Abstract
Purpose:
Patients with early-stage hormone receptor–positive (HR+) breast cancer face a prolonged risk of recurrence even after adjuvant endocrine therapy. The Breast Cancer Index (BCI) is significantly prognostic for overall (0–10 years) and late (5–10 years) distant recurrence (DR) risk in N0 and N1 patients. Here, BCI prognostic performance was evaluated in HR+ postmenopausal women from the Tamoxifen and Exemestane Adjuvant Multinational (TEAM) trial.
Experimental Design:
3,544 patients were included in the analysis (N = 1,519 N0, N = 2,025 N+). BCI risk groups were calculated using pre-specified cutoff points. Kaplan–Meier analyses and log-rank tests were used to assess the prognostic significance of BCI risk groups based on DR. Hazard ratios (HR) and confidence intervals (CI) were calculated using Cox models with and without clinical covariates.
Results:
For overall 10-year DR, BCI was significantly prognostic in Ni0 (N = 1,196) and N1 (N = 1,234) patients who did not receive prior chemotherapy (P < 0.001). In patients who were DR-free for 5 years, 10-year late DR rates for low- and high-risk groups were 5.4% and 9.3% (N0 cohort, N = 1,285) and 4.8% and 12.2% (N1 cohort, N = 1,625) with multivariate HRs of 2.25 (95% CI, 1.30–3.88; P = 0.004) and 2.67 (95% CI, 1.53–4.63; P < 0.001), respectively. Late DR performance was substantially improved using previously optimized cutoff points, identifying BCI low-risk groups with even lower 10-year late DR rates of 3.8% and 2.7% in N0 and N1 patients, respectively.
Conclusions:
The TEAM trial represents the largest prognostic validation study for BCI to date and provides a more representative assessment of late DR risk to guide individualized treatment decision-making for HR+ patients with early-stage breast cancer.
Translational Relevance.
Accurate prognostication of hormone receptor–positive (HR+) patients with early-stage breast cancer is important to inform adjuvant therapy selection. This study evaluated the Breast Cancer Index (BCI) prognostic models in a cohort of postmenopausal women from the Tamoxifen and Exemestane Adjuvant Multinational trial confirming that BCI and BCIN+ are significantly prognostic for overall and late distant recurrence (DR) in N0 and N1 patients. This study further refines the identification of women at low risk of DR, using the BCI prognostic score, who may be spared from extended endocrine therapy (EET) due to their low absolute risk and modest benefit from longer endocrine therapy. Women at high risk of late DR should receive EET based on BCI-predictive results. In summary, BCI may inform personalized decision-making for adjuvant therapies by providing independent prognostic information.
Introduction
Advancements in adjuvant endocrine therapy for early-stage hormone receptor–positive (HR+) breast cancer have led to improved survival and reduced recurrence of disease (1–3). Even so, patients with breast cancer face a substantial and prolonged risk of recurrence with over 50% of recurrences occurring after the first 5 years from diagnosis (4–7). An Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis showed that the risk of distant recurrence (DR) between 5 and 20 years after 5 years of adjuvant endocrine therapy for ER-positive breast cancer was strongly correlated with nodal status with an absolute risk of 13%, 20%, and 34% for patients with N0, N1, and N2 disease even with T1 tumors, respectively (6). Consequently, N+ patients are more likely to be treated with extended endocrine therapy (EET) than N0 patients (1). However, more intensive therapies may also expose patients to a greater risk of side effects. Genomic assays that inform prognosis and predict response to therapy can help assess risk versus benefit and have been incorporated into clinical decision-making (8).
The Breast Cancer Index (BCI) is a gene expression-based biomarker comprising two complementary functional domains that interrogate different biological pathways: The Molecular Grade Index that assesses proliferative status based on the expression levels of five cell-cycle–associated genes and the HOXB13:IL17BR expression ratio (H/I) that interrogates estrogen signaling in HR+ breast cancer. The combined index, BCI, has been shown to significantly stratify patients with N0 disease based on the risk of overall (0–10 years) and late (5–10 years) DR (9–11). For patients with breast cancer with 1 to 3 positive nodes (N1), an updated prognostic model (BCIN+) that integrates BCI with tumor size and grade was developed and validated with significantly improved prognostic performance (12). Recently, new cutoff points for both BCI and BCIN+ models that were specifically optimized for late DR were developed using N0 patients from the Trans-aTTom study (13) and N1 patients from the IDEAL trial (14), which showed improved prognostication by identifying low-risk groups with an even lower risk of late DR.
This study evaluated the BCI and BCIN+ prognostic models in a cohort of women from the Tamoxifen and Exemestane Adjuvant Multinational (TEAM) trial (registered with ClinicalTrials.gov, NCT00279448, NCT00032136, and NCT00036270; ref. 15). BCI and BCIN+ were assessed for their ability to significantly stratify HR+ patients with breast cancer based on the risk for overall (0–10 years) and late (5–10 years) DR.
Materials and Methods
Study design and patients
Tumor samples in this study were derived from patients previously enrolled in the TEAM trial. The TEAM trial is a prospective phase III trial that examined disease-free survival (DFS) after 5 years of either aromatase inhibitor (AI) monotherapy or sequential therapy, consisting of tamoxifen for 2.5–3 years followed by an AI to complete 5 years of endocrine therapy (15). Postmenopausal HR+ women (9,766 patients) were randomly assigned to AI monotherapy (4,904 patients) or TAM-AI sequential therapy (4,875 patients). Results of the study showed no difference in DFS at 5 and 10 years between the two groups of patients (15, 16). Patients from the translational pathology cohort with available RNA samples for BCI testing were assessed in this study (N = 4,086). Patients were excluded if they had received neoadjuvant chemotherapy. Hormone receptor status was defined locally at a cutoff point of 1% for ER or PR.
The TEAM trial was done in compliance with the guidelines of the Declaration of Helsinki, International Conference on Harmonization, and Good Clinical Practice. Appropriate approvals from the ethical committee were obtained. All patients provided written informed consent.
BCI assay
BCI gene expression analysis was performed by RT-PCR blinded to clinical outcome (Biotheranostics Inc., A Hologic Company), as previously described (9). BCI and BCIN+ scores were calculated for N0 and N1 patients, respectively, and pre-defined cutoff points were used to stratify patients into overall (0–10 years) risk groups: BCI-Low, BCI-Intermediate, and BCI-High for N0 patients as well as BCIN+-Low and BCIN+-High (9, 12). To evaluate late DR in N0 patients, BCI-Intermediate and BCI-High risk groups were grouped together and re-categorized as BCI-High (9, 12). In addition, new cutoff points (4.4 for N0 and 1.8 for N1) were previously optimized for late DR based on the classification of a low-risk group with <5% risk of 5–15 years late DR using N0 patients from the Trans-aTTom study (13) and N1 patients from the IDEAL trial (14) were also used to further evaluate BCI and BCIN+ prognostic performance.
Study endpoints
The primary endpoint was time to DR, defined as the time from randomization in the main trial to the first recurrence at distant sites. Contralateral disease, locoregional recurrences, and other secondary primary cancers were neither counted as events nor censored. Death before DR was treated as a censoring event. The secondary endpoint was time to recurrence, defined as the time from randomization to first locoregional or DR. The primary objective was to evaluate the prognostic performance of BCI and BCIN+ for overall (0–10 years) and late DR (5–10 years) risk in postmenopausal women with HR+ N0 and N1 breast cancer, respectively. The secondary objective was to evaluate the prognostic performance of BCI and BCIN+ in the subset of patients with HER2− disease. Overall DR risk (0–10 years) was evaluated within the subset of patients that did not receive chemotherapy and late DR risk (5–10 years) was evaluated within the subset of patients that remained DR-free for at least 5 years, independent of having received chemotherapy or not.
Statistical analyses
Analyses were prespecified in a Statistical Analysis Plan before unblinding. Kaplan–Meier analysis and log-rank test were used to compare the differences between BCI and BCIN+ risk groups for overall (0–10 years) and late (5–10 years) DR risk. Hazard ratios (HR) and the associated 95% confidence intervals (CI) were estimated using Cox proportional hazards regression analysis. Multivariate models were adjusted for standard clinicopathological variables, including age, tumor size, tumor grade, and treatment. Ten-year risk of DR, as a function of continuous BCI and BCIN+ risk score, was estimated from a Cox model based on Breslow estimate (17). A two-sided P value of less than 0.05 was considered statistically significant. All analyses were performed using SAS statistical package.
Data availability
The data analyzed in the current study are not publicly available because they contain patient data and proprietary information. Aggregated data analyzed in the study are included in the article. Qualified researchers may contact the corresponding author with reasonable requests to view additional data.
Results
Patient characteristics
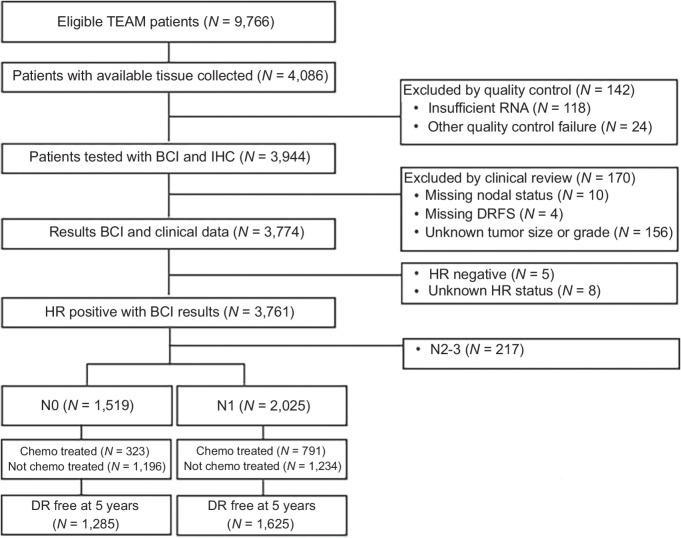
Patient and tumor characteristics for the translational cohort and the parent TEAM trial are summarized in Table 1. Of the 9,766 patients evaluated in TEAM, 4,086 had tissue available for analysis and 3,544 patients were included in the final analysis, consisting of 1,519 HR+ N0 and 2,025 N1 patients (Fig. 1).
Table 1.
Patient characteristics.
| TEAM trial (N = 9,766) | BCI cohort (N = 3,544) | |
|---|---|---|
| Age at randomization, years | ||
| <50 | 331 (3%) | 88 (3%) |
| 50–59 | 3,017 (31%) | 1,057 (30%) |
| 60–69 | 3,731 (38%) | 1,350 (38%) |
| ≥70 | 2,687 (28%) | 1,049 (30%) |
| Histological grade | ||
| Well differentiated | 1,677 (19%) | 412 (12%) |
| Moderately Differentiated | 4,797 (54%) | 1,874 (54%) |
| Poorly differentiated | 2,438 (27%) | 1,197 (34%) |
| Not known | 854 | 61 |
| Tumor size | ||
| T1 | 5,691 (58%) | 1,769 (50%) |
| T2 | 3,591 (37%) | 1,597 (45%) |
| T3–T4 | 457 (5%) | 177 (5%) |
| T0/T in situ | 6 | 0 |
| Not known | 21 | 1 |
| Nodal status | ||
| N0 | 5,113 (53%) | 1,519 (43%) |
| N1 | 4,109 (42%) | 2,025 (57%) |
| N2–3 | 478 (5%) | |
| Not known | 66 | |
| ER status | ||
| Positive | 9,585 (98%) | 3,480 (98%) |
| Negative | 176 (2%) | 63 (2%) |
| Not assessed | 5 | 1 |
| PR status | ||
| Positive | 7,301 (81%) | 2,419 (79%) |
| Negative | 1,724 (19%) | 644 (21%) |
| Not assessed | 741 | 481 |
| HER2 status | ||
| Positive | 560 (13%)a | 430 (13%) |
| Negative | 3,825 (87%)a | 2,966 (87%) |
| Not assessed | 1,735a | 148 |
| Most extensive surgery | ||
| Mastectomy | 4,333 (44%) | 1,671 (47%) |
| Wide local incision | 5,423 (56%) | 1,871 (53%) |
| No resection | 3 (<1%) | 0 (0%) |
| Not known | 7 | 2 |
| Adjuvant radiotherapy | ||
| Yes | 6,697 (69%) | 2,263 (64%) |
| No | 2,976 (31%) | 1,276 (36%) |
| Not known | 93 | 5 |
| Adjuvant chemotherapy | ||
| Yes | 3,513 (36%) | 1,110 (31%) |
| No | 6,248 (64%) | 2,430 (69%) |
| Not known | 5 | 4 |
| Histology | ||
| Ductal | 3,696 (60%) | 2,667 (75%) |
| Lobular | 809 (13%) | 498 (14%) |
| Mixed | 226 (4%) | 143 (4%) |
| Mucinous | 12 (<1%) | 8 (<1%) |
| Medullary | 3 (<1%) | 2 (<1%) |
| Papillary | 6 (<1%) | 2 (<1%) |
| Tubular | 10 (<1%) | 3 (<1%) |
| Otherb | 6 (<1%) | 3 (<1%) |
| NOSc/Missing | 1,352 (22%) | 218 (6%) |
aHER2 status only available for patients in pathology sub study (n = 6,120).
bOther histological subtypes: In situ, Bifocal, Cribiform, Multifocal, Neuroendocrine.
cNot otherwise specified.
Figure 1.
Case flow diagram.
Distributions of age, ER status, PR status, adjuvant radiotherapy and chemotherapy were similar between patients in the TEAM trial and the translational BCI cohort (Table 1). Compared with the parent TEAM trial, more patients in the translational cohort had T2 (45% vs. 37%), poorly differentiated tumors (34% vs. 27%), node-positive disease (57% vs. 47%), and mastectomy (47% vs. 44%), largely due to the exclusion of US study sites that had the lowest risk population of patients among the 9 countries that participated in the TEAM trial.
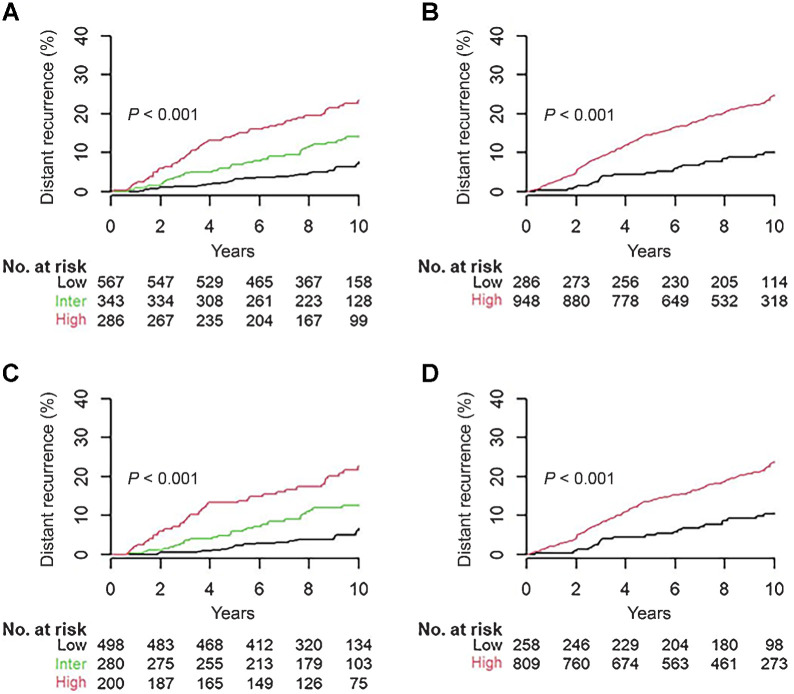
Performance of BCI and BCIN± for overall DR
Using previously established cutoff points (9, 12), BCI stratified N0 patients (N = 1,196) who did not receive chemotherapy into three distinct risk groups for overall DR: 47% (N = 567) into a BCI low-risk group with a 10-year DR rate of 7.8% (95% CI, 4.9–10.5), 29% (N = 343) BCI intermediate-risk with a 10-year DR rate of 14.1% (95% CI, 9.9–18.1), and 24% (N = 286) BCI high-risk with a 10-year DR rate of 23.5% (95% CI, 17.9–28.7; Table 2 and Fig. 2A). BCI was significantly prognostic for overall DR in N0 patients with a multivariate HR of 2.16 (95% CI, 1.32–3.52) for intermediate-risk and 3.89 (95% CI, 2.42–6.24) for high-risk versus low-risk groups, respectively (P < 0.001; Table 2 and Fig. 2A).
Table 2.
Prognostic performance of BCI and BCIN+ for overall and late DR in all patients with N0 and N1 breast cancers, as well as in HER2− subsets, respectively.
| All patients | HER2− subset | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BCI/BCIN+ groups | N | 10-y DR, % (95% CI) | Univariate HR (95% CI) | Multivariate HR (95% CI) | N | 10-y DR, % (95% CI) | Univariate HR (95% CI) | Multivariate HR (95% CI) | |
| Overall DR (0–10 years) in patients not treated with chemotherapy | |||||||||
| N0 | Low | 567 | 7.8 (4.9–10.5) | — | — | 498 | 6.8 (3.8–9.7) | — | — |
| Intermediate | 343 | 14.1 (9.9–18.1) | 2.14 (1.35–3.40) | 2.16 (1.32–3.52) | 280 | 12.6 (8.2–16.8) | 2.35 (1.36–4.04) | 2.51 (1.41–4.47) | |
| High | 286 | 23.5 (17.9–28.7) | 3.94 (2.56–6.06) | 3.89 (2.42–6.24) | 200 | 22.7 (16.1–28.9) | 4.56 (2.73–7.61) | 5.00 (2.83–8.84) | |
| N1 | Low | 286 | 10.1 (6.2–13.8) | — | — | 258 | 10.5 (6.3–14.5) | — | — |
| High | 948 | 24.6 (21.5–27.6) | 2.68 (1.77–4.07) | 2.62 (1.72–3.98) | 809 | 23.7 (20.4–27.0) | 2.46 (1.59–3.81) | 2.37 (1.53–3.67) | |
| Late DR (5–10 years) in patients DR free for 5 years | |||||||||
| N0 | Low | 633 | 5.4 (3.0–7.8) | — | — | 556 | 5.3 (2.6–7.9) | — | — |
| High | 652 | 9.3 (6.7–11.8) | 2.10 (1.26–3.50) | 2.25 (1.30–3.88) | 507 | 9.0 (6.1–11.8) | 2.18 (1.23–3.88) | 2.53 (1.37–4.67) | |
| N1 | Low | 349 | 4.8 (2.3–7.3) | — | — | 311 | 5.1 (2.3–7.8) | — | — |
| High | 1,276 | 12.2 (10.1–14.2) | 2.68 (1.54–4.66) | 2.67 (1.53–4.63) | 1,083 | 12.1 (9.9–14.3) | 2.50 (1.41–4.45) | 2.49 (1.40–4.44) | |
Note: Multivariate analysis was adjusted for age, tumor size, tumor grade, and treatment for N0 subsets, but excluded tumor grade for N1 subsets due to confounding with BCIN+.
Figure 2.
Prognostic performance of BCI and BCIN+ for overall 10-year risk of DR in N0 and N1 patients who did not receive adjuvant chemotherapy. A, BCI stratification in 1,196 N0 patients. B, BCIN+ stratification in 1,234 N1 patients. C, BCI stratification in 978 N0 HER2− patients. D, BCIN+ stratification in 1,067 N1 HER2− patients
Similarly, using previously defined cutoff points (12), BCIN+ stratified N1 patients (N = 1,234) who did not receive chemotherapy into two prognostic groups for overall DR (Table 2 and Fig. 2B). The BCIN+ low-risk group comprised 23% of patients (N = 286) with a 10-year DR rate of 10.1% (95% CI: 6.2–13.8). The BCIN+ high-risk group included 77% of patients (N = 948) with a 10-year DR rate of 24.6% (95% CI, 21.5–27.6; Table 2 and Fig. 2B). BCIN+ was significantly prognostic with a multivariate HR of 2.62 (95% CI, 1.72–3.98; P < 0.001; Table 2 and Fig. 2B).
In the N0 subset of patients who did not receive chemotherapy, 82% (N = 978) were HER2−. BCI was significantly prognostic for overall DR (P < 0.001) with a multivariate HR of 2.51 (95% CI, 1.41–4.47) for intermediate-risk and 5.00 (95% CI, 2.83–8.84) for the high-risk versus low-risk groups (Table 2 and Fig. 2C). Over half (51%, N = 498) of N0 HER2− patients were in the BCI low-risk group, with a 10-year DR rate of 6.8% (95% CI, 3.8–9.7), whereas 29% (N = 280) and 20% (N = 200) of N0 HER2− patients were in the BCI intermediate and BCI high-risk groups, with a 10-year DR rate of 12.6% (95% CI, 8.2–16.8) and 22.7% (95% CI, 16.1–28.9), respectively (Table 2 and Fig. 2C).
Among N1 patients who did not receive chemotherapy, 86% (N = 1,067) were HER2−. BCIN+ significantly stratified these patients into two risk groups with a multivariate HR of 2.37 (95% CI, 1.53–3.67) for overall DR (P < 0.001; Table 2 and Fig. 2D). The low-risk group consisted of 24% of patients (N = 258) with a 10-year DR rate of 10.5% (95% CI, 6.3–14.5) and the high-risk group included 76% of patients (N = 809) with a 10-year DR rate of 23.7% (95% CI, 20.4–27.0; Table 2 and Fig. 2D).
In both the overall and HER2− cohort, the risk of overall DR increased exponentially with higher BCI and BCIN+ scores (Supplementary Fig. S1).
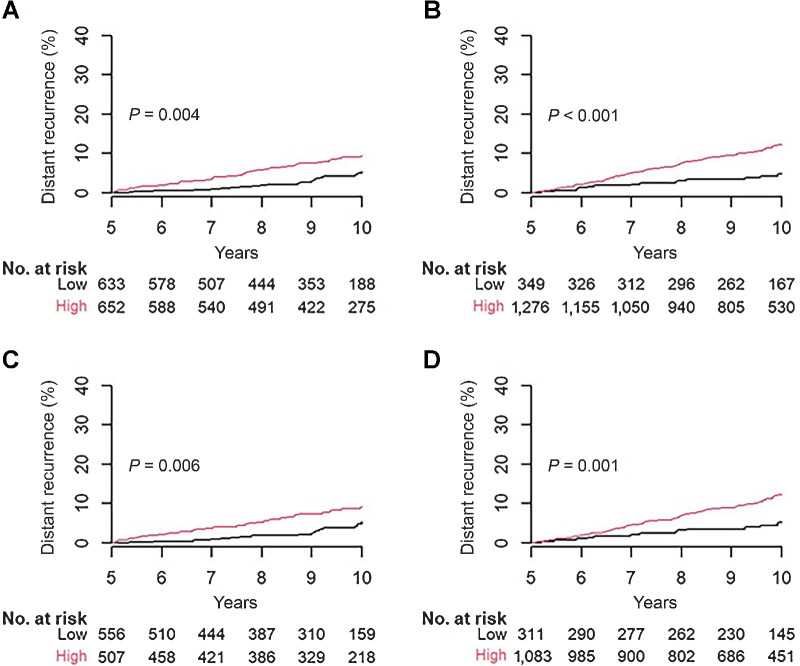
Performance of BCI and BCIN± for late DR
Among the 3,544 patients in this study, 82% (N = 2,910) were DR-free for at least 5 years, including 1,285 N0 and 1,625 N1 patients. Among these patients, 21% and 41% of the N0 and N1 patients received prior chemotherapy, respectively. For late DR, BCI significantly stratified these 1,285 N0 patients into two prognostic groups with a multivariate HR of 2.25 (95% CI, 1.30–3.89; P = 0.004; Table 2 and Fig. 3A): 49% (N = 633) were low-risk with a late DR rate of 5.4% (95% CI, 3.0–7.8) and 51% (N = 652) were high-risk with a late DR rate of 9.3% (95% CI, 6.7–11.8).
Figure 3.
Prognostic performance of BCI and BCIN+ for late DR in N0 and N1 patients who were DR free at 5 years. A, BCI stratification in 1,285 N0 patients. B, BCIN+ stratification in 1,625 N1 patients. C, BCI stratification in 1,063 N0 HER2− patients. D, BCIN+ stratification in 1,394 N1 HER2− patients.
BCIN+ significantly stratified 1,625 N1 patients with respect to risk of late DR into low- and high-risk groups with a multivariate HR of 2.67 (95% CI, 1.53–4.63; P < 0.001; Table 2 and Fig. 3B). The low-risk group, consisting of 21% of N1 patients (N = 349), had a late DR rate of 4.8% (95% CI, 2.3–7.3), whereas the high-risk group, comprising 79% of N1 patients (N = 1,276), exhibited a late DR rate of 12.2% (95% CI, 10.1–14.2; Table 2 and Fig. 3B).
In the HER2− subsets, BCI and BCIN+ remained significantly prognostic for late DR in N0 and N1 patients, respectively (P = 0.006 and P < 0.001; Fig. 3C and D). Out of 1,063 N0 HER2− patients, BCI classified 52% (N = 556) into a low-risk group with a late DR rate of 5.3% (95% CI, 2.6–7.9) and 48% (N = 507) into a high-risk group with a late DR rate of 9.0% (95% CI, 6.1–11.8), resulting in a multivariate HR of 2.53 (95% CI, 1.37–4.67; Table 2 and Fig. 3C).
In the late DR analysis, 85% of N1 patients (N = 1,394) were HER2−. BCIN+ stratified 22% of patients (N = 311) into a low-risk group with a late DR rate of 5.1% (95% CI, 2.3–7.8), and 78% patients (N = 1,083) as high-risk with a late DR rate of 12.1% (95% CI, 9.9–14.3), resulting in a multivariate HR of 2.49 (95% CI, 1.40–4.44; Table 2 and Fig. 3D).
Similar to the findings for overall DR, the risk of late DR increased with higher BCI and BCIN+ scores in both the overall and HER2− cohort (Supplementary Fig. S2).
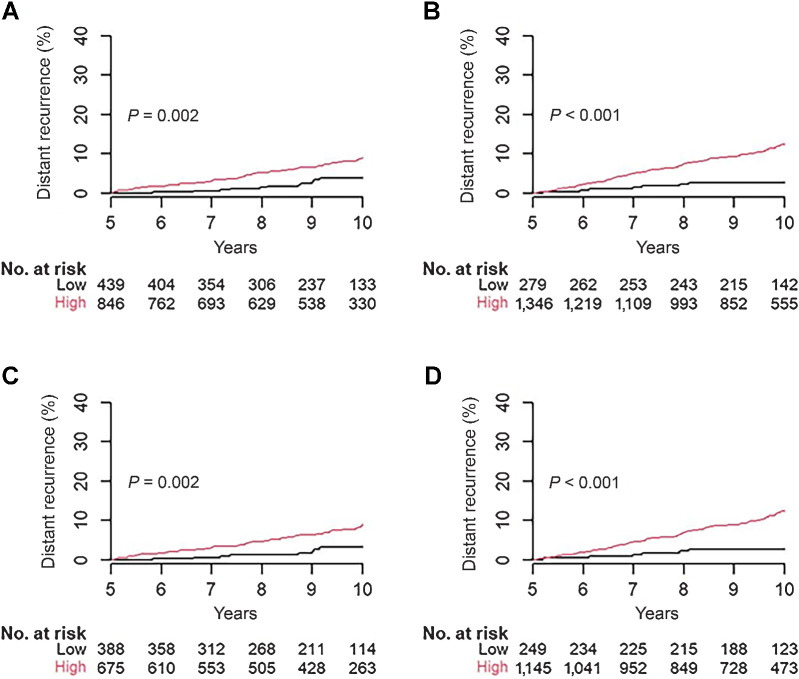
Performance of BCI and BCIN± with optimized cutoff points for late DR
Previous work demonstrated that alternative BCI cutoff points specifically optimized for late DR resulted in improved prognostic performance for both BCI and BCIN+ (13, 14). Using these optimized cutoff points, BCI significantly stratified 1,285 N0 patients into two prognostic groups (P = 0.002; Fig. 4A): a low-risk group consisting of 34% of N0 patients (N = 439) with a late DR rate of 3.8% (95% CI, 1.5–6.0) and a high-risk group, including 66% of N0 patients (N = 846) with a late DR rate of 9.1% (95% CI, 6.8–11.4) resulting in a multivariate HR of 2.63 (95% CI, 1.36–5.12; Fig. 4A; Supplementary Table S1). BCIN+ also significantly stratified the risk of late DR in 1,625 N1 patients using the optimized cutoff points (P < 0.001; Fig. 4B). The low-risk group, consisting of 16% of N1 patients (N = 279), exhibited a late DR rate of 2.7% (95% CI, 0.7–4.7), whereas the high-risk group, comprising 84% of N1 patients (N = 1,346), demonstrated a late DR rate of 12.3% (95% CI, 10.3–14.3), resulting in a multivariate HR of 4.34 (95% CI, 2.03–9.28; Fig. 4B; Supplementary Table S1).
Figure 4.
Prognostic performance for late DR with optimized BCI/BCIN+ cutoff points. A, Stratification of risk of late DR in N0 patients (N = 1,285) by BCI. B, Stratification of risk of late DR in N1 patients (N = 1,625) by BCIN+. C, Stratification of risk of late DR in N0 HER2− patients (N = 1,063) by BCI. D, Stratification of risk of late DR in N1 HER2− patients (N = 1,394) by BCIN+.
In HER2− patients, BCI and BCIN+ demonstrated statistically significant prognostication using the optimized cutoff points (P = 0.002 and P < 0.001; Fig. 4C and D). In the N0 HER2− subset (N = 1,063), BCI stratified 37% of patients (N = 388) into low-risk with a late DR rate of 3.1% (95% CI, 0.9–5.3) and 63% of patients (N = 675) into high-risk with a late DR rate of 9.0% (95% CI, 6.4–11.6), resulting in a multivariate HR of 3.22 (95% CI, 1.50–6.93; Fig. 4C; Supplementary Table S1). In the N1 HER2− subset (N = 1,394), BCIN+ stratified 17% of patients (N = 249) into a low-risk group with a late DR rate of 2.6% (95% CI, 0.5–4.7) and 83% of patients (N = 1,145) into a high-risk group with a late DR rate of 12.3% (95% CI, 10.1–14.5), with a multivariate HR of 4.44 (95% CI, 1.95–10.11; Fig. 4D; Supplementary Table S1).
Discussion
In this translational TEAM study, consistent with previous BCI validation studies (9, 12, 13), BCI and BCIN+ were confirmed to be significantly prognostic for risk of overall and late DR in postmenopausal women with N0 and N1 HR+ breast cancer, respectively. These results suggest that BCI demonstrates the ability to inform on two important clinical decision points in the management of these patients. At time of diagnosis, 24% of N0 and 77% of N1 patients were classified by BCI and BCIN+ as high-risk, who did not benefit sufficiently from endocrine therapy alone with 10-year DR risks of 23.5% and 24.6%, respectively. These patients might be a group for whom additional therapy could be considered. At the time point of 5 years after diagnosis, 49% of N0 and 21% of N1 patients were classified by BCI and BCIN+ as low-risk with a 10-year late DR risk of 5.4% and 4.8%, respectively, after receiving only 5 years of endocrine therapy. In particular, with the new alternative cutoff points optimized specifically for late DR, the low-risk patients identified by BCI and BCIN+ demonstrated a very low 10-year risk of late DR of 3.8% and 2.7% for N0 and N1 patients, respectively, suggesting 5 years of endocrine therapy might be sufficient for these patients.
Traditionally, clinical and pathologic factors such as tumor size, tumor grade, and extent of nodal involvement have been used to assess the risk of late DR (18, 19). In particular, the Clinical Treatment Score post-5 years (CTS5) has been described as a prognostic tool to estimate the risk of late DR based on age, tumor size, grade, and nodal involvement (20). However, these measures can be limited in their prognostic power. For example, in an EBCTCG meta-analysis, pN1 patients with small tumors (T1) had a risk of late DR of 7%, 14%, and 20% between years 5 to 10, 5 to 15, and 5 to 20, respectively (6). In addition, an analysis of CTS5 in the TEAM and IDEAL trials showed that CTS5 not only overestimated the risk of late DR for high-risk patients, but also did not predict the benefit of EET (21). Thus, identification of patients with a limited risk of late DR can be challenging based on clinicopathologic factors alone.
This study has shown that BCI and BCIN+ remained statistically significant for N0 and N1 patients in multivariate models adjusted for age, tumor size, tumor grade, and treatment, consistently demonstrating that BCI and BCIN+ provide independent prognostic information beyond clinicopathological factors. Two other gene expression-based classifiers have been shown to prognosticate late DR in HR+ breast cancer (22, 23). The PAM50-based ROR score identified a low-risk group with 2.3% and 3.3% risk of 5–10 years late DR for N0 and N1 patients in the combined ABCSG-8/ATAC cohorts, respectively (22). Similarly, the EPclin score was able to identify a low-risk group with 3.1% and 13.0% risk of 5–15 years late DR for N0 and N1 patients in the combined ABCSG-6/8 cohorts, respectively (23). However, neither of them has been demonstrated to be predictive of benefit from EET. On the other hand, although the 70-gene assay did not specifically conduct studies on the late DR risk in patients who remained DR-free at 5 years, it classified 16% of tamoxifen-treated patients from the STO-3 study as an “ultralow-risk” group with a 97% cumulative 20-year breast cancer–specific survival (24). In the same STO-3 study, BCI was also able to derive a minimal risk group, including 27% of tamoxifen-treated patients with a 98% cumulative 20-year breast cancer–specific survival (24). In summary, BCI provides both prognostic and predictive information in the late recurrence and extended endocrine treatment setting and has been recognized by NCCN and ASCO to predict benefit of EET (25, 26).
N1 patients are at significantly greater risk of recurrence (4–6) and have lower rates of both DFS and overall survival when compared with N0 patients (27–30). Consequently, N1 patients are recommended to receive additional treatment than N0 patients, including EET, adjuvant chemotherapy, and ovarian suppression (25, 31). However, previous BCI results in Trans-aTTom indicate that nearly half of all N1 patients did not derive significant benefit from longer duration of tamoxifen treatment (32). Furthermore, even though endocrine therapy is generally better tolerated compared with chemotherapy, adverse side effects are both common and significant, and can include vasomotor symptoms and sexual dysfunction; osteoporosis, skeletal fractures, and musculoskeletal symptoms associated with AIs; and endometrial cancer and venous thrombosis associated with tamoxifen (33–35). Thus, an accurate personalized assessment of risk and benefit for each patient, as offered by BCI, is important for adjuvant therapy decision-making.
More importantly, the BCI (H/I) ratio has been extensively validated as a predictive biomarker for the benefit of EET, including either tamoxifen or AIs (32, 36–38). On the basis of this clinical evidence, BCI has been recognized by national guidelines such as NCCN and ASCO (25, 26). When combining both BCI prognostic and BCI (H/I)-predictive results based on the optimized prognostic cutoff points, 56% of patients were BCI (H/I) predictive of low-likelihood to benefit and thus could be spared from potential toxicities of EET (Supplementary Table S2). Among these patients, those classified as BCI prognostic high-risk could consider other alternative therapeutic approaches to address their residual high risk of late DR. On the other hand for those predicted to be high-likelihood to benefit by BCI (H/I), using the predicted benefit of 58%–62% relative risk reduction estimated from previous BCI predictive studies (32, 36–38), 38% of patients were also BCI prognostic high-risk, with absolute risk of late DR between 9.1% and 12.3%, and might be able to derive an estimated 5%–9% absolute recurrence risk reduction from EET; whereas 6% of patients who were BCI prognostic low-risk might only derive a modest benefit of 2%–3% with EET, therefore, their treatment decision should be made on an individual patient basis. In summary, BCI adds important information to better manage HR+ patients with breast cancer to personalize EET decision-making.
This study has strengths and limitations. The study was a retrospective analysis of a prospective clinical trial representing the largest BCI validation study to date that included both N0 and N1 patients. In addition, the treatment regimen represented contemporary endocrine therapy in the US with 5 years of either an AI or a sequence of tamoxifen and AI for postmenopausal women. Although the analysis was retrospective, the study was prospectively defined in a Statistical Analysis Plan and BCI testing was conducted blinded to clinical outcome.
In summary, this study has confirmed the independent prognostic ability of BCI for both overall and late DR in HR+ postmenopausal patients, irrespective of nodal status, who were treated with adjuvant endocrine therapy, including an AI as part of primary adjuvant endocrine therapy. On the basis of these results, BCI provides clinically important information to facilitate the selection of treatments at two important decision points in the management of HR+ breast cancer: first at time of diagnosis for potential chemotherapy benefit and second at 5 years following diagnosis to predict the benefit from extended endocrine treatment.
Supplementary Material
Supplemental Table 1. Prognostic performance of BCI and BCIN+ optimized cut-points for late DR in all patients with N0 and N1 breast cancers, as well as in HER2- subsets, respectively.
Supplemental Table 2. Distribution of patients as defined by BCI prognostic (BCI/BCIN+) and BCI predictive (BCI (H/I)).
Supplemental Figure 1. Continuous risk curves of BCI and BCIN+ for overall 10-year DR in patients who did not receive adjuvant chemotherapy. A: risk of overall 10-year DR as a function of continuous BCI for N0 patients (N=1196); B: risk of overall 10-year DR as a function of continuous BCIN+ for N1 patients (N=1234); C: risk of overall 10-year DR as a function of continuous BCI for N0 HER2- patients (N=978); D: risk of overall 10-year DR as a function of continuous BCIN+ for N1 HER2- patients (N=1067).
Supplemental Figure 2. Continuous risk curves of BCI and BCIN+ for late 10-year DR in patients DR-free at 5 years. A: risk of 10-year late DR as a function of continuous BCI for N0 patients (N=1285); B: risk of 10-year late DR as a function of continuous BCIN+ for N1 patients (N=1625); C: risk of 10-year late DR as a function of continuous BCI for N0 HER2- patients (N=1063); D: risk of 10-year late DR as a function of continuous BCIN+ for N1 HER2- patients (N=1394).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J.M.S. Bartlett reports grants from Biotheranostics, a Hologic Company during the conduct of the study. J.M.S. Bartlett also reports grants, personal fees, and non-financial support from Biotheranostics, a Hologic Company and NanoString Technologies, Inc.; personal fees from Insight Genetics, Inc., BioNTech AG, Pfizer, Rna Diagnostics Inc., oncoXchange/MedcomXchange Communications Inc., Herbert Smith French Solicitors, Oncology Education, and OncoCyte Corporation; grants from Thermo Fisher Scientific, Amgen, Genoptix, and Stratifyer GmbH; and non-financial support from Breast Cancer Society of Canada outside the submitted work. G. Pond reports personal fees from AstraZeneca, Takeda, Merck, and Profound Medical outside the submitted work, as well as stock in Roche Ltd; in addition, G. Pond reports employment of a close family member with Roche Canada. Y. Zhang reports patents on the biomarker analyzed in this work pending and issued. M. Spears reports grants from Biotheranostics Inc. during the conduct of the study. D. Rea reports other project funding from Biotheranostics. C.A. Schnabel reports other support from Biotheranostics/Hologic during the conduct of the study; C.A. Schnabel also reports a patent for BCI pending and issued. K. Treuner reports a patent for BCI pending. J. Bayani reports grants from Biotheranostics, a Hologic Company during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
J.M.S. Bartlett: Conceptualization, resources, supervision, investigation, writing–review and editing. K. Xu: Formal analysis, writing–review and editing. J. Wong: Writing–review and editing. G. Pond: Data curation, formal analysis. Y. Zhang: Conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing–original draft. M. Spears: Writing–review and editing. R. Salunga: Formal analysis, methodology. E. Mallon: Writing–review and editing. K.J. Taylor: Writing–review and editing. A. Hasenburg: Writing–review and editing. C. Markopoulos: Writing–review and editing. L. Dirix: Writing–review and editing. C.J.H. van de Velde: Writing–review and editing. D. Rea: Writing–review and editing. C.A. Schnabel: Conceptualization, supervision, investigation, methodology, writing–original draft, writing–review and editing. K. Treuner: Conceptualization, supervision, investigation, methodology, writing–original draft, writing–review and editing. J. Bayani: Formal analysis, writing–review and editing.
References
- 1. Krauss K, Stickeler E.. Endocrine therapy in early breast cancer. Breast Care 2020;15:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crystal JS, Rand J, Johnson J, Kim S, Basho R, Amersi F, et al. Adjuvant endocrine therapy is associated with improved overall survival in elderly hormone receptor–positive breast cancer patients. Breast Cancer Res Treat 2020;184:63–74. [DOI] [PubMed] [Google Scholar]
- 3. Walsh EM, Smith KL, Stearns V. Management of hormone receptor-positive, HER2-negative early breast cancer. Semin Oncol 2020;47:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet North Am Ed 2005;365:1687–717. [DOI] [PubMed] [Google Scholar]
- 5. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135–41. [DOI] [PubMed] [Google Scholar]
- 6. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen RN, Esen BÖ, Mellemkjær L, Christiansen P, Ejlertsen B, Lash TL, et al. The incidence of breast cancer recurrence 10–32 years after primary diagnosis. J Natl Cancer Inst 2022;114:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian breast and colorectal cancer study group trial 6a. J Natl Cancer Inst 2007;99:1845–53. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Schnabel CA, Schroeder BE, Jerevall PL, Jankowitz RC, Fornander T, et al. Breast cancer index identifies early-stage estrogen receptor–positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205. [DOI] [PubMed] [Google Scholar]
- 10. Sgroi DC, Carney E, Zarrella E, Steffel L, Binns SN, Finkelstein DM, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habel LA, Sakoda LC, Achacoso N, Ma XJ, Erlander MG, Sgroi DC, et al. HOXB13: IL17BR and molecular grade index and risk of breast cancer death among patients with lymph node-negative invasive disease. Breast Cancer Res 2013;15:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Schroeder BE, Jerevall PL, Ly A, Nolan H, Schnabel CA, et al. A novel breast cancer index for prediction of distant recurrence in HR +early-stage breast cancer with one to three positive nodes. Clin Cancer Res 2017;23:7217–24. [DOI] [PubMed] [Google Scholar]
- 13. Bartlett JMS, Zhang Y, Ahmed I, Treuner K, Piper T, Pirrie SJ, et al. 11P A Breast Cancer Index (BCI) prognostic model for N0 HR +breast cancer optimized for late distant recurrence. Ann Oncol 2021;32:S25. [Google Scholar]
- 14. Liefers G-J, Noordhoek I, Zhang Y, Sgroi DC, Putter H, Treuner K, et al. 7P An optimized Breast Cancer Index node-positive (BCIN +) prognostic model for late distant recurrence in patients with hormone receptor-positive (HR +) node-positive breast cancer. Ann Oncol 2021;32:S23–4. [Google Scholar]
- 15. Van De Velde CJ, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel JM, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet North Am Ed 2011;377:321–31. [DOI] [PubMed] [Google Scholar]
- 16. Derks MGM, Blok EJ, Seynaeve C, Nortier JWR, Kranenbarg EMK, Liefers GJ, et al. Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:1211–20. [DOI] [PubMed] [Google Scholar]
- 17. Fleming TR, Harrington DP. Nonparametric estimation of the survival distribution in censored data. Commun Theory Methods 1984;13:2469–86. [Google Scholar]
- 18. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46. [DOI] [PubMed] [Google Scholar]
- 19. Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst 2008;100:1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dowsett M, Sestak I, Regan MM, Dodson A, Viale G, Thürlimann B, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor–positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol 2018;36:1941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noordhoek I, Blok EJ, Meershoek-Klein Kranenbarg E, Putter H, Duijm-De Carpentier M, Rutgers EJT, et al. Overestimation of late distant recurrences in high-risk patients with ER-positive breast cancer: validity and accuracy of the CTS5 risk score in the TEAM and IDEAL trials. J Clin Oncol 2020;38:3273–81. [DOI] [PubMed] [Google Scholar]
- 22. Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and Arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22. [DOI] [PubMed] [Google Scholar]
- 23. Filipits M, Dubsky P, Rudas M, Greil R, Balic M, Bago-Horvath Z, et al. Prediction of distant recurrence using endopredict among women with ER+, HER2− node-positive and node-negative breast cancer treated with endocrine therapy only. Clin Cancer Res 2019;25:3865–72. [DOI] [PubMed] [Google Scholar]
- 24. Kaklamani VG, Poage GM, Fornander T, Nordenskjold B, Stål O, Zhang Y, et al. Genomic stratification with BCI of ER +early breast cancer patients with limited long-term risk of breast cancer death. Am Soc Clin Oncol; 2018;36:516. [Google Scholar]
- 25. Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, gnese D, et al. NCCN Guidelines® Insights: breast cancer, version 4.2023: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2023;21:594–608. [DOI] [PubMed] [Google Scholar]
- 26. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol 2022;40:1816–37 [DOI] [PubMed] [Google Scholar]
- 27. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181–7. [DOI] [PubMed] [Google Scholar]
- 28. Hatoum HA, Jamali FR, El-Saghir NS, Musallam KM, Seoud M, Dimassi H, et al. Ratio between positive lymph nodes and total excised axillary lymph nodes as an independent prognostic factor for overall survival in patients with nonmetastatic lymph node-positive breast cancer. Ann Surg Oncol 2009;16:3388–95. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Yin Z, Wang D, Zhang J, Wang S, Zhao J, et al. Greater negative lymph node count predicts favorable survival of patients with breast cancer in the setting of neoadjuvant chemotherapy and mastectomy. Future Oncol 2019;15:3701–9. [DOI] [PubMed] [Google Scholar]
- 30. Singh D, Mandal A. The prognostic value of lymph node ratio in survival of non-metastatic breast carcinoma patients. Breast Cancer Res Treat 2020;184:839–48. [DOI] [PubMed] [Google Scholar]
- 31. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 2019;37:423–38. [DOI] [PubMed] [Google Scholar]
- 32. Bartlett JMSS, Sgroi DC, Treuner K, Zhang Y, Ahmed I, Piper T, et al. Breast cancer index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial. Ann Oncol 2019;30:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54. [DOI] [PubMed] [Google Scholar]
- 35. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet North Am Ed 2013;381:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sgroi D, Carney E, Richardson E, Steffel L, Binns SN, Finkelstein DM, et al. Prediction of late recurrences by breast cancer index in the NCIC CTG MA.17 cohort. J Clin Oncol 2011;29. [Google Scholar]
- 37. Noordhoek I, Treuner K, Putter H, Zhang Y, Wong J, Kranenbarg EMK, et al. Breast cancer index predicts extended endocrine benefit to individualize selection of patients with HR +early-stage breast cancer for 10 years of endocrine therapy. Clin Cancer Res 2021;27:311–9. [DOI] [PubMed] [Google Scholar]
- 38. Mamounas EP, Bandos H, Rastogi P, Zhang Y, Treuner K, Lucas PC, et al. Breast cancer index and prediction of extended aromatase inhibitor therapy benefit in hormone receptor-positive breast cancer from the NRG Oncology/NSABP B-42 trial. Clin Cancer Res 2024Feb 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Prognostic performance of BCI and BCIN+ optimized cut-points for late DR in all patients with N0 and N1 breast cancers, as well as in HER2- subsets, respectively.
Supplemental Table 2. Distribution of patients as defined by BCI prognostic (BCI/BCIN+) and BCI predictive (BCI (H/I)).
Supplemental Figure 1. Continuous risk curves of BCI and BCIN+ for overall 10-year DR in patients who did not receive adjuvant chemotherapy. A: risk of overall 10-year DR as a function of continuous BCI for N0 patients (N=1196); B: risk of overall 10-year DR as a function of continuous BCIN+ for N1 patients (N=1234); C: risk of overall 10-year DR as a function of continuous BCI for N0 HER2- patients (N=978); D: risk of overall 10-year DR as a function of continuous BCIN+ for N1 HER2- patients (N=1067).
Supplemental Figure 2. Continuous risk curves of BCI and BCIN+ for late 10-year DR in patients DR-free at 5 years. A: risk of 10-year late DR as a function of continuous BCI for N0 patients (N=1285); B: risk of 10-year late DR as a function of continuous BCIN+ for N1 patients (N=1625); C: risk of 10-year late DR as a function of continuous BCI for N0 HER2- patients (N=1063); D: risk of 10-year late DR as a function of continuous BCIN+ for N1 HER2- patients (N=1394).
Data Availability Statement
The data analyzed in the current study are not publicly available because they contain patient data and proprietary information. Aggregated data analyzed in the study are included in the article. Qualified researchers may contact the corresponding author with reasonable requests to view additional data.