Abstract
The blackness traits, considered an important economic factor in the black-bone chicken industry, still exhibits a common phenomenon of significant difference in blackness of breast muscle. To improve this phenomenon, this study compared growth traits, blackness traits, and transcriptome of breast muscles between the High Blackness Group (H group) and Low Blackness Group (L group) in the Xuefeng black-bone chickens. The results are as follows: 1) There was no significant difference in growth traits between the H group and the L group (P > 0.05). 2) The skin/breast muscle L values in the H group were significantly lower than those in the L group, while the breast muscle melanin content exhibited the opposite trend (P < 0.05). 3) A significant negative correlation was observed between breast muscle melanin content and skin/breast muscle L value (P < 0.05), and skin L value exhibiting a significant positive correlation with breast muscle L value (P < 0.05). 4) The breast muscle transcriptome comparison between the H group and L group revealed 831 and 405 DEGs in female and male chickens, respectively. This included 37 shared DEGs significantly enriched in melanosome, pigment granule, and the melanogenesis pathway. Seven candidate genes (DCT, PMEL, MLANA, TYRP1, OCA2, EDNRB2, and CALML4) may play a crucial role in the melanin production of breast muscle in Xuefeng black-bone chicken. The findings could accelerate the breeding process for achieving desired levels of breast muscle blackness and contribute to the exploration of the mechanisms underlying melanin production in black-bone chickens.
Key words: breast muscle, blackness variation, histomorphological, pigmentation, Xuefeng black-bone chicken
INTRODUCTION
The black-bone chicken is a highly prized breed in China due to its medicinal attributes, and it is often utilized in traditional Chinese medicinal dishes to promote health of body (Geng et al., 2010; Zhu et al., 2014; Zhang et al., 2015). Numerous studies have substantiated the medicinal qualities of black-bone chicken, attributing them to the abundant melanin content within the meat. Because melanin possesses antioxidant properties and contributes to enhanced immune function, radioprotective effects, and other beneficial functions (Chen et al., 2009; Tu et al., 2009; Premi et al., 2015). In comparison to other broiler chickens, black-bone chicken meat exhibits lower fat and cholesterol levels, along with higher protein and microelement content (Tu et al., 2009; Tian et al., 2011; Yang et al., 2019). Consequently, black-bone chicken holds substantial economic and market value in China.
The blackness traits is a pivotal economic characteristic in the black-bone chickens and occupies an important position in the breeding process. Consumers generally associate the blacker appearance with high nutritional value in black-bone chickens (Yu et al., 2018; Xu et al., 2023). However, compared to observable traits that can be directly measured or assessed instrumentally, such as feather color, skin pigmentation, body size, and more (Li et al., 2023; Lyu et al., 2023; Zheng et al., 2023), the blackness of breast muscle is not visually discernible and also cannot be directly detect in live body. And blackness variations significantly impact the commercial value of black-bone chickens, therefore, addressing this challenge remains a difficult problem in breeding processes today (Sun et al., 2022; Xu et al., 2023). RNA-seq, owing to its high-throughput and precision, demonstrates unique advantages in molecular breeding. It unravels the complexity of gene regulatory networks and facilitating the identification of key genes and pathways associated with phenotypic character (Deng et al., 2019; Saidi and Hajibarat, 2020). Several studies used transcriptome sequencing to discover gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and key genes related to meat color. This not only provides molecular markers for breeding but also establishes a theoretical foundation for understanding the functional molecular mechanisms associated with the meat color (Yu et al., 2018; Sun et al., 2022; Xu et al., 2023).
The Xuefeng Black-bone chicken is the only native black-bone chicken breed in Hunan Province of China. This breed exhibits substantial market potential within the province and its neighboring regions. Over an extended period of domestication, it has developed stable genetic traits and unique flavor. These traits encompass notable black features in the skin, bones, meat, beak, legs, cockscomb, and tongue (Deng et al., 2021; Deng et al., 2022). However, a noteworthy phenomenon was observed during the slaughter process, revealing significant variations in the blackness of breast muscles (BBM) among Xuefeng black-bone chickens. Scarce research has been conducted regarding the breeding of BBM in black-bone chickens. Does the augmentation of blackness traits impact the regular growth and development of chickens? Can observable correlated traits be leveraged to improve BBM selection? Are there molecular markers suitable for BBM breeding in black-bone chickens? Based on above problem, our objective is to expedite the breeding process of BBM by identifying molecular markers or correlated traits in black-bone chicken and enhance comprehension of the mechanism about melanin deposition. To achieve this, we conducted a comparative analysis of the growth traits, melanin deposition and transcriptomic variances between high blackness and low blackness group.
MATERIALS AND METHODS
Animals and Sample Collection
All the birds and the experimental protocols were approved by the Institutional Animal Care and Use Committee of Hunan Agricultural University, Hunan, China (approval number: 2019022). Xuefeng black-bone chicken were obtained from Hunan Yunfeifeng Agricultural Commercial Company, Hunan, China. The study population was the Xuefeng black-bone chicken F2 resource population that was produced from reciprocal crosses of the high blackness line and low blackness line. All chickens were uniformly raised under consistent management conditions, with both management and nutritional requirements being overseen by company employees in accordance with established company procedures.
A total of 405 chickens, consisting of 194 female chickens at 180 d and 211 male chickens at 130 d, were slaughtered at the market age. Approximately 0.5 g breast muscle tissue were promptly collected and snap-frozen in liquid nitrogen for subsequent RNA-seq analysis. Additionally, roughly 2 cm × 1 cm × 1 cm breast muscle were preserved in 4% paraformaldehyde for morphological assessments, while the entirety of the breast muscle was collected for melanin content determination. All samples were extracted from the right breast muscle.
Growth and Blackness Traits
All chickens recorded the initial body weight (IBW) at one-day-old and the final body weight (FBW) at the market age. Skin lightness values (SL) were measured at different stages, including 6, 12, and 18 w, using a Minolta CR-400 colorimeter (Minolta Co. Ltd., Tokyo, Japan), the breast muscle under the wing has an area with less hairs, and this is where the SL was determined, each SL value measured 3 times. Subsequently, determinations were made for eviscerated weight (EW), breast muscle weight (BMW), breast lightness value (BML), and tibia weight (TW) post-slaughter, the BML were obtained from 3 areas of the breast major muscle.
Grouping
This study primarily focusses on blackness of breast muscle (BBM) variations, so the BML results as the grouping criterion which have been obtained. The community evenness is defined as the percentage of number of individuals falling within the range of 0.9 to 1.1 times the mean value divided by the total number of individuals, indicating smaller differences among individuals in this range (Wang et al., 2017; Alfaro-Wisaquillo et al., 2021). Consequently, individuals falling outside the 0.9 to 1.1 times the mean range were categorized as extreme cases. Given the negative correlation between the lightness and the blackness, the groups were classified as follows: Female High Blackness group (FH) comprised 62 chickens with BML < 28.62, Female Low Blackness group (FL) comprised 55 chickens with BML > 34.98, Male High Blackness group (MH) included 43 chickens with BML < 30.15, and Male Low Blackness group (ML) included 37 chickens with BML > 36.73.
Determination of Melanin Content
The determination of melanin content followed the method described by Xu et al. (2023). In brief, the entire breast muscle, with the fascia removed, underwent vacuum freeze-drying (LGL-10 C, Sihuan Tech. Instrument Co. Ltd., Beijing, China). The melanin standard (CAS#8049-97-6, Sigma-Aldrich, Saint Louis, MO) was dissolved in sodium hydroxide and diluted to various concentrations to create a standard curve. The absorbance of a 10 mg sample was measured at 500 nm after dissolving in non-denaturing lysis buffer (Solarbio, Beijing, China) and sodium hydroxide for 20 min and 2 h, respectively. Melanin content was calculated using the absorbance of the sample and the melanin standard curve (y = 0.1518x + 0.0012, R2 = 0.9998).
Histology of the Breast Muscle
The breast muscle tissue fixed samples were embedded in paraffin, sectioned (4 mm), followed by hematoxylin-eosin staining. Morphological observation were performed by CaseViewer Image analysis software.
Total RNA Extraction, cDNA Library Preparation, and Sequencing
A total of 14 sample were sent to Novogene (Beijing, China) for RNA extraction, library preparation, and sequencing. Briefly, Total RNA was extracted from the breast muscles using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA). Library construction using the Next Ultra RNA Library Prep Kit for Illumina (NEB, Beijing, China) (Parkhomchuk et al., 2009). And then the library preparations were sequenced on an Illumina Novaseq platform and 150 bp paired-end reads were generated.
Analysis of RNA-seq Data
Raw data of fastq format were firstly processed through fastp software to calculated Q20, Q30 and GC content. All the downstream analyses were based on the clean data with high quality. All clean reads were mapped to the chicken genome (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000002315.6/), using Hisat2 v2.0.5, and then compiled and evaluated. featureCounts v1.5.0-p3 were used to calculate the reads and FPKM mapped to each gene. Differential expression analysis of 2 groups was performed using the DESeq2 R package (1.20.0). The resulting P-values were adjusted using the Benjamini and Hochberg's approach for controlling the false discovery rate. Genes with an adjusted P-value < = 0.05 found by DESeq2 were assigned as differentially expressed. Gene ontology (GO) enrichment analysis of differentially expressed genes was implemented by the clusterProfiler R package, in which gene length bias was corrected. Gene ontology terms with corrected P value less than 0.05 were considered significantly enriched by differential expressed genes. We used clusterProfiler R package to test the statistical enrichment of differential expression genes in KEGG pathways.
Real-Time Quantitative PCR Verification of RNA-seq Data
To verify the accuracy of the transcriptomic data, we randomly selected seven DEGs for RT-qPCR. The primers (Table 1) were synthesized by Tsingke Biotechnology Co., Ltd (Beijing, China). Three times were detected for each sample. The RNA samples from RNA-seq remained were reverse transcribed into cDNA using the cDNA Synthesis kit (Vazyme Biotech Co., Lted. Nanjing, China) according the operation manual. RT-qPCR reaction system and thermal cycling parameters are operated according to the SYBR qPCR Master Mix (Vazyme Biotech Co., Lted. Nanjing, China) instructions. Genes expression were calculated by the 2−△△Ct method, using GAPDH as an internal control.
Table 1.
Primer pairs used for real-time quantitative PCR.
| Gene | Accession number | Sequence (5′-3′) | Product length (bp) |
|---|---|---|---|
| DCT | NM_204935.2 | F: GCTGTTGGTGCACAGGAAAC R: ATCGAGGAACTGCTCCCTCT |
149 |
| PMEL | NM_205112.3 | F: AGTTCAGCATCACCGACCAG R: CCCGAAGTCCCACGAATAGG |
173 |
| MLANA | XM_046935722.1 | F: CAGGTCTGAAGGAGGGATAGGA R: AGCCGCTACGCCTTTTGTAA |
186 |
| EDNRB2 | NM_204120.2 | F: GCACTGGCATCTTCTACACCC R: TGACGAGGAGGAAACTGAGCA |
237 |
| CALML4 | NM_001277629.2 | F: CCATGGCCAAGTTTCTGTCC R: TCTGCATTGCGCTCGATCT |
190 |
| KITLG | NM_001105315.1 | F: TGAAGAAGGCACAAACTTGGA R: ATCTGTCACTGGATTCCCGC |
104 |
| EDNRB | NM_001001127.2 | F: TGGCCCTTTGGTGTCGAAAT R: CAACTGCTCGGTACCTGTCT |
112 |
| GAPDH | NM_204305.2 | F: TCGGAGTCAACGGATTTGGC R: TTCCCGTTCTCAGCCTTGAC |
181 |
Statistical Analysis
All data except RNA-seq data were analyzed by t-test using SPSS 22.0 statistical software (SPSS Institute Inc., Chicago, IL). And correlation analysis of blackness characteristics was assessed by Spearman correlation. The results are expressed as arithmetic mean ± standard deviation, P < 0.05 was considered statistically significant.
RESULTS
Growth Traits
As presented in Table 2. There are no significant differences in IBW, FBW, EW, BMW, and TW between the H group and L group of Xuefeng black-bone chickens (P > 0.05).
Table 2.
Comparative analysis of growth traits between H group and L group in Xuefeng black-bone chicken.1
| Items/g3 | Group2 |
P-value |
||||
|---|---|---|---|---|---|---|
| FH | FL | MH | ML | FH vs. FL | MH vs. ML | |
| IBW | 26.91 ± 2.36 | 26.51 ± 1.80 | 26.63 ± 2.19 | 27.34 ± 2.55 | 0.301 | 0.193 |
| FBW | 1331.94 ± 150.51 | 1337.73 ± 141.18 | 1383.33 ± 140.85 | 1406.03 ± 167.84 | 0.830 | 0.518 |
| EW | 738.36 ± 84.84 | 738.14 ± 87.45 | 861.98 ± 96.14 | 875.45 ± 111.68 | 0.989 | 0.568 |
| BMW | 69.76 ± 10.20 | 72.10 ± 10.52 | 70.87 ± 10.10 | 72.49 ± 12.84 | 0.225 | 0.537 |
| TW | 18.92 ± 5.55 | 18.65 ± 2.54 | 25.08 ± 5.20 | 25.95 ± 3.75 | 0.731 | 0.393 |
H group: high blackness group; L group: low blackness group.
FH: female high blackness group; FL: female low blackness group; MH: male high blackness group; ML: male low blackness group.
IBW: initial body weight; FBW: final body weight; EW: eviscerated weight; BMW: breast muscle weight; TW: tibia weight.
Blackness Traits
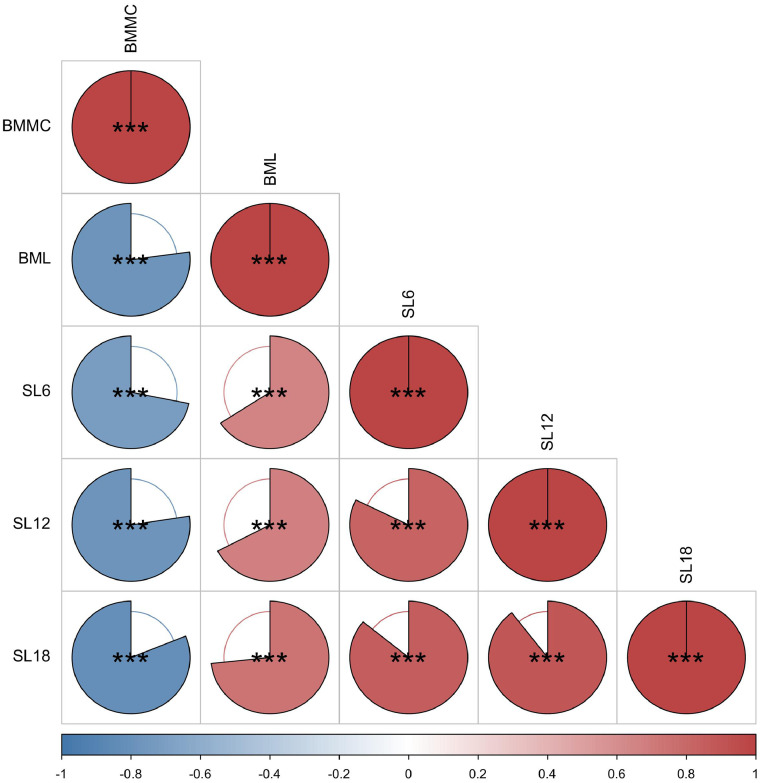
As showed in Table 3. The SL/BML in the H group were significantly lower than that in the L group while BMMC was higher than the L group (P < 0.001). And the SL increases as the chicken grows older, which indicated a decrease in skin blackness. We performed Spearman correlation to analyse the blackness traits of Xuefeng black-bone chickens (Figure 1). BML value was significantly positive correlated with different stage SL while significantly negative with BMMC (P < 0.001).
Table 3.
Comparative analysis of blackness traits between H group and L group in Xuefeng black-bone chicken.1
| Items3 | Group2 |
P-value |
||||
|---|---|---|---|---|---|---|
| FH | FL | MH | ML | FH vs FL | MH vs ML | |
| BMMC/(mg/g) | 1.82 ± 0.47A | 1.22 ± 0.11B | 1.58 ± 0.29A | 1.13 ± 0.09B | <0.001 | <0.001 |
| BML | 26.15 ± 2.02B | 37.68 ± 2.29A | 27.08 ± 2.48B | 38.80 ± 1.74A | <0.001 | <0.001 |
| SL6 | 27.45 ± 2.48B | 30.12 ± 1.84A | 27.75 ± 1.68B | 31.19 ± 2.15A | <0.001 | <0.001 |
| SL12 | 29.13 ± 2.19B | 33.06 ± 2.29A | 30.00 ± 2.47B | 34.77 ± 2.52A | <0.001 | <0.001 |
| SL18 | 31.10 ± 2.27B | 35.96 ± 2.53A | 32.46 ± 2.61B | 38.29 ± 2.82A | <0.001 | <0.001 |
Means within same sex with different superscripts differ highly significantly (P < 0.001).
H group: high blackness group; L group: low blackness group.
FH: Female high blackness group; FL: Female low blackness group; MH: Male high blackness group; ML: Male low blackness group.
BML: breast muscle L value; BMMC: melanin content of breast muscle; SL6: 6w skin L value; SL12: 12w skin L value; SL18: 18w skin L value.
Figure 1.
Correlation analysis of blackness traits in Xuefeng black-bone chicken. BML: breast muscle L value; BMMC: melanin content of breast muscle; SL6: 6w skin L value; SL12: 12w skin L value; SL18: 18w skin L value. *P < 0.05; **P < 0.01; ***P < 0.001.
Histomorphological Characteristics of Breast Muscle
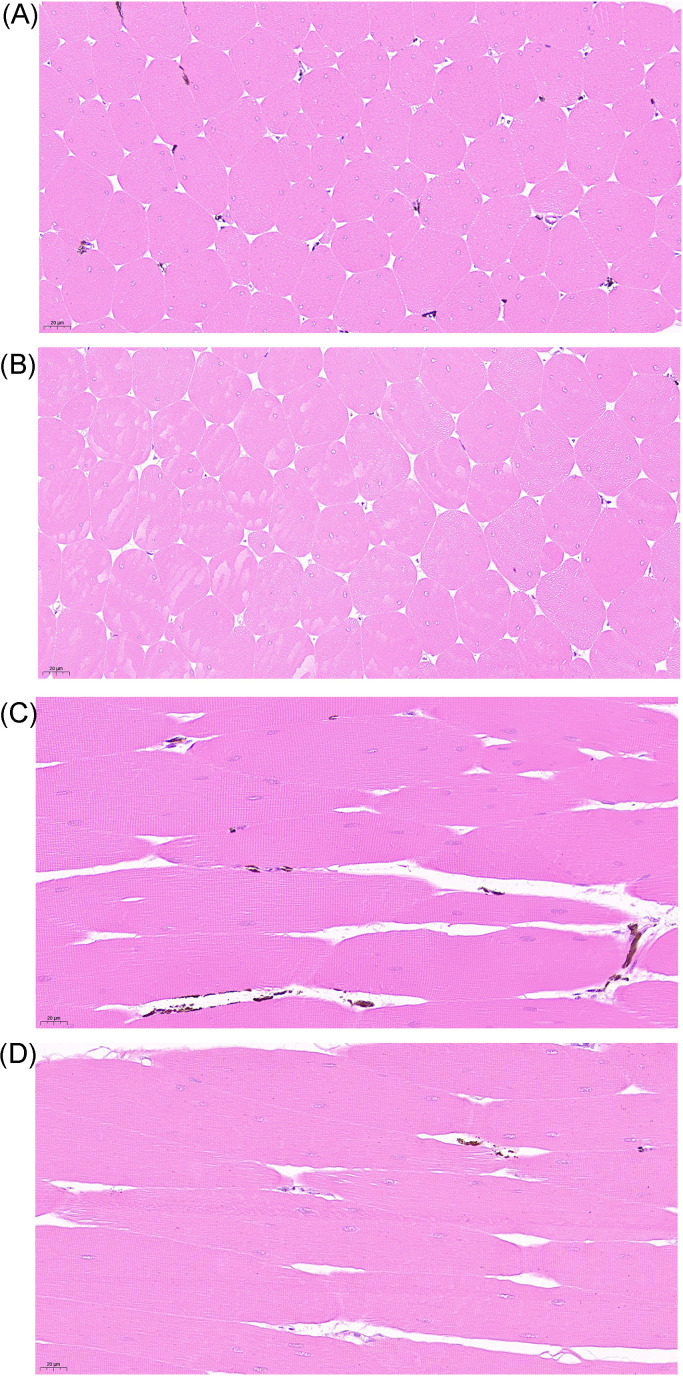
As presented at Figure 2. Melanin is primarily distributed unevenly between muscle fibers. Additionally, the H group exhibits higher melanin content and aggregation compared to the L group.
Figure 2.
Histomorphological of breast muscle in Xuefeng black-bone chickens (40 ×). (A) Cross-section of high blackness group; (B) Cross-section of low blackness group; (C) Longitudinal-section of high blackness group; (D) Longitudinal-section of low blackness group.
Sequencing Result and Differential Expression Genes
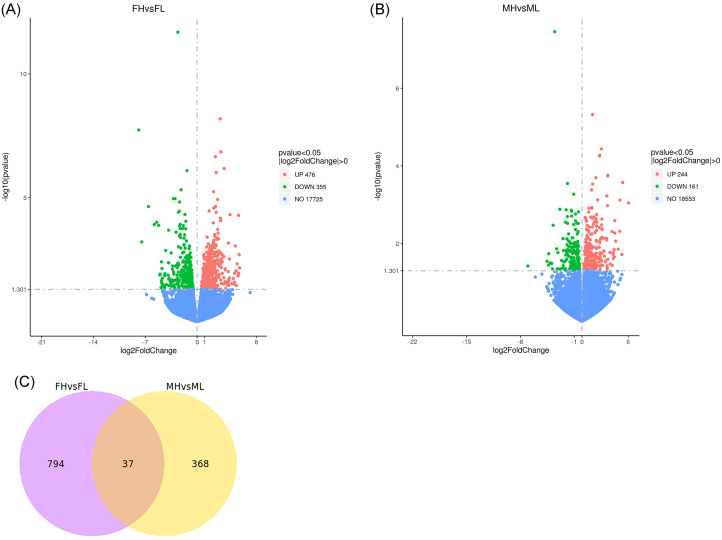
A total of 621,756,856 raw reads were obtained from 14 libraries, and the clean reads ratio was over 95% in each sample and the total map, Q20, Q30, and QC contents respectively exceeded 84, 97, 92, and 50%, which mean the the sequencing data can be used for subsequent bioinformatics analysis because of good quality and high coverage (Table 4). Meanwhile, a total of 831 differential expression genes (DEG) were discovered in FH vs. FL, including 476 upregulated genes and 355 downregulated genes, and in total of 405 DEGs consist of 244 upregulated genes and 161 downregulated genes were detected in MH vs. ML (Figure 3). In addition, 37 differential co-expressed genes were obtained from 2 comparisons (Figure 3).
Table 4.
Sequencing data for breast muscle samples in Xuefeng black-bone chickens.
| Sample1 | Raw reads | Clean reads | Total map | Q20 | Q30 | GC_pct |
|---|---|---|---|---|---|---|
| MH1 | 44168884 | 42678944 | 36355893(85.18%) | 97.26% | 93.05% | 52.35% |
| MH2 | 45267180 | 43956246 | 38929352(88.56%) | 97.63% | 93.87% | 50.32% |
| MH3 | 46332722 | 44211802 | 38744528(87.63%) | 97.88% | 94.46% | 51.76% |
| MH4 | 43878464 | 42568726 | 37089713(87.13%) | 97.48% | 93.50% | 51.33% |
| ML1 | 46953370 | 45410886 | 40154823(88.43%) | 97.66% | 93.91% | 50.60% |
| ML2 | 40939768 | 39197944 | 34157427(87.14%) | 97.49% | 93.40% | 51.50% |
| ML3 | 43945952 | 42790528 | 36608247(85.55%) | 97.49% | 93.56% | 52.16% |
| ML4 | 44817096 | 43525082 | 37198343(85.46%) | 97.10% | 92.74% | 51.89% |
| FH1 | 43630796 | 42175002 | 35775386(84.83%) | 97.44% | 93.50% | 53.38% |
| FH2 | 43940412 | 42779666 | 36912851(86.29%) | 97.25% | 92.96% | 52.31% |
| FH3 | 45499310 | 44051084 | 39182153(88.95%) | 97.44% | 93.40% | 51.38% |
| FL1 | 44083926 | 42830290 | 37937133(88.58%) | 97.59% | 93.72% | 51.49% |
| FL2 | 43931098 | 42673912 | 38245440(89.62%) | 97.61% | 93.72% | 50.23% |
| FL3 | 44367878 | 42918108 | 37657478(87.74%) | 97.69% | 93.96% | 51.59% |
FH: female high blackness group; FL: female low blackness group; MH: male high blackness group; ML: male low blackness group.
Figure 3.
The volcano plot and venn diagram of the differential expression genes. (A) FH vs. FL: the high blackness group compared with the low blackness group in female chicken; (B) MH vs. ML: the high blackness group compared with the low blackness group in male chicken; (C) Venn diagram of the differential co-expression genes in 2 comparisons (FH vs FL and MH vs. ML). Red dots mean upregulated genes; green dots mean downregulated genes; blue dots mean non-differential genes.
GO Enrichment Analysis
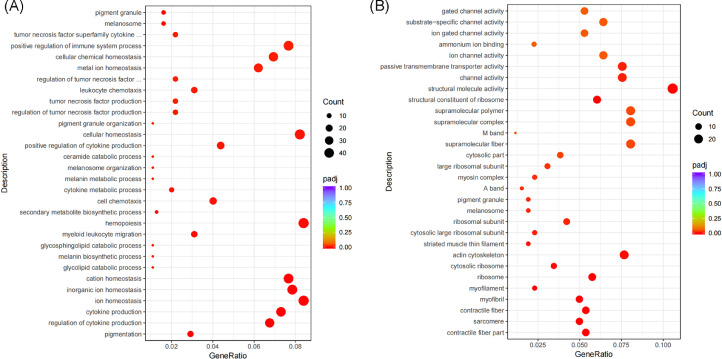
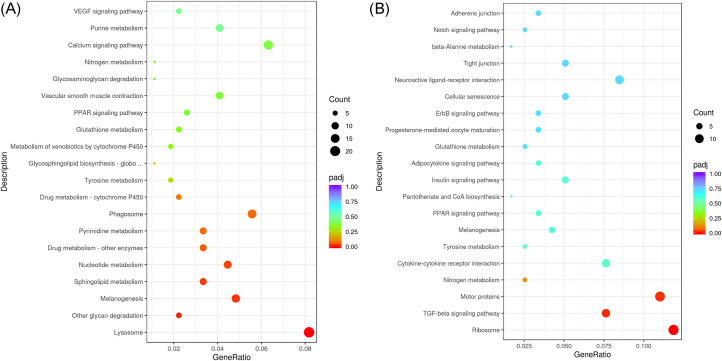
We conducted GO enrichment analyses with DEGs of 2 comparisons and listed the top 30 most significant pathway (Figure 4). In the FH vs. FL comparisons, the results indicated that these genes were enriched to terms such as pigmentation, cytokine production, melanin biosynthetic process, and melanosome etc (Figure 4A). In the MH vs. ML comparisons, the results indicated these genes were enriched to terms such as contractile fiber part, myofibril, melanosome, and pigment granule etc (Figure 4B). As presented in Table 5. We primary focus on melanin-related pathways, including pigmentation, melanin biosynthetic process, melanin metabolic process, melanosome organization, pigment granule organization, melanosome, and pigment granule. And we screened 5 upregulated genes (DCT, PEML, MLALA, TYRP1, and OCA2) in the melanin-related pathway that may influence the blackness trait of Xuefeng black bone chicken.
Figure 4.
The 30 most significant GO pathway in the 2 comparisons. (A) FH vs. FL: the high blackness group compared with the low blackness group in female chicken; (B) MH vs. ML: the high blackness group compared with the low blackness group in male chicken.
Table 5.
GO enrichment pathways related to melanin.
| Sex | Category1 | GO ID | Description | Gene name | P-adj |
|---|---|---|---|---|---|
| Female | BP | GO:0043473 | Pigmentation | Up: PMEL, OCA2, TYR, DCT, TYRP1, EDN3, SOX10, ASIP, RAB32, GPR143, FIG4, RAB17, HPS1, MC1R Down:KITLG, EDNRB |
<0.001 |
| BP | GO:0042438 | Melanin biosynthetic process | Up: OCA2, TYR, DCT, TYRP1, ASIP, MC1R | 0.002 | |
| BP | GO:0006582 | Melanin metabolic process | Up: OCA2, TYR, DCT, TYRP1, ASIP, MC1R | 0.003 | |
| BP | GO:0032438 | Melanosome organization | Up: PMEL, TYRP, ASIP, RAB32, GPR143, HPS1 | 0.003 | |
| BP | GO:0048753 | Pigment granule organization | Up: PMEL, TYRP, ASIP, RAB32, GPR143, HPS1 | 0.004 | |
| CC | GO:0042470 | Melanosome | Up: PMEL, OCA2, MLANA, TYR, DCT, TYRP1, RAB32, GPR143, RAB17 | 0.001 | |
| CC | GO:0048770 | Pigment granule | Up: PMEL, OCA2, MLANA, TYR, DCT, TYRP1, RAB32, GPR143, RAB17 | 0.001 | |
| Male | CC | GO:0042470 | Melanosome | Up: DCT, PMEL, MLANA, TYRP1, OCA2 | 0.010 |
| CC | GO:0048770 | Pigment granule | Up: DCT, PMEL, MLANA, TYRP1, OCA2 | 0.010 |
BP: biological process; CC: cellular component.
KEGG Enrichment Analysis
We also performed KEGG enrichment analyses with DEGs of 2 comparisons and listed the top 30 pathway (Figure 5). In the FH vs. FL comparisons, the results indicated that these genes were significantly enriched to terms such as lysosome, other glycan degradation, melanogenesis, sphingolipid metabolism, and nucleotide metabolism (Figure 5A). In the MH vs ML comparisons, the results indicated that these genes were significantly enriched to terms such as ribosome, TGF-beta signaling pathway, and motor proteins (Figure 5B). As showed in Table 6, we listed melanin-related pathways, including melanogenesis and tyrosine. And we screened 4 upregulated genes (DCT, EDNRB2, TYRP1, and CALML4) in the melanin-related pathway that may influence the blackness trait of Xuefeng black bone chicken. In addition, according to GO and KEGG pathway results, there are 2 downregulated genes (KITLG and EDNRB) which may be potential candidate genes for blackness trait of female chicken.
Figure 5.
The KEGG enrichment pathway. (A) FH vs. FL: the high blackness group compared with the low blackness group in female chicken; (B) MH vs. ML: the high blackness group compared with the low blackness group in male chicken.
Table 6.
KEGG enrichment pathways related to melanin.
| Sex | KEGG ID | Description | Gene name | P-adj |
|---|---|---|---|---|
| Female | gga04916 | Melanogenesis | Up: EDNRB2, CALML4, TYR, DCT, TYRP1, ASIP, CAMK2A, PRKCB, GNAO1, MC1R Down:KITLG, EDNRB, WNT9B |
0.018 |
| gga00350 | Tyrosine metabolism | Up: TYR, DCT,TYRP1 Down: ADH1C |
0.258 | |
| Male | gga04916 | Melanogenesis | Up: DCT, EDNRB2, TYRP1, CALML4 Down: CREBBP |
0.594 |
| gga00350 | Tyrosine metabolism | Up: DCT, TYRP1 | 0.566 |
Real-Time Quantitative PCR Verification
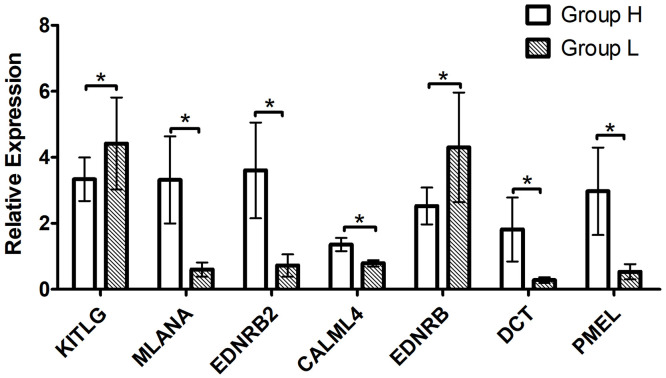
To validate RNA-seq data, the mRNA levels of 7 DEGs were analyzed by RT-qPCR (Figure 6), including 2 downregulated genes (KITLG and EDNRB) and 5 upregulated genes (MLANA, EDNRB2, CALML4, DCT, and PMEL). The expression patterns observed in the RT-qPCR data consistently aligned with the findings from RNA-Seq analysis, thus confirming the credibility and reliability of the transcriptome results.
Figure 6.
Validation of candidate DEGs using real-time quantitative PCR. Sample size in each group was seven, include female and male chickens. *P < 0.05.
DISCUSSION
As the economic trait of the black-bone chicken, the blackness traits are influenced by many factors, including environment, nutrition and genetics (Yu et al., 2018; Li et al., 2019; Cheng et al., 2023). Although the melanin formation process and some candidate genes for pigmentation have been defined, the regulatory mechanism and the key candidate genes in the breast muscle are still unclear (Singh et al., 2021; Zhou et al., 2021). Therefore, there is still a common phenomenon of significant variation of breast muscle in black-bone chicken (Yu et al., 2018; Xu et al., 2023). Our study found that the blackness variations not affect the growth performance of black-bone chicken, indicating that strengthening the breeding of blackness traits does not influence the original economic value. Meanwhile, there was a significant positive correlation between skin L value (SL) and breast muscle L value (BML). In addition, we also identified seven key candidate genes associated with melanin deposition in the breast muscle of Xuefeng black-bone chicken at market-age, and 2 potential candidate genes only for female chickens, which can be used as molecular markers for breeding. This result will help us accelerate the breeding efficiency of breast muscle blackness traits in black-bone chicken industry and understand the regulatory mechanisms of melanin deposition better.
Our studies demonstrated that there are no difference in the initial body weight (IBW), final body weight (FBW), eviscerated weight (EW), breast muscle weight (BMW), tibia wight (TW) between the high blackness group (H group) and the low blackness group (L group) in same sex. The body weight as a important economic trait can directly reflect the growth performance of chickens, so we recorded the one-day-age, the market-age and the eviscerated weight (Li et al., 2018). The breast muscle is one of the major meat-producing sites and the breast muscle rate was roughly 18.42% in Xuefeng black-bone chicken (Maharjan et al., 2021; Xu et al., 2023). And we also focused on the BMW because the most obvious changes in our research were the blackness of breast muscle (BBM), but there are no affect in BMW. The TW are positively correlated with body weight of chickens, which can react the potential growth trends of body (Kolakshyapati et al., 2019; Chew et al., 2021). Wang et al. (2021a) reported that genetics and breeding of body weight and skin color are not affect each other. There are no paper reported about the relationship between the BBM and the growth performance at present but our results showed that BBM changes can not effect the growth performance of black-bone chickens.
Melanin content is the main influencing factor that leads to the different degree of blackness (Nganvongpanit et al., 2020; Jian et al., 2021), our results maybe proof this point. Since the melanin content of breast muscle (BMMC) in the H group was significant higher than the L group. And the breast muscle histomorphological presented that melanin distributed unevenly between muscle fibers, and the H group can be observed more melanin content and aggregation compared to the L group. There are many results similar with our finding (Nganvongpanit et al., 2020; Kriangwanich et al., 2021). The SL/BML of the H group was significantly lower than the L group and the SL/BML was significantly negative correlated with the melanin content in this study. It indicates that L value can be used as one of the criteria for judging blackness in Xuefeng black-bone chicken, and the L value is easier to obtain in routine breeding process. A large number of studies evaluate the blackness of black-bone chickens by using colorimeter to measure the L value (Li et al., 2019; Wang et al., 2021a; Zi et al., 2023). And there are significantly positive correlation between the SL and BML. This suggests that perhaps the SL can be used as an indirect breeding index of the breast muscle blackness. In addition, we found an interesting phenomenon that the SL increased with the growth of black-bone chickens. It is mean the blackness was become lower along with growth, the reason maybe that the number of melanocytes and the melanin deposition ability was affected by age (Wang et al., 2021a; Zi et al., 2023).
We performed transcriptome sequencing analysis of breast muscle tissues from roosters and hens. There are 831 and 405 DEGs were discovered in FH vs. FL and MH vs. ML comparison, respectively. But only 37 differential co-expressed genes were obtained from 2 comparisons, which may be due to different market-age and sex. And these DEGs included seven candidate gene (DCT, PMEL, MLANA, TYRP1, OCA2, EDNRB2, and CALML4) were mainly enriched in the pigment granule, melanosome, and melanogenesis pathway, which involved in the regulation of melanin biosynthesis. Melanin deposition is mainly divided into the following 3 processes, melanocytes formation and development, melanin synthesis and melanin transport (Cieslak et al., 2011; Vandamme and Berx, 2019). First, the embryonal neural crest cells differentiate into melanoblast which migrate to specific sites along the dorsolateral and ventral migratory pathways and thus differentiate into melanocytes (Mort et al., 2015; Vandamme and Berx, 2019). Endothelin receptor B subtype 2 (EDNRB2) is play an important role in above process. Numerous studies have shown that aberrant EDNRB2 mainly causes hypopigmentation through restraining the migration of melanoblasts to other places (Li et al., 2015; Xi et al., 2020; Xi et al., 2021). Xi et al. (2021) revealed that EDNRB2 is the key genes influenced the duck body surface spot size, and found 2 allele locus as molecular markers. And Xi et al. (2020) demonstrated that a 14-bp insertion in EDNRB2 exon 3 related with white plumage in Chinese geese.
Melanin is synthesized and secreted by melanosomes in melanocytes, and then transported to the surrounding keratinocytes to exercise the pigment function (Crawford et al., 2020; Zhou et al., 2021). The melanosome, as a lysosome-associated organelles, mature through 4 stages including the immature step (stage I and II) and mature step (stage III and IV) (Costin and Hearing, 2007; Bissig et al., 2016). Premelanosomal protein (PMEL) as a type I transmembrane glycoprotein, it mainly maintains the structural morphology during the immature step of melanosomes (Mcglinchey et al., 2009; Knaust et al., 2020). Premelanosomal protein begin to hydrolyze form the PMEL fibrils/melanosome matrix at the stage I melanosomes, and Orderly and structured PMEL fibrils turn melanosomes into a typical ellipsoid shape at the stage II melanosomes (Bissig et al., 2016; Knaust et al., 2020). Melan-A (MLANA) also known as melanoma antigen recognized by T-cells 1 (MART-1), which could form a complex with PMEL to regulating its expression, stability, and processing (Hoashi et al., 2005). We can observe coat color faded and morphological structure of melanosomes altered in mice when silence MLANA expression or knockout this gene (Hoashi et al., 2005; Aydin et al., 2012). Oculocutaneous albinism type 2 (OCA2), encoded a melanosome-specific transmembrane protein, may participate melanosome maturation at the stage I and II. This protein forms a transmembrane channel crucial for the modulation of melanosome pH and transport of tyrosine, which is essential for melanin synthesis (Bellono et al., 2014; Klaassen et al., 2018; Le L et al., 2020). And OCA2 abnormal expressed along with the changes in the number, morphology and type of melanosomes (Park et al., 2015), but the specific mechanism is not clear. The melanogenesis process is start in stage III melanosomes, the L-tyrosine oxidation to dopaquinone and eventually generate eumelanin under the catalysis of tyrosinase, tyrosinase-related proteins1 (TYR1), dopachrome tautomerase (DCT) (Pillaiyar et al., 2017; Lu et al., 2021). Mutations in TYRP1 usually can result in oculocutaneous albinism type 3 with rufous or brown phenotype in skin, hair and irises (Patel et al., 2021). And recently studies defined DCT as a disease-causing gene in oculocutaneous albinism type 8 (Tingaud-Sequeira et al., 2022). No candidate genes related to melanin transport were found in this study, but there are lots of studies proved all of the above genes play necessary roles in the process of melanin production in animals (Bovo et al., 2023; Cheng et al., 2023; Li et al., 2023).
In addition, we found calmodulin-like protein 4 (CALML4) also involved in melanogenesis pathway, but there are no paper reported that its functional properties about melanin-related at present. Calmodulin-like protein 4, which functions as a light chain for myosin-7a, is a novel component of the intermicrovillar adhesion complex and Usher complex. It may play a significant role in both intestine and inner ear biology (Choi et al., 2020; Kapustina and Cheney, 2020). We speculated that CALML4 maybe need combined with other melanin-related proteins to form a complex that participates in melanin production, similar to how melanophilin requires binding with Rab27a and myosin Va to affect melanosomes transport (Park et al., 2019). And we found 2 potential candidate genes (KITLG and EDNRB) in female chicken, which could help us exhaustive understand the mechanisms of melanin production. Receptor tyrosine kinase (KIT) / KIT ligand (KITLG) signaling pathway is necessary for the survival of melanocytes. Ligand-induced dimerization is triggered after KITLG binds the c-KIT receptor, and it startover signal transduction through the RAS/MAPK pathway to increased melanoblast reproduce (Gorenjak et al., 2021). Mutation in KITLG are responsible for causing familial progressive hyper- and hypopigmentation in human (Wang et al., 2021b). And KITLG expression was lower in light color animals than in dark color animals (Song et al., 2017; Wu et al., 2021). Endothelin (EDN)/Endothelin Receptor Type B (EDNRB) signaling pathway was similar with KIT/KITLG signaling pathway, which mainly affecting the migration and proliferation of melanoblasts (Regazzetti et al., 2015). Therefore, abnormal expression in EDNRB cause a reduction in the number of melanoblasts and pigment dilution (Imokawa and Ishida, 2014; Bovo et al., 2023).
CONCLUSIONS
In conclusion, our research revealed that blackness variations in breast muscle do not affect growth traits in Xuefeng black-bone chicken. And the skin L value demonstrates a significant positive correlation with breast muscle L value, suggesting skin L value can serve as an indirect indicator for breast muscle breeding. Additionally, we identified seven candidate genes (DCT, PMEL, MLANA, TYRP1, OCA2, EDNRB2, and CALML4) associated with melanin production at market-age of Xuefeng black-bone chicken, and 2 potential candidate genes (KITLG and EDNRB) only for female chicken. These genes are beneficial to explore the genetic mechanism of black traits and could serve as molecular markers for breeding of breast muscle blackness. However, there maybe some shortage to deeper explore mechanism of melanin deposition due to the different market-age, we will adopt the same age black-bone chicken to research further. This study provides valuable insights for the breast muscle breeding of black-bone chicken, contributing to the advancement of the black-bone chicken industry.
Acknowledgments
ACKNOWLEDGMENTS
Integrative analysis of Genome Re-sequencing and 10 × Genomics scRNA-Seq Data Elucidate the Molecular Mechanisms Regulating Melanin Transfer of Pectoral Muscle in Black-boned Chickens (32072711) and Identification of SNPs Affected Blackness of Breast Muscle in Xuefeng Black-bone Chicken by Combining of Bulked Segregant Analysis and RNA Sequencing (2021JJ30322).
DISCLOSURES
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103691.
Appendix. Supplementary materials
REFERENCES
- Aydin I.T., Hummler E., Smit N.P., Beermann F. Coat color dilution in mice because of inactivation of the melanoma antigen MART-1. Pigment Cell Melanoma Res. 2012;25:37–46. doi: 10.1111/j.1755-148X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- Alfaro-Wisaquillo M.C., Oviedo-Rondón E.O., Cordova-Noboa H.A., Caldas J.V., Quintana-Ospina G.A., Ospina-Rojas I.C., San Martin V. Effects of amino acid levels during rearing on Cobb 500 slow-feathering broiler breeders: 1. Growth and development. Poul. Sci. 2021;100 doi: 10.1016/j.psj.2021.101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono N.W., Escobar I.E., Lefkovith A.J., Marks M.S., Oancea E. An intracellular anion channel critical for pigmentation. Elife. 2014;3:e4543. doi: 10.7554/eLife.04543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig C., Rochin L., van Niel G. PMEL amyloid fibril formation: the bright steps of pigmentation. Int. J. Mol. Sci. 2016;17:1438. doi: 10.3390/ijms17091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovo S., Ribani A., Utzeri V.J., Taurisano V., Bertarini G., Fontanesi L. Whole genome sequencing identifies candidate genes and mutations that can explain diluted and other colour varieties of domestic canaries (Serinus canaria) Anim. Genet. 2023;54:510–525. doi: 10.1111/age.13331. [DOI] [PubMed] [Google Scholar]
- Chen S.R., Jiang B., Zheng J.X., Xu G.Y., Li J.Y., Yang N. Isolation and characterization of natural melanin derived from silky fowl (Gallus gallus domesticus Brisson) Food Chem. 2009;111:745–749. [Google Scholar]
- Cheng J., Wang L., Wang S., Chen R., Zhang T., Ma H., Lu H., Yuan G. Transcriptomic analysis of thigh muscle of Lueyang black-bone chicken in free-range and caged feeding. Anim. Biotechnol. 2023;34:785–795. doi: 10.1080/10495398.2021.1993235. [DOI] [PubMed] [Google Scholar]
- Chew J., Widowski T., Herwig E., Shynkaruk T., Schwean-Lardner K. The effect of light intensity on the body weight, keel bone quality, tibia bone strength, and mortality of brown and white feathered egg-strain pullets reared in perchery systems. Poul. Sci. 2021;100 doi: 10.1016/j.psj.2021.101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.S., Graves M.J., Matoo S., Storad Z.A., El S.I.R., Weck M.L., Smith Z.B., Tyska M.J., Crawley S.W. The small EF-hand protein CALML4 functions as a critical myosin light chain within the intermicrovillar adhesion complex. J. Biol. Chem. 2020;295:9281–9296. doi: 10.1074/jbc.RA120.012820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak M., Reissmann M., Hofreiter M., Ludwig A. Colours of domestication. Biol. Rev. Camb. Philos. Soc. 2011;86:885–899. doi: 10.1111/j.1469-185X.2011.00177.x. [DOI] [PubMed] [Google Scholar]
- Costin G.E., Hearing V.J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- Crawford M., Liu N., Mahdipour E., Barr K., Heit B., Dagnino L. Integrin-linked kinase regulates melanosome trafficking and melanin transfer in melanocytes. Mol. Biol. Cell. 2020;31:768–781. doi: 10.1091/mbc.E19-09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T., Liang A., Liang S., Ma X., Lu X., Duan A., Pang C., Hua G., Liu S., Campanile G., Salzano A., Gasparrini B., Neglia G., Liang X., Yang L. Integrative analysis of transcriptome and gwas data to identify the hub genes associated with milk yield trait in buffalo. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Liu X., Yao Y., Xiao B., He C., Guo S., Tang S., Qu X. The potential role of palygorskite and probiotics complex on the laying performance and faecal microbial community in Xuefeng black-bone chicken. Ital. J. Anim. Sci. 2022;21:1660–1669. [Google Scholar]
- Deng Y., Xiong X., Liu X., He C., Guo S., Tang S., Qu X. Palygorskite combined probiotics improve the laying performance, hatching performance, egg quality, plasma antioxidative status, and immune response of broiler breeders. Ital. J. Anim. Sci. 2021;20:1292–1301. [Google Scholar]
- Geng S.S., Li H.Z., Wu X.K., Dang J.M., Tong H., Zhao C.Y., Liu Y., Cai Y.Q. Effect of Wujijing oral liquid on menstrual disturbance of women. J. Ethnopharmacol. 2010;128:649–653. doi: 10.1016/j.jep.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Gorenjak M., Fijacko N., Bogomir M.P., Zivanovic M., Potocnik U. De novo mutation in KITLG gene causes a variant of Familial Progressive Hyper- and Hypo-pigmentation (FPHH) Mol. Genet. Genomic. Med. 2021;9:e1841. doi: 10.1002/mgg3.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoashi T., Watabe H., Muller J., Yamaguchi Y., Vieira W.D., Hearing V.J. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- Imokawa G., Ishida K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014;15:8293–8315. doi: 10.3390/ijms15058293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian H., Zu P., Rao Y., Li W., Mou T., Lin J., Zhang F. Comparative analysis of melanin deposition between Chishui silky fowl and Taihe silky fowl. J. Appl. Anim. Res. 2021;49:366–373. [Google Scholar]
- Kapustina M., Cheney R.E. A new light chain for myosin-7. J. Biol. Chem. 2020;295:9297–9298. doi: 10.1074/jbc.H120.014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen H., Wang Y., Adamski K., Rohner N., Kowalko J.E. CRISPR mutagenesis confirms the role of OCA2 in melanin pigmentation in Astyanax mexicanus. Dev. Biol. 2018;441:313–318. doi: 10.1016/j.ydbio.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Knaust J., Weikard R., Albrecht E., Brunner R.M., Günther J., Kühn C. Indication of premelanosome protein (PMEL) expression outside of pigmented bovine skin suggests functions beyond eumelanogenesis. Genes (Basel) 2020;11:788. doi: 10.3390/genes11070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakshyapati M., Flavel R.J., Sibanda T.Z., Schneider D., Welch M.C., Ruhnke I. Various bone parameters are positively correlated with hen body weight while range access has no beneficial effect on tibia health of free-range layers. Poult. Sci. 2019;98:6241–6250. doi: 10.3382/ps/pez487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriangwanich W., Piboon P., Sakorn W., Buddhachat K., Kochagul V., Pringproa K., Mekchay S., Nganvongpanit K. Consistency of dark skeletal muscles in Thai native black-bone chickens (Gallus gallus domesticus) PeerJ. 2021;9:e10728. doi: 10.7717/peerj.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le L., Escobar I.E., Ho T., Lefkovith A.J., Latteri E., Haltaufderhyde K.D., Dennis M.K., Plowright L., Sviderskaya E.V., Bennett D.C., Oancea E., Marks M.S. SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation. Mol. Biol. Cell. 2020;31:2687–2702. doi: 10.1091/mbc.E20-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Wang X., Fu Y., Zhang C., Cao Y., Wang J., Zhang Y., Li Y., Chen Y., Li Z., Li W., Jiang R., Sun G., Tian Y., Li G., Kang X. Transcriptome analysis of the breast muscle of xichuan black-bone chickens under tyrosine supplementation revealed the mechanism of tyrosine-induced melanin deposition. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Han H., Lei Q., Gao J., Liu J., Liu W., Zhou Y., Li H., Cao D. Genome-wide association study of body weight in Wenshang Barred chicken based on the SLAF-seq technology. J. Appl. Genet. 2018;59:305–312. doi: 10.1007/s13353-018-0452-7. [DOI] [PubMed] [Google Scholar]
- Li L., Li D., Liu L., Li S., Feng Y., Peng X., Gong Y. Endothelin Receptor B2 (EDNRB2) gene is associated with spot plumage pattern in domestic ducks (Anas platyrhynchos) PLoS One. 2015;10 doi: 10.1371/journal.pone.0125883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wang Y., Liu Y., Li D., Tian Y., Liu X., Kang X., Li Z. Effects of SLC45A2 and GPNMB on melanin deposition based on transcriptome sequencing in chicken feather follicles. Animals. 2023;13:2608. doi: 10.3390/ani13162608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Tonissen K.F., Di Trapani G. Modulating skin colour: role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021;41 doi: 10.1042/BSR20210427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S., Arends D., Nassar M.K., Weigend A., Weigend S., Wang E., Brockmann G.A. High-density genotyping reveals candidate genomic regions for chicken body size in breeds of Asian origin. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan P., Martinez D.A., Weil J., Suesuttajit N., Umberson C., Mullenix G., Hilton K.M., Beitia A., Coon C.N. Review: Physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production. Animal. 2021;15(Suppl. 1) doi: 10.1016/j.animal.2021.100284. [DOI] [PubMed] [Google Scholar]
- McGlinchey R.P., Shewmaker F., McPhie P., Monterroso B., Thurber K., Wickner R.B. The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc. Natl. Acad. Sci. 2009;106:13731–13736. doi: 10.1073/pnas.0906509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort R.L., Jackson I.J., Patton E.E. The melanocyte lineage in development and disease. Development. 2015;142:620–632. doi: 10.1242/dev.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nganvongpanit K., Kaewkumpai P., Kochagul V., Pringproa K., Punyapornwithaya V., Mekchay S. Distribution of melanin pigmentation in 33 organs of thai black-bone chickens (Gallus gallus domesticus) Animals. 2020;10:777. doi: 10.3390/ani10050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.I., Lee J.E., Myung C.H., Jo C.S., Jang H.S., Hwang J.S. The absence of Rab27a accelerates the degradation of Melanophilin. Exp. Dermatol. 2019;28:90–93. doi: 10.1111/exd.13840. [DOI] [PubMed] [Google Scholar]
- Park S., Morya V.K., Nguyen D.H., Singh B.K., Lee H.B., Kim E.K. Unrevealing the role of P-protein on melanosome biology and structure, using siRNA-mediated down regulation of OCA2. Mol. Cell. Biochem. 2015;403:61–71. doi: 10.1007/s11010-015-2337-y. [DOI] [PubMed] [Google Scholar]
- Patel M.H., Dolinska M.B., Sergeev Y.V. TYRP1 mutant variants associated with OCA3: computational characterization of protein stability and ligand binding. Int. J. Mol. Sci. 2021;22:10203. doi: 10.3390/ijms221910203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T., Manickam M., Jung S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017;40:99–115. doi: 10.1016/j.cellsig.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Premi S., Wallisch S., Mano C.M., Weiner A.B., Bacchiocchi A., Wakamatsu K., Bechara E.J., Halaban R., Douki T., Brash D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzetti C., De Donatis G.M., Ghorbel H.H., Cardot-Leccia N., Ambrosetti D., Bahadoran P., Chignon-Sicard B., Lacour J.P., Ballotti R., Mahns A., Passeron T. Endothelial cells promote pigmentation through endothelin receptor b activation. J. Invest. Dermatol. 2015;135:3096–3104. doi: 10.1038/jid.2015.332. [DOI] [PubMed] [Google Scholar]
- Saidi A., Hajibarat Z. Application of next generation sequencing, gwas, rna seq, wgrs, for genetic improvement of potato (Solanum tuberosum L.) under drought stress. Biocatal. Agric. Biotechnol. 2020;29 [Google Scholar]
- Singh S., Nimse S.B., Mathew D.E., Dhimmar A., Sahastrabudhe H., Gajjar A., Ghadge V.A., Kumar P., Shinde P.B. Microbial melanin: recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021;53 doi: 10.1016/j.biotechadv.2021.107773. [DOI] [PubMed] [Google Scholar]
- Song X., Xu C., Liu Z., Yue Z., Liu L., Yang T., Cong B., Yang F. Comparative transcriptome analysis of mink (neovison vison) skin reveals the key genes involved in the melanogenesis of black and white coat colour. Sci. Rep. 2017;7:12461. doi: 10.1038/s41598-017-12754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Tan X., Yang X., Bai L., Kong F., Zhao G., Wen J., Liu R. Identification of candidate genes for meat color of chicken by combing selection signature analyses and differentially expressed genes. Genes. 2022;13:307. doi: 10.3390/genes13020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zhu S., Xie M., Wang W., Wu H., Gong D. Composition of fatty acids in the muscle of black-bone silky chicken (Gallus gellus demesticus brissen) and its bioactivity in mice. Food Chem. 2011;126:479–483. [Google Scholar]
- Tingaud-Sequeira A., Mercier E., Michaud V., Pinson B., Gazova I., Gontier E., Decoeur F., McKie L., Jackson I.J., Arveiler B., Javerzat S. The Dct(-/-) mouse model to unravel retinogenesis misregulation in patients with albinism. Genes. 2022;13:1164. doi: 10.3390/genes13071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y.G., Sun Y.Z., Tian Y.G., Xie M.Y., Chen J. Physicochemical characterisation and antioxidant activity of melanin from the muscles of Taihe Black-bone silky fowl (Gallus gallus domesticus Brisson) Food Chem. 2009;114:1345–1350. [Google Scholar]
- Vandamme N., Berx G. From neural crest cells to melanocytes: cellular plasticity during development and beyond. Cell. Mol. Life Sci. 2019;76:1919–1934. doi: 10.1007/s00018-019-03049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Cahaner A., Lou L., Zhang L., Ge Y., Li Q., Zhang X. Genetics and breeding of a black-bone and blue eggshell chicken line. 1. Body weight, skin color, and their combined selection. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li W., Zhou N., Liu J., Zhang S., Li X., Li Z., Yang Z., Sun M., Li M. Identification of a novel mutation in the KITLG gene in a Chinese family with familial progressive hyper-and hypopigmentation. BMC Med. Genomics. 2021;14:1–7. doi: 10.1186/s12920-020-00851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wu S., Song D., Qi G., Wang J., Zhang H., Yue H. Dietary lysine requirement of Jinghong pullets during the growing period. Chin. J. Anim. Nutr. 2017;29:1132–1140. [Google Scholar]
- Wu S., Li J., Ma T., Li J., Li Y., Jiang H., Zhang Q. MiR-27a regulates WNT3A and KITLG expression in Cashmere goats with different coat colors. Anim. Biotechnol. 2021;32:205–212. doi: 10.1080/10495398.2019.1675683. [DOI] [PubMed] [Google Scholar]
- Xi Y., Wang L., Liu H., Ma S., Li Y., Li L., Wang J., Chunchun H., Bai L., Mustafa A., He H. A 14-bp insertion in endothelin receptor B-like (EDNRB2) is associated with white plumage in Chinese geese. BMC Genomics. 2020;21:162. doi: 10.1186/s12864-020-6562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Xu Q., Huang Q., Ma S., Wang Y., Han C., Zhang R., Wang J., Liu H., Li L. Genome-wide association analysis reveals that EDNRB2 causes a dose-dependent loss of pigmentation in ducks. BMC Genomics. 2021;22:381. doi: 10.1186/s12864-021-07719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Tang S., Liu X., Deng Y., He C., Guo S., Qu X. Genes influencing deposition of melanin in breast muscle of the Xuefeng black bone chicken based on bioinformatic analysis. Genome. 2023;66:212–223. doi: 10.1139/gen-2022-0090. [DOI] [PubMed] [Google Scholar]
- Yang G., Lu H., Wang L., Zhao J., Zeng W., Zhang T. Genome-wide identification and transcriptional expression of the mettl21c gene family in chicken. Genes. 2019;10:628. doi: 10.3390/genes10080628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Wang G., Liao J., Tang M. Transcriptome profile analysis identifies candidate genes for the melanin pigmentation of breast muscle in Muchuan black-boned chicken. Poult. Sci. 2018;97:3446–3455. doi: 10.3382/ps/pey238. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu F., Cao J., Liu X. Skin transcriptome profiles associated with skin color in chickens. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhang Y., Zhang Y., Chen J., Nie R., Li J., Zhang H., Wu C. HOXB8 overexpression induces morphological changes in chicken mandibular skin: an RNA-seq analysis. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Zeng H., Huang J., Lei L., Tong X., Li S., Zhou Y., Guo H., Khan M., Luo L., Xiao R., Chen J., Zeng Q. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021;69 doi: 10.1016/j.arr.2021.101349. [DOI] [PubMed] [Google Scholar]
- Zhu W.Q., Li H.F., Wang J.Y., Shu J.T., Zhu C.H., Song W.T., Song C., Ji G.G., Liu H.X. Molecular genetic diversity and maternal origin of Chinese black-bone chicken breeds. Genet. Mol. Res. 2014;13:3275–3282. doi: 10.4238/2014.April.29.5. [DOI] [PubMed] [Google Scholar]
- Zi X., Ge X., Zhu Y., Liu Y., Sun D., Li Z., Liu M., You Z., Wang B., Kang J., Dou T., Ge C., Wang K. Transcriptome profile analysis identifies candidate genes for the melanin pigmentation of skin in tengchong snow chickens. Vet. Sci. 2023;10:341. doi: 10.3390/vetsci10050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.