Abstract
Polygonatum sibiricum polysaccharide (PSP) has demonstrated diverse medicinal properties, extensively researched for human applications. Nonetheless, there is a lack of studies investigating the potential advantages of PSP in poultry farming. The present study investigated the impact of incorporating PSP into broiler diets on their growth performance, meat quality, blood metabolites, antioxidative status, and ileal histomorphology. Two hundred and forty-one-day-old male Ross-308 broiler chicks (44.98 ± 0.79 g) were randomly assigned to 3 experimental groups, with 8 replicates of 10 birds each. The birds were fed diets supplemented with PSP at 0, 400, and 800 mg/kg (control, PSP400, and PSP800, respectively). The results revealed a linear (P > 0.05) improvement in body weight gain, European production efficiency index, and feed conversion ratio during the grower (22–35 d) and overall periods (1–35 d). The pH levels in the ingluvies, ileum, and cecum exhibited a linear reduction (P > 0.05) in the PSP800 group at d 21 and d 35, respectively. Villus height and crypt depth were increased in the PSP400 and PSP800 groups compared to the control group. PSP400 and PSP800 groups exhibited decreased hydrogen peroxide (H2O2) levels and increased total antioxidant capacity (TAC) at 21 d, while at 35 d, TAC and sulfhydryl concentrations were elevated, and H2O2 was reduced only in the PSP800 group compared to the untreated one. No significant variations between the groups at the phylum and genus levels were observed, with Bacteroidetes and Firmicutes being the dominant phyla. However, PSP supplementation notably augmented Firmicutes and Verrucomicrobiota while reducing Euryarchaeota and Proteobacteria. At the genus level, there was an increase in Akkermansia, Alistipes, CHKCI001, Erysipelatoclostridium, and a decrease in Methanobrevibacter. Conclusively, incorporating PSP into broiler diets, particularly at a dosage of 800 mg/kg, improved growth performance, antioxidant capacity, and intestinal architecture and resulted in alterations in cecal microbiota without discernible impacts on digestive function and meat quality criteria.
Key words: polysaccharide, growth, gut microbiota, histomorphometry, broiler
INTRODUCTION
Eminent progress has been made in enhancing productivity in modern intensive poultry production. Nonetheless, the utilization of antibiotic-growth-promoters (AGP), encompassing antibiotics and chemotherapeutic substances in poultry diets to boost productivity and combat diseases, has raised concerns about food and human safety (Castanon, 2007). Consequences such as drug residues and the development of resistant bacteria have been identified as substantial outcomes of these practices (Abdel-Moneim et al., 2020c). Consequently, the poultry industry is undergoing a transformation towards more sustainable and responsible approaches to meet the growing customer preference for safe, nutritious, and eco-friendly products (Abd El-Moneim et al., 2020; Chen et al., 2023; Elbaz et al., 2023; Saleh et al., 2023; Chen et al., 2024b). The transition from AGPs to alternative solutions has been motivated by concerns regarding resistance to antibiotics, as well as the increasing consumers’ preference for poultry products free from antibiotics. This shift is not limited to specific regions, as the European Union banned the use of AGPs in poultry feed in 2006, setting an example for other countries to follow (Castanon, 2007).
Alternative substances, including prebiotics (Abd El-Hack et al., 2021; Shehata et al., 2022), probiotics (Abd El-Hack et al., 2020; Abdel-Moneim et al., 2020a), essential oils (Abd El-Hack et al., 2020; Elbaz et al., 2022b), traditional Chinese medicine (TCM) (Dosoky et al., 2021; Yang et al., 2023), and herbal extracts (Elbaz et al., 2021; Mesalam et al., 2021; Ebeid et al., 2023; Chen et al., 2024a), as well as improved management and nutritional practices (Abdel-Moneim et al., 2021; Elbaz et al., 2022a; Siddiqui et al., 2022; Abdel-Moneim et al., 2023), offer avenues to improve bird health and productivity, obviating the necessity for AGPs. Traditional Chinese medicine and phytochemicals, in particular, have emerged as promising substitutes owing to their plant-derived compounds with immunomodulatory, antioxidant, and antimicrobial attributes. Integrating TCM in poultry production aligns with eco-friendly practices and disease prevention. Recent research underscores the advantageous effects of plant-derived bioactives, particularly polysaccharides, in enhancing poultry growth, meat quality, and carcass characteristics (Wang et al., 2022b; Yang et al., 2023). Extensive research focused on polysaccharides commonly found in TCM plants and their extracts, which are rich in polysaccharides, have gained attention as AGP alternatives because of their multifunctionality, low toxicity, and limited adverse effects (Shan et al., 2019; Abdel-Moneim et al., 2020b; Sun et al., 2022).
Polygonatum sibiricum polysaccharide (PSP) represents a recently identified water-soluble compound extracted from the rhizome of Polygonatum. It is primarily composed of galactose and rhamnose (Liu et al., 2018a). This compound has been shown to possess medicinal efficacy by enhancing immune function (Peng et al., 2018), as well as showcasing antitumor (Peng et al., 2018), antiviral and antiinflammatory (Lu et al., 2013), and antioxidant properties (Jiang et al., 2013). Studies indicated that PSP may have the potential in addressing conditions like diabetes, osteoporosis, acting as a neuroprotective agent, and alleviating inflammatory disorders (Zhang et al., 2015; Wang et al., 2017). In a study conducted by Shu et al. (2021), the authors underscored the significance of polysaccharides derived from Polygonatum sibiricum in mitigating cyclophosphamide-caused immunosuppression in chickens, suggesting its potential immunostimulant activity. However, while the beneficial effects of PSP have been extensively studied in traditional human medicine, limited research exists on its potential application in poultry farming.
Presently, there is a lack of research evaluating the impact of dietary supplementation of PSP in broiler chickens. Therefore, the objective of this study was to explore the influence of PSP dietary inclusion on the growth, meat quality, antioxidative status, digestive physiology, and cecal microbiota of broiler chickens.
MATERIAL AND METHODS
Ethics Statement
The procedures of the present study were reviewed and approved by the Institutional Animal Care and Use Committee of Anhui Science and Technology University, Fengyang, Anhui Province in China (ECASTU-2019-P03).
PSP Composition Analysis
The percentages of crude protein, crude ash, ether extract, neutral detergent fiber, acid detergent fiber, calcium, and phosphorus in PSP were measured as reported by da Teixeira et al. (2018). The phenol-sulfuric acid method was used to assess the polysaccharides content in PSP (Masuko et al., 2005), which was purchased from Shaanxi Hannah Biotechnology Co., Ltd. (Xi'an, China).
Experimental Design and Bird Management
Two hundred and forty-one-day-old male Ross-308 broiler chicks (44.98 ± 0.79 g) were randomly assigned into 3 experimental groups, with 8 replicates of 10 birds each. The chicks were supplied by Bengbu Dacheng Food Co., Ltd.’s hatchery (Anhui, China). They were provided with a starter (1–21 d) and grower (22–35 d) mash corn-soybean meal basal diet (Table 1) with ad libitum access to feed and fresh water. PSP was added to the diets at levels of 0, 400, and 800 mg/kg, respectively (control, PSP400, and PSP800, respectively). The chicks were reared in floor cages with a 23L:1D lighting program under controlled environmental conditions. For the first 3 d of the experiment, the indoor temperature was maintained at 35°C and gradually decreased to 20°C with a relative humidity of 58% till the end of the trial.
Table 1.
Ingredient and composition of the basal diet.
| Items | Starter (1–21 d) | Grower (22–35 d) | |
|---|---|---|---|
| Ingredients (g/kg) | |||
| Corn | 514.2 | 566.6 | |
| Corn starch | 10.0 | 10.0 | |
| Soybean meal | 386.5 | 341.0 | |
| Fish meal | 35.0 | 20.0 | |
| Soybean oil | 30.0 | 35.0 | |
| Dicalcium phosphate | 9.5 | 11.0 | |
| Ground limestone | 9.0 | 10.5 | |
| Iodine salt | 2.5 | 2.5 | |
| DL-methionine | 0.9 | 1.0 | |
| Vitamin-mineral premix1 | 2.4 | 2.4 | |
| Nutrient content2 | |||
| Crude protein | 231.2 | 204.7 | |
| AME (MJ/kg) | 11.35 | 12.46 | |
| Methionine | 4.8 | 4.3 | |
| Lysine | 12.5 | 11.4 | |
| Methionine + cysteine | 8.4 | 7.6 | |
| Non-phytate phosphorus | 4.2 | 3.7 | |
| Calcium | 10.3 | 8.8 | |
vitamin-mineral premix provided per kg diet: IU: vit. A 4,000,000, vit. D3 500,000; g: vit. E 16.7, vit. K 0.67, vit. B1 0.67, vit. B2 2, vit. B6 67, vit. B12 0.004, nicotinic acid 16.7, pantothenic acid 6.67, biotin 0.07, folic acid 1.67, choline chloride 400, Zn 23.3, Mn 10, Fe 25, Cu 1.67, I 0.25, Se 0.033, Mg 133.4
Calculated according to NRC (1994).
Growth Performance
On a pen basis, body weight and feed intake (FI) were measured on d 21 and 35. Body weight gain (BWG), European production efficiency index (EPEI), and feed conversion ratio (FCR) were calculated from the obtained data for each experimental phase as mentioned by Abdel-Moneim et al. (2022).
The Intestinal Morphology
At the end of the experiment, eight chicks per group were randomly chosen for blood and meat sampling. Birds were fasted for 12 h and then manually slaughtered. Following slaughter, the intestinal segments (duodenum, jejunum, ileum, and cecum) were gathered and weighed, and their length was measured. The boundary between the jejunum and ileum was determined by the presence of the yolk pedicle.
Gastrointestinal pH
On d 21 and 35, gastrointestinal organs, including proventriculus, ingluvies, gizzard, jejunum, duodenum, cecum, and ileum, were dissected. Subsequently, a small incision was made at the midpoint of each organ, and an electrode was promptly inserted into the organ content to measure the pH value using a pH meter (Shenzhen Jige Electromechanical Equipment Co., Ltd., Shenzhen, China).
Histomorphometric Evaluation
Ileal samples were collected (1.5 cm from the mid-ileum), were flushed with 0.9% saline, and were fixed in a 10% formalin solution. Tissue sections, 4 µm thick, were obtained from paraffin-embedded tissue blocks and were stained with hematoxylin and eosin following the protocol of Bancroft and Gamble (2002). Stained tissues were observed using a light microscope (Leica DM300 with Leica FLEXACAMC1), and representative fields were photographed for morphometric analysis using Leica LAS X dedicated software. Measurements of villus height (VH), and crypt depth (CD) were obtained by averaging data from 10 randomly chosen sections in each sample, and VH/CD ratio was calculated.
Antioxidative Status
Blood samples were collected from the wing vein at 21 d of age and after slaughtering at 35 d of age. Samples were then centrifuged for 20 min at 5℃ at 3,500 × g and the sera were separated and stored at -80℃. The serum concentrations of total antioxidant capacity (TAC), sulfhydryl, and hydrogen peroxide (H2O2) were colorimetrically measured following the manufacturing instructions of the commercial kits (NJIB, Jiangsu, China).
Meat Quality
Following the procedure documented by Hou et al. (2020), meat pH and color were determined. In brief, immediately after slaughter, the physical characteristics of pectoral and leg muscle samples were evaluated. Meat pH was measured by totally embedding the electrodes in the samples to ensure entire contact with the tissue fluid. The pH values were measured using a pH meter (Shenzhen Jige Electromechanical Equipment Co., Ltd., Shenzhen, China) and were recorded after the readings had stabilized. The CR-10 Plus chromameter (Zibo Diye Instrument Equipment Co., Ltd., Zibo, China) was used to measure color lightness (L*), yellowness (b*), and redness (a*) of pectoral and leg muscle samples.
In order to calculate drip loss (%), in triplicate, pectoral, and leg muscle meat samples were suspended in sealed plastic bags and stored at 4℃ for 24 h. The differences between the initial and final weights of the samples were calculated and expressed as relative weights to the initial weight. The calculation of cooking loss (%) was conducted in accordance with the procedure proposed by Honikel (1998). In brief, fresh slices of muscle samples measuring 4 × 3 × 1 cm³ were weighed, positioned within sealed plastic bags, and subjected to cooking until an internal temperature of 70°C was attained, which took approximately 15 min in a water bath set at 80°C. Subsequently, the slices were cooled in water, dried, and reweighed. The percentage of cooking loss was determined using the formula: (initial weight - final weight)/initial weight × 100%.
Cecal Microbiota Analysis
On d 35, cecum samples from 5 randomly selected birds from both the control and PSP800 groups (the highest level of the supplement used in the present study) were quickly collected, tightly tied with a thin thread, and stored in a -80℃ freezer for sequencing. Microbial DNA was extracted from the cecum samples using the HiPure Soil DNA Kits (Magen, Guangzhou, China), following the manufacturer's guidelines. The 16S rDNA target regions of the ribosomal RNA gene were PCR-amplified using a 50 µL mixture comprising 10 µL of 5 × Q5@ Reaction Buffer, 10 µL of 5 × Q5@ High GC Enhancer, 1.5 µL of 2.5 mM dNTPs, 1.5 µL of each primer (10 µM), 0.2 µL of Q5@ High-Fidelity DNA Polymerase, and 50 ng of template DNA. PCR reagents were sourced from New England Biolabs, USA. The purified amplicons were pooled equimolarly and subjected to paired-end sequenced (PE250) on an Illumina platform following standardized protocols. The V3-V4 hypervariable region of the bacterial 16S rRNA gene was specifically PCR-amplified using the forward primer 341F: 5′-CCTACGGGNGGCWGCAG-3′ and the reverse primer 806R: 5′-GGACTACHVGGGTATCTAAT-3′.
Reads Filtering and Assembly and Raw Tag Filtering
To obtain high-quality clean reads, we applied filtering steps to the raw reads using FASTP (version 0.18.0) based on the following criteria: 1) Removal of reads containing more than 10% of unknown nucleotides (N); 2) Discarding reads with less than 50% of bases with quality (Q-value) above 20; 3) Elimination of adapter contamination. Paired-end clean reads were merged into raw tags using FLASH (version 1.2.11) with a minimum overlap of 10 bp and a mismatch error rate of 2%. Furthermore, for obtaining high-quality clean tags, we performed additional filtering on the raw tags using the following standards: 1) Trimming raw tags starting from the first low-quality base site (default quality threshold is ≤3) until the desired length (default length is 3 bp); 2) Filtering out tags with a continuous high-quality base length of less than 75% of the tag length.
Chimera Removal and Community Composition Analysis
The clean tags were then clustered into operational taxonomic units (OTU) with a similarity threshold of 97% using the UPARSE (version 9.2.64) pipeline. Chimeric tags were removed using the UCHIME algorithm while retaining the effective tags for subsequent analysis. Within each cluster, the tag sequence with the highest abundance was selected as the representative sequence. The abundance of each taxonomic group was visualized using Krona (version 2.6). Circular layout representations depicting species abundance were generated using Circus (version 0.69-3). Additionally, a heatmap illustrating species abundance was created within the R project using the heatmap package (version 1.0.12).
Function Prediction
To predict the function of the OTUs/ASVs, we conducted a KEGG pathway analysis using Tax4Fun (version 1.0). To classify the bacterial microbiome phenotypes, BugBase was employed. The ecological functional profiles of bacteria were generated using the FAPROTAX database. For the functional grouping of fungi, FUNGuild (version 1.0) was utilized. Differences in functionality between groups were evaluated using Welch's t-test within the R project Vegan package (version 2.5.3).
Statistical Analysis
The gathered data were statistically analyzed by One-way ANOVA after conducting the tests of normality and the homogeneity of variance using SPSS software (version 19.0; SPSS Inc., IL). The statistical significance among mean differences was determined at P < 0.05 using Tukey's multiple comparison test.
RESULTS
Composition of PSP
Data in Table 2 represent the chemical composition of PSP. Crude protein, crude ash, acid detergent fiber, neutral detergent fiber, calcium, phosphorus, ether extract, and polysaccharide levels of PSP were 1.31,1.78, 3.12, 3.44, 0.42, 0.24, 1.86, and 81.95%, respectively.
Table 2.
The composition of Polygonatum sibiricum polysaccharide.
| Ingredients | Content (%) |
|---|---|
| Polysaccharide | 81.95 |
| Crude protein | 1.31 |
| Ether extract | 1.86 |
| Neutral detergent fiber | 3.44 |
| Acid detergent fiber | 3.12 |
| Crude ash | 1.78 |
| Calcium | 0.42 |
| Phosphorus | 0.24 |
Growth Performance
Table 3 presents the impact of dietary PSP incorporation on the growth performance of broiler chicks. BWG, FI, FCR, and EPEI were not affected by PSP treatment during the starter period. However, all the aforementioned parameters, except FI, were linearly improved (P > 0.05) in the PSP400 and PSP800 groups during the grower period (22–35 d). Throughout the overall period, EPEI, FCR, and BWG were linearly enhanced (P > 0.05) in the PSP800 group and numerically in the PSP400 group, while FI remained unaffected.
Table 3.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on growth performance of broiler chickens from 1 to 35 d of age.
| Parameter1 | Dietary PSP level, mg/kg |
SEM2 |
P-values |
||||
|---|---|---|---|---|---|---|---|
| 0 | 400 | 800 | PSP | Linear | Quadratic | ||
| BWG, g.bird/d | |||||||
| 1–21 d | 32.88 | 32.36 | 34.09 | 0.353 | 0.116 | 0.146 | 0.122 |
| 22–35 d | 65.46b | 70.09a | 69.68a | 0.715 | 0.003 | 0.004 | 0.029 |
| 1–35 d | 45.91b | 47.45ab | 48.33a | 0.412 | 0.038 | 0.013 | 0.652 |
| FI, g.bird/d | |||||||
| 1–21 d | 46.89 | 47.20 | 47.05 | 0.118 | 0.599 | 0.592 | 0.399 |
| 22–35 d | 129.4 | 128.4 | 129.5 | 0.346 | 0.357 | 0.848 | 0.163 |
| 1–35 d | 79.88 | 79.66 | 80.04 | 0.176 | 0.709 | 0.722 | 0.463 |
| FCR, g feed.g/gain | |||||||
| 1–21 d | 1.427 | 1.460 | 1.382 | 0.016 | 0.134 | 0.234 | 0.090 |
| 22–35 d | 1.976a | 1.833b | 1.861b | 0.022 | 0.008 | 0.013 | 0.027 |
| 1–35 d | 1.740a | 1.680ab | 1.658b | 0.016 | 0.010 | 0.041 | 0.555 |
| EPEI | |||||||
| 1–21 d | 245.8 | 237.0 | 262.8 | 5.335 | 0.130 | 0.180 | 0.119 |
| 22–35 d | 597.4b | 666.1a | 667.8a | 12.74 | 0.021 | 0.014 | 0.139 |
| 1–35 d | 271.4b | 290.7ab | 299.8a | 5.281 | 0.049 | 0.026 | 0.610 |
Means in the same row with different superscripts are significantly different.
BWG: body weight gain; FI: feed intake; FCR: feed conversion ratio, EPEI: European production efficiency index.
SEM: standard error of means. Values with different superscript letters are statistically different (P < 0.05).
Gut Morphology
Table 4 presents the influence of adding PSP to the diet on the gut structure of broiler chickens at 21 and 35 d old. The incorporation of PSP did not affect the proportional length and weight of the jejunum, duodenum, ileum, or cecum at either age.
Table 4.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on gut morphology of broiler chickens at 21 and 35 d of age.
| Parameter | Dietary PSP level, mg/kg |
SEM1 |
P-values |
||||
|---|---|---|---|---|---|---|---|
| 0 | 400 | 800 | PSP | Linear | Quadratic | ||
| 21 d | |||||||
| Intestinal segments’ relative weight, % | |||||||
| Duodenum | 17.81 | 19.79 | 19.47 | 0.511 | 0.249 | 0.192 | 0.291 |
| Jejunum | 39.32 | 38.81 | 39.15 | 0.872 | 0.975 | 0.941 | 0.836 |
| Ileum | 33.53 | 31.59 | 32.10 | 0.770 | 0.603 | 0.481 | 0.483 |
| Cecum | 9.346 | 9.807 | 9.277 | 0.343 | 0.814 | 0.940 | 0.533 |
| Total, g | 36.07 | 34.43 | 35.63 | 0.783 | 0.706 | 0.830 | 0.430 |
| Intestinal segments’ relative length, % | |||||||
| Duodenum | 14.94 | 14.75 | 15.26 | 0.280 | 0.779 | 0.665 | 0.585 |
| Jejunum | 36.27 | 36.34 | 36.65 | 0.360 | 0.915 | 0.700 | 0.885 |
| Ileum | 36.54 | 36.92 | 36.44 | 0.431 | 0.905 | 0.927 | 0.668 |
| Cecum | 12.25 | 12.00 | 11.66 | 0.370 | 0.831 | 0.553 | 0.959 |
| Total, cm | 161.4 | 161.5 | 157.7 | 1.731 | 0.625 | 0.412 | 0.623 |
| Density | 0.224 | 0.213 | 0.182 | 0.004 | 0.562 | 0.866 | 0.298 |
| 35 d | |||||||
| Intestinal segments’ relative weight, % | |||||||
| Duodenum | 16.61 | 16.34 | 16.23 | 0.246 | 0.830 | 0.561 | 0.890 |
| Jejunum | 37.53 | 35.89 | 36.64 | 0.417 | 0.297 | 0.391 | 0.193 |
| Ileum | 35.90 | 36.71 | 36.52 | 0.381 | 0.697 | 0.540 | 0.567 |
| Cecum | 9.963 | 11.06 | 10.62 | 0.301 | 0.347 | 0.386 | 0.243 |
| Total, g | 45.55 | 45.21 | 45.12 | 0.373 | 0.898 | 0.666 | 0.882 |
| Intestinal segments’ relative length, % | |||||||
| Duodenum | 13.13 | 13.32 | 13.61 | 0.197 | 0.646 | 0.364 | 0.904 |
| Jejunum | 35.16 | 35.23 | 35.71 | 0.207 | 0.521 | 0.301 | 0.655 |
| Ileum | 36.05 | 35.82 | 34.90 | 0.309 | 0.294 | 0.145 | 0.600 |
| Cecum | 15.66 | 15.63 | 15.78 | 0.255 | 0.976 | 0.868 | 0.887 |
| Total, cm | 182.2 | 181.5 | 182.0 | 1.282 | 0.977 | 0.956 | 0.837 |
| Density | 0.250 | 0.249 | 0.248 | 0.003 | 0.961 | 0.784 | 0.975 |
Means in the same row with different superscripts are significantly different
SEM: standard error of means, Density = total weight/ total length ratio
pH of the Digestive Organs
As presented in Table 5, the introduction of PSP resulted in a linear decrease (P < 0.05) in the pH levels of the ingluvies at d 21 and in the ileum and cecum at d 35 within the PSP800 group compared to the control. However, there were no notable changes in the pH of the other digestive organs at either age.
Table 5.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on digestive organs' pH of broiler chickens at 21 and 35 d of age.
| Parameter | Dietary PSP level, mg/kg |
SEM1 |
P-values |
||||
|---|---|---|---|---|---|---|---|
| 0 | 400 | 800 | PSP | Linear | Quadratic | ||
| 21 d | |||||||
| Ingluvies | 6.292a | 6.128ab | 5.724b | 0.155 | 0.030 | 0.049 | 0.718 |
| Proventriculus | 4.458 | 4.558 | 4.384 | 0.120 | 0.858 | 0.817 | 0.623 |
| Gizzard | 2.618 | 2.718 | 2.626 | 0.074 | 0.849 | 0.968 | 0.577 |
| Duodenum | 6.146 | 6.060 | 5.952 | 0.086 | 0.686 | 0.633 | 0.478 |
| Jejunum | 6.424 | 6.336 | 6.214 | 0.088 | 0.656 | 0.370 | 0.932 |
| Ileum | 6.242 | 6.196 | 6.064 | 0.078 | 0.657 | 0.518 | 0.526 |
| Cecum | 7.272 | 7.250 | 7.096 | 0.092 | 0.728 | 0.473 | 0.754 |
| 35 d | |||||||
| Ingluvies | 6.220 | 6.290 | 6.206 | 0.050 | 0.783 | 0.592 | 0.667 |
| Proventriculus | 4.310 | 4.204 | 4.148 | 0.115 | 0.863 | 0.731 | 0.684 |
| Gizzard | 3.042 | 3.106 | 3.052 | 0.051 | 0.878 | 0.642 | 0.853 |
| Duodenum | 5.986 | 6.038 | 5.960 | 0.053 | 0.849 | 0.713 | 0.671 |
| Jejunum | 6.252 | 6.422 | 6.282 | 0.059 | 0.488 | 0.270 | 0.673 |
| Ileum | 6.772a | 6.364ab | 6.100b | 0.114 | 0.039 | 0.270 | 0.673 |
| Cecum | 6.668a | 6.326ab | 6.084b | 0.121 | 0.037 | 0.050 | 0.834 |
Means in the same row with different superscripts are significantly different
SEM: standard error of means.
Histomorphometric Evaluation
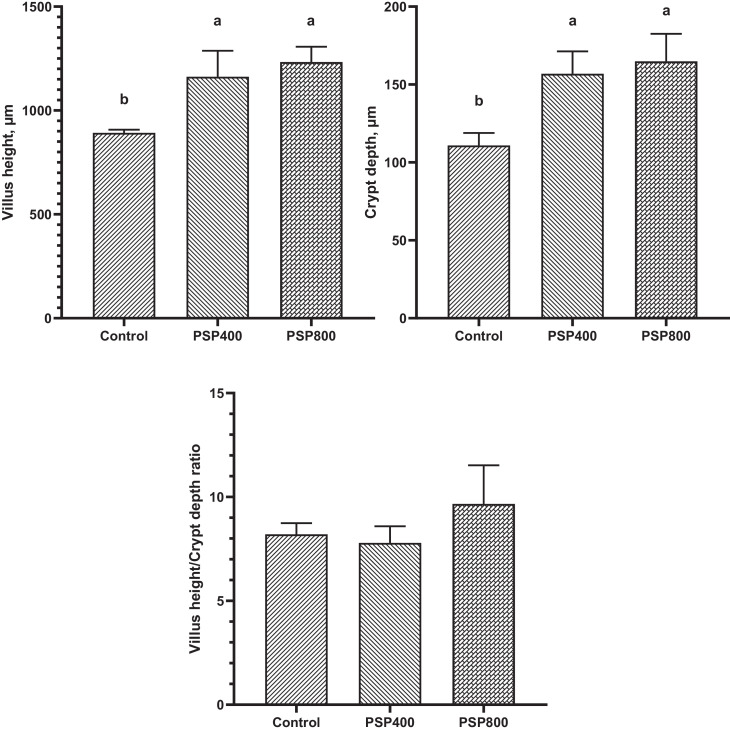
The impact of dietary PSP on the ileal histomorphometry of broilers at 35 d of age is depicted in Figure 1. VH and CD were linearly increased (P < 0.05) in the PSP400 and PSP800 groups compared to the control. The VH/CD ratio was not significantly affected.
Figure 1.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on ileal histomorphometry of broiler chickens at 35 d of age. PSP400= 400 mg PSP/kg diet, PSP800 = 800 mg PSP/kg diet. Data presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05).
Antioxidative Status
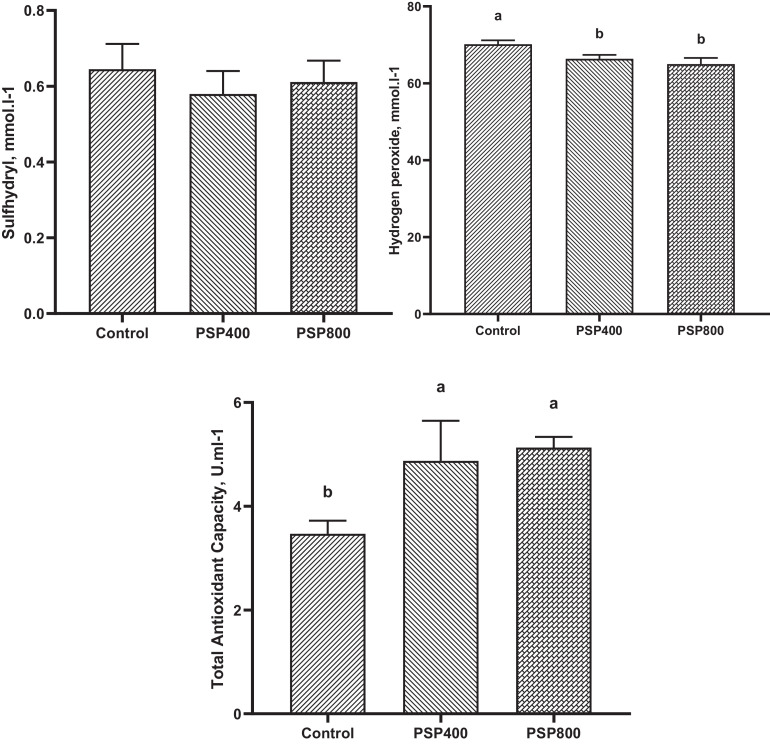
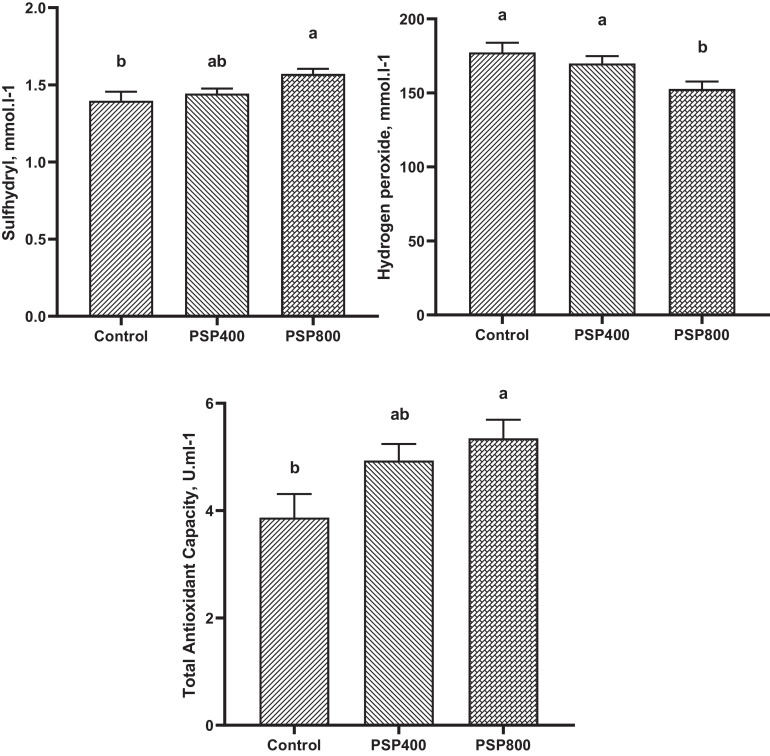
Figures 2 and 3 illustrate the impacts of dietary PSP incorporation on the antioxidant status of broilers at 21 and 35 d of age. Dietary supplementation of PSP at 400 and 800 mg/kg reduced (P < 0.05) serum level of H2O2 and increased the concentration of TAC (P < 0.05) at 21 d of age, while sulfhydryl content was not affected. However, at 35 d of age, TAC and sulfhydryl levels were elevated (P < 0.05) and H2O2 content was reduced (P < 0.05) only in the PSP800 group compared to the control.
Figure 2.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on oxidative status in the serum of broiler chickens at 21 d of age. PSP400 = 400 mg PSP/kg diet, PSP800 = 800 mg PSP/kg diet. Data presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05).
Figure 3.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on oxidative status in the serum of broiler chickens at 35 d of age. PSP400 = 400 mg PSP/kg diet, PSP800= 800 mg PSP/kg diet. Data presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05).
Meat Quality
Tables 6 and 7 show the impacts of the inclusion of PSP in broilers’ diets on meat quality criteria of pectoral and leg muscles at 35 d of age. PSP supplementation did not exert a significant effect on the pH45, pH24, color, cooking, and drip losses of the collected samples compared to the unsupplemented group. However, there was a linear decrease (P < 0.05) observed in the yellowness (b*) of the meat from birds in the PSP800 group compared to the control.
Table 6.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on meat quality traits of the pectoral muscle of broiler chickens at 35 d of age.
| Parameter | Dietary PSP level, mg/kg |
SEM1 |
P-values |
||||
|---|---|---|---|---|---|---|---|
| 0 | 400 | 800 | PSP | Linear | Quadratic | ||
| L45 min | 52.48 | 52.22 | 51.72 | 0.139 | 0.062 | 0.053 | 0.650 |
| a45 min | 11.56 | 11.95 | 12.27 | 0.163 | 0.206 | 0.082 | 0.912 |
| b45 min | 7.553 | 7.429 | 7.175 | 0.162 | 0.658 | 0.379 | 0.860 |
| pH45 min | 7.126 | 7.036 | 6.808 | 0.119 | 0.564 | 0.309 | 0.794 |
| pH24 h | 6.306 | 6.158 | 6.172 | 0.062 | 0.597 | 0.411 | 0.564 |
| Drip loss24 h/% | 4.650 | 4.970 | 5.108 | 0.120 | 0.298 | 0.137 | 0.721 |
| Cooking loss/% | 23.01 | 22.56 | 22.53 | 0.253 | 0.719 | 0.479 | 0.713 |
Means in the same row with different superscripts are significantly different,
SEM: standard error of means.
Table 7.
Effect of dietary Polygonatum sibiricum polysaccharide (PSP) on meat quality traits of leg muscle of broiler chickens at 35 d of age.
| Parameter | Dietary PSP level, mg/kg |
SEM1 |
P-values |
||||
|---|---|---|---|---|---|---|---|
| 0 | 400 | 800 | PSP | Linear | Quadratic | ||
| L45 min | 52.83 | 52.46 | 52.83 | 0.170 | 0.626 | 0.993 | 0.343 |
| a45 min | 11.57 | 11.63 | 11.92 | 0.115 | 0.439 | 0.827 | 0.215 |
| b45 min | 7.310a | 7.107ab | 7.017b | 0.171 | 0.049 | 0.047 | 0.267 |
| pH45 min | 7.108 | 7.001 | 7.082 | 0.130 | 0.948 | 0.936 | 0.757 |
| pH24 h | 6.646 | 6.332 | 6.208 | 0.148 | 0.492 | 0.257 | 0.771 |
| Drip loss24 h/% | 4.753 | 4.857 | 4.928 | 0.213 | 0.358 | 0.258 | 0.852 |
| Cooking loss/% | 22.87 | 22.69 | 22.84 | 0.189 | 0.846 | 0.587 | 0.729 |
Means in the same row with different superscripts are significantly different,
SEM: standard error of means.
Microbiota Dynamics
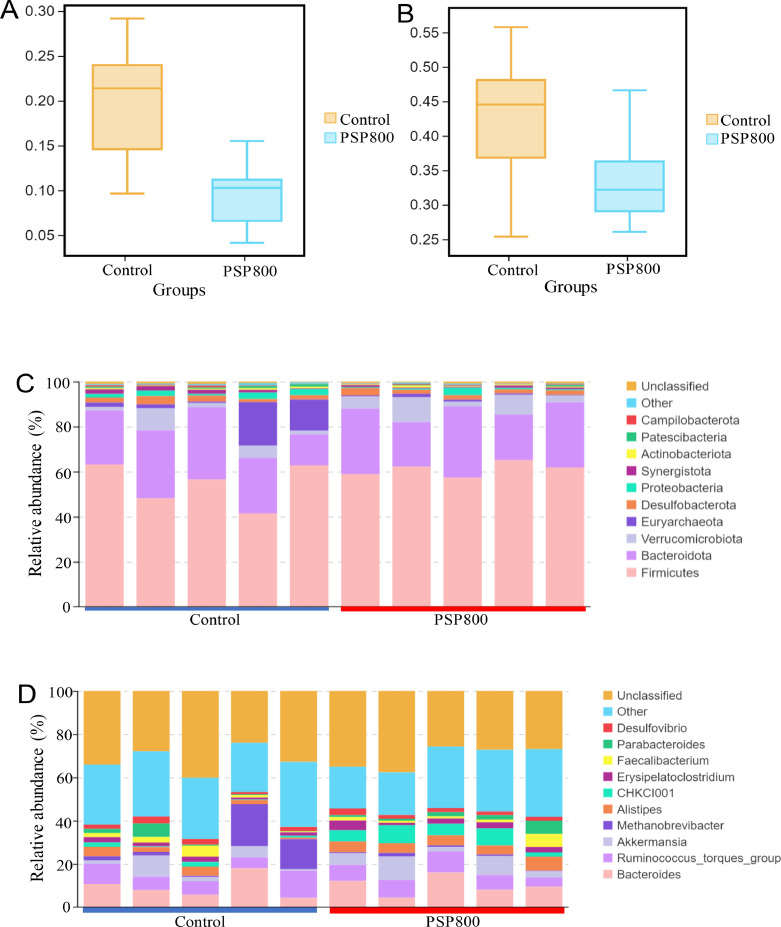
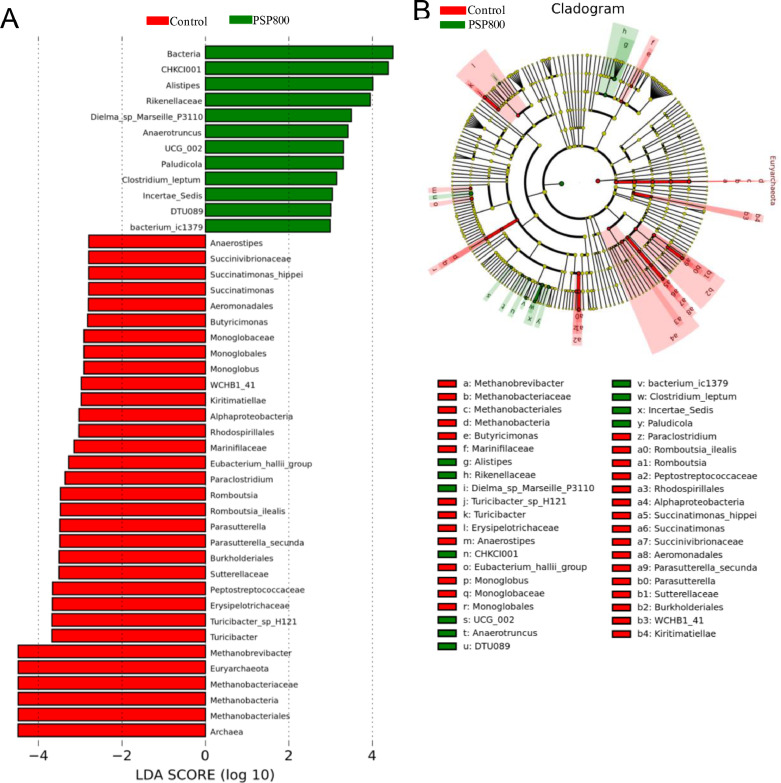
To assess the impact of PSP supplementation on caecal microbiota composition, 16S rDNA sequencing was performed on the cecal contents of both the control and PSP800 groups. The average number of raw reads was 109,518 and 121,169, while the average number of clean data was 109,440 and 121,079 for the PSP800 and control groups, respectively. Beta diversity indexes were calculated at the phylum and genus levels to assess differences in species diversity and richness differences between the treated and control groups. The results revealed no significant variations in observed species between the groups (Figures 4A and 4B; P > 0.05). Analysis of microbial abundance at the phylum and genus levels revealed that Bacteroidetes and Firmicutes were the dominant phyla, accounting for 86.99% and 79.32% of detected microbes in the PSP800 and control groups, respectively (Figure 4C). Moreover, PSP supplementation notably augmented Firmicutes and Verrucomicrobiota while reduced Euryarchaeota and Proteobacteria microbes (Figure 4C). At the genus level, an increase in Akkermansia, Alistipes, CHKCI001, Erysipelatoclostridium, and a decrease in Methanobrevibacter were found (Figure 4D). Linear discriminant analysis (LDA) identified 41 high-dimensional biomarkers, with LDA scores >2.5 from phylum to species, illustrating distinct bacterial abundance between the 2 groups (Figure 5). Notably, Bacteria, CHKCI001, and Rikenellaceae were prominent in the PSP800 group, whereas Methanobrevibacter, Euryarchaeota, Methanobacteriaceae, Methanobacteria, and Methanobacteriales prevailed in the control group.
Figure 4.
The differences in species diversity and richness in chicken cecal. Statistical tests for β diversity index at the phylum (A) and genus (B) levels. Relative microbial abundance at phylum (C) and genus (D) levels.
Figure 5.
Linear discriminant analysis (LDA) effect size (LEFSe) analysis identified microbial taxa between PSP800 (green) and control (red) groups. (A) the histogram plot from LEfSe analysis displays the LDA scores of microbial taxa with significantly different abundance between the PSP800 supplemented and control groups (LDA score > 2.5). The length of the bar columns represents the LDA score. (B) the cladogram illustrates the variances in the relative abundance of microbial taxa from phylum to genus level between the PSP800 and control groups, with circles radiating from the inner to the outer side. The red and green points indicate a clear contrast in relative abundance between the PSP800 and control groups.
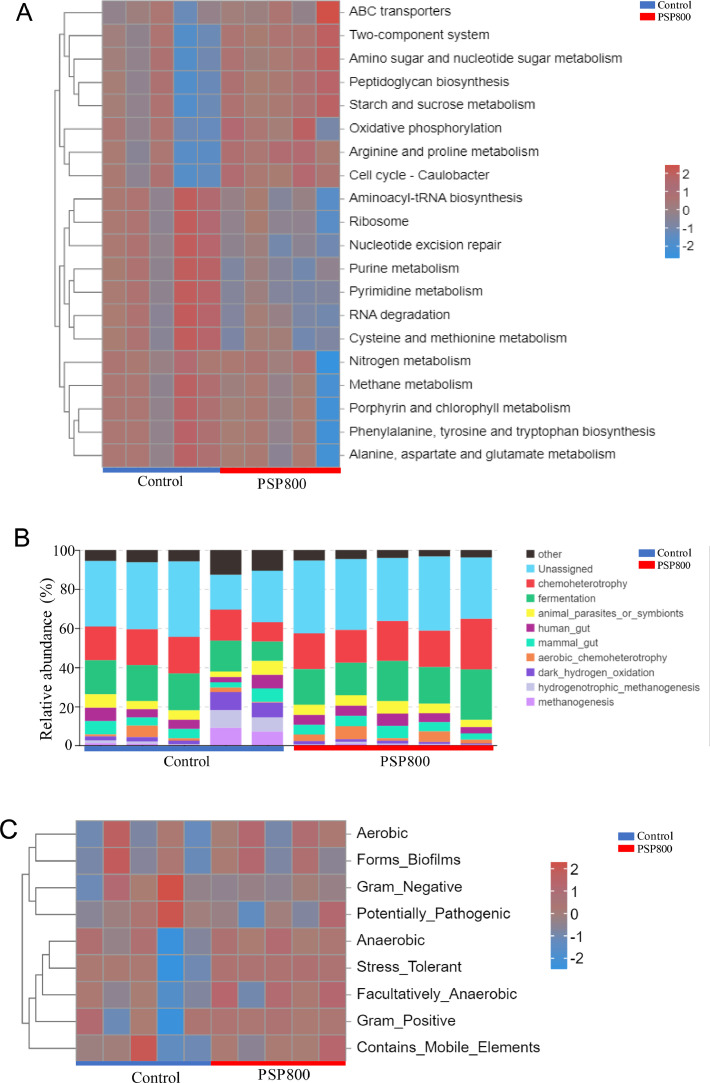
Functional and phenotypic abundance analyses were conducted to understand the pathways associated with microorganisms with varying abundance in the 2 groups. Tax4Fun functional abundance analysis (Figure 5A) revealed that these microorganisms were associated with various pathways, including ABC transporters, nitrogen metabolism, 2-component systems, methane metabolism, peptidoglycan biosynthesis, arginine and proline metabolism, cell cycle (caulobacter), cysteine, amino sugar, nucleotide sugar, and methionine metabolism, among others (Figure 6A). Additionally, PICRUSt2 functional abundance analysis revealed the involvement of these microorganisms in transcription, energy metabolism, amino acid metabolism, membrane transport, carbohydrate metabolism, signal transduction, lipid, cofactor, and vitamin metabolism, among others (Figure 6B). Furthermore, BugBase phenotypic abundance analysis demonstrated associations of these microorganisms with traits such as aerobic nature, negative biofilm formation, potentially pathogenic anaerobic features, stress tolerance, facultative anaerobic gram-positive characteristics, and the presence of mobile genetic elements (Figure 6C).
Figure 6.
Functional and phenotypic abundance analyses for gut microbial with significant differences between control and PSP800 groups. Tax4Fun (A) and PICRUSt2 (B) functional abundance analyses. (C) represent BugBase phenotypic abundance analysis.
DISCUSSION
The utilization of plant-derived polysaccharides for enhancing the productivity and general health of broilers has gained traction due to their varied biological activities, including hypoglycemic benefits, spanning improvements in immunity, antioxidant capacity, antiviral, antitumor, and anti-inflammatory properties (Wang et al., 2022b; Yang et al., 2023). The bioactive potential of polysaccharides derived from Yingshan yunwu, Radix rehmanniae praeparata, Lycium barbarum, Astragalus membranaceus, Camellia oleifera, and Ficus carica has been investigated (Liu et al., 2021b; Shu et al., 2021; Yang et al., 2023). The findings of Liu et al. (2021b) highlighted that dietary incorporation of Yingshan yunwu tea-polysaccharides improved broilers’ gut health and microbiota, meat quality, and immunity. Moreover, Shu et al. (2021) reported the immunostimulant potential of polysaccharides derived from Polygonatum sibiricum in safeguarding cyclophosphamide-immunosuppressed chickens. However, to the best of our knowledge, there is a dearth of research investigating the effects of dietary PSP incorporation as an AGP alternative on broiler chickens.
In the present study, dietary PSP incorporation improved the growth performance of broilers during the grower and overall period. The improvement in broiler growth might attributed to its capability to stimulate the expression of protease, amylase, and lipase (Long et al., 2020), thereby increasing the activities of these digestive enzymes and ultimately enhancing digestive function. Furthermore, it has been reported that plant-derived polysaccharides have the capacity to enhance intestinal permeability and improve nutrient absorption (Ren et al., 2017). These outcomes are consistent with Wu (2018) and Wang et al. (2015), who demonstrated improved growth performance in broilers treated with Astragalus polysaccharides. Additionally, Long et al. (2020) and Yang et al. (2023) observed that polysaccharides extracted from Lycium barbarum and Radix rehmanniae praeparata improved the growth performance of Arbor Acres and Cobb-500 broiler chickens.
The intestine plays a pivotal role in nutrient digestion, absorption, and immune function, significantly influencing animal health (Cui et al., 2023). It has been reported that plant-derived polysaccharides can enhance intestinal permeability and nutrient absorption (Ren et al., 2017) and stimulate the expression of digestive enzymes like protease, amylase, and lipase (Long et al., 2020). This upregulation increases the activities of these digestive enzymes, ultimately improving digestive function. Additionally, changes in small intestine length can directly affect nutrient uptake. In this study, we investigated the effect of PSP supplementation on the lengths of various intestinal segments and the pH levels of different digestive organs. The length and weight of the intestinal segment were not affected by PSP dietary supplementation. However, PSP use significantly reduced pH, specifically in the cecum and ileum. Plant-derived polysaccharides can influence intestinal pH reduction in birds through various mechanisms. Typically, these polysaccharides undergo fermentation by gut microbiota in the cecum and ileum, producing SCFA (Wahlström et al., 2016). The metabolism of SCFA leads to an acidic environment, contributing to a lowered pH in the intestines (Nogal et al., 2021). Moreover, polysaccharides’ microbial fermentation generates other secondary substances, such as succinate and lactate, further contributing to a lower intestinal pH (Wassie et al., 2021; Wang et al., 2022b). The decline in gut pH can significantly affect various physiological processes, including microbial populations, nutrient absorption, and overall gut health. The role of PSP in reducing ileal and cecal pH could additionally explain the improvement in growth traits in the present study.
Small intestine mucosa morphology is evaluated through indices like VH, CD, and the VH/CD ratio, which serve as key measures to assess small intestine nutrient absorption capacity. Higher values of these indices signify increased absorptive potential of the small intestine (Shehata et al., 2021; Li et al., 2022b). PSP exhibits the capacity to enhance intestinal architecture, contributing to its prebiotic effects by fostering beneficial intestinal bacteria (Li et al., 2009). Previous research noted significant increases in VH and VH/CD ratio in the jejunum of broilers treated with cyclophosphamide when supplemented with 600 or 900 mg/kg of gamma-irradiated Astragalus polysaccharides (Li et al., 2019). Similarly, Wang et al. (2021) reported that administering gamma-irradiated Astragalus polysaccharides at 600 mg/kg in broiler diets increased VH and VH/CD ratio in the duodenum, jejunum, and ileum. Consistent with these findings, our study observed a notable increase in VH and CD of the ileum following dietary PSP supplementation, indicating improved feed absorption. Hence, it's plausible that PSP could enhance broiler performance by positively impacting intestinal mucosal morphology and fostering intestinal health.
This study reveals the antioxidant properties of PSP, evident in the assessment of serum H2O2 and sulfhydryl levels. Hydrogen peroxide, produced by vascular and inflammatory cells, triggers oxidative stress by generating reactive oxygen species (ROS) (OH and O2−) through activating NADPH oxidase (Coyle et al., 2006) and the Fenton's reaction involving Fe2+ (Ransy et al., 2020). Conversely, sulfhydryl groups found in thiols play a pivotal role in combating ROS during amplified oxidative stress (Erkus et al., 2015). The thiol pool in the plasma mainly includes low molecular weight thiols like glutathione and protein thiols such as albumin (Turell et al., 2013). Lately, thiol/disulfide balance and thiol levels have emerged as novel markers for oxidative stress assessment (Kundi et al., 2015; Altıparmak et al., 2016; Shang et al., 2021). Notably, the present findings reveal that PSP incorporation increases serum TAC levels and reduces H2O2 concentration, indicating the potent ability of PSP to scavenge ROS effectively. Our results agree with those of Xing et al. (2023), who reported that Artemisia ordosica polysaccharide enhanced the antioxidant capacity of LPS-induced broiler chickens. Similarly, the treatment with Lycium barbarum polysaccharide improved liver and serum antioxidant indices in Arbor Acres broilers (Long et al., 2020). Wang et al. (2022a) also documented the potent antioxidant activity of Polygonatum sibiricum polysaccharides.
Measuring the pH and color of meat is crucial for assessing chicken meat quality characteristics (Juncher et al., 2001). Improving raw meat quality has become a priority to meet evolving consumer demands. Notably, this study observed a linear decrease in the yellowness (b*) of leg meat in birds from the PSP800 group compared to the control. However, other meat color parameters, pH, drip, and cooking losses remained unaffected in both breast and leg muscles. These findings are consistent with a prior study (Wang et al., 2020). Conversely, Zhao et al. (2020) noted that polysaccharides from Yingshan Yunwu tea decreased the quality of the pectoral muscle of Chongren Chicks, altering its color and pH. However, Huang et al. (2021) reported that polysaccharides of Morinda officinalis improved the meat quality criteria of broilers with tibial dyschondroplasia. Additionally, chicken meat color serves as a crucial quality attribute, and a lower b* value indicates less pale meat (Fan et al., 2013). One possible mechanism could involve accelerated synthesis of myoglobin and fat deposition in muscles, leading to a lower b* value. Additionally, the antioxidant potential of TCM polyphenols may protect muscle cell membranes and reduce lipid peroxidation injuries (Yang et al., 2011).
This study explores the interactions between intestinal microflora and polysaccharides, an area that has yet to be minimally explored despite the extensive research on poultry gut microbe composition. Bacteroidetes and Firmicutes emerged as the primary phyla in the intestinal microflora in both PSP-treated and untreated groups, aligning with previous studies by Li et al. (2020) and Yadav et al. (2021). Li et al. (2020) illustrated that Bacteroidetes and Firmicutes dominated chicken intestinal microflora irrespective of polysaccharide treatment. Meanwhile, Yadav et al. (2021) reported that Bacteroidetes, Firmicutes, and Proteobacteria comprised around 90% of phyla in commercial chickens, with Firmicutes at 63.3% and Bacteroidetes at 24.4%. Additionally, our findings indicated a notable increase in the abundance of Firmicutes following PSP supplementation, consistent with the observations of Liu et al. (2018b) on dietary Achyranthes bidentata polysaccharides. Liu et al. (2021a) noted the potential of Firmicutes in enhancing the intestinal barrier function of broiler chickens, while Lin and Lee (2020) demonstrated how Firmicutes in the ileum and cecum digesta reduced pH values and ammonia nitrogen levels, potentially favoring the growth and health of the broilers.
In addition, we observed an increase in the abundance of Verrucomicrobiota in digesta following PSP supplementation, aligning with previous studies (Liu et al., 2023a; Liu et al., 2023b). Liu et al. (2023b) demonstrated that Verrucomicrobiota has the potential to enhance various intestinal aspects, such as VH and VH/CD ratio and the length of ileum and cecum. Another study by Liu et al. (2023a) indicated the potential of Verrucomicrobiota in increasing intestinal villus height. Concurrently, our findings indicated a reduction in the relative abundance of Euryarchaeota microbes in chicken cecum due to PSP supplementation. Barrera-Rojas et al. (2023) reported that Euryarchaeota might play a role in regulating methane production and assimilation in environmental settings. Consequently, our speculation stands that PSP supplementation might decrease methane production and assimilation, potentially enhancing the utilization of feed energy in birds. Moreover, our study indicated a decline in the relative abundance of Proteobacteria microbes due to PSP supplementation, which is consistent with the findings of Li et al. (2020), where Yingshan Yunwu tea polysaccharides were observed to reduce the relative abundance of Proteobacteria in chicken cecal contents.
Interestingly, our study also revealed that PSP treatment increased the relative abundance of Akkermansia, Alistipes, and CHKCI001 while decreasing Methanobrevibacter in chicken cecum (Figure 1D). This aligns with previous studies suggesting the benefits of Akkermansia in protecting broiler chicken health by safeguarding the intestinal mucosa against injury induced by S. pullorum and promoting intestinal epithelium proliferation (Bortoluzzi et al., 2019; Zhu et al., 2020). Furthermore, Li et al. (2022a) found that the relative abundance of Alistipes in cecal contents might elevate intestinal mucosal factors such as mucin 2 while decreasing inflammatory cytokine concentrations, Bax gene expression, and the Bax/Bcl-2 ratio in the intestinal mucosa. Additionally, Deng et al. (2022) found that the relative abundance of CHKCI001 in fresh greenish-yellow faeces from Xuefeng black-bone chickens had benefits in improving laying performance and feed conversion ratio. On the other hand, increasing the abundance of Methanobrevibacter has been linked to improving energy capture and fat accumulation. Recent studies have suggested that higher levels of Methanobrevibacter are correlated with increased abdominal fat in chickens, while lower levels are associated with reduced fat accumulation (Wen et al., 2019; Xiang et al., 2021).
To the best of our knowledge, this study introduces novel evidence indicating the potential of PSP as a beneficial feed supplement in enhancing broiler growth and health. The inclusion of PSP showed potent antimicrobial and antioxidant activities, positively influencing broilers’ growth without adverse effects on meat quality and digestive physiology. The recommended inclusion levels of 800 mg/kg yielded promising results. However, further research is necessary to comprehensively understand the mechanisms involved and the optimal PSP inclusion levels in broiler production.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by Talent Introduction Program of Anhui Science and Technology University (No. DKYJ202003), The Open Project of State Key Laboratory of Tea Plant Biology and Utilization (No. SKLTOF20230121), The Open Project of Anhui Province Key Laboratory of Embryo Development and Reproductive Regulation (No. FSKFKT011) and The Open Project of Longyan University & Fujian Provincial Key Laboratory for Prevention and Control of Animal Infectious Diseases and Biotechnology (No. ZDSYS2023003). The authors extend their appreciation to the Deputyship for Research and Innovation, ”Ministry of Education” In Saudi Arabia for funding this research number (IFKSUOR3-413-2).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abd El-Hack M.E., Alagawany M., Abdel-Moneim A.-M.E., Mohammed N.G., Khafaga A.F., Bin-Jumah M., Othman S.I., Allam A.A., Elnesr S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 2020;9:210–221. doi: 10.3390/antibiotics9050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;33:1668–1677. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Moneim E.A., El-Wardany I., Abu-Taleb A.M., Wakwak M.M., Ebeid T.A., Saleh A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins. 2020;12:439–450. doi: 10.1007/s12602-019-09549-2. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Selim D.A., Basuony H.A., Sabic E.M., Saleh A.A., Ebeid T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020;52:671–680. doi: 10.1007/s11250-019-02055-1. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020;104:1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., El-Ghoul A.A., Aldhumri S.A., Gabr S.A., Mesalam N.M., Elbaz A.M. Nutritional manipulation to combat heat stress in poultry–a comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Selim D.A., El-Saadony M.T., Mesalam N.M., Saleh A.A. Spirulina platensis and biosynthesized selenium nanoparticles improve performance, antioxidant status, humoral immunity and dietary and ileal microbial populations of heat-stressed broilers. J. Therm. Biol. 2022;104 doi: 10.1016/j.jtherbio.2022.103195. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Siddiqui S.A., Shehata A.M., Biswas A., Abougabal M.S., Kamal A.M., Mesalam N.M., Elsayed M.A., Yang B., Ebeid T.A., Teng X. Impact of light wavelength on growth and welfare of broiler chickens: an overview and future perspective. Ann. Anim. Sci. 2023 [Google Scholar]
- Abdel-Moneim A.E., Elbaz A.M., Khidr R.E., Badri F.B. Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, ileum histomorphometry and microbial enumeration of broilers. Probiotics Antimicrob. Proteins. 2020;12:873–882. doi: 10.1007/s12602-019-09613-x. [DOI] [PubMed] [Google Scholar]
- Altıparmak I.H., Erkuş M.E., Sezen H., Demirbag R., Gunebakmaz O., Kaya Z., Sezen Y., Asoglu R., Dedeoglu I.H., Neselioglu S. The relation of serum thiol levels and thiol/disulphide homeostasis with the severity of coronary artery disease. Kardiologia Polska (Polish Heart J.) 2016;74:1346–1353. doi: 10.5603/KP.a2016.0085. [DOI] [PubMed] [Google Scholar]
- Bancroft J., Gamble M. Theory and Practice of Histological Techniques. 5th ed. Edinburgh Churchill Livingstone Publisher; London: 2002. [Google Scholar]
- Barrera-Rojas J., Gurubel-Tun K.J., Ríos-Castro E., López-Méndez M.C., Sulbarán-Rangel B. An initial proteomic analysis of biogas-related metabolism of euryarchaeota consortia in sediments from the Santiago river, México. Microorganisms. 2023;11:1640. doi: 10.3390/microorganisms11071640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Scapini L., Ribeiro M., Pivetta M., Buzim R., Fernandes J. Effects of β-mannanase supplementation on the intestinal microbiota composition of broiler chickens challenged with a coccidiosis vaccine. Livestock Sci. 2019;228:187–194. [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chen D., Liang J., Jiang C., Wu D., Huang B., Teng X., Tang Y. Mitochondrion participated in effect mechanism of manganese poisoning on heat shock protein and ultrastructure of testes in chickens. Biol. Trace Element Res. 2023;201:1432–1441. doi: 10.1007/s12011-022-03259-7. [DOI] [PubMed] [Google Scholar]
- Chen D., Shen F., Liu J., Tang H., Teng X., Yang F., Liu H. Luteolin enhanced antioxidant capability and induced pyroptosis through NF-κB/NLRP3/Caspase-1 in splenic lymphocytes exposure to ammonia. Sci. Total Environ. 2024;919 doi: 10.1016/j.scitotenv.2024.170699. [DOI] [PubMed] [Google Scholar]
- Chen D., Yu W., Hao Z., Qiu M., Cui J., Tang Y., Teng X., Liu Y., Liu H. Molecular mechanism of selenium against lead-induced apoptosis in chicken brainstem relating to heat shock protein, selenoproteins, and inflammatory cytokines. Ecotoxicol. Environ. Safety. 2024;272 doi: 10.1016/j.ecoenv.2024.116028. [DOI] [PubMed] [Google Scholar]
- Coyle C.H., Martinez L.J., Coleman M.C., Spitz D.R., Weintraub N.L., Kader K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radical Biol. Med. 2006;40:2206–2213. doi: 10.1016/j.freeradbiomed.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Cui J., Hao Z., Zhou Q., Qiu M., Liu Y., Liu Y., Teng X., Kang L. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol. Environ. Safety. 2023;258 doi: 10.1016/j.ecoenv.2023.114983. [DOI] [PubMed] [Google Scholar]
- da Teixeira C.S., de Carvalho G.G., Nicory I.C., Santos A.V., Dos Pina D.S., de Júnior J.E., de Araújo M.L., de Rufino L.M., Cirne L.G., Pires A.J. Evaluation of days of total collection and use of internal markers in nutritional trials with small ruminants. Trop. Anim. Health Prod. 2018;50:815–823. doi: 10.1007/s11250-017-1500-8. [DOI] [PubMed] [Google Scholar]
- Deng Y., Liu X., Yao Y., Xiao B., He C., Guo S., Tang S., Qu X. The potential role of palygorskite and probiotics complex on the laying performance and faecal microbial community in Xuefeng black-bone chicken. Italian J. Anim. Sci. 2022;21:1660–1669. [Google Scholar]
- Dosoky W.M., Zeweil H.S., Ahmed M.H., Zahran S.M., Shaalan M.M., Abdelsalam N.R., Abdel-Moneim A.-M.E., Taha A.E., El-Tarabily K.A., Abd El-Hack M.E. Impacts of onion and cinnamon supplementation as natural additives on the performance, egg quality, and immunity in laying Japanese quail. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeid T.A., Ketta M., Al-Homidan I.H., Barakat H., Abdel-Moneim A.-M.E. In ovo feeding of nutraceuticals and its role in adjusting the gastrointestinal tract, antioxidative properties, immunological response, and performance in poultry: an updated review. Czech J. Anim. Sci. 2023;68:1–16. [Google Scholar]
- Elbaz A.M., Ahmed A.M., Abdel-Maqsoud A., Badran A.M., Abdel-Moneim A.-M.E. Potential ameliorative role of Spirulina platensis in powdered or extract forms against cyclic heat stress in broiler chickens. Environ. Sci. Poll. Res. 2022;29:45578–45588. doi: 10.1007/s11356-022-19115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A.M., Ashmawy E.S., Salama A.A., Abdel-Moneim A.-M.E., Badri F.B., Thabet H.A. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022;18:1–12. doi: 10.1186/s12917-022-03530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A.M., Farrag B., Mesalam N.M., Basuony H.A., Badran A.M., Abdel-Moneim A.-M.E. Growth performance, digestive function, thyroid activity, and immunity of growing rabbits fed olive cake with or without Saccharomyces cerevisiae or citric acid. Trop. Anim. Health Prod. 2023;55:376. doi: 10.1007/s11250-023-03794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A.M., Ibrahim N.S., Shehata A.M., Mohamed N.G., Abdel-Moneim A.-M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021;53:1–10. doi: 10.1007/s11250-021-02554-0. [DOI] [PubMed] [Google Scholar]
- Erkus M.E., Altiparmak I.H., Akyuz A.R., Demirbag R., Sezen Y., Gunebakmaz O., Neselioglu S., Erel O. The association between plasma thiol levels and left ventricular diastolic dysfunction in patient with hypertension. Scand. J. Clin. Lab. Investig. 2015;75:667–673. [PubMed] [Google Scholar]
- Fan Y., Zhao L., Ma Q., Li X., Shi H., Zhou T., Zhang J., Ji C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013;59:748–753. doi: 10.1016/j.fct.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Hou L., Qiu H., Sun P., Zhu L., Chen F., Qin S. Selenium-enriched Saccharomyces cerevisiae improves the meat quality of broiler chickens via activation of the glutathione and thioredoxin systems. Poult. Sci. 2020;99:6045–6054. doi: 10.1016/j.psj.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-c., Cao Q.-q., Cao Y.-b., Yang Y.-r., Xu T.-t., Yue K., Liu F., Tong Z.-x., Wang X.-b. Morinda officinalis polysaccharides improve meat quality by reducing oxidative damage in chickens suffering from tibial dyschondroplasia. Food Chem. 2021;344 doi: 10.1016/j.foodchem.2020.128688. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Lv Y., Dai W., Miao X., Zhong D. Extraction and bioactivity of polygonatum polysaccharides. Int. J. Biol. Macromol. 2013;54:131–135. doi: 10.1016/j.ijbiomac.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Juncher D., Rønn B., Mortensen E., Henckel P., Karlsson A., Skibsted L., Bertelsen G. Effect of pre-slaughter physiological conditions on the oxidative stability of colour and lipid during chill storage of pork. Meat Sci. 2001;58:347–357. doi: 10.1016/s0309-1740(00)00156-x. [DOI] [PubMed] [Google Scholar]
- Kundi H., Ates I., Kiziltunc E., Cetin M., Cicekcioglu H., Neselioglu S., Erel O., Ornek E. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am. J. Emerg. Med. 2015;33:1567–1571. doi: 10.1016/j.ajem.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Li J., Liu Y., Niu J., Jing C., Jiao N., Huang L., Jiang S., Yan L., Yang W., Li Y. Supplementation with paraformic acid in the diet improved intestinal development through modulating intestinal inflammation and microbiota in broiler chickens. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.975056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang X., Ren L., Li J., Zhu X., Xing T., Zhang L., Gao F., Zhou G. Protective effects of γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhao X., Wang J. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Li X., Abdel-Moneim A.-M.E., Mesalam N.M., Yang B. Effects of lysophosphatidylcholine on jejuna morphology and its potential mechanism. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.911496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chen S., Zhao Z.-T., Zhao M., Han Y., Ye X.-M., An Q., Ouyang K.-H., Wang W.-J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020;155:61–70. doi: 10.1016/j.ijbiomac.2020.03.198. [DOI] [PubMed] [Google Scholar]
- Lin W.C., Lee T.T. Effects of Laetiporus sulphureus-fermented wheat bran on growth performance, intestinal microbiota and digesta characteristics in broiler chickens. Animals. 2020;10:1457. doi: 10.3390/ani10091457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Sun J., Jiang S., Jiao N., Huang L., Yuan X., Guan Q., Li Y., Yang W. Effects of dietary isoleucine supplementation on the production performance, health status and cecal microbiota of Arbor Acre broiler chickens. Microorganisms. 2023;11:236. doi: 10.3390/microorganisms11020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li L., Li C., Wang H., Zhang X., Ren Q., Zhang H., Jin N., Li C., Zhao C. Effects of Lactiplantibacillus plantarum LPJZ-658 Supplementation on the Production, Meat Quality, Intestinal Morphology, and Cecal Microbiota of Broilers Chickens. Microorganisms. 2023;11:1549. doi: 10.3390/microorganisms11061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Dong Z., Zhu X., Xu H., Zhao Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018;107:796–802. doi: 10.1016/j.ijbiomac.2017.09.051. [DOI] [PubMed] [Google Scholar]
- Liu W.-C., Guo Y., An L.-L., Zhao Z.-H. Protective effects of dietary betaine on intestinal barrier function and cecal microbial community in indigenous broiler chickens exposed to high temperature environment. Environ. Sci. Poll. Res. 2021;28:10860–10871. doi: 10.1007/s11356-020-11326-6. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li S., Wang X., Xing T., Li J., Zhu X., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021;100:273–282. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang X., Ou S., Arowolo M.A., Hou D.-X., He J. Effects of Achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polymers. 2018;10:1233. doi: 10.3390/polym10111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Kang B., Jiang Q., Chen J. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Zhang J., Zhang Y. The functional activities and application of polygonatum sibiricum polysaccharides. J. Food Safety Quality. 2013;4:273–278. [Google Scholar]
- Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S.-I., Lee Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analyt. Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mesalam N.M., Aldhumri S.A., Gabr S.A., Ibrahim M.A., Al-Mokaddem A.K., Abdel-Moneim A.-M.E. Putative abrogation impacts of Ajwa seeds on oxidative damage, liver dysfunction and associated complications in rats exposed to carbon tetrachloride. Mol. Biol. Rep. 2021;48:5305–5318. doi: 10.1007/s11033-021-06544-1. [DOI] [PubMed] [Google Scholar]
- Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., He J., Zhao J., Wu Y., Shi X., Du L., Nong M., Zong S., Zeng G. Polygonatum sibiricum polysaccharide promotes osteoblastic differentiation through the ERK/GSK-3 β/β-catenin signaling pathway in vitro. Rejuv. Res. 2018;21:44–52. doi: 10.1089/rej.2017.1956. [DOI] [PubMed] [Google Scholar]
- Ransy C., Vaz C., Lombès A., Bouillaud F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020;21:9149. doi: 10.3390/ijms21239149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Li J., Xiao Y., Zhang Y., Fan J., Zhang B., Wang L., Shen X. Polysaccharide from Lycium barbarum L. leaves enhances absorption of endogenous calcium, and elevates cecal calcium transport protein levels and serum cytokine levels in rats. J. Functional Foods. 2017;33:227–234. [Google Scholar]
- Saleh A.A., El-Tahan H.M., Shaban M., Morsy W.A., Genedy S., Alzawqari M.H., El-Tahan H.M., Shukry M., Ebeid T.A., El-Keredy A., Abdel-Moneim A.-M.E. Effect of dietary supplementation of betaine and organic minerals on growth performance, serum biochemical parameters, nutrients digestibility, and growth-related genes in broilers under heat stress. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C., Sun B., Dalloul R.A., Zhai Z., Sun P., Li M., Yang S., Luan W. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019;135 doi: 10.1016/j.micpath.2019.103621. [DOI] [PubMed] [Google Scholar]
- Shang X., Geng L., Yang J., Zhang Y., Xu W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotoxicol. Environ. Safety. 2021;225 doi: 10.1016/j.ecoenv.2021.112748. [DOI] [PubMed] [Google Scholar]
- Shehata A.M., Paswan V.K., Attia Y.A., Abdel-Moneim A.-M.E., Abougabal M.S., Sharaf M., Elmazoudy R., Alghafari W.T., Osman M.A., Farag M.R. Managing gut microbiota through in ovo nutrition influences early-life programming in broiler chickens. Animals. 2021;11:3491. doi: 10.3390/ani11123491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata A.M., Paswan V.K., Attia Y.A., Abougabal M.S., Khamis T., Alqosaibi A.I., Alnamshan M.M., Elmazoudy R., Abaza M.A., Salama E.A., Abdel-Moneim A.-M.E. In ovo inoculation of Bacillus subtilis and raffinose affects growth performance, cecal microbiota, volatile fatty acid, ileal morphology and gene expression, and sustainability of broiler chickens (Gallus gallus) Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.903847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu G., Xu D., Zhao J., Yin L., Lin J., Fu H., Tang H., Fang J., Peng X., Zhao X. Protective effect of Polygonatum sibiricum polysaccharide on cyclophosphamide-induced immunosuppression in chickens. Res. Vet. Sci. 2021;135:96–105. doi: 10.1016/j.rvsc.2020.12.025. [DOI] [PubMed] [Google Scholar]
- Siddiqui S.A., Bahmid N.A., Taha A., Abdel-Moneim A.-M.E., Shehata A.M., Tan C., Kharazmi M.S., Li Y., Assadpour E., Castro-Muñoz R. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock; purposes, techniques and applications. Adv. Colloid Interface Sci. 2022;308 doi: 10.1016/j.cis.2022.102772. [DOI] [PubMed] [Google Scholar]
- Sun Q., Liu Y., Teng X., Luan P., Teng X., Yin X. Immunosuppression participated in complement activation-mediated inflammatory injury caused by 4-octylphenol via TLR7/IκBα/NF-κB pathway in common carp (Cyprinus carpio) gills. Aquatic Toxicol. 2022;249 doi: 10.1016/j.aquatox.2022.106211. [DOI] [PubMed] [Google Scholar]
- Turell L., Radi R., Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Rad. Biol. Med. 2013;65:244–253. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlström A., Sayin S.I., Marschall H.-U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang M., Gou Z., Jiang S., Zhang Y., Wang M., Tang X., Xu B. The effect of Camellia oleifera cake polysaccharides on growth performance, carcass traits, meat quality, blood profile, and caecum microorganisms in yellow broilers. Animals. 2020;10:266. doi: 10.3390/ani10020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X., Xing T., Li J., Zhu X., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Li G., Zhang X., Wang Y., Qiang Y., Wang B., Zou J., Niu J., Wang Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydrate Polymers. 2022;291 doi: 10.1016/j.carbpol.2022.119524. [DOI] [PubMed] [Google Scholar]
- Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol. 2015;76:188–194. doi: 10.1016/j.ijbiomac.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wang Y., Qin S., Pen G., Chen D., Han C., Miao C., Lu B., Su C., Feng S., Li W. Potential ocular protection and dynamic observation of Polygonatum sibiricum polysaccharide against streptozocin-induced diabetic rats’ model. Exp. Biol. Med. 2017;242:92–101. doi: 10.1177/1535370216663866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sun W., Wu E., Wang K., Chen X., Cui Y., Zhang G., Lv F., Wang Y., Peng X. Polysaccharides from Abrus cantoniensis Hance modulate intestinal microflora and improve intestinal mucosal barrier and liver oxidative damage induced by heat stress. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.868433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie T., Lu Z., Duan X., Xie C., Gebeyew K., Yumei Z., Yin Y., Wu X. Dietary Enteromorpha polysaccharide enhances intestinal immune response, integrity, and caecal microbial activity of broiler chickens. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.783819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Yan W., Sun C., Ji C., Zhou Q., Zhang D., Zheng J., Yang N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019;13:1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Xiang H., Gan J., Zeng D., Li J., Yu H., Zhao H., Yang Y., Tan S., Li G., Luo C. Specific microbial taxa and functional capacity contribute to chicken abdominal fat deposition. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.643025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Zheng Y., Yang S., Zhang L., Guo S., Shi L., Xu Y., Jin X., Yan S., Shi B. Artemisia ordosica polysaccharide ameliorated LPS-induced growth inhibition and intestinal injury in broilers through enhancing immune-regulation and antioxidant capacity. J. Nutr. Biochem. 2023;115 doi: 10.1016/j.jnutbio.2023.109284. [DOI] [PubMed] [Google Scholar]
- Yadav S., Caliboso K.D., Nanquil J.E., Zhang J., Kae H., Neupane K., Mishra B., Jha R. Cecal microbiome profile of Hawaiian feral chickens and pasture-raised broiler (commercial) chickens determined using 16S rRNA amplicon sequencing. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Li X., Baran A.M., Abdel-Moneim A.-M.E. Effects of dietary incorporation of Radix rehmanniae praeparata polysaccharide on growth performance, digestive physiology, blood metabolites, meat quality, and tibia characteristics in broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Sun X., Li C., Wu X., Yao J. Effects of copper, iron, zinc, and manganese supplementation in a corn and soybean meal diet on the growth performance, meat quality, and immune responses of broiler chickens. J. Appl. Poult. Res. 2011;20:263–271. [Google Scholar]
- Zhang H., Cao Y., Chen L., Wang J., Tian Q., Wang N., Liu Z., Li J., Wang N., Wang X. A polysaccharide from Polygonatum sibiricum attenuates amyloid-β-induced neurotoxicity in PC12 cells. Carbohydrate Polymers. 2015;117:879–886. doi: 10.1016/j.carbpol.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-T., Ye X.-M., Ouyang K.-H., Wang W.-J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020;155:61–70. doi: 10.1016/j.ijbiomac.2020.03.198. [DOI] [PubMed] [Google Scholar]
- Zhu L., Lu X., Liu L., Voglmeir J., Zhong X., Yu Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020;51:1–9. doi: 10.1186/s13567-020-00755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]