Abstract
The burden of cardiovascular disease (CVD) in patients with metabolic dysfunction-associated steatohepatitis (MASH) is poorly characterized, particularly vs other liver diseases including metabolic dysfunction-associated steatotic liver disease (MASLD). To identify available evidence, Embase, MEDLINE, and Cochrane database searches (main search: 2011–September 6, 2021; additional ad hoc search [MEDLINE only]: September 7, 2021–February 15, 2023), plus manual searches (2019–September 2021), were performed. Studies reporting CVD outcomes (angina, coronary artery disease [CAD], heart failure, myocardial infarction, peripheral artery disease, stroke, venous thromboembolic disease, and CV mortality) in adults with histologically confirmed MASH and MASLD or other liver diseases were identified, with studies of MASLD without confirmed MASH excluded. Of 8732 studies, 21 were included. An increased incidence or prevalence of CVD in patients with MASH vs other conditions was reported in 12 studies; odds ratios (OR), where reported, ranged from 3.12 (95 % CI: 1.33–5.32) to 4.12 (95 % CI: 1.91–8.90). The risk of CAD was increased in people with MASH in 6 of 7 studies, while the risk of stroke was increased in 6 of 6 studies, and heart failure in 2 of 4 studies. Three of 6 studies provided evidence of increased CVD-related mortality in patients with MASH vs those without. In conclusion, this literature review suggests that CVD is prevalent in patients with MASH and may contribute to increased mortality. Accordingly, cardiovascular risk factors should be aggressively managed in this population. Whether the CVD burden in patients with MASH is a direct consequence of MASH itself requires further study.
Keywords: Cardiovascular disease, Epidemiology, Mortality, Non-alcoholic steatohepatitis
Highlights
-
•
The burden of CVD in MASH is not well characterized vs comparators such as MASLD.
-
•
This literature review identified studies that reported CVD outcomes in populations with MASH vs comparators.
-
•
The results suggest CVD is prevalent in patients with MASH.
-
•
Patients with MASH have a greater CVD risk than those with other liver disease etiologies.
-
•
CV risk factors should be carefully and proactively managed in patients with MASH.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), now known as metabolic dysfunction-associated steatotic liver disease (MASLD) [1], is a leading cause of liver disease globally, affecting approximately 1/4 of the adult population [2]. The prevalence of MASLD has increased dramatically in recent decades and this trend is predicted to continue [3]. In the USA, MASLD cases are forecasted to increase to >100 million by 2030 [4].
MASLD encompasses a spectrum of liver diseases ranging from isolated steatosis in the presence of cardiometabolic risk factors to the progressive, severe subtype of non-alcoholic steatohepatitis (NASH), now known as metabolic dysfunction-associated steatohepatitis (MASH) [2]. Up to 20 % of patients with MASLD have MASH, and these patients may progress to advanced fibrosis, cirrhosis, and/or hepatocellular carcinoma [2]. MASH is associated with a reduction in health-related quality of life and imposes a substantial healthcare burden [2,5]. MASH has also been linked to increased risk of cardiovascular (CV) disease (CVD), and CVD is the leading cause of morbidity and mortality in patients with MASLD/MASH [6,7]. However, many aspects of the burden of CVD among patients with MASH remain poorly characterized.
Although several literature reviews and meta-analyses have investigated the burden of CVD in patients with NAFLD/MASLD, there is a lack of such studies specifically focusing on patient populations with MASH. This systematic literature review was conducted to identify the available evidence and gain a better understanding of the epidemiological burden of CVD in patients with MASH.
2. Materials and methods
2.1. Data sources and searches
To identify relevant studies, searches of the electronic databases Embase (Elsevier), MEDLINE (PubMed), and The Cochrane Library (Wiley) were conducted on September 6, 2021, through the Ovid platform. A further ad hoc search of MEDLINE was performed to identify studies published between September 7, 2021, and February 15, 2023. Other key resources, including conference proceedings, reference lists of included publications and relevant reviews, and selected websites (Supplementary Table SI) were searched manually to identify studies not captured in the electronic database searches. A broad approach was used for the electronic database search strategy (Supplementary Table SII). As this review was conducted prior to the MASLD/MASH nomenclature update [1], NAFLD/NASH terms were included in the search strategy, and therefore these terms have been retained when reporting the methods and results of the current review.
2.2. Study selection
Studies were required to have included adults with histological confirmation of NASH and report CVD outcomes (Table I).
Table I.
Eligibility criteria for studies included in this review.
| Criteria | Include | Exclude |
|---|---|---|
| Population | Adult patients with NASH reporting CVD outcomes | Patients with any other disease |
| Intervention/Comparator | Population with NAFLD or other liver disease etiologies as comparator | Other interventions |
| Outcomes | Outcomes evaluated included: angina, CAD, HF, MI, PAD, stroke, venous thromboembolic disease, and CV mortality, reported alone or in combination | Other outcomes |
| Study design/setting |
|
|
| Language of publication | English language publications | Studies published in languages other than English |
| Date of publication |
|
Prior to 2011 |
| Countries | No restrictions | – |
CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease; HSUV, health state utility value; HF, heart failure; MI, myocardial infarction; NAFLD, non-alcoholic fatty liver disease;
NASH, non-alcoholic steatohepatitis; PAD, peripheral artery disease; QoL, quality of life; SLR, systematic literature review.
Outcomes of focus included angina, coronary artery disease (CAD), heart failure, myocardial infarction, peripheral artery disease, stroke, venous thromboembolic disease, and CV mortality. A total of 2 independent analysts used a bespoke database to remove duplicates and screened abstracts using the pre-defined eligibility criteria. An independent expert resolved any discrepancies. Full-text publications were obtained for abstracts included after the first-pass screening, and these were further screened by 2 independent analysts using the eligibility criteria. The final studies for inclusion were verified by the project lead and any disputes regarding eligibility were resolved by an independent expert.
2.3. Data extraction and quality assessment
A data extraction template in Microsoft® Excel was used to collect data on the design, patient characteristics, and outcomes of the included studies. Data extraction was conducted by a single analyst and reviewed and quality checked by the project lead. The reported methodological quality of the included studies was assessed using the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies [8]. Given our focus on the burden of CVD in patients with NASH, the resulting studies were further selected to include data on the incidence and/or prevalence of CVD in patients with NASH. Furthermore, to place the reported prevalence/incidence in context, selection of studies was restricted to only those that reported on a comparator arm. This was done following a literature review that revealed several analyses characterizing the CVD burden in NAFLD, but a lack of such studies specifically focusing on patient populations with NASH.
3. Results
3.1. Search results
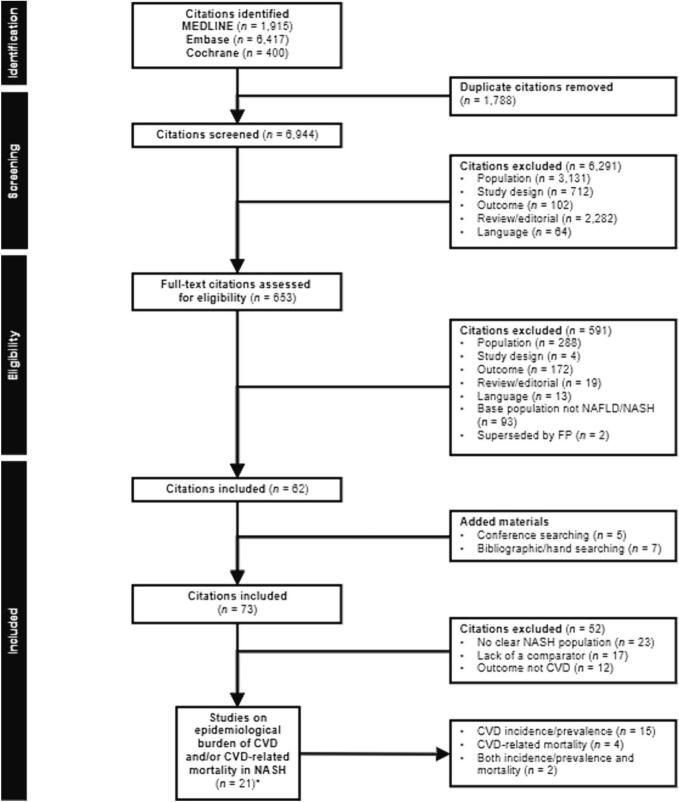
The main electronic database search identified 8732 studies and the ad hoc search identified 12 additional studies (Fig. 1).
Fig. 1.
PRISMA flow diagram.
*A total of 2 citations reported data from the same study.
CVD, cardiovascular disease; FP, full publication; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
After removing duplicates, the remaining 6944 citations were screened using titles and abstracts. Of these, 653 studies had a full assessment of eligibility based on full-text publications.
After excluding 591 publications, 62 were eligible for inclusion. A further 12 publications were added based on searching of conference abstracts (n = 5) and additional manual searches (n = 7). A total of 73 unique studies underwent a complete systematic synthesis, with 52 studies excluded based on the lack of a clearly defined NASH population (n = 23), lack of a comparator (n = 17) or because the outcome was a proxy for CVD rather than CVD itself, or the focus was on the economic and humanistic burden of NASH rather than clinical outcomes (n = 12). Among the 21 remaining eligible studies, 15 provided CVD incidence/prevalence information [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]], 4 provided CVD-related mortality information [[24], [25], [26], [27]], and 2 provided both [28,29] (Fig. 1). Most studies included were assessed as being of moderate to strong quality (Supplementary Table SIII).
3.2. Relative epidemiological burden of CVD in patients with NASH
A total of 17 studies reported data on the relative epidemiological burden of CVD in patients with NASH vs a comparator population (summarized in Table II), with 12 studies reporting an increased incidence or prevalence of CVD in patients with NASH vs those without.
Table II.
Summary of epidemiological studies reporting on the burden of CVD in patients with NASH.
| Author, year (country) | Database | Study design | Population | Comparator/subpopulation (cirrhosis/fibrosis etc.) and comorbidities | Sample size, n | CV/cerebrovascular events (%) | Limitations |
|---|---|---|---|---|---|---|---|
| Studies comparing patients with NASH vs patients with NAFLD | |||||||
| Ampuero, 2018 [9] (Spain) |
HEPAmet Spanish Registry | Prospective cohort study using registry analysis Mean follow-up: 2.6 years |
Patients with biopsy-proven NAFLD (NASH/fibrosis defined according to Steatosis, Activity, and Fibrosis score) Obesity: 56.7 % HTN: 46.3 % T2D: 31.7 % Hypertriglyceridemia: 38.8 % Dyslipidemia: 32.4 % |
NASH | 265 | CVD: 5.2 (P = 0.4 vs NAFLD) | Data available from abstract only; data on comorbidities only reported for the overall population and not for the individual subpopulations |
| NAFLD | 303 | CVD: 2.7 | |||||
| Domanski, 2012 [11] (USA) |
Brooke Army Medical Center within the Gastro-enterology and Hepatology clinic | Retrospective chart review | Biopsy-confirmed NAFLD (including NASH and non-NASH fatty liver) | NASH Obesity: 72.1 % DM: 47.5 % (P < 0.01 vs non-NASH fatty liver) |
219 | CVD: 6.8a HF: 1.8 MI: 3.2 Unstable angina: 3.2 Stroke: 1.4 Revascularization: 4.6 |
Low number of patients identified with CVD, increasing the potential for a type 2 error |
| Non-NASH fatty liver Obesity: 67.7 % DM: 22.8 % |
158 | CVD: 6.3 HF: 1.9 MI: 1.3 Unstable angina: 1.3 Stroke: 1.3 Revascularization: 2.5 |
|||||
| Park 2021 [16] (Republic of Korea) |
Boramae NAFLD registry (NCT 02206841) | Retrospective cross-sectional analysis | Biopsy-confirmed NAFLD (NASH: 45.7 %, NAFL: 54.3 %) Liver biopsy performed if ≥2 risk factors were present (central obesity, triglycerides ≥150 mg/dL, HDL-C <40 mg/dL [male]/<50 mg/dL [female], presence of HTN or insulin resistance, or clinically suspected NASH or liver fibrosis) |
NASH Mean BMI: 27.6 ± 3.6 kg/m2 Obesity: 41.2 % HTN: 42.4 % DM: 46.8 % |
182 | 10-year risk of atherosclerotic CVD: OR [95 % CI] 4.07 [1.40–11.88]; P = 0.014 vs non-NAFLD group |
CV event rates not reported; the instrument used to estimate CVD risk may underestimate this in the NAFLD and non-NAFLD subpopulations |
| NAFL Mean BMI: 27.0 ± 3.1 kg/m2 Obesity: 48.5 % HTN: 41.6 % DM: 43.6 % (All P < 0.05 vs NASH) |
216 | 10-year risk of atherosclerotic CVD: OR [95 % CI] 1.46 [0.55–3.88]; |
|||||
| Non-NAFLD Mean BMI: 24.3 ± 3.3 kg/m2 Obesity: 10.4 % HTN: 16.0 % DM: 9.6 % (All P < 0.05 vs NASH) |
102 | 10-year risk of atherosclerotic CVD: OR [95 % CI] 1 [reference] |
|||||
| Viglino, 2018 [21] (France) |
Grenoble Alps University Hospital | Prospective longitudinal cohort study 5-year follow-up |
Patients with COPD (liver damage evaluated using the Fibromax® algorithm that combines 3 tests: FibroTest, SteatoTest, and NashTest) BMI: 26 kg/m2 T2D: 74 % |
NASH | 41 | During 5 years of follow-up: CV event: 31.7 (P = 0.48 vs no NASH) Arrhythmia: 4.9 (P = 0.32 vs no NASH) MI: 2.4 (P = 0.37 vs no NASH) Stroke: 2.4 (P = 1 vs no NASH) PAD: 26.8 (P = 0.22 vs no NASH) VTD: 0 (P = 1 vs no NASH) |
Small and specific study sample that might not reflect the overall NASH population; data on BMI and T2D only reported for the overall population and not for individual subpopulations; study design may be limited by potential selection bias of including only patients with COPD; no information provided on how the ‘fibrosis’ cohort differed from the ‘NASH’ cohort |
| Moderate-to-severe steatosis | 46 | During 5 years of follow-up: CV event: 30.4 Arrhythmia: 13.0 MI: 0 Stroke: 0 PAD: 21.7 VTD: 0 |
|||||
| Fibrosis | 68 | During 5 years of follow-up: CV event: 33.8 (P = 0.08 vs no fibrosis) Arrhythmia: 11.8 MI: 1.5 Stroke: 1.5 PAD: 23.5 VTD: 0 |
|||||
| No liver disease | 28 | During 5 years of follow-up: CV event: 14.3 Arrhythmia: 7.1 MI: 0 Stroke: 0 PAD: 14.3 VTD: 3.6 |
|||||
| Weingarten 2011 [22] (USA) |
EMR | Retrospective analysis | Patients with medically complicated obesity who underwent first-time laparoscopic bariatric surgery with intraoperative liver biopsies (biopsy-proven NASH) | No liver disease or simple steatosis DM: 31.2 % HTN: 54.6 % |
141 | CV event within 30 postoperative days: 2.1 Baseline CAD: 9.9 Severe dysrhythmia event within 30 days: 0.7 Baseline CHF: 2.1 MI event within 30 days: 0 |
Short follow-up period of 30 days; study design may be limited by potential selection bias as ‘healthier’ people are usually selected for bariatric surgery, thus the baseline prevalence of CVD is lower than expected for this population |
| Mild NASH DM: 28.5 % HTN: 57.0 % |
151 | CV event within 30 postoperative days: 2.6 Baseline CAD: 7.9 Severe dysrhythmia event within 30 days: 1.3 Baseline CHF: 1.3 MI event within 30 days: 0.7 |
|||||
| Advanced NASH DM: 58.3 % (P < 0.0007 vs mild or no NASH) HTN: 64.6 % |
48 | CV event within 30 postoperative days: 2.1 Baseline CAD: 6.3 (P = 0.8 vs mild or no NASH) Severe dysrhythmia event within 30 days: 0 Baseline CHF: 0 (P = 0.7 vs mild or no NASH) MI event within 30 days: 0 |
|||||
| Studies comparing patients with NASH vs cirrhosis due to other etiologies | |||||||
| Danford, 2019 [10] (USA) |
EMR from Beth Israel Deaconess Medical Center | Retrospective cohort study | NASH (on listing for LT) (documented as recorded by the transplant hepatologist at the initial evaluation) | NASH Obesity: 63.5 % (P = NS vs non-NASH) DM: 51.4 % (P < 0.001 vs non-NASH) HTN: 46.0 % (P = 0.01 vs non-NASH) |
74 | Baselined: 23 % (P = 0.002 vs no NASH) | Baseline prevalence of reported without adjustment for the presence of other parameters; study design may be limited by potential selection bias of including only patients undergoing LT |
| Non-NASH Obesity: 58.5 % DM: 23.4 % HTN: 30.8 % |
881 | Baselined: 11 % | |||||
| Gologorsky 2013 [12] (USA) |
OPTN | Retrospective multicenter analysis | LT recipients | NASH DM: 49.6 % HTN: 33.8 % |
605 | CAD: 7.4 PVD: 1.0 |
Study design may be limited by a potential selection bias of including only patients undergoing LT |
| HCV DM: 19.7 % HTN: 19.9 % |
6000 | CAD: 2.7 PVD: 1.0 |
|||||
| HBV DM: 17.5 % HTN: 19.1 % |
653 | CAD: 2.3 PVD: 1.5 |
|||||
| Alcoholic cirrhosis DM: 18.8 % HTN: 20.4 % |
3168 | CAD: 2.9 PVD: 1.3 |
|||||
| Biliary cirrhosis DM: 10.6 % HTN: 12.3 % |
1541 | CAD: 1.7 PVD: 0.5 |
|||||
| Herndon, 2020 [13] (USA) |
University of Alabama | Retrospective chart review | Patients at high risk for CAD with a coronary evaluation as part of workup for LT | Non-NASH cirrhosis BMI: 29.66 kg/m2 DM: 33.85 % |
65 transplanted | 90 days post-transplant: Arrhythmia: 14.29 MI: 3.17 Stroke: 4.76 |
Data available from abstract only; study design may be limited by potential selection bias of including only patients undergoing LT |
| NASH cirrhosis BMI: 32.65 kg/m2 DM: 63.46 % |
52 transplanted | 90 days post-transplant: Arrhythmia: 9.80 (P = 0.5 vs non-NASH cirrhosis) MI: 3.92 (P = 0.8 vs non-NASH cirrhosis) Stroke: 5.88 (P = 0.8 vs non-NASH cirrhosis) |
|||||
| Kwong, 2020 [14] (USA) |
REALT consortium; EMR | Retrospective multicenter cohort study | LT recipients aged ≥65 years with or without NASH (NASH was considered the primary etiology if the clinical suspicion for the primary cause of chronic liver disease was NASH or if there was a dual diagnosis of HCC and NASH) | Non-NASHb DM without chronic complications: 25.4 % HTN: 54.0 % Hyperlipidemia: 23.8 % |
816 | Baseline CAD: 20.6 Within 12 months of LT: AF: 13.8 HF: 6.2 MI: 2.9 Stroke: 5.0 |
Study design may be limited by a potential selection bias of including only patients undergoing LT |
| NASH DM without chronic complications: 58.5 % HTN: 66.3 % Hyperlipidemia: 46.3 % |
207 | Baseline CAD: 36.7 (P < 0.001 vs no NASH) Within 12 months of LT (all P = NS vs no NASH): AF: 13.7 HF: 6.9 MI: 2.5 Stroke: 7.8 |
|||||
| Park 2011 [15] (USA) |
Transplant Institute at Hawaii Medical Center | Retrospective cohort study | Patients with and without NASH referred for LT Patients divided into NASH and non-NASH groups based on etiology of their end-stage liver disease |
NASH Obesity: 53.4 % (P < 0.001 vs other chronic liver disease) DM: 69.0 % (P < 0.001 vs other chronic liver disease) HTN: 55.2 % (P < 0.001 vs other chronic liver disease) |
71 (including 11 patients with initial diagnoses of ‘cryptogenic cirrhosis’) | Baseline cardiac disease: 22.4 (P < 0.001 vs other chronic liver disease) | Study design may be limited by potential selection bias of including only patients undergoing LT |
| Other chronic liver disease Obesity: 8.0 % DM: 19.9 % HTN: 24.6 % |
472 | Baseline cardiac disease: 4.9 | |||||
| Patel, 2018 [18] (USA) |
Virginia Common-wealth University | Prospective study (follow-up period not stated) |
Patients undergoing elective coronary angiography as part of LT evaluation (patients with cryptogenic cirrhosis: diagnosis of NASH suspected if they had a prior liver biopsy showing steatosis or components of metabolic syndrome in the presence of a negative serological workup for chronic liver disease) | Biopsy-proven NASH cirrhosis Mean BMI: 32.7 ± 6.7 kg/m2 DM: 69.8 % HTN: 62.3 % Obesity: 67.9 % Dyslipidemia: 43.4 % |
53 | At time of LT evaluation: CAD: 52.8 % (P = 0.004 for difference between groups) Non-obstructive CAD: 22.6 % (P = 0.02 for difference between groups) Obstructive CAD: 30.2 % (P = 0.06 for difference between groups) Single-vessel CAD: 15.1 % (P = 0.02 for difference between groups) 3-vessel CAD: 9.4 % (P = 0.001 for difference between groups) Independent predictor of significant CAD: OR [95 % CI] 3.121 [1.332–5.321]; P = 0.005 |
Study design may be limited by a potential selection bias of including only patients undergoing LT |
| HCV Mean BMI: 29.4 ± 5.9 kg/m2 DM: 19.3 % HTN: 37.6 % Obesity: 42.2 % Dyslipidemia: 18.3 % |
109 | At time of LT evaluation: CAD: 39.4 % Non-obstructive CAD: 20.2 % Obstructive CAD: 19.3 % Single-vessel CAD: 4.6 % 3-vessel CAD: 0.9 % |
|||||
| Alcoholic cirrhosis Mean BMI: 28.7 ± 9.5 kg/m2 DM: 24.4 % HTN: 33.3 % Obesity: 31.1 % Dyslipidemia: 24.4 % |
45 | At time of LT evaluation: CAD: 20.0 % Non-obstructive CAD: 8.9 % Obstructive CAD: 11.1 % Single-vessel CAD: 6.6 % 3-vessel CAD: 0 % |
|||||
| Patel, 2019 [17] (USA) |
NA (primary data collection) | Prospective study Median follow-up (range): 4.5 years (0–11 years) |
Patients undergoing LT for NASH, HCV, or alcoholic cirrhosis | HCV | Overall 495 |
Baseline CAD – obstructive: 8.4, non-obstructive: 19.8 |
Data available from poster only; study design may be limited by potential selection bias of including only patients undergoing LT |
| Alcoholic cirrhosis | Not stated | Baseline CAD – obstructive: 5.1, non-obstructive: 21.5 |
|||||
| NASH | Not stated | Baseline CAD – obstructive: 12.8, non-obstructive: 25.6 | |||||
| Piazza, 2016 [28] (USA) |
Medical records | Retrospective cohort study | LT recipients with NASH or alcoholic cirrhosis (liver biopsy or explant histology results and/or if other causes of liver disease could be excluded, as well as the presence of metabolic syndrome. Alcohol use of <10 g/day was considered consistent with diagnosis of NASH-related liver disease) | LT recipients with alcoholic cirrhosis Median BMI: 29 ± 5 kg/m2 DM: 26 % HTN: 35 % |
65 | CVD 1-year: 6.1, CVD 3-year: 13.8 Baseline CAD: 12 Baseline AF: 3 Baseline arrhythmia: 0 Baseline CHF: 3 Baseline MI: 1 Baseline stroke: 1 |
Small sample size; study design may be limited by potential selection bias of including only patients undergoing LT |
| LT recipients with NASH Median BMI: 34 ± 7 kg/m2 DM: 58 % HTN: 54 % |
78 | CVD 1-year: 7.7 (P = 0.5 vs alcoholic cirrhosis), CVD 3-year: 14.1 (P = 0.9 vs alcoholic cirrhosis) Baseline CAD: 15 Baseline AF: 4 Baseline arrhythmia: 5 Baseline CHF: 3 Baseline MI: 0 Baseline stroke: 3 |
|||||
| van den Berg, 2018 [19] (The Netherlands) |
Medical records | Retrospective cohort study | LT recipients with or without NASH (NASH defined as: [1] exclusion of other liver disease; [2] histological evidence of NASH based on liver biopsy before LT or on explant histology after LT; or [3] pre-cirrhotic imaging demonstrating hepatic steatosis) | LT recipients with NASH Median (IQR) BMI: 31.5 (28.6–36.4) kg/m2 (P < 0.01 vs no NASH) T2D: 73.5 % (P < 0.01 vs no NASH) HTN: 60.6 % (P < 0.01 vs no NASH) METS: 83.3 % (P < 0.01 vs no NASH) |
34 | Baseline CVDc: 29.4 (P < 0.01 vs no NASH) | Small sample size; study design may be limited by potential selection bias of including only patients undergoing LT |
| LT recipients without NASH Median (IQR) BMI: 25.3 (23.4–28.1) kg/m2 T2D: 20.0 % HTN: 30.0 % METS: 37.8 % |
135 | Baseline CVDc: 11.1 | |||||
| VanWagner, 2012 [20] (USA) |
Two Chicago medical centers | Retrospective review | Patients undergoing LT for NASH or alcoholic cirrhosis (NASH: biopsy-proven or explant histology demonstrating a diagnosis of NASH cirrhosis) | NASH cirrhosis Mean BMI: 32.1 ± 7.4 kg/m2 DM or fasting glucose >110 mg/dL: 51 % BP >130/85 mmHg or anti-HTN treatment: 53 % Hypertriglyceridemia (>150 mg/dL): 25 % |
115 | Any CV event within 1 year of transplant: 26 (OR [95 % CI] 4.12 [1.91–8.90]; P < 0.001 vs alcoholic cirrhosis) Baseline CAD: 20 (P = 0.05 vs alcoholic cirrhosis) Baseline AF: 6 (P = NS vs alcoholic cirrhosis) New-onset AF within 1 year of transplant: 10 (P = NS vs alcoholic cirrhosis) Baseline HF: 7 (P = NS vs alcoholic cirrhosis) Acute HF within 1 year of transplant: 3 Baseline MI: 6 (P = NS vs alcoholic cirrhosis) Non-STEMI within 1 year of transplant: 2 (P = NS vs alcoholic cirrhosis) STEMI MI within 1 year of transplant: 1 Stable ventricular tachycardia within 1 year of transplant: 2 (P = NS vs alcoholic cirrhosis) Supraventricular tachycardia within 1 year of transplant: 2 (P = NS vs alcoholic cirrhosis) Baseline stroke: 8 (P = NS vs alcoholic cirrhosis) Stroke within 1 year of transplant: 5 (P = NS vs alcoholic cirrhosis) |
Study design may be limited by potential selection bias of including only patients undergoing LT |
| Alcoholic cirrhosis Mean BMI: 28.3 ± 6.6 kg/m2 DM or fasting glucose >110 mg/dL: 52 % BP >130/85 mmHg or anti-HTN treatment: 38 % Hypertriglyceridemia (>150 mg/dL): 6 % |
127 | Any CV event within 1 year of transplant: 8 Baseline CAD: 9 Baseline AF: 4 New-onset AF within 1 year of transplant: 8 Baseline HF: 6 Acute HF within 1 year of transplant: 8 Baseline MI: 2 Non-STEMI within 1 year of transplant: 2 STEMI MI within 1 year of transplant: 1 Stable ventricular tachycardia within 1 year of transplant: 0 Supraventricular tachycardia within 1 year of transplant: 1 Baseline stroke: 2 Stroke within 1 year of transplant: 6 |
|||||
| VanWagner, 2015 [29] (USA) |
OPTN database | Retrospective database study | LT recipients Primary or secondary indication for LT |
NASH Mean BMI: 32.0 ± 5.8 kg/m2 Obesity: 64.0 % (P < 0.001 vs all other groups) HTN: 35.7 % (P < 0.001 vs all other groups) DM: 57.1 % |
5057 | Baseline CVD: 37.7 (P < 0.001 vs all other groups) Baseline angina: 7.0 (P < 0.001 vs all other groups) Baseline cerebrovascular disease: 1.2 (P ≤ 0.04 vs all other groups) Baseline PVD: 1.8 (P < 0.001 vs HCV) |
Study design may be limited by potential selection bias of including only patients undergoing LT |
| HCV Mean BMI: 28.3 ± 5.3 kg/m2 Obesity: 33.6 % HTN: 18.6 % DM: 22.8 % |
14,820 | Baseline CVD: 19.8 Baseline angina: 3.0 Baseline cerebrovascular disease: 0.5 Baseline PVD: 0.9 |
|||||
| Alcohol-induced Mean BMI: 28.0 ± 5.4 kg/m2 Obesity: 32.2 % HTN: 20.9 % DM: 22.5 % |
6998 | Baseline CVD: 22.8 Baseline angina: 3.2 Baseline cerebrovascular disease: 0.7 Baseline PVD: 1.3 |
|||||
| Other (drug-induced, autoimmune, other viral hepatitis, cholestatic, metabolic) Mean BMI: 27.3 ± 5.4 kg/m2 Obesity: 26.5 % HTN: 15.6 DM: 20.5 % |
21,485 | Baseline CVD: 17.0 Baseline angina: 2.7 Baseline cerebrovascular disease: 0.6 Baseline PVD: 0.8 |
|||||
| Wong, 2014 [23] (USA) |
United Network for Organ Sharing registry/OTPN | Retrospective analysis | LT recipients with NASH, HCV, or ALD | HCV BMI: 28.3 ± 5.3 kg/m2 (P = 0.02 vs HCV and ALD) DM: 13.6 % (P < 0.001 vs HCV and ALD) |
20,901 | Baseline CVD: 2.6 | Study design may be limited by potential selection bias of including only patients undergoing LT |
| NASH BMI: 30.5 ± 6.1 kg/m2 DM: 35.3 % |
7100 | Baseline CVD: 6.3 (P < 0.001 vs other groups) | |||||
| ALD BMI: 28.0 ± 5.4 kg/m2 DM: 12.6 % |
7962 | Baseline CVD: 4.0 | |||||
| HCC BMI: 28.2 ± 5.2 kg/m2 DM: 24.0 % |
5326 | Baseline CVD: 3.2 | |||||
Obesity was defined as BMI ≥30 kg/m2, except for Ampuero, 2018 [9] and Patel, 2018 [18] (both not specified), Danford, 2019 [10] (BMI >30 kg/m2), and Park, 2021 [16] (central obesity according to waist circumference ≥90 cm for males and ≥80 cm for females).
AF, atrial fibrillation; ALD, alcoholic liver disease; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CT, computed tomography; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes; EMR, electronic medical record; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; —HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HTN, hypertension; ICD-10, 10th revision of the International Statistical Classification of Diseases and Related Health Problems; IQR, interquartile range; LT, liver transplant; METS, metabolic syndrome; MI, myocardial infarction; NA, not available; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NS, non-significant; OR, odds ratio; OTPN, Organ Procurement Transplantation Network; PAD, peripheral arterial disease; PVD, peripheral vascular disease; REALT, Re-Evaluating Age Limits in Transplantation; STEMI, ST-segment elevation myocardial infarction; T2D, type 2 diabetes; VTD, venous thromboembolic disease.
CHF, unstable angina, MI, stroke, and revascularization.
Patients with viral hepatitis, alcohol-related liver disease, autoimmune or cholestatic liver disease, or another/unspecified etiology.
Confirmed MI, CAD, angina pectoris, CHF, AF, stroke, or transient ischemic attack.
Presence of CAD, CHF, arrhythmia, peripheral arterial disease, or cerebrovascular disease.
3.2.1. Incidence and prevalence
3.2.1.1. Studies comparing patients with NASH vs patients with NAFLD
Among 5 studies comparing patients with NASH and NAFLD, only 1 noted an increased risk in patients with NASH. A cross-sectional analysis of registry data from patients with or without biopsy-confirmed NAFLD (N = 500) found that the 10-year risk of atherosclerotic CVD (calculated from age, sex, race, systolic blood pressure, use of antihypertensives, levels of total and high-density lipoprotein cholesterol, smoking status, and history of diabetes) was significantly increased for patients with NASH vs those with no NAFLD (odds ratio [OR] 4.07; 95 % confidence interval [CI]: 1.40–11.88), whereas this was not the case for patients with NAFLD but no NASH vs non-NAFLD (OR 1.46; 95 % CI: 0.55–3.88) [16]. The prevalence of hypertension, diabetes, metabolic syndrome, and dyslipidemia were all elevated in patients with NASH compared with those with NAFLD [16]. However, it should be noted that this analysis did not report estimated or observed CV event rates in the NASH and NAFLD groups [16].
The 4 remaining studies found no significant difference in between-group risk of CV events; however, trends for increases in patients with NASH vs comparators were observed. For instance, over a mean follow-up of 2.6 years, CV events occurred in 5.2 % of patients with biopsy-proven NASH vs 2.7 % of patients with NAFLD but without NASH in a Spanish registry study, but the difference did not reach statistical significance [9]. Similarly, a single-center chart review of patients with biopsy-confirmed NAFLD found a numerically but not statistically significant difference in the prevalence of a combined CVD endpoint between patients with NASH (58.1 % of 219 patients) and non-NASH fatty liver (41.9 % of 158 patients). Prevalence rates of the individual conditions were also similar between the 2 groups (Table II) [11]. In a prospective study of patients with chronic obstructive pulmonary disease, including those with NASH (n = 41), simple liver steatosis (n = 46), or liver fibrosis (n = 68 [n = 9 NASH with liver fibrosis]), NASH or steatosis were not associated with an increased rate of CV events. Nevertheless, after 5 years of follow-up, the rate of CV events was significantly higher in patients with liver fibrosis vs without, and this risk remained after adjustment for confounders [21]. Among patients with complicated obesity who underwent first-time laparoscopic bariatric surgery, the baseline prevalence of CAD and congestive heart failure was not significantly different in patients with no liver disease or simple steatosis (n = 141), mild NASH (n = 151), and advanced NASH (n = 48); at 30 days post-surgery, rates of CV events were similar in all groups [22].
3.2.1.2. Studies comparing patients with NASH vs cirrhosis due to other etiologies
Of 12 studies evaluating patients for liver transplantation who had either NASH or other liver disease etiologies, 10 reported that NASH was associated with a higher prevalence of CAD (e.g. 3-vessel disease)/CVD.
In a prospective study, rates of obstructive and non-obstructive CAD were highest in those with biopsy-proven NASH cirrhosis (n = 53) vs hepatitis C virus (HCV; n = 109) or alcoholic cirrhosis (n = 45) [18]. Patients with NASH cirrhosis were more likely to have single-vessel CAD (15.1 %) and 3-vessel disease (9.4 %) vs those with HCV or alcoholic cirrhosis, and NASH was an independent predictor of significant CAD (OR 3.12; 95 % CI: 1.332–5.321) [18]. A database study reported a higher prevalence of CAD in pre-transplant patients with NASH vs hepatitis B or C, alcoholic cirrhosis, or biliary cirrhosis (7.4 % vs 1.7–2.9 %), and a higher prevalence of hypertension (34 % vs 12–20 %) and diabetes (50 % vs 11–20 %) [12]. Among patients undergoing liver transplant for NASH, HCV, or alcoholic cirrhosis, a higher baseline prevalence of both obstructive and non-obstructive CAD was observed with NASH than the other 2 etiologies [17]. In another study of patients who underwent liver transplant for NASH (n = 115) or alcohol-induced cirrhosis (n = 127), the pre-transplant prevalence of CAD was higher in the NASH group, with no significant between-group differences in rates of myocardial infarction, congestive heart failure, atrial fibrillation, or stroke 1-year post-transplant [20]. Patients with NASH were more likely than those with alcoholic cirrhosis to experience a CV event (26 %) within 1 year of transplant, after adjustment for confounders (OR 4.12; 95 % CI: 1.91–8.90). However, there was no difference between the 2 groups in the incidence of myocardial infarction, stable ventricular or supraventricular tachycardia, new-onset heart failure, or stroke [20].
In patients undergoing a primary or secondary liver transplant owing to underlying NASH (n = 5057), HCV (n = 14,820), alcohol-induced liver disease (n = 6998), or other causes (n = 21,485), the pre-transplant prevalence of CVD was significantly higher in the NASH vs all non-NASH groups. The prevalence of angina and cerebrovascular disease was also higher in the NASH group [29]. A single-center, retrospective database analysis revealed a higher prevalence of CVD (as well as higher body mass index and rates of type 2 diabetes [T2D], hypertension, and metabolic syndrome) in the pre-transplant medical history of patients with NASH than those without [19]. Similarly, among liver transplant recipients with NASH (n = 7100), HCV (n = 20,901), alcoholic liver disease (ALD) (n = 7962), or HCC (n = 5326), the baseline prevalence of cardiac disease was significantly higher in patients with NASH vs those without (BMI and diabetes prevalence were also significantly higher) [23]. A further study of liver transplant recipients aged ≥65 years with (n = 207) or without (n = 816) NASH reported higher rates of diabetes, hypertension, and hyperlipidemia, and a greater relative baseline prevalence of CVD, in those patients with NASH vs those without. However, at 12 months post-transplant, no significant between-group differences were noted in rates of atrial fibrillation, myocardial infarction, heart failure, or stroke [14].
A cohort study found a significantly higher baseline prevalence of CVD in patients with (n = 74) vs without (n = 881) NASH [10], while another single-center cohort study observed that the baseline prevalence of cardiac disease was significantly higher in patients with NASH (n = 71) vs those with other chronic liver diseases (n = 472), as were rates of obesity, diabetes, and hypertension [15].
In contrast, no statistical difference in the prevalence of CV events in patients with NASH vs without was observed in a chart review of liver transplant recipients with NASH (n = 78) or alcoholic cirrhosis (n = 65) [28]. At the time of pre-transplant evaluation, the prevalence of CV and cerebrovascular diseases was highest for CAD and ≤5 % in both groups for atrial fibrillation, arrhythmia, congestive heart failure, myocardial infarction, and stroke. There were no between-group differences in the frequency of post-transplant CV events at 1 and 3 years [28]. Regarding individual outcomes of interest, a chart review of patients at high risk for CAD found no significant difference between patients with NASH (n = 52) and non-NASH cirrhosis (n = 65) at 90 days post-liver transplant for myocardial infarction, arrhythmia, or stroke [13].
3.2.2. CVD mortality
A total of 6 retrospective studies reported data on the risk of CVD mortality in patients with NASH vs a comparator (Table III).
Table III.
CVD-related mortality in patients with NASH.
| Author, year (country) | Database | Study design | Population | Comparator/subpopulation (cirrhosis/fibrosis etc.) and comorbidities | Sample size, n | CVD mortality, % (unless otherwise stated) | Limitations |
|---|---|---|---|---|---|---|---|
| Studies comparing patients with NASH vs patients with NAFLD | |||||||
| Stepanova, 2013 [27] (USA) |
Forces Institute of Pathology databases | Retrospective cohort | Biopsy-proven NAFLD with and without NASH | NASH Obesity: 43.9 % T2D: 28.1 % Hyperlipidemia: 27.2 % |
171 | Over ~12 months of follow-up: 8.8 (P = 0.09 vs no NASH) |
Data not available for various major CVD risk factors and confounders (e.g. smoking status, hypertension, visceral obesity and insulin resistance), which may have contributed to the high mortality rates observed |
| Non-NASH Obesity: 49.2 % T2D: 22.9 % Hyperlipidemia: 37.5 % |
118 | Over ~12 months of follow-up: 15.3 | |||||
| Studies comparing patients with NASH vs cirrhosis due to other etiologies | |||||||
| Kennedy, 2012 [24] (USA) |
Internal transplant database | Retrospective chart review | LT patients with a diagnosis of NASH or without NASH (NASH defined as no other forms of liver disease and pre-transplant biopsy consistent with NASH or pre-cirrhotic imaging demonstrating hepatic steatosis or met criteria for the NASH phenotype) | NASH Obesity: 68 % DM: 59 % HTN: 75 % Hypercholesterolemia: 22 % |
129 | 19 Post 4 months LT: 8.5 |
Overall number of mortality events was low, which significantly raises the possibility of a type 2 error. Furthermore, the identification of pre-transplant risk factors that may specifically predict a cardiac or sepsis-related cause of death was limited by the low number of events |
| Non-NASH Obesity: 28 % DM: 17 % HTN: 41 % Hypercholesterolemia: 12 % |
775 | 7 Post 4 months LT: 4.2 |
|||||
| Nagai, 2019 [25] (USA) |
OPTN registry | Retrospective registry analysis | LT patients with NASH, HCV, or ALD (diagnoses of cirrhosis: cryptogenic [idiopathic] or cirrhosis; etiology unknown were not considered NASH) | HCV BMI ≥30 kg/m2: 34.6 % DM: 25.0 % |
17,037 | 7.0 (deaths within 1 year of LT) | OPTN registry lacks detailed clinical information, e.g. on diabetes control and pretransplant history of CVD, which could affect post-transplant outcomes; follow-up times differ among eras. An endpoint of 1-year patient and liver graft survival was chosen to minimize bias secondary to different follow-up times |
| ALD BMI ≥30 kg/m2: 34.7 % DM: 21.4 % |
9279 | 9.6 (deaths within 1 year of LT) | |||||
| NASH BMI ≥30 kg/m2: 61.7 % DM: 59.3 % |
6344 | 11.5 (deaths within 1 year of LT; P < 0.001 vs HCV and ALD) Mortality rate: 1 vs 3 vs 5 year: 1.4 vs 1.8 vs 2.8 Multivariate analyses: NASH vs HCV: HR [95 % CI] 1.30 [1.04–1.61]; P = 0.02 NASH vs ALD: HR [95 % CI] 1.34 [1.06–1.69]; P = 0.01 |
|||||
| Piazza, 2016 [28] (USA) |
Medical records | Retrospective cohort study | LT patients with NASH or alcoholic cirrhosis (NASH diagnosed by liver biopsy or explant histology results and/or if other causes of liver disease could be excluded, as well as the presence of metabolic syndrome. Alcohol use of <10 g/day was considered consistent with diagnosis of NASH-related liver disease) | Alcoholic cirrhosis Median BMI: 29 ± 5 kg/m2 DM: 26 % HTN: 35 % |
65 | 8 deaths occurred with 2 due to CV events | Study design may be limited by potential selection bias of including only patients undergoing LT |
| NASH Median BMI: 34 ± 7 kg/m2 (P < 0.0001 vs alcoholic cirrhosis) DM: 58 % (P = 0.0002 vs alcoholic cirrhosis) HTN: 54 % (P = 0.03 vs alcoholic cirrhosis) |
78 | 10 deaths occurred with 1 due to CV events | |||||
| Satapathy, 2017 [26] (USA) |
UNOS STAR dataset | Retrospective cohort study | LT patients with and without NASH (NASH patients were identified using the primary diagnosis numeric code for NASH in the dataset. Cryptogenic cirrhosis with BMI ≥30 was included in the NASH group) | NASH BMI ≥30 kg/m2: 72.05 % (P < 0.0001 vs non-NASH groups) DM: 49.72 % (P < 0.0001 vs non-NASH groups) |
3170 | NASH vs non-NASH group for CV-related death: aHR [95 % CI] 0.648 [0.531–0.791]; P < 0.0001 NASH vs non-NASH HCV+: aHR [95 % CI] 0.491 [0.396–0.609]; P < 0001 NASH vs non-NASH HCV−: aHR [95 % CI] 0.892 [0.711–1.121]; P = 0.3276 |
Prevalence of NASH during the first 2 years may be underestimated; study combined NASH diagnoses with cryptogenic cirrhosis |
| Non-NASH HCV+ BMI ≥30 kg/m2: 35.09 % DM: 20.92 % |
3012 | ||||||
| Non-NASH HCV− BMI ≥30 kg/m2: 28.30 % DM: 21.59 % |
3159 | ||||||
| VanWagner, 2015 [29] (USA) |
OPTN database | Retrospective database | LT patients with and without NASH (NASH defined as a diagnosis of cryptogenic cirrhosis with ≥1 component of the metabolic syndrome) | NASH cirrhosis Obesity (BMI ≥30 kg/m2): 64.0 % HTN: 35.7 % DM: 57.1 % |
5057 | 4.5 NASH patients more likely to die from CVD-related death vs non-NASH patients over mean 1.62 years of follow-up HR [95 % CI] 1.42 [1.23–1.63]; P < 0.001 |
|
| Non-NASH (included HCV, alcohol-induced, other) Obesity (BMI ≥30 kg/m2): 29.8 % HTN: 8.1 % DM: 21.6 % |
43,303 | 3.4 | |||||
ALD, alcoholic liver disease; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes; HCV, hepatitis C virus; (a)HR, (adjusted) hazard ratio; HTN, hypertension; LT, liver transplant; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OPTN, Organ Procurement Transplantation Network; STAR, Standard Transplant Analysis and Research; T2D, type 2 diabetes; UNOS, United Network for Organ Sharing.
3.2.2.1. Studies comparing mortality in patients with NASH vs patients with NAFLD
Findings from a single cohort study suggested that CVD-related mortality was not increased in patients with NASH vs those with NAFLD. Among patients with biopsy-proven NAFLD followed for approximately 12 years, cardiac-related deaths occurred in 15 of 171 patients with NASH (8.8 %) compared with 18 of 118 patients without NASH (15.3 %); both groups had non-significant differences in rates of obesity, T2D, and hyperlipidemia [27].
3.2.2.2. Studies comparing mortality in patients with NASH vs cirrhosis due to other etiologies
Data on CVD-related mortality were equivocal from 2 studies comparing patients with NASH vs those with cirrhosis due to other etiologies. Among patients with NASH (n = 6344), HCV (n = 17,037), or ALD (n = 9279), NASH was associated with an increased risk of 1-year mortality compared with the HCV (hazard ratio [HR] 1.30) and ALD (HR 1.34) groups; the proportions of patients with obesity and diabetes in the NASH group were higher than those observed in the HCV and ALD groups [25]. In contrast, a single-center chart review found no increase in CVD-related mortality in liver transplant recipients with NASH vs those with alcoholic cirrhosis at 3-year follow-up, despite the former group having a significantly higher body mass index, and significantly higher rates of diabetes and hypertension [28].
Two of 3 studies in liver transplant recipients comparing populations with NASH vs those with cirrhosis due to other etiologies suggested an increase in CVD-related mortality in the former group. Over a mean 1.62 years of post-transplant follow-up, patients with NASH (n = 5057) were more likely to die from CVD than patients without NASH (n = 43,303; HCV, alcohol-induced, and other causes) (HR 1.42) [29]. A single-center chart review found that patients with NASH (n = 129) had significantly higher baseline rates of CAD and cerebrovascular disease, and higher comorbidity rates compared with patients without NASH (n = 775) [24]. Deaths due to a cardiac event occurred in 19 % of patients with NASH compared with 7 % of those without NASH, with 75 % of these deaths occurring within 4 months of transplantation [24]. In both studies, rates of obesity, hypertension, and diabetes were markedly greater in patients with NASH than their non-NASH counterparts [24,29].
Conversely, a US cohort study of primary liver transplant recipients (NASH, n = 3170; non-NASH HCV+, n = 3012; non-NASH HCV−, n = 3159) found that although the prevalence of obesity and diabetes was significantly higher in the NASH vs non-NASH groups at the time of transplant, the risk of CVD-related mortality was significantly lower (adjusted HR 0.65) [26]. A significantly lower risk of CV-related death was observed in patients with NASH vs patients without NASH but with HCV (adjusted HR 0.49), but not vs those without both NASH and HCV [26].
4. Discussion
This systematic review adds to the existing literature by documenting that CVD is highly prevalent in patients with MASH, who have an elevated CVD risk compared with patients with other advanced liver disease etiologies. Comparisons between these disease states are challenging based on published studies owing to the varying prevalence of CV risk factors in patients classified as having other conditions and the heterogeneity of disease severity assessments. Several of the identified studies reporting no significant difference in CVD risk between MASH and a comparator group were conducted in populations that have a similarly high CVD risk profile to MASH, notably patients who have had bariatric surgery. It is likely that cardiometabolic risk factors play a greater role in the development of CVD than MASH, which could explain the similar risks of CV events found in many of these studies, especially those comparing populations with MASH vs MASLD.
Although we believe this systematic literature analysis is unique in focusing on patients with a histological diagnosis of MASH, our findings are in line with similar recent meta-analyses evaluating the risk of CV adverse events in patients with or without MASLD. MASLD was associated with an increased risk of myocardial infarction, ischemic stroke, atrial fibrillation, and heart failure compared with no MASLD in a systematic review and meta-analysis of 20 studies, with the strength of these associations moderated by age and male sex [30]. A second meta-analysis of 11 longitudinal cohort studies with data on >11 million middle-aged individuals followed over a median of 10 years found that MASLD was associated with a 1.5-fold higher long-term risk of new-onset heart failure, compared with no MASLD, regardless of the presence of diabetes, hypertension, and other common CV risk factors [31]. However, these studies did not specifically report on populations with MASH. It remains unclear whether MASLD with or without biopsy-proven MASH has a true association with CVD, and further research is required.
Many patients included in the identified studies had other CV risk factors in addition to MASH. In particular, MASH was often comorbid with T2D, which is commonly the case as the 2 conditions share several risk factors, notably insulin resistance and obesity [32], and the presence of both increases the risk of CV events [33]. The relationship between T2D and MASLD is thought to be bidirectional, in that T2D promotes the progression of MASLD to MASH and cirrhosis, and increases all-cause and liver-related mortality, while MASLD leads to insulin resistance and poor glycemic control [32]. Other comorbidities, such as overweight/obesity and lipid imbalances, also likely mediate the relationship between MASH and CVD risk, and indeed MASH is considered as the liver manifestation of the metabolic syndrome [2,34]. At present, published data are insufficient to permit definitive conclusions to be drawn on whether the increased CVD risk in patients with MASH compared with their counterparts without MASH is driven by MASH itself and/or its associated comorbidities, and if the presence of MASH on a background of certain comorbidities may have an incremental or synergistic effect on CVD risk.
It was unclear from the studies included in our literature review whether co-existence of MASH and CVD led to increased CVD-related mortality vs the co-existence of CVD with other liver etiologies, because patients in these studies did not necessarily have confirmed CVD; rather, they were included as they did not have advanced heart disease as a condition of receiving a liver transplant. Nevertheless, CVD is a major cause of death in patients with more advanced liver disease [6,7], including patients with MASH, particularly among those with metabolic comorbidities, and these patients are likely to benefit from aggressive CV risk factor modification both pre- and post-liver transplant. For example, statin use may be beneficial, because it reduces CVD risk and appears to be well tolerated in this patient population [35,36]. However, statins should only be used with caution in patients with decompensated cirrhosis. Further studies of liver and mortality outcomes with statins in patients with compensated cirrhosis are ongoing [37]. We can hypothesize that treatments that positively affect cardiometabolic risk in other at-risk populations, e.g. glucagon-like peptide-1 receptor agonist therapy or sodium-glucose cotransporter-2 inhibitors for people with T2D, may be effective in reducing CVD risk in patients with MASH, but this requires careful prospective evaluation. Large studies of the safety and efficacy of such treatments in MASH are ongoing [38,39].
A key strength of this systematic review is that it focused exclusively on reports from biopsy-proven MASH cohorts. This review also has several limitations, notable among which is the fact that most studies did not adjust for the presence of CV risk factors, meaning firm conclusions cannot be made regarding whether increased CVD and mortality risks are driven by MASH itself or a higher prevalence of cardiometabolic risk factors in this population. Some of the epidemiological studies included had very small patient populations and thus their findings could be subject to selection bias or chance. Additionally, causality between MASH and CVD could not be assessed in the identified studies because most were retrospective in design. Furthermore, the prevalence of cardiometabolic risk factors known to significantly impact the occurrence of CVD was vastly lower in the comparator arm in most of the studies, therefore any noted differences in the prevalence of CVD could be driven exclusively by the excess prevalence of cardiometabolic risk factors. A total of 12 studies were conducted in patients who were listed for, or had received, a liver transplant, 1 study enrolled patients who had undergone bariatric surgery, and another enrolled patients with chronic obstructive pulmonary disease; these specialized populations may not be representative of the general population with MASH and the presence of confounding clinical factors that may influence CV-related mortality in this group should be noted. Interpretation of the transplant literature is complicated by fact that patients have extensive cardiac evaluation prior to transplant and only those with a relatively manageable cardiac status are allowed to proceed to transplant, therefore this represents a selection bias where it is likely that patients with only low-to-moderate heart disease were included. None of the studies considered possible time trends in the incidence, prevalence, or mortality associated with comorbid CVD. Finally, although studies involving biopsy-confirmed MASH were included, no staging of MASH was documented, nor were patients classified by presence or absence of cirrhosis and/or hepatic decompensation.
Because it is difficult to pinpoint which patients have MASH in most cohorts, it is important that future research characterizes types of heart disease in patients with MASLD, stratified according to fibrosis severity (as currently defined by the Fibrosis-4 Index for Liver Fibrosis or elastography). This may reveal whether there are differences in the prevalence of atherosclerotic CVD, heart failure with preserved ejection fraction, or atrial fibrillation across different stages of the disease.
Recently, the new nomenclature for NAFLD/NASH was proposed by EASL-AASLD-APASL in a multi-society Delphi consensus statement [1]. With a focus on positive metabolic criteria, MASLD encompasses patients who have hepatic steatosis diagnosed histologically or by imaging and have ≥1 of 5 cardiometabolic risk factors such as T2D and obesity. Persons with MASLD and steatohepatitis are designated as having MASH [1]. As previously noted, the new MASLD/MASH criteria were not included as search terms in the current literature review; however, it is likely that most of the patient populations in the included studies would retrospectively fulfil these criteria. In the current review, we have generally assumed that both old and new terms are used interchangeably but retained NAFLD/NASH when reporting the results, given all included studies were conducted prior to the nomenclature updates [1].
In conclusion, CVD is prevalent in patients with MASH, particularly those with more severe liver disease, and is likely to contribute to mortality. Accordingly, CV risk factors should be carefully and proactively managed in this population. Whether the CVD burden in patients with MASH is a direct consequence of MASH itself, or a reflection of the underlying cardiometabolic abnormalities that lead both to CVD and MASH, remains to be resolved in future studies.
Ethical statement
All authors ensure that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association and the manuscript is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
Funding source
Novo Nordisk A/S.
CRediT authorship contribution statement
Arun J. Sanyal: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Mansoor Husain: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Crystel Diab: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Kamal Kant Mangla: Conceptualization; Investigation; Methodology; Project administration; Roles/Writing - original draft; Writing - review & editing. Ahsan Shoeb: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Ildiko Lingvay: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Elliot B. Tapper: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Arun J. Sanyal is President of Sanyal Biotechnology and has stock options in Exhalenz, Genfit, HemoShear, Durect, Indalo, Northsea, Tiziana, and Rivus. He has served as a consultant to Genfit, Gilead, Malinckrodt, Pfizer, Salix, Boehringer Ingelheim, Novartis, Bristol Myers Squibb, Merck, HemoShear, Eli Lilly, Novo Nordisk, Terns, Albireo, Jannsen, Poxel, 89 Bio, Siemens, AstraZeneca, NGM Bio, Amgen, Regeneron, Genentech, Alnylam, Roche, Madrigal, Inventiva, Covance, ProSciento, Histoindex, and Path AI. His institution has received grant support from Gilead, Malinckrodt, Boehringer Ingelheim, Novartis, Bristol Myers Squibb, Merck, Eli Lilly, Novo Nordisk, Fractyl, Madrigal, and Inventiva. He receives royalties from Elsevier and UptoDate.
Mansoor Husain has received research grants from AstraZeneca, Merck, and Novo Nordisk; consultancy fees for participation in advisory board meetings from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Roche; speaker fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck, and Novo Nordisk; and holds 2 patents relating to glucagon-like peptides.
Ildiko Lingvay has received research funding (paid to their institution) from Boehringer Ingelheim, Merck, Mylan, Novo Nordisk, Pfizer, and Sanofi; and advisory/consulting fees and/or other support from AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, GI Dynamics, Intarcia, Intercept, Johnson and Johnson, MannKind, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Shionogi, Structure Therapeutics, Target Pharma, Valeritas, WebMD, and Zealand Pharma.
Elliot B. Tapper reports grants/contracts from Madrigal and Bausch; consulting fees from Takeda.
Crystel Diab, Kamal Kant Mangla, and Ahsan Shoeb are employees of Novo Nordisk A/S, and Kamal Kant Mangla has shares in the company.
Acknowledgments
The authors thank Sunita Nair (strategic advisor), Palvi Gupta (project lead), Charlotte Fleming (systematic literature reviewer 1), and Ashvitha Shet (systematic literature reviewer 2) for their roles in the systematic literature review. The authors also thank Margarida Augusto and Katrine Grau of Novo Nordisk A/S, Denmark, for their contributions to the project. Medical writing and editorial support were provided by Stephen Purver, MChem, and Liam Gillies, PhD, of Apollo, and Sarah Stowell, PhD, a contract writer working on behalf of Apollo, OPEN Health Communications, and funded by Novo Nordisk, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2024.100386.
Appendix A. Supplementary data
Supplementary material
References
- 1.Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus J.V., Mark H.E., Anstee Q.M., Arab J.P., Batterham R.L., Castera L., et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022;19(1):60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 3.Le M.H., Yeo Y.H., Li X., Li J., Zou B., Wu Y., et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022;20(12):2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balp M.M., Krieger N., Przybysz R., Way N., Cai J., Zappe D., et al. The burden of non-alcoholic steatohepatitis (NASH) among patients from Europe: a real-world patient-reported outcomes study. JHEP Rep. 2019;1(3):154–161. doi: 10.1016/j.jhepr.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shroff H., VanWagner L.B. Cardiovascular disease in nonalcoholic steatohepatitis: screening and management. Curr. Hepatol. Rep. 2020;19(3):315–326. doi: 10.1007/s11901-020-00530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G., Byrne C.D., Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 8.Effective Public Healthcare Panacea Project. Quality Assessment Tool for Quantitative Studies. 2010. [Google Scholar]
- 9.Ampuero J.A., Aller R., Gallego-Durán R., Banales J., et al. Clinical outcomes in biopsy-proven NAFLD patients from the HEPAmet Spanish Registry. J. Hepatol. 2018:S833. (Suppl) [Google Scholar]
- 10.Danford C.J., Iriana S., Shen C., Curry M.P., Lai M. Evidence of bias during liver transplant evaluation of non-alcoholic steatohepatitis cirrhosis patients. Liver Int. 2019;39(6):1165–1173. doi: 10.1111/liv.14080. [DOI] [PubMed] [Google Scholar]
- 11.Domanski J.P., Park S.J., Harrison S.A. Cardiovascular disease and nonalcoholic fatty liver disease: does histologic severity matter? J. Clin. Gastroenterol. 2012;46(5):427–430. doi: 10.1097/MCG.0b013e31822fb3f7. [DOI] [PubMed] [Google Scholar]
- 12.Gologorsky E., Pretto E.A., Jr., Fukazawa K. Coronary artery disease and its risk factors in patients presenting for liver transplantation. J. Clin. Anesth. 2013;25(8):618–623. doi: 10.1016/j.jclinane.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Herndon J., Ravi S., Thiyagarajan A., Devabhaktuni D., Shipley L., Wooten J., et al. 1725 Invasive cardiac risk stratification of NASH patients prior to liver transplant. Hepatology. 2020;72(S1) [Google Scholar]
- 14.Kwong A.J., Devuni D., Wang C., Boike J., Jo J., VanWagner L., et al. Outcomes of liver transplantation among older recipients with nonalcoholic steatohepatitis in a large multicenter US cohort: the re-evaluating age limits in transplantation consortium. Liver Transpl. 2020;26(11):1492–1503. doi: 10.1002/lt.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park C.W., Tsai N.T., Wong L.L. Implications of worse renal dysfunction and medical comorbidities in patients with NASH undergoing liver transplant evaluation: impact on MELD and more. Clin. Transpl. 2011;25(6):E606–E611. doi: 10.1111/j.1399-0012.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Koo B.K., Kim W., Kim W.H. Innovative target exploration of NC. Histological severity of nonalcoholic fatty liver disease is associated with 10-year risk for atherosclerotic cardiovascular disease. Hepatol. Int. 2021;15(5):1148–1159. doi: 10.1007/s12072-021-10209-3. [DOI] [PubMed] [Google Scholar]
- 17.Patel S., Rodriguez V., Siddiqui M.B., Faridnia M., Clinton J., Lin F.-P., et al. FRI-389: impact of coronary artery disease on long term mortality after liver transplantation. J. Hepatol. 2019;70(1, Supplement) [Google Scholar]
- 18.Patel S.S., Nabi E., Guzman L., Abbate A., Bhati C., Stravitz R.T., et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl. 2018;24(3):333–342. doi: 10.1002/lt.25012. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg E.H., Douwes R.M., de Meijer V.E., Schreuder T., Blokzijl H. Liver transplantation for NASH cirrhosis is not performed at the expense of major post-operative morbidity. Dig. Liver Dis. 2018;50(1):68–75. doi: 10.1016/j.dld.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 20.VanWagner L.B., Bhave M., Te H.S., Feinglass J., Alvarez L., Rinella M.E. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56(5):1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 21.Viglino D., Plazanet A., Bailly S., Benmerad M., Jullian-Desayes I., Tamisier R., et al. Impact of non-alcoholic fatty liver disease on long-term cardiovascular events and death in chronic obstructive pulmonary disease. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-34988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weingarten T.N., Swain J.M., Kendrick M.L., Charlton M.R., Schroeder B.J., Lee R.E., et al. Nonalcoholic steatohepatitis (NASH) does not increase complications after laparoscopic bariatric surgery. Obes. Surg. 2011;21(11):1714–1720. doi: 10.1007/s11695-011-0521-z. [DOI] [PubMed] [Google Scholar]
- 23.Wong R.J., Chou C., Bonham C.A., Concepcion W., Esquivel C.O., Ahmed A. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002-2012 United Network for Organ Sharing data. Clin. Transpl. 2014;28(6):713–721. doi: 10.1111/ctr.12364. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy C., Redden D., Gray S., Eckhoff D., Massoud O., McGuire B., et al. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford) 2012;14(9):625–634. doi: 10.1111/j.1477-2574.2012.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai S., Collins K., Chau L.C., Safwan M., Rizzari M., Yoshida A., et al. Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Clin. Gastroenterol. Hepatol. 2019;17(13):2759–2768.e5. doi: 10.1016/j.cgh.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Satapathy S.K., Jiang Y., Eason J.D., Kedia S.K., Wong E., Singal A.K., et al. Cardiovascular mortality among liver transplant recipients with nonalcoholic steatohepatitis in the United States-a retrospective study. Transpl. Int. 2017;30(10):1051–1060. doi: 10.1111/tri.13001. [DOI] [PubMed] [Google Scholar]
- 27.Stepanova M., Rafiq N., Makhlouf H., Agrawal R., Kaur I., Younoszai Z., et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig. Dis. Sci. 2013;58(10):3017–3023. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 28.Piazza N.A., Singal A.K. Frequency of cardiovascular events and effect on survival in liver transplant recipients for cirrhosis due to alcoholic or nonalcoholic steatohepatitis. Exp. Clin. Transplant. 2016;14(1):79–85. doi: 10.6002/ect.2015.0089. [DOI] [PubMed] [Google Scholar]
- 29.VanWagner L.B., Lapin B., Skaro A.I., Lloyd-Jones D.M., Rinella M.E. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int. 2015;35(12):2575–2583. doi: 10.1111/liv.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alon L., Corica B., Raparelli V., Cangemi R., Basili S., Proietti M., et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022;29(6):938–946. doi: 10.1093/eurjpc/zwab212. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A., Petracca G., Csermely A., Beatrice G., Bonapace S., Rossi A., et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022 doi: 10.1136/gutjnl-2022-327672. gutjnl-2022-327672 (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Hassouneh R., Siddiqui M.S., Bhati C. Risk of cardio-nephro-metabolic disease from NAFLD to MAFLD: fact or fiction? Metab. Target Organ Damage. 2021;1(2):4. [Google Scholar]
- 33.Caussy C., Aubin A., Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr. Diab. Rep. 2021;21(5):15. doi: 10.1007/s11892-021-01383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muzurovic E., Peng C.C., Belanger M.J., Sanoudou D., Mikhailidis D.P., Mantzoros C.S. Nonalcoholic fatty liver disease and cardiovascular disease: a review of shared cardiometabolic risk factors. Hypertension. 2022;79(7):1319–1326. doi: 10.1161/HYPERTENSIONAHA.122.17982. [DOI] [PubMed] [Google Scholar]
- 35.Athyros V.G., Boutari C., Stavropoulos K., Anagnostis P., Imprialos K.P., Doumas M., et al. Statins: an under-appreciated asset for the prevention and the treatment of NAFLD or NASH and the related cardiovascular risk. Curr. Vasc. Pharmacol. 2018;16(3):246–253. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 36.Sigler M.A., Congdon L., Edwards K.L. An evidence-based review of statin use in patients with nonalcoholic fatty liver disease. Clin. Med. Insights Gastroenterol. 2018;11 doi: 10.1177/1179552218787502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan D.E., Mehta R., Garcia-Tsao G., Albrecht J., Aytaman A., Baffy G., et al. SACRED: effect of simvastatin on hepatic decompensation and death in subjects with high-risk compensated cirrhosis: statins and cirrhosis: reducing events of decompensation. Contemp. Clin. Trials. 2021;104 doi: 10.1016/j.cct.2021.106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yabut J.M., Drucker D.J. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr. Rev. 2023;44(1):14–32. doi: 10.1210/endrev/bnac018. [DOI] [PubMed] [Google Scholar]
- 39.Androutsakos T., Nasiri-Ansari N., Bakasis A.D., Kyrou I., Efstathopoulos E., Randeva H.S., et al. SGLT-2 inhibitors in NAFLD: expanding their role beyond diabetes and cardioprotection. Int. J. Mol. Sci. 2022;23(6):3107. doi: 10.3390/ijms23063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material