Summery
Background
Immune checkpoint inhibitors are the standard of care for first-line treatment of metastatic renal cell carcinoma, yet optimized treatment of patients whose disease progresses after these therapies is unknown. The aim of this study was to determine if adding atezolizumab to cabozantinib delayed disease progression and prolonged survival in patients with disease progression on or after prior immune checkpoint inhibitor treatment.
Methods
In this phase III, randomized, open-label trial, patients with locally advanced or metastatic renal cell carcinoma whose disease progressed with immune checkpoint inhibitors were randomly assigned 1:1 to atezolizumab (1200 mg intravenously every 3 weeks) plus cabozantinib (60 mg orally once daily) or cabozantinib alone. Multiple primary efficacy end points were progression-free survival per blinded independent central review and overall survival. Secondary end points included safety. The trial is registered with ClinicalTrials.gov, NCT04338269, and is closed to further accrual.
Findings
From July 28, 2020, to December 27, 2021, 522 patients in 15 countries were assigned to receive atezolizumab-cabozantinib (263 patients) or cabozantinib (259 patients). At data cutoff (January 3, 2023), median follow-up was 15·2 months (interquartile range, 10.7 to 19.3). Median progression-free survival was 10·6 months (95% confidence interval [CI], 9·8 to 12·3) with atezolizumab-cabozantinib and 10·8 months (95% CI, 10·0 to 12·5) with cabozantinib (hazard ratio for disease progression or death, 1·03; 95% CI, 0·83 to 1·28; P=0·78). The hazard ratio for death with atezolizumab-cabozantinib compared with cabozantinib was 0·94 (95% CI, 0·70 to 1·27; P=0·69). Serious adverse events occurred in 48·1% of 262 patients in the atezolizumab-cabozantinib group and 32·8% of 256 patients in the cabozantinib group; adverse events leading to death occurred in 6·5% and 3·5%, respectively.
Interpretation
The addition of atezolizumab to cabozantinib did not improve clinical outcomes and led to increased toxicity. These results should discourage sequential use of immune checkpoint inhibitors in renal cell carcinoma outside of clinical trials.
Keywords: renal cell carcinoma, cabozantinib, atezolizumab, metastases, PD-L1 inhibitor
Introduction
Checkpoint inhibitors now represent a cornerstone of cancer therapy. Inhibitors of programmed death-ligand 1 (PD-L1) and its cognate receptor programmed death-1 (PD-1) have garnered approvals across multiple cancer types as monotherapy or combination therapy, however, there are slight permutations in the way checkpoint inhibitors are applied across distinct tumour types. In advanced melanoma, PD-1 inhibitors may be used alone or with cytotoxic T-lymphocyte associated protein 4 (CTLA4) or lymphocyte activation gene-3 (LAG3) inhibitors.1–4 In non-small cell lung cancer, patients lacking actionable mutations may be offered the combination of cytotoxic chemotherapy with PD-L1 or PD-1 (collectively, PD-(L)1) inhibitors based on PD-L1 expression.5–7 In advanced renal cell carcinoma, like melanoma, the combination of CTLA4 and PD-1 inhibition is an established standard in the first line setting.8 However, unique to this disease are multiple phase III studies have compared combinations of vascular endothelial growth factor (VEGF) receptor–targeting agents with PD-(L)1 inhibitors against a control arm of sunitinib, a VEGF-tyrosine kinase inhibitor (VEGF-TKI). Studies separately evaluating combinations of the VEGF-TKIs cabozantinib, lenvatinib, and axitinib or the CTLA4 inhibitor ipilimumab with PD-1 inhibitors as first-line treatment have demonstrated improvements in both progression-free and overall survival relative to sunitinib.8–11
Across many malignancies, the introduction of checkpoint inhibitors in the frontline setting has led to questions around optimal second-line therapy. In advanced renal cell carcinoma, prospective evidence supports VEGF-TKI use. Most recently, the VEGF-TKI cabozantinib, which also inhibits the TAM family of kinases, has demonstrated potent activity in patients previously treated with contemporary checkpoint inhibitor–based regimens while maintaining a manageable safety profile.12,13 Cabozantinib monotherapy is approved in many countries in first-, second-, or later-line settings based on randomized trials, although these studies largely preceded widespread implementation of checkpoint inhibitors.14,15 Activity has been observed with other VEGF-TKIs, including tivozanib and axitinib, in patients with prior checkpoint inhibitor exposure, although conclusive, prospective data have not been produced for these agents following first-line combination therapy.16,17
Based on patterns-of-care data, VEGF-TKIs appear to be the mainstay of therapy in renal cell carcinoma following progression after checkpoint inhibitors, but a potential practice is continuation of checkpoint inhibitors after initial progression.18 This practice is not limited to renal cell carcinoma, as multiple published series document this phenomenon in other cancers19–22 despite the absence of level 1 evidence. To rigorously assess this paradigm, we conducted a phase III study comparing cabozantinib with or without immune-checkpoint inhibition with the PD-L1 inhibitor atezolizumab in the post–checkpoint inhibitor setting. Although atezolizumab does not have an approved renal cell carcinoma indication, it has shown activity as monotherapy in previously untreated patients with advanced renal cell carcinoma23 and in combination with cabozantinib in patients with renal cell carcinoma previously treated with checkpoint inhibitors.24 The CONTACT-03 trial evaluated the safety and efficacy of atezolizumab-cabozantinib compared with cabozantinib alone in patients with metastatic renal cell carcinoma who experienced progression during or after previous immune checkpoint inhibitor treatment.
Methods
Study design and participants
This open-label, phase III, randomized, international trial was designed by academic advisors, employees of the sponsor (F. Hoffmann-La Roche), and employees of Exelixis. The trial protocol was approved by independent review boards or ethics committees at each of 135 study sites in 15 countries. The trial sponsor and collaborators provided all investigational medicinal products (atezolizumab and cabozantinib). The trial was conducted according to Good Clinical Practice guidelines of the International Conference on Harmonisation and principles of the Declaration of Helsinki. The trial protocol and statistical analysis plan are available in the Supplementary Appendix.
Eligible patients were ≥18 years of age with Karnofsky performance status score ≥70 and had histologically confirmed, locally advanced or metastatic renal cell carcinoma. Patients had clear-cell or non–clear-cell (papillary, chromophobe, or unclassifiable only) renal cell carcinoma with or without sarcomatoid features. Patients experienced radiographic tumour progression either during or after immune checkpoint inhibitor therapy in the first- or second-line locally advanced or metastatic settings, or during or within 6 months after the last dose of adjuvant immune checkpoint therapy. Immune checkpoint inhibitor therapy was defined as anti–PD-L1 or anti–PD-1 antibody, and patients must have received ≥2 cycles in the immediately preceding line of therapy. Patients with prior treatment with VEGF-TKIs other than cabozantinib were allowed. Key exclusion criteria were prior treatment with cabozantinib or an inhibitor of mechanistic target of rapamycin kinase in any setting and prior treatment with >1 immune checkpoint inhibitor or >2 lines of therapy in the locally advanced or metastatic setting. A full list of eligibility and exclusion criteria is available in the protocol. All patients provided written informed consent.
Randomization and masking
Patients were randomly assigned in a 1:1 ratio to receive atezolizumab-cabozantinib or cabozantinib alone. Randomization was stratified by International Metastatic Renal Cell Carcinoma Database Consortium risk group (0 vs 1–2 vs ≥3); line of prior immune checkpoint inhibitor therapy (adjuvant vs first- vs second-line); and renal cell carcinoma histology (dominant clear-cell without sarcomatoid vs dominant non–clear-cell without sarcomatoid vs any sarcomatoid component). Randomization was performed through an interactive voice-response or Web-response system in permuted blocks (block size 4). An open-label study design was chosen for this trial, but the Sponsor was blinded during conduct of the study.
Procedures
Patients received cabozantinib 60 mg administered orally once daily with or without atezolizumab 1200 mg administered intravenously once every 3 weeks. Treatment continued until patients experienced unacceptable toxicity or loss of clinical benefit; treatment beyond progression was allowed in both arms. No dose reduction of atezolizumab was allowed. Dose reduction of cabozantinib and interruption or discontinuation of atezolizumab and/or cabozantinib was allowed per study guidelines. Crossover from the cabozantinib group to the atezolizumab-cabozantinib group was not allowed. Tumour assessments by computed tomography or magnetic resonance imaging scan occurred at baseline, every 9 weeks for the first 18 months, and every 12 weeks thereafter.
Outcomes
The two primary study end points were progression-free survival and overall survival. Progression-free survival was determined as duration from randomization to disease progression as assessed by blinded independent central review or death from any cause, whichever occurred first. Overall survival was defined as the duration from randomization to death from any cause. Secondary end points were progression-free survival per investigator, objective response rate (defined as the proportion of patients with complete or partial response on two consecutive occasions ≥4 weeks apart) per central review and investigator, and duration of response (defined as time from first occurrence of documented objective response to disease progression or death) per central review and investigator. All tumour-response assessments were performed per Response Evaluation Criteria in Solid Tumours, version 1·1. Time to response was assessed as a prespecified exploratory end point and was defined as the time from randomization to first complete or partial response. Safety was evaluated according to Common Terminology Criteria for Adverse Events, version 5·0, of the National Cancer Institute. Additional end points, including exploratory end points, are described in the protocol.
Statistical analysis
The study was designed to enroll approximately 500 patients. The primary analysis of progression-free survival, reported here, was planned for when the prespecified 325 events (65% of 500 patients) occurred in the intention-to-treat population. This resulted in 90% power to detect an improvement in progression-free survival with a hazard ratio of 0·67 at the 0·02 level of significance (two-sided). In order to control the overall type I error rate at α=0·05, the remaining alpha of 0·03 if the progression-free survival hypothesis testing is not statistically significant or otherwise an α of 0·05 with recycling the 0·02 from a positive progression-free survival hypothesis testing will be allocated with the Lan-DeMets O’Brien-Fleming function to two interim and one final overall survival analysis. The first interim analysis of overall survival was performed as 176 death events had occurred this time and is reported here with an allocated α of 0·0019 (two-sided) as a result of the negative progression-free survival hypothesis testing.
Efficacy was assessed in the intention-to-treat population, defined as all randomized patients. The safety-evaluable population comprised all patients who received ≥1 dose of atezolizumab or cabozantinib.
Progression-free and overall survival were compared between treatment groups with a stratified log-rank test, and the estimate of the hazard ratio between groups was calculated using a stratified Cox proportional hazards model. The International Metastatic RCC Database Consortium risk group was the only stratification factor applied in all stratified analysis models. The Schoenfeld test was used to assess the proportional hazard assumption. The Kaplan-Meier method was used to estimate the median survival time for time-to-event endpoints with the Brookmeyer-Crowley method for 95% CI calculation. All subgroup analyses of progression-free and overall survival were prespecified for key clinically relevant baseline characteristics. Hazard ratios reported for subgroup analyses are unstratified. Objective response rates for each arm were estimated with its 95% CI being calculated by the Clopper-Pearson method.
SAS version 9·4 software (SAS Institute, Cary, NC) was used for statistical analyses. The full statistical analysis plan is provided as supplementary material.
The trial is registered with ClinicalTrials.gov, NCT04338269.
Role of the funding source
The sponsor conducted the data analyses, and results were provided to the authors. All authors had full access to the data. The sponsor and authors vouch for the accuracy and completeness of the data and verify that the trial was conducted according to the protocol. Academic authors of this Article collaborated with F. Hoffmann-La Roche and Genentech in all elements of the trial, including study design, data collection, analysis, and interpretation. A first draft of the manuscript was prepared by the first and last authors. Subsequent drafts were developed by the authors with the assistance of a professional medical writer funded by the sponsor. All authors accept responsibility to submit for publication.
Results
From July 28, 2020, to December 27, 2021, 522 patients at 135 study sites in 15 countries were enrolled and comprised the intention-to-treat population, with 263 assigned to atezolizumab-cabozantinib and 259 to cabozantinib. There were 262 patients in the atezolizumab-cabozantinib group and 256 in the cabozantinib group who received ≥1 dose of study drug and comprised the safety-evaluable population (Figure 1). Major protocol deviations are listed in Table S1 in the supplementary appendix. As of clinical data cutoff (January 3, 2023), median duration of follow-up was 15·2 months (interquartile range [IQR], 10.7 to 19.3). Median follow-up was 15·6 months (IQR, 11.8 to 19.3) in the atezolizumab-cabozantinib group; 15·0 (IQR, 11.8 to 19.2) months in the cabozantinib group. Baseline characteristics were generally well balanced between treatment groups (Table 1; Table S2). Among the intention-to-treat population, 10% had renal cell carcinoma with a sarcomatoid component, and 53% had received immune checkpoint inhibitor therapy as first-line therapy. Details on the representativeness of the population are in Table S3.
Figure 1. Trial profile.
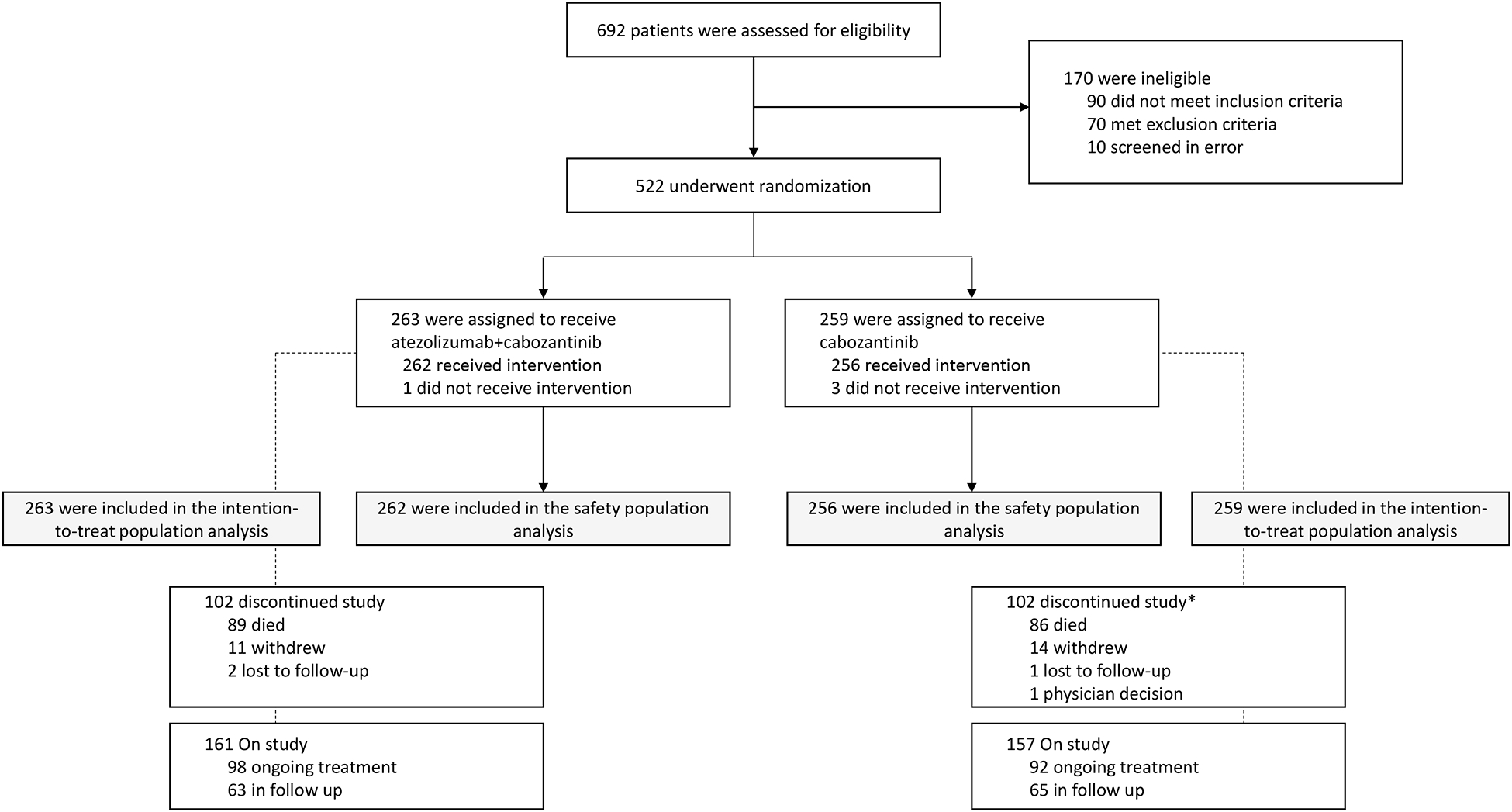
All efficacy analyses were performed in the intention-to-treat population (all patients who were randomly assigned; n=522), and all safety analyses were performed in patients who received at least one dose of study drug (n=518).
Table 1.
Characteristics of the Patients at Baseline (Intention-to-Treat Population).
| Characteristic | Atezolizumab plus cabozantinib (N=263) | Cabozantinib (N=259) |
|---|---|---|
| Age | ||
| Median (range), years | 62 (20–85) | 63 (18–89) |
| ≥65 years | 110 (42%) | 115 (44%) |
| Male sex | 204 (78%) | 197 (76%) |
| Race | ||
| White | 219 (83%) | 213 (82%) |
| Asian | 33 (13%) | 23 (9%) |
| Other | 11 (4%) | 23 (9%) |
| Most recent immune checkpoint inhibitor therapy | ||
| Adjuvant | 1 (<1%) | 1 (<1%) |
| Locally advanced or metastatic; first line | 144 (55%) | 132 (51%) |
| Locally advanced or metastatic; second line | 118 (45%) | 124 (48%) |
| None | 0 | 2 (1%) |
| Histology | ||
| Dominant clear cell without sarcomatoid | 207 (79%) | 200 (77%) |
| Dominant non–clear cell without sarcomatoid | 30 (11%) | 31 (12%) |
| Any sarcomatoid | 25 (10%) | 28 (11%) |
| Missing | 1 (<1%) | 0 |
| IMDC score | ||
| 0 | 49 (19%) | 69 (27%) |
| 1–2 | 172 (65%) | 153 (59%) |
| ≥3 | 41 (16%) | 36 (14%) |
| Missing | 1 (<1%) | 1 (<1%) |
| PD-L1 immune cell expression | ||
| <1% | 149 (57%) | 161 (62%) |
| ≥1% and <5% | 66 (25%) | 60 (23%) |
| ≥5% | 19 (7%) | 10 (4%) |
| Missing data | 29 (11%) | 28 (11%) |
| Prior VEGF-TKI use | ||
| 0 | 93 (35%) | 95 (37%) |
| 1 | 166 (63%) | 159 (61%) |
| 2 | 4 (2%) | 5 (2%) |
| Prior first-line treatment * | n=262 | n=258 |
| Ipilimumab + nivolumab | 80 (31%) | 70 (27%) |
| Sunitinib | 77 (29%) | 72 (28%) |
| Pazopanib | 36 (14%) | 43 (17%) |
| Axitinib + pembrolizumab | 36 (14%) | 28 (11%) |
| Prior second-line treatment * | n=119 | n=125 |
| Nivolumab | 104 (87%) | 116 (93%) |
Data are n (%), unless otherwise stated. IMDC denotes International Metastatic Renal Cell Carcinoma Database Consortium, PD-L1 programmed death-ligand 1, TKI tyrosine kinase inhibitor, VEGF vascular endothelial growth factor.
Treatments were mutually exclusive within each line of therapy, and patients could have received agents for more than one line of treatment. Treatments included were those in ≥10% of patients in either treatment arm.
The most common prior systemic cancer treatments, including immune checkpoint inhibitor therapy, in the first line was ipilimumab + nivolumab, and in the second line, single-agent nivolumab. Prior immune checkpoint inhibitor exposure by line of therapy is summarized in Table S4. Follow-up therapies are summarized in Table S5.
A total of 171 patients (65%) receiving atezolizumab-cabozantinib and 166 patients (64%) receiving cabozantinib had disease progression per central review or died. Progression-free survival per central review was not significantly different with atezolizumab-cabozantinib vs cabozantinib alone (median, 10·6 months [95% confidence interval (CI), 9·8 to 12·3] vs 10·8 months [95% CI, 10·0 to 12·5]; stratified hazard ratio for progression or death, 1·03; 95% CI, 0·83 to 1·28; P=0·78; [Figure 2A]; unstratified hazard ratio, 1·04; 95% CI, 0·84 to 1·29). Progression-free survival across key subgroups is shown in Figure 2B.
Figure 2. Progression-Free Survival Per Central Review.
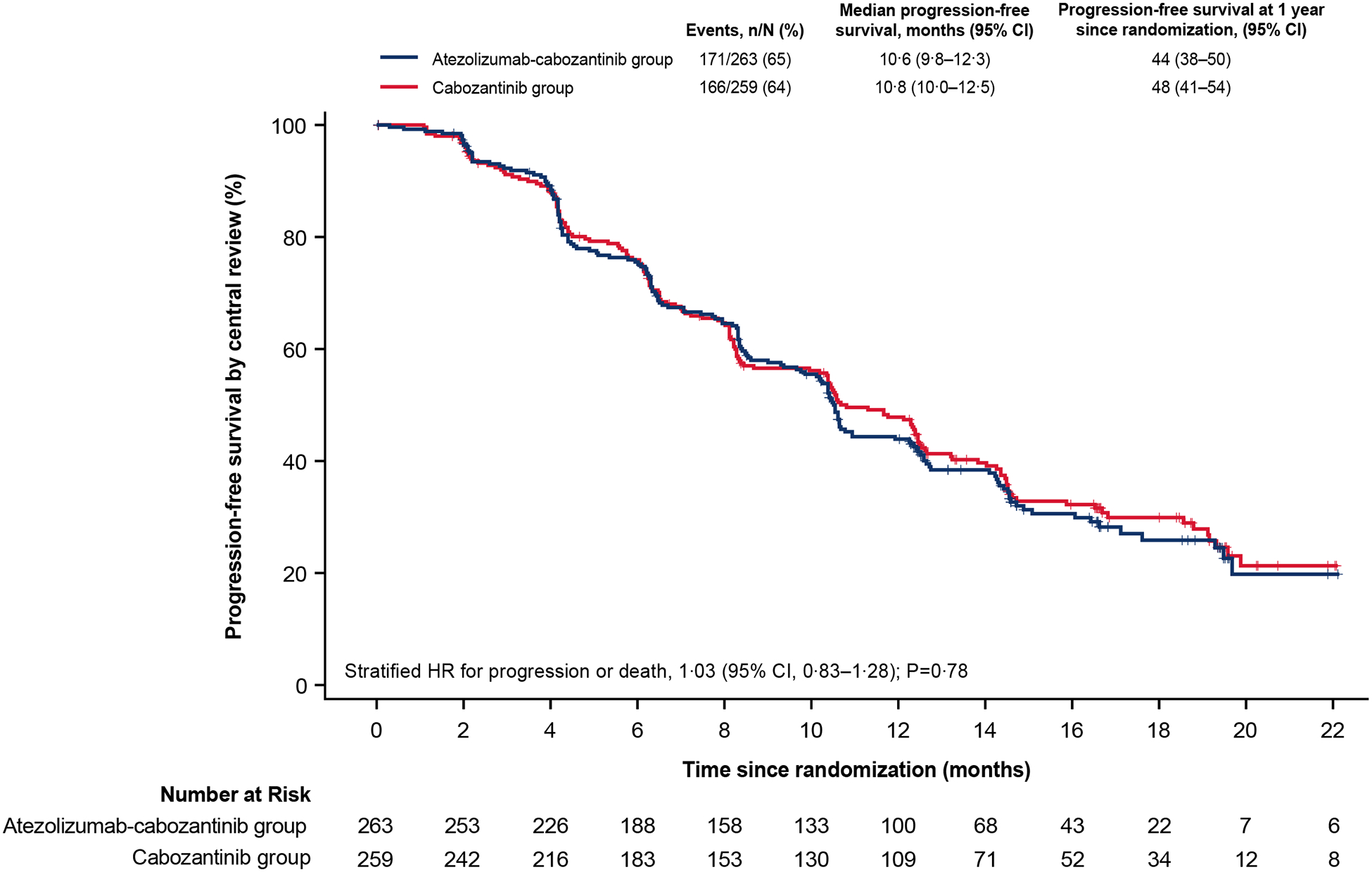
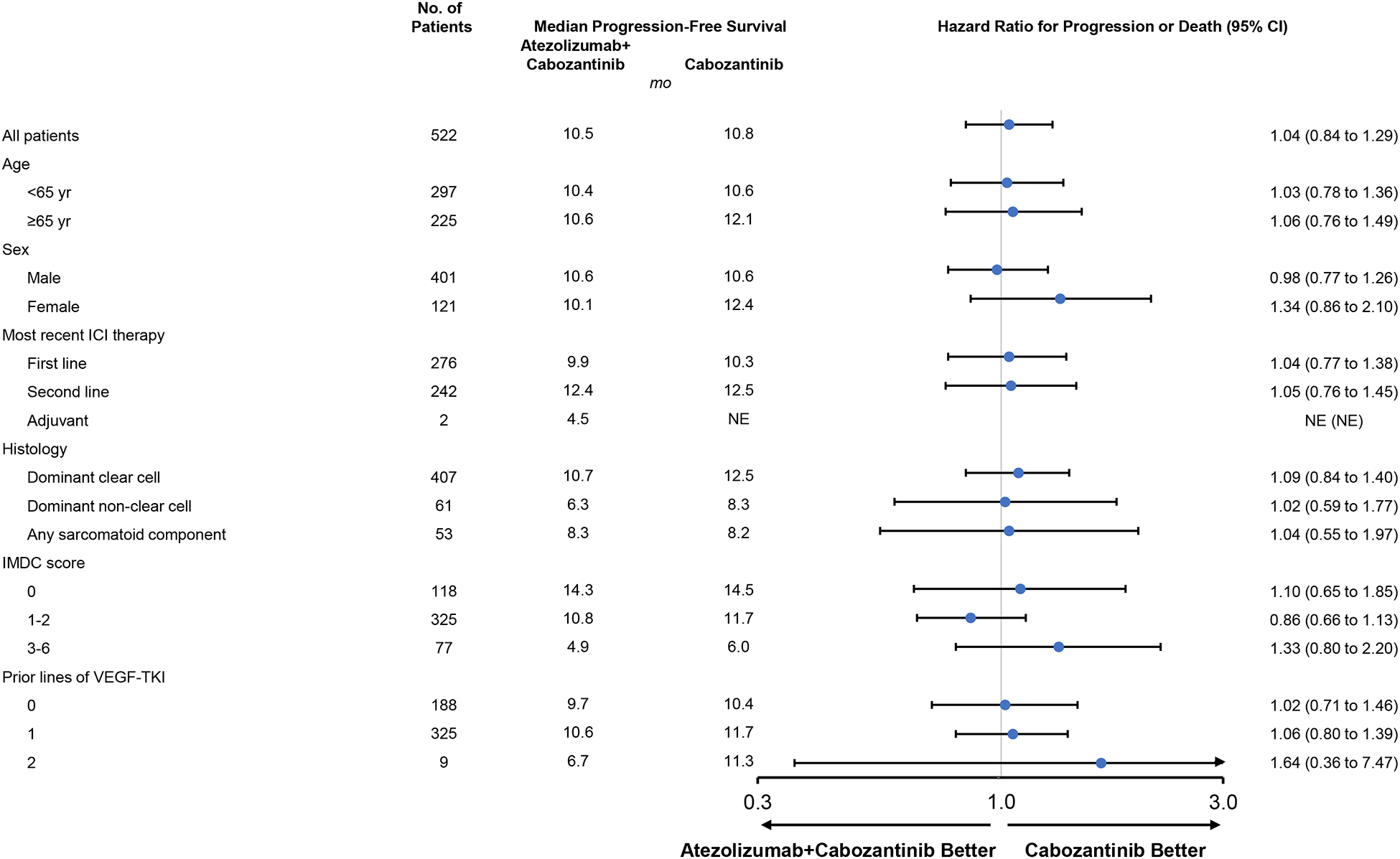
Kaplan-Meier estimates of progression-free survival as assessed by blinded central review board in the intention-to-treat population (A) and in key subgroups (B). HR values in the subgroup analysis were unstratified. CI denotes confidence interval, HR, hazard ratio, ICI immune checkpoint inhibitor, IMDC International Metastatic Renal Cell Carcinoma Database Consortium, TKI tyrosine kinase inhibitor, VEGF vascular endothelial growth factor.
A total of 89 patients (34%) in the atezolizumab-cabozantinib group and 87 (34%) in the cabozantinib group died (stratified hazard ratio for death, 0·94; 95% CI, 0·70 to 1·27; P=0·69; Figure 3; unstratified hazard ratio, 0·96; 95% CI, 0·71 to 1·29). Overall survival across key subgroups is shown in Figure S1.
Figure 3. Kaplan-Meier estimate of overall survival in the intention-to-treat population.
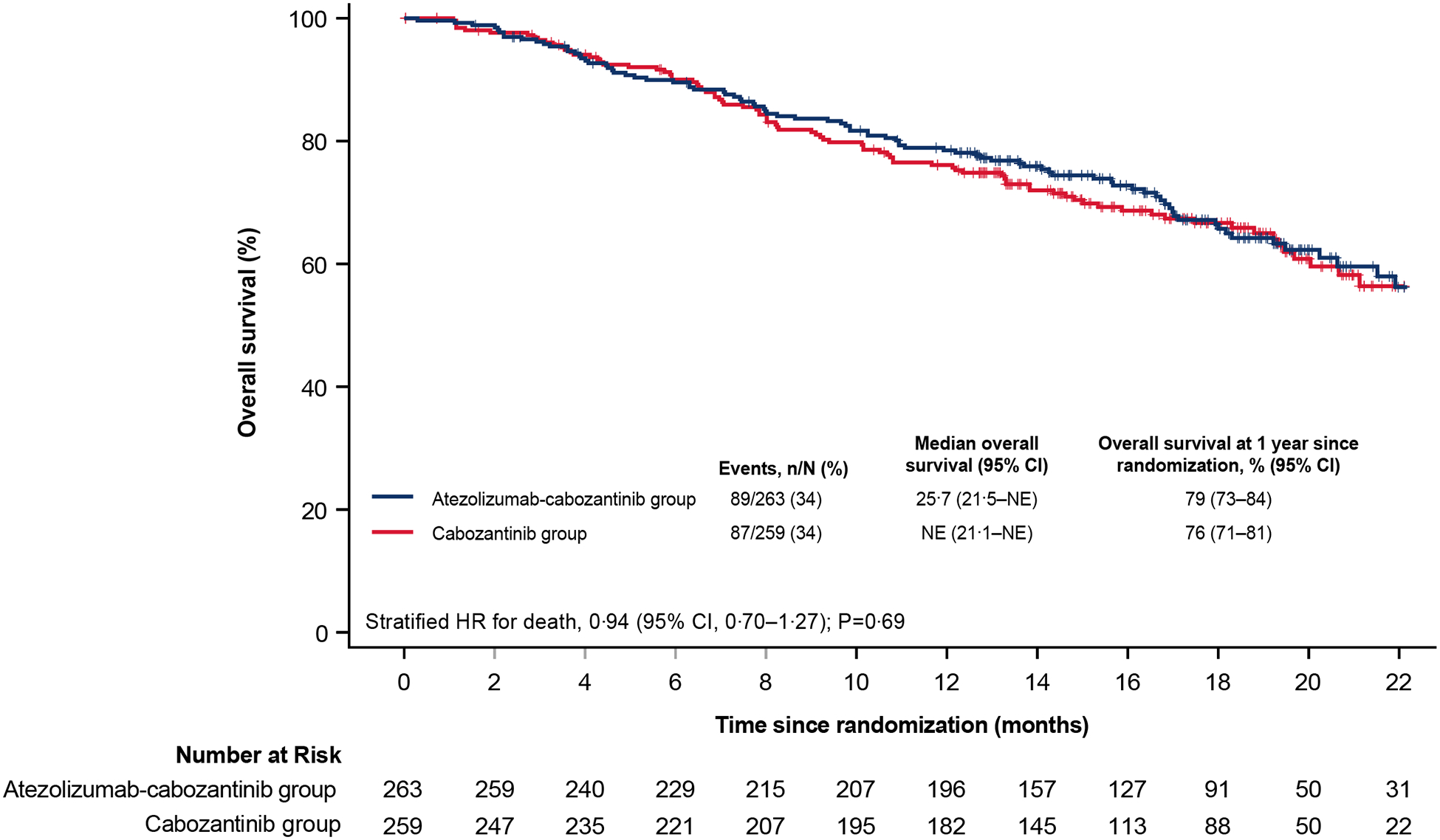
CI denotes confidence interval, HR, hazard ratio, NE not evaluable.
A total of 178 patients (68%) receiving atezolizumab-cabozantinib and 174 patients (67%) receiving cabozantinib had disease progression per investigator or died (Figure S2). No significant difference was seen in progression-free survival per investigator between the atezolizumab-cabozantinib and cabozantinib groups (median, 10·4 months [95% CI, 8·4 to 10·9] vs 10·4 months [95% CI, 8·5 to 12·3]. Objective response rates were assessed by RECIST 1·1 by central review and investigator (Table 2). Confirmed objective response rates were 41% (95% CI, 35 to 47) with atezolizumab-cabozantinib and 41% (95% CI, 35 to 47) with cabozantinib alone per central review, with complete response in no and two patients (1%), respectively. Among patients with confirmed response, median duration of response was 12·7 months (95% CI, 10·5 to 17·4) in the atezolizumab-cabozantinib group and 14·8 months (11·3 to 20·0) in the cabozantinib group; median time to response was 2·1 months (range, 1·3 to 8·4) and 2·1 months (range, 1·4 to 12·5), respectively. Similar response data were observed by investigator assessment (Table 2).
Table 2.
Secondary Efficacy Outcomes.*
| RECIST 1·1 per central review | RECIST 1·1 per investigator | |||
|---|---|---|---|---|
| Atezolizumab plus cabozantinib (N=259) | Cabozantinib (N=254) | Atezolizumab plus cabozantinib (N=263) | Cabozantinib (N=259) | |
| Confirmed objective response, n (% [95% CI]) | 105 (41% [35%–47%])† |
104 (41% [35%–47%])† |
100 (38% [32%–44%])‡ |
108 (42% [36%–48%])‡ |
| Complete response, n (%) | 0 | 2 (1%) | 4 (2%) | 2 (1%) |
| Partial response, n (%) | 105 (41%) | 102 (40%) | 96 (37%) | 106 (41%) |
| Stable disease, n (%) | 131 (51%) | 121 (48%) | 127 (48%) | 120 (46%) |
| Progressive disease, n (%) | 11 (4%) | 13 (5%) | 24 (9%) | 17 (7%) |
| Not evaluable or missing, n (%) | 12 (5%) | 16 (6%) | 12 (5%) | 14 (5%) |
| Ongoing objective response at data cutoff, n/N (%)§ | 53/105 (50%) | 55/104 (53%) | 58/100 (58%) | 48/108 (44%) |
| Median duration of response (95% CI), mo | 12·7 (10·5–17·4) | 14·8 (11·3–20·0) | NE (10·4–NE) | 12·2 (9·7–14·5) |
| Median duration of response range, mo | 2·1+ to 22·9+ | 2·3+ to 25·6+ | 2·1+ to 23·2+ | 2·1+ to 25·6+ |
Included are patients who presented with measurable disease according to RECIST 1·1, as assessed by either a central review facility or by investigators.
The estimated difference in objective response rate per central review between the atezolizumab-cabozantinib group and the cabozantinib group was −0·4 (95% CI, −9·3 to 8·5).
The estimated difference in objective response rate per investigator assessment between the atezolizumab-cabozantinib group and the cabozantinib group was −3·7(−12·5 to 5·1).
Included are patients with complete or partial response who did not experience disease progression or death.
CI denotes confidence interval, NE not evaluable, RECIST Response Evaluation Criteria in Solid Tumours, version 1·1. The plus sign indicates a censored value.
Overall, 518 patients received ≥1 dose of study treatment and were included in the safety analysis population. In the atezolizumab-cabozantinib group, median duration of atezolizumab was 10·5 months (range, 0 to 28), and median duration of cabozantinib was 11·4 months (range, 0 to 29). In the cabozantinib group, median duration of cabozantinib was 11·1 months (range, 0 to 29). In the atezolizumab-cabozantinib group, the median dose intensity of atezolizumab was 97% (range, 38 to 105) and of cabozantinib was 65% (range, 8 to 103). In the cabozantinib group, the median dose intensity of cabozantinib was 69% (range 7 to 105). The most common adverse events of any grade or cause were diarrhea (65%), palmar-plantar erythrodysesthesia syndrome (39%), and decreased appetite (38%) in the atezolizumab-cabozantinib group, and diarrhea (71%), palmar-plantar erythrodysesthesia syndrome (41%), decreased appetite (38%), and hypothyroidism (38%) in the cabozantinib group (Table S6). Grade 3–4 adverse events of any cause occurred in 68% of patients in the atezolizumab-cabozantinib group and 62% of patients in the cabozantinib group (Table 3, Table S7).
Table 3.
Adverse Events.
| Characteristic | Atezolizumab plus cabozantinib (N=262) | Cabozantinib (N=256) |
|---|---|---|
| Any-cause adverse event | 262 (100%) | 254 (99%) |
| Any-cause adverse event related to treatment | 252 (96%) | 249 (97%) |
| Grade 3 or 4 event | 177 (68%) | 158 (62%) |
| Grade 3 or 4 event related to treatment | 145 (55%) | 121 (47%) |
| Death due to adverse event | 17 (6%) | 9 (4%) |
| Death due to adverse event related to treatment | 3 (1%) | 0 |
| Serious adverse event | 126 (48%) | 84 (33%) |
| Serious adverse event related to treatment | 63 (24%) | 30 (12%) |
| Adverse event leading to withdrawal from a trial drug | 41 (16%) | 10 (4%) |
| Adverse event leading to withdrawal from atezolizumab | 29 (11%) | 0 |
| Adverse event leading to withdrawal from cabozantinib | 25 (10%) | 10 (4%) |
| Adverse event leading to interruption or reduction of a trial drug | 240 (92%) | 223 (87%) |
| Adverse event leading to interruption of atezolizumab | 159 (61%) | 0 |
| Adverse event leading to interruption or reduction of cabozantinib | 234 (89%)* | 223 (87%)† |
Data are n (%).
Due to an adverse event, 103 (39%) patients had dose reductions to a lowest dose of cabozantinib 40 mg, and 98 (37%) had a lowest dose of 20 mg
Due to an adverse event, 104 (41%) patients had dose reductions to a lowest dose of cabozantinib 40 mg, and 77 (30%) had a lowest dose of 20 mg.
In total, 48% of patients in the atezolizumab-cabozantinib group experienced a serious adverse event vs 33% in the cabozantinib group. Serious adverse events that occurred in >2% of patients in either arm were pulmonary embolism (4% in the atezolizumab-cabozantinib group and 2% in the cabozantinib group) and pyrexia (3% and 1%, respectively). Additionally, adverse events leading to discontinuation of any study treatment occurred in 16% of patients in the atezolizumab-cabozantinib group and 4% of patients in the cabozantinib group (Table S8). Adverse events leading to dose modification or interruption of any treatment component occurred in 92% of patients in the atezolizumab-cabozantinib group and 87% of patients in the cabozantinib group.
A total of 252 patients (96%) who received atezolizumab-cabozantinib and 249 (97%) who received cabozantinib experienced ≥1 adverse event deemed related to treatment by investigators (Table 3). Grade 3–4 adverse events related to treatment occurred in 55% of patients who received atezolizumab-cabozantinib and 47% of those who received cabozantinib (Table 3, Table S9).
Twenty-six deaths occurred due to adverse events, 17 (6%) in the atezolizumab-cabozantinib group and nine (4%) in the cabozantinib group (Table S10). In the atezolizumab-cabozantinib group, investigators deemed three deaths related to treatment: renal failure related to atezolizumab, enterocolitis related to atezolizumab, and gastrointestinal perforation related to cabozantinib (n=1 each). No deaths in the cabozantinib group were deemed related to treatment.
Adverse events of special interest for atezolizumab occurred in 216 (82%) patients in the atezolizumab-cabozantinib arm (grade 3–4 in 47 [18%]; grade 5 in 1 [<1%]) and 208 (82%) in the cabozantinib arm (grade 3–4 in 27 (11%); grade 5 in 0 patients) (Table S11). Adverse events of special interest for cabozantinib occurred in 190 (73%) patients in the atezolizumab-cabozantinib arm (grade 3–4 in 77 [29%]; grade 5 in 8 [3%]) and 192 (75%) in the cabozantinib arm (grade 3–4 in 73 [29%]; grade 5 in 2 [1%]) (Table S12).
Discussion
To our knowledge, CONTACT-03 is the first randomized, phase III trial in cancer to examine the safety and efficacy of rechallenge with a PD-L1 inhibitor following progression on prior PD-(L)1 therapy. Results demonstrate a lack of clinical benefit and increased toxicity with continuation of PD-L1 inhibitor use in patients receiving TKI therapy for checkpoint inhibitor–resistant renal cell carcinoma, suggesting that routine use of checkpoint inhibitor rechallenge outside of clinical trials should be discouraged.
Few phase III trials are ongoing to test PD-(L)1 inhibitor continuation, with none by addition to a control arm serving as backbone therapy. For instance, a trial of lenvatinib or sorafenib with or without atezolizumab in patients with hepatocellular carcinoma and prior bevacizumab/atezolizumab is ongoing.25 There are examples of other, less-uniform comparisons; in advanced lung cancer, the combination of ramucirumab with pembrolizumab was compared with investigator’s choice of chemotherapy in a randomized phase II study in patients with prior progression on checkpoint inhibitors.26 Estimating the relative contribution of immunotherapy in such a comparison poses a greater challenge.
Enthusiasm for paired PD-(L)1 and TKI therapy in checkpoint inhibitor–pretreated metastatic renal cell carcinoma stemmed from promising phase II data.27 Recent, real-world pattern-of-care studies suggest that >20% of US patients receive checkpoint inhibitor rechallenge.28 Furthermore, real-world treatment patterns in other cancers suggest similar rates of sequential immunotherapy use.29,30 Our data underscore the critical value of randomization. In addition to a lack of improved efficacy, adding atezolizumab led to an absolute increase of 15% in the rate of serious adverse events and an absolute increase of 8% in the rate of treatment-related Grade 3–4 adverse events. In addition, deaths due to adverse event occurred in nearly twice the number of patients who received the combination versus those who received cabozantinib alone (17 and 9, respectively). Despite the increased toxicity with addition of atezolizmaub, the rates of Grade 3–4 adverse events and fatal adverse events in the combination arm were similar to those reported for nivolumab plus cabozantinib10 and pembrolizumab plus axitinib,9 and rates in the cabozantinib arm were similar to those in other monotherapy studies.15,31 Additional clinic visits due to adverse events and infusions resulting from use of checkpoint inhibitors have been shown to increase treatment costs and resource utilization.32
The addition of atezolizumab to cabozantinib did not improve response dynamics; no differences in time to response, duration of response, or complete response rates between treatment groups were observed. No benefit was seen in key subgroups, including patients with clear-cell histology, those with prior TKI exposure, or those randomized after one prior line of therapy. A further subpopulation of interest is patients who received shorter durations of prior PD-(L)1–directed therapy: pharmacodynamic studies suggest that these agents may have protracted biological effects.33 Mechanism of action, specifically PD-L1 inhibition, could account for uniformly negative study results. However, atezolizumab monotherapy has demonstrated reasonable activity, with response rates of 25% as a single agent in a first-line, randomized trial and in combination with bevacizumab as second-line treatment after progression on atezolizumab.23,34 Furthermore, the ongoing phase III TiNivo study—comparing tivozanib, a VEGF-TKI, with or without the PD-1 inhibitor nivolumab in patients with metastatic renal cell carcinoma with prior checkpoint inhibitor exposure—will provide additional data.35
Limitations to our study include the broad eligibility criteria, encompassing a diverse array of patients including those with non-clear cell histology. The decision to include this population was based on evidence from the COSMIC-021 study where encouraging response rates were observed;24 in that trial, patients with metastatic papillary RCC had a response rate of 47%, more than double the response rate of 23% observed with cabozantinib alone in the prospective PAPMET study.36 Our study also allowed for the inclusion of patients with prior PD-(L)1 treatment in the adjuvant setting. Ultimately, only two patients were enroled, limiting our ability to assess implications in this patient population. Another limitation was the conduct of the trial during the COVID-19 pandemic. Though this did not seem to challenge enrolment, it could have impeded clinical and radiographic follow-up. However, it would not be expected to preferentially affect one study arm. Although the impact of the pandemic is difficult to quantify, it is notable that relatively few deviations were noted in the study, and few were attributed to COVID-19. The study sponsor implemented risk mitigation and management measures and worked proactively with sites to minimize disruption to the study during the COVID-19 pandemic. Of note, in our study, the median progression-free survival in the cabozantinib monotherapy arm surpassed the expectation of 8.0 months, formulated on the basis of trials such as METEOR.15 Our results are mirrored in other contemporary trials; for example, in the CANTATA study, median progression-free survival with cabozantinib monotherapy as a control arm was 9.2 months.37 The better performance of cabozantinib monotherapy in this and other recent trials may be due to many factors, such as the inclusion of patients naive to angiogenesis inhibitors (in contrast to older studies that included patients with 1 or 2 prior VEGF-TKIs) and any potentiation of effect from prior immunotherapy. Further, in CONTACT-03 there was a higher proportion of patients with favourable risk disease status in the control arm (27%) compared with the combination arm (19%).
In conclusion, our data do not support the addition of atezolizumab to targeted therapy beyond progression on prior checkpoint inhibition in renal cell carcinoma, despite encouraging phase II data supporting this approach. The data herein highlight the importance of randomized, prospective assessment of re-challenge with checkpoint inhibitors in renal cell carcinoma and other tumour types.
Data sharing
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Supplementary Material
Research in context.
Evidence before this study
Checkpoint inhibitors represent a diverse class of therapies that stimulate an antitumour immune response. They are a cornerstone of modern cancer treatment, and the most frequently utilized class of these agents abrogate signaling through programmed death-1 or programmed death-ligand 1 (collectively, PD-[L]1). A potential strategy is to rechallenge patients with PD-(L)1 inhibitors after prior progression on these agents, but the clinical benefit associated with this practice is unknown. We conducted a search of PubMed and major oncology congresses, identifying articles related to rechallenge with PD-(L)1 inhibitors following prior anti-PD-L(1) therapy in patients with advanced cancer. We searched original research articles and meeting abstracts published or reported between April 1, 2013, and April 1, 2023, using MeSH terms “cancer”, “programmed death-1”, “programmed death-ligand 1”, “second-line”, and “re-challenge”. The bulk of evidence supporting PD-(L)1 inhibitor rechallenge constituted single-arm phase II trials or retrospective experiences, spanning histologies including but not limited to advanced non-small cell lung cancer, hepatocellular cancer, melanoma, and kidney cancer. We identified no randomized, phase III trials to support this practice, however.
Added value of this study
The results of CONTACT-03 are the first to provide evidence for the strategy of PD-(L)1 re-challenge in solid tumours. There are randomized studies in other histologies underway to further explore this strategy, including in non-small cell lung cancer and hepatocellular cancer, but none with direct addition of PD-(L)1 therapy to a single, standard control arm.
Implications of all the available evidence
The results of CONTACT-03 are notable for not just a lack of clinical benefit with the addition of atezolizumab to cabozantinib, but substantial added toxicity. These data should deter the practice of PD-(L)1 inhibitor rechallenge in advanced kidney cancer. Results of ongoing phase III studies exploring PD-L(1) inhibitor rechallenge in a multitude of other cancer types are eagerly awaited. The results of this study underscore the importance (across cancer types) of prospective assessment of immunotherapy rechallenge before it is adopted as a standard of care.
Acknowledgments
This study was sponsored by F. Hoffmann-La Roche and Exelixis was a study collaborator. F. Hoffmann-La Roche Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
We thank the patients and their families and the investigators and clinical study sites for making this trial possible. Medical writing assistance for this manuscript was provided by Scott Battle, PhD, of Health Interactions, Inc, funded by F. Hoffmann-La Roche.
SKP reports their institution received grants from or has contracts with Exelixis, Xencor, Pfizer, Allogene Therapeutics, AstraZeneca, Genentech, and CRISPR Therapeutics; reports payment or honoraria for lectures, presentations, or speakers bureaus from EMD Serono and Pfizer; and reports meeting or travel support from CRISPR Therapeutics and Roche. LA L reports their institution received consulting fees from Astellas, Ipsen, BMS, Janssen, Merck, MSD, Pfizer, Eisai, and Roche; and reports travel support from BMS, MSD, and Ipsen. PT, AM, HM, CK, TO, and MA and have nothing to disclose. CS reports grants from or contracts with AB Science, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca AB, Bayer, Blueprint Medicines, Boehringer Ingelheim Espana, BMS, Clovis Oncology, Exelixis, Genentech, GlaxoSmithKline, F. Hoffmann-La Roche, Novartis, Pfizer, and Sanofi-Aventis; speakers bureau fees from Astellas Pharma, BMS, F. Hoffmann La-Roche, Ipsen, and Pfizer; participation on a data safety monitoring board or advisory board for Astellas Pharma, Bayer, BMS, Eusa Pharma, F. Hoffmann-La Roche, Ipsen, MSD, Novartis, Pfizer, and Sanofi-Aventis. MV reports grants from or contracts with Pfizer; consulting fees from Aveo, Calithera, Eisai, Exelixis, Pfizer, Genentech, Oncorena, MICURx, and Merck; and participation on a data safety monitoring board or advisory board with AstraZeneca, Affimed, Genentech, and Merck. GdV reports consulting fees from Pfizer, MSD, BMS, Roche, Merck, Bayer, Astellas, AstraZeneca, and Ipsen; reports payment or honoraria for lectures, presentations, or speakers bureaus from Pfizer, MSD, BMS, Roche, Merck, Astellas, and Ipsen; and reports travel support from MSD, Pfizer, and Roche. JC reports grants from or contracts with Pfizer; and participation on a data safety monitoring board or advisory board with Aveo, Exelixis, and Pfizer; and is a board member with VHL Alliance. GP reports consulting fees from Astellas, Roche, and Pfizer; and reports payment or honoraria for lectures, presentations, or speakers bureaus from AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, MSD, Novartis, and Gilead Sciences. FZ reports consulting fees from Apogepha Pharma, Astellas Pharma, AstraZeneca Germany, Bayer Vital, Bristol Myers Squibb, Ipsen, Janssen-Cilag, Merck, Pfizer, and Roche; reports payment or honoraria for lectures, presentations, or speakers bureaus from Astellas, Bayer Vital, Ipsen, Janssen-Cilag, Merck, Pfizer, and Sanofi-Aventis; and reports travel support from Astellas, Ipsen, Janssen-Cilag, and Pfizer. SG, BL, MH are employees of Genentech. OK is an employee of F. Hoffmann-La Roche. GB and MK are employees of F. Hoffmann-La Roche and report stock ownership. CS is an employee of Exelixis. TP reports grants from or contracts with AstraZeneca, BMS, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seagen, Merck Serono, Astellas, Johnson & Johnson, Eisai, and Roche; reports payment or honoraria for lectures, presentations, or speakers bureaus from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, MSD, Novartis, Pfizer, Seagen, Merck Serono, Astellas, Johnson & Johnson, Eisai, Roche, and Mash Up Ltd; and reports travel support from Roche, Pfizer, MSD, AstraZeneca, and Ipsen. TKC reports support for the present manuscript from Alkermes, AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi-Aventis, Surface Oncology, Takeda, Tempest, UpToDate, Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing, and others; reports their institution received grants from or has contracts with AstraZeneca, Aveo, Arcus, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi-Aventis, and Takeda; reports consulting fees from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi-Aventis, Surface Oncology, Takeda, Tempest, UpToDate, Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, and Caribou Publishing; reports participation on a data safety monitoring board or advisory board with Aravive; reports leadership or fiduciary role with KidneyCan, ASCO, ESMO, NCCN, GU, and NCI; has stock or stock options in Pionyr, Tempest, Precede Bio, Osel, and Curesponse; and declares other financial or nonfinancial interests in Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School, the Trust family, Michael Brigham, Pan-Mass Challenge, Hinda L and Arthur Marcus Foundation, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017; 377: 1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36: 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30: 582–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022; 386: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 7.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 924–37. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–27. [DOI] [PubMed] [Google Scholar]
- 10.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021; 384: 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choueiri TK, Eto M, Motzer R, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol 2023; 24: 228–38. [DOI] [PubMed] [Google Scholar]
- 12.Albiges L, Powles T, Sharma A, et al. CaboPoint: Interim results from a phase 2 study of cabozantinib after checkpoint inhibitor (CPI) therapy in patients with advanced renal cell carcinoma (RCC). J Clin Oncol 2023; 41 (suppl 6): 606. [Google Scholar]
- 13.Procopio G, Claps M, Pircher C, et al. A multicenter phase 2 single arm study of cabozantinib in patients with advanced or unresectable renal cell carcinoma pre-treated with one immune-checkpoint inhibitor: The BREAKPOINT trial (Meet-Uro trial 03). Tumori 2023; 109: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol 2017; 35: 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 917–27. [DOI] [PubMed] [Google Scholar]
- 16.Ornstein MC, Pal SK, Wood LS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol 2019; 20: 1386–94. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol 2020; 21: 95–104. [DOI] [PubMed] [Google Scholar]
- 18.Hall JP, Zanotti G, Kim R, et al. Treatment patterns, outcomes and clinical characteristics in advanced renal cell carcinoma: a real-world US study. Future Oncol 2020; 16: 3045–60. [DOI] [PubMed] [Google Scholar]
- 19.Giaj Levra M, Cotte FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer 2020; 140: 99–106. [DOI] [PubMed] [Google Scholar]
- 20.Olson DJ, Eroglu Z, Brockstein B, et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol 2021; 39: 2647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makrakis D, Bakaloudi DR, Talukder R, et al. Treatment rechallenge with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer 2023; 21: 286–94. [DOI] [PubMed] [Google Scholar]
- 22.Scheiner B, Roessler D, Phen S, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep 2023; 5: 100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018; 24: 749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal SK, McGregor B, Suarez C, et al. Cabozantinib in combination with ztezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J Clin Oncol 2021; 39: 3725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. A study of atezolizumab with lenvatinib or sorafenib versus lenvatinib or sorafenib alone in hepatocellular carcinoma previously treated With atezolizumab and bevacizumab (IMbrave251). Bethesda, MD: US National Library of Medicine, March 2023. (https://clinicaltrials.gov/ct2/show/NCT04770896.) [Google Scholar]
- 26.Reckamp KL, Redman MW, Dragnev KH, et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J Clin Oncol 2022; 40: 2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021; 22: 946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah NJ, Sura SD, Shinde R, et al. Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment era. Eur Urol Open Sci 2023; 49: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betof Warner A, Palmer JS, Shoushtari AN, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol 2020; 38: 1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins MB, Julian C, Secrest MH, Lee J, Abajo-Guijarro AM, McKenna E. Real-world treatment patterns and overall survival in BRAF-mutant melanoma patients treated with immunotherapy or targeted therapy. Future Oncol 2022; 18: 2233–45. [DOI] [PubMed] [Google Scholar]
- 31.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George S, Bell EJ, Zheng Y, et al. The impact of adverse events on health care resource utilization, costs, and mortality among patients treated with immune checkpoint inhibitors. Oncologist 2021; 26: e1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2023; 41: 715–23. [DOI] [PubMed] [Google Scholar]
- 34.Powles T, Atkins MB, Escudier B, et al. Efficacy and safety of atezolizumab plus bevacizumab following disease progression on atezolizumab or sunitinib monotherapy in patients with metastatic renal cell carcinoma in IMmotion150: A randomized phase 2 clinical trial. Eur Urol 2021; 79: 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Study to Compare Tivozanib in Combination With Nivolumab to Tivozanib Monotherapy in Subjects With Renal Cell Carcinoma. Bethesda, MD: US National Library of Medicine, March 2023. (https://clinicaltrials.gov/ct2/show/NCT04987203). [Google Scholar]
- 36.Pal SK, Tangen C, Thompson IM Jr., et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021; 397: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tannir NM, Agarwal N, Porta C, et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: the CANTATA randomized clinical trial. JAMA Oncol 2022; 8: 1411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


