Abstract
Background
The effect of analgesic modalities on short‐term outcomes in acute pancreatitis remains unknown. However, preclinical models have raised safety concerns regarding opioid use in patients with acute pancreatitis.
Objective
This study aimed to assess the association between analgesics, particularly opioids, and severity and mortality in hospitalised patients with acute pancreatitis.
Methods
This prospective multicentre cohort study recruited consecutive patients admitted with a first episode of acute pancreatitis between April 1 and 30 June 2022, with a 1‐month follow‐up. Data on aetiology, clinical course, and analgesic treatment were collected. The primary outcome was the association between opioid analgesia and acute pancreatitis severity, which was analysed using univariate and multivariate analyses.
Results
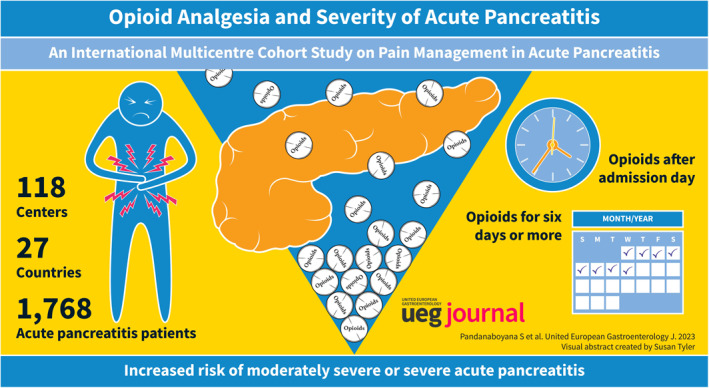
Among a total of 1768 patients, included from 118 centres across 27 countries, 1036 (59%) had opioids administered on admission day, and 167 (9%) received opioids after admission day. On univariate analysis, moderately severe or severe acute pancreatitis was associated with male sex, Asian ethnicity, alcohol aetiology, comorbidity, predicted severe acute pancreatitis, higher pain scores, longer pain duration and opioid treatment (all p < 0.001). On multivariate analysis, comorbidity, alcohol aetiology, longer pain duration and higher pain scores increased the risk of moderately severe or severe acute pancreatitis (all p < 0.001). Furthermore, opioids administered after admission day (but not on admission day) doubled the risk of moderately severe or severe disease (OR 2.07 (95% CI, 1.29–3.33); p = 0.003). Opioid treatment for 6 days or more was an independent risk factor for moderately severe or severe acute pancreatitis (OR 3.21 (95% CI, 2.16–4.79; p < 0.001). On univariate analysis, longer opioid duration was associated with mortality.
Conclusion
Opioid treatment increased the risk of more severe acute pancreatitis only when administered after admission day or for 6 days or more. Future randomised studies should re‐evaluate whether opioids might be safe in acute pancreatitis.
Keywords: acute pancreatitis, alcoholic, analgesia, morbidity, mortality, opioid, pain, severity
Key summary.
Summarize the established knowledge on this subject
Severe epigastric pain is the primary presenting symptom of acute pancreatitis.
Management of acute pancreatitis pain varies across different centres, and there is a lack of guidelines.
Preclinical studies have raised safety concerns regarding the use of opioids in the treatment of acute pancreatitis.
What are the significant and/or new findings of this study?
This international multicentre dataset is the largest to date to specifically explore the association of analgesia with severity, morbidity, and mortality of acute pancreatitis.
Upon admission, 1036 (59%) patients had opioids administered.
Higher pain severity, longer pain duration prior to admission, and opioid treatment were associated with moderately severe or severe acute pancreatitis.
On multivariate analysis, opioid treatment increased the risk of moderately severe or severe disease when administered after admission day or for 6 days or more.
INTRODUCTION
Acute pancreatitis (AP) is a common gastrointestinal disease with increasing incidence worldwide. 1 The primary presenting symptom of AP patients is intense epigastric pain warranting hospital admission. As such, the recently published James Lind Alliance Priority Setting Partnerships in Acute Pancreatitis has identified pain management in AP as the top priority. 2 However, the approach to pain management varies across different centres, and high‐quality evidence and guidelines are lacking. 3 A recent large cohort study from California found that opioids were administered on admission to 79% of patients with AP. 4 Although some preclinical evidence suggests that opioids may worsen the disease process, it is not known whether their use worsens AP severity and outcomes. 5
There are many potentially harmful effects of opioids on the human gastrointestinal tract, which could influence the course and outcome of AP. 6 , 7 , 8 Opioids can cause intestinal dysmotility, ileus, and bacterial overgrowth as well as increased intestinal permeability, which in turn might increase the risk of bacterial translocation, infected pancreatic necrosis, and worsen the severity of AP. 9 Furthermore, opioid administration may contribute to immunosuppression, which may promote infections. 10 Opioids can also influence the sphincter of Oddi tone and pancreatic secretions, resulting in a decreased wash‐out of activated pancreatic enzymes and inflammatory cytokines. 8
We hypothesised that opioid treatment may be associated with an increased risk of moderately severe or severe pancreatitis, morbidity, and mortality in AP patients. This prospective multicentre study aimed to investigate the effect of analgesic modalities, particularly opioids, on the severity, morbidity, and mortality of AP in hospitalised patients.
MATERIALS AND METHODS
Study design, ethics approval, and protocol
This was an international prospective multicentre collaborative cohort study of consecutive patients admitted with the first AP presentation for 3 months (April 1–30 June 2022) with 1‐month follow‐up. Data were collected online using the Research Electronic Data Capture (REDCap) database, which was maintained and monitored by the Newcastle Joint Research Office. No identifiable patient information was available. The study was registered in the clinical effectiveness register in the Research and Development (R&D) Department at Newcastle upon Tyne Hospitals NHS Trust (ID 14212). Each centre obtained local R&D approval prior to patient recruitment. Research ethics approval was not required for this study, and this was confirmed by the online National Research Ethics Service decision tool (http://www.hra‐decisiontools.org.uk/research/). Inclusion in this study did not affect individual patients' clinical care. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies were followed and adhered to as much as possible. There was no patient or public involvement in the study design or conduct.
Participating centres
This study was initiated and developed at Freeman Hospital, Newcastle upon Tyne, UK. International centres with expertise in managing patients with AP and who have previously contributed to multicentre AP studies and all centres attending patients with AP in the UK were invited to participate in the study. 11 , 12 The European Pancreatic Club endorsed the study as part of an initiative on pain management in AP led by the corresponding author, SP.
Conceptual model
In this study, we aimed to investigate the effect of opioid treatment on AP severity (Figure 1). We excluded patients with established AP severity upon admission (local complications or organ failure). Given this exclusion of patients, AP severity was undetermined for the remaining patients on the admission day, as it takes at least 48 h for assessment of AP severity according to the Revised Atlanta Classification (RAC). 13 Accordingly, we decided to stratify the timing of opioid treatment based on the day of admission to separate exposure (opioid treatment) and outcome (AP severity).
FIGURE 1.
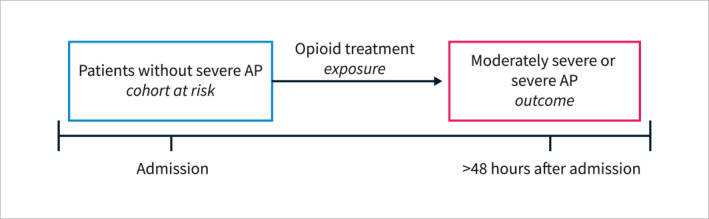
Conceptual model explaining the temporal association between exposure and outcomes in this study. AP = acute pancreatitis.
Patients and procedures
Consecutive adult patients (aged ≥18 years) admitted with the first presentation of AP were included. Only patients admitted directly to our recruiting sites were eligible and as such patients referred from other hospitals were excluded. The exclusion criteria were known chronic pancreatitis, recurrent AP, chronic pancreatitis, and pregnancy. We excluded patients who received epidural analgesia or acupuncture and those with missing data on exposure (analgesic treatment), outcome (AP severity), age, or sex. Furthermore, we excluded patients with established moderately severe or severe AP on admission (local complications or organ failure) since disease severity was our main outcome. Multiple variables were collected, including demographic data, presenting symptoms, blood parameters, aetiology of AP, predicted severity of AP, the severity of AP (based on the RAC(13)), pain duration and pain severity on admission, endoscopic or surgical interventions, intensive care treatment, length of admission, and 30‐day mortality (Case report form, Supplementary File S2).
Definitions
Acute pancreatitis was diagnosed according to the RAC. 13
Predicted mild or severe AP was defined based on Glasgow‐Imrie Criteria for Severity of Acute Pancreatitis, 14 Ranson's Criteria for Pancreatitis Mortality, 15 SIRS (Systemic Inflammatory Response Syndrome (SIRS) Criteria, 16 BISAP (Bedside Index of Severity in Acute Pancreatitis (BISAP) score, 17 or the Acute Physiology and Chronic Health Evaluation II (APACHE II) score. 18 Since different centres used different prediction methods, the locally used scoring system was recorded and used to classify patients into predicted mild or predicted severe AP.
The severity of AP was graded as mild, moderately severe, or severe, according to the RAC. 13 Following data collection, patients were further grouped into two groups:1) mild AP (no complications) and 2) moderately severe AP combined with severe AP (local complications, exacerbation of previous comorbidity, or organ failure).
Local complications were defined according to the definitions given in the RAC. 13
The analgesic modalities 6 were categorised as:
simple analgesic (e.g., paracetamol, non‐steroidal anti‐inflammatory drugs)
weak opioid (e.g., codeine, dihydrocodeine, tramadol)
strong opioids (e.g., morphine, diamorphine, oxycodone, hydromorphone, buprenorphine, fentanyl, and tapentadol)
adjuvant analgesics (e.g., tricyclic antidepressants, gabapentin, venlafaxine, duloxetine, and antispasmodics).
The opioid type was further categorised based on the type of opioid administered: strong opioids at any point were classified as “strong opioid” (see above); weak opioids only were classified as “weak opioid” (see above); and no opioids were classified as “no opioid."
Duration of opioid treatment was defined as the longest duration of any opioid administered. Patients who did not receive opioids were assigned an opioid treatment duration of 0.
Exposure data
Analgesia‐related data that were collected included analgesia prescribed prior to admission, analgesic treatment initiated during admission, including timing (which drug was given first, second, third, etc.), and for each analgesia administered: dosage and duration.
Based on this information, we identified the first administration of weak or strong opioids and recorded the corresponding day. Subsequently, we categorised patients as having no opioid use, opioid use on admission, and opioid use after admission day.
Outcome measures
The primary outcome measure was moderately severe or severe AP graded using the RAC. 13 The secondary outcome measures were admission to the intensive care unit, length of hospital stay, development of acute pancreatic fluid collections, acute necrotic collections (pancreatic necrosis and/or peripancreatic fat necrosis), pseudocysts, walled‐off necrosis, pancreatic ascites, pancreaticopleural fistula, peripancreatic vein thrombosis (portal, mesenteric, or splenic vein), and organ failure (single or multiple) and 30‐day mortality.
Statistical analysis
Categorical data were presented as numbers and percentages (%). Continuous data are presented as mean and standard deviation (SD) or median and interquartile range (IQR), depending on the data distribution. We compared mild pancreatitis and moderately severe/severe pancreatitis in relation to baseline demographic and clinical variables using Student's t‐test, Wilcoxon Rank Sum test, or Fischer's exact test/Chi‐squared test (univariate analysis). We compared all three RAC severity groups using one‐way ANOVA, Kruskal‐Wallis test, or Fischer's exact test/chi‐squared test, as appropriate. Next, we performed multivariate regression analyses according to the TRIPOD guidelines 19 using binary logistic regression, including variables significantly associated with AP severity in univariate analyses. In this multivariate model, we opted to exclude the prediction models for AP severity due to the dependence on various scoring systems and omitted opioid type and duration due to challenges in determining morphine equivalent doses. However, to elaborate on this issue, we subsequently repeated the multivariate model with opioid duration instead of opioid timing.
Furthermore, we conducted four subgroup analyses in matched cohorts of patients with 1). Biliary AP; 2). Alcoholic AP; 3). Longer pain duration prior to admission (more than 24 h), and 4). severe pain upon admission (numeric rating scale 7–10). The results were presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). The performance of the model was tested using the Area Under the Curve of the Receiver Operating Characteristic (AUC‐ROC). Risk factors for mortality were investigated by univariate analysis as described for AP severity. Mortality was not subjected to multivariate analysis due to low mortality numbers. Statistical significance was set at p < 0.05. For statistical analysis, we used STATA software packages (StataCorp LP, College Station, version 17.0) and R (version 4.3).
Data validation
After recruitment was completed, data validation was conducted using a representative sample of centres based on the number of patients contributed and the location of the centre. Independent validators (e.g., doctors, nurses, and medical students) who were not involved in the original data collection undertook data validation and sampled data accuracy. The following representative subset of variables was selected for further validation: sex, smoking status, aetiology of AP, AP severity, mortality, duration of pain prior to admission, the maximum number of days on opioids, epidural use, and analgesia side effects. We confirmed that the validated data were representative of the entire dataset with respect to the sampled patients per unit. Continuous variable data accuracy was assessed using Spearman's correlation coefficient. The accuracy of categorical variables was assessed using Cohen's kappa for robustness of comparison of categorical variables. Agreement between the primary and validated data was summarised using Cohen's kappa coefficient. The categories relating to the degree of agreement from the value of Cohen's Kappa coefficient were poor (<0.2), fair (0.2–0.4), moderate (0.41–0.6), good (0.61, 0.8), and very good (0.81, 1.0).
RESULTS
A total of 2119 consecutive patients with an index AP episode from 118 centres across 27 countries were included in the database. After excluding patients receiving epidural analgesia or acupuncture, those with missing data on exposure, outcome, age, or sex, and those with established moderately severe or severe AP on admission, the final patient cohort consisted of 1768 patients (Figure 2).
FIGURE 2.
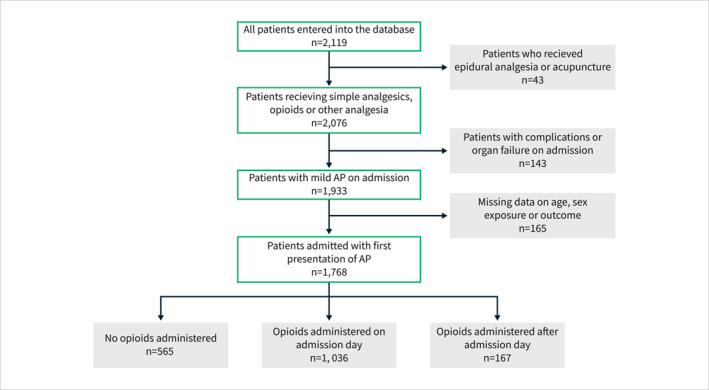
Flowchart for the patients included in the study and stratification based on the timing of opioid administration.
Demographics and clinical characteristics
The demographic and clinical characteristics are reported in Table 1. There were 885 (50%) male patients, and the overall median age was 56 years (IQR, 41–72). The most common aetiology of AP was gallstones (59%), followed by alcohol‐induced AP (19%). Other aetiologies, which were present in 121 (7%) patients, comprised idiopathic, drug‐induced, autoimmune, and pancreatic cancer‐associated AP. Median pain severity on admission was 7 (IQR, 5–8), and duration of pain prior to admission was more than 24 h in 778 (45%) patients and less than 12 h in 467 (27%) patients. Opioids were administered on admission day in 1036 (59%) patients, whereas 167 (9%) patients received opioids after the admission day. Among patients who received opioids after admission day, 97 (58%) patients received opioids the day after admission, 46 (28%) patients received opioids two or three days after admission and 24 (14%) patients received opioids later than day three. Most patients (50%) received strong opioids, whereas 314 (18%) patients received weak opioids, and 565 (32%) patients did not receive any opioid treatment during admission. Only 84 (5%) patients received adjuvant analgesics during admission.
TABLE 1.
Demographic and clinical characteristics of patients included in the study (n = 1768).
| Data completeness, n (%) | ||
|---|---|---|
| Sex, n (%) | 1768 (100) | |
| Male | 885 (50) | |
| Female | 883 (50) | |
| Median age, years (IQR) | 56 (41–72) | 1768 (100) |
| Age category, n (%) | 1768 (100) | |
| <30 | 154 (9) | |
| 30–40 | 238 (13) | |
| 40–50 | 270 (15) | |
| 50–60 | 339 (19) | |
| 60–70 | 263 (15) | |
| 70–80 | 263 (15) | |
| >80 | 241 (14) | |
| Continent, n (%) | 1768 (100) | |
| Asia | 226 (13) | |
| Africa | 17 (1) | |
| Australia | 177 (10) | |
| Europe | 1316 (75) | |
| North America | 25 (1) | |
| South America | 7 (0) | |
| Ethnicity | 1728 (98) | |
| Asian | 312 (18) | |
| Black | 45 (3) | |
| Caucasian | 1250 (72) | |
| Other | 121 (7) | |
| Aetiology, n (%) a | 1768 (100) | |
| Biliary | 1041 (59) | |
| Alcohol | 332 (19) | |
| Post ERCP | 59 (3) | |
| Hypertriglyceridemia | 56 (3) | |
| Other | 345 (20) | |
| Predicted severity at admission, n (%) | 1369 (77) | |
| Mild AP | 1068 (78) | |
| Severe AP | 301 (22) | |
| Median CCI‐score (IQR) | 2 (0–4) | 1722 (97) |
| CCI Category, n (%) | 1722 (97) | |
| 0 | 582 (34) | |
| 1–2 | 496 (29) | |
| >2 | 644 (37) | |
| Median pain severity at admission, NRS (IQR) | 7 (5–8) | 1465 (83) |
| Pain duration prior to admission, n (%) | 1743 (99) | |
| <12 h | 467 (27) | |
| 12–24 h | 498 (28) | |
| >24 h | 778 (45) | |
| Timing of opioid treatment, n (%) | 1768 (100) | |
| No opioid given during admission | 565 (32) | |
| Opioid administered on admission day | 1036 (59) | |
| Opioid administered after admission day | 167 (9) | |
| Opioid administered during admission, n (%) | 1768 (100) | |
| No opioid administered | 565 (32) | |
| Weak opioid | 314 (18) | |
| Strong opioid | 889 (50) | |
| Median duration of opioid treatment, days (IQR) | 2 (0–4) | 1735 (98) |
Abbreviations: CCI, Charlson Comorbidity Index; IQR, interquartile range; NRS, numeric rating scale.
A subset of patients had overlapping aetiological risk factors.
Acute pancreatitis severity, morbidity, and mortality
In total, 235 (13%) patients developed moderately severe AP, and 107 (6%) developed severe AP (Table 2). Among patients with organ failure (n = 160), 106 (66%) patients had respiratory failure, 93 (58%) patients had renal failure, whereas cardiac failure was present in 32 (20%) patients, and 53 (33%) patients had multiorgan failure. Among patients with local complications (n = 221), the most common complication was acute pancreatic fluid collection present in 129 (58%) patients and acute necrotic collection in 99 (45%) patients. The 30‐day mortality rate is 2%.
TABLE 2.
Clinical outcomes of patients included in the study (n = 1798).
| Data completeness, n (%) | ||
|---|---|---|
| AP severity, n (%) a | 1768 (100) | |
| Mild AP | 1426 (81) | |
| Moderately severe AP | 235 (13) | |
| Severe AP | 107 (6) | |
| Organ failure and type, n (%) | 1747 (99) | |
| No organ failure | 1587 (91) | |
| Organ failure | 160 (9) | |
| Type of organ failure b | ||
| Respiratory | 106 (66) | |
| Renal | 93 (58) | |
| Cardiac | 32 (20) | |
| Multiorgan | 53 (33) | |
| Local complications and type, n (%) | 1755 (99) | |
| No local complications | 1534 (87) | |
| Local complications | 221 (13) | |
| Type of complication c | ||
| Acute fluid collection | 129 (58) | |
| Pseudocyst | 21 (10) | |
| Acute necrotic collection | 99 (45) | |
| Walled‐off necrosis | 22 (10) | |
| Portal vein thrombosis | 11 (5) | |
| Splenic vein thrombosis | 26 (12) | |
| Median length of admission, days (IQR) | 6 (4–10) | 1753 (99) |
| 30‐day mortality, n (%) | 1749 (99) | |
| No | 1708 (98) | |
| Yes | 41 (2) |
Abbreviations: AP, acute pancreatitis; SD, standard deviation.
According to revised Atlanta criteria.
Patients with multiorgan failure are also categorised based on affected organs.
A subset of patients had multiple complications.
Parameters associated with moderately severe and severe acute pancreatitis
On univariate analysis, male sex (p = 0.001), Asian ethnicity (p < 0.001), alcohol‐induced AP (p < 0.001), and a higher Charlson Comorbidity Index (p < 0.001) were associated with moderately severe or severe AP (Table 3). As expected, patients with predicted severe AP had an increased risk of developing moderately severe or severe AP (P < 0.001). Higher pain severity scores (p < 0.001) and longer pain durations (p < 0.001) were also associated with moderately severe or severe AP (Figure 3). Finally, patients who received opioids were at an increased risk of moderately severe or severe AP regardless of the timing (p < 0.001) and duration of opioid administration (p < 0.001) (Figure 3). These associations were consistent for all parameters except pain severity when comparing the three RAC severity groups (Supplementary Table S1). 13
TABLE 3.
Association between demographic and clinical characteristics and risk of moderately severe or severe acute pancreatitis.
| Mild pancreatitis | Moderately severe/Severe pancreatitis | p‐value | |
|---|---|---|---|
| Sex, n (%) | 0.001 | ||
| Male | 686 (48) | 199 (58) | |
| Female | 740 (52) | 143 (42) | |
| Median age, years (IQR) | 56 (41–72) | 56.5 (41–73) | 0.642 |
| Age category, n (%) | 0.624 | ||
| <30 | 123 (9) | 31 (9) | |
| 30–40 | 196 (14) | 42 (12) | |
| 40–50 | 217 (15) | 53 (15) | |
| 50–60 | 272 (19) | 67 (20) | |
| 60–70 | 213 (15) | 50 (15) | |
| 70–80 | 220 (15) | 43 (13) | |
| >80 | 185 (13) | 56 (16) | |
| Ethnicity | <0.001 | ||
| Asian | 217 (16) | 95 (28) | |
| Black | 31 (2) | 14 (4) | |
| Caucasian | 1033 (74) | 217 (65) | |
| Other | 111 (8) | 10 (3) | |
| Aetiology, n (%) a | |||
| Biliary | 851 (60) | 190 (56) | 0.164 |
| Alcohol | 238 (17) | 94 (27) | <0.001 |
| Post ERCP | 47 (3) | 12 (4) | 0.867 |
| Hypertriglyceridemia | 42 (3) | 14 (4) | 0.301 |
| Other | 293 (21) | 52 (15) | 0.027 |
| Predicted severity at admission, n (%) | <0.001 | ||
| Mild AP | 923 (85) | 145 (51) | |
| Severe AP | 164 (15) | 137 (49) | |
| Median CCI‐score (IQR) | 1 (0–4) | 2 (0–4) | 0.005 |
| CCI Category, n (%) | 0.016 | ||
| 0 | 481 (35) | 101 (30) | |
| 1–2 | 411 (29) | 85 (26) | |
| >2 | 496 (36) | 148 (44) | |
| Median pain severity at admission, NRS (IQR) | 7 (5–8) | 8 (6–9) | <0.001 |
| Pain duration prior to admission, n (%) | <0.001 | ||
| <12 h | 397 (28) | 70 (21) | |
| 12–24 h | 415 (30) | 83 (24) | |
| >24 h | 592 (42) | 186 (55) | |
| Timing of opioid treatment, n (%) | <0.001 | ||
| No opioids given during admission | 486 (34) | 79 (23) | |
| Administered on admission day | 819 (57) | 217 (64) | |
| Administered after admission day | 121 (9) | 46 (13) | |
| Opioid administered during admission, n (%) | <0.001 | ||
| No opioids | 486 (34) | 79 (23) | |
| Weak opioids | 239 (17) | 75 (22) | |
| Strong opioids | 701 (49) | 188 (55) | |
| Median duration of opioid treatment, days (IQR) | 2 (0–4) | 3 (1–7) | <0.001 |
A subset of patients had overlapping aetiological risk factors. Continuous variables were tested using Student's t‐test or Wilcoxon Rank Sum test, depending on normality. Categorical variables were tested using Chi‐squared test or Fischer's exact test in case of low numbers.
FIGURE 3.
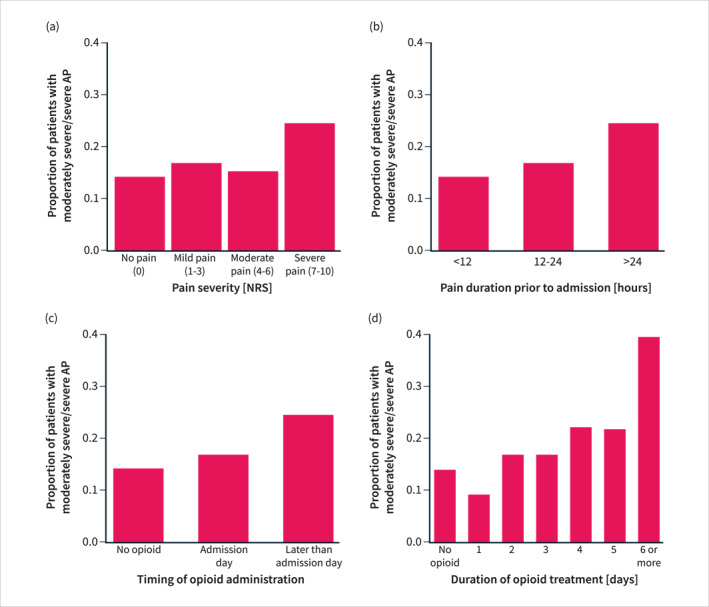
The proportion of patients with moderately severe or severe AP stratified according to pain severity (a), pain duration (b), timing of opioid administration (c) and the duration of opioid treatment (d). AP = acute pancreatitis.
On multivariate analysis, higher Charlson Comorbidity Index scores (p = 0.002), alcohol aetiology (p < 0.001), longer pain duration on admission (p < 0.001), higher admission pain scores (p < 0.001), and opioid treatment (p = 0.003) were all independently and significantly associated with an increased risk of developing moderately severe or severe AP (Supplementary Table S2) (Figure 4). For opioid treatment, this finding was significant for late administration of opioids (2.07 (95% CI, 1.29–3.33), p = 0.003), but not on the day of admission (1.38 (1.00–1.90), p = 0.052). The AUC‐ROC for this multivariate model was 0.68 (95% CI, 0.64–0.71). These results for the timing of opioid treatment were reproduced in two of the subgroup analyses conducted in patients with biliary AP and patients with pain duration >24 h prior to admission, respectively (Supplementary Table S3 and S5). In patients with alcoholic AP, opioid treatment did not increase the risk of moderately severe or severe AP (Supplementary Table S4). In the subgroup analysis of patients with severe pain upon admission, opioid treatment was also a risk factor for moderately severe or severe AP when administered upon admission day (1.96 (95% CI 1.26–3.03), p = 0.003) (Supplementary Table S6).
FIGURE 4.
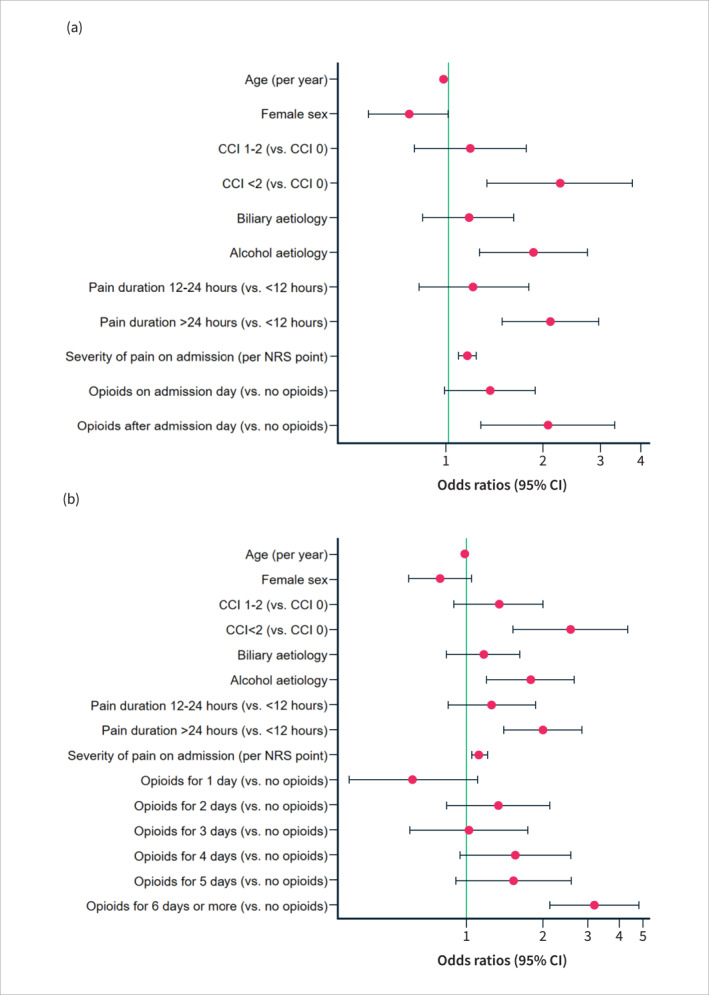
Forest plot illustrating the odds ratios for the risk of developing moderately severe or severe acute pancreatitis (AP) based on the timing of opioid administration (a) and the duration of opioid treatment (b) (multivariate analyses). CCI, Charlson Comorbidity Index; NRS, Numeric Rating Scale; CI, confidence interval.
In the multivariate model, including opioid duration, higher Charlson Comorbidity Index scores (p < 0.001), alcohol aetiology (p = 0.004), longer pain duration on admission (p < 0.001), higher admission pain severity (p = 0.001) were also independent risk factors for moderately severe or severe AP (Supplementary Table S7) (Figure 4). Opioid treatment increased the risk of moderately severe or severe AP only when the duration was six days or more (3.21 (95% CI, 2.16–4.79), p < 0.001). The AUC‐ROC for this model was 0.70 (95% CI, 0.67–0.74).
Parameters associated with 30‐day mortality after acute pancreatitis
Older age (p < 0.001), higher Charlson Comorbidity Index (p < 0.001), predicted severe AP (p < 0.001) and longer duration of opioid treatment (p = 0.023) were associated with mortality on univariate analysis (Supplementary Table S8). Mortality did not differ between the patients stratified according to the timing and type of opioid treatment.
Data validation
Validation of the accuracy of the data was performed in a random selection of 575 patients from 14 countries and 34 hospitals out of the originally recruited 2119 patients before patient exclusion. The distribution of validated samples per centre was comparable to the distribution over the entire primary dataset (Supplementary Figure S1). The only continuous variable was the number of days of opioid treatment. The primary and validated values were highly correlated (Supplementary Figure S2; Spearman's correlation coefficient = 0.81, p < 0.0001). The remaining 13 validated variables were categorical and showed very good or good (12 variables) and moderate (one variable) correlations (Supplementary Table S9).
DISCUSSION
In this international multicentre prospective cohort study of 1768 patients with AP, we found that male sex, alcohol aetiology, increased comorbidity, higher pain scores, and opioid treatment were associated with increased severity of AP. Multivariate analysis confirmed that higher Charlson comorbidity index scores, alcohol‐induced AP, longer pain duration, higher admission pain severity, late opioid administration and longer duration of opioid treatment were independent risk factors for moderately severe or severe AP. Opioid administration on the day of admission for AP and shorter duration of opioid treatment did not appear to be associated with worsening of the severity of AP. Finally, mortality due to AP was associated with older age, increased comorbidity, predicted severe AP, and longer duration of opioid treatment while there were no associations between mortality and timing or type of opioid treatment.
The composition of the patient cohort was comparable to previous findings on first‐time AP with regard to age, aetiology, necrosis rates, and 30‐day mortality. 13 , 20 , 21 In our study, we observed a lower occurrence of moderately severe AP (13%) and severe AP (6%) compared to previous reporting. 21 However, we did exclude 143 patients presenting with complications or organ failure upon admission. A retrospective study of hospitalised AP patients found that 79.9% were treated with opioids upon admission, 4 which is higher than our finding of 1036 (59%) patients receiving opioids on admission day. Again, this may be attributed to the exclusion of patients with severe disease, but geographical differences may also play a role. 22
The present study showed an increased risk of moderately severe and severe AP when opioids were administered late and when the duration of opioid treatment was long. This finding may be due to confounding by indication (patients with more severe disease tend to require opioid interventions). It may be due to disease severity, including inflammation and/or necrosis, rather than the direct effect of opioids. In contrast, preclinical data previously reported an increased severity of experimental AP in morphine‐, fentanyl‐ or buprenorphine‐treated mice. 5 , 23 On the other hand, the latter study found both exacerbation and alleviation of experimental AP depending on the type and timing of the opioid. 23 Clinical studies have also reported safety concerns regarding the use of opioids in AP patients. As such, retrospective studies have shown that opioid treatment increased the risk of 30‐day mortality, invasive ventilation, vasopressor treatment, abdominal surgery, increased morphological severity of AP, and longer length of admission. 13 , 24 , 25 Furthermore, peripheral μ‐opioid receptor antagonists are currently being tested in patients with predicted severe and recurrent AP 7 , 26 due to their potential to counteract opioid‐induced alterations to the gastrointestinal and immune systems.
In this study, we also showed that pain severity and duration prior to admission were independent risk factors for moderately severe or severe AP. These factors are not consistently and systematically included in the assessment of patients on admission or used to tailor treatments for improved management. The necessity of opioid analgesia has been questioned, as adequate pain relief has been reported with non‐opioid analgesia. 6 , 27 However, some patients will find non‐opioid analgesics inadequate and require an alternative for optimal pain relief. While optimal pain relief is important for mobilisation and recovery 28 there is also a concern, at least theoretically, that without it, there is an increased risk of central neuronal sensitisation and chronic pain. 29
This study has several strengths and limitations. Our dataset is the largest to date in the literature to specifically explore the impact of analgesia on AP severity, morbidity, and mortality. The study design was international, multicentred, and prospective, which improved the generalizability of the findings. Another strength of this study was the completeness of the data and data validation, showing a good correlation between the primary and validated variables. The use of opioids was available from the day of admission, allowing temporal assessment of outcomes in relation to opioid use. Unfortunately, our dataset did not enable us to calculate morphine equivalent doses and as such we could not explore any potential dose‐response relationship between opioid administration and development of moderately severe or severe AP. Furthermore, we could not infer whether repetitive opioid administration might be more harmful than single doses or whether the route of opioid administration impacted the severity of AP. One of the limitations of this study was the relatively low proportion of patients with moderately severe and severe AP (19%) and the low overall mortality (2%), that did not allow us to perform multivariate analysis for the risk of mortality. The snap‐shot design and pragmatic 1‐month follow‐up meant that some late complications (e.g., infected pancreatic necrosis and mortality) might not have been captured. Another limitation was the variation in the use of scoring systems for assessing the severity of AP; however, the severity of AP as an outcome was uniformly reported using the RAC. Finally, we did not register if opioid treatment was combined with peripheral μ‐opioid receptor antagonists, which may potentially alter the effects of opioids on AP. However, we expect that this would concern a small patient group since combining opioids with peripheral μ‐opioid receptor antagonists is not first‐line treatment.
In this international, multicentre cohort study, opioid treatment was associated with a higher risk of severe acute pancreatitis only when administered after admission day or for 6 days or more. However, no causal inference can be drawn. Definitive proof that opioid treatment might be safe in patients with acute pancreatitis would require a robust and adequately powered randomised controlled trial comparing opioid and non‐opioid analgesic protocols.
CONFLICT OF INTEREST STATEMENT
None to declare.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The full list of PAINAP collaborators is available in Supplementary File S1. We acknowledge the Newcastle Joint Research Office for developing and maintaining the REDCap database.
Pandanaboyana S, Knoph CS, Olesen SS, Jones M, Lucocq J, Samanta J, et al. Opioid analgesia and severity of acute pancreatitis: an international multicentre cohort study on pain management in acute pancreatitis. United European Gastroenterol J. 2024;12(3):326–338. 10.1002/ueg2.12542
Sanjay Pandanaboyana and Cecilie Siggaard Knoph are Joint first authors
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta‐analysis. Gastroenterology. 2022;162(1):122–134. 10.1053/j.gastro.2021.09.043 [DOI] [PubMed] [Google Scholar]
- 2. Mitra V, Munnelly S, Grammatikopoulos T, Mole D, Hopper A, Ryan B, et al. The top 10 research priorities for pancreatitis: findings from a James Lind Alliance priority setting partnership. Lancet Gastroenterol Hepatol. 2023;8(9):780–782. 10.1016/s2468-1253(23)00151-6 [DOI] [PubMed] [Google Scholar]
- 3. Thavanesan N, White S, Lee S, Ratnayake B, Oppong KW, Nayar MK, et al. Analgesia in the initial management of acute pancreatitis: a systematic review and meta‐analysis of randomised controlled trials. World J Surg. 2022;46(4):878–890. 10.1007/s00268-021-06420-w [DOI] [PubMed] [Google Scholar]
- 4. Wu BU, Butler RK, Chen W. Factors associated with opioid use in patients hospitalized for acute pancreatitis. JAMA Netw Open. 2019;2(4):e191827. 10.1001/jamanetworkopen.2019.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barlass U, Dutta R, Cheema H, George J, Sareen A, Dixit A, et al. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut. 2018;67(4):600–602. [DOI] [PubMed] [Google Scholar]
- 6. Pandanaboyana S, Huang W, Windsor JA, Drewes AM. Update on pain management in acute pancreatitis. Curr Opin Gastroenterol. 2022;38(5):487–494. 10.1097/mog.0000000000000861 [DOI] [PubMed] [Google Scholar]
- 7. Knoph CS, Cook ME, Fjelsted CA, Novovic S, Mortensen MB, Nielsen LBJ, et al. Effects of the peripherally acting μ‐opioid receptor antagonist methylnaltrexone on acute pancreatitis severity: study protocol for a multicentre double‐blind randomised placebo‐controlled interventional trial, the PAMORA‐AP trial. Trials. 2021;22(1):940. 10.1186/s13063-021-05885-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farmer AD, Drewes AM, Chiarioni G, De Giorgio R, O'Brien T, Morlion B, et al. Pathophysiology and management of opioid‐induced constipation: European expert consensus statement. United Eur Gastroenterol J. 2019;7(1):7–20. 10.1177/2050640618818305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, et al. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46((Suppl 1)):46–51. 10.1097/mcg.0b013e3182652096 [DOI] [PubMed] [Google Scholar]
- 10. Plein LM, Rittner HL. Opioids and the immune system ‐ friend or foe. Br J Pharmacol. 2018;175(14):2717–2725. 10.1111/bph.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, et al. SARS‐CoV‐2 infection in acute pancreatitis increases disease severity and 30‐day mortality: COVID PAN collaborative study. Gut. 2021;70(6):1061–1069. 10.1136/gutjnl-2020-323364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Podda M, Pacella D, Pellino G, Coccolini F, Giordano A, Di Saverio S, et al. coMpliAnce with evideNce‐based cliniCal guidelines in the managemenT of acute biliaRy pancreAtitis): the MANCTRA‐1 international audit. Pancreatology. 2022;22(7):902–916. 10.1016/j.pan.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 13. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 14. Blamey SL, Imrie CW, O’Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25(12):1340–1346. 10.1136/gut.25.12.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139(1):69–81. [PubMed] [Google Scholar]
- 16. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of chest physicians/society of critical care medicine. Chest. 1992;101(6):1644–1655. 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 17. Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population‐based study. Gut. 2008;57(12):1698–1703. 10.1136/gut.2008.152702 [DOI] [PubMed] [Google Scholar]
- 18. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 19. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13(1):1–10. 10.1016/j.eururo.2014.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spanier BWM, Dijkgraaf MGW, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract Res Clin Gastroenterol. 2008;22(1):45–63. 10.1016/j.bpg.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 21. Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, et al. Acute pancreatitis: diagnosis and treatment. Drugs. 2022;82(12):1251–1276. 10.1007/s40265-022-01766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholten WK, Christensen A.‐E, Olesen AE, Drewes AM. Quantifying the adequacy of opioid analgesic consumption globally: an updated method and early findings. Am J Public Health. 2019;109(1):52–57. 10.2105/ajph.2018.304753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bálint ER, Fűr G, Kui B, Balla Z, Kormányos ES, Orján EM, et al. Fentanyl but not morphine or buprenorphine improves the severity of necrotizing acute pancreatitis in rats. Int J Mol Sci. 2022;23(3):1–23. 10.3390/ijms23031192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elias A, Korytny A, Klein A, Khoury Y, Ben Hur D, Braun E, et al. The association between opioid use and opioid type and the clinical course and outcomes of acute pancreatitis. Pancreas. 2022;51(5):523–530. 10.1097/mpa.0000000000002052 [DOI] [PubMed] [Google Scholar]
- 25. Ashok A, Faghih M, Azadi JR, Parsa N, Fan C, Bhullar F, et al. Morphologic severity of acute pancreatitis on imaging is independently associated with opioid dose requirements in hospitalized patients. Dig Dis Sci. 2022;67(4):1362–1370. 10.1007/s10620-021-06944-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook ME, Knoph CS, Fjelsted CA, Frøkjær JB, Bilgrau AE, Novovic S, et al. Effects of a peripherally acting µ‐opioid receptor antagonist for the prevention of recurrent acute pancreatitis: study protocol for an investigator‐initiated, randomized, placebo‐controlled, double‐blind clinical trial (PAMORA‐RAP trial). Trials. 2023;24(1):301. 10.1186/s13063-023-07287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gülen B, Dur A, Serinken M, Ö K, Sönmez E. Pain treatment in patients with acute pancreatitis: a randomized controlled trial. Turkish J Gastroenterol. 2016;27(2):192–196. 10.5152/tjg.2015.150398 [DOI] [PubMed] [Google Scholar]
- 28. Zang K, Chen B, Wang M, Chen D, Hui L, Guo S, et al. The effect of early mobilization in critically ill patients: a meta‐analysis. Nurs Crit Care. 2020;25(6):360–367. 10.1111/nicc.12455 [DOI] [PubMed] [Google Scholar]
- 29. Arendt‐Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress H, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain. 2018;22(2):216–241. 10.1002/ejp.1140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


