Abstract
The NR4A nuclear receptor family (NR4As), encompassing NR4A1, NR4A2, and NR4A3, exerts pivotal roles in cellular processes through intricate expression patterns and interactions. Despite the influence of some NR4As on anterior pituitary functions regulated by the hypothalamus, their physiological expression patterns remain unclear. In our prior work, we demonstrated the specific upregulation of NR4A3 in the rat anterior pituitary gland during the proestrus afternoon, coinciding with a gonadotropin surge. In this study, we investigated changes in pituitary Nr4a gene expression throughout the estrous cycle in rats and a gonadotropin surge-induced model. Nr4a1 and Nr4a2 gene expression significantly increased during proestrus, aligning with previous observations for Nr4a3. Furthermore, prolactin gene expression increased sequentially with rising Nr4a gene expression, while thyroid-stimulating hormone beta gene expression remained stable. Immunohistochemistry revealed a widespread and differential distribution of NR4A proteins in the anterior pituitary, with NR4A1 and NR4A3 being particularly abundant in thyrotrophs, and NR4A2 in gonadotrophs. In estrogen-treated ovariectomized rats, elevated luteinizing hormone secretion corresponded to markedly upregulated expression of Nr4a1, Nr4a2, and Nr4a3. In gonadotroph and somatomammotroph cell lines, gonadotropin- and thyrotropin-releasing hormones transiently and dose-dependently increased the expression of Nr4a genes. These findings suggest that hypothalamic hormone secretion during proestrus may induce the parallel expression of pituitary Nr4a genes, potentially influencing the pituitary gene expression program related to endocrine functions before and after ovulation.
Keywords: Anterior pituitary gland, Estrous cycle, NR4A1, NR4A2, NR4A3
The NR4A nuclear receptor family (NR4As), comprising NR4A1 (Nur77), NR4A2 (Nurr1), and NR4A3 (Nor1), consists of orphan receptors with unknown physiological ligands. Nr4as, as immediate early response genes, respond to various stimuli and exhibit both overlapping and distinct functions [1, 2]. They are frequently co-regulated in parallel across different cell types, including hepatocytes, skeletal muscle cells, thymocytes, macrophages, ovarian granulosa cells, and pituitary corticotroph cells [3,4,5,6,7,8].
NR4As play crucial roles in diverse cellular processes [9]. Their activity primarily relies on changes in gene expression, post-translational modifications, and interactions with co-regulatory proteins, rather than ligand binding. NR4A1, for instance, is implicated in endocrine signaling, as it can interact with activated estrogen receptor alpha, androgen receptor, and glucocorticoid receptor, inhibiting their DNA binding and activity [10,11,12,13]. This interaction suggests that NR4As are involved in endocrine feedback regulation at the hypophyseal and pituitary levels.
Comprising three domains, NR4As exhibit an exceptionally high degree of sequence homology within their DNA binding domain and approximately 60% homology within their ligand-binding domain [9]. NR4As function as monomers when binding to the NGFI-B response element sequence [14]. They also bind to palindromic Nur77 response element (NurRE) sequences as homodimers or heterodimers of NR4As [9]. The first functional NurRE was identified in the promoter of the pro-opiomelanocortin (Pomc) gene in pituitary-derived AtT-20 cells [15]. Notably, the heterodimer activated Pomc transcription more potently than the NR4A1 homodimer, indicating an interdependence among NR4As in activating their target genes [16]. Furthermore, NR4As can potentially regulate transcriptional activity by interacting with other transcription factors, either by directly enhancing or inhibiting the activities of these transcription factors [9]. Therefore, exploring the intricate relationship between NR4As and their interactions with other factors is crucial to elucidate their multifaceted roles.
The hypothalamus-hypophyseal system critically regulates endocrine-controlled biological functions, such as reproduction, metabolism, growth, and stress responses, by integrating internal and external signals. Hypothalamic hormones, such as corticotropin-releasing hormone and thyrotropin-releasing hormone (TRH), influence the expression of NR4As in anterior pituitary endocrine cells [17,18,19]. Pituitary NR4As may also participate in regulating Pomc gene expression and providing feedback control for the hypothalamic-pituitary-adrenal axis during stress responses [13, 16,17,18, 20]. However, limited data are available on changes in pituitary NR4A expression under different physiological conditions. In a recent study, we observed a significant transient upregulation of Nr4a3 expression in the rat anterior pituitary during the afternoon of proestrus [21]. We further demonstrated that this increase in Nr4a3 expression was induced by gonadotropin-releasing hormone (GnRH) and TRH [21, 22]. During this period, elevated levels of 17β-estradiol (E2) secreted from developing follicles triggered positive feedback effects on the hypothalamus, activating hypothalamic hormone secretion and resulting in gonadotropin surges and the activated secretion of pituitary hormones [23, 24]. These observations strongly suggest that the secretion of hypothalamic hormones activated by E2 induces pituitary NR4As, including NR4A3.
In the present study, we investigated the kinetics of Nr4a gene expression during the estrous cycle and the inducibility of Nr4a genes in an E2-induced gonadotropin surge model and cell lines stimulated with GnRH and TRH. We compared the expression patterns of Nr4a genes and pituitary hormone genes to examine the involvement of NR4As in pituitary hormone synthesis.
Materials and Methods
Animals
Female Wistar–Imamichi rats (200–300 g) were obtained from SLC Japan (Shizuoka, Japan) and were housed under controlled conditions (temperature: 23 ± 3°C; light: 14 h; dark: 10 h; lights on at 0500 h). Rats had ad libitum access to laboratory chow and tap water. All animal protocols were approved by the Animal Care and Use Committee of Kitasato University School of Veterinary Medicine (approval no. 19-173).
Determination of the estrous cycle
The estrous cycle stages (proestrus, estrus, and diestrus) were determined based on specific vaginal cytological characteristics. Vaginal smears were monitored daily for at least 2 weeks before the experiments. Female rats with regular 4-day estrous cycles were selected. Animals were euthanized by decapitation, and anterior pituitary tissues were promptly collected and frozen in liquid nitrogen. For immunohistochemistry, rats under isoflurane anesthesia were perfused with 4% paraformaldehyde in 50 mM phosphate buffer using a peristaltic pump, and pituitary tissues were collected and fixed overnight at 4°C in 4% paraformaldehyde in 50 mM phosphate buffer.
Estrogen-primed gonadotropin surge-induced rat model
Ovariectomized rats were continuously treated with E2 (Sigma–Aldrich, St. Louis, MO, USA) to induce an evening gonadotropin surge. Bilateral ovariectomy was performed under isoflurane anesthesia. One week post-surgery, ovariectomized rats were subcutaneously implanted with a 3 cm long Silastic capsule (100-2N; Kaneka Medix Co., Osaka, Japan) containing either 0.1 μg/100 μl or 10 μg/100 μl E2 in sesame oil. Simultaneously, ongoing cytological evaluation of vaginal smears monitored estrus status. Three days post-implantation, anterior pituitary and trunk blood samples were collected at 1100 h, 1400 h, and 1700 h.
Cell culture
The LβT2 mouse gonadotroph cell line [25] was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco Fetal Bovine Serum, Thermo Fisher Scientific) at 37°C in a 5% CO2 environment. The GH3 rat somatomammotroph cell line [26] was cultured in DMEM/F12 (Thermo Fisher Scientific) with 15% normal horse serum (Gibco Horse Serum, Thermo Fisher Scientific) and 2.5% fetal bovine serum under the same conditions. LβT2 and GH3 cells underwent treatment with GnRH (Peptide Institute Inc., Osaka, Japan) or TRH (Sigma–Aldrich) at a concentration of 100 nM for various durations (0, 0.5, 1, 2, 4, and 8 h) or various concentrations (0, 0.1, 1, 10, and 100 nM) for 1 h. Subsequently, post-culture, the cells were harvested using ISOGEN (Nippon Gene, Tokyo, Japan) and stored at –80°C.
Reverse transcription-quantitative PCR
Total RNA was extracted from pituitary tissues and cells using ISOGEN and reverse transcribed to cDNA using a High-Capacity cDNA Synthesis Kit (Thermo Fisher Scientific). Primer sequences for all reverse transcription-quantitative PCR (RT-qPCR) assays are listed in Table 1. qPCR was performed using THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan) on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s protocol. The cycle-threshold (Ct) values were set at 0.5 delta Rn value, and relative gene expression levels were calculated using the ΔΔCT method with ribosomal protein L19 (Rpl19) as the internal control for normalization.
Table 1. Sequences of the primers used in this study.
| Species | Gene | Forward primer (5'–3') | Reverse primer (5'–3') |
|---|---|---|---|
| Rat | Nr4a1 | ATTTGGACTCCGGGCCTAAC | GGAGCCAGAGAGCAAGTCAT |
| Rat | Nr4a2 | TGTCAGCATTACGGTGTTCG | ACCGACAGTACTGACAACGAT |
| Rat | Nr4a3 | AAAGACGGAACCTCCACAGAA | GTCGGGATAGGCGAAGCA |
| Rat | Prl | AAGGTGTCGAAGGTTTATAAAGTCA | ATCATCAGCAGGAGGAGTGT |
| Rat | Tshb | CAGCATTAACTCGCCAGTGC | GATGACACTTGCCCACAAGC |
| Rat | Rpl19 | GGAAGCCTGTGACTGTCCAT | ATCCTTCGCATCCAGGTCAC |
| Mouse | Nr4a1 | GTGGTGACAATGCTTCGTGT | GCAGATGTACTTGGCGCTTT |
| Mouse | Nr4a2 | TGTCGGTTTCAGAAGTGCCT | CTTCGGCTTCGAGGGTAAAC |
| Mouse | Nr4a3 | CCGAGCTTTAACAGATGCAA | AGCTTCTGGACACGTCAATG |
| Mouse | Lhb | GTCTGCATCACCTTCACCAC | GTAGGTGCACACTGGCTGAG |
| Mouse | Rpl19 | AGCCTGTGACTGTCCATTCC | GCATTGGCAGTACCCTTCCT |
Determination of luteinizing hormone levels
Plasma luteinizing hormone (LH) concentrations were measured using a time-resolved fluorometric immunoassay (DELFIA System; PerkinElmer, Branchburg, NJ, USA). Purified rat LH (LH-I-9, National Institute of Diabetes and Digestive and Kidney Diseases, NIDDK, Torrance, CA, USA) was labeled with europium (Eu) using a DELFIA Eu-Labeling Kit (PerkinElmer, Waltham. MA, USA). Anti-rabbit gamma globulin goat serum was prepared in our laboratory and purified via ammonium sulfate precipitation.
A 96-well immunoplate (Nunc, Tokyo, Japan) was coated with anti-rabbit gamma globulin and overlaid with a 1:4,000 dilution of NIDDK anti-rat LH-S-11 (NIDDK). Diluted plasma samples and LH standards (rat LH-RP-3, NIDDK) were incubated at 4°C overnight, and optimally diluted Eu-labeled hormones were added for 2 h at 20–25°C. After washing, the plates were treated with DELFIA Enhancement Solution (PerkinElmer), and specific fluorescence (excitation and emission wavelengths of 340 and 613 nm, respectively) was measured using a Multilabel Reader 2030 ARVO X4 (PerkinElmer). The intra- and inter-assay coefficients of variation for the LH levels were 6.5% and 9.9%, respectively.
Immunohistochemistry
Each NR4A protein was detected using double-fluorescence immunohistochemistry with a monoclonal antibody and the tyramide signal amplification technique. The anti-NR4A2 mouse monoclonal antibody (#66878-1-Ig; Proteintech Japan, Tokyo, Japan) and anti-NR4A3 mouse monoclonal antibody (#PP-H7833-00; Perseus Proteomics, Tokyo, Japan) were biotinylated using biotin-LC-LC-NHS (Tokyo Chemical Industry, Tokyo, Japan) and purified in 10 kDa MWCO Amicon Ultra-0.5 Centrifugal Filter Units (Merck Millipore, Dublin, Ireland).
The fixed pituitary tissues underwent dehydration using a graded ethanol series, were cleared in xylene, embedded in paraffin, and sectioned at 4 μm with a microtome. Sections were dewaxed in xylene and rehydrated using a graded ethanol series. Heat-induced antigen retrieval was performed using a KOS Histostation Microwave Multifunctional Tissue Processor (Milestone Medical, Sorisole, Italy) with a 10 mM citrate buffer solution (pH 6.0) at 98°C for 25 min.
Endogenous peroxidase was quenched for 30 min with 1% H2O2 in methanol, followed by blocking of endogenous avidin and biotin using an Avidin/Biotin Blocking Kit (Abcam, Tokyo, Japan), as per the manufacturer’s instructions. Sections were incubated with 5% normal goat serum in phosphate-buffered saline (PBS) with 0.1% Triton X-100 for 30 min and then with biotinylated NR4A2 antibody (0.1 ng/ml), biotinylated NR4A3 antibody (1 ng/ml), or anti-NR4A1 rabbit monoclonal antibody (1:1,000; #MA5-32647; Thermo Fisher Scientific) overnight at 4°C.
Sections were incubated with HRP-Conjugated Streptavidin (1:4,000; Thermo Fisher Scientific) or goat anti-rabbit IgG HRP conjugate (0.02 μg/ml; #G-21234; Thermo Fisher Scientific) in PBS with 0.5% bovine serum albumin (Sigma–Aldrich) and 0.1% Tween 20 for 30 min. They were then incubated with 50 μM Biotinyl-tyramide (Sigma–Aldrich) in PBS with 0.0015% H2O2 and 0.1% Tween 20 for 15 min. The sections were labeled with fluorescent-labeled streptavidin (1:1,000; Streptavidin, Alexa Fluor 488 Conjugate, ThermoFisher Scientific) for 30 min.
All antibodies (guinea pig antiserum) against the anterior pituitary hormones were purchased from NIDDK. Sections were incubated with primary antibody (LH beta, AFP344191, 1:2,000,000; prolactin (PRL), AFP450191, 1:1,000; thyroid-stimulating hormone (TSH) beta, AFP967793, 1:100,000; growth hormone (GH), AFP12121390, 1:4,000; adrenocorticotropic hormone (ACTH), AFP71111591, 1:1,000) for 60 min and secondary antibody (1:1,000; goat anti-guinea pig IgG secondary antibody, Alexa Fluor 568, #A110175; Thermo Fisher Scientific) for 60 min. Finally, slides were mounted with Vectashield H1200 mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA), and images were captured using a confocal laser scanning microscope LSM710 (Carl Zeiss, Oberkochen, Germany) with a 63× lens.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Differences were analyzed using a one-way analysis of variance, followed by the Tukey–Kramer test. All statistical analyses were performed using JMP Pro 17.0.0 (SAS Institute, Cary, NC, USA).
Results
Proestrus-specific expression of Nr4a genes in the anterior pituitary gland of rats
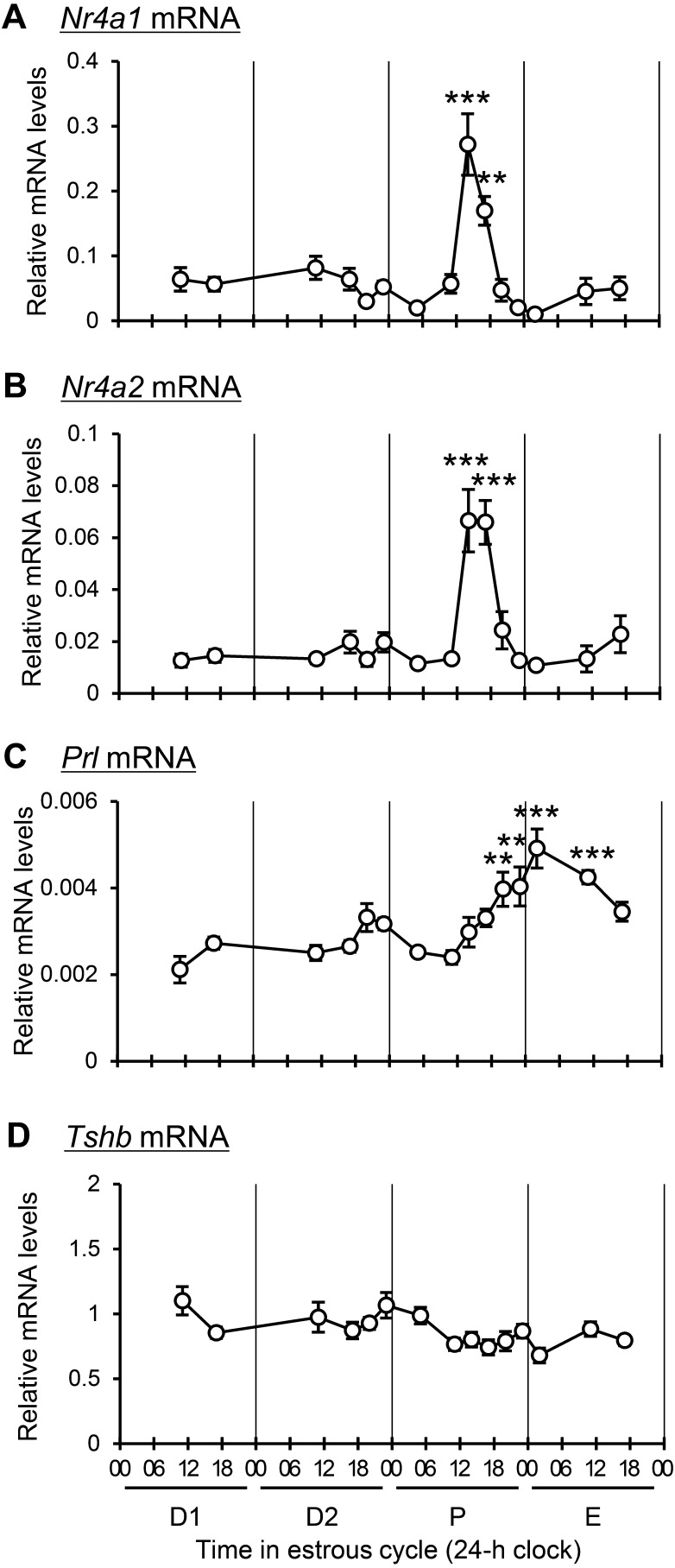
In alignment with our previous findings indicating specific upregulation of pituitary Nr4a3 mRNA between 1400 and 1700 h during proestrus [21], the current study explored the expression kinetics of Nr4a1 (Fig. 1A) and Nr4a2 (Fig. 1B) throughout the rat pituitary gland across the estrous cycle. Both genes exhibited a significant increase in mRNA expression between 1400 and 1700 h during proestrus. This pattern was contrasted with the expression of the prolactin (Prl) gene in lactotrophs and the thyroid-stimulating hormone beta (Tshb) gene in thyrotrophs (both stimulated by TRH). Prl mRNA expression gradually increased from 1400 h in proestrus, with a significant rise observed from 2000 h in proestrus to 1100 h in estrus (Fig. 1C). Conversely, no significant change was noted in Tshb mRNA expression throughout the cell cycle (Fig. 1D).
Fig. 1.
Changes in the expression levels of Nr4a1, Nr4a2, Prl, and Tshb mRNA during the estrous cycle. Relative mRNA expression levels of Nr4a1 (A), Nr4a2 (B), Prl (C), and Tshb (D) in the anterior pituitary gland were measured during diestrus 1 (D1; 1100 and 1700 h), diestrus 2 (D2; 1100, 1700, 2000, and 2300 h), proestrus (P; 0500, 1100, 1400, 1700, 2000, and 2300 h), and estrus (E; 0200, 1100, and 1700 h) by RT-qPCR. Data are presented as mean ± SEM (n = 5). Statistical analyses were performed using the Tukey–Kramer test; ** P < 0.01, *** P < 0.001 versus proestrus at 1100 h.
Differential distribution of NR4As in pituitary endocrine cells of rats
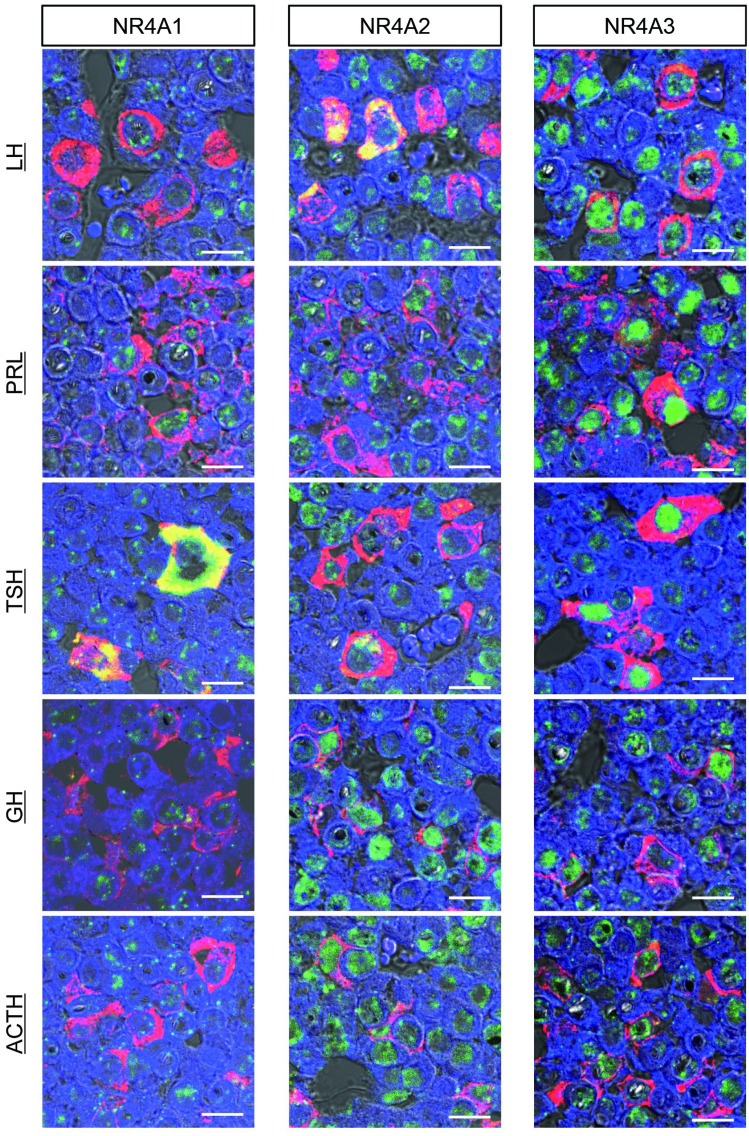
To characterize NR4A-expressing cells with high expression levels in the pituitary, double-fluorescence immunostaining for NR4As and anterior pituitary hormones was conducted on the rat anterior pituitary obtained at 1400 h in proestrus rats (Fig. 2). NR4A1 exhibited abundance in the cytoplasm of TSH-producing cells, with additional expression detected in the nuclei of LH- and PRL-producing cells, and comparatively lower expression in GH- and ACTH-producing cells. NR4A2, in contrast, displayed widespread expression patterns across all cell types, with strong cytoplasmic distribution in LH-producing cells and some TSH-producing cells. In other cell types, NR4A2 primarily localized to the nucleus and showed high expression in GH- and ACTH-producing cells. NR4A3 predominantly localized in the nucleus, exhibiting high expression in TSH-producing cells. Additionally, some PRL-, LH-, and GH-producing cells displayed high expression levels of NR4A3.
Fig. 2.
Expression of NR4A proteins in the anterior pituitary gland of proestrus rats. Double-fluorescence immunohistochemistry on rat pituitary gland biopsies obtained at 1400 h during proestrus. NR4As (NR4A1, NR4A2, and NR4A3; green) and pituitary hormones (LH, PRL, TSH, GH, and ACTH; red) were observed by confocal laser scanning microscopy. Colocalized images are shown in yellow, and DAPI (blue) was used as a nuclear counterstain. Scale bars indicate 10 μm.
Time-dependent induction of Nr4a genes in an estrogen-primed, gonadotropin surge-induced rat model
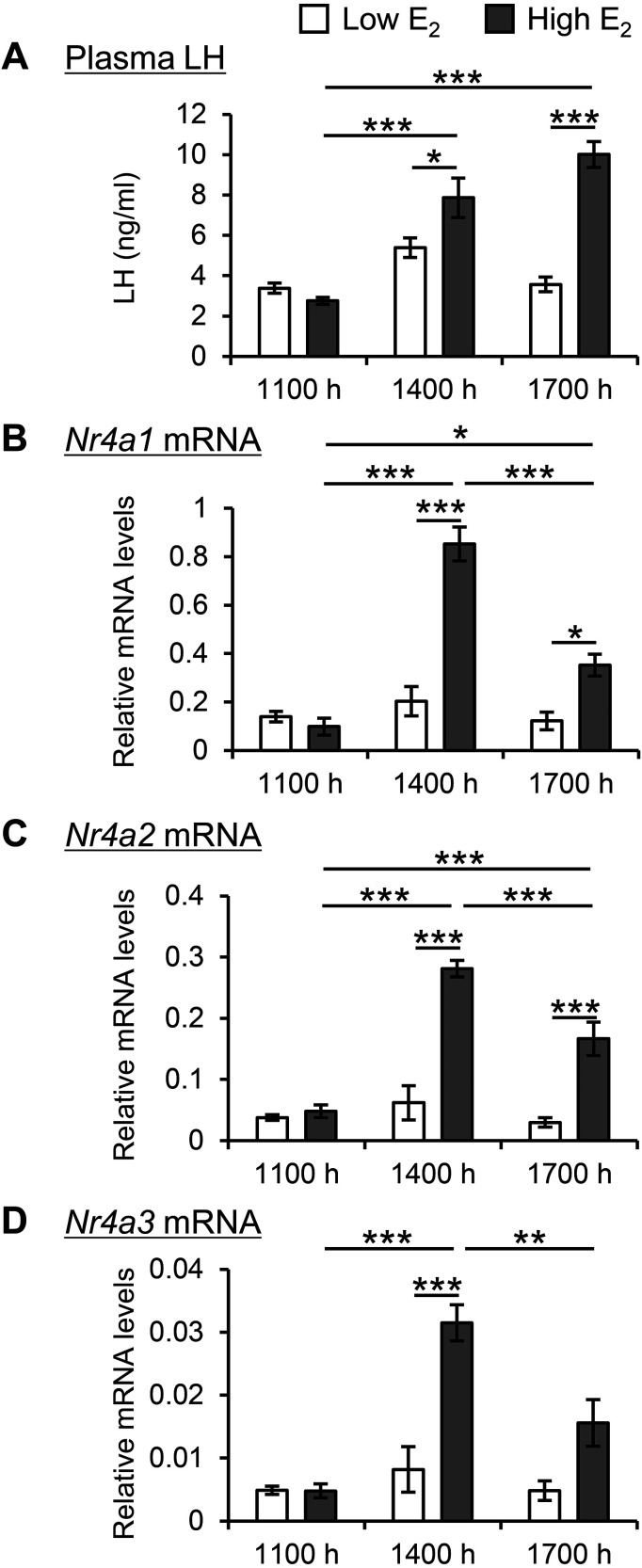
In investigating the potential correlation between pituitary Nr4a expression and hypothalamic activation, ovariectomized rats were continuously exposed to E2. Rats exposed to high E2 concentrations displayed surge-like increases in LH secretion at both 1400 and 1700 h (Fig. 3A). Low E2 concentrations failed to elicit this response. Similarly, the mRNA expression of Nr4a1 (Fig. 3B), Nr4a2 (Fig. 3C), and Nr4a3 (Fig. 3D) in the pituitary glands of rats exposed to high E2 concentrations increased at 1400 h and subsequently declined at 1700 h.
Fig. 3.
Changes in Nr4a expression in estrogen-primed rats. Ovariectomized rats were implanted with silastic capsules containing either 0.1 μg/100 μl (Low) or 10 μg/100 μl (High) E2 for 3 days. Anterior pituitary tissue and blood samples were collected at 1100, 1400, and 1700 h. Plasma LH concentration (A) and Nr4a1 (B), Nr4a2 (C), and Nr4a3 (D) mRNA expression were measured. Relative mRNA levels were compared to those in the Low E2 group at 1100 h. Data are presented as mean ± SEM (n = 5). Statistical analyses were performed using the Tukey–Kramer test; * P < 0.05, ** P < 0.01, and *** P < 0.001.
Induction of Nr4a genes by GnRH and TRH in LβT2 and GH3 cells
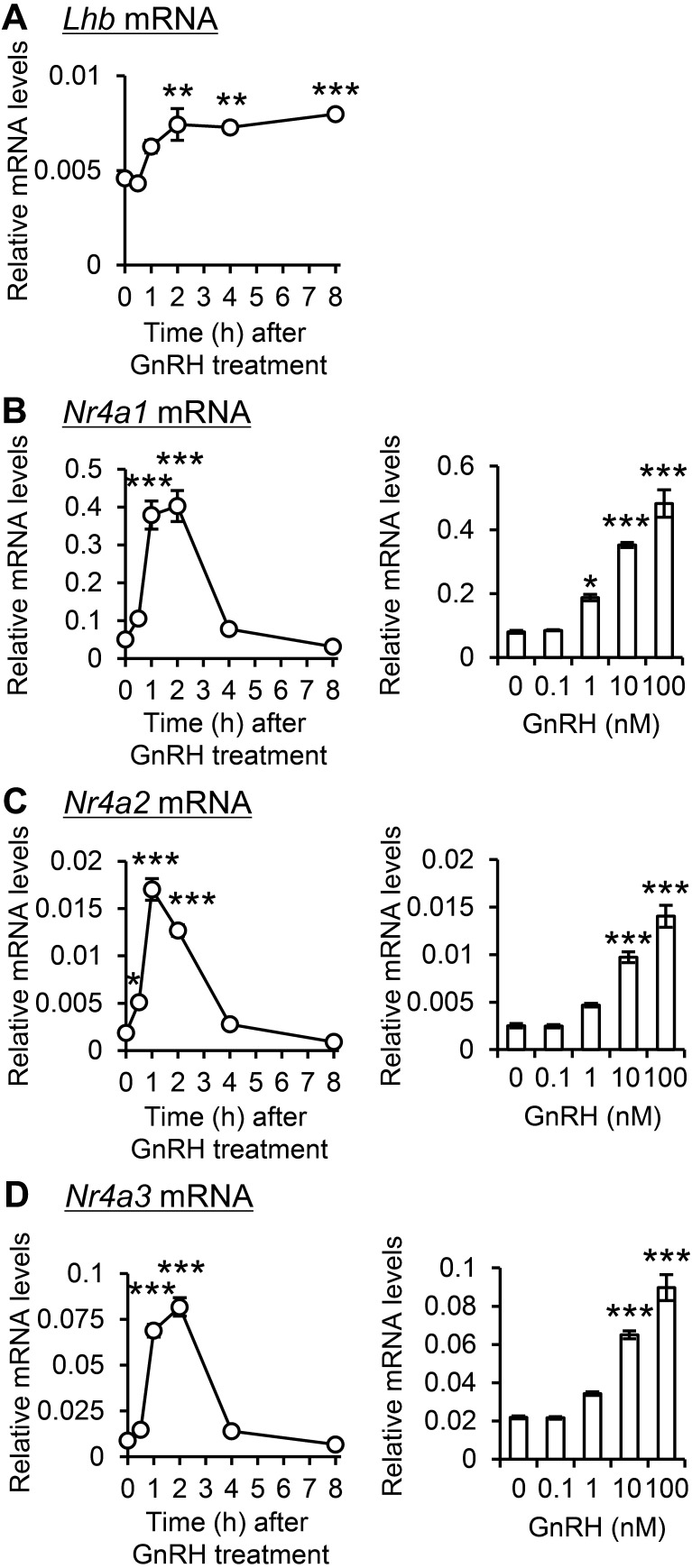
To validate the impact of GnRH on Nr4a gene expression, LβT2 gonadotroph cells were exposed to GnRH, resulting in a significant increase in luteinizing hormone beta (Lhb) mRNA expression (Fig. 4A). Simultaneously, transient peaks in Nr4a1 (Fig. 4B), Nr4a2 (Fig. 4C), and Nr4a3 (Fig. 4D) mRNA expression were observed within 1–2 h. Gene expression also exhibited a dose-dependent increase 1 h after GnRH administration, especially at concentrations of 10 nM or higher.
Fig. 4.
Effect of GnRH on Nr4a genes expression in LβT2 gonadotroph cells. LβT2 cells were incubated with 100 nM GnRH for 0 to 8 h or 0 to 100 nM GnRH for 1 h. Lhb (A), Nr4a1 (B), Nr4a2 (C), and Nr4a3 (D) mRNA expression was measured. Data are presented as mean ± SEM (n = 4). Statistical analysis was performed using the Tukey–Kramer test; * P < 0.05, *** P < 0.001 versus 0 h or 0 nM.
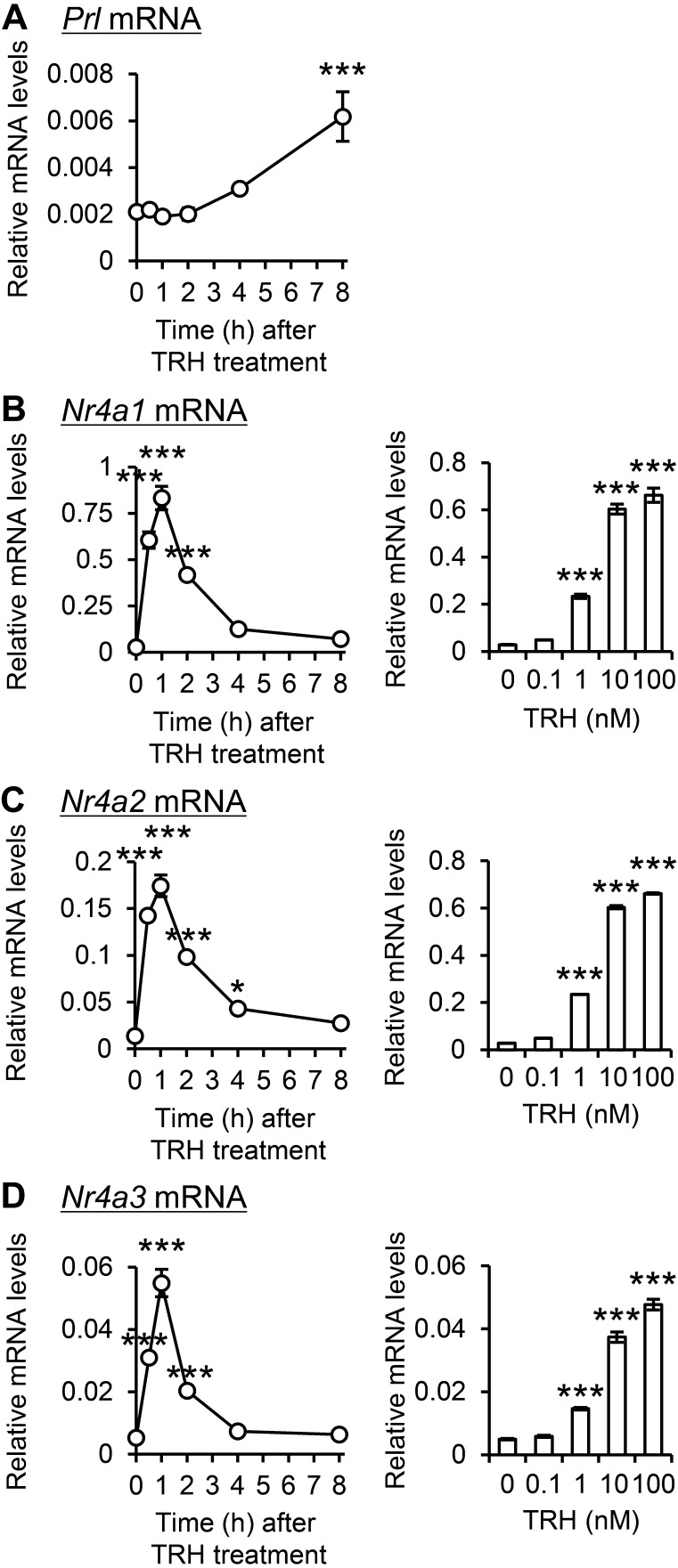
Given the impact of hypothalamic TRH on Nr4a3 expression during proestrus afternoons, the effect of TRH on Nr4a genes was examined in TRH-sensitive GH3 somatomammotrophic cells. TRH treatment significantly increased Prl mRNA expression at 8 h (Fig. 5A). TRH transiently induced Nr4a1 (Fig. 5B), Nr4a2 (Fig. 5C), and Nr4a3 (Fig. 5D) mRNA expression, peaking after 1 h. Furthermore, the expression of these genes 1 h after treatment exhibited a dose-dependent augmentation at TRH concentrations exceeding 1 nM.
Fig. 5.
Effect of TRH on expression of Nr4a genes in TRH-sensitive GH3 somatomammotroph cells. GH3 cells were incubated with 100 nM TRH for 0–8 h or with 0–100 nM TRH for 1 h. Prl (A), Nr4a1 (B), Nr4a2 (C), and Nr4a3 (D) mRNA expression were measured. Relative mRNA levels were compared with those in the 0 h and 0 nM groups. Data are presented as mean ± SEM (n = 4). Statistical analysis was performed using the Tukey–Kramer test; * P < 0.05, *** P < 0.001 versus 0 h or 0 nM.
Discussion
Considering the suggested cooperative or antagonistic interactions between NR4A proteins, this study compared their expression patterns. Previous research confirmed specific upregulation of pituitary Nr4a3 expression in response to GnRH and TRH during proestrus. The current study extended this assessment to Nr4a1 and Nr4a2, revealing synchronized expression patterns linked to preovulatory hypothalamic activation in proestrus rats. This indicates potential interactions among NR4As in the pituitary gland. Insights from a previous study on double-knockout mice lacking both NR4A1 and NR4A3 emphasized compensatory functions within the NR4A family [27]. These mice displayed heightened acute leukemia severity and reduced lifespan compared to single-gene knockout mice, highlighting the importance of maintaining functional balance within the NR4A network. The existence of mechanisms ensuring the multifaceted functions of NR4As underscores the significance of synchronized pituitary Nr4a regulation.
Our results underscore the pivotal role of high-level estrogen-dependent activation of the hypothalamus in regulating pituitary Nr4a genes during proestrus. Subsequent experiments with cell lines demonstrated that physiological levels of GnRH and TRH induce synchronized Nr4a expression. Established evidence indicates time-dependent and extensive GnRH release in E2-primed ovariectomized rats [28]. While increased TRH secretion is noted during proestrus [29], its association with E2 feedback remains unconfirmed. The heightened activities of GnRH and TRH during proestrus enhance Nr4a3 expression [21, 22], strongly suggesting that the positive feedback effect of E2 stimulates the secretion of both GnRH and TRH, subsequently inducing Nr4a gene expression.
Our investigation unveiled differentially expressing cells and subcellular distribution patterns of NR4A proteins in the anterior pituitary. This suggests divergent roles and mechanisms of action of NR4As in regulating pituitary endocrine secretion. While tentative comparisons indicate that Nr4a1 gene expression may be the highest and Nr4a3 gene expression may be the lowest in the entire anterior pituitary, caution is warranted due to the use of different primer sets. Additionally, variations in gene expression among cell lines were observed, supporting the idea that different cell types have distinct functional NR4A profiles. However, it is crucial to exercise caution as the present data lack a substantial comparison of expression levels, and the cell lines may not fully replicate physiological cellular differences. Considering NR4A3’s impact on Fshb expression in gonadotroph cell lines [30] and the known interactions and compensatory mechanisms among NR4As [27], dismissing their involvement in functional regulation based solely on lower expression levels in specific cell types would be premature. A more comprehensive assessment is imperative to determine the relative contribution of each NR4A to pituitary functionality.
Among anterior pituitary cells, the expression of NR4As, particularly NR4A1 and NR4A3, was prominent in thyrotrophs, indicating a potential inductive effect of TRH. Previous studies have shown reduced NR4A1 protein distribution in the thyrotrophs of TRH-deficient mice [19], supporting TRH-induced upregulation of NR4As in these cells. The sensitivity of lactotrophs to TRH [31] suggests its potential involvement in the induction of NR4As in this cell type. Additionally, the clear distribution of NR4A2, along with NR4A3, in gonadotrophs suggests the potential induction of its expression by GnRH.
The prevailing consensus suggests that NR4As are predominantly induced by shared signaling pathways, and our study revealed that both GnRH and TRH amplify Nr4a genes with comparable expression ratios despite different basal levels. This finding suggests the common involvement of intracellular signaling pathways in the induction of Nr4a genes by GnRH and TRH.
Notably, there have been multiple reported inductive effects of GnRH and TRH on Nr4a1, accompanied by conflicting evidence. Experiments using the PRL-producing cell line GH4C1 demonstrated that TRH induces Nr4a1 expression not through calcium (Ca2+) or protein kinase A (PKA) but via protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) signaling pathways [19]. Conversely, earlier research on LβT2 cells revealed that GnRH induces Nr4a1 through the PKA and Ca2+ signaling pathways, excluding the involvement of PKC or MAPK [32]. However, conflicting evidence from other groups, utilizing a gonadotroph-derived cell line (αT3-1) and primary pituitary cultured cells from knockout mice for signaling factors, implicated PKC and MAPK in Nr4a1 induction [33]. These observations suggest that, at least under physiological conditions, the PKC and MAPK signaling pathways are likely primary regulators. Although information on Nr4a2 and Nr4a3 remains limited, it is plausible that the PKC and MAPK signaling pathways are the principal regulators of these members of the NR4A family.
Significant changes in pituitary endocrine secretion, such as notable gonadotropin and PRL surges, have been observed during proestrus in rats [34]. Our previous research demonstrated that NR4A3 expression in gonadotrophs suppresses Fshb expression, suggesting a regulatory role for upregulated NR4As, particularly in Fshb gene expression [30]. Fshb gene expression significantly increases following a gonadotropin surge, leading to a secondary FSH surge [35]. Although the precise significance of this secondary surge remains unclear, it may affect follicular development during the subsequent estrous cycle [36]. Additionally, in this study, we observed a concurrent increase in Prl expression following the upregulation of Nr4a genes, suggesting the possible involvement of NR4As in Prl expression during this period. Increased Prl expression persisted for half a day to one day, consistent with previous observations [37]. Prl expression is believed to ensure adequate PRL secretion during early pregnancy and the post-ovulation estrous cycle. Therefore, the induction of pituitary NR4As during proestrus may play a role in establishing pregnancy and/or influence the subsequent normal estrous cycle by modulating several pituitary hormone genes. Furthermore, although its specific involvement in Tshb expression remains unknown, it is noteworthy that the significant expression of NR4As in thyrotrophs may also contribute to TSH secretory function.
In summary, this is the first demonstration of the synchronized upregulation of Nr4a1, Nr4a2, and Nr4a3 in the anterior pituitary gland during the afternoon of proestrus. This coordinated response is orchestrated by the estrogen-dependent activation of the hypothalamic system, with strong implications for GnRH and TRH in this regulatory process. The increased expression of these NR4As in the anterior pituitary gland during proestrus may affect the sexual cycle and pregnancy by modulating the secretory capacity of pituitary hormones, including FSH, PRL, and TSH. Further investigations into the diverse actions and intricate interaction mechanisms of NR4As will provide potential insights into fertility and reproductive health.
Conflicts of interests
None of the authors have any potential conflicts of interest associated with this research.
Acknowledgments
This work was supported by the Kitasato University Research Grant for Young Researchers and JSPS KAKENHI Grant Number JP23K05595.
References
- 1.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 2006; 4: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 2010; 24: 1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 2006; 12: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 4.Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology 2008; 149: 2853–2865. [DOI] [PubMed] [Google Scholar]
- 5.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell 2002; 109(Suppl): S57–S66. [DOI] [PubMed] [Google Scholar]
- 6.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem 2005; 280: 29256–29262. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Ghosh S, Nishi Y, Yanase T, Nawata H, Hu Y. The orphan nuclear receptors NURR1 and NGFI-B modulate aromatase gene expression in ovarian granulosa cells: a possible mechanism for repression of aromatase expression upon luteinizing hormone surge. Endocrinology 2005; 146: 237–246. [DOI] [PubMed] [Google Scholar]
- 8.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol 2002; 16: 1638–1651. [DOI] [PubMed] [Google Scholar]
- 9.Kurakula K, Koenis DS, van Tiel CM, de Vries CJ. NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys Acta 2014; 1843: 2543–2555. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Park E, Kim SC, Ahn RS, Ko C, Lee K. ERα/E2 signaling suppresses the expression of steroidogenic enzyme genes via cross-talk with orphan nuclear receptor Nur77 in the testes. Mol Cell Endocrinol 2012; 362: 91–103. [DOI] [PubMed] [Google Scholar]
- 11.Song CH, Gong EY, Park J, Lee K. Testicular steroidogenesis is locally regulated by androgen via suppression of Nur77. Biochem Biophys Res Commun 2012; 422: 327–332. [DOI] [PubMed] [Google Scholar]
- 12.Philips A, Maira M, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol 1997; 17: 5952–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin LJ, Tremblay JJ. The nuclear receptors NUR77 and SF1 play additive roles with c-JUN through distinct elements on the mouse Star promoter. J Mol Endocrinol 2009; 42: 119–129. [DOI] [PubMed] [Google Scholar]
- 14.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 1991; 252: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 15.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 1997; 17: 5946–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol 1999; 19: 7549–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroth N, Liu Y, Aguilera G, Eiden LE. Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol 2011; 23: 944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helbling JC, Minni AM, Pallet V, Moisan MP. Stress and glucocorticoid regulation of NR4A genes in mice. J Neurosci Res 2014; 92: 825–834. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima Y, Yamada M, Taguchi R, Shibusawa N, Ozawa A, Tomaru T, Hashimoto K, Saito T, Tsuchiya T, Okada S, Satoh T, Mori M. NR4A1 (Nur77) mediates thyrotropin-releasing hormone-induced stimulation of transcription of the thyrotropin β gene: analysis of TRH knockout mice. PLoS One 2012; 7: e40437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol 1997; 11: 39–47. [DOI] [PubMed] [Google Scholar]
- 21.Terashima R, Laoharatchatathanin T, Kurusu S, Kawaminami M. Sequential preovulatory expression of a gonadotropin-releasing hormone-inducible gene, Nr4a3, and its suppressor Anxa5 in the pituitary gland of female rats. J Reprod Dev 2021; 67: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terashima R, Tani T, Sakakibara K, Kurusu S, Kawaminami M. Thyrotropin-releasing hormone stimulates NR4A3 expression in the pituitary thyrotrophs of proestrus rats. Endocr J 2023; 70: 805–814. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology 1969; 85: 103–112. [DOI] [PubMed] [Google Scholar]
- 24.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 25.Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 1996; 122: 3319–3329. [DOI] [PubMed] [Google Scholar]
- 26.Tashjian AH, Jr, Yasumura Y, Levine L, Sato GH, Parker ML. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology 1968; 82: 342–352. [DOI] [PubMed] [Google Scholar]
- 27.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med 2007; 13: 730–735. [DOI] [PubMed] [Google Scholar]
- 28.Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci 2022; 16: 953252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fink G, Koch Y, Ben Aroya N. Release of thyrotropin releasing hormone into hypophysial portal blood is high relative to other neuropeptides and may be related to prolactin secretion. Brain Res 1982; 243: 186–189. [DOI] [PubMed] [Google Scholar]
- 30.Terashima R, Saigo T, Laoharatchatathanin T, Kurusu S, Brachvogel B, Pöschl E, Kawaminami M. Augmentation of Nr4a3 and suppression of Fshb expression in the pituitary gland of female Annexin A5 null mouse. J Endocr Soc 2020; 4: bvaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanasaki H, Oride A, Mijiddorj T, Kyo S. Role of thyrotropin-releasing hormone in prolactin-producing cell models. Neuropeptides 2015; 54: 73–77. [DOI] [PubMed] [Google Scholar]
- 32.Hamid T, Malik MT, Millar RP, Kakar SS. Protein kinase A serves as a primary pathway in activation of Nur77 expression by gonadotropin-releasing hormone in the LbetaT2 mouse pituitary gonadotroph tumor cell line. Int J Oncol 2008; 33: 1055–1064. [PubMed] [Google Scholar]
- 33.Bliss SP, Navratil AM, Xie J, Miller A, Baccarini M, Roberson MS. ERK signaling, but not c-Raf, is required for gonadotropin-releasing hormone (GnRH)-induced regulation of Nur77 in pituitary gonadotropes. Endocrinology 2012; 153: 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975; 96: 219–226. [DOI] [PubMed] [Google Scholar]
- 35.Haisenleder DJ, Ortolano GA, Jolly D, Dalkin AC, Landefeld TD, Vale WW, Marshall JC. Inhibin secretion during the rat estrous cycle: relationships to FSH secretion and FSH beta subunit mRNA concentrations. Life Sci 1990; 47: 1769–1773. [DOI] [PubMed] [Google Scholar]
- 36.Hoak DC, Schwartz NB. Blockade of recruitment of ovarian follicles by suppression of the secondary surge of follicle-stimulating hormone with porcine follicular field. Proc Natl Acad Sci USA 1980; 77: 4953–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haisenleder DJ, Ortolano GA, Landefeld TD, Zmeili SM, Marshall JC. Prolactin messenger ribonucleic acid concentrations in 4-day cycling rats and during the prolactin surge. Endocrinology 1989; 124: 2023–2028. [DOI] [PubMed] [Google Scholar]