucose-6-phosphatase (g6pca1) | F: CTTATGACTTTCTATTTCCTGTCGTTTG | XM_044177989.1 |
| R: GTTCTTGTCTTCTCTCTTTCCCTTG | ||
| fructose-1,6-bisphosphatase 1b (fbp1b) | F: TCCCAGTTCCACACCTGACA | XM_044175013.1 |
| R: GTTAGCGATCCCAGCCTTCC | ||
| phosphoenolpyruvate carboxykinase (pepck) | F: CTGAGTTTGTGAAGAGAGCGG | XM_044176969.1 |
| R: GTCCTTTGGGTCTGTGCGT | ||
| glycogen synthase kinase (gsk) | F: CTCGGTGTGTCTTTCCACGA | XM_044171890.1 |
| R: GCTGCCGCTCATATCTCTGT | ||
| glycogen phosphorylase (gp) | F: CTCAGAACAAAACCTGCCCAC | XM_044165888.1 |
| R: CCGCAACATTCTCCACTCCC | ||
| sterol regulatory element binding protein (srebp1) | F: CTCCCTCCTTTCTGTCGGCTC | XM_044180668.1 |
| R: TCATTTGCTGGCAGTCGTGG | ||
| acetyl-CoA carboxylase (accα) | F: TATGCCCACTTACCCAAATGC | XM_044221969.1 |
| R: TGCCACCATACCAATCTCGTT | ||
| fatty acid synthase (fas) | F: ATGGAAATCACCCCTGTAATCTT | XM_044177556.1 |
| R: CTTATCTGACTACGGAATGAATCG | ||
| lipoprotein lipase (lpl) | F: TTACCCCAATGGAGGCACTT | XM_044199514.1 |
| R: CGGACCTTGTTGATGTTGTAG | ||
| peroxisome proliferator-activated receptor-a (pparα) | F: GGGTGTGCTCAGACAAGGCT | XM_044185657.1 |
| R: GTTGCGGTTCTTCTTTTGGAT |
2.7. Statistical analysis
Statistical analysis was conducted using SPSS 22.0 software (SPSS Inc., USA). One-way ANOVA analysis was conducted, and Duncan's multiple comparisons test was carried out to compare between the groups. The comparisons test was performed between the NC and HC groups, as well as between the HC and HC + BET groups. Results are showed as mean ± standard error of the mean (SEM). P value < 0.05 indicated statistically significant.
3. Results
3.1. Plasma lipid metabolites and enzyme activities in the liver
The impacts of dietary betaine on plasma lipid metabolites and liver function enzyme activities are summarized in Table 3. The results suggested that the HC group had significantly higher plasma TG levels compared to those of the NC group (P < 0.05). No remarkable variations were found in plasma T-CHO, LDL-C, and HDL-C levels between all groups (P > 0.05). Nevertheless, the fish in the HC + BET groups showed significantly higher plasma T-CHO levels compared to the fish in the HC group (P < 0.05). In terms of liver function indicators including AKP and ACP in the liver of mandarin fish, no significant variations were found between all groups (P > 0.05).
Table 3.
Plasma metabolites and liver enzyme activities in mandarin fish.
| Items | NC | BET | HC | HC + BET | NC vs HC | HC vs HC + BET |
|---|---|---|---|---|---|---|
| Plasma | ||||||
| TG (mmol/L) | 1.84 ± 0.18 | 2.27 ± 0.19 | 2.33 ± 0.26 | 2.38 ± 0.27 | * | ns |
| T-CHO (mmol/L) | 2.41 ± 0.11 | 2.62 ± 0.09 | 2.33 ± 0.10 | 2.51 ± 0.11 | ns | ns |
| LDL-C (mmol/L) | 5.03 ± 0.76 | 6.08 ± 0.53 | 4.86 ± 0.35 | 6.28 ± 0.67 | ns | ns |
| HDL-C (mmol/L) | 2.32 ± 0.11 | 2.39 ± 0.08 | 2.42 ± 0.10 | 2.05 ± 0.22 | ns | ns |
| Liver | ||||||
| T-CHO (mmol/gprot) | 0.19 ± 0.01 | 0.16 ± 0.01 | 0.20 ± 0.01 | 0.29 ± 0.02 | ns | * |
| AKP (U/L/gprot) | 180.27 ± 13.35 | 192.93 ± 27.22 | 192.33 ± 25.69 | 233.23 ± 11.53 | ns | ns |
| ACP (U/L/gprot) | 2078.35 ± 66.16 | 1956.23 ± 74.49 | 1947.63 ± 58.38 | 2101.32 ± 102.95 | ns | ns |
3.2. Feed intake and hypothalamic gene expression involved in appetite regulation
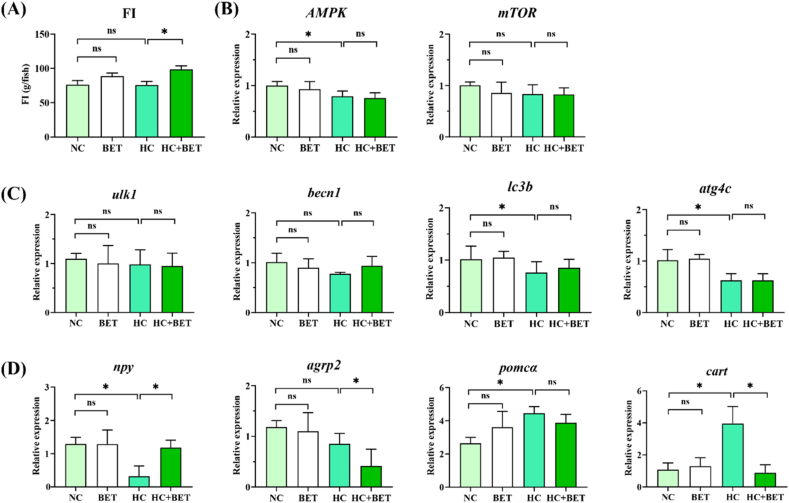
As shown in Fig. 1A, the application of betaine in the HC diet significantly increased the feed intake (FI) of mandarin fish than the HC group (P < 0.05). Between the other groups, no remarkable variations in FI were observed (Fig. 1A; P > 0.05). For the transcriptional mRNA levels in the hypothalamus, the HC diet resulted in a significant reduction in AMPK, lc3b and atg4c mRNA levels in mandarin fish than the NC group (Fig. 1B and C; P < 0.05). While the hypothalamic mTOR, ulk1, and becn1 mRNA levels remained consistent between all groups (Fig. 1B and C; P > 0.05). Additionally, the HC diet significantly lowered the npy mRNA levels but elevated the pomcα and cart mRNA levels in the hypothalamus (Fig. 1D; P < 0.05). On the other hand, as compared to the HC group, dietary betaine usage in the HC diet significantly increased the npy and agrp2 mRNA levels while decreased the cart mRNA levels in the HC + BET group (Fig. 1D; P < 0.05).
Fig. 1.
Effects of dietary betaine on feed intake and associated gene expressions in hypothalamus of mandarin fish. (A) FI, feed intake; (B) mRNA levels of AMPK and mTOR in the hypothalamus; (C) transcriptional expression of autophagy genes in the hypothalamus; (D) transcriptional expression of appetite genes in the hypothalamus. Data are expressed as mean ± SEM (n = 6). * indicates significant difference between groups (P < 0.05). AMPK: AMP-activated protein kinase; mTOR: mammalian target of rapamycin; ulk1: unc-51 like autophagy activating kinase 1; becn1: beclin 1; lc3b: microtubule-associated protein 1 light chain 3b; atg4c: autophagy related 4c; npy: neuropeptide y; agrp2: agouti gene-related protein 2; pomca: pro-opiomelanocortin a; cart: cocaine and amphetamine regulated transcript.
3.3. Glucose and glycogen metabolism in the liver
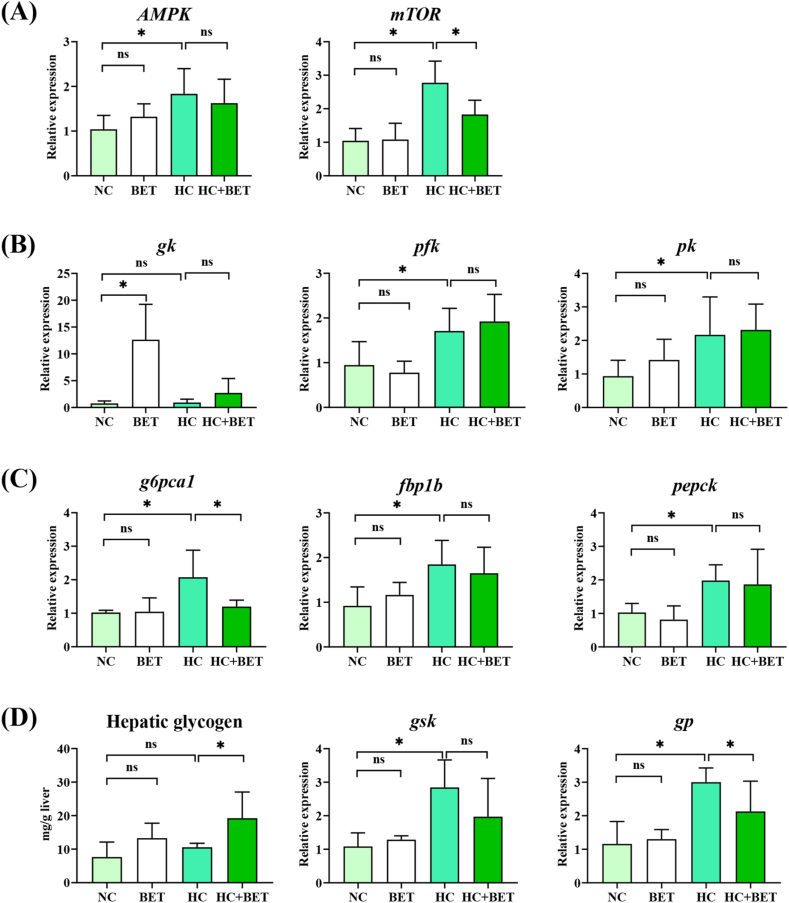
Fig. 2 presents the hepatic mRNA levels of the regulators and genes related to glucose metabolism in mandarin fish. The HC diet significantly increased hepatic AMPK and mTOR mRNA levels in mandarin fish than the NC diet (Fig. 2A; P < 0.05). Compared with the HC group, the hepatic mRNA level of mTOR diminished significantly in the HC + BET group (Fig. 2A; P < 0.05).
Fig. 2.
Effects of dietary betaine on glucose and glycogen metabolism in the liver of mandarin fish. (A) mRNA levels of AMPK and mTOR in the liver; (B) hepatic glycolysis gene expression; (C) hepatic gluconeogenesis gene expression; (D) hepatic glycogen and associated metabolic genes. Data are expressed as mean ± SEM (n = 6). * indicates significant difference between groups (P < 0.05). gk: glucokinase; pfk: 6-phosphofructokinase; pk: pyruvate kinase; g6pca1: glucose-6-phosphatase; fbp1b: fructose-1,6-bisphosphatase 1b; pepck: phosphoenolpyruvate carboxykinase; gsk: glycogen synthase kinase; gp: glycogen phosphorylase.
For genes involved in glycolysis, the HC group showed significantly higher pfk and pk mRNA levels, while the BET group showed higher hepatic gk mRNA levels, as compared to the NC group (Fig. 2B; P < 0.05). In addition, the HC diet group stimulated higher transcriptional levels for gluconeogenesis-related genes, including g6pca1, fbp1b, and pepck in mandarin fish (Fig. 2C; P < 0.05). Compared to the HC group, dietary betaine usage significantly decreased the hepatic g6pca1 mRNA levels in mandarin fish (Fig. 2C; P < 0.05). Moreover, the HC + BET group had significant higher glycogen levels in the liver than the HC group (Fig. 2D; P < 0.05). Similarly, in the HC group, both gsk and gp mRNA levels were significantly higher than in the NC group in the liver of mandarin fish (Fig. 2D; P < 0.05). Additionally, hepatic gp mRNA levels were significantly decreased by betaine supplementation in the HC diet, in comparison to the HC diet alone (Fig. 2D; P < 0.05).
3.4. Lipid contents and associated metabolic events in the plasma and liver
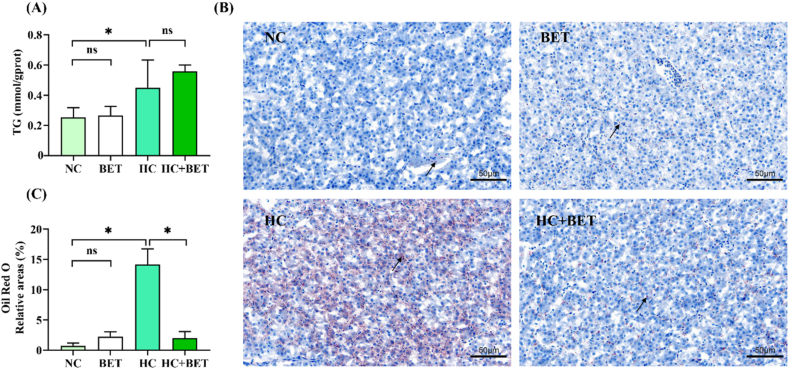
The HC diet showed significantly higher liver TG contents than the NC group (Fig. 3A). In comparison to the NC group, the lipid droplets and relative areas as shown by the Oil Red O staining images, which represent the portion of liver lipid droplets in mandarin fish, increased significantly in the HC group, (Fig. 3B and C; P < 0.05).
Fig. 3.
Effects of dietary betaine on plasma triglycerides and lipid deposition in the liver of mandarin fish. (A) TG: triglycerides in plasma; (B) photomicrographs of representative Oil Red O stained liver sections; (C) relative area of lipid droplets (LD). Lipid droplets was red-colored and nuclei was blue-colored. Data are expressed as mean ± SEM (n = 6). * indicates significant difference between groups (P < 0.05).
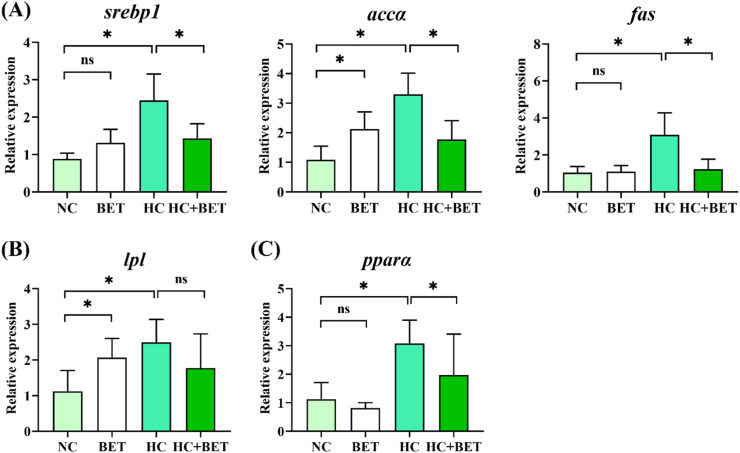
Hepatic mRNA levels of genes related to lipogenesis such as srebp1, accα and fas were significantly increased in the HC group (Fig. 4A; P < 0.05). Conversely, the HC + BET diet suppressed the transcriptional level of the aforementioned genes (Fig. 4A; P < 0.05). The lpl and pparα mRNA levels were both stimulated in the HC group for lipid catabolism, as compared to the NC group (Fig. 4B and C; P < 0.05). In addition, the HC + BET group had significantly lower pparα mRNA levels than the HC group (Fig. 4C; P < 0.05). Despite the higher accα and lpl mRNA levels in the BET group than NC group, no remarkable variations were found between the other groups.
Fig. 4.
Effects of dietary betaine on lipid metabolism in the liver of mandarin fish. (A) expression of lipogenesis genes in the liver; (B) expression of lipolysis gene in the liver; (C) expression of gene related to fatty acid β-oxidation in the liver. Data are expressed as mean ± SEM (n = 6). * indicates significant difference between groups (P < 0.05). srebp1: sterol regulatory element binding protein; accα: acetyl-CoA carboxylase; fas: fatty acid synthase; lpl: lipoprotein lipase; pparα: peroxisome proliferator-activated receptor-a.
4. Discussion
4.1. AMPK/mTOR might regulate appetite through autophagy in the hypothalamus of mandarin fish
The AMPK/mTOR signaling pathway is known to function as a fuel gauge that modulate cellular energy status and regulate food intake in the hypothalamus of mammals [11]. AMPK and mTOR may be regulators of autophagy through direct phosphorylation of Ulk1, a homologue of yeast ATG1 and an autophagy-initiating kinase [11,17]. In general, autophagy is a process that degrades cellular components to maintain essential activity and viability under nutrient limitation. Therefore, hypothalamic autophagy can be considered a nutrient-responsive processes that regulates food intake [16]. In fish, it has been demonstrated that the activation or inhibition of mTOR signaling can restrain or trigger food intake by modifying the anorexigenic POMC protein levels [28]. The HC diet has also been reported to reduce ingestion in fish including rainbow trout (Oncorhynchus mykiss Walbaum) and mandarin fish [5,6]. In this study, although no significant variations concerning feed intake (FI) were found between mandarin fish fed the NC and HC diet, some events linking the gene expression of AMPK/mTOR signaling, autophagy and appetite regulation and the phenotype of feed intake displayed positively related to some extent. In mice, the 5-aminoimidazole-4-carboxamide ribonucleotide stimulated the activation of AMPK by intracerebroventricular administration, resulting in a significant increase in food intake [13]. In current research, the HC diet significantly inhibited the AMPK transcript levels in mandarin fish hypothalamus than the NC diet treatment. In addition, the inhibition of AMPK by the HC diet led to upregulation of anorexigenic genes cart and pomc and downregulation of orexigenic genes npy in the hypothalamus, resulting in inhibition of appetite in mandarin fish. Importantly, such inhibition of appetite regulation was positively correlated with the decreased mRNA levels of lc3b and atg4c, which is related to autophagy. Taken the decreased mRNA levels of AMPK together, it suggests that the AMPK/mTOR signaling pathway may modulate appetite genes through autophagy in the mandarin fish hypothalamus.
Betaine is a well-known feeding stimulant for fish, and it is commonly used to enhance the flavor of chemical substances in feed, thereby increasing feed intake [29]. Dietary supplementation of betaine at an appropriate level has been reported to enhance feed intake in various aquatic species, such as grass carp (Ctenopharyngodon idella), black seabream (Acanthopagrus schlegelii), and Nile tilapia (Oreochromis niloticus) [21,30,31]. In this study, compared to the HC group, a stimulation of food intake was observed in the HC + BET diet group of mandarin fish. Accordingly, the orexigenic genes npy and agrp2 mRNA levels was significantly stimulated, and the anorexigenic genes cart mRNA levels was significantly suppressed in the hypothalamus of mandarin fish. These findings indicate that dietary supplementation of betaine affects the transcriptional expression of appetite genes in the hypothalamus, thereby improving feed intake of mandarin fish. These results further support the feed-enhancing effects of betaine in aquatic species.
4.2. The HC diet stimulated hepatic glucose metabolism and induced lipid deposition in mandarin fish
Dietary carbohydrates, when provided at an appropriate level, can promote growth, save protein, and provide metabolic intermediates for aquatic animals [1,3]. Nevertheless, prolonged ingestion of high carbohydrate diets can lead to metabolic disorders in glucose homoeostasis in aquatic species [32]. As a typical carnivorous fish species, mandarin fish are thought to have a restrained ability to efficiently utilize the dietary carbohydrates [25,33]. Despite the HC diet-induced oxidative status and apoptotic responses, it also significantly elevated the hepatic AMPK and mTOR mRNA levels in mandarin fish. Moreover, the HC diet significantly increased the hepatic glycolysis-related gene mRNA levels, such as pfk, and pk in mandarin fish. Accompanied with the activated glycolysis, significantly higher hepatic transcriptional expression of g6pc1, fbp1b, and pepck were observed in mandarin fish of the HC diet group. Generally, a diet rich in carbohydrates always stimulates the glycolysis process to convert glucose to pyruvate, releasing ATP for energy expenditure for fish [3,34]. Due to the limited capability to regulate the hepatic glucose metabolism, carnivorous fish were particularly unable to inhibit gluconeogenesis when consumed a high-carbohydrate diet [35]. Therefore, the activated glycolysis and gluconeogenesis pathways could be considered typical glucose metabolic activities in mandarin fish exposed to the HC diet. In this study, the glycogen levels in the liver were stable between the NC and HC diet group, which could be ascribed to increased levels of glycogen synthesis regulated by glycogen synthase kinase (gsk) as well as increased glycogen catabolism regulated by glycogen phosphorylase (gp). Consequently, the HC diet stimulated molecular event related to glucose metabolism but maintained a constant glycogen level in the liver of mandarin fish.
In addition, glucose and lipid metabolism are closely interconnected processes in the liver, which is important for converting redundant dietary carbohydrates into triglycerides [36]. Ingestion of a high-carbohydrate diet has been reported to lead to hepatic lipid deposition in various fish species, including grass carp (Ctenopharyngodon idella), gibel carp (Carassius gibelio), as well as mandarin fish [3,4,24]. Consistent with this, compared to mandarin fish fed a NC diet, those fed HC diets showed significantly higher plasma TG levels and lipid droplets in their livers. The sterol regulatory element binding proteins 1 (srebp1) is reported to be crucial regulator of lipid anabolism in fish by converting excess glucose into fatty acids [37]. Additionally, the lipoprotein lipase (lpl) is responsible for hydrolyzing triglycerides, while the nuclear receptor peroxisome proliferator-activated receptor alpha (pparα) initiates degradation of fatty acids through fatty acid β oxidation. Although significantly higher lpl and pparα mRNA levels of lipid catabolic genes were observed in the HC group, the upregulated srebp1, accα, and fas mRNA levels ultimately contributed to liver lipid deposition in mandarin fish. Thus, the HC diet induced lipogenesis via upregulating srebp1 signaling and stored the excess dietary carbohydrate through hepatic lipid deposition in mandarin fish.
4.3. Betaine usage in the HC diet altered the energy storage form in the liver of mandarin fish
Recently, there has been growing evidence that dietary betaine has hepatoprotective effects in both mammals and fish, as it reduces fat accumulation and alleviates hepatic steatosis [22,38]. In black seabream (Acanthopagrus schlegelii), dietary betaine supplementation worked as a lipid-lowering substance to alleviate high-fat diet-induced liver steatosis and mitigate inflammatory responses in fish [22]. In blunt snout bream (Megalobrama amblycephala), dietary betaine reduced liver lipid accumulation by improving bile acid metabolism [32]. In this study, significantly lower srebp1, accα and fas mRNA levels was found in fish fed HC + BET diet in comparison to the HC diet. Subsequently, the significantly lower lipid droplets levels as evidenced by the Oil-Red O staining confirmed the results that dietary betaine alleviated the HC-diet induced liver lipid deposition in mandarin fish. Generally, glycogen and lipid are deposited as energy storage in fish in response to excess dietary carbohydrate, depending on their feeding habits and nutrition [4]. For instance, golden pompano (T. ovatus) and largemouth bass (Micropterus salmoides) exhibit storage of liver glycogen with increasing dietary carbohydrates [39,40]. But, overload amounts of ingested glucose could also be converted into lipid storage in the body of some fish species [3,4]. This study suggested that incorporating betaine into the HC diet led to accumulation of the hepatic glycogen, as compared to fish fed the HC diet alone. This might be attributed to the reduced catabolism of glycogen, as evidenced by the decreased hepatic mRNA levels of gp in mandarin fish. Therefore, dietary betaine may alter the energy storage to hepatic glycogen rather than lipid, thus alleviating the HC diet-induced hepatic lipid over-deposition in mandarin fish.
5. Conclusion
Overall, we explored the impacts and regulatory mechanisms of dietary betaine on appetite regulation and glucose-lipid intermediary metabolism in mandarin fish. The findings revealed that betaine (at a concentration of 1%) enhanced appetite in mandarin fish fed a HC diet. This regulatory effect is likely mediated through the AMPK/mTOR signaling pathway, which targets autophagy in the mandarin fish hypothalamus. In addition, the HC diet stimulated both glycolysis and gluconeogenesis, contributing to the maintenance of stable levels of hepatic glycogen in mandarin fish. Meanwhile, the incorporating betaine into the HC diet relieved the HC-induced hepatic lipid deposition and led to changes of the energy storage form. As a conclusion, the findings suggest that betaine can serve as a viable additive for aquafeed to regulate intermediary metabolism in aquatic species.
Ethics statement
All animal studies were allowed by the Institutional Animal Care and Use Ethics Committee of the Chinese Academy of Fishery Sciences (No. LAEC-PRFRI-2022-08-58).
Data availability statement
Datasets are available on request.
CRediT authorship contribution statement
Hongyan Li: Writing – review & editing, Writing – original draft, Funding acquisition. Yanzhi Zeng: Formal analysis, Conceptualization. Guangjun Wang: Conceptualization. Kai Zhang: Investigation. Wangbao Gong: Validation. Zhifei Li: Investigation. Jingjing Tian: Data curation. Yun Xia: Data curation. Wenping Xie: Project administration. Jun Xie: Validation. Shouqi Xie: Conceptualization. Ermeng Yu: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by Key Laboratory of Breeding Biotechnology and Sustainable Aquaculture, Chinese Academy of Sciences (2023FB02), the Guangdong Basic and Applied Basic Research Foundation (2023A1515010008), Science and Technology Program of Guangzhou (2024A04J4584), Scientific Innovation Fund, PRFRI (2023CXYC2, 2024CXYC1), the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2023TD62), the Fujian Province Key Laboratory of Special Aquatic Formula Feed (TMKJZ2205), and the Special Financial Fund of Foshan in 2023-Cooperation project for high-level agricultural science and technology demonstration city construction in Guangdong Province.
Contributor Information
Jun Xie, Email: xiejunhy01@126.com.
Shouqi Xie, Email: sqxie@ihb.ac.cn.
Ermeng Yu, Email: yem@prfri.ac.cn.
References
- 1.Kamalam B.S., Medale F., Panserat S. Utilisation of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture. 2017;467:3–27. doi: 10.1016/j.aquaculture.2016.02.007. [DOI] [Google Scholar]
- 2.Kamalam B.S., Medale F., Kaushik S., Polakof S., Skiba-Cassy S., Panserat S. Regulation of metabolism by dietary carbohydrates in two lines of rainbow trout divergently selected for muscle fat content. J. Exp. Biol. 2012;215:2567–2578. doi: 10.1242/jeb.070581. [DOI] [PubMed] [Google Scholar]
- 3.Li H., Xu W., Jin J., Zhu X., Yang Y., Han D., Liu H., Xie S. Effects of dietary carbohydrate and lipid concentrations on growth performance, feed utilization, glucose, and lipid metabolism in two strains of gibel carp. Front. Vet. Sci. 2019;6:165. doi: 10.3389/fvets.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su J., Gong Y., Mei L., Xi L., Chi S., Yang Y., Jin J., Liu H., Zhu X., Xie S., Han D. The characteristics of glucose homoeostasis in grass carp and Chinese longsnout catfish after oral starch administration: a comparative study between herbivorous and carnivorous species of fish. Br. J. Nutr. 2020;123:627–641. doi: 10.1017/S0007114519003234. [DOI] [PubMed] [Google Scholar]
- 5.Polakof S., Míguez J.M., Soengas J.L. Dietary carbohydrates induce changes in glucosensing capacity and food intake of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R478–R489. doi: 10.1152/ajpregu.00176.2008. [DOI] [PubMed] [Google Scholar]
- 6.You J.J., Ren P., He S., Liang X.F., Xiao Q.Q., Zhang Y.P. Histone methylation of H3K4 involved in the anorexia of carnivorous Mandarin fish (Siniperca chuatsi) after feeding on a carbohydrate-rich diet. Front. Endocrinol. 2020;11:323. doi: 10.3389/fendo.2020.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado M.J., Cerdá-Reverter J.M., Soengas J.L. Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: involvement in the control of food intake. Front. Neurosci. 2017;11:354. doi: 10.3389/fnins.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh T.S., Cho H., Cho J.H., Yu S.W., Kim E.K. Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy. 2016;12:2009–2025. doi: 10.1080/15548627.2016.1215382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soengas J.L. Contribution of glucose- and fatty acid sensing systems to the regulation of food intake in fish. a review. Gen. Comp. Endocrinol. 2014;205:36–48. doi: 10.1016/j.ygcen.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Peters H.P.F., Ravestein P., van der Hijden H.T.W.M., Boers H.M., Mela D.J. Effect of carbohydrate digestibility on appetite and its relationship to postprandial blood glucose and insulin levels. Eur. J. Clin. Nutr. 2011;65:47–54. doi: 10.1038/ejcn.2010.189. [DOI] [PubMed] [Google Scholar]
- 11.Cota D., Proulx K., Smith K.A.B., Kozma S.C., Thomas G., Woods S.C., Seeley R.J. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 12.Hu X., Kong L., Xiao C., Zhu Q., Song Z. The AMPK-mTOR signaling pathway is involved in regulation of food intake in the hypothalamus of stressed chickens. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2021;258 doi: 10.1016/j.cbpa.2021.110979. [DOI] [PubMed] [Google Scholar]
- 13.Minokoshi Y., Alquier T., Furukawa N., Kim Y.B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M.J., Stuck B.J., Kahn B.B. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 14.Claret M., Schneeberger M. Recent insights into the role of hypothalamic AMPK signaling cascade upon metabolic control. Front. Neurosci. 2012;6:185. doi: 10.3389/fnins.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik S., Rodriguez-Navarro J.A., Arias E., Kiffin R., Sahu S., Schwartz G.J., Cuervo A.M., Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metabol. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R. Autophagy in the control of food intake. Adipocyte. 2012;1:75–79. doi: 10.4161/adip.18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan W., Kim H.K., Moon E.Y., Kim S.S., Choi C.S., Komatsu M., Jeong Y.T., Lee M.K., Kim K.W., Kim M.S. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Neuroendocrinology. 2012;153:1817–1826. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 19.Alagawany M., Elnesr S.S., Farag M.R., El-Naggar K., Taha A.E., Khafaga A.F., Madkour M., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E. Betaine and related compounds: chemistry, metabolism and role in mitigating heat stress in poultry. J. Therm. Biol. 2022;104 doi: 10.1016/j.jtherbio.2021.103168. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Fang J., Liang X.F., Alam M.S., Liu L., Yuan X. Effect of feeding stimulants on growth performance, feed intake and appetite regulation of Mandarin fish. Siniperca chuatsi. Aquacult. Nutr. 2019;50:3684–3691. doi: 10.1111/are.14327. [DOI] [Google Scholar]
- 21.Ejaz A., Martinez-Guino L., Goldfine A.B., Ribas-Aulinas F., De Nigris V., Ribó S., Gonzalez-Franquesa A., Garcia-Roves P.M., Li E., Dreyfuss J.M., Gall W., Kim J.K., Bottiglieri T., Villarroya F., Gerszten R.E., Patti M.E., Lerin C. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes. 2016;65:902–912. doi: 10.2337/db15-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin M., Shen Y., Pan T., Zhu T., Li X., Xu F., Betancor M.B., Jiao L., Tocher D.R., Zhou Q. Dietary Betaine mitigates hepatic steatosis and inflammation induced by a high-fat-diet by modulating the Sirt1/Srebp-1/Pparɑ pathway in juvenile black seabream (Acanthopagrus schlegelii) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.694720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D.W., Gu Y.Q., Pang Q.X., Yu H.R., Zhang J.J. Dietary betaine regulates the synthesis of fatty acids through mTOR signaling in the muscle of zebrafish. J. Funct.Foods. 2021;85 doi: 10.1016/j.jff.2021.104610. [DOI] [Google Scholar]
- 24.Zhang Y., Liang X.F., He S., Wang J., Li L., Zhang Z., Li J., Chen X., Li L., Alam M.S. Metabolic responses of Chinese perch (Siniperca chuatsi) to different levels of dietary carbohydrate. Fish Physiol. Biochem. 2021;47:1449–1465. doi: 10.1007/s10695-021-00965-2. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Zeng Y., Zheng X., Wang G., Tian J., Gong W., Xia Y., Zhang K., Li Z., Xie W., Xie J., Yu E. Dietary betaine attenuates high-carbohydrate-diet-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in Mandarin fish (Siniperca chuatsi) Antioxidants. 2023;12:1860. doi: 10.3390/antiox12101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Xu W., Wu L., Dong B., Jin J., Han D., Zhu X., Yang Y., Liu H., Xie S. Distinct dietary cadmium toxic effects and defense strategies in two strains of gibel carp (Carassius gibelio) revealed by a comprehensive perspective. Chemosphere. 2020;261 doi: 10.1016/j.chemosphere.2020.127597. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K., Zhang Z., Li J., Xie S., Shi L.J., He Y.H., Liang X.F., Zhu Q.S., He S. Different regulation of branched-chain amino acid on food intake by TOR signaling in Chinese perch (Siniperca chuatsi) Aquaculture. 2021;530 doi: 10.1016/j.aquaculture.2020.735792. [DOI] [Google Scholar]
- 29.Lim L.S., Lai S.K.J., Yong A.S.K., Shapawi R., Kawamura G. Evaluation on the potential of betaine, taurine, nucleotide and nucleoside as feeding stimulant for juvenile marble goby Oxyeleotris marmoratus through behavioural assays. Int. Aquat. Res. 2016;8:161–167. doi: 10.1007/s40071-016-0131-4. [DOI] [Google Scholar]
- 30.Luo Z., Tan X.Y., Liu X.J., Wen H. Effect of dietary betaine levels on growth performance and hepatic intermediary metabolism of GIFT strain of Nile tilapia Oreochromis niloticus reared in freshwater. Aquacult. Nutrition. 2011;17:361–367. doi: 10.1111/j.1365-2095.2010.00805.x. [DOI] [Google Scholar]
- 31.Sun H., Jiang W.D., Wu P., Liu Y., Jiang J., Yang Q.H., Kuang S.Y., Tang L., Zhou X.Q., Feng L. Betaine supplementations enhance the intestinal immunity of on-growing grass carp (Ctenopharyngodon idella): partly related to TOR and NF-κB signaling pathways. Aquaculture. 2020;518 doi: 10.1016/j.aquaculture.2019.734846. [DOI] [Google Scholar]
- 32.Callet T., Hu H., Larroquet L., Surget A., Liu J., Plagnes-Juan E., Maunas P., Turonnet N., Mennigen J.A., Bobe J., Burel C., Corraze G., Panserat S., Marandel L. Exploring the impact of a low-protein high-carbohydrate diet in mature broodstock of a glucose-intolerant teleost, the rainbow trout. Front. Physiol. 2020;11:303. doi: 10.3389/fphys.2020.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson R.P. Utilization of dietary carbohydrate by fish. Aquaculture. 1994;124:67–80. doi: 10.1016/0044-8486(94)90363-8. [DOI] [Google Scholar]
- 34.Panserat S., Médale F., Blin C., Brèque J., Vachot C., Plagnes-Juan E., Gomes E., Krishnamoorthy R., Kaushik S.J. Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout (Oncorhyncus mykiss), gilthead seabream (Sparus aurata) and common carp (Cyprinus carpio) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R1164–R1170. doi: 10.1152/ajpregu.2000.278.5.R1164. [DOI] [PubMed] [Google Scholar]
- 35.Panserat S., Plagnes-Juan E., Breque J., Kaushik S. Hepatic phosphoenolpyruvate carboxykinase gene expression is not repressed by dietary carbohydrates in rainbow trout (Oncorhynchus mykiss) Exp. Biol. 2001;204:359–365. doi: 10.1242/jeb.204.2.359. [DOI] [PubMed] [Google Scholar]
- 36.Uyeda K., Yamashita H., Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem. Pharmacol. 2002;63:2075–2080. doi: 10.1016/S0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 37.Gong Y., Xi L., Liu Y., Lu Q., Zhang Z., Liu H., Jin J., Yang Y., Zhu X., Xie S., Han D. Sequential activations of Chrebp and Srebp1 signals regulate the high carbohydrate diet-induced hepatic lipid deposition in gibel carp (Carassius Gibelio) Front. Nutr. 2022;9 doi: 10.2139/ssrn.4377513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Yao T., Pini M., Zhou Z., Fantuzzi G., Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G634–G642. doi: 10.1152/ajpgi.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C., Ge X., Niu J., Lin H., Huang Z., Tan X. Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus. Aquaculture. 2015;437:390–397. doi: 10.1016/j.aquaculture.2014.12.016. [DOI] [Google Scholar]
- 40.Ma H.J., Mou M.M., Pu D.C., Lin S.M., Chen Y.J., Luo L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus salmoides. Aquaculture. 2019;498:482–487. doi: 10.1016/j.aquaculture.2018.07.039. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available on request.