Abstract
Tumor-associated tertiary lymphoid structures (TLS) have been associated with favorable clinical outcomes and response to immune checkpoint inhibitors in many cancer types, including non–small cell lung cancer. Although the detailed cellular and molecular mechanisms underlying these clinical associations have not been fully elucidated, growing preclinical and clinical studies are helping to elucidate the mechanisms at the basis of TLS formation, composition, and regulation of immune responses. However, a major challenge remains how to exploit TLS to enhance naïve and treatment-mediated antitumor immune responses. Here, we discuss the current understanding of tumor-associated TLS, preclinical models that can be used to study them, and potential therapeutic interventions to boost TLS formation, with a particular focus on lung cancer research.
Introduction
Immune checkpoint inhibitors (ICI) have significantly revolutionized the clinical management of many human malignancies, including non–small cell lung cancer (NSCLC). Despite these advantages, most patients with NSCLC normally fail to respond to treatment with ICIs irrespectively of PD-L1 expression or tumor mutational burden (TMB; refs. 1–3). Although several efforts have been made in this field, the identification of reliable predictors of response to ICIs, as well as of approaches to improve clinical outcomes, is still a major challenge (4). In this context, although most of the studies have been focused on T-cell populations as mainly mediators of treatment-naïve and ICI-mediated antitumor immune responses, mounting evidence reported a favorable impact of tumor-infiltrating B cells and plasma cells (which can be collectively referred as TIL-B) on prognosis and response to ICIs across many cancers (5–9), including NSCLC (10).
TIL-Bs normally reside in the tumor microenvironment (TME) in organized structures, known as tertiary lymphoid structures (TLS; refs. 11, 12). TLS can be generally defined as lymphoid aggregates surrounded by a stromal network and located in close proximity to specialized blood vessels [called high endothelial venules (HEV)] involved in lymphocyte trafficking and whose alteration in density or morphology can result in immune activation (13). In TLS, the coordinated presentation of neighboring tumor antigens by dendritic cells (DC), activation of T cells with cytotoxic function, and maturation of B cells toward antibody-secreting plasma cells, can generate both local and systemic antitumor immune responses, thus resulting in the clearance of adjacent tumor cells or distant metastases (12, 14).
Noteworthy, although TLS are mostly composed of T cells, B cells and DCs, other immune populations, such as regulatory T cells (Treg) and macrophages, have been found in tumor-associated TLS in some settings (15–17), and impact of TLS on survival and response to ICIs reportedly depends on both their cellular components and maturation stage, which can differ among cancer types (6, 17–19). Complicating this scenario, other factors seem to affect the impact of TLS on tumor progression (12), including (but not limited to) the local environment or TLS location, throughout mechanisms that are still poorly understood.
Overview of TLS Presence and Composition in Lung Cancer
In the setting of treatment-naïve human NSCLC, high density of TLS characterized by the presence of lysosome-associated membrane protein 3 (LAMP3)+ antigen-presenting mature DCs, together with T cells skewed toward a T helper 1 (Th1) and CD8+ cytotoxic T lymphocyte (CTL) phenotype (20), or follicular B cells (21), has been correlated to prolonged survival.
So far, some factors have been proposed to influence TLS development in lung cancers. In particular, IHC analysis of NSCLC specimens showed a predominant accumulation of PD-1+ CD8+ exhausted T cells in TLS near to T follicular helper (Tfh) cell and B-cell areas, proposing them as potential active players in the recruitment of immune cells by secreting C-X-C motif chemokine ligand 13 (CXCL13; ref. 22). In addition, data obtained by the TME characterization of untreated human lung squamous cell carcinoma (LSCC) tissues suggested that the lung parenchyma may provide a favorable environment for TLS development and germinal center (GC) B-cell maturation, involving CXCL13+ perivascular and TLS-associated stromal cells, C-C motif chemokine ligand 21 (CCL21)+ TLS-associated HEVs, and CXCL12+ hyperplastic alveolar epithelial cells (23). However, direct experimental evidence is needed to verify these assumptions.
Conversely, the presence of Tregs in tumor-associated TLS (but also in non-TLS areas) has been associated to reduced survival in patients with NSCLC (16), and to suppression of TLS-mediated antitumor immunosurveillance in a preclinical model of lung adenocarcinoma (LUAD; ref. 15). Interestingly, TLS formation and maturation can also be impaired by therapies with immunosuppressive properties, such as corticosteroids (23), which are administered to manage comorbidities or side effects of coadministered neoadjuvant chemotherapy. On the other hand, higher abundance of mature TLS have been observed in NSCLC tissues from patients treated with neoadjuvant chemoimmunotherapy, compared with both untreated patients or patients treated with neoadjuvant chemotherapy only (17), thus suggesting that ICIs may foster TLS formation. Supporting these clinical findings, treatment with a PD-1 blocker increased TLS abundance and Tfh-mediated B-cell activation in a subcutaneous mouse model of LSCC (24).
Finally, fascinating preclinical findings unraveling mechanisms by which TLS-associated B cells mediate naïve and ICI-mediated antitumor immunity in LUAD have been recently published by Ng and collaborators (25). In particular, by harnessing a novel mouse model of immunogenic LUAD the authors demonstrated that lung resident B cells associated to TLS contribute to response to immunotherapy through the production of antibodies targeting tumor antigens derived from endogenous retroviruses (ERV), and that ICI efficacy can be augmented by inducing TLS formation in a CXCL13-dependent manner (25).
Overall, these findings suggest that antitumor immunity mediated by TLS relies on CTL-mediated responses, as well as on GC B-cell maturation and production of tumor antigen–specific antibodies, at least in the setting of lung cancer. However, mechanisms by which TLS promote ICI efficacy still need to be fully elucidated. In this review, we discuss the latest interesting preclinical and clinical findings that have been made in the attempt to address these knowledge gaps and all the exciting potential developments for the management of cancer (in particular of NSCLC).
Appropriate Mouse Models to Study Tumor-Associated TLS
Immunocompetent mouse models are crucial for the investigation of anticancer immune responses, the preclinical development of immunotherapies, as well as the identification of mechanisms by which TLS mediate antitumor immunity. However, few studies reported the spontaneous development of tumor-associated TLS so far (Table 1), thus making it difficult to dissect TLS role in antitumor immunity and to identify effective therapeutic interventions able to foster their formation, maturity, and function, that could be successfully translated to the clinic. This is probably due to the intrinsic limitations of the vast majority of the available mouse models naturally hampering the study of both TLS development and immunotherapy efficacy (26).
Table 1.
Summary of preclinical models investigated for the spontaneous formation of tumor-associated TLS.
| Tumor model | Origin of the tumor | Tumor location | Time of TLS assessment | Method for TLS detection | Definition of TLS | Notes | Ref |
|---|---|---|---|---|---|---|---|
| Transplanted models | |||||||
| Metastatic B16-OVA melanoma | 4 × 105 B16-OVA cells injected i.v. in WT mice | Lung | 20 days after tumor injection | IF with antibodies directed against B220 (B cells), CD3 (T cells) and PNAd (endothelial cells) | Aggregates of ≥50 B cells in juxtaposition to PNAd+ vasculature | TLS formation not observed in s.c. tumors | (28) |
| Metastatic B16-OVA melanoma | 4 × 105 B16-OVA cells injected i.p. in WT mice | Peritoneal cavity | 14 days after tumor injection | IF with antibodies directed against B220, CD3 and PNAd | Aggregates of ≥50 B cells in juxtaposition to PNAd+ vasculature | Only less than 30% TLS show discrete T- and B-cell zones, a percentage that increases after treatment with ICIs | (28) |
| Metastatic LLC-OVA Lewis lung carcinoma | 4 × 105 LLC-OVA cells injected i.p. in WT mice | Peritoneal cavity | 14 days after tumor injection | IF with antibodies directed against B220, CD3 and PNAd | Aggregates of ≥50 B cells in juxtaposition to PNAd+ vasculature | TLS formation not observed in s.c. tumors | (28) |
| Metastatic MC38-OVA CCR | 4 × 105 MC38-OVA cells injected i.p. in WT mice | Peritoneal cavity | 14 days after tumor injection | IF with antibodies directed against B220, CD3 and PNAd | Aggregates of ≥50 B cells in juxtaposition to PNAd+ vasculature | TLS formation not observed in s.c. tumors | (28) |
| Metastatic UPK10 ovarian cancer | 6 × 106 UPK10 cells injected i.p. in CD4CreSatb1flox/flox mice | Peritoneal cavity | 14 days after tumor injection | IF with antibodies directed against CD19 (B cells), CD3 and PNAd | Aggregates of >0.1 mm2 with B cell zones adjacent to T cell zones in juxtaposition to PNAd+ areas and with a central core of T cells | Satb1-competent mice show small and poorly organized lymphoid aggregates, and conditional deletion of Satb1 in CD4+ T cells enhances TLS formation | (30) |
| Orthotopic CT-2A glioma | 5 × 104 CT-2A cells injected i.c. in WT mice | Brain | Ethical end point | IF with antibodies directed against B220, CD3 and Ki67 (proliferating cells) | Tight cluster of CD45+ cells containing B220+ B cells and CD3+ T cells | TLS are located within the meningeal regions in proximity to the tumor, and their formation is enhanced upon agonistic CD40 therapy or LIGHT-based therapy | (31, 32) |
| Orthotopic GL261 glioma | 2 × 104 GL261 cells injected i.c. in WT mice | Brain | Ethical end point | IF with antibodies directed against B220, CD3 and Ki67 | Tight cluster of CD45+ cells containing B220+ B cells and CD3+ T cells | TLS are located within meningeal regions, in proximity to the tumor, and their formation is enhanced upon LIGHT-based therapy | (31, 32) |
| Orthotopic KPAR LUAD | 1.5 × 105 KPARa cells injected i.v. in WT mice | Lung | Ethical end point | IF with antibodies directed against CD3, CD20 (B cells), and PNA (GC B cells) | Mature TLS defined as lymphoid aggregates with the presence of segregated T- and B-cell areas, as well as active GC responses (PNA+) | TLS formation is increased after treatment with a KRASG12C inhibitor; reported sensitivity to treatment with ICIs | (25) |
| Orthotopic KPC PDAC | 103 KPCb cells injected into the pancreas of WT mice | Pancreas | Not specified | IF with antibodies directed against CD3, B220 and CD21 (FDCs) | Copresence of B cells, T cells and FDCs in a compact organization | TLS develop in approximately 10% of orthotopic PDAC tumors and this percentage increases upon administration of CXCL13 and CCL21 | (34) |
| Subcutaneous KLN205 LSCC | KLN205 cells injected s.c. in WT mice | Flank | 14 days after treatment initiation | IHC with antibodies directed CD4 (CD4+ T cells) and CD20 | Dense and localized lymphocyte infiltrations inside tumors or in the invasive margins | Abundance of TLS increases upon treatment with an anti-PD-1 antibody | (24) |
| Tissue-specific GEMMs | |||||||
| KP LUAD KrasLSL-G12D/+ Trp53flox/flox | Induced by i.t. injection of Cre lentiviral vectors | Lung | 20 weeks after i.t. infection | IF with antibodies directed against CD3, CD20 and NKX2.1 (cancer cells) | B-cell clusters of >10 cells directly associated with T cells | TLS are rich of Tregs and TLS area increases after local Tregs depletion | (15) |
| KP-NINJA LUAD KrasLSL-G12D/+ Trp53flox/flox R26-NINJA/NINJACCSP-rtTA+ | Induced by i.t. injection of Cre lentiviral vectors (followed by systemic injection of Dox/Tam for antigen expression) | Lung | 8 weeks after i.t. infection | IF with antibodies directed against CD3, CD20 and Ninja (cancer cells) | B-cell clusters of ≥ 20 cells directly associated with T cells | Conditional expression of neoantigens increases the immunogenicity of KP LUADs and their sensitivity to treatment with ICIs | (33) |
| KPC PDAC KrasLSL-G12D/+ Trp53R172H/+ Pdx-1-Cre | De novo from 4–6 weeks of age | Pancreas + metastases to liver and lungs | Not specified | IF with antibodies directed against CD3, B220 and CD21 | Copresence of B, T, and FDCs in a compact organization | TLS develop in approximately 50% of KPC tumors | (34) |
| Germline GEMMs | |||||||
| Gastric cancer Gp130757flox/flox | De novo from approximately 6 weeks of age | Stomach | 3–6 months of age | IHC with antibodies directed against CD3 and B220 | Dense accumulations of T and B cells | TLS develop in the gastric submucosa | (35) |
| Carcinogen-induced models | |||||||
| AOM/DSS-induced inflammatory CRC | Chemically induced by sequential administration of AOM and DSS | Intestine | 9–12 weeks after AOM | IF with antibodies directed against CD4, CD19 and CD11c (DCs) | Dense accumulations of cells containing clear T- and B-cell zones | TLS mainly develop in normal tissue adjacent to the tumor and their maturation improves upon colonization with immunogenic intestinal bacteria | (38, 39) |
Abbreviations: AOM, azoxymethane; CRC, colorectal cancer; Dox/Tam, doxycycline/tamoxifen; DSS, dextran sodium sulfate; FDCs, follicular dendritic cells; IF, immunofluorescence; i.c., intracranially; i.p., intraperitoneally; i.t., intratracheal; i.v., intravenously; GC, germinal center; LSCC, lung squamous cell carcinoma; NINJA, inversion inducible joined neoantigen; PNA, peanut agglutinin; PNAd, peripheral node addressin; s.c., subcutaneous; WT, wild-type.
aKPAR cells were obtained from a tumor driven by the expression of oncogenic KRASG12D, deletion of Trp53, and the expression of a human APOBEC3B minigene (A3Bi) in the Rosa26 locus in lung epithelial cells in immunodeficient mice [i.e., KrasLSL-G12D/+;Trp53flox/flox;Rosa26A3Bi;Rag1−/− (KPAR) mice].
bKPC cells were obtained from a tumor established in KPC (KrasLSL-G12D/+;Trp53R172H/+;Pdx-1-Cre) mice.
Models based on the subcutaneous, orthotopic (to mimic the anatomic location of the disease), or systemic (intraperitoneally or intravenously—to monitor their metastatic spread) injection of murine-derived cancer cell lines in syngeneic immunocompetent mice, for example, are normally characterized by a rapid growth (faster than in cancer patients) that might not allow the time window necessary for the organization of lymphocytes in tumor-associated TLS. In addition, syngeneic tumor models do not allow the follow-up of the spontaneous course of the disease in the context of a proficient native immune microenvironment, as cell lines have already evaded immunosurveillance in their original host (26, 27).
So far, the development of spontaneous tumor-associated TLS in transplanted tumors has been reported in models of subcutaneously injected LSCC cells (24), or of intraperitoneally implanted murine melanoma, colon adenocarcinoma, and Lewis lung carcinoma (LLC) cells overexpressing ovalbumin (OVA; ref. 28), a tumor-specific antigen well known for its ability to enhance tumor immunogenicity. Interestingly, TLS were also observed in melanoma tumors established into the lung of syngeneic immunocompetent mice, but not subcutaneously, thus suggesting that TLS development might depend on the local environment rather than on the growth rate of transplanted cells, at least in this setting (28). The spontaneous assembly of well-organized TLS in peritoneal tumors has also been described for a model of metastatic ovarian cancer obtained by the intraperitoneal injection of Kras/Trp53-mutant UPK10 cells, only when Satb1 [encoding special AT-rich sequence binding protein 1, a genomic organizer implicated in regulating the phenotype and differentiation of different immune cells, including T-cell activation (29)], was knocked-out in CD4+ T cells (CD4CreSatb1flox/flox mice; ref. 30). Among orthotopic models, TLS occurrence has been reported in models of glioma obtained by the intracranial injection of Kras/Trp53-mutant GL261 cells or Pten-deficient CT2A cells (31, 32). Intriguingly, similarly to data obtained in human glioma and glioblastoma tissues, TLS were found in close proximity to the meningeal tissue in these models, rather than in the tumor mass or in the brain of tumor-free mice (31, 32).
The spontaneous development of tumor-associated TLS has also been described in some genetically engineered mouse models (GEMM), such as models of (i) LUAD and (ii) pancreatic ductal adenocarcinoma (PDAC) driven by the tissue-specific expression of KrasG12D mutation in combination with Trp53 deletion or mutations (15, 33, 34), as well as (iii) a mouse model of gastric cancer driven by a germline knock-in mutation in Il6st (also known as Gp130; gp130757flox/flox mice), encoding a mutated form of the IL6 receptor subunit and resulting in the hyperactivation of STAT3 (35).
Compared with transplanted models, GEMMs better recapitulate tumor development and progression as they develop spontaneous autochthonous tumors in a natural immune microenvironment (26, 27). However, transgene-driven tumors are mostly resistant to natural immunosurveillance and immunotherapy, due to their low TMB as compared with their human counterpart (36, 37). These limitations could be addressed by using carcinogen-induced tumor models, as they provide a higher level of genomic instability, resulting in the spontaneous development of more clinically relevant and heterogeneous tumors characterized by a high mutational load (27, 37). However, to the best of our knowledge, the occurrence of tumor-associated TLS has been only described in a model of colitis-associated colorectal cancer induced by azoxymethane and dextran sodium sulfate (38, 39). Notably, in this model, TLS form in normal tissue adjacent to the tumor and not within the tumor bed (38, 39).
In the context of lung cancer research, KrasLSL-G12D/+;Trp53flox/flox mice (hereafter referred to as KP mice) have been widely used as GEMMs that recapitulate key features of human LUAD. However, although the spontaneous formation of tumor-associated TLS has been described in KP mice bearing lung tumors (15), the intrinsic resistance to ICIs of this model (40) did not allow to study TLS involvement in mediating ICI efficacy. To address these issues, Ng and collaborators recently set out to study TLS formation and humoral responses by harnessing a novel immunogenic model of LUAD established by the orthotopic transplantation of LUAD (KPAR) cells into the lung of immunocompetent syngeneic wild-type mice (25). This model was previously generated and characterized by the same group by deriving KPAR cells from a single-cell clone of a lung tumor driven by the expression of the oncogene KrasG12D and Trp53 deletion, expressing the apolipoprotein B mRNA-editing catalytic subunit 3B (APOBEC3B) enzyme [a DNA cytosine deaminase responsible for inducing high mutational burden in human lung cancers (41)], and developed in an immune-deficient background [i.e., KrasLSL-G12D/+;Trp53flox/flox;Rosa26A3Bi;Rag1−/− (KPAR) mice] to avoid immunoediting (42). Differently to the widely used KP cells (i.e., KPB6 cells, derived from lung tumors established in KP mice), which differ from human LUADs for the low number of clonal somatic single-nucleotide variants, their knowingly resistance to treatment with ICIs and their nonimmunogenic phenotype (40), KPAR cells have higher TMB, increased immunogenicity, and are sensitive to therapy with PD-1, PD-L1, and CTLA4 blockers (25, 42). By harnessing this novel immunogenic LUAD model, Ng and collaborators were able to demonstrate the formation of perivascular mature TLS in proximity of KPAR tumors by immunofluorescence, while TLS were not detectable in commonly used KP (KPB6) tumors (25). Consistent with these findings, the presence of peritumoral TLS, linked to improved sensitivity to dual CTLA4 and PD-1 blockade, has also been described in mice bearing KP tumors made more immunogenic by inducing neoantigen expression in malignant cells (i.e., KP-NINJA mice; ref. 33).
Thus, the selection of an adequate mouse model that recapitulates key aspects of human immunogenic cancers (such as NSCLC) is fundamental to better understand (i) the cross-talk between tumor and immune cells, (ii) to investigate cellular and molecular mechanisms underlying TLS formation, composition, and their involvement in naïve and immunotherapy-driven antitumor immune responses, as well as (iii) to assess the antitumor efficacy of novel combinatorial immunologic interventions.
Current Understanding on TLS Role in Mediating Antitumor Immune Responses
Antigen-driven differentiation of GC B cells and B-T-cell collaboration
The presence of TLS-associated TIL-Bs has been associated with extended overall survival in patients with melanoma, soft-tissue sarcomas, renal cell carcinoma, and NSCLC, treated with ICIs (5, 6, 8–10), thus suggesting a role for TLS and humoral immunity in mediating ICI responses in these settings. Generally, TIL-Bs have been proposed to mediate antitumor immunity in both antibody- and cell-dependent ways, by producing antibodies that recognize tumor antigens and redirect cytotoxic cells [such as natural killer (NK) cells and macrophages] against tumor cells, or cross-presenting antigens to T cells and activating them (43). Ex vivo production of IgG and IgA antibodies against tumor antigens has been demonstrated for B cells isolated from human NSCLC and breast cancer biopsies (21, 44). Of note, B cells have been associated with the presence of TLS and improved disease outcomes in these settings (21, 44), and recent evidence suggested that TLS could be the drivers for the in situ generation of B cell–mediated responses (45). In particular, spatial transcriptomics of human clear-cell renal cell carcinomas showed all B-cell maturation stages (including the differentiation in IgG- and IgA-producing plasma cells) in tumor-associated TLS, and that plasma cells can disseminate into the tumor bed along fibers formed of CXCL12+ fibroblasts (6). TLS+ tumors also showed a higher infiltration of IgG-producing plasma cells linked to a higher number of IgG-labeled and apoptotic cancer cells, as well as macrophages, thus suggesting an antitumor effector activity for these antibodies (6).
In line with these findings, GC B-cell maturation is paralleled by increased levels of IgG and IgA antibodies specific for tumor antigens (specifically ERV envelope glycoproteins) in the serum of mice bearing immunogenic LUADs obtained by the orthotopic injection of KPAR cells, and transferred serum from donor KPAR-bearing mice to recipient KPAR-bearing mice significantly extended the overall survival of recipient mice (25). Interestingly, prolonged survival of recipient mice is linked to increased tumor infiltration by NK cells and antibody-dependent cellular cytotoxicity (ADCC)-mediated by NK cells, an effect that is abrogated by NK cell (but not CD8+ T cell) depletion, thus confirming the effector activity of tumor-specific antibodies and suggesting an independency on CD8+ T cell–mediated responses in this model (25). An absent cross-talk between B and CD8+ T cells has also been suggested by clinical findings obtained from the profiling of pretreatment tumors of patients with NSCLC progressed following platinum-based chemotherapy from randomized phase II (NCT01903993) and III (NCT02008227) trials evaluating the efficacy of the PD-L1 blocker atezolizumab versus docetaxel in NSCLC (10). Although B cells have been found in close proximity to CD8+ T cells organized in TLS in NSCLC specimens, Patil and colleagues (10) found that increased plasma cell signatures are predictive of extended overall survival for patients treated with PD-L1 blockade (but not with chemotherapy), independently of the presence of CD8+ T cells. Similarly, improved objective response (OR) rate to ICIs has been correlated to the presence of mature TLS (as defined by the presence of CD23+ follicular DCs) in several human cancers, including NSCLC, independently of CD8+ T-cell density and PD-L1 expression (18).
The contribution of B-cell responses to the efficacy of ICIs against NSCLC has also been investigated in the immunogenic mouse model of LUAD by Ng and collaborators. In particular, the authors showed that the antitumor activity of PD-L1 inhibition is linked to augmented avidity of serum IgG and IgA binding to lung cancer cells, as well as to increased levels of GC B cells in the lungs of mice bearing LUAD tumors (25). Interestingly, the expansion of GC B cells is always linked to enhanced CD4+ Tfh cell responses in this model, thus suggesting a potential B-Tfh-cell collaboration in mediating both naïve and ICI-mediated antitumor immune responses (25). However, the eventual collaboration between B and Tfh cells (as well as the impact of other cellular components) on TLS formation and ICI efficacy has not been investigated in this model (25). These questions have been partially answered by previous findings obtained in another mouse model of LUAD obtained by the subcutaneous injection of engineered KP cells expressing a fusion protein that can be recognized by both B and T cells (KP-HELLO cells; ref. 46). The introduction of tumor neoantigens in KP tumors led to the stimulation of specific B cells necessary for the differentiation of CD4+ Tfh cells, which in turn promoted antitumor CD8+ T-cell responses via IL21 secretion, thus providing the necessary stimuli to further support B-cell differentiation in plasma cells (46). However, these interactions occurred in tumor draining lymph nodes of mice bearing KP-HELLO tumors (46), thus B-T-cell interactions in LUAD-associated TLS, as well as whether T cell–mediated responses in KPAR tumors are elicited by the cross-presentation of tumor antigens by B cells or independently of B cells, still need to be elucidated. Along similar lines, Tfh cell–mediated B-cell activation, with consequent production of antitumor antibodies and enhanced T-cell priming, has also been described in a subcutaneous mouse model of LSCC and in a GEMM of triple-negative breast cancer (TNBC) characterized by a high TMB, after treatment with ICIs (24, 47). Noteworthy, while PD-1 blockade also increased TLS formation in LSCC tumors (24), the occurrence of TLS has not been investigated in the TNBC model (47).
Despite some mechanisms at the basis of TLS function in LUAD tumors still need additional investigations, the preclinical findings we discussed above nicely demonstrate potential mechanisms by which TLS are drivers of the in situ activation and maturation of T cells, B cells, and antibody-producing plasma cells, and how TLS may mediate tumor control by favoring T-B cell collaboration (Fig. 1A), thus significantly improving our understanding on LUAD immunology.
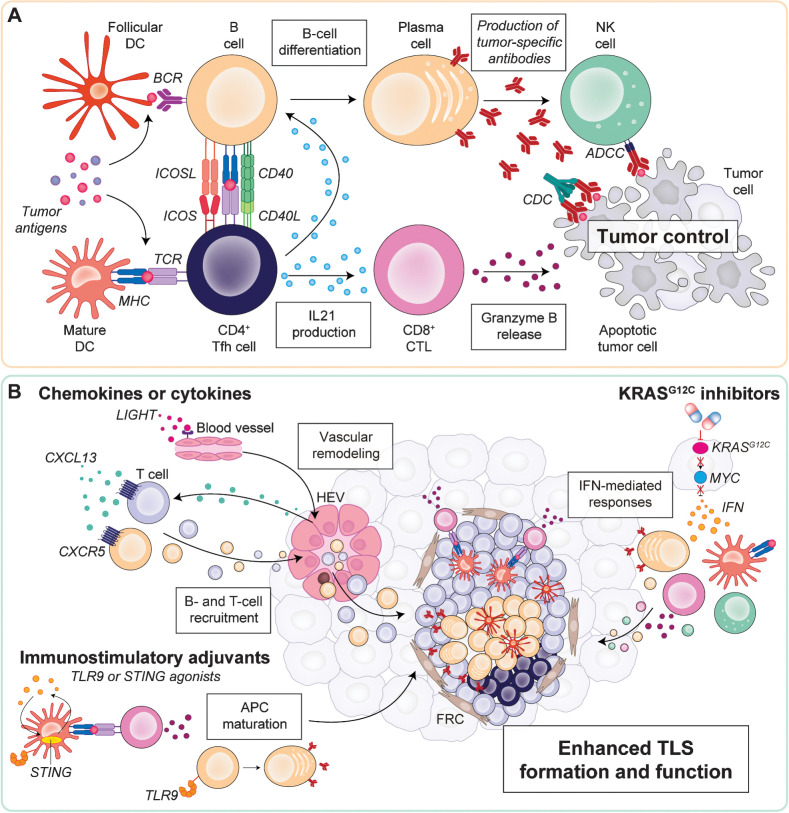
Figure 1.
A, Summary of mechanisms by which TLS mediate antitumor immunity. On the one hand, GC B cells recognize tumor antigens (potentially presented by follicular DCs) and differentiate to plasma cells producing tumor-specific antibodies, that in turn propagate in the tumor bed and mediate CDC or NK cell–mediated ADCC of cancer cells. On the other hand, mature DCs present antigenic peptides to Tfh cells, activating them, and in turn stimulating CD8+ CTLs against tumor cells. Subsequent interaction of activated Tfh cells with B cells in TLS provide additional activating signals for Tfh cells by B cells (such us, antigen peptides presented on MHC class II molecules and ICOSL signals), and vice versa for B cells by Tfh cells (i.e., CD40–CD40 L interactions and IL21 production). B, Potential strategies to boost TLS formation and function. TLS neogenesis can be induced by the administration of chemoattractant chemokines (e.g., CXCL13), or cytokines (e.g., LIGHT) able to induce HEVs, thus boosting the influx of endogenous B and T cells. Immunostimulatory adjuvants (e.g., TLR9 or STING agonists) can promote TLS functions by mediating APC maturation, such as B-cell differentiation in antibody-producing plasma cells or maturation of DCs and subsequent cross-priming of CD8+ CTLs. Finally, KRASG12C inhibitors have demonstrated ability to promote tumor-associated TLS formation by potentially derepressing tumor-intrinsic IFN-mediated effects upon MYC inhibition, and subsequently upregulating MHC class II molecules on DCs and promoting the infiltration of activated CD8+ CTLs, B cells, and NK cells into the tumor. APC, antigen-presenting cells; BCR, B-cell receptor; CDC, complement-dependent cytotoxicity; FRC, fibroblastic reticular cells; TCR, T-cell receptor.
CXCL13 as the main driver of TLS formation
Accumulating evidence proposed CXCL13 as one of the main initiators of TLS formation (12, 14). In addition, transcriptional upregulation of CXCL13 has been associated with improved survival for patients treated with immunotherapy in different cancer settings (48–50), including LUAD (22, 50–52), thus suggesting a role for CXCL13 in mediating ICI efficacy by promoting the recruitment of CXC chemokine receptor 5 (CXCR5)+ lymphocytes and their organization in tumor-associated TLS. In line with these findings, recombinant CXCL13 improved PD-1 blockade efficacy in subcutaneous murine models of ovarian cancer, colorectal carcinoma, and NSCLC, an effect linked to increased levels of tumor-infiltrating CD8+ T cells (48, 52). In addition to CD8+ T cells, CXCL13 reportedly improved ICI efficacy against mouse LUADs by recruiting B cells (25). Indeed, the survival benefit offered by PD-L1 blockade was further improved by intranasal treatment with an expression vector encoding Cxcl13 (resulting in increased CXCL13 expression in LUAD tumors) in mice bearing immunogenic LUAD tumors, and conversely abrogated by CXCL13 neutralization or B-cell depletion (25). These results are in line with data obtained in a preclinical model of PDAC where the coinjection of CXCL13 with CCL21 into orthotopic tumors promoted the recruitment of both B and T cells and their organization in tumor-associated TLS, as well as the ability of the chemotherapeutic gemcitabine in delaying PDAC growth (34). Accordingly, the delivery of the cytokine LIGHT (also known as TNF superfamily member 14, TNFSF14) specifically to tumor vessels promoted the de novo TLS assembly in PDAC tumors, by inducing the production of CCL21 in tumor endothelial cells, and ultimately sensitized resistant PDAC tumors to treatment with PD-1 and CTLA4 blockers (53).
Of note, CXCL13 can be secreted by multiple cellular sources depending on the type of cancer, including stromal cells, follicular DCs, and Tfh cells (12, 14). In this context, Thommen and colleagues (22) suggested tumor-infiltrating PD1+ T cells as cellular source for CXCL13-mediated B-cell recruitment in NSCLC-associated TLS. However, although they demonstrated a proximity between PD1+CD8+ T cells and B cells in intratumoral and peritumoral TLS in human NSCLC specimens (22), functional experiments confirming this hypothesis are missing. Along similar lines, single-cell RNA sequencing (scRNA-seq) of human nasopharyngeal carcinomas identified PD1+CD4+ T cells as potential drivers of B-cell recruitment in tumor-associated TLS and differentiation in plasma cells, via CXCL13 and IL21 secretion (54). Consistent with these data, findings obtained by harnessing a mouse model of metastatic ovarian cancer (30) and scRNA-seq of treatment-naïve human PDAC specimens (55) showed that TGFβ impairs Treg formation in favor of CXCL13+ Tfh cell differentiation, in turn promoting B-cell recruitment and activation in a CXCL13-dependent manner, and ultimately resulting in the assembly of intratumoral TLS. Intriguingly, activated Tfh cells also secreted high levels of LIGHT, which in turn, by triggering the secretion of CCL21 by endothelial cells (53), may contribute to the initial recruitment of lymphocytes into the tumor bed (30). Finally, evidence obtained in a murine model of B16-OVA melanoma suggested CD8+ T cells as the initiators of tumor-associated TLS aggregation through the promotion of a reticular network of cancer-associated fibroblasts (CAF), which in turn foster B-cell recruitment by CXCL13 secretion, and subsequently B cells drive CAFs proliferation and TLS expansion via lymphotoxin β receptor (LTβR) signaling, thus pointing to CAFs as important players in TLS formation (28). Interestingly, treatment with ICIs induced the formation of larger and well-organized TLS in this model, an effect also linked to improved disease control by ICIs (28).
Of note, CAFs are a group of heterogeneous cell subtypes, with different phenotypes and functions depending on the cancer type. Although CAFs are being generally considered “oncogenic” players, accumulating evidence ascribes them also antitumor and drug sensitizing abilities (56). In the context of lung cancer, CAFs are the major components of the TME and their characterization by scRNA-seq identified five subtypes with different functions (including tumor progression and both immunosuppressive and immunostimulatory functions), with differences in term of frequency among lung cancer histologic subtypes and tumor stages, and different impact on survival (57–60). In vitro experiments suggested that CAFs are implicated both in resistance and response to tyrosine kinase inhibitors in a context-dependent manner (61), and secretion of TGFβ by CAFs has been reported to limit T-cell infiltration by promoting the expression of laminin γ2 by cancer cells, as well as the efficacy of PD-1 blockers, in mouse models of lung cancer (54). So far, the incomplete understanding of CAFs heterogeneity has hampered the development of strategies that (by targeting CAFs or promoting their immunostimulatory effects) efficiently improve clinical outcomes of a variety of malignant diseases, including NSCLC (56).
Overall, although recent preclinical findings point to CXCL13 as a key player in TLS formation in lung cancers, further experiments are needed to better understand mechanisms at the basis of CXCL13-dependent recruitment and organization of immune cells in NSCLC-associated TLS, as well as to define the potential role of CAFs in promoting B-cell activation in NSCLC, to efficiently translate these preclinical findings to the clinic.
Potential Strategies to Exploit TLS Formation and Function for Improving ICI Response and Clinical Outcomes
Despite significant advancements in targeted anticancer treatments and immunotherapies, NSCLC remains a leading global cause of cancer-related deaths. Responses to ICIs vary among patients with NSCLC, with only a limited subset of patients exhibiting sustained responses, while most patients develop intrinsic or acquired resistance to currently used immunotherapies (1–4). Currently, PD-L1 overexpression and high TMB are the most extensively studied predictive markers for ICI response. However, they cannot be considered clear-cut biomarkers, as the intertumor and intratumor heterogeneity, coupled with diverse methods applied for their identification, and often challenging reproducibility in clinical practice, renders their predictive value variable and not always reliable across different studies (4). For these reasons, several efforts are being made to understand the mechanisms underlying intrinsic and acquired resistance to currently used cancer therapies, as well as for the identification of biomarkers to accurately predict response to anticancer treatments and of novel druggable targets or combinatorial treatments to efficiently improve survival rates among patients with cancer. In this context, TLS are emerging as reliable biomarkers of response to ICIs (as extensively reviewed in refs. 12, 45) independently of commonly known markers of response to ICIs, such as CD8+ T-cell density and PD-L1 expression (18). In addition, accumulating preclinical evidence suggests that the therapeutic modulation of TLS development, composition, and function (Fig. 1B) could represent a potential strategy to enhance antitumor immune responses and thus to improve clinical outcomes of patients with NSCLC refractory to available FDA-approved treatments.
Induction of TLS by chemokines, cytokines, or immunostimulatory adjuvants
Preclinical findings recently published by Ng and colleagues (25) suggested a potential combinatorial approach with a PD-L1 blocker and an intranasal vector encoding Cxcl13 to enhance TLS formation in mouse LUADs and to further delay tumor growth. However, the translational potential of these findings has not been discussed by the authors, a thing that need to be taken in consideration because both antitumor and protumor activities have been associated to the CXCL13/CXCR5 axis in different cancer settings, including NSCLC (62, 63). In addition, the authors did not investigate why LUAD-bearing mice eventually relapse to treatment with ICIs and to combination therapy after an initial response (25). Thus, the investigation of immunosuppressive cellular and molecular mechanisms potentially hampering antitumor immune responses in this setting is missing. Previous findings by other groups described the presence of immunosuppressive cells in TLS associated to mouse and human lung tumors (15, 16). For instance, TLS-associated Tregs have been correlated to poor clinical outcome in patients with NSCLC (16), and Tregs reportedly suppressed DC-mediated activation and T-cell expansion in TLS (and thus TLS-mediated antitumor immune responses) in LUADs established in KP mice (15). Of note, in the latter model although Treg depletion led to increased TLS area and percentage of proliferating T cells in TLS, it did not affect B-cell infiltration in LUAD-associated TLS (15). Conversely, a negative correlation between B cells (high) and Tregs (low) infiltrating TLS has been reported in NSCLC specimens from patients with better clinical outcomes (21). Thus, the cross-talk between Tregs and B cells associated to TLS in NSCLC, and the potential depletion/target of Tregs to potentiate TLS-driven humoral responses to ICIs, needs further investigations.
Contextually, reduced activation of Tregs, paralleled by (i) increased expression of activation markers on B cells and DCs, (ii) enhanced effector functions of CD4+ and CD8+ T cells, and (iii) de novo formation of tumor-associated TLS, has been observed in lung metastases established in mice by the subcutaneous injection of TNBC 4T1 cells, and treated with intranasal injection of SD-101, a Toll-like receptor 9 (TLR9) agonist (64). In addition, combination of SD-101 with PD-1 blockade further increased tumor-infiltrating levels of CD8+ CTLs, ultimately resulting in durable rejection of lung tumors in this model (64). Interestingly, combination of intratumor injection of SD-101 with pembrolizumab in a phase II trial (NCT02521870) induced OR (OR rate was 24%, including two complete and 10 partial responses) and led to increased levels of CD8+ CTLs and B cells in biopsies obtained from responder patients with recurrent/metastatic head and neck squamous cell carcinoma (65). On the basis of these findings, combination of TLR9 agonists and ICIs for the treatment of human NSCLC warrants investigation.
Finally, another issue that needs to be addressed is to investigate whether TLS generation in NSCLC could be enhanced by boosting lymphocyte infiltration into tumors throughout the therapeutic induction of intratumoral HEVs, as demonstrated in other preclinical settings. Indeed, TLS neogenesis has been observed after tumor vessel remodeling and HEV induction upon the administration of a stimulator of interferon genes (STING) agonist or of the cytokine LIGHT, respectively in mouse models of melanoma (66), or model of PDAC (53) and glioma (31). Interestingly, both STING agonist and LIGHT reportedly synergized with PD-1 blockade in delaying the growth of melanoma lung metastases, by respectively remodeling lung metastases' TME via the promotion of type I IFN signaling (67), or the induction of metastatic HEVs (68). Notably, whereas STING agonists entered clinical evaluation (69), LIGHT-based therapies are still under preclinical development (70).
Induction of TLS by KRASG12C inhibitors
An important finding recently emerged by harnessing a novel immunogenic model of LUAD is the impact of the highly selective KRASG12C inhibitor adagrasib [which has been granted FDA approval only in 2022 for locally advanced or metastatic NSCLC harboring KRASG12C mutation and who have received at least one prior systemic therapy (71)] on LUAD-associated TLS (25). In particular, the authors showed increased TLS formation in mice bearing KrasG12C-mutant LUADs upon treatment with adagrasib, thus highlighting a novel immunomodulatory effect for KRASG12C inhibitors in LUAD (25). Interestingly, the same effects were not observed in mice treated with the MAPK (also known as MEK) inhibitor trametinib (25), thus suggesting that antitumor B cell–mediated responses in this model may be hampered by the ubiquitous inhibition of MEK, which is highly upregulated in KRASG12C-mutant cancer cells, but also in B cells as it plays a key role in B-cell differentiation (72). The mechanisms by which TLS contribute to the response to KRASG12C inhibitors, however, remain to be clarified, as well as whether the presence of tumor-associated TLS could represent a potential indicator of response to KRASG12C inhibitors for patients with NSCLC.
These preclinical findings open to the possibility of combining FDA-approved KRASG12C inhibitors with ICIs for the treatment of NSCLC to promote TLS formation and improve therapy-mediated antitumor immune responses. Supporting this hypothesis and partially filling in the missing gaps regarding the mechanisms underlying KRASG12C inhibitor-mediated TLS formation in LUADs (25), a study published in 2022 by the same group tested the synergy between a KRASG12C inhibitor analog for adagrasib (i.e., MRTX1257) and an anti-PD-1 antibody in mice bearing KrasG12C-mutant lung tumors (73). In particular, MRTX1257 has been shown to promote antitumor immunity and to potentiate the therapeutic efficacy of PD-1 inhibition in immunogenic KrasG12C-mutant (KPAR) LUADs (73). The same synergy, however, has not been replicated in mice bearing nonimmunogenic (3LL) tumors knockout for Nras or KrasG12C-mutant conventional KP (KPB6) LUADs, which are highly sensitive to KRASG12C inhibitors, but intrinsically resistant to therapy with PD-1 blockers (42), even if MRTX1257 was able to induce a profound remodeling of the TME in all these models, turning them from “cold” to “hot” tumors (73). A potential limiting factor in the latter models (i.e., 3LL and KPB6 tumors) could be the paralleled increase in Treg infiltration observed following KRASG12C inhibition, that might (i) inhibit the activity of CTLs, thus hampering the synergy between PD-1 blockers and KRASG12C inhibitor (73), but also (ii) impair the development of tumor-associated TLS, as TLS formation has not been observed in KPB6 tumors (25) and Treg depletion have been shown to increase TLS formation in lung tumors established in KP mice (15). Mechanistically, the KRASG12C inhibitor has been proposed to remodel lung TME through the upregulation of IFN pathways in cancer cells via MYC inhibition, in turn leading to enhanced antigen presentation and intratumoral recruitment of cytotoxic NK cells, CD8+ CTLs (73), and potentially B cells [as described in a preclinical model of PDAC upon derepression of IFN signaling by MYC inhibition (74)].
Overall, extrapolating from the preclinical studies discussed above, patients with TLS+ NSCLC are more likely to benefit from combinations of KRASG12C inhibitors with immunotherapies. Of note, the efficacy of KRASG12C inhibitors in combination with ICIs in NSCLC is under clinical investigation in phase I–III trials (NCT04613596, NCT03785249, NCT05609578, NCT05472623, NCT05920356, NCT05848843; classic.clinicaltrials.gov), and it will be very interesting the retrospective evaluation of the impact of TLS in response to combinatorial treatment for patients enrolled in those studies.
Of note, supporting the clinical combination of KRASG12C inhibitors with ICIs, similarly to data discussed above, the synergy between KRASG12C inhibitors and PD-1 blockers has also been reported in other preclinical cancer models, such as mice subcutaneously injected with syngeneic KrasG12C-mutant colon carcinoma CT26 cells and in a models of KrasG12C-driven LUAD, by three independent studies (75–77). The ability of KRASG12C inhibitors to enhance the therapeutic activity of PD-1 blockade has been correlated to increased tumor-infiltrating levels of proinflammatory M1-like macrophages, DCs, CD4+ and CD8+ T cells, paralleled by reduced levels of immunosuppressive cells, such as anti-inflammatory M2-like macrophages and myeloid-derived suppressor cells (75). Interestingly, the TME of CT26 tumors treated with adagrasib used as single agent or combined with an anti-PD-1 antibody (but not with anti-PD-1 alone) showed also increased levels of B cells, thus suggesting a potential involvement of humoral immunity also in this model (75). Whereas the immunomodulatory effects for ASP2453 (a novel selective KRASG12C inhibitor still under preclinical investigation) have not been investigated yet (77), sotorasib reportedly remodels the TME of KrasG12C-mutant colon carcinomas established in mice by promoting the upregulation of MHC class I molecules, as well as by inducing immunogenic cell death (ICD) in cancer cells, an effect consequently reflected by the upregulation of IFN signaling (76).
Similarly to KRASG12C inhibitors, radiotherapy and chemotherapy have the ability to exert immunostimulatory effects mostly by inducing ICD in cancer cells (78). Of note, only few reports have studied the correlation between the use of these conventional cancer treatments with lung cancer–associated TLS so far, and their impact on TLS formation and function is still not clear. In particular, by harnessing KP mice bearing LUADs, hypofractionated radiotherapy has been shown to temporally decrease the size of tumor-associated TLS, and that TLS size reincreased 14 days after irradiation, accompanied by a higher number of infiltrating Tregs compared with baseline (79). The impact of chemotherapy on TLS formation is controversial. Indeed, chemotherapy alone seems to impair TLS maturation in human NSCLC (17) and LSCC (23), whereas mature TLS have been observed in a higher number of specimens of human NSCLC treated with neoadjuvant platinum-based chemotherapy in combination with PD-1 blockers (17).
Noteworthy, one of the advantages in the use of KRASG12C inhibitors is that they exert a tumor cell–restricted activity, thus allowing the investigation of combinations of therapies without resulting in excessive toxicities. However, despite the demonstrated benefit of KRASG12C inhibitors in the management of KRASG12C-mutant NSCLC relapsing to platinum-based chemotherapy or PD-1/PD-L1 blockers, the overall survival of patients treated with KRASG12C inhibitors appears disappointingly similar when compared with the standard-of-care treatment (i.e., docetaxel) used for over 20 years in this setting (80), potentially due to poorly investigated mechanisms of intrinsic and acquired resistance, but also to the lack of predictive biomarkers of response (81). A way to improve therapy responses to KRASG12C inhibitors could be represented by the combination (other than with ICIs, as discussed above) with targeted agents with demonstrated immunostimulatory effects (82), and that could have a positive impact on TLS formation and function. As an example, a potential approach warrants preclinical and clinical investigation is the combination of KRASG12C inhibitors with agents targeting cyclin-dependent kinase 4 and 6 (CDK4/6). Indeed, CDK4/6 inhibitors could (i) limit the immunosuppressive effects that have been observed in a preclinical model of LUAD following KRASG12C inhibition (73), as well as (ii) increase NSCLC immunogenicity by augmenting tumor antigen expression (and thus improving antibody-dependent B-cell responses against NSCLC), thanks respectively to their demonstrated ability in repressing Treg proliferation and inducing ERV expression in cancer cells (83, 84).
Further investigations in combinatorial treatments able to augment response to currently used anticancer therapies (such as ICIs and KRASG12C inhibitors) by promoting immunostimulatory effects (including the enhancement of TLS formation and function), will potentially unveil new promising opportunities for developing more effective cancer therapies for patients with cancer.
Conclusions
Growing evidence is rapidly corroborating TIL-Bs and TLS as prognostic and predictive factors of response to ICIs in multiple human malignancies (5, 6, 8, 9), including NSCLC (10). However, the investigation of cellular and molecular mechanisms governing the development of TLS and their involvement in mediating ICI responses has been limited so far, due to intrinsic limitation of most of the available preclinical mouse models for immuno-oncology–related studies (26, 27). In this context, the recent development of more advanced preclinical models of lung cancer, resembling key features of human LUAD (i.e., high TMB, increased immunogenicity, and sensitivity to ICIs; refs. 25, 46) (i) significantly improved our understanding on mechanisms at the basis of TLS formation and on their involvement in mediating naïve and treatment-mediated antitumor responses (Fig. 1A), and (ii) suggested potential interesting approaches to exploit TLS genesis and function (Fig. 1B), to ultimately improve response to ICIs and clinical outcomes of patients with NSCLC. Among these, the enhancement of TLS formation by KRASG12C inhibitors comes up as the most interesting translational finding emerging from recent preclinical studies, with exciting implications for the clinical practice. In the future, more preclinical and clinical studies are necessary to fully understand (i) the mechanisms underlying TLS generation, composition, and function, as well as (ii) the therapeutic opportunities these mechanisms will provide to foster immune-mediated tumor control and maximize (immuno)therapy efficacy in a higher percentage of patients with cancer.
Authors' Disclosures
No disclosures were reported.
References
- 1. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. [DOI] [PubMed] [Google Scholar]
- 2. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- 3. Fancelli S, Caliman E, Mazzoni F, Paglialunga L, Gatta Michelet MR, Lavacchi D, et al. KRAS G12 isoforms exert influence over up-front treatments: a retrospective, multicenter, Italian analysis of the impact of first-line immune checkpoint inhibitors in an NSCLC real-life population. Front Oncol 2022;12:968064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol 2019;16:341–55. [DOI] [PubMed] [Google Scholar]
- 5. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meylan M, Petitprez F, Becht E, Bougoüin A, Pupier G, Calvez A, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 2022;55:527–41. [DOI] [PubMed] [Google Scholar]
- 7. Lu H, Lou H, Wengert G, Paudel R, Patel N, Desai S, et al. Tumor and local lymphoid tissue interaction determines prognosis in high-grade serous ovarian cancer. Cell Rep Med 2023;4:101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556–60. [DOI] [PubMed] [Google Scholar]
- 9. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020;577:561–5. [DOI] [PubMed] [Google Scholar]
- 10. Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 2022;40:289–300. [DOI] [PubMed] [Google Scholar]
- 11. Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer 2022;22:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307–25. [DOI] [PubMed] [Google Scholar]
- 13. Vella G, Hua Y, Bergers G. High endothelial venules in cancer: regulation, function, and therapeutic implication. Cancer Cell 2023;41:527–45. [DOI] [PubMed] [Google Scholar]
- 14. Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science 2022;375:eabf9419. [DOI] [PubMed] [Google Scholar]
- 15. Joshi NS, Akama-Garren EH, Lu Y, Lee D-Y, Chang GP, Li A, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 2015;43:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoïd structures and are associated with poor clinical outcome in NSCLC. Commun Biol 2022;5:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X, et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J Immunother Cancer 2022;10:e005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanhersecke L, Brunet M, Guégan J-P, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer 2021;2:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lynch KT, Young SJ, Meneveau MO, Wages NA, Engelhard VH, Slingluff CL, et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J Immunother Cancer 2021;9:e002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705–15. [DOI] [PubMed] [Google Scholar]
- 21. Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014;189:832–44. [DOI] [PubMed] [Google Scholar]
- 22. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018;24:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res 2018;78:1308–20. [DOI] [PubMed] [Google Scholar]
- 24. Sánchez-Alonso S, Setti-Jerez G, Arroyo M, Hernández T, Martos MI, Sánchez-Torres JM, et al. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J Immunother Cancer 2020;8:e001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng KW, Boumelha J, Enfield KSS, Almagro J, Cha H, Pich O, et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature 2023;616:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olson B, Li Y, Lin Y, Liu ET, Patnaik A. Mouse models for cancer immunotherapy research. Cancer Discov 2018;8:1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buqué A, Galluzzi L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 2018;4:599–601. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep 2021;36:109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stephen TL, Payne KK, Chaurio RA, Allegrezza MJ, Zhu H, Perez-Sanz J, et al. SATB1 expression governs epigenetic repression of PD-1 in tumor-reactive T cells. Immunity 2017;46:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 2022;55:115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramachandran M, Vaccaro A, van de Walle T, Georganaki M, Lugano R, Vemuri K, et al. Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma. Cancer Cell 2023;41:1134–51. [DOI] [PubMed] [Google Scholar]
- 32. van Hooren L, Vaccaro A, Ramachandran M, Vazaios K, Libard S, van de Walle T, et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat Commun 2021;12:4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitzgerald B, Connolly KA, Cui C, Fagerberg E, Mariuzza DL, Hornick NI, et al. A mouse model for the study of anti-tumor T cell responses in Kras-driven lung adenocarcinoma. Cell Rep Methods 2021;1:100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delvecchio FR, Fincham REA, Spear S, Clear A, Roy-Luzarraga M, Balkwill FR, et al. Pancreatic cancer chemotherapy is potentiated by induction of tertiary lymphoid structures in mice. Cell Mol Gastroenterol Hepatol 2021;12:1543–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill DG, Yu L, Gao H, Balic JJ, West A, Oshima H, et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int J Cancer 2018;143:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, et al. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc Natl Acad Sci U S A 2016;113:E6409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Westcott PMK, Halliwill KD, To MD, Rashid M, Rust AG, Keane TM, et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature 2015;517:489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z, Lu Z, Lin S, Xia J, Zhong Z, Xie Z, et al. Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity 2022;55:1067–81.e8. [DOI] [PubMed] [Google Scholar]
- 39. Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 2021;54:2812–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016;44:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet 2013;45:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boumelha J, de Carné Trécesson S, Law EK, Romero-Clavijo P, Coelho MA, Ng KW, et al. An immunogenic model of KRAS-mutant lung cancer enables evaluation of targeted therapy and immunotherapy combinations. Cancer Res 2022;82:3435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fridman WH, Meylan M, Petitprez F, Sun C-M, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol 2022;19:441–57. [DOI] [PubMed] [Google Scholar]
- 44. Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight 2019;5:e129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fridman WH, Meylan M, Pupier G, Calvez A, Hernandez I, Sautès-Fridman C. Tertiary lymphoid structures and B cells: an intratumoral immunity cycle. Immunity 2023;56:2254–69. [DOI] [PubMed] [Google Scholar]
- 46. Cui C, Wang J, Fagerberg E, Chen P-M, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 2021;184:6101–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 2019;179:1191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang M, Lu J, Zhang G, Wang Y, He M, Xu Q, et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J Immunother Cancer 2021;9:e001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groeneveld CS, Fontugne J, Cabel L, Bernard-Pierrot I, Radvanyi F, Allory Y, et al. Tertiary lymphoid structures marker CXCL13 is associated with better survival for patients with advanced-stage bladder cancer treated with immunotherapy. Eur J Cancer 2021;148:181–9. [DOI] [PubMed] [Google Scholar]
- 50. Litchfield K, Reading JL, Puttick C, Thakkar K, Abbosh C, Bentham R, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021;184:596–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park S, Cha H, Kim HS, Lee B, Kim S, Kim TM, et al. Transcriptional upregulation of CXCL13 is correlated with a favorable response to immune checkpoint inhibitors in lung adenocarcinoma. Cancer Med 2023;12:7639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sorin M, Karimi E, Rezanejad M, Yu MW, Desharnais L, McDowell SAC, et al. Single-cell spatial landscape of immunotherapy response reveals mechanisms of CXCL13 enhanced antitumor immunity. J Immunother Cancer 2023;11:e005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol 2017;18:1207–17. [DOI] [PubMed] [Google Scholar]
- 54. Li J-P, Wu C-Y, Chen M-Y, Liu S-X, Yan S-M, Kang Y-F, et al. PD-1+CXCR5-CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer 2021;9:e002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut 2023;72:1927–41. [DOI] [PubMed] [Google Scholar]
- 56. Saw PE, Chen J, Song E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer 2022;8:527–55. [DOI] [PubMed] [Google Scholar]
- 57. Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277–89. [DOI] [PubMed] [Google Scholar]
- 58. Hu H, Piotrowska Z, Hare PJ, Chen H, Mulvey HE, Mayfield A, et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021;39:1531–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ren Q, Zhang P, Lin H, Feng Y, Chi H, Zhang X, et al. A novel signature predicts prognosis and immunotherapy in lung adenocarcinoma based on cancer-associated fibroblasts. Front Immunol 2023;14:1201573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Q, Zhang X, Du K, Wu X, Zhou Y, Chen D, et al. Machine learning identifies characteristics molecules of cancer associated fibroblasts significantly correlated with the prognosis, immunotherapy response and immune microenvironment in lung adenocarcinoma. Front Oncol 2022;12:1059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Remsing Rix LL, Sumi NJ, Hu Q, Desai B, Bryant AT, Li X, et al. IGF-binding proteins secreted by cancer-associated fibroblasts induce context-dependent drug sensitization of lung cancer cells. Sci Signal 2022;15:eabj5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hussain M, Adah D, Tariq M, Lu Y, Zhang J, Liu J. CXCL13/CXCR5 signaling axis in cancer. Life Sci 2019;227:175–86. [DOI] [PubMed] [Google Scholar]
- 63. Singh R, Gupta P, Kloecker GH, Singh S, Lillard JW. Expression and clinical significance of CXCR5/CXCL13 in human non-small cell lung carcinoma. Int J Oncol 2014;45:2232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gallotta M, Assi H, Degagné É, Kannan SK, Coffman RL, Guiducci C. Inhaled TLR9 agonist renders lung tumors permissive to PD-1 blockade by promoting optimal CD4+ and CD8+ T-cell interplay. Cancer Res 2018;78:4943–56. [DOI] [PubMed] [Google Scholar]
- 65. Cohen EEW, Nabell L, Wong DJ, Day T, Daniels GA, Milhem M, et al. Intralesional SD-101 in combination with pembrolizumab in anti-PD-1 treatment-naïve head and neck squamous cell carcinoma: results from a multicenter, phase II trial. Clin Cancer Res 2022;28:1157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J Immunother Cancer 2021;9:e001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nakamura T, Sato T, Endo R, Sasaki S, Takahashi N, Sato Y, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer 2021;9:e002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. He B, Johansson-Percival A, Backhouse J, Li J, Lee GYF, Hamzah J, et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep 2020;30:714–24. [DOI] [PubMed] [Google Scholar]
- 69. Le Naour J, Zitvogel L, Galluzzi L, Vacchelli E, Kroemer G. Trial watch: STING agonists in cancer therapy. Oncoimmunology 2020;9:1777624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Skeate JG, Otsmaa ME, Prins R, Fernandez DJ, Da Silva DM, Kast WM. TNFSF14: LIGHTing the way for effective cancer immunotherapy. Front Immunol 2020;11:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 2022;387:120–31. [DOI] [PubMed] [Google Scholar]
- 72. Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. J Exp Med 2010;207:607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mugarza E, van Maldegem F, Boumelha J, Moore C, Rana S, Llorian Sopena M, et al. Therapeutic KRASG12C inhibition drives effective interferon-mediated antitumor immunity in immunogenic lung cancers. Sci Adv 2022;8:eabm8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Muthalagu N, Monteverde T, Raffo-Iraolagoitia X, Wiesheu R, Whyte D, Hedley A, et al. Repression of the type I interferon pathway underlies MYC- and KRAS-dependent evasion of NK and B cells in pancreatic ductal adenocarcinoma. Cancer Discov 2020;10:872–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Briere DM, Li S, Calinisan A, Sudhakar N, Aranda R, Hargis L, et al. The KRASG12C inhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol Cancer Ther 2021;20:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019;575:217–23. [DOI] [PubMed] [Google Scholar]
- 77. Nakayama A, Nagashima T, Nishizono Y, Kuramoto K, Mori K, Homboh K, et al. Characterisation of a novel KRAS G12C inhibitor ASP2453 that shows potent anti-tumour activity in KRAS G12C-mutated preclinical models. Br J Cancer 2022;126:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis 2020;11:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boivin G, Kalambaden P, Faget J, Rusakiewicz S, Montay-Gruel P, Meylan E, et al. Cellular composition and contribution of tertiary lymphoid structures to tumor immune infiltration and modulation by radiation therapy. Front Oncol 2018;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. de Langen AJ, Johnson ML, Mazieres J, Dingemans A-MC, Mountzios G, Pless M, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet 2023;401:733–46. [DOI] [PubMed] [Google Scholar]
- 81. Di Federico A, Ricciotti I, Favorito V, Michelina SV, Scaparone P, Metro G, et al. Resistance to KRAS G12C inhibition in non-small cell lung cancer. Curr Oncol Rep 2023;25:1017–29. [DOI] [PubMed] [Google Scholar]
- 82. Petroni G, Buqué A, Coussens LM, Galluzzi L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat Rev Drug Discov 2022;21:440–62. [DOI] [PubMed] [Google Scholar]
- 83. Petroni G, Formenti SC, Chen-Kiang S, Galluzzi L. Immunomodulation by anticancer cell cycle inhibitors. Nat Rev Immunol 2020;20:669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]