Abstract
Despite the great body of research done on Alzheimer's disease, the underlying mechanisms have not been vividly investigated. To date, the accumulation of amyloid‐beta plaques and tau tangles constitutes the hallmark of the disease; however, dysregulation of the mammalian target of rapamycin (mTOR) seems to be significantly involved in the pathogenesis of the disease as well. mTOR, as a serine–threonine protein kinase, was previously known for controlling many cellular functions such as cell size, autophagy, and metabolism. In this regard, mammalian target of rapamycin complex 1 (mTORC1) may leave anti‐aging impacts by robustly inhibiting autophagy, a mechanism that inhibits the accumulation of damaged protein aggregate and dysfunctional organelles. Formation and aggregation of neurofibrillary tangles and amyloid‐beta plaques seem to be significantly regulated by mTOR signaling. Understanding the underlying mechanisms and connection between mTOR signaling and AD may suggest conducting clinical trials assessing the efficacy of rapamycin, as an mTOR inhibitor drug, in managing AD or may help develop other medications. In this literature review, we aim to elaborate mTOR signaling network mainly in the brain, point to gaps of knowledge, and define how and in which ways mTOR signaling can be connected with AD pathogenesis and symptoms.
Keywords: Alzheimer's disease, mammalian target of rapamycin, pathogenesis, rapamycin
mTOR signaling and Alzheimer's disease (AD). Upregulation of mTOR activity appears to be involved in the main pathological cascade of AD, and mTOR inhibition or targeting its downstream substrates could supply attractive avenues for AD treatment.
1. INTRODUCTION
Dementia is a clinical syndrome characterized by a progressive decline in various cognitive domains which disrupt independent functions. 1 The World Alzheimer Report 2019 claims that approximately 50 million running cases of dementia exist in the world and will almost be threefold by 2050. 2 Alzheimer's disease (AD), the most common cause of dementia, is becoming a global age‐related health issue. 3 The World Health Organization predicts that by 2040, neurodegenerative diseases such as AD and other causes of dementia will outdo cancer in death rates and become the second leading cause of death in the world. 4 Regarding the dramatic growth of the older population, it is expected that 8%–10% of the older people in Iran will be affected by AD over the next 2–3 decades. 5 In addition to the increasing average age, as an important public health concern in many communities, high care costs for AD highlight the need to discover efficient treatment approaches for AD. To this end, unraveling the underlying molecular and cellular mechanisms involved in the pathogenesis and prognosis of AD appears essential. Despite the various valuable research conducted so far on investigating the pathogenesis of AD, there are still many gaps to be filled and questions to be answered.
More than 95% of cases of AD occur sporadically, without any familial history. It is important to mention that familial forms of AD result from specific gene mutations in amyloid precursor protein (APP), presenilin 1 (PS1), PS2, and apolipoprotein E (APOE). 6 AD is characterized by the presence of extracellular amyloid β (Aβ) plaques, the accumulation of hyper‐phosphorylated tau in the form of intracellular neurofibrillary tangles (NFTs), neural cell death, and loss of synaptic connections. 7 Furthermore, scientific literature suggests that dysregulated cell survival and death mechanisms may also contribute to the development of AD. For example, disturbances in mammalian/mechanistic target of rapamycin (mTOR) signaling pathways could initiate a series of events that lead to AD pathogenesis. 8
mTOR interacts with several molecules and is contained within at least two major specific complexes named the mTOR complex 1 (mTORC1) and the mTOR complex 2 (mTORC2). Today, the medical scientific community is experiencing an explosion in the information generated on the role of mTOR. Although a lot has been learned about the physiological involvement of mTORC1, we are still way behind and in the early stages of discovering the role and participation of mTORC2 in various diseases. It would be interesting to learn how a soil sample leading to the discovery of a drug named rapamycin led to the whole discovery of mTOR. In this regard, rapamycin was first discovered in 1964 in a soil sample from the island of Rapa Nui (Easter Island). Sehgal and his colleagues isolated a new macrolide with potent antifungal properties from a Streptomyces hygroscopicus soil bacterium, which they named “rapamycin.” 9 In 1984, Eng et al. 10 discovered the inhibitory effect of rapamycin on tumor growth at any stage. Years later, the immunosuppressant and chemotherapeutic properties of rapamycin became FDA‐approved. 11
To the best of available knowledge, rapamycin, as an mTOR inhibitor drug, inhibits mTORC1. Although mTORC2 is insensitive to acute rapamycin treatment, some evidence proposes that chronic treatment with rapamycin can inhibit mTORC2 as well as mTORC1 and its consequential stream. 12 Animal studies suggest that mTOR inhibitor drugs, such as rapamycin, may aid to lower the AD progression or ameliorate cognitive impairments of AD 12 , 13 ; however, based on the data retrieved from clinicaltrial.gov, there is no terminated clinical trial on elucidating the effects of rapamycin on delaying the progression of AD to date. A clinical trial entitled “Cognition, Age, and RaPamycin Effectiveness—Downregulation of the mTOR Pathway” NCT number: NCT04200911 is in the active stage. Additionally, a clinical trial entitled “Rapamycin—Effects on Alzheimer's and Cognitive Health” NCT number: NCT04200911 is in the recruiting stage. In all, the specific role of mTOR in AD onset and pathogenesis, and the role of rapamycin, in delaying the progression of AD remain elusive and much work remains to be done.
The present review focuses on mTOR signaling network, its role in normal aging, and underlying mechanisms involved in AD pathology. We also summarized preclinical studies addressing therapeutic potentials of mTOR network targeting in animal models of AD. Finally, we discussed the clinical prospects regarding the translation of mTOR targeting.
2. mTOR AND BRAIN PHYSIOLOGY
mTOR, a serine–threonine protein kinase, plays a crucial role in regulating various fundamental cellular functions in both neural and non‐neural cells. These functions encompass cell size, mitochondria, protein synthesis, autophagy, energy metabolism, lysosome biogenesis, and lipid metabolism. 14 The roles of mTOR are achieved by merging extracellular instructions with information on metabolic resources; therefore, mTOR signaling controls the rate of catabolism and anabolism. Generally, mTORC1 controls growth, autophagy, and protein synthesis, and mTORC2 is involved in apoptosis, 15 osmotic homeostasis maintenance, 16 somatic and cancer cells' survival, and cytoskeletal organization. 11 Furthermore, specific functions of mTOR in the brain include axonal regeneration and spouting, myelination, and channel expression. Moreover, nutrients and neurotransmitters that suppress autophagy and enhance protein synthesis and neurotrophic factors may activate mTOR signaling pathways. Since these processes have a role in neural growth by promoting neural elongation and branching, synaptic plasticity, and neural differentiation; therefore, dysregulated mTOR signaling may impair neural regeneration and development. It can be expected that dysfunctional mTOR pathway is associated with neurological disorders. In particular, inhibiting mTOR signaling may bring about neurodegeneration. On the other hand, mTOR signaling hyperactivation may damage the development of neurons and glia and lead to brain malformation. 17 In this context, inhibition of mTORC1 can be useful in treating some acute neurological conditions including epilepsy, brain tumors, and cognitive impairment. 14
Studies have demonstrated that increasing mTORC1 signaling by upregulating a constitutively active form of Akt or removing PTEN, a negative regulator of the Akt/mTOR pathway, can have neuroprotective effects. 18 , 19 These effects may be attributed to the reduction in endoplasmic reticulum (ER) stress, decreased production of reactive oxygen species (ROS), enhanced mitochondrial biogenesis, and elevated energy metabolism stimulated by mTORC1. 14 By promoting these beneficial processes, mTORC1 activation can potentially safeguard neurons and contribute to their overall well‐being. 14 In various models of central nervous system (CNS) injury, the PTEN/AKT/mTOR pathway tends to become inactive. However, functional recovery in injured neurons, such as through axonal sprouting and regeneration, can be achieved by upregulating mTOR signaling. 18 Furthermore, Akt/mTOR signaling has been associated with neurogenesis. Recent research demonstrated the expression of Akt3 protein in neuroblasts, mature newborn neurons, and progenitor cells. The study revealed that Akt3‐mTOR signaling was closely linked to neurite growth, proliferation of neural progenitor cells, and neurite outgrowth in the hippocampal dentate gyrus. 20 Moreover, mTORC1 activity is closely linked to CNS myelination, whereas mTORC2 plays a negligible role in this process. Maintaining balanced mTORC1 activity in oligodendrocytes is crucial for efficient CNS myelination. 21 Genetic studies have shown that Rheb1, through activating mTORC1, plays a vital role in myelination and the differentiation of oligodendrocyte precursor cells (OPCs). 22 However, hyper‐activated mTOR signaling may be detrimental in proteinopathies where an increased activity level of autophagy is needed to suppress the pathogenesis. 14 It is important to note that the functions of mTOR signaling in the brain extend beyond synaptic plasticity, circadian rhythm, memory, and cognition. 14 The mTOR complexes, their signal transduction pathways, and their various functions are summarized in the subsequent sections of this review.
2.1. mTOR complex
mTOR belongs to the phosphatidylinositol‐3 kinases (PI3K)‐related kinase (PIKK) family and is a dual‐specificity protein kinase that can phosphorylate serine, threonine, and tyrosine residues. mTOR constitutes several complexes including mTORC1 and mTORC2. Common components of mTORC1 and mTORC2 include mTOR itself, the target of rapamycin complex subunit LST8 (mLST8 also known as GβL), and DEP domain‐containing protein 6 (Deptor). Specific components of mTORC1 are listed as the regulatory protein associated with mTOR (Raptor) and the 40 kDa proline‐rich Akt substrate (PRAS40). Alternatively, the unique components within mTORC2 include rapamycin‐insensitive companion of mammalian target of rapamycin (Rictor), mammalian stress‐activated protein kinase‐interacting protein 1 (MAPKAP1 or mSIN1), and protein observed with Rictor‐1 (Protor‐1). 23 All the main components of mTORC and their functions are summarized in Table 1. The recent evidence suggests that mTOR can be found in at least two other complexes. The recent research focuses on a putative novel mTOR complex involving G‐protein‐coupled receptor kinase‐interacting protein 1 (GIT1) lacking Raptor or Rictor, which is identified in mouse astrocytes and is crucial for mTOR‐mediated astrocyte survival. 24 Furthermore, another complex named mTORC3 is present in many cancer cell types which mTORC3 lacks Raptor, Rictor, mSIN1, and mSLT8; however, E26 transformation specific (ETS) translocation variant 7 (ETV7) transcription factor and mTOR seem to be the core components within mTORC3. Appealingly, despite the inhibitory effect of mTOR kinase inhibitors (TORKIs) on mTORC3, the in vitro kinase activity of mTORC3 seems to be resistant to rapamycin. 25
TABLE 1.
The main mTOR complexes components and their functions.
| mTORC component | Function |
|---|---|
| mTOR | Kinase: serine–threonine protein kinase |
| mLST8 | Positive regulator: acts as positive regulator to promote the mTORC1 signaling |
| Deptor | Negative regulator: suppresses mTOR activity and negatively regulates both mTORC1 and mTORC2 |
| Raptor | Substrate recognizing component: Scaffold protein determining mTORC1 specific substrate regulates the assembly and the localization, promotes the mTORC1 activity, essential for rapamycin sensitivity |
| PRAS40 | Negative regulator: As both component and substrate of mTORC1, suppressing TORC1 activity through its association with Raptor, while its phosphorylation by mTORC1 enhances mTORC1 signaling |
| Rictor | Substrate recognizing component: Scaffold protein determining mTORC2 specific substrate and regulates the assembly, promotes the mTORC2 activity |
| mSIN1 | Positive regulator: Scaffold protein regulating the assembly, essential for the integrity of mTORC2, necessary for mTORC2 to activate Akt |
| Protor‐1 | Positive regulator: It may play a role in enabling mTORC2 to efficiently activate SGK‐1 |
2.2. The mTOR signaling
The mTORC1 activation is regulated by different signals such as growth factors, insulin, amino acids, nutrients, energy status, and cellular stressors including hypoxia, osmotic stress, and oxidative stress. 26 The upstream components of the signaling pathways of mTOR as well as the downstream targets have been explained below.
2.2.1. Upstream signaling
The mTORC1 signaling pathway in the brain is triggered through extracellular activators including insulin, insulin‐like growth factor 1 (IGF1), brain‐derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), glutamate, and guidance molecules that inhibit center upstream regulator of mTORC1 signaling called tuberous sclerosis complex (TSC). 17 , 27 TSC is a heterotrimeric complex comprising TSC1 (hamartin), TSC2 (tuberin), and TBC1D7 which acts as a GTPase‐activating protein (GAP) to inhibit Ras homolog enriched in the brain (Rheb). To illustrate, Rheb is a small GTPase that binds to mTORC1 on the surface of lysosomes and activates it. 28 Although there is a functional GAP domain in the TSC2 protein, TSC1 is required to stabilize TSC2 and both are involved in the functionality of this heterodimer. 27
Activation of growth factors by binding to tyrosine kinase receptors leads to induction of Akt‐dependent phosphorylation of TSC2. This multi‐site phosphorylation inhibits TSC1/TSC2 complex by dissociating it from the lysosomal membrane. In addition to PI3K‐Akt, multiple upstream signaling pathways, including extracellular‐regulated kinase (ERK), ribosomal S6 kinase (p90‐RSK), and glycogen synthase kinase 3β (GSK‐3β), promote mTORC1 activity through phosphorylation of GAP and consequently suppression of TSC2. 15 In neurons, G‐protein coupled receptors (GPCRs), including metabotropic glutamate μ‐opioid and cannabinoid receptors, transduce signals to Akt and/or MAPK and stimulate mTORC1 activation. 17 The mTORC1 responds to intracellular stressors such as inflammation, hypoxia, low level of ATP, and DNA damage which can activate the TSC complex to suppress the mTORC1 pathway. 26
The mTORC1 network is well described, but mTORC2 signaling is relatively poorly defined. The mTORC2 is primarily stimulated by insulin/PI3K signaling. Although growth factors trigger mTORC2 activation by the PI3K pathway, energy status and amino acids are not able to stimulate the mTORC2 sufficiently. 28 , 29 It has been shown that mTORC2 phosphorylation by Akt is accomplished on the surface of the endoplasmic reticulum, 30 and insulin‐PI3K signaling stimulates mTORC2‐ribosome association. 31 While TSC1/TSC2 is a vital negative regulator of mTORC1, this complex may promote the mTORC2 activity in a manner independent of its GAP activity toward Rheb. 32 Furthermore, there is a negative feedback regulation of insulin/PI3K and mTORC2 signaling by mTORC1 which brings about the control of mTORC2 network by mTORC1. 33
2.2.2. Downstream signaling
The mTORC1 regulates protein synthesis through phosphorylation of the eukaryotic initiation factor 4E‐binding protein 1 (4E‐BP1) and the p70 ribosomal S6 kinase 1 (S6K1). The phosphorylation of S6K1 by mTORC1 and activation of several substrates through S6K1 promote the initiation of mRNA translation, elongation, and also ribosome biogenesis. The phosphorylation of 4E‐BP1 by mTORC1 causes dissociation of 4E‐BP1 from eIF‐4E, binding eIF‐4E to eIF‐4G, and finally promoting cap‐dependent translation. 15
The mTORC1 impacts autophagosome biogenesis through phosphorylation of unc‐51‐like kinase 1 (ULK1) and its interacting proteins including the autophagy‐related protein 13 (ATG13) and the focal adhesion kinase family interacting protein of 200 kDa (FIP200) which in turn leads to suppression of autophagy process. 34 The mTORC1 activates sterol‐responsive element binding protein‐1,2 (SREBP‐1,2) and peroxisome proliferator‐activated receptor‐γ (PPAR‐γ), two transcription factors which regulate the expression of metabolic genes encoding proteins involved in de novo lipid synthesis. Besides controlling the protein and lipid synthesis by mTORC1, mTORC1 affects ribosome biogenesis and anabolic growth by promoting the production of required nucleotides for RNA and DNA synthesis. 35
The mRTC2 impacts cell survival and proliferation through phosphorylating AGC (PKA/PKG/PKC) family of protein kinases and serum‐ and glucocorticoid‐induced kinase 1 (SGK‐1). This function is mainly achieved by Akt phosphorylation and causing its maximal activation. 36 Akt prevents the pro‐apoptotic characteristics of Forkhead box protein O1 (FOXO1) and FOXO3 by phosphorylating them. 28 PKCα, PKCδ, and PKCζ, as several members of the PKC family, are substrates of mTORC2 which contribute to different aspects of the regulation of cytoskeletal organization and cell migration. 27 Furthermore, mTORC2 phosphorylates and activates SGK1, a molecule involved in the regulation of neuronal functions, and has inhibitory effects on FOXO1/3 activities. 37
3. mTOR IN NORMAL AGING
It is evident that the functions of mTOR exceed proliferation and the signaling participates in coordinating the cell‐tailored metabolic programs to regulate cell growth and various biological processes such as cellular senescence, lifespan, and aging. To the present date, rapamycin, as an mTOR inhibitor pharmacological substance, is the substance that can postpone aging in all model organisms tested, as well as the only one in mammals. 38 The first evidence that mTOR is a cellular lifespan regulator comes from the study on the fruit fly Drosophila melanogaster 39 and nematode Caenorhabditis elegans. 40 Later, in 2009, Harrison et al. 41 suggested that when rapamycin, as an mTOR inhibitor drug, is administered to genetically heterogeneous mice from 600 days of age (relatively equivalent to 60 years of age in humans), it can prolong the median and maximal longevity. Considering age with 90% mortality, administration of rapamycin led to elevated lifespan: about 14% in female mice and 9% in males. A great body of further studies confirmed that inhibition of mTOR by specific chemicals can prolong life 42 ; however, the impact of mTOR inhibitor drug on longevity was indicated dose‐dependent and not as robust as expected. 43 Animal studies indicated that deletion of S6K1 protects against age‐related pathologies and increases lifespan. 44 The life‐increasing effects of mTOR inhibitor drugs may be related to the inhibition of mTORC1, and the inhibition of mTORC2 may even leave dire effects on the inhibitory effect of insulin‐mediated hepatic gluconeogenesis which is controlled by mTORC2. 45 , 46
It is now well‐accepted that mTOR inhibition can delay age‐related diseases. However, the putative anti‐aging effects of mTOR inhibitor drugs and the underlying mechanisms are yet to be investigated.
It is now widely acknowledged that mTOR inhibition has the potential to delay age‐related diseases, but the specific anti‐aging effects of mTOR inhibitor drugs and their underlying mechanisms still require further investigation. 38 Aging is characterized by several hallmarks, including genome instability, accumulation of misfolded proteins, telomere shortening, epigenetic changes, disruption of nutrient‐sensing pathways, cellular senescence, altered intracellular communication, stem cell exhaustion, and impaired mitochondrial function. 47 mTORC1 is known for regulating mRNA translation by phosphorylating 4E‐BP1 and 4E‐BP2, and ribosomal activity by phosphorylating S6K1 and S6K2, suggesting that the overall decline in mRNA translation caused by mTORC1 inhibition delays aging. 38 , 48 Furthermore, mTORC1 has a strong inhibitory effect on autophagy, a cellular mechanism that helps prevent the accumulation of damaged proteins and dysfunctional organelles, thereby exerting anti‐aging effects. 49 Recent research has also indicated that the mTORC1/p70S6K pathway interacts with stress‐induced senescence of mesenchymal stem cells (MSCs), which contributes to age‐related tissue dysfunction. 50 Moreover, chronic mTOR inhibition by rapamycin could increase chaperones levels which in turn maintain proteostasis, while its failure leads to aging as well as even AD development. 51 Although significant amount of animal studies have confirmed that mTOR inhibitor drugs can prolong lifespan; yet, the underlying mechanisms and the actual lifespan‐enhancing effects of mTOR inhibitor drugs need to be further studied. If the aging can be postponed by mTOR inhibitor drugs more specifically, then the putative effects of these drugs on AD can be more easily elucidated.
4. mTOR AND AD PATHOLOGY
mTOR signaling plays a significant role in the pathogenic processes of AD, and its underlying mechanisms are discussed below. The amyloid hypothesis is dominating AD pathogenesis and guiding treatment development. In this regard, the imbalanced Aβ production and clearance seem to initiate cascades that result in increasing Aβ levels, and consequent oligomer and plaque aggregation. The failure of synapses and the inflammation they trigger lead to altered neuronal ionic homeostasis, tau hyperphosphorylation, oxidative injury, and intracellular neurofibrillary tangle formation 52 Although available literature suggests that Aβ deposition is occurred many years before when the AD symptoms are manifested, 53 , 54 researchers have not been able yet to clearly define a chronological cascade in pathologic events of AD due to multiple uninvestigated interactions 55 , 56 ; however, piled‐up toxic proteins and protein misfolding are mainly considered as causative events resulting in neuronal loss and neurodegenerative disease. 56 In this regard, in topics below we discussed the main pathologic events in separate sections but elaborating their interaction with other mechanisms involved in AD pathology.
The mTOR network comprises several crucial molecular targets that have been identified as 53 potential therapeutic targets for various neurological disorders. Pharmacological manipulation of these targets within the mTOR network has shown promise as a therapeutic approach. Several animal studies focusing on AD have demonstrated the beneficial effects of targeting the mTOR network, as summarized in Table 2. These studies provide valuable insights into the potential of mTOR‐related interventions in the treatment of AD.
TABLE 2.
Effect of rapamycin in animal models of AD.
| AD model | Intervention (route of delivery) | Treatment protocol | Key results | Ref. |
|---|---|---|---|---|
| Human amyloid precursor protein (PDAPP) mouse model | Chow supplemented with either encapsulated rapamycin (14 ppm) | For 10 months | Restored BBB integrity through upregulating specific tight junction proteins | [132] |
| Low‐density lipoprotei receptor‐null (LDLR_/_) mice modeling vascular cognitive impairment | Starting at 5–6 months of age | Downregulated matrix metalloproteinase‐9 activity | ||
| PDAPP mouse model | Chow containing either microencapsulated rapamycin (2.24 mg/kg) | For 16 weeks | Increased chaperones levels which, in turn, maintained proteostasis | [51] |
| Starting at 4 months of age | ||||
| PDAPP mouse model | Chow containing either microencapsulated rapamycin (2.24 mg/kg) | For 13 weeks | Prevented AD‐like cognitive deficits | [70] |
| Starting at 4 months of age | Improved spatial learning and memory | |||
| Decreased Aβ42 levels | ||||
| Increased autophagy | ||||
| Senescence accelerated mouse prone 8 (SAMP8) | Treatment of neurons taken from SAMP8 mice with either 0.5 or 1.0 μM rapamycin | Incubation for 3 days | Reduced the levels of phosphorylated tau and p70S6K (pT389) in SAMP8 mice | [80] |
| Inhibited mTOR signaling | ||||
| Promoted autophagy | ||||
| Decreased the protein expression level of Bcl‐2 in primary neurons | ||||
| APOE4 transgenic mice | Chow containing either microencapsulated rapamycin (2.24 mg/kg) | For 6 months | Restored cerebral blood flow, BBB integrity, and glucose metabolism | [133] |
| Restored brain vascular functions and metabolic functions | ||||
| Attenuated spatial learning deficits | ||||
| No change in anxiety level | ||||
| 3xTg‐AD mice | Chow containing either microencapsulated rapamycin (2.24 mg/kg) | For 10 weeks | Reduced Aβ42 levels and deposition | [67] |
| Rescued cognitive deficits | ||||
| Ameliorated Aβ and tau pathology by increasing autophagy | ||||
| PDAPP mouse model | Chow containing either microencapsulated rapamycin (2.24 mg/kg) | For 16 weeks | Improved spatial learning and memory | [131] |
| Starting at 7 months of age | Increased vascular density | |||
| Reduced cerebrovascular amyloid angiopathy and Aß Plaques | ||||
| Induced nitric oxide (NO)‐dependent vasodilation | ||||
| No change in glucose metabolism was observed | ||||
| Ts65Dn mouse model of Down syndrome | Intranasal rapamycin dose of 1 μg/mouse | For 12 weeks | Improved cognitive performances | [153] |
| Starting at 6 months of age | Decreased mTOR hyper‐activation | |||
| Induced autophagy | ||||
| Reduced aberrant APP levels and APP processing | ||||
| Diminished tau hyper‐phosphorylation | ||||
| Reduced oxidative stress markers | ||||
| Adeno‐associated viral vector‐based mouse model of early‐stage AD‐type tauopathy (AAV‐hTauP301) | Intraperitoneal injections of rapamycin at 15 mg/kg/day | 3 times a week for either 3 or 5 weeks | Reduced mTOR activity and stimulated a marker of autophagic flux | [84] |
| Protected against the tau‐induced neuronal loss, synaptotoxicity, reactive microgliosis, and astrogliosis | ||||
| No changes in human tau mRNA or total protein levels were observed | ||||
| 3xTg‐AD mice | Microencapsulated rapamycin was added at a concentration of 14 mg/kg to mouse chow 14 ppm | For 16 months, Starting at 2 months of age | Ameliorated learning and memory deficits | [71] |
| For 3 months, Starting at 15 months of age | Reduced Aβ plaques and NFTs formation | |||
| Reduced microglia activation | ||||
| Late rapamycin administration did not change AD‐like pathology and cognitive deficits | ||||
| P301S Tau transgenic mice | Intraperitoneal injection of rapamycin (15 mg/kg/week) | Twice weekly from the age of 3 weeks to 5.5 months of age | Reduced cortical tau tangles, tau hyper‐phosphorylation, and insoluble tau in the forebrain | [154] |
| Decreased astrogliosis | ||||
| PDAPP mouse model | Restored neurovascular coupling and concomitantly reduced cortical amyloid‐beta levels | [155] | ||
| Effectively treated memory deficits | ||||
| APP/PS1 mouse | Intraperitoneal injection of rapamycin (1 mg/kg) | For 2 months | Increased parkin‐mediated mitophagy and promoted fusion of mitophagosome and lysosome | [144] |
| Starting at 4 months of age | Enhanced learning and memory viability, synaptic plasticity, and the expression of synapse‐related proteins | |||
| Decreased apoptosis and oxidative status | ||||
| Recovered mitochondrial function | ||||
| 3xTg‐AD mice | Intracerebroventricular injection of everolimus (0.167 μg/μL) | For 4 weeks | Reduced human APP/Aβ and human tau levels | [72] |
| Starting at 4 months of age | Improved cognitive function and depressive‐like phenotype |
4.1. mTOR and amyloid‐beta
The amyloid hypothesis provides a solid framework to explain the Aβ mediated AD pathogenesis especially in the early disease process. 57 The accumulation of Aβ may be evident 15–20 years before the clinical manifestation. 58 Aβ aggregations, ranging from oligomers formation to fibrils deposition, are involved in AD progression including synaptic dysfunction, mitochondrial dysregulation, formation of NFTs, oxidative stress, glutamate excitotoxicity, calcium dysregulation, inflammatory responses, and finally neuronal loss. 57 , 59
The connection between mTOR and Aβ has been widely explored. Aβ plaques and both inhibition and activation of mTOR signaling counteract through known and unknown mechanisms. 60 Research on transgenic mice models presents conflicting results. At the early stages of AD, activating mTOR signaling highly impacts Aβ production. 61 On the other hand, an early report showed that phosphorylated forms of mTOR and p70S6k are decreased in the cortex of APP/PS1 transgenic mice compared to control. The authors also found that murine neuroblastoma cells treated with 20 μM Aβ1‐42 for 24 h exhibited reduced mTOR signaling. 62 Caccamo et al. 63 showed that in 3xTg mice brains, an animal model of AD, and cell lines stably transfected with mutant APP, the mTOR signaling and activity are significantly increased. Additionally, the authors observed that by genetically preventing the accumulation of Aβ, the mTOR activity levels in 3xTg mice could be close to the levels in wild‐type ones. Accordingly, intrahippocampal injections of an anti‐Aβ antibody normalized the mTOR signaling and decreased Aβ levels, suggesting that in 3xTg mice elevated levels of Aβ are essential to mTOR hyperactivity. Moreover, intrahippocampal injection of naturally secreted Aβ in the brains of wild‐type mice increased mTOR signaling.
To illustrate the underlying mechanisms, Aβ is known to interact with protein PRAS40, a component of the mTORC1, which directly binds to mTOR and decreases the mTOR activity. 64 Although the mechanisms remain poorly understood, research shows that Aβ upregulates mTOR activity by phosphorylating PRAS40, since blocking the phosphorylation of PRAS40 can prevent the Aβ‐mediated mTOR activity upregulation. 63 Accordingly, the brains of AD patients and 3xTg‐AD mice display significantly higher phosphorylated PRAS40. 65 PRAS40 phosphorylation, in turn, is regulated by Pim 1, a protein kinase of the proto‐oncogene family. In this context, administering Pim1i, a selective Pim1 inhibitor, in 3xTg‐AD mice yielded interesting results. The Pim1i‐treated 3xTg‐AD mice exhibited better performance in spatial reference and working memory than their non‐transgenic and vehicle‐treated counterparts through declines in phosphorylated PRAS40 levels as well as Aβ reduction. 65
Previous research has provided conflicting findings regarding the relationship between mTOR signaling and AD. Hyper‐activated mTOR signaling was initially reported in 9‐month‐old APP/PS1 mice 66 ; however, later research found lower levels of mTOR signaling in 12‐month‐old APP/PS1 mice compared to age‐matched wild‐type mice. 62 Similarly, in 3xTg‐AD mice, the increase in mTOR signaling was found to depend on both age and brain region. 63 , 67 Post‐mortem studies on AD brains consistently demonstrated upregulated mTOR signaling, which aligned with the results observed in 3xTg‐AD models. 63 , 68 Confocal microscopy data showed a direct interaction between mTOR signaling and Aβ42 (a hallmark protein in AD) within neurons. 69 The co‐localization of Aβ42 with mTOR and p70S6K (a downstream effector of mTOR) in neurites of APP transgenic mice suggested that components of the mTOR signaling pathway are regulated by intraneuronal Aβ. 69 While there is evidence supporting a direct interaction between mTOR and Aβ, it is important to note that the concept is complex, and in vitro and in vivo studies often yield conflicting results. However, the mTOR pathway can be considered one of the pathways through which Aβ toxicity is exerted, supporting the notion that reducing mTOR signaling in AD may be an effective treatment strategy. In line with this, a study by Spilman et al. 70 demonstrated that chronic treatment with rapamycin, an mTOR inhibitor, for 13 weeks could reduce Aβ42 levels in the brains of a transgenic mouse model of AD. Additionally, in a study using the 3xTg‐AD mice, early administration of microencapsulated rapamycin was found to reduce amyloid plaque load and levels of soluble and insoluble Aβ40 and Aβ42, while late administration had less effect. 71 Intracerebroventricular infusion of everolimus, an mTORC1 inhibitor, also showed reduced levels of APP/Aβ and tau in brain regions of symptomatic 3xTg‐AD mice. 72
4.2. mTOR and tau
Tau is a microtubule‐associated protein and is mainly based on axons to ensure neural connections and microtubule stability. However, when hyper‐phosphorylated, its functions are impaired. The production of toxic tau oligomers, and further NFTs, also known as tauopathies, will play a key role in AD pathogenesis. 73 The evidence linking tau and mTOR is less controversial. In general, mTOR‐related pathways are involved in the formation, hyper‐phosphorylation, degradation, and aggregation of NFTs. 74 It is thought that the upregulation of the mTOR pathway can increase the level of hyper‐phosphorylated tau. To illustrate, mTOR activation impairs autophagy, a mechanism which reduces the accumulation of Aβ. When autophagy is impaired, the higher levels of piled‐up Aβ stimulate hyper‐phosphorylation of tau and the formation of NFTs. 75 Various kinases phosphorylate tau including cAMP‐dependent protein kinase, Cyclin‐dependent protein kinase 5 (Cdk5), stress‐activated protein kinases (SAPK), AMPK, GSK3, mitogen‐activated protein kinases, and Protein Kinase A. According to a great body of research, AMPK activation precedes tauopathy, suggesting its involvement in hyper‐phosphorylation of tau and subsequent formation of NFTs. 76 mTOR activity can drive tau accumulation in AD by increasing translation of tau mRNA via S6K1 activation as well as inhibiting protein phosphatase 2A (PP2A), as the major tau phosphatase. 77 mTOR activity upregulation increases cytosolic tau, intracellular accumulation, and tau translocation to various cellular compartments, including endoplasmic reticulum, mitochondria, and Golgi apparatus, as seen in AD brains and human SH‐SY5Y neuroblastoma cells. 78
In cellular and animal models, it has been shown that autophagy activators, for example, mTOR inhibitor drugs, can attenuate hyperphosphorylated tau and other aggregated misfolded proteins through stimulating the autophagy of NFTs and Aβ plaques. 79 Wang et al. 80 showed that upregulation of mTOR, phosphorylated 70S6 kinase (p70S6K) as mTOR substrate, and the protein expression levels of tau (pS396 or pS199) were reported elevated in hippocampus tissues isolated from senescence‐accelerated mouse prone 8 (SAMP8) newborn mice compared to control‐strain SAMR1 mice. Accordingly, administration of 0.5 μM rapamycin for 3 days dramatically decreased phosphorylated Tau protein (pS396 or pS199) expression levels as well as p70S6K in SAMP8 neurons in comparison with the untreated neurons‐SAMP8 group. 80 The findings of the mentioned study suggested that the administration of rapamycin could ameliorate AD pathology by reducing the levels of p70S6K and phosphorylated tau. Interestingly, in contrast to this evidence, Hodges et al. proposed that mTOR could protect tau deposition. The authors aimed to find out how the levels of neuropathological proteins of AD were altered in the hippocampus of subset‐specific deletion of PTEN (NS‐PTEN) mice, manifesting with hyper‐activated mTOR activity. The authors observed reduced levels of GSK3α, GSK3β, tau, and APP in hippocampi of NS‐PTEN KO mice compared to wild‐type mice. Although mTOR inhibitor therapy could protect tau deposition, the authors note that a drastic reduction in mTOR activity could have a negative impact on the brain's memory and cognition as well. 8
4.3. mTOR, gliosis, and inflammation
Glial cells, particularly activated microglia and reactive astrocytes, appear to play critical and interactive roles in AD pathogenesis. Neuroinflammation, including glia cell activation, is a remarkable characteristic of AD. 81 For instance, elevated expression of inflammatory mediators has been reported in postmortem AD samples. 82 , 83 The level of Glial cell activation and their interplay are associated with the extent of brain atrophy and cognitive impairment; therefore, it might be expected that neuroinflammatory responses led by glial cells, and those of microglia and astrocyte in particular, exacerbate the neurodegeneration associated with AD. 81 Rapamycin could putatively protect the entorhinal cortex and perforant pathway projection from reactive gliosis in a mouse model of early‐stage AD‐type tauopathy. 84 Additionally, it is previously known that metformin has neuroprotective effects by upregulating fibroblastic growth factor 21 (Fgf21), leading to the AMPK/mTOR pathway activation and inhibition of tau phosphorylation inhibition. However, some research suggests that metformin may lead to cognitive impairments and enhanced gliosis. 85
Neuro‐inflammation is considered one of the hallmarks in the brains of AD patients which is associated with inflammatory responses including enhancement in activated microglia and astrocytes, activated complement proteins, cytokines, and free radicals. 86 Inflammation and oxidative stress promote initiation and progression of AD through facilitating Aβ production and NFTs formation which, in turn, contribute to worsening cognitive impairments. 87
It has been revealed that rapamycin could exert neuroprotective effects via its anti‐inflammatory properties. 88 , 89 In this regard, chronic treatment with rapamycin could alleviate age‐dependent cognitive deficits in mice through downregulating interleukin‐1β (IL‐1β) and upregulating NMDA signaling in the hippocampus. 90 Following lipopolysaccharide‐induced neuroinflammation response, rapamycin could suppress the expression of key proteins and cytokines including IL‐1β, IL‐6, hypoxia‐inducible factor‐1α (HIF‐1α), nuclear factor κB (NFκB) and modulate the neuronal inflammatory response in vitro and in vivo. 91 The mTOR‐mediated phosphorylation of 4E‐BP1 and S6K1 pathways amplify in the hippocampus of AD rats. Conversely, suppressed mTOR signaling by rapamycin could increase pro‐inflammatory cytokines including IL‐6 and tumor necrosis factor‐α (TNF‐α) signaling pathways, as well as caspase‐3, and worsen learning performance in AD rats. Worthy to note that this discrepancy can be attributed to dosages of rapamycin, delivery route, animal species, and the range of age. 92
4.4. mTOR and autophagy/apoptosis
Hyper‐ and hypoactivation of autophagy are involved in neurodegenerative diseases. 14 AD is associated with neural death, which is an outcome or concomitant with Aβ and tau deposition. 93
The relationship between the autophagy and the development of AD has garnered significant attention. In physiological conditions, autophagy operates consistently and effectively in normal neurons, but abnormal autophagy is involved in the pathogenesis of AD. 94 The regulation of autophagy involves two pathways: mTOR‐dependent pathway and mTOR‐independent pathway. Nevertheless, both of these pathways were found to be abnormal in AD. It appears there is a bi‐directional relationship between AD pathology and autophagy. 95 It has been established that autophagy induction can foster the clearance and degradation of Aβ and Tau in AD patients and animal models. 96 On the other hand, as AD advances, autophagy becomes aberrant, and this process is accompanied by elevated Aβ and Tau levels which leads to impaired autophagy and mitophagy in AD. 97 , 98
mTORC1 regulates cell autophagy by recycling intracellular organelles through lysosomal degradation. 99 , 100 Accordingly, the involvement of mTOR signaling in AD pathogenesis is more implicated in the identified autophagy dysfunction in neurodegenerative diseases. Regarding the issue, the role of autophagy in Aβ pathogenesis should not be overlooked. Autophagy induces Aβ clearance and Aβ degradation. Moreover, autophagy is downregulated by mTOR activity; thus, dysregulation of autophagic processes is often expected in individuals with AD, which leads to facilitation of Aβ production and failure of its clearance. 61 , 101 mTORC1 activation reduces autophagy through phosphorylation of distinct substrates such as ULK1, which is involved in initiating the early steps of autophagy. Other substrates that mTORC1 phosphorylates include death‐associated protein 1 (DAP1), the nuclear translocation of the transcription factor EB (TFEB), and ATG13, all of which contribute to the inhibition of autophagy. 102 , 103 ULK1 phosphorylation by mTORC1 prevents AMPK from activating ULK1 in nutrient‐replete conditions. 104 In various cellular conditions, the relative activity of mTORC1 and AMPK dictates the degree of autophagy. Moreover, phosphorylation and inhibition of TFEB by mTORC1 can partially control autophagy as TFEB controls the expression of genes engaged in lysosomal biogenesis. 105 , 106 , 107 However, the exact pathways in which mTORC1 inhibits autophagy still remain ambiguous.
Over the past decade, the dysregulation of microRNAs (miRNAs) expression has been demonstrated in cellular, animal models, and even human AD subjects. 108 , 109 Several studies have shown that the expression of miR‐128 is dysregulated in AD brains. 110 , 111 In this respect, it has been shown that miR‐128 can promote autophagic flux by directly inhibiting mTOR, resulting in elevated Aβ clearance and reduced Aβ levels. 112 Rapamycin binds to intracellular receptor, the FK506‐binding protein (FKBP12). The rapamycin‐FKBP12 forms an inhibitory complex that directly interacts with mTOR blocking its activity, which leads to autophagy induction. 113 It has been shown that a novel small molecule named TH2849 from these derivative compounds has a significant binding connection with mTOR. TH2849 may induce autophagy most likely due to its suppression of the activation of mTOR, which subsequently dephosphorylates ULK1 and results in ULK1 complex formation. 114
Consistently, mTOR hyperactivation can attenuate autophagy and apoptosis in individuals with AD, since mTOR is responsible for controlling cell growth and death by sensing environmental and nutritional status. Overactivation of PI3K/Akt/mTOR axis can downregulate autophagy, and functional autophagy protects neurons from apoptosis. Research on animal models shows that inhibition of mTOR pathway and its downstream signals by rapamycin may improve cognitive impairment by preventing neural apoptosis. 115 Consistently, administration of Magnolol, an autophagy regulator compound, to transgenic mice model of AD inhibited apoptosis and decreased AD‐related pathologies by activating the AMPK/mTOR/ULK1 pathway. 116 Moreover, autophagy induces Aβ clearance and Aβ degradation. 117 Postmortem AD samples represent the alteration of mTOR signaling and autophagy at the early stages of AD. On the other hand, it seems that elevated Aβ levels are associated with reduced autophagy. 68 Thus, dysregulation of autophagic processes is often expected in individuals with AD, which can result in facilitation of the process of Aβ production and failure of its clearance. 61 , 101
Given that apoptosis and autophagy are extremely linked together through various intrinsic and extrinsic pathways, alteration in apoptosis level is also crucial in the pathophysiology of AD. 118 Caspase‐dependent apoptosis aligns with activating special pathways that bring about activation of specific proteases. 118 Caspase 3 and apoptosis are activated when cultured hippocampal neurons are exposed to Aβ. 119 Caspase 3, as a dominant caspase, contributes to amyloidogenic cleavage of the APP. 120 , 121 Moreover, caspase 3 can cleave pathologic soluble Tau hyper‐phosphorylated at Ser396 and Ser404 122 ; thus, leading to NFTs formation. Similarly, Aβ‐induced caspase 3 activation can impair normal tau processing. 118 Research on 3 transgenic AD mouse models, revealed that the anti‐apoptotic Bcl‐2 gene resulted in the inhibition of caspase‐dependent cleavage of tau, inhibition of plaques and tangles formation, and improvement of memory. 123 Paquet et al. 124 conducted a clinical trial aiming to investigate the effect of previous Aβ immunization on the expression of various apoptotic proteins in post‐mortem human brain tissue. Thirteen cortexes of AD patients were immunized by administering AN1792 (iAD) and 27 cortexes of nonimmunized AD patients were immunolabeled to determine the pro‐apoptotic proteins engaged in AD. The results indicated that apoptosis is downregulated in residual neural and other cells.
Moreover, Bryostatin administration leads to the inhibition of apoptosis. Bryostatin also induces anti‐Aβ oligomers, anti‐hyper‐phosphorylated tau, and synaptogenesis. Farlow et al. 125 conducted a double‐blind, placebo‐controlled, phase II study on 150 AD severe patients for 12 weeks to assess the safety, tolerability, and efficacy of Bryostatin in the treatment of moderate to severe AD. The result suggested that Bryostatin might be favor compared to placebo. To sum up, inhibiting the intrinsic apoptotic pathways may be beneficial to AD progression. However, more research is required to unravel the connection between apoptosis, anti‐apoptotic drugs, mTOR signaling, and AD physiopathology.
4.5. mTOR and vascular dysfunction
Vascular dysfunction can be the source of various pathogenic pathways that lead to neuronal damage, dementia, and AD development according to the vascular hypothesis of AD that was postulated in 1993. 126 In vascular aging, disruption in endothelium‐dependent vasodilation chronically declines cerebral blood flow (CBF). The vascular dysfunction including cerebral amyloid angiopathy (CAA) and pathological alterations in vessel cell function, blood–brain barrier (BBB) permeability, aberrant immune cell recruitment, and direct vascular contribution to inflammation are related to neurodegenerative changes. On the other hand, these vascular impairments in turn take part in amyloid deposition, neurotoxicity, glial cell activation, and metabolic deficits in the AD brain. 127 Consequently, the formation of feedback cycle and remarkable overlap between cerebrovascular dysfunction and AD leads to progressive exacerbation of neuronal and vascular pathology indicating synergistic/additive effects of both compartments on cognitive decline. 128
mTOR signaling through specific mechanisms active in endothelial and smooth muscle cells of vasculature regulates vascular function acutely and chronically. 129 Elevated mTOR signaling has been found in AD, and the mTOR network contributes to cerebrovascular dysfunction, subsequence CBF deficits, and cognitive decline. 130 Chronic attenuation of mTOR pathway by rapamycin treatment could not only restore CBF and cerebrovascular density but also decrease CAA and micro‐hemorrhages, amyloid burden, and finally can block the progression of cognitive deficits in AD mouse brains through nitric oxide synthase (NOS) activation. 131 Rapamycin or other rapalogs exerts protective effects on BBB integrity in transgenic APP mice and vascular cognitive impairment models through downregulating matrix metalloproteinase‐9 activity and upregulating tight junction proteins present in cellular connections. 132 The ApoE 4 transgenic mice treated with rapamycin exhibited restoration in CBF, BBB integrity, and glucose metabolism compared to wild‐type controls in which preservation of vasculature and metabolism were associated with amelioration of learning deficits. 133 Furthermore, mTOR is engaged in cerebrovascular and cognitive dysfunctions associated with atherosclerosis. In this context, rapamycin‐mediated inhibition of mTOR pathway could restore neurovascular functions and cardiovascular health in mice model of atherosclerosis. 134 Available literature shows that rapamycin treatment of obese mice via inhibition of Akt/mTORC2 axis activity brings about vascular senescence suppression, endothelial sprouting, endothelial NOS activity, and vasodilation. 135
Studies have shown that the APOE ε4 allele imposes a great risk for developing dementia, since it regulates several key genes which are involved in function of brain. 136 A cohort study conducted by Robb et al. 137 on 246 APOE ε4 carriers and non‐carriers aimed to test whether APOE ε4 status could impact cerebral oxygen homeostasis and cognitive performance. The authors found that APOE4 carriers are often manifested with lower cerebral oxygen metabolism, interacting with the pathogenesis of AD and related neurodegeneration. Additionally, the cognitive decline associated with AD may be caused by cerebral hypoperfusion, 138 which is even more significant in APOE ε4 carriers. 139 A longitudinal study was conducted on 950 APOE ε4 carriers and non‐carriers who were cognitively intact. Findings of the study suggested an interactive effect between and sex on cerebral perfusion trajectory during later pathological stages of AD. 140 To further elaborate on the relation between APOE ε4, oxygen homoeostasis, and mTOR signaling, it is worthy to mention the research conducted by Lin et al. using multimodal in‐vivo imaging. The authors aimed to test if an early intervention using rapamycin could prevent the progression of AD‐like symptoms in pre‐symptomatic APOE ε4 transgenic mice by restoring neurometabolic and neurovascular functions. Compared to wild‐type controls, rapamycin‐treated mice showed improved cerebral blood flow and less disrupted blood‐barrier integrity accompanied by ameliorated learning deficits. The link mentioned above between mTOR signaling and abnormal brain metabolism and cerebral blood flow in AD, especially in APOE4 carriers, may add to the rationale of rapamycin clinical trials previously discussed in the paper. 133
4.6. mTOR and synaptic plasticity
The synaptic impairment is an early event of AD and develops before neurodegeneration. The synaptic loss underlies the memory impairment in the initial phase of AD, and there is a solid correlation between the level of synaptic loss and dementia severity. 87 Many evidence has indicated that prior to plaque formation, soluble oligomeric forms of Aβ lead to neurotoxicity at synapses, especially glutamatergic synaptic transmission which triggers synaptic failure and memory impairment. 129 , 141
The mTOR is involved in long‐lasting forms of memory formation and synaptic plasticity. mTOR is engaged in protein synthesis related to synaptic plasticity through 4E‐BP and S6K. 130 Interestingly, mTOR signaling pathway is inhibited in hippocampal slices of AD transgenic mice as well as in hippocampal slices of wild‐type mice treated with Aβ1‐42, suggesting that mTOR dysregulation is correlated to synaptic plasticity impairment. In this regard, up‐regulation of mTOR pathway through pharmacological and genetic manners could restore long‐term potentiation and prevent Aβ‐induced impairment in synaptic plasticity. 69 Pretreatment of cultured hippocampal neurons with rapamycin could inhibit BDNF‐induced synaptic plasticity and reduce late‐phase LTP expression induced by high‐frequency stimulation. Moreover, the localization pattern of several key components including mTOR, eIF‐4E, 4E‐BP1, and 4E‐BP2 proposes that these proteins are involved in coupling synaptic events with the protein translational machinery. 142 However, mechanisms underlying the relationship between synaptic plasticity and mTOR signaling also remain controversial.
4.7. mTOR and learning/memory
Inhibiting mTOR signaling with rapamycin not only protects against Aβ and tau pathology but also may prevent memory impairments. Simen et al. 84 evaluated the impact of the rapamycin, an mTOR inhibitor and autophagy stimulator, on the tau‐mediated neurodegeneration and synaptic loss in a mouse model of early‐stage AD‐type tauopathy. The results of the study displayed that intraperitoneal injection of rapamycin can alleviate neuronal, axonal, and synaptic loss in perforant pathway projection and the entorhinal cortex. The perforant starting from layer II of the entorhinal cortex ending in the hippocampal dentate gyrus has a central role in long‐term memory and is precisely delicate to damages caused by tauopathy. 84 , 143
In the study conducted by Wang et al., 144 it was observed that rapamycin not only improved cognitive deficits but also enhanced memory viability, learning, and the expression of synapse proteins in APP/PS1 mice. The authors suggested that cognitive function in the APP/PS1 group showed a direct correlation with autophagy activation and an inverse correlation with mTOR activity and Aβ plaque levels, while the levels of soluble or insoluble Aβ were not significantly associated with cognitive function. These results indicated a significant involvement of mTOR signaling in the cognitive performance of APP/PS1 mice. Similarly, Cassano et al. 72 reported that the administration of everolimus, in their study led to improvements in cognitive functions such as novel object recognition, spatial memory, inhibitory passive avoidance, and depressive‐like phenotype in 3xTg‐AD mice.
On the other hand, given that balanced mTOR activity has a pivotal role in learning and memory, chronic inhibition of mTOR activity by rapamycin or other mTOR inhibitors may harm cognition in the long term. 145 Sui et al. 146 found that systemically administered rapamycin in mice can cause deficits in spatial memory retrieval but not acquisition. 147 Moreover, when rapamycin was infused into the basolateral amygdala or dorsal hippocampus of the rat, novel object recognition became impaired. 148 , 149 The study on an 8‐month‐old APP/PS1 transgenic mice model for AD revealed that astaxanthin (Ast), as a carotenoid with potent antioxidant and neuroprotective properties, can activate the mTOR pathway and can ameliorate cognitive impairment, and suggests that the Ast may be beneficial for the treatment of cognitive impairments in AD. The authors suggest that Ast diminishes cognitive deficits of AD by enhancing the mTOR‐dependent mitochondrial dynamics, decreasing Aβ accumulation, and ameliorating synaptic damage. 150 L‐3‐n‐Butylphthalide (L‐NBP), a compound extracted from the seeds of Chinese celery, can activate mTOR/AKT signaling and ameliorate learning and memory deficits following repeated cerebral ischemia–reperfusion injuries. 151 In this regard, results from a study on rat models of vascular dementia (VD) revealed that the neuroprotective characteristics of NBP on learning/memory impairments are mainly derived through activation of the mTOR pathway which suppresses ischemia‐induced autophagy. 152 Available data on the effects of mTOR inhibitor/activator drugs on learning/memory point to the critical involvement of mTOR signaling in learning and memory. However, much further work would be required to elucidate the exact association between learning/memory impairments in AD and mTOR signaling pathway.
5. THERAPEUTIC POTENTIAL
In the past two decades, much research has indicated the massive complexity of the mTOR signaling network in mammalian brains. 156 In general, hyperactivated mTOR signaling contributes to aging and AD progression. In this context, independent of AD pathology, rapamycin treatment may extend life span and delay aging per se, mediated through targeting of downstream signaling of mTORC1, enhancing autophagy, and reducing protein translation. 28 As shown in Figure 1, administration of mTOR inhibitor drugs such as rapamycin may be beneficial in preventing the progression of AD, as mTOR signaling is involved in AD pathogenesis including the formation of NFTs and Aβ plaques. 75 , 84 Additionally, based on animal studies, administration of mTOR inhibitor drugs seems to be a potential pharmaceutical approach in patients with AD to ameliorate cognitive decline. 157 Nonetheless, due to poor understanding of the underlying mechanisms, connections, and correlation between mTOR signaling and AD pathogenesis long‐term activation or inhibition of mTOR signaling might leave dire impacts.
FIGURE 1.
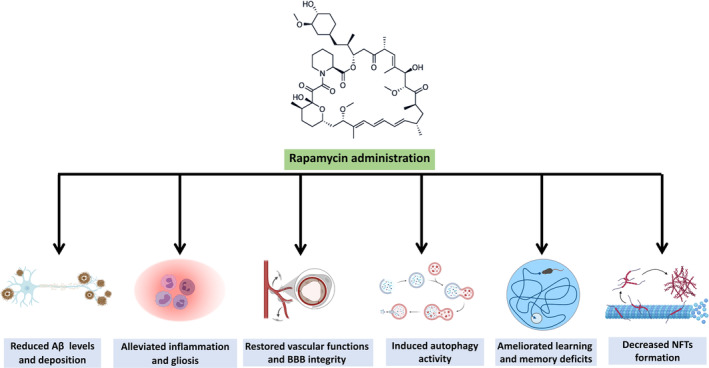
The therapeutic effects of rapamycin administration in Alzheimer's disease models.
The beneficial effects of rapamycin on AD pathology are paradoxical for synaptic plasticity and memory, and mTOR inhibition signaling might impair synaptic plasticity in the AD brain. As regards, balanced mTOR signaling is required for synaptic plasticity and memory, whereas chronic and complete inhibition of mTOR might exert deleterious effects on plasticity and long‐term memory. 69 On the other hand, different concentrations of Aβ could result in differential effects on mTOR signaling. 145 Shi et al. 158 observed that in the 5XFAD AD mouse model, mTOR activation was accompanied by decreased dendrite loss, enhanced Aβ clearance, and improved cognitive function. Extended administration of rapamycin even elicited further decrease of mTOR functioning and elevation of Aβ levels. This study contradicts previous studies that showed reducing mTORC1 could benefit the treatment of Alzheimer's disease. Although we believe that the controversial findings may stem from the different AD mouse models use, there may be multiple factors at play in the controversial findings. Low mTORC1 signaling is likely to be desirable in healthy state or early progression of the disease, while high levels are likely to be needed for brain health maintenance and microglial homeostasis at later stages. 159 Thus, clinical risks of chronic treatment with mTOR inhibitors should be considered, and for translation into AD patients, it is essential to determine optimal dosage of rapamycin. 28
It is important to note that the effects of rapamycin can vary between different animal models. 160 A prophylactic dose of rapamycin significantly reduced plaques, tangles, and cognitive deficits in 2‐month‐old 3xTg‐AD mice. The induction of autophagy, however, had no effects on AD‐like pathology and cognitive deficits in 15‐month‐old 3xTg‐AD mice that had plaques and tangles. 71 In the mouse hippocampus of APP/PS1, rapamycin helps promote Parkin‐mediated mitophagy. With rapamycin treatment, APP/PS1 mice showed enhanced recovery of cognitive function, synaptic plasticity, synapse‐related proteins, and immunity to cytochrome C‐mediated apoptosis. 144 Rapamycin treatment in mouse model of early‐stage AD‐type tauopathy resulted in a reduction in mTOR kinase activity in the brain. This led to a decrease in phosphorylation of the mTOR substrate P70S6 kinase. Additionally, rapamycin inhibited the trans‐synaptic transfer of human tau expression to the dentate granule neuron targets, potentially preventing neurodegeneration and synapse loss in the perforant pathway. 84 The complex nature of AD and the limitations of animal models necessitate further research to fully understand the potential benefits and risks of rapamycin in treating Alzheimer's disease in humans.
Although rapamycin is widely recognized as an inhibitor of mTOR, the off‐target effects of it should not be overlooked and the exploration of other targets of rapamycin will further elucidate its underlying mechanisms of action. Le Sun et al. demonstrated that STAT3 as a direct functional protein target of rapamycin. They showed that rapamycin, a macrolide compound with inhibitory function for mTOR, directly binds to STAT3 inhibiting its transcription factor activity. Furthermore, another transcription factor, c‐Myc, that up to now has been undruggable, is inhibited by rapamycin. Long‐term rapamycin treatment of xenograft mice results in a decrease of both STAT3 and c‐Myc expression. Therefore, rapamycin may serve as a lead compound for oncology drug development efforts targeting STAT3. 161 In addition, geroprotective effects of chronic rapamycin treatment can be obtained with a brief pulse of the drug in early adulthood in female Drosophila and mice, without the adverse effects sometimes seen with chronic, long‐term dosing. 162
6. CONCLUSIONS AND FUTURE PROSPECTS
Currently, there is no terminated clinical trial assessing the efficacy of mTOR inhibitor drugs in delaying or preventing the onset or progression of AD. The experimental studies have provided solid evidence for the contribution of mTOR signaling to AD pathogenesis. Upregulation of mTOR activity appears to be involved in the pathological cascade of AD, and mTOR inhibition or targeting its downstream substrates could supply attractive avenues for AD treatment. It has been also shown to be the most efficient pharmacologic compound directly attenuating the aging process and prolonging life span in animal models. Research on animal models shows that anti‐aging properties of rapamycin exceed increasing life span to inhibit age‐related dysfunctions or diseases such as cognitive decline and muscle loss. 12 Additionally, treatment of different AD models with rapamycin led to improved autophagy and/or amyloidosis. 77 However, the role of mTOR activation in AD seems complex.
Despite recent advances in understanding mTOR functioning, many questions regarding the application in the clinical setting remain to be answered. Furthermore, there are ongoing debates that rapamycin treatment may not be an efficient pharmacological approach in humans, since the efficacy of reducing the neuropathological hallmarks of AD is tested in the early phases of AD progression and not when the dire symptoms are present in patients. After the clinical diagnosis in humans, the lysosomal system is impaired, and treatment with rapamycin may even aggravate the impairment. 163 Taken together, further efforts should be applied to optimize the therapeutic potential of mTOR inhibitors and minimize side effects. Furthermore, although beneficial effects of rapamycin have been proven in animal models of AD, it remains to be defined whether these results could be extended to AD patients as well. In this respect, determining the favorable dosing, timing, and treatment duration will be of dominant significance.
AUTHOR CONTRIBUTIONS
Samin Davoody and Afsaneh Asgari Taei: Performed the literature review, wrote the first draft of the manuscript, designed the tables and figure, revised and edited the manuscript; Pariya Khodabakhsh: performed the literature review, revised and edited the manuscript; Leila Dargahi: overviewed the latest state of knowledge, revised and edited the manuscript, supervised the review process. All authors read and approved the final manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
All the authors declare that they have no conflicts of interest.
CONSENT FOR PUBLICATION
All authors whose names appear on the submission approved the final manuscript and agreed to be published.
ACKNOWLEDGMENTS
We would like to thank Prof. Mohammad Hossein Pourgholami for his kind assistance in this study.
Davoody S, Asgari Taei A, Khodabakhsh P, Dargahi L. mTOR signaling and Alzheimer's disease: What we know and where we are? CNS Neurosci Ther. 2024;30:e14463. doi: 10.1111/cns.14463
The first two authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Duong S, Patel T, Chang F. Dementia: what pharmacists need to know. Can Pharm J (Ott). 2017;150(2):118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt J, Comas Herrera A, Amico F, et al. The World Alzheimer Report 2019: Attitudes to dementia 2019. Alzheimers & Dementia. 2020;16. [Google Scholar]
- 3. Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease. Mol Neurodegener. 2020;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gammon K. Neurodegenerative disease: brain windfall. Nature. 2014;515(7526):299‐300. [DOI] [PubMed] [Google Scholar]
- 5. Zali H, Seyyedi SS, Rashidy Pour AI, Rezaei Tavirani M. Epidemiology and etiology of Alzheimer's disease. Koomesh. 2014;16(2):119‐127. [Google Scholar]
- 6. Chakrabarti S, Kumar Khemka V, Banerjee A, Chatterjee G, Ganguly A, Biswas A. Metabolic risk factors of sporadic Alzheimer's disease: implications in the pathology, pathogenesis and treatment. Aging Dis. 2015;6(4):282‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ittner LM, Götz J. Amyloid‐β and tau—a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12(2):67‐72. [DOI] [PubMed] [Google Scholar]
- 8. Hodges SL, Reynolds CD, Smith GD, Jefferson TS, Nolan SO, Lugo JN. Molecular interplay between hyperactive mammalian target of rapamycin signaling and Alzheimer's disease neuropathology in the NS‐Pten knockout mouse model. Neuroreport. 2018;29(13):1109‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY‐22,989), a new antifungal antibiotic. I. Taxonomy of the producing Streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28(10):721‐726. [DOI] [PubMed] [Google Scholar]
- 10. Eng C, Sehgal S, Vezina C, Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY‐22,989) against transplanted tumors. J Antibiot (Tokyo). 1984;37:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 11. Jhanwar‐Uniya M, Wainwright JV, Mohan AL, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. 2019;72:51‐62. [DOI] [PubMed] [Google Scholar]
- 12. Kaeberlein M, Galvan V. Rapamycin and Alzheimer's disease: time for a clinical trial? Sci Transl Med. 2019;11(476):eaar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding Y, Liu H, Cen M, Tao Y, Lai C, Tang Z. Rapamycin ameliorates cognitive impairments and Alzheimer's disease‐like pathology with restoring mitochondrial abnormality in the hippocampus of Streptozotocin‐induced diabetic mice. Neurochem Res. 2021;46(2):265‐275. [DOI] [PubMed] [Google Scholar]
- 14. Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95(4):1157‐1187. [DOI] [PubMed] [Google Scholar]
- 15. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eltschinger S, Loewith R. TOR complexes and the maintenance of cellular homeostasis. Trends Cell Biol. 2016;26(2):148‐159. [DOI] [PubMed] [Google Scholar]
- 17. Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci. 2014;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun T, Duan L, Li J, Guo H, Xiong M. Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA‐21‐mediated PTEN/AKT/mTOR pathway. Int J Mol Med. 2021;48(2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang H, Jiang X, Li B, et al. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature. 2017;552(7685):368‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang T, Ding H, Wang Y, et al. Akt3‐mTOR regulates hippocampal neurogenesis in adult mouse. J Neurochem. 2021;159:498‐511. [DOI] [PubMed] [Google Scholar]
- 21. Lebrun‐Julien F, Bachmann L, Norrmen C, et al. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J Neurosci. 2014;34(25):8432‐8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou Y, Jiang W, Wang J, et al. Oligodendrocyte precursor cell‐intrinsic effect of Rheb1 controls differentiation and mediates mTORC1‐dependent myelination in brain. J Neurosci. 2014;34(47):15764‐15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smithson LJ, Gutmann DH. Proteomic analysis reveals GIT1 as a novel mTOR complex component critical for mediating astrocyte survival. Genes Dev. 2016;30(12):1383‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiarini F, Evangelisti C, Lattanzi G, McCubrey JA, Martelli AM. Advances in understanding the mechanisms of evasive and innate resistance to mTOR inhibition in cancer cells. Biochim Biophys Acta, Mol Cell Res. 2019;1866(8):1322‐1337. [DOI] [PubMed] [Google Scholar]
- 26. Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11(9):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84(2):275‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang C, Yu JT, Miao D, Wu ZC, Tan MS, Tan L. Targeting the mTOR signaling network for Alzheimer's disease therapy. Mol Neurobiol. 2014;49(1):120‐135. [DOI] [PubMed] [Google Scholar]
- 29. Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10(14):2305‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boulbés DR, Shaiken T, Sarbassov DD. Endoplasmic reticulum is a main localization site of mTORC2. Biochem Biophys Res Commun. 2011;413(1):46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757‐768. [DOI] [PubMed] [Google Scholar]
- 32. Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1‐TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28(12):4104‐4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu Y, Yoon SO, Poulogiannis G, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332(6035):1322‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dossou AS, Basu A. The emerging roles of mTORC1 in macromanaging autophagy. Cancer. 2019;11(10):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589‐3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo Y, Xu W, Li G, Cui W. Weighing in on mTOR complex 2 signaling: the expanding role in cell metabolism. Oxidative Med Cell Longev. 2018;2018:7838647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu W, Hall MN. Regulation of mTORC2 signaling. Genes (Basel). 2020;11(9):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini‐review. Gerontology. 2018;64(2):127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vellai T, Takacs‐Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans . Nature. 2003;426(6967):620. [DOI] [PubMed] [Google Scholar]
- 41. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller RA, Harrison DE, Astle CM, et al. Rapamycin‐mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson S, Yanos ME, Bitto A, et al. Dose‐dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front Genet. 2015;6:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selman C, Tullet JMA, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lamming DW, Ye L, Katajisto P, et al. Rapamycin‐induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamming DW, Mihaylova MM, Katajisto P, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13(5):911‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Connell AE, Gerashchenko MV, O'Donohue MF, et al. Mammalian Hbs1L deficiency causes congenital anomalies and developmental delay associated with Pelota depletion and 80S monosome accumulation. PLoS Genet. 2019;15(2):e1007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bensalem J, Fourrier C, Hein LK, Hassiotis S, Proud CG, Sargeant TJ. Inhibiting mTOR activity using AZD2014 increases autophagy in the mouse cerebral cortex. Neuropharmacology. 2021;190:108541. [DOI] [PubMed] [Google Scholar]
- 50. Liu H, Huang B, Xue S, et al. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress‐induced senescence of MSCs. Stem Cell Res Ther. 2020;11(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pierce A, Podlutskaya N, Halloran JJ, et al. Over‐expression of heat shock factor 1 phenocopies the effect of chronic inhibition of TOR by rapamycin and is sufficient to ameliorate Alzheimer's‐like deficits in mice modeling the disease. J Neurochem. 2013;124(6):880‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D'Andrea L, Stringhi R, Di Luca M, Marcello E. Looking at Alzheimer's disease pathogenesis from the nuclear side. Biomolecules. 2021;11(9):1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Welikovitch LA, do Carmo S, Maglóczky Z, et al. Early intraneuronal amyloid triggers neuron‐derived inflammatory signaling in APP transgenic rats and human brain. Proc Natl Acad Sci U S A. 2020;117(12):6844‐6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vickers JC, Mitew S, Woodhouse A, et al. Defining the earliest pathological changes of Alzheimer's disease. Curr Alzheimer Res. 2016;13(3):281‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Musardo S, Therin S, Pelucchi S, et al. The development of ADAM10 endocytosis inhibitors for the treatment of Alzheimer's disease. Mol Ther. 2022;30(7):2474‐2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de la Fuente AG, Pelucchi S, Mertens J, di Luca M, Mauceri D, Marcello E. Novel therapeutic approaches to target neurodegeneration. Br J Pharmacol. 2023;180(13):1651‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vijayan D, Chandra R. Amyloid Beta hypothesis in Alzheimer's disease: major culprits and recent therapeutic strategies. Curr Drug Targets. 2020;21(2):148‐166. [DOI] [PubMed] [Google Scholar]
- 58. Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84(3):608‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen G‐f, Xu TH, Yan Y, et al. Amyloid beta: structure, biology and structure‐based therapeutic development. Acta Pharmacol Sin. 2017;38(9):1205‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Z, Wang X, Zhang D, Liu Y, Li L. Geniposide‐mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer's disease. Aging (Albany NY). 2019;11(2):536‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cai Z, Chen G, He W, Xiao M, Yan LJ. Activation of mTOR: a culprit of Alzheimer's disease? Neuropsychiatr Dis Treat. 2015;11:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lafay‐Chebassier C, Paccalin M, Page G, et al. mTOR/p70S6k signalling alteration by Aβ exposure as well as in APP‐PS1 transgenic models and in patients with Alzheimer's disease. J Neurochem. 2005;94(1):215‐225. [DOI] [PubMed] [Google Scholar]
- 63. Caccamo A, Maldonado MA, Majumder S, et al. Naturally secreted amyloid‐beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40‐mediated mechanism. J Biol Chem. 2011;286(11):8924‐8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Velazquez R, Shaw DM, Caccamo A, Oddo S. Pim1 inhibition as a novel therapeutic strategy for Alzheimer's disease. Mol Neurodegener. 2016;11(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou XW, Tanila H, Pei JJ. Parallel increase in p70 kinase activation and tau phosphorylation (S262) with Abeta overproduction. FEBS Lett. 2008;582(2):159‐164. [DOI] [PubMed] [Google Scholar]
- 67. Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid‐beta, and tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107‐13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tramutola A, Triplett JC, di Domenico F, et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre‐clinical AD, amnestic mild cognitive impairment and late‐stage AD. J Neurochem. 2015;133(5):739‐749. [DOI] [PubMed] [Google Scholar]
- 69. Ma T, Hoeffer CA, Capetillo‐Zarate E, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9):e12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid‐β levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cassano T, Magini A, Giovagnoli S, et al. Early intrathecal infusion of everolimus restores cognitive function and mood in a murine model of Alzheimer's disease. Exp Neurol. 2019;311:88‐105. [DOI] [PubMed] [Google Scholar]
- 73. Chang H‐Y, Sang T‐K, Chiang A‐S. Untangling the Tauopathy for Alzheimer's disease and parkinsonism. J Biomed Sci. 2018;25(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lafay‐Chebassier C, Paccalin M, Page G, et al. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP‐PS1 transgenic models and in patients with Alzheimer's disease. J Neurochem. 2005;94(1):215‐225. [DOI] [PubMed] [Google Scholar]
- 75. Tramutola A, Lanzillotta C, Di Domenico F. Targeting mTOR to reduce Alzheimer‐related cognitive decline: from current hits to future therapies. Expert Rev Neurother. 2017;17(1):33‐45. [DOI] [PubMed] [Google Scholar]
- 76. Mueed Z, Tandon P, Maurya SK, Deval R, Kamal MA, Poddar NK. Tau and mTOR: the hotspots for multifarious diseases in Alzheimer's development. Front Neurosci. 2019;12:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Querfurth H, Lee H‐K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tang Z, Ioja E, Bereczki E, et al. mTor mediates tau localization and secretion: implication for Alzheimer's disease. Biochim Biophys Acta. 2015;1853(7):1646‐1657. [DOI] [PubMed] [Google Scholar]
- 79. Silva MC, Nandi GA, Tentarelli S, et al. Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun. 2020;11(1):3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Ma Q, Ma X, Zhang Z, Liu N, Wang M. Role of mammalian target of rapamycin signaling in autophagy and the neurodegenerative process using a senescence accelerated mouse‐prone 8 model. Exp Ther Med. 2017;14(2):1051‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chun H, Marriott I, Lee CJ, Cho H. Elucidating the interactive roles of glia in Alzheimer's disease using established and newly developed experimental models. Front Neurol. 2018;9:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Morimoto K, Horio J, Satoh H, et al. Expression profiles of cytokines in the brains of Alzheimer's disease (AD) patients compared to the brains of non‐demented patients with and without increasing AD pathology. J Alzheimers Dis. 2011;25(1):59‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Siman R, Cocca R, Dong Y. The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early‐stage Alzheimer‐type Tauopathy. PLoS One. 2015;10(11):e0142340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kuhla A, Brichmann E, Rühlmann C, Thiele R, Meuth L, Vollmar B. Metformin therapy aggravates neurodegenerative processes in ApoE−/− mice. J Alzheimers Dis. 2019;68(4):1415‐1427. [DOI] [PubMed] [Google Scholar]
- 86. Millington C, Sonego S, Karunaweera N, et al. Chronic neuroinflammation in Alzheimer's disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int. 2014;2014:309129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cai Z, Yan L‐J. Rapamycin, autophagy, and Alzheimer's disease. J Biochem Pharmacol Res. 2013;1(2):84‐90. [PMC free article] [PubMed] [Google Scholar]
- 88. Chen WQ, Zhong L, Zhang L, et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol. 2009;156(6):941‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen H‐C, Fong TH, Hsu PW, Chiu WT. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti‐inflammation, and neuroprotection. J Surg Res. 2013;179(1):e203‐e210. [DOI] [PubMed] [Google Scholar]
- 90. Majumder S, Caccamo A, Medina DX, et al. Lifelong rapamycin administration ameliorates age‐dependent cognitive deficits by reducing IL‐1β and enhancing NMDA signaling. Aging Cell. 2012;11(2):326‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mengke NS, Hu B, Han QP, et al. Rapamycin inhibits lipopolysaccharide‐induced neuroinflammation in vitro and in vivo. Mol Med Rep. 2016;14(6):4957‐4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang X, Li GJ, Hu HX, et al. Cerebral mTOR signal and pro‐inflammatory cytokines in Alzheimer's disease rats. Transl Neurosci. 2016;7(1):151‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reggiani AM, Simoni E, Caporaso R, et al. In vivo characterization of ARN14140, a Memantine/Galantamine‐based multi‐target compound for Alzheimer's disease. Sci Rep. 2016;6:33172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schmukler E, Pinkas‐Kramarski R. Autophagy induction in the treatment of Alzheimer's disease. Drug Dev Res. 2020;81(2):184‐193. [DOI] [PubMed] [Google Scholar]
- 95. Kuang H, Tan CY, Tian HZ, et al. Exploring the bi‐directional relationship between autophagy and Alzheimer's disease. CNS Neurosci Ther. 2020;26(2):155‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen Y, Xu S, Wang N, et al. Dynasore suppresses mTORC1 activity and induces autophagy to regulate the clearance of protein aggregates in neurodegenerative diseases. Neurotox Res. 2019;36(1):108‐116. [DOI] [PubMed] [Google Scholar]
- 97. Reddy PH, Oliver DM. Amyloid beta and phosphorylated tau‐induced defective autophagy and mitophagy in Alzheimer's disease. Cell. 2019;8(5):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ntsapi C, Lumkwana D, Swart C, du Toit A, Loos B. New insights into autophagy dysfunction related to amyloid beta toxicity and neuropathology in Alzheimer's disease. Int Rev Cell Mol Biol. 2018;336:321‐361. [DOI] [PubMed] [Google Scholar]
- 99. Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol. 2010;2(12):a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2011;8(2):108‐117. [DOI] [PubMed] [Google Scholar]
- 101. Zhao H, Wang ZC, Wang KF, Chen XY. Aβ peptide secretion is reduced by radix polygalae‐induced autophagy via activation of the AMPK/mTOR pathway. Mol Med Rep. 2015;12(2):2771‐2776. [DOI] [PubMed] [Google Scholar]
- 102. Hosokawa N, Hara T, Kaizuka T, et al. Nutrient‐dependent mTORC1 association with the ULK1‐Atg13‐FIP200 complex required for autophagy. Mol Biol Cell. 2009;20(7):1981‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Koren I, Reem E, Kimchi A. Autophagy gets a brake: DAP1, a novel mTOR substrate, is activated to suppress the autophagic process. Autophagy. 2010;6(8):1179‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8(6):903‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Roczniak‐Ferguson A, Petit CS, Froehlich F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5(228):ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Settembre C, Zoncu R, Medina DL, et al. A lysosome‐to‐nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31(5):1095‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wei W, Wang ZY, Ma LN, Zhang TT, Cao Y, Li H. MicroRNAs in Alzheimer's disease: function and potential applications as diagnostic biomarkers. Front Mol Neurosci. 2020;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yoon S, Kim SE, Ko Y, et al. Differential expression of microRNAs in Alzheimer's disease: a systematic review and meta‐analysis. Mol Psychiatry. 2022;27(5):2405‐2413. [DOI] [PubMed] [Google Scholar]
- 110. Liu Y, Zhang Y, Liu P, et al. MicroRNA‐128 knockout inhibits the development of Alzheimer's disease by targeting PPARγ in mouse models. Eur J Pharmacol. 2019;843:134‐144. [DOI] [PubMed] [Google Scholar]
- 111. Zhang M, Han W, Xu Y, Li D, Xue Q. Serum miR‐128 serves as a potential diagnostic biomarker for Alzheimer's disease. Neuropsychiatr Dis Treat. 2021;17:269‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li S, Poon CH, Zhang Z, et al. MicroRNA‐128 suppresses tau phosphorylation and reduces amyloid‐beta accumulation by inhibiting the expression of GSK3β, APPBP2, and mTOR in Alzheimer's disease. CNS Neurosci Ther. 2023;29(7):1848‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926‐1945. [DOI] [PubMed] [Google Scholar]
- 114. Ding L, Nan WH, Zhu XB, et al. Rapamycin and FK506 derivative TH2849 could ameliorate neurodegenerative diseases through autophagy with low immunosuppressive effect. CNS Neurosci Ther. 2019;25(4):452‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zheng G, Wang L, Li X, Niu X, Xu G, Lv P. Rapamycin alleviates cognitive impairment in murine vascular dementia: the enhancement of mitophagy by PI3K/AKT/mTOR axis. Tissue Cell. 2021;69:101481. [DOI] [PubMed] [Google Scholar]
- 116. Wang X, Jia J. Magnolol improves Alzheimer's disease‐like pathologies and cognitive decline by promoting autophagy through activation of the AMPK/mTOR/ULK1 pathway. Biomed Pharmacother. 2023;161:114473. [DOI] [PubMed] [Google Scholar]
- 117. Li HJ, Wang TZ, Hou C, et al. Artemether attenuates Aβ25‐35‐induced cognitive impairments by downregulating Aβ, BACE1, mTOR and tau proteins. Clin Lab. 2021;67(10). [DOI] [PubMed] [Google Scholar]
- 118. Favaloro B, Allocati N, Graziano V, di Ilio C, de Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012;4(5):330‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rohn TT. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities. Apoptosis. 2010;15(11):1403‐1409. [DOI] [PubMed] [Google Scholar]
- 120. Gervais F, Xu D, Robertson GS, et al. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid‐β precursor protein and amyloidogenic Aβ peptide formation. Cell. 1999;97:395‐406. [DOI] [PubMed] [Google Scholar]
- 121. Shang Y, Chong ZZ, Wang S, Maiese K. Prevention of β‐amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3‐K/mTOR pathway, bad, and Bcl‐xL. Aging. 2012;4:187‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Quntanilla RA, Tapia‐Monsalves C. The role of mitochondrial impairment in Alzheimer's disease neurodegeneration: the tau connection. Curr Neuropharmacol. 2020;18(11):1076‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rohn T, Vyas V, Hernandez‐Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer's disease after overexpression of the anti‐apoptotic protein Bcl‐2. J Neurosci. 2008;28:3051‐3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Paquet C, Nicoll JAR, Love S, et al. Downregulated apoptosis and autophagy after anti‐Aβ immunotherapy in Alzheimer's disease. Brain Pathol. 2018;28(5):603‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Farlow MR, Thompson RE, Wei LJ, et al. A randomized, double‐blind, placebo‐controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer's disease. J Alzheimers Dis. 2019;67(2):555‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rius‐Pérez S, Tormos AM, Pérez S, Taléns‐Visconti R. Vascular pathology: cause or effect in Alzheimer disease? Neurología (Engl Ed). 2018;33(2):112‐120. [DOI] [PubMed] [Google Scholar]
- 127. Govindpani K, McNamara LG, Smith NR, et al. Vascular dysfunction in Alzheimer's disease: a prelude to the pathological process or a consequence of it? J Clin Med. 2019;8(5):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease‐lessons from pathology. BMC Med. 2014;12(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Galvan V, Hart MJ. Vascular mTOR‐dependent mechanisms linking the control of aging to Alzheimer's disease. Biochim Biophys Acta. 2016;1862(5):992‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Uddin MS, Rahman MA, Kabir MT, et al. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of Alzheimer's disease. IUBMB Life. 2020;72(9):1843‐1855. [DOI] [PubMed] [Google Scholar]
- 131. Lin A‐L, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J Cereb Blood Flow Metab. 2013;33(9):1412‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. van Skike C, Jahrling JB, Olson AB, et al. Inhibition of mTOR protects the blood–brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693‐H703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lin A‐L, Jahrling JB, Zhang W, et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre‐symptomatic Alzheimer's disease. J Cereb Blood Flow Metab. 2017;37(1):217‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jahrling JB, Lin AL, DeRosa N, et al. mTOR drives cerebral blood flow and memory deficits in LDLR−/− mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab. 2018;38(1):58‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang C‐Y, Kim HH, Hiroi Y, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2(62):ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community‐based cohort study. Lancet Neurol. 2018;17(5):434‐444. [DOI] [PubMed] [Google Scholar]
- 137. Robb WH, Khan OA, Ahmed HA, et al. Lower cerebral oxygen utilization is associated with Alzheimer's disease‐related neurodegeneration and poorer cognitive performance among apolipoprotein E ε4 carriers. J Cereb Blood Flow Metab. 2022;42(4):642‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Okonkwo OC, Xu G, Oh JM, et al. Cerebral blood flow is diminished in asymptomatic middle‐aged adults with maternal history of Alzheimer's disease. Cereb Cortex. 2014;24(4):978‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Michels L, Warnock G, Buck A, et al. Arterial spin labeling imaging reveals widespread and Aβ‐independent reductions in cerebral blood flow in elderly apolipoprotein epsilon‐4 carriers. J Cereb Blood Flow Metab. 2016;36(3):581‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang R, Oh JM, Motovylyak A, et al. Impact of sex and APOE ε4 on age‐related cerebral perfusion trajectories in cognitively asymptomatic middle‐aged and older adults: a longitudinal study. J Cereb Blood Flow Metab. 2021;41(11):3016‐3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. Soluble amyloid‐b oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front Cell Neurosci. 2015;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin‐sensitive signaling pathway contributes to long‐term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99(1):467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid‐beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang H, Fu J, Xu X, Yang Z, Zhang T. Rapamycin activates mitophagy and alleviates cognitive and synaptic plasticity deficits in a mouse model of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2021;76:1707‐1713. [DOI] [PubMed] [Google Scholar]
- 145. Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed). 2012;4:941‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sui L, Wang J, Li BM. Role of the phosphoinositide 3‐kinase‐Akt‐mammalian target of the rapamycin signaling pathway in long‐term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15(10):762‐776. [DOI] [PubMed] [Google Scholar]
- 147. Deli A, Schipany K, Rosner M, et al. Blocking mTORC1 activity by rapamycin leads to impairment of spatial memory retrieval but not acquisition in C57BL/6J mice. Behav Brain Res. 2012;229(2):320‐324. [DOI] [PubMed] [Google Scholar]
- 148. Myskiw JC, Rossato JI, Bevilaqua LRM, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89(3):338‐351. [DOI] [PubMed] [Google Scholar]
- 149. Jobim PF, Pedroso TR, Christoff RR, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012;97(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 150. Huang C, Wen C, Yang M, et al. Astaxanthin improved the cognitive deficits in APP/PS1 transgenic mice via selective activation of mTOR. J Neuroimmune Pharmacol. 2020;16:609‐619. [DOI] [PubMed] [Google Scholar]
- 151. Xu J, Huai Y, Meng N, et al. L‐3‐n‐butylphthalide activates Akt/mTOR signaling, inhibits neuronal apoptosis and autophagy and improves cognitive impairment in mice with repeated cerebral ischemia‐reperfusion injury. Neurochem Res. 2017;42(10):2968‐2981. [DOI] [PubMed] [Google Scholar]
- 152. Tian A, Ma X, Li H, Zhang R. Dl‐3n‐butylphthalide improves spatial learning and memory in rats with vascular dementia by reducing autophagy via regulation of the mTOR signaling pathway. Exp Ther Med. 2020;19(3):1940‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tramutola A, Lanzillotta C, Barone E, et al. Intranasal rapamycin ameliorates Alzheimer‐like cognitive decline in a mouse model of down syndrome. Transl Neurodegener. 2018;7(1):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ozcelik S, Fraser G, Castets P, et al. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One. 2013;8(5):e62459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Van Skike CE, Hussong SA, Hernandez SF, Banh AQ, DeRosa N, Galvan V. mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer's disease. J Neurosci. 2021;41(19):4305‐4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Switon K, Kotulska K, Janusz‐Kaminska A, Zmorzynska J, Jaworski J. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112‐153. [DOI] [PubMed] [Google Scholar]
- 157. Vartak RS, Rodin A, Oddo S. Differential activation of the mTOR/autophagy pathway predicts cognitive performance in APP/PS1 mice. Neurobiol Aging. 2019;83:105‐113. [DOI] [PubMed] [Google Scholar]
- 158. Shi Q, Chang C, Saliba A, Bhat MA. Microglial mTOR activation upregulates Trem2 and enhances β‐amyloid plaque clearance in the 5XFAD Alzheimer's disease model. J Neurosci. 2022;42(27):5294‐5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bensalem J, Hein LK, Hassiotis S, et al. Modifying dietary protein impacts mTOR signaling and brain deposition of amyloid β in a knock‐in mouse model of Alzheimer disease. J Nutr. 2023;153:1407‐1419. [DOI] [PubMed] [Google Scholar]
- 160. Zhang W, Wang J, Yang C. Celastrol, a TFEB (transcription factor EB) agonist, is a promising drug candidate for Alzheimer disease. Autophagy. 2022;18(7):1740‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sun L, Yan Y, Lv H, et al. Rapamycin targets STAT3 and impacts c‐Myc to suppress tumor growth. Cell Chem Biol. 2022;29(3):373‐385.e6. [DOI] [PubMed] [Google Scholar]
- 162. Juricic P, Lu YX, Leech T, et al. Long‐lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat Aging. 2022;2(9):824‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Carosi JM, Sargeant TJ. Rapamycin and Alzheimer disease: a double‐edged sword? Autophagy. 2019;15(8):1460‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


