Abstract
INTRODUCTION
People with Down syndrome (DS) have a 75% to 90% lifetime risk of Alzheimer's disease (AD). AD pathology begins a decade or more prior to onset of clinical AD dementia in people with DS. It is not clear if plasma biomarkers of AD pathology are correlated with early cognitive and functional impairments in DS, and if these biomarkers could be used to track the early stages of AD in DS or to inform inclusion criteria for clinical AD treatment trials.
METHODS
This large cross‐sectional cohort study investigated the associations between plasma biomarkers of amyloid beta (Aβ)42/40, total tau, and neurofilament light chain (NfL) and cognitive (episodic memory, visual–motor integration, and visuospatial abilities) and functional (adaptive behavior) impairments in 260 adults with DS without dementia (aged 25–81 years).
RESULTS
In general linear models lower plasma Aβ42/40 was related to lower visuospatial ability, higher total tau was related to lower episodic memory, and higher NfL was related to lower visuospatial ability and lower episodic memory.
DISCUSSION
Plasma biomarkers may have utility in tracking AD pathology associated with early stages of cognitive decline in adults with DS, although associations were modest.
Highlights
Plasma Alzheimer's disease (AD) biomarkers correlate with cognition prior to dementia in Down syndrome.
Lower plasma amyloid beta 42/40 was related to lower visuospatial abilities.
Higher plasma total tau and neurofilament light chain were associated with lower cognitive performance.
Plasma biomarkers show potential for tracking early stages of AD symptomology.
Keywords: adaptive behavior, adults, cognitive performance, functional abilities, neurofilament light chain, plasma amyloid beta, plasma total tau, trisomy 21
1. INTRODUCTION
Adults with Down syndrome (DS) have a 75% to 90% likelihood of developing Alzheimer's disease (AD), with an average age of clinical dementia onset in the mid‐50s. 1 , 2 DS is characterized by the full or partial triplication of chromosome 21, which includes the amyloid precursor protein (APP) gene. The overexpression of APP is posited to be a driver of the near‐universal presence of AD pathology in individuals with DS by the fourth decade of life. 3 , 4 , 5 , 6 As in autosomal dominant AD 7 and sporadic late onset AD, 8 AD pathology begins a decade or more prior to the onset of clinical dementia in DS. 9 , 10 To advance clinical AD interventions in DS, there is a need to identify biomarkers of early disease progression that correspond to initial cognitive or functional impairments and could predict time to dementia onset. Blood biomarkers are appealing given that they are minimally invasive and of low cost relative to neuroimaging and cerebrospinal fluid (CSF) biomarkers. 11
The AT(N) framework was developed to stage biomarkers of AD progression in autosomal dominant and sporadic late onset AD. 12 In this framework, the aggregation of amyloid beta (Aβ; A) is followed by the accumulation of tau (T) and then neurodegeneration (N). 12 , 13 This framework has shown utility for modeling the progression of biomarkers of AD pathology in DS. 14 In DS, neuroimaging‐derived positron emission tomography (PET), magnetic resonance imaging (MRI), and CSF biomarkers indicating elevated Aβ, tau, and presence of neurodegeneration have been shown to be associated with clinical AD dementia status. 15 , 16 In addition, prior to clinical dementia onset, biomarkers of AT(N) have been shown to be correlated with early cognitive decline in DS. 17 For example, higher neocortical PET Aβ ([C‐11]Pittsburgh compound B) was reported to be associated with a faster rate of decline per year in episodic memory, visual attention, and visuospatial performance. 18 , 19 Recent work has also shown that change in CSF and PET biomarkers of Aβ have a similar trajectory in DS as has been found in autosomal dominant AD. 20 , 21 , 22
Less is known about the ability of blood‐based AT(N) biomarkers to predict the time course of unfolding of AD clinical symptomology in DS. In non‐DS populations, plasma biomarkers of Aβ40 and 42, tau, and neurofilament light chain (NfL) distinguish individuals with versus without a clinical status of AD dementia. 23 , 24 A combination of plasma Aβ42/40, phosphorylated tau (p‐tau)217, and NfL also predicted cognitive decline and progression to AD dementia over a 6‐year interval. 24 In DS, higher plasma total tau and NfL were reported to be able to distinguish adults with DS with and without clinical AD dementia. 15 , 25 However, to date, only a handful of studies have examined whether plasma AD biomarkers correlate with early cognitive or functional impairment prior to dementia onset. Lower plasma Aβ42 and Aβ42/40 was associated with cognitive decline over a 2‐year interval 26 and conversion to AD over a 4‐year interval; 27 , 28 however, other studies reported that higher plasma Aβ42 concentrations were associated with AD dementia in people with DS. 28 , 29 Plasma biomarkers of neurodegeneration in DS research have focused on NfL, a non‐specific measure of neuroaxonal injury. 30 Cross‐sectional models of age trajectories suggest that plasma NfL increases early in the progression toward AD, aligning in time with decreases in CSF Aβ and increases in CSF p‐tau and plasma tau in DS. 10 , 31 In a sample of 24 teens and adults with DS (aged 16–57 years), higher plasma NfL was associated with decline across 1 year in overall everyday living skills. 32
The current study investigated whether plasma Aβ42/40, total tau, and NfL were associated with AD‐related cognitive and functional impairments prior to dementia onset in DS. Analyses involved 260 adults with DS without dementia from the Alzheimer Biomarker Consortium–Down Syndrome (ABC‐DS). In line with prior research in DS, 15 , 25 , 26 a lower plasma Aβ42/Aβ40 ratio and higher total tau and NfL concentrations were hypothesized to be associated with lower cognitive performance and functional ability.
RESEARCH IN CONTEXT
Systematic review: Blood‐based biomarkers can distinguish individuals with Down syndrome (DS) with versus without a clinical status of Alzheimer's disease (AD) dementia. Yet, few studies have examined the association between plasma AD biomarkers and early cognitive and functional impairments prior to dementia in DS.
Interpretation: Our findings identified an association between plasma biomarkers of amyloid beta 42/40, total tau, and neurofilament light chain and cognitive performance in adults with DS without dementia.
Future directions: Longitudinal studies are needed to examine changes in cognition and functional ability over time in relation to change in plasma AD biomarker levels. Incorporating additional plasma biomarkers and establishing thresholds for different stages of disease progression is an important next step in establishing utility of plasma biomarkers for predicting time to dementia onset in DS.
2. METHODS
2.1. Participants
Participants were 260 adults with DS, aged 25 to 81 years old, who participated in a baseline study visit from the multisite ABC‐DS. 33 Informed consent or assent was obtained prior to study activities. Inclusion criteria for the broader study were age ≥ 25 years, genetic confirmation of DS (trisomy 21, mosaicism, or translocation), and no conditions that precluded brain imaging. A research team involving a psychologist, physician, and at least one other team member determined AD diagnostic status in a case consensus meeting that used information from cognitive testing, caregiver report, physical examination, and medical history (see Handen et al. for details on consensus process). 33 AD diagnostic status categories included cognitively stable, mild cognitive impairment–Down syndrome (MCI‐DS), AD dementia, and unable to determine. The current study included cognitively stable (n = 219) and MCI‐DS (n = 41) groups (total n = 260). Adults with DS with a diagnostic status of AD dementia or unable to be determined were excluded. Table 1 displays participant demographics.
TABLE 1.
Descriptive statistics for participant demographics and plasma biomarkers.
| M (SD)/ n (%) | ||||
|---|---|---|---|---|
| Total sample (n = 260) | Cognitively stable (n = 219) | MCI‐DS (n = 41) | χ2 or t value | |
| Chronological age | 43.74 (9.51) | 42.14 (9.06) | 52.27 (7.10) | −7.99 ** |
| Sex, n (%) male | 144 (55.4%) | 115 (52.5%) | 29 (70.7%) | 4.64 * |
| Race | ||||
| White | 249 (95.7%) | 211 (96.3%) | 38 (92.7%) | 6.48 * |
| Black | 3 (1.2%) | 1 (0.5%) | 2 (4.9%) | |
| Asian | 3 (1.2%) | 2 (0.9%) | 1 (2.4%) | |
| Unreported | 5 (1.9%) | 5 (2.3%) | – | |
| Ethnicity | ||||
| Non‐Hispanic | 248 (95.4%) | 207 (94.5%) | 41 (100%) | 2.36 |
| Hispanic | 12 (4.6%) | 12 (5.5%) | – | |
| Down syndrome type | ||||
| Trisomy 21 | 223 (85.8%) | 189 (86.3%) | 34 (82.9%) | 2.45 |
| Mosaicism | 9 (3.5%) | 6 (2.7) | 3 (7.3%) | |
| Translocation | 18 (6.9%) | 16 (7.3%) | 2 (4.9%) | |
| Unreported | 10 (3.8%) | 8 (3.7%) | 2 (4.9%) | |
| Lifetime ID level | ||||
| Mild | 141 (54.2%) | 123 (56.2%) | 18 (43.9%) | 4.22 |
| Moderate | 97 (37.3%) | 76 (34.7%) | 21 (51.2%) | |
| Severe | 22 (8.5%) | 20 (9.1%) | 2 (4.9%) | |
| Cognitive/functional measures | ||||
| mCRT | 29.02 (9.17) | 31.17 (7.01) | 17.51 (10.75) | 7.84 ** |
| VMI | 15.79 (3.58) | 16.19 (3.43) | 13.68 (3.69) | 4.24 ** |
| Block Design (with Haxby) | 24.24 (11.14) | 25.49 (10.30) | 17.59 (13.12) | 3.65 ** |
| Vineland‐3 ABC | 47.47 (18.54) | 49.48 (18.42) | 36.73 (15.39) | 4.17 ** |
| Plasma biomarkers | ||||
| Plasma Aβ42/40 | 0.034 (0.004) | 0.034 (0.004) | 0.033 (0.005) | 1.45 |
| Plasma total tau (pg/mL) | 2.29 (0.77) | 2.21 (0.70) | 2.73 (0.97) | −3.96 ** |
| Plasma NfL (pg/mL) | 16.92 (10.72) | 15.31 (9.73) | 25.92 (11.62) | −6.09 ** |
Abbreviations: ABC, Adaptive Behavior Composite; Aβ, amyloid beta; ID, intellectual disability; mCRT, modified Cued Recall Test; MCI‐DS, mild cognitive impairment–Down syndrome; NfL, neurofilament light chain; SD, standard deviation; VMI, visual motor integration.
P < 0.05.
P < 0.01.
2.2. Procedures
During a multiday visit, participants completed a battery of directly administered cognitive assessments and a blood draw analyzed for Aβ40, Aβ42, total tau, and NfL concentrations. A study partner accompanied the participant to the study visit and completed a series of measures including the Vineland‐3 comprehensive caregiver interview, which assesses everyday functional abilities. A full listing of ABC‐DS procedures is described in Handen et al. 33
2.3. Measures
2.3.1. Cognitive performance
Three assessments were selected that have been shown to measure cognitive domains affected early in the course of dementia in DS. 34 , 35 These tests measured episodic memory (modified Cued Recall Test [mCRT]), visual–motor integration (Beery‐Buktenica Developmental Test of Visual‐Motor Integration Sixth Edition [VMI]), and visuospatial abilities (Block Design with Haxby extension). Rigorous training and certification processes ensured standardization of administration and scoring of measures across sites. 33
The mCRT 36 measures episodic memory. The task involves learning the names of 12 black‐and‐white line drawings of simple objects along with an associated semantic cue (e.g., “fruit” for the picture of grapes). Participants are then asked to freely recall as many items as possible and are provided the semantic cue for items not recalled. The total score is the sum of items remembered in the free recall and cued recall conditions over three trials (maximum score of 36). The mCRT total score is reliable and highly sensitive to AD dementia clinical status in adults with DS (area under the curve = 0.955). 34 , 36
The VMI 37 assesses integration of visual and motor abilities. Geometric forms are presented in a workbook and participants are required to copy each shape or design, with figures becoming increasingly more complex. The VMI has excellent test–retest reliability (α = 0.94) and shows decline in individuals that corresponds with dementia onset in people with DS. 35
Visuospatial construction was assessed with the Wechsler Memory Scale Fourth Edition (WISC‐IV) Block Design subtest and Haxby extension, 38 , 39 a downward extension of the WISC‐IV. Participants are asked to re‐create various set designs of blocks (from easy to more challenging) with scores based on accuracy of block placement/design within a time limit. Among people with DS, the WISC‐IV Block Design subtest has excellent test–retest reliability (α = 0.94) 35 and performance is sensitive to presence of AD dementia. 40 A total visuospatial construction score was obtained by adding the raw scores from the WISC‐IV and the Haxby extension.
2.3.2. Functional ability
The Vineland‐3 Comprehensive Caregiver Interview 41 is an informant‐based assessment of functional behavior across three domains: communication, daily living skills, and socialization. An overall Adaptive Behavior Composite (ABC) is calculated from the three domain scores. Caregivers respond using a three‐point Likert scale to indicate if the adult with DS could independently complete daily functional activities often, sometimes, or never. Vineland‐3 ABC standard scores have a mean of 100 (standard deviation [SD] = 15). The communication domain includes receptive, expressive, and written language. The daily living skills component comprises personal, domestic, and community subdomains. Socialization evaluates interpersonal relationships, play and leisure, and coping skills. The Vineland‐3 is commonly used in studies including adults with DS 42 and has high internal consistency (α = 0.90–0.98) and test–retest reliability (r = 0.76–0.89). 41
2.3.3. Plasma biomarkers
ABC‐DS blood collection and processing methods were harmonized across all eight ABC‐DS clinical sites. Blood was collected into a 10 mL ethylenediaminetetraacetic acid tube, then centrifuged for 10 minutes at 2000 × g at 4°C. The plasma fraction was aliquoted in 0.25 mL units to individual 0.5 mL siliconized cryovials and stored at −80°C at local ABC‐DS clinical performance sites. The vials were shipped from the local ABC‐DS sites on dry ice via overnight courier to the Institute for Translational Research at the University of North Texas Health Science Center for analysis. International samples were shipped using World Courier with dry ice replenished throughout shipping to ensure samples remained frozen. Assays were run using single‐molecule array (Simoa) technology on the HD‐1 analyzer with commercially available kits (Quanterix). Frozen samples were thawed, vortexed, and spun at 10,000 × g for 5 minutes. Next, the supernatant was transferred to a 96‐well plate and processed according to kit procedures. The time in storage from blood draw to analysis for all samples was < 3 years. Detailed plasma assay information can be found in Petersen et al. 25
2.3.4. Sociodemographic data
Participant age and biological sex were reported by the study partner. DS was established by blood karyotyping (trisomy 21, mosaicism, or translocation). Lifetime intellectual disability (ID) level was coded based on standardized IQ scores derived by the Stanford–Binet, Fifth Edition abbreviated battery IQ or medical records that documented IQ or intellectual level from testing during early adulthood (i.e., age ≥ 21 years) and prior to MCI‐DS. ID level was coded: mild (1), moderate (2), or severe/profound (3) based on IQ standard scores (mild: 50–69, moderate: 35–49, and severe/profound: < 35) or estimated age equivalent scores (mild: 9–14 years, moderate: 4–8 years, and severe/profound: ≤ 3 years).
2.3.5. Analysis plan
Dependent variables were examined for normality using qq plots, histograms, and descriptive statistics. The mCRT required a reversed natural log transformation due to skewness (ln [36 – total score] +1; skewness after transformation = 0.26). Participant characteristics, cognition/functional measures, and plasma biomarkers of the cognitively stable and MCI‐DS groups were compared using t tests and chi‐squared tests (as appropriate). Pearson correlations, Kruskal–Wallis tests, and point biserial correlations (as appropriate) were used to examine the association between sociodemographic variables and primary variables of interest (cognitive skills, functional abilities, and plasma AD biomarkers). Sociodemographic variables (biological sex, age, and ID) that were significantly associated with primary variables were controlled for in subsequent analyses. Site differences were also controlled for in models in line with best practice for multisite research. Generalized linear models, controlling for any relevant sociodemographic and site effects, were then conducted in SAS version 9.4 to examine the associations among plasma Aβ42/40, total tau, and NfL and cognitive and functional ability measures (mCRT, VMI, Block Design, and Vineland‐3 ABC). Secondary analyses were conducted to examine models in the cognitively stable group only (excluding those with MCI‐DS) to further understand how plasma AD biomarkers were associated with cognitive and functional ability before any cognitive decline was detectable.
3. RESULTS
Review of histograms and descriptive data indicated significant (i.e., ≥ 3 SDs from mean) outliers in the plasma biomarkers. This resulted in removal of two (1.0%) plasma Aβ42/40 cases, four (1.5%) plasma total tau cases, and three (1.2%) plasma NfL cases. Participants in the MCI‐DS group were older, had lower scores on the cognition/functional measures, and higher values for two of the plasma biomarkers (plasma total tau and NfL; Table 1). Cognitive scores (mCRT, VMI, Block Design) and functional ability scores (Vineland‐3 ABC) were significantly associated with age and lifetime ID level (Table 2). Plasma NfL was also associated with age. Additionally, there were significant differences in biological sex for the mCRT and plasma total tau, such that on average females scored 1.90 points higher than males on the mCRT and total tau concentrations were higher in females compared to males (0.19 mean difference). There were no other significant differences identified based on biological sex (Table 2). Thus, all subsequent analyses controlled for age, ID level, biological sex, and site.
TABLE 2.
Demographic variable Pearson correlations, Kruskal–Wallis tests, and point biserial correlations with cognitive skills, functional ability, and plasma AD biomarkers, n = 260.
| mCRT | VMI | Block design | Vineland‐3 ABC | Plasma Aβ42/40 | Plasma total tau | Plasma NfL | |
|---|---|---|---|---|---|---|---|
| Chronological age | −0.46 ** | −0.30 ** | −0.22 ** | −0.28 ** | −0.11 | 0.12 | 0.64 ** |
| Lifetime ID level a | 18.56 ** | 43.67 ** | 49.27 ** | 62.22 ** | 2.19 | 5.82 | 0.83 |
| Biological sex b | 0.14 * | −0.01 | −0.06 | 0.05 | 0.05 | 0.13 * | −0.01 |
Abbreviations: ABC, Adaptive Behavior Composite; Aβ, amyloid beta; AD, Alzheimer's disease; ID, intellectual disability; mCRT, modified Cued Recall Test; NfL, neurofilament light chain; VMI, visual motor integration.
P < 0.05.
P < 0.01.
Kruskal–Wallis test used due to categorical variable.
Point biserial correlations used due to dichotomous variable.
Table 3 shows the results of the generalized linear models examining the associations among plasma Aβ42/40, total tau, and NfL and cognitive and functional ability measures (mCRT, VMI, Block Design, and Vineland‐3 ABC), controlling for age, ID level, biological sex, and site. Lower plasma Aβ42/40 was related to lower Block Design performance, higher plasma total tau was significantly related to lower mCRT performance, and higher plasma NfL was significantly associated with lower Block Design and mCRT performance. Figure 1 shows partial correlation graphs adjusted for site, age, lifetime ID, and biological sex for all significant associations between the plasma biomarkers and cognitive measures.
TABLE 3.
General linear model results predicting cognitive skills and functional abilities in adults with DS prior to dementia, n = 260.
| β Estimate [95% CI] | ||||
|---|---|---|---|---|
| mCRT a | VMI | Block Design | Vineland‐3 ABC | |
| Site (vs. site 1) | ||||
| Site 2 | 0.279 [−0.188, 0.746] | −1.603 [−3.236, 0.030] | −5.227 ** [−10.083, −0.370] | −11.568 ** [−19.033, −4.103] |
| Site 3 | 0.048 [−0.388, 0.485] | −0.604 [−2.131, 0.922] | −0.540 [−5.078, 3.999] | −12.463 ** [−19.439, −5.487] |
| Site 4 | 0.842 ** [0.438, 1.246] | −1.757 [−3.169, −0.344] | −7.332 ** [−11.533, −3.131] | −4.451 [−10.908, 2.007] |
| Site 5 | 0.793 [−0.506, 2.092] | −0.281 [−4.827, 4.265] | 3.357 [−10.162, 16.877] | 8.097 [−12.682, 28.876] |
| Site 6 | 0.132 [−0.271, 0.535] | −0.686 [−2.096, 0.723] | 0.119 [−4.073, 4.311] | 0.879 [−5.564, 7.322] |
| Site 7 | −0.003 [−0.365, 0.359] | 0.283 [−0.984, 1.549] | −1.466 [−5.233, 2.301] | −1.480 [−7.270, 4.310] |
| Age | 0.042 ** [0.027, 0.057] | −0.072 ** [−0.126, −0.019] | −0.147 [−0.307, 0.013] | −0.383 ** [−0.628, −0.137] |
| Lifetime ID level (vs. mild) | ||||
| Moderate | 0.405 ** [0.164, 0.647] | −1.732 ** [−2.576, −0.887] | −6.590 ** [−9.101, −4.078] | −16.127 ** [−19.987, −12.267] |
| Severe/profound | 1.081 ** [0.663, 1.498] | −4.280 ** [−5.741, −2.820] | −15.994 ** [−20.337, −11.650] | −28.125 ** [−34.802, −21.449] |
| Biological sex (vs. male) | −0.236 * [−0.465, −0.008] | −0.316 [−1.114, 0.482] | −1.760 [−4.134, 0.614] | 0.558 [−3.091, 4.207] |
| Plasma Aβ42/40 | 7.339 [−18.406, 33.085] | 57.615 [−32.458, 147.689] | 280.604 * [12.726, 548.482] | 122.718 [−289.009, 534.444] |
| Site (vs. site 1) | ||||
| Site 2 | 0.268 [−0.187, 0.724] | −1.567 [−3.212, 0.078] | −5.452 ** [−10.354, −0.549] | −12.400 ** [−20.115, −4.685] |
| Site 3 | 0.091 [−0.331, 0.513] | −0.743 [−2.267, 0.781] | −2.185 [−6.726, 2.356] | −13.148 ** [−20.294, −6.001] |
| Site 4 | 0.824 ** [0.430, 1.218] | −1.798 [−3.221, −0.375] | −7.643 ** [−11.883, −3.403] | −4.680 [−11.352, 1.992] |
| Site 5 | 0.996 [−0.276, 2.269] | −0.269 [−4.863, 4.325] | 3.731 [−9.961, 17.422] | 8.126 [−13.419, 29.670] |
| Site 6 | 0.151 [−0.254, 0.557] | ‐0.827 [−2.291, 0.637] | 0.223 [−4.140, 4.586] | 0.761 [−6.104, 7.627] |
| Site 7 | 0.027 [−0.327, 0.381] | 0.200 [−1.077, 1.477] | −1.803 [−5.609, 2.003] | −1.672 [−7.661, 4.317] |
| Age | 0.038 ** [0.023, 0.053] | −0.077 ** [−0.131, −0.024] | −0.136 [−0.296, 0.024] | −0.289 * [−0.541, −0.038] |
| Lifetime ID level (vs. mild) | ||||
| Moderate | 0.378 ** [0.141, 0.614] | −1.777 ** [−2.630, −0.923] | −6.507 ** [−9.051, −3.964] | −15.185 ** [−19.187, −11.183] |
| Severe/profound | 0.983 ** [0.572, 1.395] | −4.154 ** [−5.640, −2.668] | −15.491 ** [−19.919, −11.063] | −27.074 ** [−34.042, −20.107] |
| Biological sex (vs. male) | −0.284 * [−0.511, −0.058] | −0.247 [−1.065, 0.570] | −1.578 [−4.014, 0.858] | 0.596 [−3.238, 4.430] |
| Plasma total tau | 0.271 ** [0.125, 0.417] | −0.102 [−0.631, 0.427] | −0.559 [−2.135, 1.016] | −1.673 [−4.152, 0.806] |
| Site (vs. site 1) | ||||
| Site 2 | 0.263 [−0.201, 0.728] | −1.651 * [−3.276, −0.026] | −5.211 ** [−10.045, −0.377] | −13.454 ** [−21.231, −5.677] |
| Site 3 | −0.034 [−0.465, 0.397] | −0.562 [−2.070, 0.947] | −0.724 [−5.211, 3.764] | −13.377 ** [−20.597, −6.157] |
| Site 4 | 0.781 ** [0.377, 1.185] | −1.857 * [−3.269, −0.444] | −6.953 ** [−11.155, −2.751] | −5.702 [−12.463, 1.058] |
| Site 5 | 0.497 [−0.810, 1.805] | 0.072 [−4.501, 4.645] | 8.885 [−4.717, 22.487] | 7.738 [−14.146, 29.622] |
| Site 6 | 0.109 [−0.290, 0.508] | −0.730 [−2.127, 0.667] | 0.242 [−3.912, 4.397] | 0.244 [−6.440, 6.928] |
| Site 7 | −0.038 [−0.399, 0.322] | 0.320 [−0.941, 1.581] | −1.020 [−4.770, 2.731] | −2.162 [−8.196, 3.872] |
| Age | 0.031 ** [0.013, 0.049] | −0.065 * [−0.128, −0.001] | 0.023 [−0.165, 0.212] | −0.318 * [−0.622, −0.015] |
| Lifetime ID level (vs. mild) | ||||
| Moderate | 0.373 ** [0.135, 0.610] | −1.704 ** [−2.535, −0.874] | −6.332 ** [−8.802, −3.862] | −15.358 ** [−19.332, −11.383] |
| Severe/profound | 1.041 ** [0.629, 1.452] | −4.190 ** [−5.630, −2.750] | −14.967 ** [−19.250, −10.685] | −27.989 ** [−34.879, −21.099] |
| Biological sex (vs. male) | −0.238 * [−0.465, −0.011] | −0.259 [−1.053, 0.534] | −1.449 [−3.808, 0.911] | 0.411 [−3.385, 4.207] |
| Plasma NfL | 0.015 * [0.001, 0.028] | −0.012 [−0.060, 0.036] | −0.228 ** [−0.371, −0.086] | 0.047 [−0.182, 0.277] |
Abbreviations: ABC, Adaptive Behavior Composite; Aβ, amyloid beta; CI, confidence interval; DS, Down syndrome; ID, intellectual disability; mCRT, modified Cued Recall Test; NfL, neurofilament light chain.
P < 0.05.
P < 0.01.
Reversed natural log transformation, positive coefficient means an inverse relationship.
FIGURE 1.
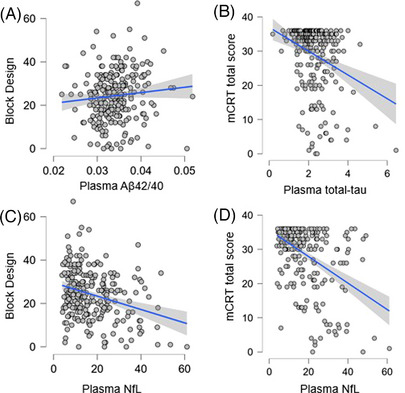
A, Plasma Aβ42/40 and Block Design. B, Plasma total tau and mCRT. C, Plasma NfL and Block Design. D, Plasma NfL and mCRT. Aβ, amyloid beta; mCRT, modified Cued Recall Test; NfL, neurofilament light chain
Secondary analyses were then conducted using the same generalized linear models described above but conducted with only the participants with a clinical status of cognitively stable (n = 219). When examining the cognitive stable group only, there was a significant negative association between plasma NfL and Block Design (F = 4.652, P = 0.032).
4. DISCUSSION
The current study drew on a large cohort of adults with DS without dementia and found that plasma biomarkers of Aβ42/40, total tau, and NfL had associations with cognitive abilities, after adjusting for age, lifetime ID level, and biological sex, and controlling for site effects. Higher plasma Aβ42/40 was related to higher visuospatial performance (Block Design), higher plasma total tau was associated with lower episodic memory performance (mCRT), and higher plasma NfL was related to both lower visuospatial performance (Block Design) and episodic memory performance (mCRT). Impairments in these cognitive domains have been shown in prior work to be among the earliest observed in the progression toward AD dementia in DS and in longitudinal studies the rate of decline in these cognitive domains has been shown to be similar for people with DS with mild versus moderate ID. 18 , 34 , 43 , 44 These findings build on recent efforts to establish imaging, CSF, and plasma AT(N) biomarkers related to early symptomology (i.e., cognitive and functional impairments) in the progression toward clinical dementia in people with DS. 10 , 18 , 19 , 32 , 45 , 46
The MCI‐DS group had significantly higher mean levels of plasma total tau and NfL than the cognitively stable group, and significantly lower cognitive scores. The relation between our plasma biomarkers and cognitive impairments was driven by differences between adults with DS with a clinical status of cognitively stable versus MCI‐DS, as only the association between higher plasma NfL and lower visuospatial performance remained significant when adults with MCI‐DS were excluded from analyses. This finding could signal that a higher NfL captures visuospatial ability differences unrelated to early AD pathology, in line with the idea that elevated plasma NfL is a non‐specific biomarker that has been linked to several neurodegenerative conditions. 30 Alternatively, cross‐sectional evidence from other large DS cohort studies 10 has suggested that plasma NfL increases could be part of the early trajectory toward AD dementia in DS. Future longitudinal research is needed to fully understand when and if early NfL changes have predictive value in understanding AD disease progression. In contrast to NfL, our cross‐sectional findings suggest that associations between plasma Aβ42/40 and total tau and cognitive impairments may not be evident until individuals with DS progress to MCI‐DS.
Our findings also suggest that impairments in visuospatial ability and episodic memory may be among the earliest in the progression to AD dementia in DS, given their association with plasma biomarkers prior to onset of AD dementia. Prior work from the ABC‐DS cohort identified episodic memory, and to a lesser extent visuospatial declines, to be closely associated with PET biomarkers of Aβ. 18 In the current study, higher plasma total tau was associated with lower episodic memory when examining adults with DS with a clinical status of cognitively stable and MCI‐DS, which also mirrors prior work showing that biomarkers of PET tau are associated with episodic memory declines prior to AD dementia in DS. 19 Functional ability has previously been found to be related to concentrations of plasma Aβ in young adults with DS. 47 However, we did not find significant associations between plasma markers and the Vineland‐3, and functional declines are not consistently described as being part of the early unfolding of AD in adults with DS, 9 albeit functional behavior is not always given sufficient attention.
The strength of the association between plasma Aβ42/40, total tau, and NfL and cognitive and functional impairments was modest. These modest associations may reflect that adults with DS are only expected to experience subtle cognitive impairments in the early stages of AD progression, prior to clinical dementia. PET imaging biomarkers of Aβ and tau have similarly been found to have meaningful but modest associations with cognitive performance prior to dementia onset in adults with DS. 18 , 19 As shown in Figure 1, there were cases in which low cognitive performance did not correspond with plasma Aβ42/40, total tau, and NfL values that would indicate risk for AD. For example, although overall performance on the Block Design was lower for adults with DS who had higher plasma NfL, there were individuals who had similarly low Block Design scores with low plasma NfL (Figure 1C). While this variance in test scores may be attributed to limitations of cognitive testing or the overall ID levels of adults with DS, another possible interpretation is that a single plasma biomarker (Aβ42/40, total tau, or NfL) alone is unlikely to be sufficient as an inclusion criterion or targeting engagement for clinical AD trials. Additional plasma AD biomarkers should be explored in future studies, as recent findings identify plasma p‐tau217 as an accurate indicator of PET Aβ and tau brain pathology. 48
This study has several strengths, including a large cohort of adults with DS and use of well‐established measures of cognitive performance and functional skills in the DS population. There were also limitations that should be considered when interpreting results. First, the study design was cross‐sectional and future studies would benefit from examining additional time points to determine changes in cognition and functional abilities over time and in relation to plasma AD biomarker levels. Second, the study relied on caregiver report of functional abilities and although the Vineland‐3 is the gold standard for assessing functional abilities in individuals with ID, there may have been variation in the amount of time each caregiver spent with the adult with DS that affected the accuracy of responses. Additionally, the participants in the study were primarily White and non‐Hispanic and future studies should investigate the relation between plasma AD biomarkers and cognition/functional ability in a sample that is more representative of the population of adults with DS. Future studies should incorporate imaging biomarkers of AD (e.g., PET Aβ and tau; MRI hippocampal volume) to corroborate and better understand their connection to plasma Aβ42/40, tau, and NfL. Studies are also warranted that investigate alternative plasma tau assays such as p‐tau181 or p‐tau217, as these biomarkers have recently been found to distinguish adults with DS with versus without clinical AD dementia. 48 , 49 It is important to note that participants with plasma biomarker values that were ≥ 3 SD from the sample mean were removed from analyses. However, these values may reflect valid data points, as sample and individual assay confidence intervals were within acceptable ranges. An understanding of expected variability in these biomarkers using larger DS populations is needed to guide future plasma biomarker research. Finally, in a recent DS study, plasma NfL differed by biological sex; 50 however, biological sex differences were only found with plasma total tau in the current analyses. It is not clear if this difference is due to differences in age range of study participants or other factors, but should be examined in future studies and samples.
In conclusion, plasma AD biomarkers are low burden and low cost relative to neuroimaging and CSF biomarkers 11 and thus are appealing for use in future clinical AD intervention trials in DS. Our cross‐sectional findings suggest that plasma Aβ42/40, total tau, and NfL have modest associations with early impairments in episodic memory and visuospatial ability prior to the onset of AD dementia and beyond what would be expected with age and lifetime ID level. Future longitudinal studies should determine whether these plasma biomarkers have utility in predicting timelines of within‐person cognitive declines and transition to clinical dementia in people with DS.
CONFLICT OF INTEREST STATEMENT
No authors have conflicts of interest to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Informed consent and/or assent was obtained for all participants.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to the ABC‐DS study participants, their families and care providers, and the ABC‐DS research and support staff for their contributions to this study. This manuscript has been reviewed by ABC‐DS investigators for scientific content and consistency of data interpretation with previous ABC‐DS study publications. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIHR, or Department of Health and Social Care. The Alzheimer Biomarkers Consortium–Down Syndrome (ABC‐DS) is funded by the National Institute on Aging and the National Institute for Child Health and Human Development (U01 AG051406, U01 AG051412, U19 AG068054). The work contained in this publication was also supported through the following National Institutes of Health Programs: The Alzheimer's Disease Research Centers Program (P50 AG008702, P30 AG062421, P50 AG16537, P50 AG005133, P50 AG005681, P30 AG062715, and P30 AG066519), the Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Program (U54 HD090256, U54 HD087011, and P50 HD105353), the National Center for Advancing Translational Sciences (UL1 TR001873, UL1 TR002373, UL1 TR001414, UL1 TR001857, UL1 TR002345), the National Centralized Repository for Alzheimer Disease and Related Dementias (U24 AG21886), and DS‐Connect (The Down Syndrome Registry) supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Research reported in this publication was also supported by NICHD under award number T32 HD007489. All research at the Department of Psychiatry in the University of Cambridge was supported by the NIHR Cambridge Biomedical Research Centre (NIHR203312) and the NIHR Applied Research Collaboration East of England.
Schworer EK, Handen BL, Petersen M, et al.; the Alzheimer Biomarker Consortium‐Down Syndrome . Cognitive and functional performance and plasma biomarkers of early Alzheimer's disease in Down syndrome. Alzheimer's Dement. 2024;16:e12582. 10.1002/dad2.12582
REFERENCES
- 1. Mccarron M, Mccallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61(9):843‐852. [DOI] [PubMed] [Google Scholar]
- 2. Iulita MF, Garzón Chavez D, Klitgaard Christensen M, et al. Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Netw Open. 2022;5(5):e2212910‐e2212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiseman FK, Al‐Janabi T, Hardy J, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16(9):564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonarakis SE, Skotko BG, Rafii MS, et al. Down syndrome. Nat Rev Dis Primers. 2020;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doran E, Keator D, Head E, et al. Down syndrome, partial trisomy 21, and absence of Alzheimer's disease: the role of APP. J Alzheimers Dis. 2017;56(2):459‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prasher VP, Farrer MJ, Kessling AM, et al. Molecular mapping of Alzheimer‐type dementia in Down's syndrome. Ann Neurol. 1998;43(3):380‐383. [DOI] [PubMed] [Google Scholar]
- 7. Mcdade E, Wang G, Gordon BA, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91(14):e1295‐e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchie K, Ritchie CW, Yaffe K, Skoog I, Scarmeas N. Is late‐onset Alzheimer's disease really a disease of midlife? Alzheimers Dement. 2015;1(2):122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019;15(3):135‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fortea J, Vilaplana E, Carmona‐Iragui M, et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross‐sectional study. Lancet North Am Ed. 2020;395(10242):1988‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montoliu‐Gaya L, Strydom A, Blennow K, Zetterberg H, Ashton NJ. Blood biomarkers for Alzheimer's disease in Down syndrome. J Clin Med. 2021;10(16):3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rafii MS, Ances BM, Schupf N, et al. The AT (N) framework for Alzheimer's disease in adults with Down syndrome. Alzheimers Dement. 2020;12(1):e12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortea J, Carmona‐Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross‐sectional study. Lancet Neurol. 2018;17(10):860‐869. [DOI] [PubMed] [Google Scholar]
- 16. Keator DB, Doran E, Taylor L, et al. Brain amyloid and the transition to dementia in Down syndrome. Alzheimers Dement. 2020;12(1):e12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Annus T, Wilson LR, Hong YT, et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement. 2016;12(5):538‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartley SL, Handen BL, Devenny D, et al. Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer's disease in Down syndrome. Alzheimers Dement. 2020;12(1):e12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartley SL, Handen BL, Tudorascu D, et al. Role of tau deposition in early cognitive decline in Down syndrome. Alzheimers Dement. 2022;14(1):e12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagan AM, Henson RL, Li Y, et al. Comparison of CSF biomarkers in Down syndrome and autosomal dominant Alzheimer's disease: a cross‐sectional study. Lancet Neurol. 2021;20(8):615‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boerwinkle AH, Gordon BA, Wisch J, et al. Comparison of amyloid burden in individuals with Down syndrome versus autosomal dominant Alzheimer's disease: a cross‐sectional study. Lancet Neurol. 2023;22(1):55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henson RL, Doran E, Christian BT, et al. Cerebrospinal fluid biomarkers of Alzheimer's disease in a cohort of adults with Down syndrome. Alzheimers Dement. 2020;12(1):e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simrén J, Leuzy A, Karikari TK, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. 2021;17(7):1145‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petersen ME, Rafii MS, Zhang F, et al. Plasma total‐tau and neurofilament light chain as diagnostic biomarkers of Alzheimer's disease dementia and mild cognitive impairment in adults with Down syndrome. J Alzheimers Dis. 2021;79(2):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iulita MF, Ower A, Barone C, et al. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: relation to cognitive decline and longitudinal evaluation. Alzheimers Dement. 2016;12(11):1132‐1148. [DOI] [PubMed] [Google Scholar]
- 27. Schupf N, Zigman WB, Tang M‐X, et al. Change in plasma Aß peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75(18):1639‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersen ME, O'bryant SE. Blood‐based biomarkers for Down syndrome and Alzheimer's disease: a systematic review. Dev Neurobiol. 2019;79(7):699‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schupf N, Patel B, Pang D, et al. Elevated plasma beta‐amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64(7):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zetterberg H. Neurofilament light: a dynamic cross‐disease fluid biomarker for neurodegeneration. Neuron. 2016;91(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 31. Hendrix JA, Airey DC, Britton A, et al. Cross‐sectional exploration of plasma biomarkers of Alzheimer's disease in Down syndrome: early data from the longitudinal investigation for enhancing Down syndrome research (LIFE‐DSR) study. J Clin Med. 2021;10(9):1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinomoto M, Kasai T, Tatebe H, et al. Plasma neurofilament light chain: a potential prognostic biomarker of dementia in adult Down syndrome patients. PLoS One. 2019;14(4):e0211575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Handen BL, Lott IT, Christian BT, et al. The Alzheimer's biomarker consortium‐Down syndrome: rationale and methodology. Alzheimers Dement. 2020;12(1):e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krinsky‐Mchale SJ, Hartley S, Hom C, et al. A modified cued recall test for detecting prodromal AD in adults with Down syndrome. Alzheimers dement. 2022;14(1):e12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krinsky‐McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer's dementia in adults with Down syndrome. Alzheimers Dement. 2020;12(1):e12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Devenny DA, Zimmerli EJ, Kittler P, Krinsky‐Mchale SJ. Cued recall in early‐stage dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46(Pt 6):472‐483. [DOI] [PubMed] [Google Scholar]
- 37. Beery KE, Buktenica NA, Beery NA. Beery‐Buktenica Developmental Test of Visual‐Motor Integration. 6th ed. Pearson Assessments; 2010. [Google Scholar]
- 38. Wechsler D. Wechsler Memory Scale, Fourth Edition: Administration and Scoring Manual. The Psychological Corporation; 2004. [Google Scholar]
- 39. Haxby JV. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non‐demented old adults. J Ment Defic Res. 1989;33:193‐210. [DOI] [PubMed] [Google Scholar]
- 40. Schapiro MB, Haxby JV, Grady CL. Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging. 1992;13(6):723‐734. [DOI] [PubMed] [Google Scholar]
- 41. Sparrow SS, Cicchetti DV, Saulnier CA. Vineland Adaptive Behavior Scales. 3rd ed. Pearson; 2016. [Google Scholar]
- 42. Hamburg S, Lowe B, Startin CM, et al. Assessing general cognitive and adaptive abilities in adults with Down syndrome: a systematic review. J Neurodev Disord. 2019;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aschenbrenner AJ, Baksh RA, Benejam B, et al. Markers of early changes in cognition across cohorts of adults with Down syndrome at risk of Alzheimer's disease. Alzheimers Dement. 2021;13(1):e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Videla L, Benejam B, Pegueroles J, et al. Longitudinal clinical and cognitive changes along the Alzheimer disease continuum in Down syndrome. JAMA Netw Open. 2022;5(8):e2225573‐e2225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartley SL, Handen BL, Devenny DA, et al. Cognitive functioning in relation to brain amyloid‐β in healthy adults with Down syndrome. Brain. 2014;137(9):2556‐2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zammit MD, Tudorascu DL, Laymon CM, et al. PET measurement of longitudinal amyloid load identifies the earliest stages of amyloid‐beta accumulation during Alzheimer's disease progression in Down syndrome. Neuroimage. 2021;228:117728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Del Hoyo L, Xicota L, Sánchez‐Benavides G, et al. Semantic verbal fluency pattern, dementia rating scores and adaptive behavior correlate with plasma Aβ42 concentrations in Down syndrome young adults. Front Behav Neurosci. 2015;9:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janelidze S, Christian BT, Price J, et al. Detection of brain tau pathology in Down syndrome using plasma biomarkers. JAMA Neurol. 2022;79(8):797‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lleó A, Zetterberg H, Pegueroles J, et al. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021;12(1):4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dang LHT, Krinsky‐McHale SJ, O'Bryant S, et al. Sex differences in levels of plasma neurofilament light and total tau in adults with Down syndrome. Alzheimers Dement. 2021;17:e055785. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


