Abstract
Several members of the seven-transmembrane chemokine receptor family have been shown to serve, with CD4, as coreceptors for entry by human immunodeficiency virus type 1 (HIV-1). While coreceptor usage by HIV-1 primary isolates has been studied by several groups, there is only limited information available concerning coreceptor usage by primary HIV-2 isolates. In this study, we have analyzed coreceptor usage of 15 primary HIV-2 isolates, using lymphocytes from a donor with nonfunctional CCR5 (CCR5 −/−; homozygous 32-bp deletion). Based on the infections of PBMCs, seven of these primary isolates had an absolute requirement for CCR5 expression, whereas the remaining eight exhibited a broader coreceptor usage. All CCR5-requiring isolates were non-syncytium inducing, whereas isolates utilizing multiple coreceptors were syncytium inducing. Blocking experiments using known ligands for chemokine receptors provided indirect evidence for additional coreceptor utilization by primary HIV-2 isolates. Analysis of GHOST4 cell lines expressing various chemokine receptors (CCR1, CCR2b, CCR3, CCR4, CCR5, CXCR4, BONZO, and BOB) further defined specific coreceptor usage of primary HIV-2 isolates. The receptors used included CXCR4, CCR1-5, and the recently described receptors BONZO and BOB. However, the efficiency at which the coreceptors were utilized varied greatly among the various isolates. Analysis of V3 envelope sequences revealed no specific motif that correlated with coreceptor usage. Our data demonstrate that primary HIV-2 isolates are capable of using a broad range of coreceptors for productive infection in vitro. Additionally, our data suggest that expanded coreceptor usage by HIV-2 may correlate with disease progression.
Human immunodeficiency virus type 2 (HIV-2) infection is mostly confined to West African countries, including Guinea Bissau, Gambia, Senegal, and Ivory Coast (17, 45, 52). While HIV-2-infected individuals have also been identified on other continents, they are generally epidemiologically linked to individuals of West African origin (17, 52). HIV-2 shares several characteristics with HIV-1, including similar genome structure, replication properties, and tropism for CD4-positive cells (32, 45, 46, 55, 64, 65). Despite these similarities, there is evidence that HIV-1 and HIV-2 differ in their natural courses of infection. Most HIV-2-infected individuals exhibit longer clinical latency periods and progress more slowly toward AIDS (17, 37, 46, 49). Likewise, both vertical and horizontal transmission rates are significantly lower for HIV-2 than for HIV-1 (4, 23, 37). The factors responsible for these differences remain to be determined and may, in part, be linked to differences in target cell tropism between the two virus types.
Both HIV-1 and HIV-2 infect cells by a membrane fusion process that requires the interaction of the external envelope glycoprotein with the cellular receptor CD4 (44, 47, 57). In addition, certain chemokine receptor molecules have recently been identified as important coreceptors for HIV-1 entry. The seven-transmembrane (7TM) G-protein-coupled chemokine receptor CCR5 serves as a coreceptor for the non-syncytium-inducing (NSI), macrophage-tropic HIV-1 strains (10, 13, 20, 25, 29, 63, 66, 68). Further, dualtropic HIV-1 isolates as well as primary HIV-1 isolates with syncytium-inducing (SI) phenotypes can use both CXCR4 and CCR5 for entry (22, 24, 40, 60, 68). Additional members of the chemokine receptor family, such as CCR3 and CCR2b, can be utilized by some HIV-1 isolates (5, 13, 22, 24). The natural ligands for these α- and β-chemokine receptors block entry of HIV-1 into susceptible target cells (2, 6, 14, 48).
Previous studies have shown that lab-adapted HIV-2 isolates can use CXCR4 to efficiently enter CD4-negative cells (27, 50, 53). More recent studies have demonstrated that chemokine receptors can also be used for cell entry by HIV-2 primary isolates (33, 61). In addition, it was shown that simian immunodeficiency virus (SIV) isolates SIVMAC, SIVSM, and SIVCPZ can use CCR5 but not CXCR4 as a coreceptor (9, 26). However, SIVMAC and SIVSM isolates were shown to infect the CCR5-negative cell line CEMx174, suggesting utilization of an additional coreceptor (9, 39). This led to the identification of two new members of the 7TM family, BONZO/STRL33 and BOB/GPR15, which were shown by an envelope pseudotyping assay to serve as coreceptors for viral entry of HIV-1, HIV-2, SIVMAC, and SIVAGM (3, 21, 28, 42). Furthermore, both BOB and BONZO are expressed in lymphoid tissues and therefore may play an important role in HIV pathogenesis (21, 42).
In this study, we have used peripheral blood mononuclear cells (PBMCs) from a donor with nonfunctional CCR5 (homozygous 32-bp deletion) and GHOST4 cell lines which express eight different 7TM receptor genes to examine coreceptor usage by primary HIV-2 isolates. Our data demonstrate that like HIV-1 isolates, primary HIV-2 isolates use CCR5 and CXCR4 as coreceptors. However, we also show that primary HIV-2 isolates are capable of using a wide variety of additional receptors, including CXCR4, CCR4, CCR3, CCR2b, CCR1, and the recently described receptors BONZO and BOB.
MATERIALS AND METHODS
HIV-2 primary isolates.
Fifteen primary HIV-2 isolates from various West African countries including Ivory Coast, Senegal, and Guinea-Bissau were used to study coreceptor usage. The demographic characteristics of the patients from whom the isolates were generated are shown in Table 1. All virus isolates were established by cocultivation of patient PBMCs with phytohemagglutinin (PHA)-stimulated uninfected donor PBMCs (30). All viral stocks were expanded in PHA-stimulated PBMCs for 7 to 10 days, filtered through 0.22-μm-pore-size filters, tested for reverse transcriptase (RT) activity, aliquoted, and stored at −70°C. (Most viral stocks have been submitted to the NIH AIDS Research and Reference Reagent Program, Rockville, Md.) On the day viral stocks were harvested, cells were also collected for PCR analysis. Cell culture-adapted T-cell-tropic (LAI) and macrophage-tropic (BAL) laboratory isolates of HIV-1 were used as controls.
TABLE 1.
CCR5 coreceptor usage of primary HIV-2 isolates
| Isolate | Country | Clinical statusa | Subtype (env) | Infectibility of PBMCsb
|
MT-2 syncytia (day 10)c | |
|---|---|---|---|---|---|---|
| CCR5 +/+ | CCR5 −/− | |||||
| A1958 | Senegal | AIDS | A | >784 | 0 | — |
| A2267 | Senegal | Asymptomatic | A | 522 | 0 | — |
| SLRHC | Guinea-Bissau | Asymptomatic | Ad | 801 | 0 | — |
| A2270 | Senegal | Asymptomatic | A | 481 | 0 | — |
| 310072 | Ivory Coast | Blood donor | B | 250 | 0 | — |
| 310340 | Ivory Coast | Blood donor | NDe | >784 | 0 | — |
| 60415K | Senegal | Asymptomatic | Ad | >784 | 0 | — |
| 7312A | Ivory Coast | Lymphadenopathy | A/Bf | 503 | 79 | + |
| GB87 | Guinea-Bissau | Hospitalized TB patient | A | 100 | 250 | + |
| 310248 | Ivory Coast | Blood donor | A | 270 | 55 | + |
| 310342 | Ivory Coast | Blood donor | ND | >784 | 513 | + |
| 310319 | Ivory Coast | Blood donor | B | 383 | 154 | ++ |
| 7924A | Guinea-Bissau | AIDS | Ad | 99 | 96 | ++ |
| 77618 | Ivory Coast | AIDS | A | 193 | 100 | ++ |
| GB122 | Guinea-Bissau | AIDS | A | 110 | 174 | +++ |
For blood donors, actual clinical status is unknown. TB, tuberculosis.
Results are based on two independent experiments, and the values given are nanograms of p27 antigen per milliliter from day 14 of one representative experiment.
—, no visible syncytia; +, ++, and +++, increasing numbers of syncytia relative to cultures with + designation.
Subtype was determined based on sequence analysis of two different genome regions (i.e., gag [31]) or the primer binding site (data not shown).
ND, not determined.
A/B recombinant (based on full-length genome).
Determination of viral phenotype.
Infection of the MT-2 cell line was performed to determine the viral phenotype. MT-2 cells were infected with virus stocks equivalent to 20,000 RT counts per 106 cells. The cultures were observed on days 1, 3, 7, and 10 postinfection for the presence of syncytia.
HIV-2 infection of PBMCs.
PBMCs were isolated from healthy donors by leukopheresis and the standard Ficoll-Paque (Pharmacia, Inc., Piscataway, N.J.) density gradient centrifugation. The CCR5 genotype was determined by a previously described PCR-based assay (43). Prior to infection, PBMCs were depleted of CD8+ T cells by incubation with magnetic beads coated with anti-CD8 antibody (Dynabeads; Dynal, Lake Success, N.Y.) as instructed by the manufacturer. The CD4-enriched PBMCs were stimulated with PHA (0.1%) for 2 to 3 days. The cells were then plated in 24-well plates at 2 × 106 cells/well in a total volume of 2 ml of RPMI 1640 supplemented with 10% fetal calf serum and 10% interleukin-2 (C-RPMI). The PHA-stimulated CD4+ cells were infected with the various HIV-2 isolates by using 20,000 RT cpm per 106 cells. Half of the culture supernatant was collected twice a week and replaced with C-RPMI. Levels of p27 antigen in the culture supernatants were determined by using immunoassay kits (Coulter Immunology, Hialeah, Fla.). HIV-associated RT activity was measured by a standard procedure (62). Two independent infection experiments were performed.
When indicated, cells were cultured in the absence or presence of stromal cell-derived growth factor 1 (SDF-1; 1 μg/ml; ligand for CXCR4) (chemically synthesized peptide) or a cocktail of the chemokines RANTES (200 ng/ml), eotaxin (100 ng/ml), and MCP-3 (100 ng/ml) (ligands for β-chemokine receptors) (R & D Systems, Minneapolis, Minn.) as blocking reagents. The chemokines were added to the cells prior to the addition of virus and were maintained in the cultures throughout the experiment.
Sequence analysis of the V3 region.
Cell pellets corresponding to virus stocks used for infection were lysed with lysis buffer (50 mM KCl, 10 mM Tris-HCl, 0.01% gelatin, 0.45% Nonidet P-40, 0.45% Tween 20, 0.1 mg of proteinase K per ml [pH 8.3]) at 56°C for 2 h. After the proteinase K was heat inactivated, the V3 to V4 region of the envelope protein (513 bp corresponding to nucleotides 6827 to 7340) was amplified by PCR, using previously described primers (1). The PCR fragment was directly sequenced by using dye terminators with an automated DNA Sequenator. DNA sequences (300 to 350 bp) were aligned by using CLUSTAL (34, 35) with minor manual adjustments, bearing in mind the predicted protein sequence. Pairwise evolutionary distances were estimated by using Kimura’s two-parameter method (38) to correct for superimposed hits (alignments were gap-stripped). Phylogenetic relationships were computed by the neighbor-joining method (56).
Infection of GHOST4 cells coexpressing various chemokine receptors.
The GHOST4 cells (kindly provided by Dan Littman), which are human osteosarcoma (HOS) cells transfected with the human CD4 gene maintained by G418 selection and further modified by introduction of the various coreceptor genes by infection with the pBABEpuro vector, were used to further elucidate specific coreceptor usage by HIV-2 primary isolates. The GHOST4 cells expressing CXCR4, CCR5, CCR4, CCR3, CCR2b, CCR1, BOB, and BONZO were maintained in Dulbecco modified Eagle medium with 10% fetal calf serum, puromycin (1 μg/ml), hygromycin B (100 μg/ml), and G418 (500 μg/ml). The medium for the parental GHOST4 cells did not contain puromycin. The HOS cells were plated at 4 × 104 cells per well in 24-well plates and infected with 40,000 RT counts of virus stock. All cultures were washed three times with 1.5 ml of PBS following a 6- to 18-h incubation with virus. The cultures were maintained in 2 ml of Dulbecco modified Eagle medium supplemented as described above. Supernatants (1 ml) were collected from the cultures every 3 to 4 days and tested for p27 antigen (Coulter, Hialeah, Fla.).
RESULTS
CCR5 coreceptor usage of primary HIV-2 isolates and comparison with biological phenotype.
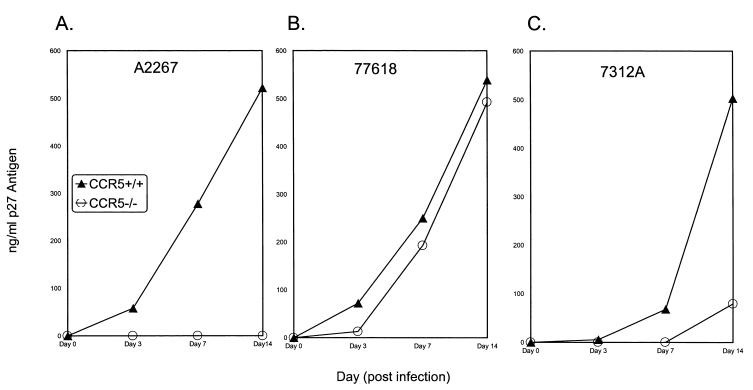
The CCR5 coreceptor usage of HIV-2 primary isolates was examined by infecting lymphocytes from a donor homozygous for 32-bp deletion in the CCR5 gene (CCR5 −/−) and compared with an infection of PBMCs from a wild-type CCR5 (CCR5 +/+) donor. As shown in Table 1, infection of the CCR5 +/+ and CCR5 −/− PBMCs with primary HIV-2 isolates demonstrated that 7 of the 15 primary isolates (A1958, A2267, SLRHC, A2270, 310072, 310340, and 60415K) had an absolute requirement for CCR5, since they did not infect lymphocytes from the CCR5 −/− donor. The remaining eight isolates infected both CCR5 +/+ and CCR5 −/− lymphocytes, demonstrating utilization of a coreceptor other than CCR5. However, the kinetics and quantities of antigens produced varied among the isolates as shown in Fig. 1. A limited analysis of macrophage infection with the primary isolates demonstrated that some of these (e.g., 77618) could also infect macrophages from both CCR5 +/+ and CCR5 −/− donors (data not shown).
FIG. 1.
Infection of CCR5 +/+ and CCR5 −/− normal donor PBMCs by primary HIV-2 isolates. (A) Isolate A2267 was not capable of replicating in CCR5 −/− PBMCs. The data shown are representative of the remaining six isolates (A1958, SLRHC, A2270, 310072, 310340, and 60415K) that require CCR5 for replication. (B) Isolate 77618 showed comparable replication abilities in CCR5 +/+ and CCR −/− donor PBMCs. The data are representative of isolates 7924A, GB122, GB87, and 310319. (C) Isolate 7312A showed delayed kinetics and decreased antigen production in the CCR5 −/− donor PBMCs. This was also observed with isolate 310248.
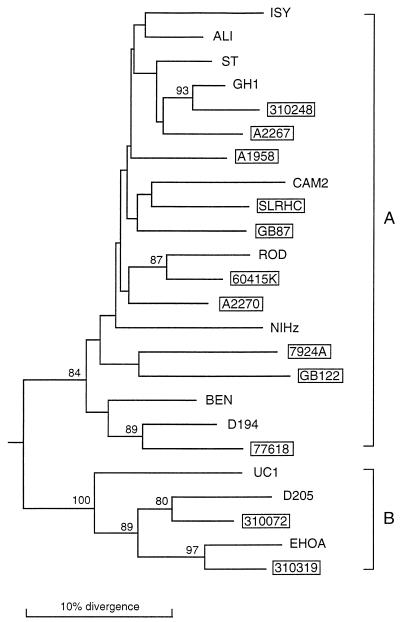
A phylogenetic analysis of PCR-derived envelope sequences allowed the isolates to be classified as either HIV-2 subtype A or B (31, 54) or a recombinant between A and B (Fig. 2). Thus, geographically divergent strains of HIV-2 representing isolates from both subtypes can utilize more than one coreceptor. We next analyzed the relationship between coreceptor usage and viral phenotype, using a standard MT-2 phenotype assay. The seven isolates which had an absolute requirement of CCR5 for viral entry were NSI, whereas all of the isolates that had broader coreceptor usage were SI in the MT-2 cell line (Table 1).
FIG. 2.
Phylogenetic relationships of the newly derived HIV-2 isolates with representatives of HIV-2 subtypes A and B. The tree was constructed from partial env nucleotide sequences in the C2/V3 region (consensus alignment, 300 to 350 bp). Phylogenetic relationships were determined by the neighbor-joining method as described in Materials and Methods. Horizontal branch lengths are drawn to scale, while vertical branches are for clarity only. The numbers on the nodes represent the percentage bootstrap samples with which the cluster to the right is supported; only values over 80% are shown. The tree was rooted by using SIVMAC 251 as an outgroup. Brackets denote HIV-2 subtypes as reported previously (31). Newly derived isolates are in boxes.
Blocking HIV-2 infection with chemokines.
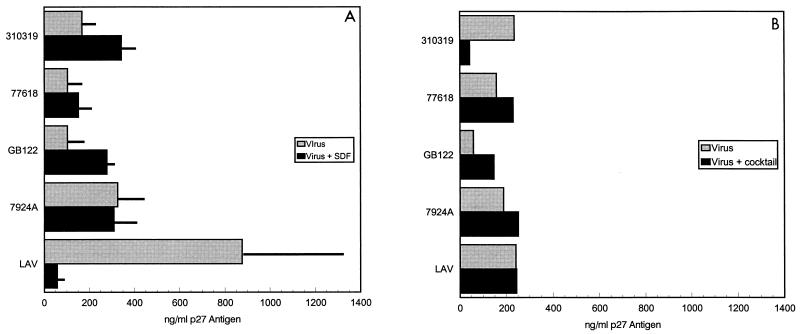
To determine if infection by HIV-2 isolates could be blocked by specific chemokine ligands, we conducted blocking experiments using SDF-1, which should bind to CXCR4, or a cocktail of chemokines (eotaxin, RANTES, and MCP-3) which should bind to the β-chemokine receptors CCR1 to CCR5 (51). To rule out CCR5 utilization in the experiments, the CCR5 −/− lymphocytes were used in the blocking experiments. The HIV-1 isolate LAI, used as a control in these experiments, was inhibited by SDF-1 and unaffected by the addition of the chemokine mixture. Addition of SDF-1 resulted in various degrees of inhibition of isolates 7312A, 310248, 310342, and GB87 ranging from 40 to >90%, suggesting that they use CXCR4 to infect the CCR5 −/− lymphocytes (data not shown). In contrast, four isolates (310319, 77618, 7924A, and GB122) were either unaffected or enhanced by the addition of SDF-1, suggesting that these isolates could use other receptors in addition to CXCR4 and CCR5 (Fig. 3). The addition of the chemokine mixture (eotaxin, RANTES, and MCP-3) resulted in the inhibition of viral replication by the isolate 310319. None of the other broadly tropic strains tested (77618, 7924A, and GB122) could be blocked by these chemokines (Fig. 3), suggesting again that these isolates could utilize additional coreceptors.
FIG. 3.
Chemokine effects on infection with HIV-2 primary isolates. CCR5 −/− donor PBMCs were infected with HIV-2 in the presence or absence of SDF-1 (A) or a cocktail of RANTES, eotaxin, and MCP-3 (B). The values shown are p27 antigen concentrations from day 7 of culture. The values in panel A represent means ± standard errors of the means of two independent experiments.
HIV-2 primary isolates can use various chemokine receptors, including BONZO and BOB.
We next used GHOST4 cells which express a functional CD4 molecule along with various chemokine receptors to further elucidate specific coreceptor usage of these broadly tropic HIV-2 primary isolates. Infection of GHOST4 cells coexpressing various coreceptors demonstrated that all eight isolates were able to utilize CXCR4 in addition to CCR5 (Table 2). Furthermore, some isolates could use CCR1, CCR2b, CCR3, and CCR4, as well as recently identified receptors BONZO and BOB (Table 2). None of these primary isolates were able to infect HOS cells or the parental GHOST4 cell lines (no p27 antigen production through 15 days of culture), suggesting that all isolates required expression of a coreceptor in addition to CD4.
TABLE 2.
Infection of GHOST4 cells expressing chemokine receptors
| Virus | p27 antigen levela
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CCR1 | CCR2b | CCR3 | CCR4 | CCR5 | CXCR4 | BONZO | BOB | |
| 310342 | + | − | − | − | +++ | + | + | − |
| 7312A | − | − | − | + | ++ | + | − | +++ |
| GB87 | + | ++ | − | ++ | +++ | ++ | ++ | +++ |
| 310248 | + | − | − | + | + | + | − | − |
| 310319 | − | +++ | +++ | ++ | + | +++ | +++ | +++ |
| 7924A | − | +++ | +++ | − | + | +++ | + | +++ |
| 77618 | − | +++ | +++ | − | + | +++ | ++ | +++ |
| GB122 | − | +++ | ++ | − | + | + | − | +++ |
From day 15 of culture. +, 50 to 100 pg/ml; ++, 100 to 200 pg/ml; +++, >200 pg/ml; −, no measurable p27 antigen. The parental GHOST4 cell line expressed no p27 antigen with any isolate.
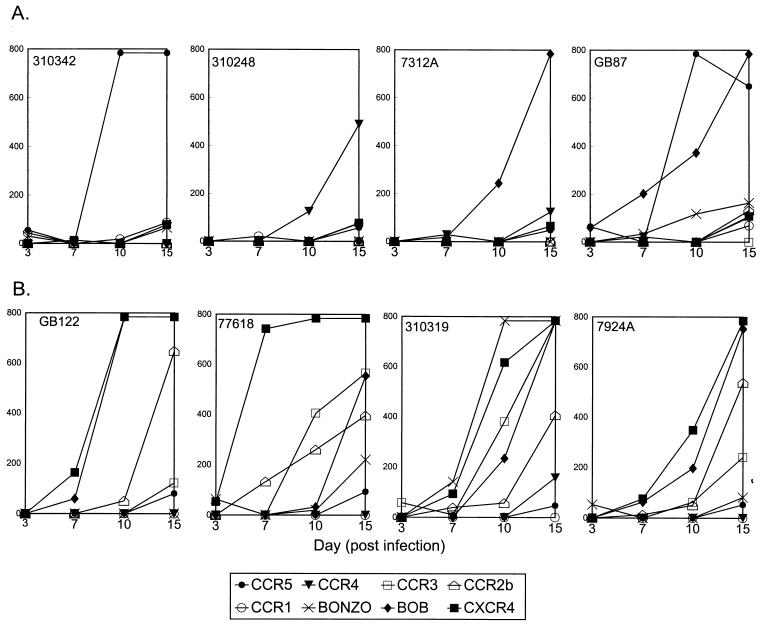
While various coreceptors could be used by primary HIV-2 isolates, the quantity of virus produced (based on p27 antigen production from day 10 of the culture) from the cells expressing different coreceptors varied greatly among the isolates. Infection of various cell lines with equal quantities of virus (based on RT counts) yielded widely varying amounts of p27 antigen production (Fig. 4). For example, 310342 produced more virus in the CCR5-expressing cell line, while 7312A produced more virus from the BOB-expressing cell line; isolate 310248 yielded the largest quantity of virus from the CCR4-expressing line. Isolate GB87 produced comparable levels of virus from both CCR5- and BOB-expressing cells. In contrast, 77618, 7924A, and GB122 produced the greatest quantities of p27 antigen in the GHOST4 cells expressing CXCR4. Isolate 310319 was different from all others, with the highest quantity of virus being produced in the BONZO-expressing line (Fig. 4). However, unlike isolates 310342, 310248, and 7312A, which produced virus well from only one coreceptor-expressing cell line, isolates 77618, 7924A, GB122, and 310319 produced virus from cell lines expressing CXCR4, BOB, and CCR2b and, in the case of 77618, the CCR3-expressing cell line.
FIG. 4.
Kinetics of p27 antigen production by HIV-2 isolates in GHOST4 cells expressing various coreceptors. The values given are p27 antigen (picograms/milliliter) present in the supernatants of cultures on days 3, 7, 10, and 15 from different GHOST4 cell lines infected with the same infectious dose of virus. Values given as 784 pg/ml actually represent antigen values which were above the linear scale of the assay. (A) Isolates which produce large quantities of antigen in only a few cell lines; (B) isolates which produce large quantities of antigen in many cell lines.
Sequence analysis of the envelope V3 region.
Since specific amino acid sequences in the V3 region of the HIV-1 envelope have been shown to correlate with coreceptor usage in HIV-1 (66), we analyzed the V3 region of HIV-2 primary isolates to examine whether we could delineate a consensus motif that might correlate with specific coreceptor usage. In contrast to HIV-1 V3 sequences, there was considerable conservation among the V3 sequences from the HIV-2 isolates (Table 3). No consensus motif could be discerned between isolates that required CCR5 or could utilize other coreceptors. Some isolates which used multiple coreceptors including CXCR4, CCR2b, and BOB efficiently had an additional valine at position 25 of V3 and contained an arginine in place of glutamine at position 23. However, the significance of these amino acid changes remains unclear.
TABLE 3.
Amino acid sequences of the V3 regions of primary HIV-2 isolates
| Isolate | V3 sequencea |
|---|---|
| 1 11 21 31 | |
| A1958 | CRRPGNKTVV PVTLMSGLVF HSQP-INTRP RQAWC |
| A2267 | .K.......L .I........ ....-..... ..... |
| SLRHC | .......... .I......I. ....-..K.. ..... |
| A2270 | .K........ .I........ ....-..... ..... |
| 310072 | .K........ .I.V....I. ....-..... ..... |
| 60415K | .K.......T .I........ ....-..... ..... |
| 7312A | .K........ .I........ ....-..... ..... |
| GB87 | .......... .I..F..... ....-..... ..... |
| 310248 | .......... .I........ ....-..K.. ..... |
| 310319 | .......... .IRTV...L. ...A-..KK. K.... |
| 7924A | .K.......K .......YK. ..R.V..E.. K.... |
| 77618 | .K.......L .I.....QK. ..R.V..KK. K.... |
| GB122 | .K........ .M.....QSY .FR.V..DK. ..... |
., identical amino acid; -, no amino acid.
DISCUSSION
It is now well established that HIV-1 and HIV-2 enter cells by interacting with both CD4 and a coreceptor at the cell surface. The emerging evidence with coreceptor specificities has made it clear that different strains of HIV-1 can use divergent members of the 7TM chemokine receptor families (13, 22, 24, 66). However, less is known about the coreceptor utilization of primary HIV-2 isolates, although some data suggest that lab-adapted isolates of HIV-2 can utilize CXCR4 in a CD4- independent manner (27, 50, 53). Here we provide evidence that divergent strains of primary HIV-2 isolates, representing various geographic distributions and genetically different subtypes, can utilize CCR5 as well as other coreceptors, including all β-chemokine receptors (CCR1 to CCR5), CXCR4, and the recently described receptors BONZO and BOB.
All of our primary isolates which had an absolute requirement of CCR5 for viral entry failed to induce syncytia. This phenomenon is most likely explained by the fact that MT-2 cells do not express CCR5 and thus are not infectable by isolates which require CCR5 for viral entry. In contrast, isolates that could use multiple coreceptors were SI. However, the size and number of syncytia produced varied from isolate to isolate. Thus, we provide evidence that HIV-2 isolates that preferentially use CCR5 are predominantly NSI, whereas those with broader coreceptor specificities are SI. These results are in general agreement with what has been reported for HIV-1 (10, 13, 22, 24, 25, 60, 66, 68) and HIV-2 (33).
The specific coreceptor requirement of primary HIV-2 isolates was further examined by blocking experiments using ligands for α- and β-chemokines receptors. Low levels of inhibition by SDF-1 or a cocktail of β-chemokines suggested multiple coreceptor usage by several isolates, and this was further confirmed by GHOST4 infection experiments. Interestingly, two isolates (GB122 and 310319) that utilized multiple coreceptors had significantly increased antigen production when SDF-1 was added, suggesting a different interaction with the coreceptor. Further studies are ongoing to clarify this observation. The inability of specific ligands to inhibit replication or increase viral production by isolates that are capable of utilizing various receptors demonstrates one of the limitations of using chemokines for therapy of infected individuals.
A wide range of coreceptor utilization by HIV-2 isolates was demonstrated in assays using GHOST4 cells expressing various chemokine coreceptors. The usage of CCR5 was most common for NSI isolates, while the coreceptor utilization of SI isolates was much broader. We provide evidence that primary HIV-2 isolates can use a wide variety of coreceptors, including the β-chemokine receptors (CCR1 to CCR5), CXCR4, and the recently described receptors BONZO and BOB, for productive infection of GHOST4 cells. Additionally, four of the isolates were able to infect the GHOST4 cells which expressed CCR4; this is the first evidence of CCR4 being utilized as a coreceptor that allows productive infection. Recent studies utilizing different primary HIV-2 isolates have provided evidence for usage of CCR5, CXCR4, and CCR3 (33, 61) as well as CCR1 and CCR2b (33). Promiscuous use of the CC and CXC chemokine receptors CCR1, CCR2, CCR3, CCR4, CCR5, CXCR2, and CXCR4 in viral pseudotype assays or cell-to-cell fusion assays has recently been reported for the lab-adapted strain HIV-2ROD (8). Using 15 different HIV-2 isolates, we confirm and extend these studies, showing that in addition to CCR5, CXCR4, CCR1, CCR2b, and CCR3, CCR4 can allow viral entry and replication. More importantly, the newly described coreceptors BONZO and BOB appear to be important coreceptors for primary HIV-2 isolates since five of eight utilized BONZO and six of eight utilized BOB. With the exception of one isolate, 310319, infection of the BOB-expressing cell line results in higher viral production than the BONZO-expressing cells. While it is clear that different isolates produce various amounts of virus from different cell lines, the cause for this has yet to be determined. One potential explanation is that virus strains have different binding affinities for distinct coreceptors and thus are more capable of entering cells expressing certain coreceptors. An additional explanation for the observed data is that a process downstream of viral entry affects the quantity of virus produced. Alternatively, the nonclonal nature of the GHOST4 cells used could also potentially affect virus production due to the possibility of various levels of coreceptor expression.
While the factors responsible for switching to broader coreceptor usage are not known, it is clear that broad coreceptor usage by HIV-1 isolates is correlated with a progressive CD4 loss, followed by rapid progression to AIDS (16). Our data suggest that expanded coreceptor usage also appears to occur in HIV-2 isolates from patients who are in late-stage disease. While we do not have clinical staging data on all of our patients, it is interesting that three of the isolates (77618, GB122, and 7924A) which produced the greatest amounts of syncytium or cell death in MT-2 cells and efficiently utilized a broad range of coreceptors (including BOB and BONZO) were isolated from patients who had been diagnosed with AIDS. In contrast, four of the seven isolates which required CCR5 were isolated from asymptomatic individuals. Only one isolate (A1958) which required CCR5 is known to have originated from an infected patient with AIDS. Taken together, these data suggest that viral adaptation to utilize a broad range of coreceptors correlates with disease progression for both HIV-1 and HIV-2. Recent data from our lab suggest that sequential isolates, derived from HIV-1-infected patients who rapidly progressed to AIDS, not only are able to utilize CC and CXC coreceptors but also utilize BOB toward the end stage of disease (67). While broad coreceptor usage appears to correlate with disease progression, an actual cause-and-effect relationship between coreceptor usage and disease progression for both HIV-1 and HIV-2 has yet to be proven.
Another important finding in our study as well as the other reports on HIV-2 coreceptor usage (33, 61) is the fact that CXCR4 is used by primary HIV-2 isolates. This finding is in direct contrast to the data presented thus far for SIVMAC, SIVSM, and SIVCPZ isolates, which cannot utilize CXCR4 as a coreceptor for entry (9, 26, 39). These results are somewhat surprising, given the genomic and structural homologies between HIV-2 and SIVSM/SIVMAC and the fact that HIV-2 is predicted to be the result of cross-species transmission of SIVSM. Whether such differences could potentially be due to lab adaptation of SIV isolates or to differences in the basic biology of the various viruses needs to be further investigated.
While CD4 binding is mediated essentially by a conserved domain of gp120, the hypervariable V3 loop of HIV-1 gp120 seems to be required for the interaction with CCR5 or CXCR4 (5, 7, 36). As a first step toward defining the genomic regions of HIV-2 involved in coreceptor usage, we analyzed the V3 sequences of 13 primary HIV-2 isolates. Analysis of the V3 sequences from the isolates demonstrated considerable conservation of amino acids in this region, in contrast to the extreme variability observed for HIV-1 isolates (11, 18, 19). No specific motif that would correlate with specific coreceptor usage could be discerned in the V3 region. However, certain amino acid changes, at positions 18, 19, and 23, which resulted in an increase in the charge of the V3 loop, as well as the presence of an additional valine at position 25, were observed in some isolates that used a broad range of coreceptors. Substitution of basic amino acids at positions 18 and 19 of the HIV-2 V3 loop has recently been shown to correlate with the SI viral phenotype (1). Likewise, an increase in charge in the V3 region of HIV-1 gp120 appears to correlate with a switch from exclusive usage of CCR5 as a coreceptor to usage of CXCR4 or multiple coreceptors (41, 58, 59). In addition, we recently identified a motif in the V3 loop of HIV-1 where substitution of basic amino acids resulted in broader coreceptor utilization (66). While the interactions between envelope glycoproteins and membrane coreceptors are conformationally complex (5, 7), recent studies suggest a direct interaction of envelope glycoproteins of HIV-1, HIV-2, and SIVMAC with CCR5 that is markedly increased in the presence of CD4 (36). The relatively conserved nature of the V3 region and the inability to correlate a specific sequence with specific coreceptor usage by our HIV-2 isolates suggest that other regions of the HIV-2 genome may play an important role in coreceptor usage by primary HIV-2 isolates.
Our study clearly shows that like HIV-1 isolates, primary HIV- 2 isolates can utilize various members of the 7TM chemokine family as coreceptors for virus entry. Additionally, this is the first study to demonstrate usage of the chemokine receptors CCR4, BONZO, and BOB by HIV-2 primary isolates for viral entry and replication. Sequence analysis of 350 bp spanning the V3 to V4 region of the genome indicated that multiple coreceptor use is found among isolates of both the A and B subtypes and is not confined to one geographic region. Additional sequence data from different regions of the envelope from these HIV-2 isolates are needed to elucidate the exact viral sequences that are important for HIV-2 coreceptor utilization. Further epidemiologic studies are necessary to confirm the suggested correlation between disease state of an individual and expanded coreceptor usage by HIV-2 and to determine what role coreceptor use plays in HIV-2 pathogenesis.
ACKNOWLEDGMENTS
We thank Dan Littman, Howard Hughes Medical Institute of Rockefeller University, for providing the GHOST4 cell lines and Julie Decker for excellent technical assistance.
This work was supported in part by grants from the NIH (AI37466 and AI25291) to B.H.H.
REFERENCES
- 1.Albert J, Stalhandske P, Marquina S, Karis J, Fouchier R A, Norby E, Chiodi F. Biological phenotype of HIV type 2 isolates correlates with V3 genotype. AIDS Res Hum Retroviruses. 1996;12:821–828. doi: 10.1089/aid.1996.12.821. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, and MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Liao F, Berger E A, Farber J M, Pedan K W C. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 4.Andreasson P A, Dias F, Naucler A, Andersson S, Biberfeld G. A prospective study of vertical transmission of HIV-2 in Bissau, Guinea-Bissau. AIDS. 1993;7:989–993. [PubMed] [Google Scholar]
- 5.Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A): S3–S16. [PubMed]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71:4744–4751. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron R, Klasse P J, Wilkinson D, Clapham P R, Pelchen-Matthews A, Power C, Wells T N C, Kim J, Peiper S C, Hoxie J A, Marsh M. Promiscuous use of the CC and CXC chemokine receptors in cell-to-cell fusion mediated by a human immunodeficiency virus type 2 envelope protein. J Virol. 1997;71:8405–8415. doi: 10.1128/jvi.71.11.8405-8415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesebro B, Wehrly K, Nishio J, Perryman S. Mapping of independent V3 envelope determinants of human immunodeficiency virus type 1 macrophage tropism and syncytium formation in lymphocytes. J Virol. 1996;70:9055–9059. doi: 10.1128/jvi.70.12.9055-9059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico A L, Garzino-Demo L, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Cocchi F, DeVico A L, Garzino-Demo L, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 16.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1- infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Cock K M, Adjorlolo G, Ekpini E, Sibailly T, Kouadio J, Maran M, Brattegaard K, Vetter K M, Doorly R, Gayle H D. Epidemiology and transmission of HIV-2: why there is no HIV-2 pandemic. JAMA. 1993;270:2083–2086. doi: 10.1001/jama.270.17.2083. [DOI] [PubMed] [Google Scholar]
- 18.De Jong J-J, De Ronde A, Keulen W, Tersmette M, Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992;66:6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong J-J, Goudsmit J, Keulen W, Klaver B, Krone W, Tersmette M, de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992;66:757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng D, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Sutton R E, Littman D R, Landau N L. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–665. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 21.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 22.Dittmar M T, McKnight A, Simmons G, Clapham P R, Weiss R A, Simmonds P. HIV-1 tropism and coreceptor use. Nature. 1997;385:495–496. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly C, Leisenring W, Kanki P, Awerbuch T, Sandberg S. Comparison of transmission rates of HIV-1 and HIV-2 in a cohort of prostitutes in Senegal. Bull Math Biol. 1993;55:731–743. doi: 10.1007/BF02460671. [DOI] [PubMed] [Google Scholar]
- 24.Doranz B J, Rucker J, Yi Y, Smith R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptor CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 25.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 26.Edinger A L, Amedee A, Miller K, Doranz B J, Endres M, Sharon M, Samson M, Lu Z-H, Clements J E, Murphy-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage tropic and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 28.Farzan M, Choe H, Martin K, Marcon L, Hofman W, Karlson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 30.Feorino P M, Kalyanaraman V S, Haverkos H W, Cabradilla C D, Warfield D T, Jaffe H W, Harrison A K. Lymphadenopathy associated virus infection of a blood donor-recipient pair with acquired immunodeficiency syndrome. Science. 1984;255:69–72. doi: 10.1126/science.6328663. [DOI] [PubMed] [Google Scholar]
- 31.Gao F, Yue L, Robertson D L, Hill S C, Hui H, Biggar R J, Neequaye A E, Whelan T M, Ho D D, Shaw G M, Sharp P M, Hahn B H. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn B H. Viral genes and their products. In: Broder S, Merigan T, Bolognesi D, editors. Textbook on AIDS medicine. Baltimore, Md: Williams & Wilkins; 1994. pp. 21–43. [Google Scholar]
- 33.Heredia A, Vallejo V, Soriano V, Epstein J S, Hewlett I K. Chemokine receptors and HIV-2. AIDS. 1997;11:1198–1199. doi: 10.1097/00002030-199709000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple alignment on a microcomputer. Comp Appl Biosci. 1992;5:593–604. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 35.Higgins D G, Sharp P M. Clustal: a package for performing multiple sequence alignments on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 36.Hill C M, Deng H, Unutmaz D, KewalRamani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanki P J, Travers K U, M’Boup S, Hsieh C C, Marlink R G, Gueye-N’Diaye A, Siby T, Thior I, Hernandez-Avila M, Sankale J L, N’Doye I, Essex M E. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343:943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 38.Kimura M. The neutral theory of molecular evolution. Cambridge, England: Cambridge University Press; 1983. [Google Scholar]
- 39.Kirchhoff F, Pohlmann S, Hamacher M, Means R E, Kraus T, Uberla K, Di Marizio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuiken C L, de Jong J-J, Baan E, Keulen W, Tersmette M, Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992;66:4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a new novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T-cell-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu R, Oaxton W A, Choe S, Ceradini D, Martin S R, Horuk R, McDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 44.Maddon P J, McDougal J S, Clapham P R, Dalgleish A G, Jamal S, Weiss R A, Axel R. HIV infection does not require endocytosis of its receptor CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 45.Markovitz D M. Infection with the human immunodeficiency virus type 2. Ann Intern Med. 1993;118:211–218. doi: 10.7326/0003-4819-118-3-199302010-00010. [DOI] [PubMed] [Google Scholar]
- 46.Marlink R G, Ricard D, Boup S M, Kanki P J, Romet-Lemonne J L, Doye I N, Diop K, Simpson M A, Greco F, Chou M J, Degruttola V, Hsieh C C, Boye C, Barin F, Denis F, McLane M F, Essex M. Clinical, hematological, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2) AIDS Res Hum Retroviruses. 1988;4:137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- 47.McDougal J S, Kennedy S M, Sligh J M, Cort S P, Mawle A, Nicholson J K A. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986;231:382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- 48.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizer J-L, Arenzana-Seisdedos F, Schwartz O, Heard J, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 49.Pepin J, Morgan G, Dunn D, Gevao S, Mendy M, Gaye I, Scollen N, Tedder R, Whittle H. HIV-2-induced immunosuppression among asymptomatic West African prostitutes: evidence that HIV-2 is pathogenic, but less so than HIV-1. AIDS. 1991;5:1165–1172. [PubMed] [Google Scholar]
- 50.Potempa S, Picard L, Reeves J D, Wilkinson D, Weiss R A, Talbot S J. CD4-independent infection by human immunodeficiency virus type 2 strain ROD/B: the role of the N-terminal domain of CXCR4 in fusion and entry. J Virol. 1997;71:4419–4424. doi: 10.1128/jvi.71.6.4419-4424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Premack B A, Schall T J. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 52.Quinn T C. Population migration and the spread of types 1 and 2 human immunodeficiency viruses. Proc Natl Acad Sci USA. 1994;91:2407–2414. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD-4 independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR3 and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 54.Robertson D L, Hahn B H, Sharp P M. Recombination in AIDS viruses. J Mol Evol. 1995;40:249–259. doi: 10.1007/BF00163230. [DOI] [PubMed] [Google Scholar]
- 55.Romieu I, Marlik R, Kanki P, Boup S M, Essex M. HIV-2 link to AIDS in West Africa. J Acquired Immune Defic Syndr. 1990;3:220–230. [PubMed] [Google Scholar]
- 56.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 57.Sattentau Q J, Clapham P R, Weiss R A, Beverley P C L, Montagnier L, Alhalabi M F, Gluckman J-C, Klatzmann D. The human and simian immunodeficiency viruses HIV-1, HIV-2 and SIV interact with similar epitopes on their cellular receptor, the CD4 molecule. AIDS. 1988;2:101–105. doi: 10.1097/00002030-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M A, Schattenkerk J K M E, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shioda T, Ley J A, Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sol N, Ferchal F, Braun J, Pleskoff O, Treboute C, Ansart I, Alizon M. Usage of the coreceptors CCR-5, CCR3, and CXCR-4 by primary and cell line-adapted human immunodeficiency virus type 2. J Virol. 1997;71:8237–8244. doi: 10.1128/jvi.71.11.8237-8244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Telesnitsky A, Blain S, Goff S P. Assays for retroviral reverse transcriptase. Methods Enzymol. 1995;262:347–351. doi: 10.1016/0076-6879(95)62029-x. [DOI] [PubMed] [Google Scholar]
- 63.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–188. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 64.Valentin A, Albert J, Fenyo M, Asjo M. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss R A. Cellular receptors and viral glycoproteins involved in retrovirus entry. In: Levy J A, editor. The Retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 1–108. [Google Scholar]
- 66.Xiao L, Owen S M, Goldman I, Lal A A, deJong J J, Goudsmit J, Lal R B. CCR5-coreceptor usage of non-syncytium-inducing primary HIV-1 is independent of phylogenetically distinct global HIV-1 isolates: delineation of consensus motif in the V3 domain that predicts CCR-5 usage. Virology. 1998;240:83–92. doi: 10.1006/viro.1997.8924. [DOI] [PubMed] [Google Scholar]
- 67.Xiao, L., D. L. Rudolph, S. M. Owen, T. J. Spira, and R. B. Lal. Adaptation to promiscuous usage of CC and CXC chemokine coreceptors in vivo correlates with HIV-1 disease progression. Submitted for publication. [DOI] [PubMed]
- 68.Zhang L, Huang Y, He T, Cao Y, Ho D D. HIV-1 subtype and second receptor use. Nature. 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]