Abstract
Sleep deprivation refers to an intentional or unintentional reduction in sleep time, resulting in insufficient sleep. It is often caused by sleep disorders, work demands (e.g., night shifts), and study pressure. Sleep deprivation promotes Aβ deposition and tau hyperphosphorylation, which is a risk factor for the pathogenesis and progression of Alzheimer's disease (AD). Recent research has demonstrated the potential involvement of sleep deprivation in both the pathogenesis and progression of AD through glial cell activation, the glial lymphatic system, orexin system, circadian rhythm system, inflammation, and the gut microbiota. Thus, investigating the molecular mechanisms underlying the association between sleep deprivation and AD is crucial, which may contribute to the development of preventive and therapeutic strategies for AD. This review aims to analyze the impact of sleep deprivation on AD, exploring the underlying pathological mechanisms that link sleep deprivation to the initiation and progression of AD, which offers a theoretical foundation for the development of drugs aimed at preventing and treating AD.
Keywords: Sleep deprivation, Risk factor, Alzheimer's disease, Mechanisms, β-amyloid, Tau protein, Disease progression
1. Introduction
Sleep accounts for approximately one-third of human life. It is greatly significant for growth and development, emotion regulation, clearance of metabolic waste generated during wakefulness, reduction of energy loss, repair of oxidative stress damage, and consolidation of memory [[1], [2], [3], [4]]. Approximately one-third of the global population is affected by sleep deprivation (SD) [5]. SD refers to an intentional or unintentional reduction in sleep duration, resulting in insufficient sleep. This may be attributed to work needs, learning pressure, poor sleep habits, or other sleep disorders. SD is the normal state among individuals of various occupations such as doctors, soldiers, workers, and other people on night shifts) [5,6]. Especially in the elderly population, SD becomes very common because of certain diseases (such as Parkinson's disease and sleep apnea syndrome), neuroendocrine disorders, circadian rhythms, and other factors. SD increases the risk of age-related diseases, such as dementia and stroke. Thus, the co-occurrence of SD and Alzheimer's disease (AD) is commonly observed in certain sleep disorders such as sleep apnea, as well as in certain occupations such as night-shift workers. Research has demonstrated that the detrimental effects of SD are profound even after one night of inadequate sleep. These effects include emotional stress, reduced attention span, impairments in learning and memory, and increased daytime sleepiness. Furthermore, prolonged periods of SD significantly increase the risk of various conditions, including heart disease, hypertension, obesity, stroke, diabetes, and AD [2,7]. Additionally, SD leads to industrial, traffic, and medical accidents, resulting in a decline in production and work efficiency, and causing serious economic and social losses [5].
Recently, notable advancements have been made in the field of research. Research on the relationship between SD and neurodegenerative diseases has attracted considerable attention. SD has been extensively studied and has been reported to have detrimental effects on learning and memory. A bidirectional relationship between SD and AD has been thoroughly documented in studies involving both humans and animals. More studies have demonstrated that SD contributes to the deposition of β-amyloid (Aβ) and the hyperphosphorylation of tau, which are the underlying pathological mechanisms of AD. Hence, SD has been identified as a risk factor for the onset and progression of AD [8].
AD is a neurodegenerative disease characterized by progressive cognitive decline. This disease has several clinical manifestations, including memory decline and impairment of visuospatial skills and orientation. Individuals with AD commonly experience various behavioral disorders such as changes in personality and social interactions. SD is frequently observed, along with symptoms of anxiety, depression, and other neuropsychiatric disorders [9,10]. According to statistics, a person with dementia is diagnosed every 3 s globally. Approximately 50 million people are affected by dementia worldwide. The global prevalence of this condition is projected to increase significantly, reaching an estimated 152 million individuals by 2050 [11]. In China, the estimated prevalence of AD among individuals aged >65 years is approximately 3.21% [12], and in Europe, the estimated prevalence of AD is approximately 5.05% [13]. With the current prevalence of 5.8 million individuals living with AD in the United States, the number of patients with AD in the country will soar to 13.8 million by 2050. AD imposes significant economic and social burdens. In 2018, the global economic and social costs associated with AD were approximately $1 trillion. This figure is projected to increase significantly, reaching approximately $2 trillion by 2030 [11]. The pathogenesis of AD is complex, and results from the comprehensive action of neurobiological, psychological, and social factors. The pathogenesis of AD has been extensively studied, and multiple hypotheses have been proposed to explain its underlying mechanisms. Among them, the Aβ hypothesis has the most extensive and far-reaching influence and has been at the core of the pathogenesis of AD for a long time. Aβ is a precursor protein of amyloid protein, which is produced by β-secretase cleavage. Aβ hypothesis believes that the imbalance between Aβ production and clearance is the initial event leading to AD, and its mechanism is that oligomeric Aβ causes neuronal toxic damage and abnormal deposition, forming neuroinflammatory plaques [14]. The tau theory holds that hyperphosphorylated tau proteins disrupt the stability of tubulin within the skeletal structure of neurons. This disruption ultimately leads to the formation of neurofibrillary tangles, which impair the functions of neurons and synapses [14,15]. Currently, the medical treatments for AD primarily focus on temporarily stabilizing cognitive decline and alleviating neuropsychiatric symptoms. Additionally, the early stages of drug discovery and development present significant challenges for accurate prediction and identification of potential therapeutic agents [16]. Therefore, to prevent and delay the progression of AD, investigating the risk factors that contribute to its onset and it is crucial. Moreover, the underlying causes of the imbalance between the formation and clearance of Aβ and tau proteins need to be explored. This study shapes the future of AD treatment and management.
SD causes Aβ metabolic disorders and excessive aggregation of tau protein, which increase the risk of AD. Investigating the effects of SD on AD could establish a crucial theoretical foundation for the prevention and treatment of this neurodegenerative condition. This review will primarily concentrate on exploring the intricate relationship between SD and AD while delving into the underlying mechanisms through which SD influences the pathogenesis and progression of AD.
2. SD, Aβ deposition, and tau protein hyperphosphorylation
2.1. What is sleep and loss of which sleep structure has an important role in Aβ and tau formation?
Sleep is a reversible behavior in which the body loses its awareness and response to the surrounding environment. It probably occurs in all vertebrates [17]and is greatly important for growth, development, and memory consolidation. According to different physiological activity parameters, sleep is divided into two stages: non-rapid eye movement (NREM) and rapid eye movement (REM) [18]. REM sleep is also known as paradoxical sleep (PS) or fast-wave sleep. Based on the sleep depth, NREM sleep is divided into three stages: NREM1 (N1), NREM2 (N2), and NREM3 (N3). N1–2 is light NREM sleep, whereas N3 is deep NREM sleep (also known as slow wave sleep, SWS) [17,19]. Human sleep architecture is closely related to age, with significant changes in sleep architecture and depth occurring in older adults because of frequent awakenings, especially during N3 sleep [20]. Aging increases the incidence of sleep-related diseases (such as stroke, AD, hypertension, and coronary heart disease), especially AD, which is characterized by cognitive decline. AD is a common neurodegenerative disease in the elderly population. Therefore, sleep quality should be paid close attention in the elderly to prevent and delay the onset and progression of AD. Studies have demonstrated that sleep contributes to memory consolidation, particularly during SWS. While REM sleep may enhance this process at the molecular and synaptic levels [17,21]. Clinical research has demonstrated that slow wave activity is a significant predictor of Aβ plaque accumulation rates; its frequencies <1 Hz were negatively correlated with Aβ deposition [[22], [23], [24], [25]]. An animal experiment has revealed that amyloid precursor protein (APP)/presenilin 1 (PS1) mice exhibited more severe accumulation of Aβ and worse spatial learning and memory ability after REM SD [26]. Several studies have demonstrated that sleep is essential for cognition, and SD promotes Aβ deposition and tau hyperphosphorylation, which leads to the decline of cognitive functions such as learning and memory, and increases the risk of AD.
2.2. SD promotes Aβ deposition and tau protein hyperphosphorylation
Recent studies have revealed that Aβ levels in the interbrain fluid (ISF) exhibit an elevation during wakefulness, and a decline during sleep, in both human and mouse subjects, and chronic SD significantly increases Aβ formation [27]. Meanwhile, the accumulation of Aβ can also contribute to sleep loss and sleep fragmentation [28] and cause learning and memory impairment [29]; An animal experiment has demonstrated that 2-month SD mice exhibited changes in Aβ precursor protein, increased levels of phosphorylated tau protein, more senile plaques in the cortex and hippocampus, and impaired learning and memory function, which provided experimental evidence that SD is a known risk factor for AD [30]. Various studies have indicated that SD significantly enhances the activity of β-amyloid precursor protein (APP) cleaving enzyme 1 (BACE1, also known as β-secretase). This increase in BACE1 activity promotes the aggregation of Aβ and tau protein by modulating the metabolic processes associated with these proteins, thus the progression of AD is accelerated [31]. Rats with chronic sleep restriction (CSR) for 21 days exhibited diminished learning and memory abilities in the Morris water maze test, along with heightened accumulation of Aβ42 in both the hippocampus and prefrontal cortex. This outcome could potentially be attributed to an augmented production of Aβ caused by upregulation of BACE1 pathway and the impairment of Aβ clearance affecting Aβ transport in the brain [32]. Chronic SD in a mouse model of AD, known as the APP/PS1/tau triple transgenic AD model (3xTg-AD), resulted in multiple cognitive impairments such as recognition and working memory. Meanwhile, this condition led to an elevated deposition of Aβ and triggered the activation of microglia [33].
Measurement of brain Aβ burden in adults after one night of SD using PET and (18)F-florbetaben revealed that one night of SD resulted in a significant increase in Aβ burden in the right hippocampus and thalamus [34]. The evaluation of cerebrospinal fluid (CSF) in patients with SD, along with the assessment of Pittsburgh Sleep Quality Index (PSQI), revealed a significant correlation between levels of Aβ40 and Aβ42 and PSQI score. SD can disrupt the metabolism of Aβ in the brain, thus potentially increasing the risk of AD [35]. Further, acute SD has been demonstrated to elevate tau levels in the ISF of mouse brains and CSF of humans. Chronic SD accelerates the spread of tau aggregates within neural networks [36].
Human and animal experiments have fully demonstrated that regardless of the association of acute or chronic SD with cognitive decline, SD will affect Aβ and tau metabolic process, increasing Aβ and tau protein aggregation, thereby increasing the risk of AD.
3. Aβ deposition and tau protein hyperphosphorylation are the key pathological basis of AD
AD is a neurodegenerative disease with various characteristics. These include cognitive impairment, presence of extracellular amyloid plaques, formation of intracellular neurofibrillary tangles, and loss of synapses and neurons [37]. Extensive evidence has demonstrated that the accumulation of Aβ in the brain plays a vital role in the pathogenesis of AD, and this accumulation may result from an imbalance between the production and clearance of Aβ [38]. The accumulation of Aβ is a concentration-dependent process [27,37], which has toxic effects on synapses and neurons around it, and can destroy synapses and eventually cause nerve cell death. The preclinical stage of AD is marked by several key features: the formation of amyloid plaques due to the aggregation of Aβ, phosphorylation of the tau protein, and subsequent aggregation of tau into neurofibrillary tangles. Currently, an increasing number of AD studies have focused on the preclinical stage and conducted in-depth research on its risk factors and pathogenesis. Mounting evidence supports a bidirectional relationship between SD and AD. Sleep changes are observed during the preclinical stages of AD and often occur several years before the onset of cognitive decline. Long-term SD has been linked to an increased deposition of amyloid plaques, which impacts the onset and progression of AD [39,40]. Meanwhile, the accumulation of Aβ, a key protein associated with AD, can disrupt normal sleep–wake cycle [41], and they interact with each other. Therefore, the deposition of Aβ and hyperphosphorylation of tau proteins are the key pathological processes underlying the pathogenesis of AD. SD leads to Aβ deposition and tau protein aggregation, which are risk factors for the progression of AD. Meanwhile, aggregation of Aβ and tau proteins can disrupt the sleep–wake cycle. The interaction between SD and AD results in a vicious cycle. Therefore, intervention and treatment of SD are crucial to effectively prevent and treat AD.
4. Mechanism of Aβ deposition and tau protein hyperphosphorylation caused by SD
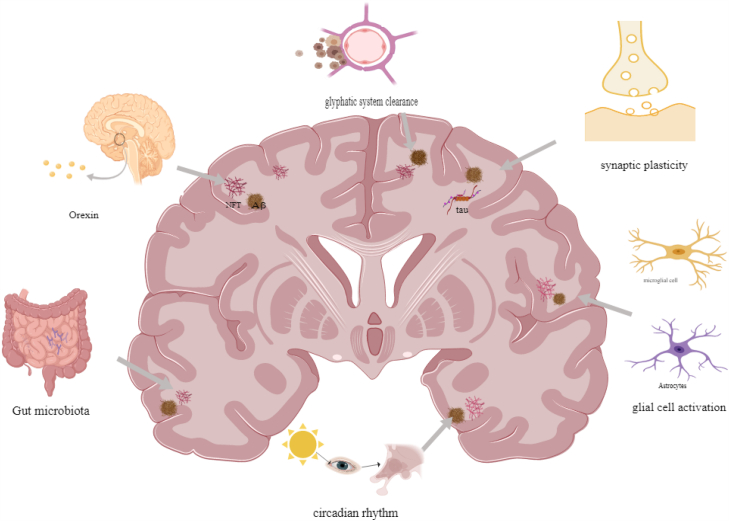
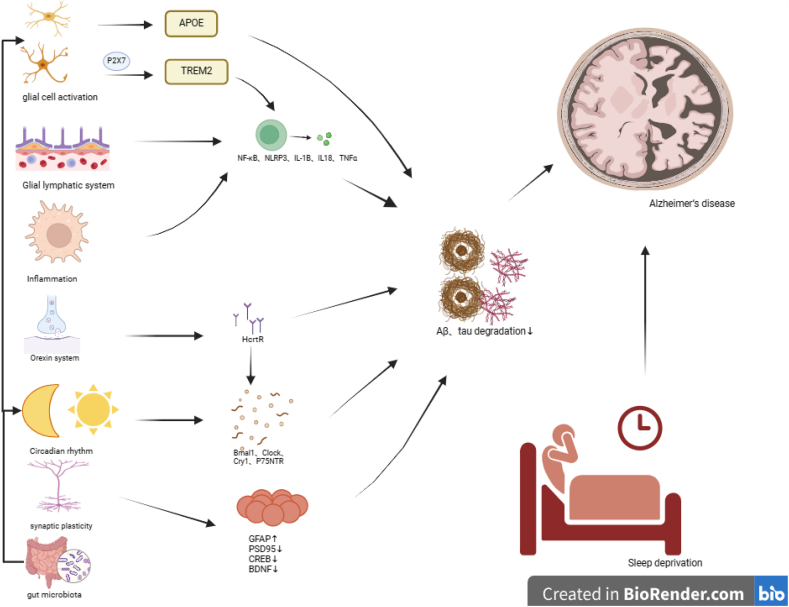
SD is known to increase the risk of various neurological disorders, including AD, Parkinson's disease, and stroke. Identifying and appropriately treating sleep problems may reduce their risk [42]. SD is well documented to promote Aβ and tau aggregation, Aβ and tau are key pathogenic step in the progression of AD. To analyze why SD promotes Aβ deposition and tau aggregation, why SD leads to a decline in cognitive functions such as learning and memory, and the underlying mechanisms, current research focuses on the activation of glial cells, Aβ and tau clearance by the glial lymphatic system, circadian rhythms, orexin, synaptic plasticity, inflammation, and neurotrophic factors, and gut microbiota.
4.1. SD induces glial cell activation
Glial cells, including astrocytes, microglia, and oligodendrocytes play important roles in phagocytosis, support, and nutrition. Microglia primarily function in immune surveillance and become activated when affected by the pathological factors of AD [43]. In the initial phases of AD, microglia can phagocytose Aβ. However, with long-term exposure to AD and certain risk factors, its phagocytosis is weakened, and Aβ is overdeposited, which further stimulates glial cells activation, leading to a cascade of inflammatory immune responses [[44], [45], [46]]. Astrocytes are pivotal in clearing Aβ and tau, the activation of astrocytes can also cause the production of various inflammatory factors, and start the downstream harmful cascade reaction to aggravate the damage of nerve cells [47]. Glial cells have long been associated with AD progression, and recent studies have indicated their involvement in regulating sleep homeostasis. However, the precise mechanisms by which glial cells influence the balance between sleep and wakefulness and how SD can initiate or exacerbate AD have become significant areas of research [48].
Studies have indicated that astrocytes and microglia play critical regulatory roles in both SD and AD. After acute and chronic SD, the phagocytic activity of astrocytes increased compared to that during normal sleep and wakefulness. In addition, microglia are activated and phagocytic activity increases [[48], [49], [50]]. One study has reported that REM-SD and total SD were associated with worse memory abilities, greater microglial activation, and more severe Aβ accumulation [26]. Specific knockdown of Grin2a in hippocampal astrocytes exacerbated cognitive decline, increased Aβ levels, and attenuated SD increased autophagic flux, suggesting a novel neuroprotective role of astrocyte GluN2A in SD [51]. Activation of astrocytes and microglia involves apolipoprotein E-induced Aβ clearance in astrocytes, enhanced Aβ deposition in microglia in a TREM2-dependent manner [50,52], and astrocytes and microglia activation can trigger an inflammatory response that promotes Aβ deposition. Activation of microglial cells triggers activation of the P2X7 receptor (P2X7R), resulting in the downregulation of TREM2. P2X7R activation leads to an increase in calcium influx and the release of inflammatory cytokines, whereas decreased TREM2 levels impair microglial phagocytosis of Aβ, thereby increasing the accumulation of Aβ in the brain parenchyma. Additionally, microglial activation is associated with the activation of NF-κB and NLRP3 inflammasomes, which in turn activate caspase-1 and stimulate the secretion of proinflammatory cytokines such as interleukin (IL)-1β and IL-18 [53], thereby triggering a series of downstream cascades. Astrocytes are also involved in the transcellular transport of Aβ and play a key role in the clearance of Aβ by regulating cellular degradation and Aβ enzyme degradation through low-density lipoprotein receptor-associated proteins [48]. Therefore, linking glial cells with SD and AD has great clinical significance in gaining insight into the intricate relationship between AD and SD, ultimately facilitating the development of effective prevention and treatment strategies for AD [54].
4.2. SD reduces brain clearance mechanisms through the glial lymphatic system
The glymphatic system is a rapid exchange flow system between CSF and interstitial fluids. This exchange is mediated by aquaporin-4 (AQP4), which is located on astrocyte endfeet. It cleans brain tissue fluid of metabolites such as lactate and abnormal proteins such as Aβ. These substances exit the brain via the perivenous space adjacent to the cranial and spinal nerves. Lymphatic vessels in the meninges and the surrounding soft tissues of the skull play vital roles in transporting abnormal proteins and metabolites from the central nervous system, effectively clearing harmful substances from the brain [55]. The concentrations of Aβ and tau in brain parenchyma and CSF follow circadian patterns, being highest during wakefulness and lowest during sleep. SD has been associated with an increased burden of Aβ, a protein implicated in AD. This change in the protein concentration suggests that the glymphatic system, which plays a role in waste clearance from the brain, may be activated during sleep [56]. Aβ clearance through the glymphatic system holds the potential to lower the risk of AD or enhance cognitive function among individuals with AD [57,58]. Recently, the glymphatic system has been reported to be the most active during SWS and plays a vital role in the clearance of Aβ. SD leads to glymphatic system dysfunction and is closely related to AD [39,[59], [60], [61]]. Animal studies have demonstrated that during sleep, the glymphatic system demonstrates a remarkable boost in efficiency, clearing harmful metabolites at a rate twice as fast as that during wakefulness. Similarly, circadian fluctuations of Aβ have been observed in human studies. After SD, Aβ accumulates in the brain and the clearance rate is reduced, indicating that the glymphatic system is primarily active during sleep [55,62,63]. The discovery of the glymphatic system provides a new perspective for the study of the pathological mechanisms and treatment of brain diseases. Currently, the mechanism by which sleep-mediated glymphatic system regulates Aβ and tau clearance remains unclear. Although it is closely related to AQP4 in astrocytic endfeet, advancing our understanding of AD and effectively developing prevention and treatment strategies are necessary to explore its specific molecular mechanisms. This exploration will identify precise targets to combat this disease.
4.3. Involvement of orexin in the pathological process of AD
Orexin, a neuropeptide with multifunctional roles, is secreted by lateral hypothalamic neurons. The regulatory role of orexin spans various physiological processes, such as sleep–wake cycles, eating behavior, reward systems, anxiety modulation, cognition, and even neuroprotection. It is divided into two subtypes: orexin A and orexin B, also known as hypocretin1 (Hcrt1) and hypocretin2 (Hcrt2), respectively. Orexin neurons project excitatory signals to the entire nervous system, except for the cerebellum [[64], [65], [66], [67]]. The bidirectional neuronal projections between the monoaminergic neurons are as follows: norepinephrine in locus coeruleus, 5-HT in the raphe nucleus, dopamine in the substantia nigra, acetylcholine in the basal forebrain, and γ-aminobutyric acid neurons in the sleep center of the human body to regulate the state of sleep–wake cycle [[68], [69], [70], [71], [72], [73]]. The impact of orexin on Aβ clearance is attributed to its regulation of sleep–wake cycles, as well as its inhibition of autophagic flow. In vitro studies have demonstrated that it disrupts autophagosome–lysosome fusion, leading to impaired degradation of Aβ specifically in BV2 cells [74]. Upregulation of orexin and its receptor in the CSF and disruption of the blood–brain barrier (BBB) can also contribute to the acceleration of AD [75]. Dysregulation of the orexin system may affect patients with both SD and AD. Consequently, targeting the orexin system through interventions could serve as a valuable treatment strategy for AD.
4.4. Involvement of circadian rhythm disruption in the pathogenesis of AD
Emerging studies have demonstrated the prevalence of circadian rhythm disorders in patients with AD. In mice, chronic SD disrupts the expression of genes associated with circadian rhythm regulation. This disruption specifically affects genes such as Bmal1, Clock, and Cry1, which play crucial roles in maintaining the normal function of circadian rhythms within the nuclei responsible for their regulation. In addition, chronic SD negatively affects learning and memory, and these alterations are more pronounced in mice with AD, further exacerbating disease progression [76]. The neurotrophin receptor p75 (p75NTR) has been identified as a circadian regulator that plays a role in mediating cognitive impairment associated with SD, and its soluble extracellular domain p75NTR (p75ECD) exhibits a neuroprotective effect in AD. When mice with SD were administered a high dose of p75Ecd-Fc (10 mg/kg) peripherally, the Aβ and p75ECD levels in the hippocampus normalized. This intervention successfully addressed the cognitive deficits observed in SD mice [77]. Potential interaction between SD and disruption of circadian rhythm can impede the clearance of Aβ and tau proteins through the glial-vascular-lymphatic system. This impaired clearance mechanism can lead to an accumulation of Aβ and tau, contributing to the development of AD. Furthermore, SD and circadian rhythm disruption can lead to increased oxidative stress in the brain and decreased melatonin levels in the bloodstream. Collectively, these factors contribute to the increased risk of AD progression [78]. Circadian dysfunction is widely recognized as a significant contributor to the pathology of AD, characterized by the accumulation of Aβ plaques and tau tangles. Recent studies have revealed that the phosphorylation of tau, a key protein associated with AD, follows a circadian rhythm influenced by body temperature and sleep. Recognizing early symptoms of AD, such as alterations in sleep patterns and disrupted circadian rhythms, may enable intervention in the early stages to prevent the formation of Aβ plaques, neurofibrillary tangles, and neuronal degeneration. Intervention at this stage may mitigate AD progression. Therefore, circadian rhythms may be an effective target to counter Aβ pathology [37,79,80].
SD has various negative impacts on the brain, such as disrupting the circadian rhythm, hindering the efficient removal of Aβ and tau proteins, and potentially increasing the exocytosis of Aβ and tau. The interplay of these factors can exacerbate the load of Aβ and tau proteins in the ISF, leading to their aggregation and subsequent pathological changes. SD can alter the REDOX chemical state of neurons and glial cells, triggering oxidative stress and inflammatory responses. These processes additionally facilitate the aggregation of pathological substances linked to AD, including Aβ and tau [78]. Further research on the correlation between circadian rhythms and AD is crucial for the early diagnosis, prevention, and management of AD.
4.5. Relationship between SD, synaptic plasticity, and AD
Studies have demonstrated that the expression levels of presynaptic marker proteins and postsynaptic density proteins significantly decrease after long-term SD, suggesting that SD may affect memory consolidation by interfering with long-term potentiation (LTP), resulting in a reduced density of hippocampal dendritic spines. Meanwhile, SD leads to alterations in dendritic length and branching of pyramidal neurons in the prefrontal cortex (PFC). In AD mice, the PFC undergoes early changes at the electrophysiological, molecular, and morphological levels, which are initiated by a decrease in two important factors, cAMP response element-binding protein and brain-derived neurotrophic factor (BDNF) [81], which promote the occurrence and progression of AD. Animal experiments have demonstrated that SD can significantly inhibit hippocampal LTP in APP/PS1 mice, reduce the expression of post-synaptic density protein 95 in the hippocampus, increase the level of glial fibrillary acidic protein, and increase Aβ deposition and microglia overactivation, thereby impairing the synaptic plasticity, worsening cognitive dysfunction, and contributing to the onset and progression of AD [82,83]. Therefore, correcting SD and improving the synaptic plasticity damage caused by SD may prevent the onset of AD or delay its progression.
4.6. SD, inflammation, and AD
SD can continuously activate an inflammatory response, which plays a key role in the pathogenesis of metabolic and neurodegenerative diseases associated with SD [84,85]. A previous study has indicated that SD significantly increases the expression of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase, and nitric oxide (NO) in the brain. Additionally, the levels of inflammatory factors are positively correlated with the deposition of Aβ42. Furthermore, IL-1β, TNF-α, and NO plasma levels increase after CSR. Thus, both brain and plasma inflammatory responses may be involved in SD-induced Aβ accumulation [38], .and SD may participate in the pathogenesis and progression of AD by mediating inflammatory responses. Further research has revealed that SD increases the levels of LPS and activates the TLR4/NF-κB signaling pathway, leading to the activation of downstream mediators such as NF-κB. This activation increases the production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. In addition, SD can cause dysbiosis of the gut microbiota. Dysbiosis of the gut microbiota can promote peripheral and central inflammatory processes as well as cognitive deficits caused by SD [86,87]. One study has reported that 72 h of SD disrupts the circadian rhythm of the colon, accompanied with gut microbiota dysbiosis, inflammation induction, and impaired intestinal barrier integrity. Inflammatory signals activate the expression of microglia and chemokines, aggravating inflammation and psychiatric disorders [88]. In conclusion, SD can induce inflammatory reactions and participate in the pathogenesis and progression of AD, and inhibiting inflammatory reactions may provide potential intervention measures for delaying the pathogenesis and progression of AD caused by SD.
4.7. Correlation of SD, gut microbiota, and AD
In recent years, the role of the gut microbiota in neurodegenerative diseases has attracted increased interest. Research has indicated a bidirectional relationship between inflammation, sleep, and the gut microbiota. SD can increase inflammatory responses, and inflammation, in certain circumstances, can be mediated by the gut microbiota [87]. The gut microbiota can participate in peripheral and central inflammatory processes as well as cognitive deficits induced by SD through the brain–gut–microbiota axis, thereby promoting the onset and progression of AD. The reshaped gut microbiota due to SD serves as a driving factor for neuroinflammation and adverse consequences associated with SD [86,89]. A study explored whether SD alters the gut microbiota and whether these changes are associated with increased inflammation and cognitive impairment resulting from SD. The findings revealed that SD can affect the gut microbiota, cognitive impairment, and activation of the TLR4/NF-κB signaling pathway. These results highlight the potential pathogenic role of the gut microbiota in the inflammation and cognitive impairment induced by SD [86]. SD can lead to changes in the gut microbiota, disrupting the balance between Firmicutes and Bacteroidetes [90], compromising the intestinal barrier and further affecting central immunity by increasing BBB permeability. This activation triggers signaling pathways in microglia and astrocytes, promoting inflammatory and oxidative stress responses, resulting in the production and accumulation of Aβ [86]. The gut microbiota also affects neurotrophic factors such as N-methyl-d-aspartate receptors and BDNF, which ultimately contribute to the pathogenesis and progression of AD [90]. Moreover, dysbiosis of the gut microbiota causes excessive secretion of amyloid and lipopolysaccharide by gut bacteria, resulting in increased permeability of both the gut and BBB, affecting signaling pathways related to the pathogenesis of AD and promoting neuroinflammation, thereby mediating the onset and progression of AD [91,92]. Recent studies have demonstrated the molecular mimiculation ability of bacterial amyloid, leading to Aβ misfolding and aggregation. This process spreads through the gut–brain axis [93] and triggers the activation of microglia, which play a significant role in the pathogenesis and progression of AD. Further research has suggested that SD may disrupt the gut microbiota and increase intestinal permeability through dysfunction of the circadian clock genes. This disruption can exacerbate inflammation and neuroimmune reactions in the brain. Thus, adequate sleep and a healthy gut microbiota may play protective roles in disease by restoring the intestinal epithelial barrier function and reducing inflammation [88]. In conclusion, SD leads to changes in the gut microbiota and participates in the pathogenesis and progression of AD through neuroendocrine and neuroimmunity, providing potential targets and ideas for the prevention and treatment of AD.
5. Conclusion
Previous studies have revealed that SD and disturbances in the circadian rhythm manifest in individuals with AD prior to the onset and diagnosis of the condition [94,95], and that changes in sleep patterns and abnormal circadian rhythms may be the initial symptoms of AD. The identification of these initial symptoms may provide biomarkers for the intervention and prevention of AD and contribute to the initial diagnosis of AD [96]. A growing body of research has suggested the association of SD with Aβ deposition and tau protein hyperphosphorylation. These abnormalities serve as the underlying pathology and are crucial contributors to AD progression. Hence, SD serves as a significant risk factor for both the onset and progression of AD, and its mechanism is related to glial cell activation, glymphatic system clearance, the orexin system, circadian disruption, synaptic plasticity, inflammation, and the gut microbiota (Fig. 1, Fig. 2). SD is linked to the initiation of AD and can potentially influence its symptoms and progression. This association deserves more attention in research, and a thorough understanding can play a pivotal role in the prevention of AD by implementing comprehensive screening measures and adopting suitable management approaches [97]. Although AD remains incurable, ongoing research efforts have elucidated its pathology and underlying risk factors, providing opportunities to leverage these factors to effectively prevent disease onset and progression [8]. However, certain studies have failed to identify any evidence supporting the notion that chronic exposure to work-related SD cause cognitive decline or early symptoms of dementia [98], CSF concentrations of AD-related biomarkers such as Aβ40, Aβ42, tau, and neurofilament light chains also did not change in healthy adults who received SD for 5 consecutive days [60,99]. Therefore, the exact association between SD and AD remains unknown. However, the role and mechanisms of action of SD in AD remain unclear. In future research, prioritizing the investigation of the intricate connections between SD and AD will be important. This involves exploring the specific relationship between SD and AD as well elucidating the role that SD plays and the underlying mechanisms affecting the development of AD. These studies will play a crucial role in not only establishing a clearer understanding of the relationship between SD and AD but also in identifying more effective therapeutic strategies for preventing and treating AD.
Fig. 1.
Possible machanism of the Onset and Progression of Alzheimer's disease induced by sleep deprivation. SD serves as a significant risk factor in both the onset and progression of AD, its mechanism is related with glial cell activation, glymphatic system clearance, orexin system, circadian disruption, synaptic plasticity and gut microbiota.
Fig. 2.
Molecular mechanism of the Onset and Progression of Alzheimer's disease induced by sleep deprivation.
Disclosure statement
The authors declare that they have no conficts of interest.
Ethics statement
Not applicable. because this is a review based on published literature.
Data available statement
No data was used in this review.
CRediT authorship contribution statement
Zhengyun Han: Writing – original draft, Software, Resources, Methodology, Investigation, Conceptualization. Xingmao Yang: Writing – original draft, Visualization, Validation. Shuiqing Huang: Writing – review & editing, Validation, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Xu M., Liu X., Wang Q., et al. Phosphoproteomic analysis reveals the effects of sleep deprivation on the hippocampus in mice. Mol Omics. 2022;18(7):677–685. doi: 10.1039/d2mo00061j. [DOI] [PubMed] [Google Scholar]
- 2.Dissel S. Drosophila as a model to study the relationship between sleep, plasticity, and memory. Front. Physiol. 2020;11:533. doi: 10.3389/fphys.2020.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman A., Languille S., Lamberty Y., et al. Sleep deprivation impairs spatial retrieval but not spatial learning in the non-human primate grey mouse lemur. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei M., Zhao B., Huo K., et al. Sleep deprivation induced plasma amyloid-β transport disturbance in healthy young adults. J Alzheimers Dis. 2017;57(3):899–906. doi: 10.3233/JAD-161213. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Wheaton A.G., Chapman D.P., et al. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65(6):137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay A., Sigua N.L. What is sleep deprivation? Am. J. Respir. Crit. Care Med. 2019;199(6):P11–p12. doi: 10.1164/rccm.1996P11. [DOI] [PubMed] [Google Scholar]
- 7.Estrada C., Cuenca L., Cano-Fernandez L., et al. Voluntary exercise reduces plasma cortisol levels and improves transitory memory impairment in young and aged Octodon degus. Behav. Brain Res. 2019;373 doi: 10.1016/j.bbr.2019.112066. [DOI] [PubMed] [Google Scholar]
- 8.Delic V., Ratliff W.A., Citron B.A. Sleep deprivation, a link between post-traumatic stress disorder and alzheimer's disease. J Alzheimers Dis. 2021;79(4):1443–1449. doi: 10.3233/JAD-201378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer's disease. Mol. Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havekes R., Heckman P.R.A., Wams E.J., et al. Alzheimer's disease pathogenesis: the role of disturbed sleep in attenuated brain plasticity and neurodegenerative processes. Cell. Signal. 2019;64 doi: 10.1016/j.cellsig.2019.109420. [DOI] [PubMed] [Google Scholar]
- 11.World alzheimer report. 2018. https://www.alzint.org/resource/world-alzheimer-report-2018/
- 12.Jia J., Wang F., Wei C., et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10(1):1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Niu H., Alvarez-Alvarez I., Guillen-Grima F., et al. Prevalence and incidence of Alzheimer's disease in Europe: a meta-analysis. Neurologia. 2017;32(8):523–532. doi: 10.1016/j.nrl.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari S., Atluri V., Kaushik A., et al. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 16.Cassé-Perrot C., Lanteaume L., Deguil J., et al. Neurobehavioral and cognitive changes induced by sleep deprivation in healthy volunteers. CNS Neurol. Disord.: Drug Targets. 2016;15(7):777–801. doi: 10.2174/1871527315666160518125156. [DOI] [PubMed] [Google Scholar]
- 17.Rasch B., Born J. About sleep's role in memory. Physiol. Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bon O. Relationships between REM and NREM in the NREM-REM sleep cycle: a review on competing concepts. Sleep Med. 2020;70:6–16. doi: 10.1016/j.sleep.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann S., Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr. Neurol. Neurosci. Rep. 2014;14(2):430. doi: 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- 20.Gulia K.K., Kumar V.M. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18(3):155–165. doi: 10.1111/psyg.12319. [DOI] [PubMed] [Google Scholar]
- 21.Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 22.Ngo H.V., Claassen J., Dresler M. Sleep: slow wave activity predicts amyloid-β accumulation. Curr. Biol. 2020;30(22):R1371–r1373. doi: 10.1016/j.cub.2020.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Winer J.R., Mander B.A., Kumar S., et al. Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr. Biol. 2020;30(21):4291–4298.e4293. doi: 10.1016/j.cub.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chylinski D., Van Egroo M., Narbutas J., et al. Timely coupling of sleep spindles and slow waves linked to early amyloid-β burden and predicts memory decline. Elife. 2022;11 doi: 10.7554/eLife.78191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varga A.W., Wohlleber M.E., Giménez S., et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep. 2016;39(11):2041–2048. doi: 10.5665/sleep.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S., Meng Y., Wang N., et al. Disturbance of REM sleep exacerbates microglial activation in APP/PS1 mice. Neurobiol. Learn. Mem. 2023;200 doi: 10.1016/j.nlm.2023.107737. [DOI] [PubMed] [Google Scholar]
- 27.Liao F., Zhang T.J., Mahan T.E., et al. Effects of growth hormone-releasing hormone on sleep and brain interstitial fluid amyloid-β in an APP transgenic mouse model. Brain Behav. Immun. 2015;47:163–171. doi: 10.1016/j.bbi.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabuchi M., Lone S.R., Liu S., et al. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr. Biol. 2015;25(6):702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daulatzai M.A. Pharmacotherpy and alzheimer's disease: the M-drugs (melatonin, Minocycline, Modafinil, and Memantine) Approach. Curr. Pharmaceut. Des. 2016;22(16):2411–2430. doi: 10.2174/1381612822666160203142111. [DOI] [PubMed] [Google Scholar]
- 30.Qiu H., Zhong R., Liu H., et al. Chronic sleep deprivation exacerbates learning-memory disability and alzheimer's disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J Alzheimers Dis. 2016;50(3):669–685. doi: 10.3233/JAD-150774. [DOI] [PubMed] [Google Scholar]
- 31.Chen L., Huang J., Yang L., et al. Sleep deprivation accelerates the progression of alzheimer's disease by influencing Aβ-related metabolism. Neurosci. Lett. 2017;650:146–152. doi: 10.1016/j.neulet.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Zhao B., Liu P., Wei M., et al. Chronic sleep restriction induces aβ accumulation by disrupting the balance of aβ production and clearance in rats. Neurochem. Res. 2019;44(4):859–873. doi: 10.1007/s11064-019-02719-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Cao X., Yin J., et al. [Chronic sleep deprivation exacerbates cognitive and pathological impairments in APP/PS1/tau triple transgenic Alzheimer's disease model mice] Sheng Li Xue Bao. 2021;73(3):471–481. [PubMed] [Google Scholar]
- 34.Shokri-Kojori E., Wang G.J., Wiers C.E., et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D.W., Wang J., Zhang L.L., et al. Cerebrospinal fluid amyloid-β levels are increased in patients with insomnia. J Alzheimers Dis. 2018;61(2):645–651. doi: 10.3233/JAD-170032. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Holtzman D.M. Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104–120. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin M.S., Sumsuzzman D.M., Jeandet P., et al. Deciphering the interacting mechanisms of circadian disruption and alzheimer's disease. Neurochem. Res. 2021;46(7):1603–1617. doi: 10.1007/s11064-021-03325-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu P., Zhao B., Wei M., et al. Activation of inflammation is associated with amyloid-β accumulation induced by chronic sleep restriction in rats. J Alzheimers Dis. 2020;74(3):759–773. doi: 10.3233/JAD-191317. [DOI] [PubMed] [Google Scholar]
- 39.Cordone S., Annarumma L., Rossini P.M., et al. Sleep and β-amyloid deposition in alzheimer disease: insights on mechanisms and possible innovative treatments. Front. Pharmacol. 2019;10:695. doi: 10.3389/fphar.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim M.M., Gerstner J.R., Holtzman D.M. The sleep-wake cycle and Alzheimer's disease: what do we know? Neurodegener. Dis. Manag. 2014;4(5):351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh J.H., Jiang H., Finn M.B., et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J. Exp. Med. 2014;211(13):2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma J.A., Urrestarazu E., Iriarte J. Sleep loss as risk factor for neurologic disorders: a review. Sleep Med. 2013;14(3):229–236. doi: 10.1016/j.sleep.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Solito E., Sastre M. Microglia function in Alzheimer's disease. Front. Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G., Wang Z., Hu H., et al. Microglia in alzheimer's disease: a target for therapeutic intervention. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.749587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf S.A., Boddeke H.W., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 46.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer's disease. J. Cell Biol. 2018;217(2):459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leng K., Rose I.V.L., Kim H., et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat. Neurosci. 2022;25(11):1528–1542. doi: 10.1038/s41593-022-01180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunkaria A., Bhardwaj S. Sleep disturbance and alzheimer's disease: the glial connection. Neurochem. Res. 2022;47(7):1799–1815. doi: 10.1007/s11064-022-03578-0. [DOI] [PubMed] [Google Scholar]
- 49.Bellesi M., de Vivo L., Chini M., et al. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci. 2017;37(21):5263–5273. doi: 10.1523/JNEUROSCI.3981-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao S.Y., Liu Y.J., Lu W., et al. Possible neuropathology of sleep disturbance linking to alzheimer's disease: astrocytic and microglial roles. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.875138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W., Chen X., Du Z., et al. Knockdown of astrocytic Grin2a exacerbated sleep deprivation-induced cognitive impairments and elevation of amyloid-beta. Sleep Med. 2022;100:280–290. doi: 10.1016/j.sleep.2022.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Parhizkar S., Gent G., Chen Y., et al. Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. Sci. Transl. Med. 2023;15(693) doi: 10.1126/scitranslmed.ade6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Ghraiybah N.F., Wang J., Alkhalifa A.E., et al. Glial cell-mediated neuroinflammation in alzheimer's disease. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farca Luna A.J., Perier M., Seugnet L. Amyloid precursor protein in Drosophila glia regulates sleep and genes involved in glutamate recycling. J. Neurosci. 2017;37(16):4289–4300. doi: 10.1523/JNEUROSCI.2826-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmussen M.K., Mestre H., Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nedergaard M., Goldman S.A. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shetty A.K., Zanirati G. The interstitial system of the brain in health and disease. Aging Dis. 2020;11(1):200–211. doi: 10.14336/AD.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy O.C., van der Werf Y.D. The sleeping brain: harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020;10(11) doi: 10.3390/brainsci10110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohela T.J., Lilius T.O., Nedergaard M. The glymphatic system: implications for drugs for central nervous system diseases. Nat. Rev. Drug Discov. 2022;21(10):763–779. doi: 10.1038/s41573-022-00500-9. [DOI] [PubMed] [Google Scholar]
- 60.Olsson M., Ärlig J., Hedner J., et al. Sleep deprivation and plasma biomarkers for Alzheimer's disease. Sleep Med. 2019;57:92–93. doi: 10.1016/j.sleep.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 61.Ahmadian N., Hejazi S., Mahmoudi J., et al. Tau pathology of alzheimer disease: possible role of sleep deprivation. Basic Clin. Neurosci. 2018;9(5):307–316. doi: 10.32598/bcn.9.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brzecka A., Leszek J., Ashraf G.M., et al. Sleep disorders associated with alzheimer's disease: a perspective. Front. Neurosci. 2018;12:330. doi: 10.3389/fnins.2018.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nesse R.M., Finch C.E., Nunn C.L. Does selection for short sleep duration explain human vulnerability to Alzheimer's disease? Evol Med Public Health. 2017;2017(1):39–46. doi: 10.1093/emph/eow035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson L.H., Hoyer D., de Lecea L. Hypocretins (orexins): the ultimate translational neuropeptides. J. Intern. Med. 2022;291(5):533–556. doi: 10.1111/joim.13406. [DOI] [PubMed] [Google Scholar]
- 65.Fronczek R., Schinkelshoek M., Shan L., et al. The orexin/hypocretin system in neuropsychiatric disorders: relation to signs and symptoms. Handb. Clin. Neurol. 2021;180:343–358. doi: 10.1016/B978-0-12-820107-7.00021-5. [DOI] [PubMed] [Google Scholar]
- 66.Berteotti C., Liguori C., Pace M. Dysregulation of the orexin/hypocretin system is not limited to narcolepsy but has far-reaching implications for neurological disorders. Eur. J. Neurosci. 2021;53(4):1136–1154. doi: 10.1111/ejn.15077. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S., Behl T., Sehgal A., et al. Exploring the role of orexinergic neurons in Parkinson's disease. Neurotox. Res. 2021;39(6):2141–2153. doi: 10.1007/s12640-021-00411-4. [DOI] [PubMed] [Google Scholar]
- 68.Sakurai T., Nagata R., Yamanaka A., et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Li Y., Gao X.B., Sakurai T., et al. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36(6):1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 70.Soya S., Takahashi T.M., McHugh T.J., et al. Orexin modulates behavioral fear expression through the locus coeruleus. Nat. Commun. 2017;8(1):1606. doi: 10.1038/s41467-017-01782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang C., Zhang L., Hao H., et al. Serotonergic neurons in the dorsal raphe nucleus mediate the arousal-promoting effect of orexin during isoflurane anesthesia in male rats. Neuropeptides. 2019;75:25–33. doi: 10.1016/j.npep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Ishibashi M., Gumenchuk I., Kang B., et al. Orexin receptor activation generates gamma band input to cholinergic and serotonergic arousal system neurons and drives an intrinsic Ca(2+)-dependent resonance in LDT and PPT cholinergic neurons. Front. Neurol. 2015;6:120. doi: 10.3389/fneur.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen Y.C., Sun X., Li L., et al. Roles of neuropeptides in sleep-wake regulation. Int. J. Mol. Sci. 2022;23(9) doi: 10.3390/ijms23094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.An H., Cho M.H., Kim D.H., et al. Orexin impairs the phagocytosis and degradation of amyloid-β fibrils by microglial cells. J Alzheimers Dis. 2017;58(1):253–261. doi: 10.3233/JAD-170108. [DOI] [PubMed] [Google Scholar]
- 75.Guo P., Zhang W.J., Lian T.H., et al. Alzheimer's disease with sleep insufficiency: a cross-sectional study on correlations among clinical characteristics, orexin, its receptors, and the blood-brain barrier. Neural Regen Res. 2023;18(8):1757–1762. doi: 10.4103/1673-5374.360250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niu L., Zhang F., Xu X., et al. Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer's disease neuropathology. Brain Pathol. 2022;32(3) doi: 10.1111/bpa.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Y., Gao M., Huang H., et al. p75NTR ectodomain ameliorates cognitive deficits and pathologies in a rapid eye movement sleep deprivation mice model. Neuroscience. 2022;496:27–37. doi: 10.1016/j.neuroscience.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Wu H., Dunnett S., Ho Y.S., et al. The role of sleep deprivation and circadian rhythm disruption as risk factors of Alzheimer's disease. Front. Neuroendocrinol. 2019;54 doi: 10.1016/j.yfrne.2019.100764. [DOI] [PubMed] [Google Scholar]
- 79.Guisle I., Gratuze M., Petry S., et al. Circadian and sleep/wake-dependent variations in tau phosphorylation are driven by temperature. Sleep. 2020;43(4) doi: 10.1093/sleep/zsz266. [DOI] [PubMed] [Google Scholar]
- 80.Ahmad F., Sachdeva P., Sarkar J., et al. Circadian dysfunction and Alzheimer's disease - an updated review. Aging Med (Milton) 2023;6(1):71–81. doi: 10.1002/agm2.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabassum S., Misrani A., Tabassum S., et al. Disrupted prefrontal neuronal oscillations and morphology induced by sleep deprivation in young APP/PS1 transgenic AD mice. Brain Res. Bull. 2021;166:12–20. doi: 10.1016/j.brainresbull.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Wang C., Gao W.R., Yin J., et al. Chronic sleep deprivation exacerbates cognitive and synaptic plasticity impairments in APP/PS1 transgenic mice. Behav. Brain Res. 2021;412 doi: 10.1016/j.bbr.2021.113400. [DOI] [PubMed] [Google Scholar]
- 83.Di Meco A., Joshi Y.B., Praticò D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiol. Aging. 2014;35(8):1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 84.Tobaldini E., Costantino G., Solbiati M., et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017;74(Pt B):321–329. doi: 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Shi L., Chen S.J., Ma M.Y., et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med. Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z., Chen W.H., Li S.X., et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatr. 2021;26(11):6277–6292. doi: 10.1038/s41380-021-01113-1. [DOI] [PubMed] [Google Scholar]
- 87.Matenchuk B.A., Mandhane P.J., Kozyrskyj A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020;53 doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 88.Yang D.F., Huang W.C., Wu C.W., et al. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023;268 doi: 10.1016/j.micres.2022.127292. [DOI] [PubMed] [Google Scholar]
- 89.Hashim H.M., Makpol S. A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.1007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benedict C., Vogel H., Jonas W., et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metabol. 2016;5(12):1175–1186. doi: 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang C., Li G., Huang P., et al. The gut microbiota and alzheimer's disease. J Alzheimers Dis. 2017;58(1):1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 92.Kesika P., Suganthy N., Sivamaruthi B.S., et al. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118627. [DOI] [PubMed] [Google Scholar]
- 93.Kowalski K., Mulak A. Brain-gut-microbiota Axis in alzheimer's disease. J Neurogastroenterol Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uddin M.S., Ashraf G.M. Advances in Dementia Research: IntechOpen. 2018. Alzheimer's disease—the most common cause of dementia. [Google Scholar]
- 95.Urrestarazu E., Iriarte J. Clinical management of sleep disturbances in Alzheimer's disease: current and emerging strategies. Nat. Sci. Sleep. 2016;8:21–33. doi: 10.2147/NSS.S76706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uddin M.S., Tewari D., Mamun A.A., et al. Circadian and sleep dysfunction in Alzheimer's disease. Ageing Res. Rev. 2020;60 doi: 10.1016/j.arr.2020.101046. [DOI] [PubMed] [Google Scholar]
- 97.Sadeghmousavi S., Eskian M., Rahmani F., et al. The effect of insomnia on development of Alzheimer's disease. J. Neuroinflammation. 2020;17(1):289. doi: 10.1186/s12974-020-01960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas J., Overeem S., Claassen J. Long-term occupational sleep loss and post-retirement cognitive decline or dementia. Dement. Geriatr. Cogn. Disord. 2019;48(1–2):105–112. doi: 10.1159/000504020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olsson M., Ärlig J., Hedner J., et al. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer's disease. Sleep. 2018;41(5) doi: 10.1093/sleep/zsy025. [DOI] [PubMed] [Google Scholar]