Abstract
Mutations in mitochondrial DNA (mtDNA) are maternally inherited and have the potential to cause severe disorders. Mitochondrial replacement therapies, including spindle, polar body, and pronuclear transfers, are promising strategies for preventing the hereditary transmission of mtDNA diseases. While pronuclear transfer has been used to generate mitochondrial replacement mouse models and human embryos, its application in non-human primates has not been previously reported. In this study, we successfully generated four healthy cynomolgus monkeys (Macaca fascicularis) via female pronuclear transfer. These individuals all survived for more than two years and exhibited minimal mtDNA carryover (3.8%–6.7%), as well as relatively stable mtDNA heteroplasmy dynamics during development. The successful establishment of this non-human primate model highlights the considerable potential of pronuclear transfer in reducing the risk of inherited mtDNA diseases and provides a valuable preclinical research model for advancing mitochondrial replacement therapies in humans.
Keywords: Non-human primates, Mitochondrial replacement, Female pronuclear transfer
INTRODUCTION
Mitochondrial diseases are a group of inherited disorders characterized by dysfunction of mitochondrial oxidative phosphorylation, arising from mutations in nuclear DNA or mitochondrial DNA (mtDNA), commonly leading to neuronal and muscle system pathologies (Greenfield et al., 2017). In diseases induced by mtDNA mutations, the prevalence of mutant mitochondria is positively correlated with the likelihood of disease manifestation. Due to the substantially lower quantity of sperm-derived mitochondria compared to oocytes, mtDNA diseases predominantly follow maternal inheritance patterns (Kaneda et al., 1995). To reduce the risk of a woman carrying abnormal mitochondria giving birth to a child afflicted with mitochondrial disease, various strategies have been used to prevent or minimize the transmission of abnormal mitochondria. Mitochondrial replacement therapy (MRT) is a commonly used therapeutic strategy, involving the transfer of nuclear DNA from an oocyte harboring defective mitochondria into an enucleated oocyte with healthy mitochondria (Tang et al., 2022; Zhang et al., 2017). The primary techniques of MRT include spindle transfer (ST) (Wang et al., 2001), polar body transfer (PBT) (Wakayama et al., 1997; Wakayama & Yanagimachi, 1998), and pronuclear transfer (PNT) (Mann & Lovell-Badge, 1984). However, MRT cannot entirely eliminate maternal defective mitochondria, leading to residual carryover of maternal mtDNA (Lee et al., 2012). Therefore, both the safety and efficacy of MRT approaches require further evaluation.
Non-human primates (NHPs), which show strong genetic and physiological similarities to humans, can serve as highly suitable models for the preclinical safety evaluation of MRT. While monkeys have been successfully generated through ST and PBT techniques (Tachibana et al., 2009, 2013; Wang et al., 2021), with subsequent analyses of mtDNA carryover in their first-generation (F1) offspring providing a comprehensive reference for clinical application (Ma et al., 2021), the production of monkeys via PNT remains unrealized. Previous studies employing mouse models have demonstrated that PNT between mouse zygotes can generate mice with a corrected mtDNA-related phenotype. However, these mice retain 5%–44% mutant mtDNA at 300 days after birth (Sato et al., 2005), which, despite being much lower than non-transferred controls (~80%), raises concerns regarding the potential transmission of inherited mtDNA variants to subsequent generations. Regarding human-based PNT research, several approaches have been explored (Craven et al., 2010; Hyslop et al., 2016; Wu et al., 2017). However, ethical constraints and safety considerations prevent the transfer of PNT embryos into surrogates. Consequently, analyses are restricted to the detection of mtDNA carryover in pre-implantation embryos or embryonic stem cells (ESCs), with the dynamics of mtDNA heteroplasmy during the development of PNT-treated individuals remaining unexplored. As such, the utilization of NHP models is crucial for understanding mtDNA changes during postnatal development and assessing health outcomes.
In this study, we successfully generated mitochondrial replacement cynomolgus macaques (Macaca fascicularis) using reconstructed embryos through female pronucleus transfer (FPNT), an innovative PNT technique characterized by reduced cytoplasmic carryover. These FPNT-derived monkeys were born in good health and have survived for more than two years, exhibiting a unique genetic lineage derived from three distinct parental genomes. Furthermore, the monkeys contain minimal maternal mtDNA carryover (3.8%–6.7%), with stable maintenance of the initial proportion of maternal mtDNA heteroplasmy. Overall, this research provides a valuable NHP model for the assessment of methods aimed at improving the efficiency and safety of MRT, offering an important preclinical model for the application of MRT in humans.
MATERIALS AND METHODS
Animal ethics statement
For this study, healthy female monkeys (5 to 12 years old) were selected. All animals were accommodated in a conditional environment (temperature: 22±1°C; humidity: 50%±5%) with a 12 h light/ 12 h dark cycle (light on time 0700h to 1900h). All animals were given commercial monkey diet (Jiangsu Xietong, JiangSu, China) twice a day and fed fruits once daily, and with free access to water. The care and use of all animals were conducted in accordance with the guidelines established by the Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, which granted ethical approval (ION-2018004).
Monkey oocyte collection
The oocyte collection procedures have been described previously (Wang et al., 2021). In brief, cynomolgus monkeys with regular menstrual cycles and ranging in age from 5 to 12 years were chosen for superovulation. These monkeys were injected with 25 IU recombinant human follitropin twice daily from day 3 of their menstrual cycle, followed by 1 000 IU human chorionic gonadotropin (hCG) on day 11. Oocytes were then aspirated from the follicles 36 h later. Metaphase II-arrested oocytes were used for manipulation.
Monkey FPNT
In the FPNT procedure, oocytes from two different monkeys were divided into two groups. MII oocytes from a donor monkey were placed in manipulation drops containing HEPES-buffered Tyrode’s lactate medium (TH3) with 5 μg/mL cytochalasin B (CB, Absin, China) in a glass bottom dish to rapidly perform spindle-chromosome complex (SCC) removal using a piezo-driven pipette under polarized light imaging (Oosight Imaging System, Cri, Hamilton Thorne, USA). Sperm were then injected into the enucleated oocytes 1 h later. Concurrently, oocytes from another donor monkey underwent parthenogenetic activation in 5 μmol/L ionomycin (Sigma, USA) for 5 min and were then transferred to 7.5 μg/mL cycloheximide (Sigma, USA) medium for 5–6 h (I/CHX). After this, successful oocyte activation in each group was confirmed by the presence of a single pronucleus. 5–10 I/CHX-activated embryos were then transferred to manipulation drops containing TH3 with 5 μg/mL CB and 5 μg/mL nocodazole (MedChemExpress, USA). A laser objective (Hamilton Thorne, USA) was used to create a hole in the zona pellucida of the embryos, allowing for the precise extraction of the female pronucleus and surrounding cytoplasm using a pipette with a 15–18 μm outer diameter (Supplementary Movie S1). After brief incubation with hemagglutinating virus of Japan envelope (HVJ-E) medium (Cosmo Bio, Japan) at 37°C for 10 s, the female pronucleus were introduced into the zona pellucida space of sperm-activated embryos (Supplementary Movie S2). This process resulted in the successful creation of a reconstructed embryo harboring genetic material from three distinct genomes. All embryos were cultured in HECM-9 (H9) medium at 37°C under 5% CO2.
Embryo transfer
Healthy female monkeys with synchronous menstrual cycles were used as surrogates. Two to three cleaved embryos at the 2–8-cell stage were transferred into the oviduct of each surrogate. Approximately 20–30 days after embryo transfer, pregnancy was confirmed by ultrasonographic diagnosis.
Mitochondrial staining
The I/CHX-activated embryos containing a female pronucleus were stained with MitoTracker (Life Technologies, USA) and Hoechst 33342 (Sigma, USA), as described previously (Liu et al., 2018). In brief, embryos were stained with 250 nmol/L MitoTracker at 37°C for 10 min, washed with H9 three times, and incubated in 1 mg/mL Hoechst 33342 at room temperature for 10 min. Imaging of embryos was performed using a laser-scanning inverted confocal microscope (Olympus F10I, Japan).
Short tandem repeat (STR) analysis
Extracted genomic DNA was amplified using locus-specific primers with fluorescent dye. The amplicons were then subjected to fragment analysis using a genetic analyzer (ABI PRISM 3730, Thermo Fisher, USA), with data analyzed using Gene Marker (v.2.2.0).
Single nucleotide polymorphism (SNP) analysis
Extracted DNA from blood or ear tissue samples was amplified with specific primers in mtDNA. Polymerase chain reaction (PCR) products were used for Sanger sequencing, with results analyzed using Chromas (v.2.6.6.0).
MtDNA copy number analysis by digital PCR (dPCR)
The pronuclei were isolated and collected using a micropipette. The isolated single pronucleus (n=7), double pronuclei (n=6), and activated oocytes (n=8) were washed with phosphate-buffered saline (PBS) and transferred to PCR tubes containing 3 μL of PBS, followed by the addition of 3.5 μL of freshly prepared Buffer D2 (QIAGEN, 150343, Germany). The PCR tubes were placed in a thermal cycler and incubated at 65°C for 10 min, with a final hold at 10°C. Subsequently, 3 μL of Stop Solution was added to each tube. The pronuclei and oocyte lysates were then directly used for dPCR. The dPCR mixture (16 μL) included 8 μL of Digital PCR Master Mix, 0.2 μL of forward primer (10 μmol/L), 0.2 μL of reverse primer (10 μmol/L), 0.25 μL of TaqMan Probe (10 μmol/L), 1 or 2 μL of DNA sample, and 5.35 or 4.35 μL of water. A lysate-buffer mix was used as the negative control. MtDNA copy number was quantified using dPCR, targeting a segment spanning nt13523–nt13606 of the mitochondrial ND5 gene. The primer sequences were CGAAGCCACAAACACGTCATAT (forward) and GTGCTGTAGGCGCTTGTTAGG (reverse). The TaqMan probe sequence was 5-FAM-TGAGCCCTATTTATTACTCTC-MGB-3 (Invitrogen, USA).
MtDNA heteroplasmy analysis by pyrosequencing
Total genomic DNA extraction of ear tissues or blood samples from FPNT-derived infants was performed using a Genomic DNA Extraction Kit (TIANGEN, Germany) according to the manufacturer’s instructions. For FPNT-1 and FPNT-2, the SNP used to detect heteroplasmy was C>T, the primer sequences were forward TCTTACTTTTAACCAGTGAAATTGA and reverse Biotin-TAATAGATTAAAGCTCCATAGGGTC, and the pyrosequencing primer was AAGAGGCGG ACATAAAA. For FPNT-3 and FPNT-4, the SNP used to detect heteroplasmy was G>A, the primer sequences were forward CGAATACGTTAGGTCAAGGTGT and reverse Biotin-CCTGGTTCAATTAAGCACTCTAT, and the pyrosequencing primer was AAAACCCTTATG AAACTTA. The PCR for pyrosequencing was performed at 95°C for 5 min, with 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 50 s, followed by one cycle at 72°C for 8 min. Pyrosequencing was performed using the PyroMark Q96 ID platform according to the manufacturer’s instructions (QIAGEN, Germany).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (v.9). Analyses were conducted using two-tailed Student’s t-test. All graphs display mean±standard error of the mean (SEM).
RESULTS
Isolated single pronucleus carried less mitochondria
Traditional biparental pronuclei transfer strategy tends to result in karyoplast with higher cytoplasmic carryover from the donor oocyte (Craven et al., 2010; Wang et al., 2014), leading to the generation of offspring with very high levels of mtDNA carryover (Sato et al., 2005). Consequently, minimizing cytoplasmic carryover during PNT is a critical consideration. Here, we opted for a single pronucleus transfer strategy, which may result in reduced cytoplasmic carryover from parthenogenetic donor oocytes compared to tranditional PNT.
Initially, we examined mitochondrial distribution in parthenogenetically activated monkey embryos using MitoTracker and Hoechst staining. In the I/CHX-activated embryos, we found that the mitochondria were uniformly distributed throughout the cytoplasm (Figure 1A). Subsequent dPCR analysis of isolated pronuclei and whole activated oocytes revealed that the average mtDNA copy number in isolated single pronucleus was 3 145 (n=7), in isolated double pronuclei was 6 249 (n=6), and in I/CHX-activated oocytes was approximately 1 300 000 (n=8) (Figure 1B; Supplementary Table S1). These findings indicate that a single pronucleus carries fewer mitochondria relative to whole oocytes, and less than that observed with double pronuclei, demonstrating the potential feasibility of employing a single pronucleus transfer approach.
Figure 1.
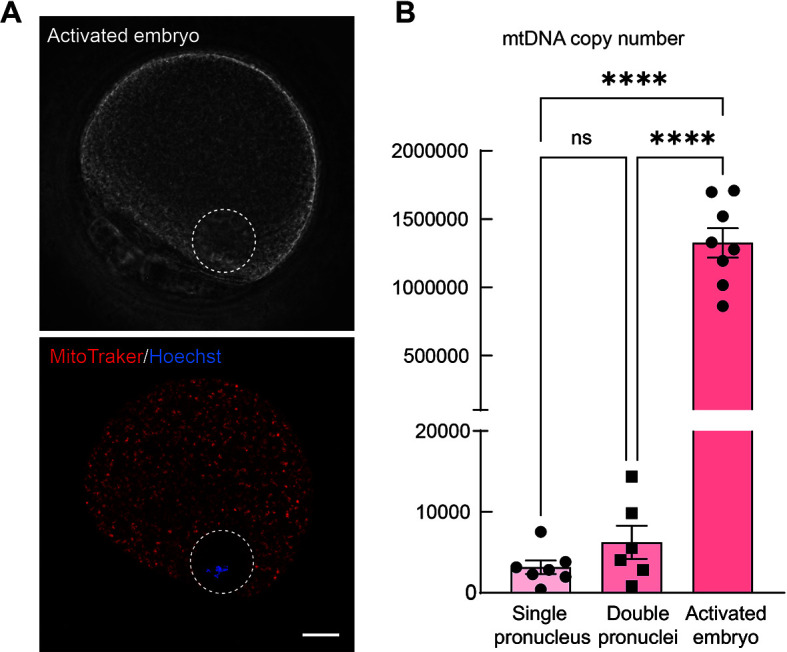
Mitochondrial carryover in isolated pronuclei is much lower than that in intact oocytes
A: Confocal microscopy images showing parthenogenetic-activated embryo stained with MitoTracker to indicate mitochondria and Hoechst to indicate chromosomes. Scale bar: 20 μm. B: Digital PCR analysis results showing mtDNA copy number in isolated single or double pronuclei and parthenogenetic-activated embryos. Each dot indicates one biological replicate. Data are presented as mean±SEM. Statistical analysis was performed using two-sided Student's t-test, ns: Not significant; ****: P<0.0001.
Generation of mitochondrial replacement monkeys by FPNT
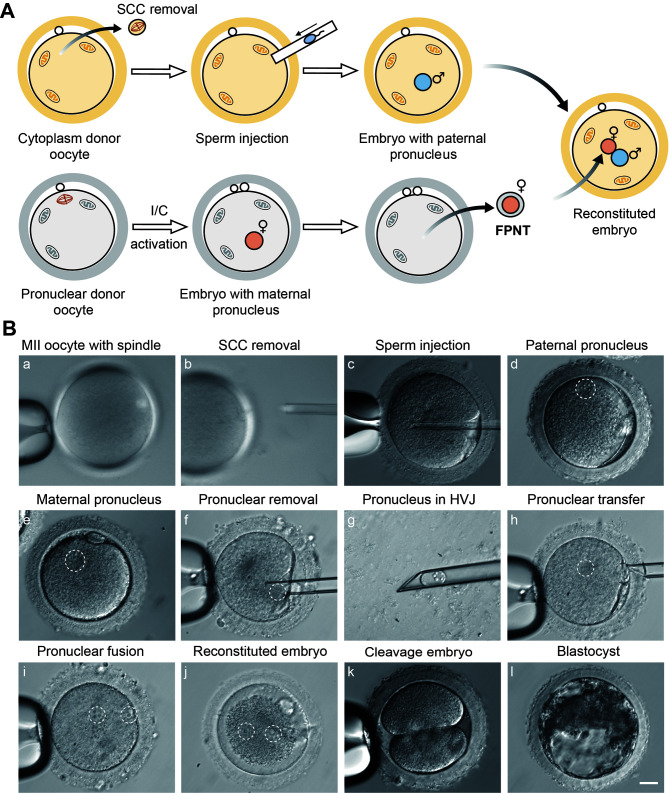
MII oocytes were divided into two groups: one designated for cytoplasm donor oocytes and the other for female pronucleus (FPN) donor oocytes, with each cohort sourced from different macaques. In the cytoplasm donor oocyte group, SCC was removed under polarized-light imaging, followed by the injection of sperm into the enucleated oocytes after recovery. In the FPN donor oocyte group, the mature oocytes were subjected to I/CHX activation. At 5 h post-activation, embryos from both groups exhibited a single pronucleus, with embryos from the cytoplasm donor group containing a male pronucleus (MPN) and embryos from the FPN donor group containing an FPN. Subsequently, the FPN was carefully extracted from the embryo, and, after brief incubation in HVJ-E, was transferred into the perivitelline space of a cytoplasm donor embryo. After 1 h of fusion, FPNT embryos were successfully reconstructed (Figure 2A, B; Supplementary Movies S1, S2).
Figure 2.
Generation of reconstructed embryos through FPNT
A: Experimental scheme showing FPNT process. B: Representative image of FPNT process and developmental stages of reconstructed embryo. Scale bar: 20 μm. (a, b) SCC removal of cytoplasm donor oocyte. (c) Sperm injection into SCC-removed oocyte. (d) Cytoplasm donor embryo with only MPN. (e) I/CHX-activated embryo with only FPN. (f–i) Transfer of FPN from I/CHX-activated embryo into embryo with only MPN. (j) Reconstructed embryo with biparental pronuclei. (k–l) Reconstructed embryo at two-cell and blastocyst stage.
Using the above approach, a total of 35 reconstructed FPNT embryos were obtained using 125 MII mature oocytes and transferred into 15 macaque surrogates. Pregnancy was confirmed in three surrogates after approximately four weeks (pregnancy rate 20%), with one pregnancy carrying twins, similar to the pregnancy rate in normal ICSI embryos (28.95%) (Table 1; Supplementary Table S2). Four healthy infants were delivered by caesarean section at full-term (Figure 3A), all of which have survived for more than 2 years.
Table 1. Summary of development of FPNT embryos.
| Oocytes | Cytoplasm donor oocytes |
Female pronuclear donor oocytes |
Reconstructed FPNT embryos |
Embryos transferred |
Surrogates | Pregnancies | Live births |
| 125 | 65 | 60 | 35 | 35 | 15 | 3 | 4 |
Figure 3.
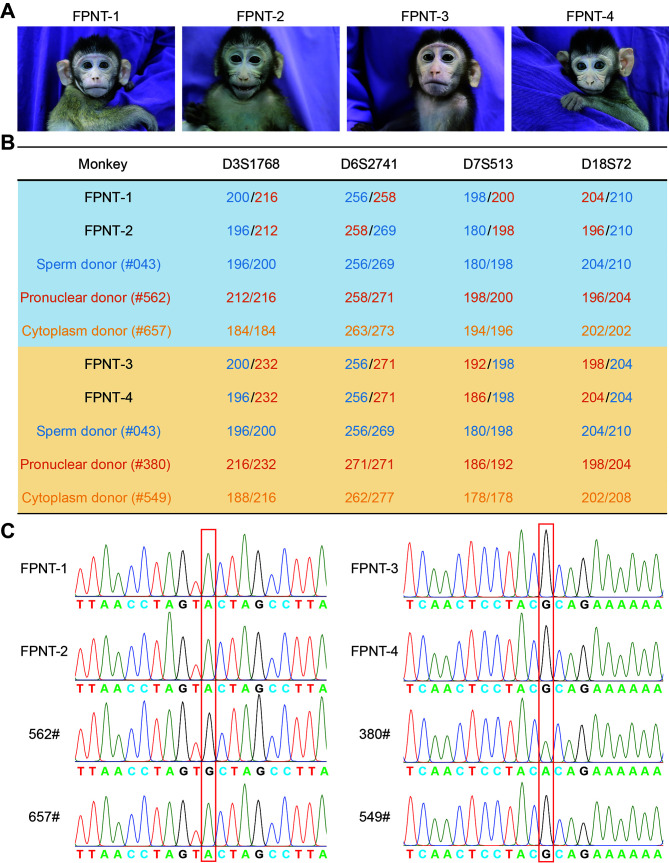
Genomic inheritances of FPNT-derived monkeys
A: Images of four FPNT monkeys. B: STR analysis of nuclear DNA origin in FPNT offspring. STR results showed that the female nuclear DNA of offspring was identical to that of the FPN oocyte donor but different from that of the cytoplasm oocyte donor. Further examples are shown in Supplementary Figure S1. C: SNP analysis of mtDNA origin in FPNT offspring. Results showed that the mtDNA of offspring was identical to that of the cytoplasm oocyte donor but different from the pronuclear oocyte donor. Further examples are shown in Supplementary Figure S1.
Genetic origin of FPNT monkeys
To determine the genetic lineage of the FPNT-derived monkeys, genomic DNA was extracted from blood cells for the identification of mtDNA and nuclear DNA origins by STR and SNP analyses. STR analysis of 27 loci demonstrated that all monkeys inherited their maternal genome from the FPN donor monkeys and paternal genome from the sperm donor monkeys (Figure 3B; Supplementary Figure S1A and Table S3). Furthermore, SNP analysis, conducted by direct sequencing of the PCR-amplified mitochondria D-loop regions and ND5 gene, indicated that the mtDNA of FPNT-derived monkeys predominantly originated from the cytoplasm donor monkeys (Figure 3C; Supplementary Figure S1B).
Postnatal development of FPNT-derived monkeys
Although PNT and early PNT (ePNT) have been performed in human embryos, confirming the technical feasibility of these approaches, questions remain regarding whether the manipulation process could induce damage causing later developmental disorders in PNT individuals (Craven et al., 2010; Hyslop et al., 2016; Wu et al., 2017). To address this concern, we conducted a two-year follow-up on the four FPNT-produced monkeys.
The health status of the FPNT monkey infants was carefully tracked, with physical examinations conducted every two months to assess body weight (Figure 4A), head circumference, and body length (Supplementary Figure S2A). In comparison to age-matched monkeys conceived via intracytoplasmic sperm injection (ICSI), the FPNT-derived monkeys showed normal postnatal growth. Viral testing for colony-excluded viruses (i.e., simian immunodeficiency virus, simian T-cell leukemia virus, simian retrovirus, tubercle bacillus, and herpes B virus) was uniformly negative for all four FPNT monkeys. Furthermore, none exhibited any serious health issues during their development.
Figure 4.
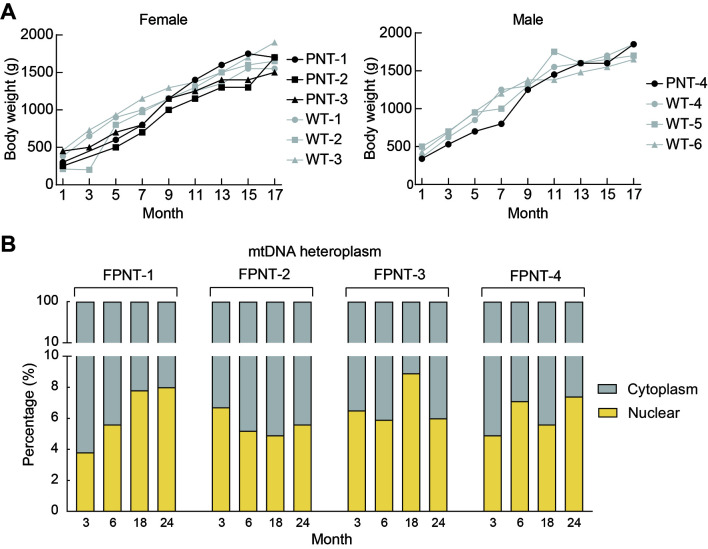
mtDNA carryover and postnatal development of FPNT monkeys
A: No significant differences in body weight were observed between FPNT and control monkeys. B: Percentage of mitochondria derived from FPN donors did not increase with development in the four FPNT-derived monkeys, as determined by pyrosequencing analysis at 3, 6, 18, and 24 months of age. Yellow bar represents mitochondria from the female PN donor, gray bar represents mitochondria from the cytoplasm donor.
MtDNA heteroplasmy dynamics in FPNT-derived monkeys
Similar to the detection of mtDNA copies in pronuclei (Figure 1B), a small but measurable fraction of cytoplasm from the FPN donor oocytes is unaviodably co-transferred with the FPN, resulting in the presence of residual FPN donor mtDNA in FPNT monkeys. Thus, we investigated mtDNA carryover in the blood cells of the four FPNT macaque infants at 3 months post-birth using pyrosequencing. Analysis revealed that, while the monkeys predominantly carried mtDNA from the cytoplasm donor monkeys, a small proportion of mtDNA from the FPN donor monkeys was also present, ranging from 3.8%–6.7% (Supplementary Figure S2B).
In addition to monitoring health and growth rates, we also conducted a two-year follow-up to assess mtDNA carryover dynamics from the FPN donor oocytes. Based on pyrosequencing at 3, 6, 18, and 24 months after birth, mtDNA carryover from the FPN donors remained relatively stable throughout the development of the FPNT-derived macaques (Figure 4B). Among the four macaques, only FPNT-1 showed an increasing trend in mtDNA carryover as it matured; in the remaining three monkeys, the initially elevated mtDNA carryover levels observed to decline (Figure 4B).
DISCUSSION
This study is the first to report on the generation of healthy mitochondrial replacement NHPs through the transfer of FPN into androgenetic embryos. As an effective MRT method, PNT has been used to generate mitochondrial replacement human embryos and mouse models (Craven et al., 2010; Wang et al., 2014). However, in human PNT research, due to ethical and safety concerns, reconstructed embryos are only used to generate ESC lines rather than for embryo transfer (Hyslop et al., 2016; Wu et al., 2017). Additionally, PNT-derived mouse models exhibit high mtDNA carryover levels (23.7%±11.1% or 5%–44%), attributed to the high cytoplasmic carryover, may compromising the effective prevention of mitochondrial disease transmission (Sato et al., 2005; Wang et al., 2014). Thus, to achieve minimal cytoplasmic carryover from donor oocytes, we employed FPNT to construct mitochondrial replacement monkeys and detect postnatal dynamics of mtDNA heteroplasmy.
The FPNT strategy differs from the traditional PNT approaches applied in mice or humans, achieving lower mtDNA carryover and eliminating the ambiguity often associated with differentiating between male and female pronuclei. Using this strategy, we successfully produced four healthy mitochondrial replacement cynomolgus monkeys from a total of 35 reconstructed embryos, yielding a birth rate of 11.43%. These monkeys have all survived beyond two years, with relatively stable mtDNA carryover during postnatal development.
At present, there are no definitive treatments for mitochondrial diseases, with management focusing primarily on palliative support. As such, various strategies have been devised to reduce the likelihood of transmitting abnormal mitochondria from mothers to their offspring. Despite the development of mtDNA gene editors such as DdCBE three years ago (Mok et al., 2020), which have since been applied in human embryo gene editing (Chen et al., 2022; Wei et al., 2022), MRT is still considered the most effective strategy for preventing the transmission of mutant mtDNA to the next generation. However, the safety of MRT and its effects on progeny remain critical concerns in the field. Therefore, to gain a deeper understanding of the long-term effects of FPNT, additional longitudinal research is required, focusing on health, fertility, and the transmission and segregation of FPN donor mtDNA across different tissues and organs in these monkeys and their offspring. As embryos or infants produced via MRT inherently carry maternal mtDNA, future investigations should also focus on combining MRT with mtDNA gene editing to minimize mtDNA carryover to an undetectable level.
In summary, this study provides a valuable NHP model for MRT preclinical research, as well as longitudinal safety assessments. Furthermore, integrating this approach with CRISPR technology will enable the generation of allele-specific gene-editing models, which can be used to screen imprinted gene functions or construct lethal bi-allelic mutation models.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTIONS
Q.S. designed and supervised the project. C.Y.L. and X.C.L. performed the embryo experiments. Y.Z.L. and X.C.L. performed the molecular experiments and statistical analyses. Y.W., Y.H.N., Y.T.X., X.T.Z., and Y.L. performed monkey-assisted reproductive experiments and physical examinations. X.C.L., Y.Z.L., C.Y.L., and Q.S. wrote the manuscript with contributions from all other authors. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Zhen Liu from the Institute of Neuroscience, Chinese Academy of Sciences for helpful suggestion, and all veterinarians from the Songjiang Non-Human Primate Facility, Institute of Neuroscience, Chinese Academy of Sciences, for help with animal breeding and care.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82021001, 31825018), National Key Research and Development Program of China (2022YFF0710901), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32060100), Biological Resources Program of Chinese Academy of Sciences (KFJ-BRP-005), and National Science and Technology Innovation 2030 Major Program 2021ZD0200900
References
- Chen XX, Liang D, Guo JY, et al DdCBE-mediated mitochondrial base editing in human 3PN embryos. Cell Discovery. 2022;8(1):8. doi: 10.1038/s41421-021-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven L, Tuppen HA, Greggains GD, et al Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465(7294):82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield A, Braude P, Flinter F, et al Assisted reproductive technologies to prevent human mitochondrial disease transmission. Nature Biotechnology. 2017;35(11):1059–1068. doi: 10.1038/nbt.3997. [DOI] [PubMed] [Google Scholar]
- Hyslop LA, Blakeley P, Craven L, et al Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016;534(7607):383–386. doi: 10.1038/nature18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda H, Hayashi J, Takahama S, et al Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4542–4546. doi: 10.1073/pnas.92.10.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ma H, Juanes RC, et al Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Reports. 2012;1(5):506–515. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Cai YJ, Wang Y, et al Cloning of macaque monkeys by somatic cell nuclear transfer. Cell. 2018;172(4):881–887.e7. doi: 10.1016/j.cell.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Ma H, Van Dyken C, Darby H, et al Germline transmission of donor, maternal and paternal mtDNA in primates. Human Reproduction. 2021;36(2):493–505. doi: 10.1093/humrep/deaa308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JR, Lovell-Badge RH Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature. 1984;310(5972):66–67. doi: 10.1038/310066a0. [DOI] [PubMed] [Google Scholar]
- Mok BY, de Moraes MH, Zeng J, et al A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583(7817):631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kono T, Nakada K, et al Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(46):16765–16770. doi: 10.1073/pnas.0506197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, et al Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493(7434):627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, et al Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461(7262):367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Boel A, Castelluccio N, et al Human germline nuclear transfer to overcome mitochondrial disease and failed fertilization after ICSI. Journal of Assisted Reproduction and Genetics. 2022;39(3):609–618. doi: 10.1007/s10815-022-02401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Hayashi Y, Ogura A Participation of the female pronucleus derived from the second polar body in full embryonic development of mice. Journal of Reproduction and Fertility. 1997;110(2):263–266. doi: 10.1530/jrf.0.1100263. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R The first polar body can be used for the production of normal offspring in mice. Biology of Reproduction. 1998;59(1):100–104. doi: 10.1095/biolreprod59.1.100. [DOI] [PubMed] [Google Scholar]
- Wang MK, Chen DY, Liu JL, et al In vitro fertilisation of mouse oocytes reconstructed by transfer of metaphase II chromosomes results in live births. Zygote. 2001;9(1):9–14. doi: 10.1017/S0967199401001022. [DOI] [PubMed] [Google Scholar]
- Wang T, Sha HY, Ji DM, et al Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell. 2014;157(7):1591–1604. doi: 10.1016/j.cell.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Li YZ, Yang XF, et al Mitochondrial replacement in macaque monkey offspring by first polar body transfer. Cell Research. 2021;31(2):233–236. doi: 10.1038/s41422-020-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YH, Xu CL, Feng H, et al Human cleaving embryos enable efficient mitochondrial base-editing with DdCBE. Cell Discovery. 2022;8(1):7. doi: 10.1038/s41421-021-00372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Chen TL, Huang SX, et al Mitochondrial replacement by pre-pronuclear transfer in human embryos. Cell Research. 2017;27(6):834–837. doi: 10.1038/cr.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Luo SY, et al Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reproductive BioMedicine Online. 2017;34(4):361–368. doi: 10.1016/j.rbmo.2017.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.