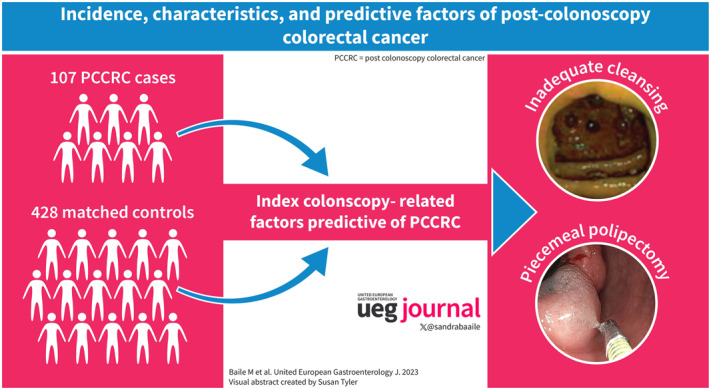
Abstract
Background
Post‐colonoscopy colorectal cancer (PCCRC) is colorectal cancer (CRC) diagnosed after a colonoscopy in which no cancer is found.
Objective
As PCCRC has become an important quality indicator, we determined its rates, characteristics, and index colonoscopy‐related predictive factors.
Methods
We carried out a multicenter, observational, retrospective study between 2015 and 2018. Rates were calculated for PCCRC developing up to 10 years after colonoscopy. PCCRC was categorized according to the most plausible explanation using World Endoscopy Organization methodology. Our PCCRC population was compared to a control cohort without CRC matched 1:4 by sex, age, index colonoscopy date, indication, endoscopist, and hospital.
Results
One hundred seven PCCRC and 2508 detected CRC were diagnosed among 101,524 colonoscopy (0.1%), leading to rates of 0.4%, 2.2%, 3.1%, and 4.1% at 1, 3, 5, and 10 years, respectively. PCCRC was in right (42.4%), left (41.4%), and transverse (16.4%) colon with 31.5% at stage I, 24.7% stage II, 32.6% stage III, and 11.2% stage IV. Twenty point three percent were classified as incomplete resection, 5.4% as unresected lesions, 48.6% as missed lesions with adequate colonoscopy, and 25.7% as missed lesions with inadequate colonoscopy. The median time from colonoscopy to PCCRC was 42 months. Previous inadequate preparation (OR 3.05, 95%CI 1.73–5.36) and piecemeal polypectomy (OR 19.89, 95%CI 8.67–45.61) were independently associated with PCCRC.
Conclusions
In our population, 4.1% of CRC cases were PCCRC. Most of these lesions were in right colon and attributable to lesions not visualized despite adequate bowel cleansing. Previous inadequate cleansing and piecemeal polypectomy were associated with PCCRC.
Keywords: colonoscopy, colorectal cancer, colorectal neoplasms, incidence
Key Summary.
Summarize the established knowledge on this subject
The post‐colonoscopy colorectal cancer (PCCRC) rate has become one of the main quality indicators for colonoscopy because it reflects its efficacy in detecting and preventing cancer.
A recent World Endoscopy Organization (WEO) consensus statement offered recommendations on classification and rate calculation for benchmark services.
What are the significant and/or new findings of this study?
We estimated a 3‐year PCCRC rate of 2.2% over a 4‐year period, attributed mostly to missed lesions despite an adequate index colonoscopy.
Inadequate bowel preparation and piecemeal polypectomy at index colonoscopy were independently associated with subsequent PCCRC development.
Our findings may help identify opportunities for improved colonoscopy performance and help other services initiate monitoring and review of their PCCRC cases and rates.
INTRODUCTION
Post‐colonoscopy colorectal cancer (PCCRC) is defined as cancers appearing after a colonoscopy in which no cancer is diagnosed, 1 , 2 , 3 and a recent consensus by the World Endoscopy Organization (WEO) formulated recommendations on classification and rate calculation of these lesions. 4 Reported rates of PCCRC according to large population‐based studies range between 2.9% and 14%. 5 , 6
Potential reasons for PCCRC include missed lesions due to inadequate bowel preparation or incomplete colonoscopy, as well as endoscopist‐dependent factors, such as short withdrawal time, a suboptimal inspection technique with a low adenoma detection rate (ADR), or incomplete polypectomy. 7 In this regard, patients attended by endoscopists with lower ADR have a higher risk of interval cancer. 8 , 9 However, index colonoscopy‐related characteristics associated with the subsequent development of PCCRC have not been adequately investigated.
Most of these lesions could be avoided and indicate opportunities for improved colonoscopy performance, leading to PCCRC becoming an important quality indicator in endoscopy services. Therefore, we performed this multicenter observational retrospective study to assess the PCCRC rates in a regional group of secondary and tertiary centers, classify PCCRC according to the most plausible explanation following the WEO algorithm, and identify the index colonoscopy‐related factors that lead to PCCRC using a case‐control design.
MATERIALS AND METHODS
Study design
We performed an observational, retrospective, multicenter study to identify and review the PCCRC cases at eight hospitals in the province of Alicante in Spain (Hospital General Universitario Dr. Balmis, Hospital Universitario del Vinalopó, Hospital General Universitario de Elche, Hospital General Universitario de Elda, Hospital Universitario de San Juan de Alicante, Hospital Universitario Vega Baja, Hospital Universitario Marina Baixa, and Hospital Universitario de Torrevieja). This study was approved by the institutional ethics committee. A waiver of informed consent was obtained given the study's observational nature.
Using the Pathology Department databases, we searched all biopsies tagged with International Classification of Diseases, 10th Revision (ICD‐10) codes for malignant neoplasm of the colon (ICD‐10 C18.0‐C18.9), rectosigmoid (ICD‐10 C19), and rectum (ICD‐10 C20) and selected all colorectal adenocarcinomas diagnosed between January 2015 and December 2018. Neuroendocrine tumors or squamous cell carcinomas of the anorectum and adenocarcinoma of the appendix were excluded.
Case and control selection
All CRC cases were matched with the Endoscopy Unit database and individually reviewed to identify which patients had an index colonoscopy negative for CRC in the preceding 6–120 months (10 years), which were selected as PCCRC cases. CRCs diagnosed at the colonoscopy or within 6 months were considered detected cancers 4 and not included as PCCRC. Patients with a personal history of CRC diagnosed with a new CRC in the same bowel segment were considered a recurrence and not included as PCCRC. In addition, patients with inflammatory bowel disease or hereditary CRC syndromes were excluded.
A case‐control study design was used to examine the association between factors related to index colonoscopy and the risk of PCCRC in the subsequent 6 months to 10 years. Controls were patients who underwent a colonoscopy negative for CRC between 2005 and 2018 and were without a CRC diagnosis at the time of their selection as controls between 2021 and 2022. Controls were matched to cases for birth year (±1 year), sex, date of index colonoscopy (±1 year), endoscopist, indication (primary CRC screening, fecal immunochemical test (FIT) positive screening, post‐polypectomy surveillance or symptoms), and center. Four matched controls were assigned for each PCCRC case.
Data collection
The clinical record and pathology, radiology, and endoscopy reports were reviewed to obtain the patient sex, age, index colonoscopy‐related factors (date, endoscopist, indication, bowel preparation adequacy, extent of the examination, polyp presence, number, size, morphology, 10 location, method and completeness of excision), and pathology findings (adenomatous or serrated, villous component, grade of dysplasia). For each CRC, information on the size, location, histology, and TNM stage was collected.
An adequate colonoscopy was defined as a complete procedure with adequate bowel preparation. Inadequate bowel preparation was described as a Boston Bowel Preparation Scale (BBPS) score of 0–1 in any colonic segment; adequate bowel preparation was considered a BBPS score of 2–3 in every colonic segment. An incomplete colonoscopy was defined as a colonoscopy that did not reach the cecum. If the adequacy of the bowel preparation or extent of the examination was not described, it was assumed that the preparation was adequate, and the examination was complete for the main analyses.
Endoscopists were classified according to their endoscopy volume. Physicians with full‐time dedication to endoscopy were considered high‐volume endoscopists, whereas other gastroenterologists with part‐time dedication to endoscopy were considered low‐volume endoscopists.
Non‐advanced adenoma (NAA) was defined as tubular adenomas <10 mm with low‐grade dysplasia. Advanced adenoma (AA) was defined as an adenoma ≥10 mm, containing ≥25% villous component, or high‐grade dysplasia (HGD). A proximal polyp was a polyp in the cecum, ascending colon, hepatic flexure, or transverse colon. CRC was defined as an invasion of malignant cells through the muscularis mucosa. Normal colonoscopy referred to colonoscopy in which no neoplasia was found.
Outcome definitions
The primary outcome was to estimate the PCCRC rates at 1, 3, 5, and 10 years. The secondary outcomes were to identify the most plausible explanation for PCCRC according to the WEO algorithm and to determine which index colonoscopy characteristics are related to the development of PCCRC.
According to the WEO consensus, 4 true‐positive colonoscopy was a colonoscopy in which CRC was detected during that procedure or within 6 months; false‐negative colonoscopy was a colonoscopy in which no CRC was found but PCCRC was detected between 6 and 120 months after the procedure. The unadjusted PCCRC rate was calculated as the number of PCCRC cases divided by the total number of PCCRC cases plus the number of detected CRCs, expressed as a percentage (i.e., false‐negative colonoscopies divided by true‐positive colonoscopies + false‐negative colonoscopies expressed as a percentage). If a case of CRC fit into both categories (PCCRC and detected cancer), it was accounted for in both groups. If a patient had more than one colonoscopy in the 120 months prior to the PCCRC diagnosis, only the closest false‐negative colonoscopy was included.
Root‐cause analysis
A root‐cause analysis was performed for each PCCRC case following the algorithm proposed by the WEO 4 to classify PCCRC according to the most plausible explanation using the following categories: (A) Possible missed lesion, prior examination adequate; (B) Possible missed lesion, prior examination negative but inadequate; (C) Detected lesion, not resected; and (D) Likely incomplete resection of the previously identified lesion.
A case fit into category A if the patient had an adequate colonoscopy within the last 4 years that did not detect cancer and no AA was identified in the same bowel segment, category B was considered if the patient had an inadequate colonoscopy within the last 4 years that did not detect cancer where no AA was identified in the same bowel segment, category C if the patient had a colonoscopy within the last 4 years in which AA was identified but not resected in the same bowel segment, and category D if the patient had a colonoscopy within the last 4 years in which AA was resected from the same bowel segment but there was no endoscopic/histological confirmation of complete resection. PCCRC appearing 4 years after the index colonoscopy was categorized as likely new cancer.
Data analysis
The patients' endoscopy reports were analyzed via the electronic institutional database (Endobase, Olympus Europe,). Categorical variables were described as numbers and frequencies (n, %), and quantitative variables as the mean and standard deviation (SD) or median and interquartile range (IQR) depending on their distribution. The parametric distribution of the quantitative variables was determined using the Kolmogorov‐Smirnov test. In the univariate analysis, categorical variables were compared using chi‐squared, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by multivariate logistic regression. All tests were two‐sided. A p‐value <0.05 was considered significant. All analyses were performed using IBM SPSS Statistics 24 (SPSS Inc.).
RESULTS
PCCRC rates
During the 4‐year study period, from January 2015 to December 2018, we detected 107 PCCRC cases and 2508 detected CRCs out of 101,524 colonoscopies (0.1%). Of these PCCRC cases, 11 were diagnosed within 6 and 12 months after the false‐negative colonoscopy, 56 within 6 and 36 months, 81 within 6 months and 5 years, and 107 within 6 months and 10 years. These results led to the following rates (Figure 1): 1‐year PCCRC rate of 0.4% (95% CI 0.2–0.6), 3‐year PCCRC rate of 2.2% (95% CI 1.6–2.8), 5‐year PCCRC rate of 3.1% (95% CI 2.5–3.8), and 10‐year PCCRC rate of 4.1% (95% CI 3.3–4.8).
FIGURE 1.
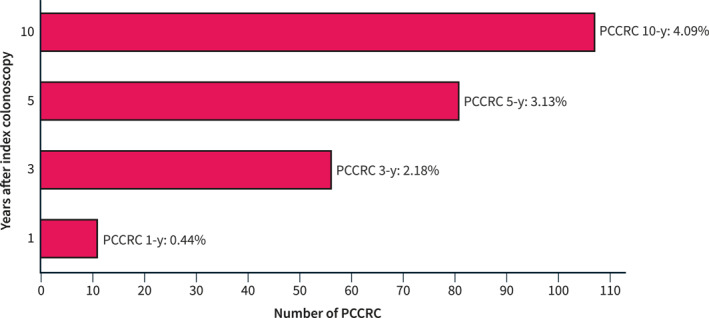
Time of diagnosis of PCCRC with respect to index colonoscopy and 1‐, 3‐, 5‐, and 10‐year rates. PCCRC, post‐colonoscopy colorectal cancer.
PCCRC characteristics
The characteristics of the PCCRC cases are described in Table 1. Seventy‐one (66.4%) PCCRCs occurred in male patients, and the mean (±SD) age at the time of PCCRC diagnosis was 72.1 ± 11.1 years. The median delay between index colonoscopy and PCCRC diagnosis was 34 months (IQR 38 months). Most PCCRCs were in the right colon (46.7%). The distribution of PCCRC among the eight included hospitals is shown in Figure 2.
TABLE 1.
Characteristics of the PCCRC population.
|
||
|---|---|---|
| Age at PCCRC diagnosis (years) | Mean (SD) | 72.1 (11.1) |
| Sex | Male | 71 (66.4) |
| Female | 36 (33.6) | |
| Indication of PCCRC colonoscopy | Direct screening | 9 (8.4) |
| +FIT | 5 (4.7) | |
| Surveillance | 33 (30.8) | |
| Symptoms | 48 (44.9) | |
| Unknown | 12 (11.2) | |
| Size of PCCRC (mm) | Median (IQR) | 30 (22) |
| Previous CRC in a different colonic segment | Yes | 4 (3.7) |
| No | 103 (96.3) | |
| Location of PCCRC | Right colon | 50 (46.7) |
| Transverse colon | 11 (10.3) | |
| Left colon | 18 (16.8) | |
| Rectum | 25 (23.4) | |
| Unknown | 3 (2.8) | |
| Stage of PCCRC | I | 28 (26.2) |
| II | 22 (20.6) | |
| III | 29 (27.1) | |
| IV | 10 (9.3) | |
| Unknown | 18 (16.8) |
Abbreviations: FIT, fecal immunochemical test; PCCRC, post‐colonoscopy colorectal cancer.
FIGURE 2.
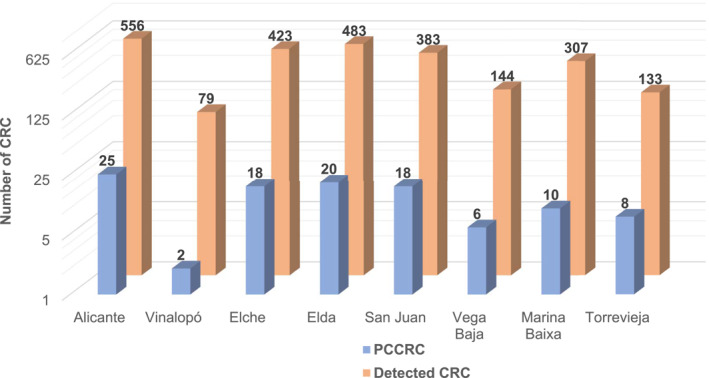
Distribution of PCCRC and detected CRC among participating centers. CRC, colorectal cancer; PCCRC, post‐colonoscopy colorectal cancer.
Root‐cause analysis
Of the 107 PCCRC cases, 33 (30.8%) appeared >4 years after the index colonoscopy and were categorized as likely new cancers; 74 were diagnosed within 6–48 months of the index colonoscopy and classified according to their most plausible explanation as follows: 36 (48.6%) category A, 19 (25.7%) category B, 4 (5.4%) category C, and 15 (20.3%) category D (Figure 3).
FIGURE 3.
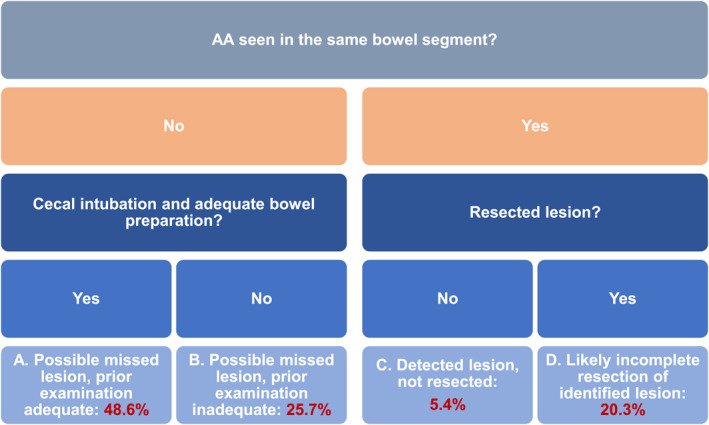
Root‐cause classification of PCCRC according to the most plausible explanation following the WEO algorithm. PCCRC, post‐colonoscopy colorectal cancer; WEO, World Endoscopy Organization.
Index colonoscopy‐related predictive factors for PCCRC
For each of the 107 PCCRC cases with index colonoscopy, 4 controls without CRC matched by age, sex, date of index colonoscopy, endoscopist of index colonoscopy, indication of index colonoscopy, and center were selected. The characteristics of the cases, controls, and matched colonoscopy examinations are detailed in Table 2.
TABLE 2.
Index colonoscopy characteristics and univariate and multivariate analyses.
|
|
OR (95% CI) | p | OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 69.1 (11.2) | 68.6 (10.7) | 1.16 (−1.80–2.76) | 0.678 | ||
| Male sex | 71 (66.4) | 284 (66.4) | 1.00 (0.64–1.57) | 0.999 | ||
| Indication | ||||||
| Direct screening | 8 (7.5) | 32 (7.5) | 1.00 (0.04–3.64) | 0.999 | ||
| +FIT | 9 (8.4) | 36 (8.4) | ||||
| Surveillance | 31 (29.0) | 124 (29) | ||||
| Symptoms | 54 (50.5) | 216 (50.5) | ||||
| Unknown | 5 (4.7) | 20 (4.7) | ||||
| Low‐volume endoscopist | 56 (52.3) | 224 (52.3) | 1.00 (0.50–2.65) | 0.999 | ||
| No fecal intubation | 13 (12.3) | 23 (5.4) | 2.46 (1.20–5.04) | 0.011 | 1.93 (0.89–4.15) | 0.121 |
| Inadequate bowel preparation | 28 (26.4) | 44 (10.3) | 3.13 (1.84–5.34) | <0.001 | 3.05 (1.73–5.36) | 0.001 |
| Presence of polyps | 67 (62.6) | 187 (43.7) | 2.16 (1.40–3.34) | <0.001 | 1.46 (0.706–3.03) | 0.685 |
| Presence of adenomas | 55 (51.4) | 139 (32.5) | 2.20 (1.43–3.38) | <0.001 | 1.75 (0.85–3.59) | 0.674 |
| Presence of AA | 30 (28.0) | 72 (16.8) | 1.93 (1.18–3.15) | 0.008 | 0.58 (0.23–1.45) | 0.242 |
| Presence of serrated polyps | 14 (13.1) | 48 (11.2) | 1.19 (0.63–2.25) | 0.589 |
| PCCRC (N = 55) | Controls (N = 139) | OR (95% CI) | p | OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| ≥3 adenomas | 18 (32.7) | 29 (21.0) | 1.83 (0.91–3.67) | 0.087 | ||
| Flat morphology | 6 (10.9) | 19 (13.7) | 0.77 (0.29–2.05) | 0.605 | ||
| Size ≥10 mm | 24 (43.6) | 56 (40.3) | 1.14 (0.61–2.15) | 0.669 | ||
| Size ≥20 mm | 12 (21.8) | 14 (10.1) | 2.49 (1.07–5.80) | 0.030 | 0.85 (0.24–3.01) | 0.536 |
| Proximal adenoma | 28 (50.9) | 71 (51.1) | 0.99 (0.53–1.86) | 0.983 | ||
| Villous component | 15 (27.3) | 21 (15.1) | 2.11 (1.02–4.47) | 0.049 | 1.47 (0.49–4.36) | 0.376 |
| HGD | 7 (12.7) | 9 (6.5) | 2.11 (0.74–5.97) | 0.154 | ||
| Piecemeal polypectomy | 37 (67.3) | 13 (9.4) | 19.92 (8.93–44.42) | <0.001 | 19.89 (8.67–45.61) | <0.001 |
Note: Controls comprised a cohort of colonoscopies in patients without CRC matched 1:4 by sex, age, endoscopist, date, indication of index colonoscopy, and center. Significant values are bolded.
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HGD, high‐grade dysplasia; PCCRC, post‐colonoscopy colorectal cancer.
In the multivariate analysis, the characteristics of the index colonoscopy that were significantly associated with the subsequent development of PCCRC were inadequate bowel preparation (OR 3.05, 95% CI 1.73–5.36; p = 0.001) and piecemeal polypectomy (OR 19.89, 95% CI 8.67–45.61; p < 0.001) (Table 2).
Since some of the index colonoscopies included were performed more than 15 years ago and may not be representative of current quality standards, a second analysis was performed including only those PCCRCs diagnosed within 3 years from the index colonoscopy and their matched controls, and therefore included only index colonoscopies performed between 2012 and 2018 (Supplementary Table S1). In this analysis, inadequate bowel preparation and piecemeal polypectomy remain the only factors independently associated with the subsequent development of PCCRC.
DISCUSSION
A total of 107 PCCRC cases were described and categorized according to their most plausible explanation following the WEO algorithm. In addition, our PCCRC population was compared to a control cohort without CRC adjusted for important confounders to help identify the index colonoscopy‐related factors associated with the development of PCCRC. Our PCCRC rates were slightly lower than those described in previous studies. Regarding the root‐cause analysis, we found that possibly missed lesions are the main cause of PCCRC, especially with a prior examination that was considered adequate. Importantly, in our case‐control study, we found inadequate bowel preparation and a history of piecemeal polypectomy to be independent risk factors for PCCRC.
The WEO consensus proposes using the 3‐year PCCRC rate to benchmark services. Our 3‐year PCCRC rate was 2.2%, which is similar to the rates reported in Canada (3.4%), 7 Utah, USA (3.5%), 11 and Córdoba, Spain (3.6%), 12 but less than the rates reported in England (4.7%), 13 Sweden (7%), 14 and Belgium (7.6%). 15 Differences in methodology used to calculate the PCCRC rate, with different time frames and inclusion or exclusion criteria, could explain these variations. 16 For example, in our study, we exclude high‐risk populations, such as patients with IBD or hereditary nonpolyposis CRC, whereas these patients are often included in previous studies, 11 , 12 , 13 , 14 , 15 which could explain part of the differences. Furthermore, some studies were published before the WEO consensus and do not use a standardized and uniform method to calculate PCCRC. 4
Similar to previous studies, most of the PCCRCs in our series (55.8%) were located in the proximal colon. 6 This right‐side predominance could be explained by the higher rate of inadequate bowel preparation in proximal segments or by right‐sided lesions being more frequently serrated or flat, which make them more difficult to detect and remove completely. 17 In line with previous studies, 13 , 15 we found a relatively high proportion of stage I cancer (26.2%). However, despite the predominance of this early stage, shorter survival times have been reported for PCCRC compared to detected CRC, especially when controlling for both lead time bias and immortal time bias. 14 , 15 The association of proximal tumor location, an earlier stage at diagnosis, and survival disadvantage compared with detected CRCs suggests differences in tumor biology, and further research is needed to clarify whether the mismatch repair or serrated pathways of tumorigenesis play a determining role in the pathogenesis of these lesions. 18 , 19 , 20
In our population, more than 45% of PCCRCs appearing in the first 6–48 months after index colonoscopy were attributed to missed lesions in a prior adequate colonoscopy. Previous studies have already described missed lesions as the main explanation for PCCRC, 21 , 22 and two studies reported up to 66%–70% of PCCRC cases being attributable to missed lesions in a prior adequate examination. 22 , 23 In addition, some difficulties in classifying PCCRC according to the four categories proposed by the WEO were noted. First, to consider a PCCRC for categories C (detected lesion, not resected) or D (likely incomplete resection), we required the identification of an AA in the same bowel segment at index colonoscopy, which was challenging in some cases due to the index colonoscopy report being unclear about the location or the size of the lesion or the inability to recover the piece for pathology analysis. Second, there were cases in which PCCRCs appeared after index colonoscopy in which a NAA or serrated lesion was resected on the same segment, but as these lesions did not fulfill the WEO definition, these PCCRCs were categorized as A or B (possible missed lesion). Finally, some PCCRCs, especially those in the left bowel, were categorized as B (possible missed lesion, prior examination inadequate) because the index colonoscopy had poor bowel preparation or was incomplete, even though the bowel segment where the PCCRC appeared had been adequately examined.
Previous studies have suggested several potential risk factors for PCCRC, including patient factors, such as comorbidity, older age, female sex, diverticular disease, 24 and personal or family history of CRC 11 ; colonoscopy factors such as preparation quality 25 ; endoscopist factors, such as endoscopy volume, colonoscopy completion rate, ADR, or withdrawal time 25 ; and tumor molecular characteristics, such as mismatch repair deficiency. 26 However, the vast majority of these studies compared PCCRC with detected cancers. In our results comparing PCCRC cases' index colonoscopy with a control cohort with colonoscopies without CRC adjusted for age, sex, endoscopist, indication, center, and date, inadequate bowel preparation and prior piecemeal polypectomy were the only factors independently associated with the subsequent development of PCCRC. Regarding inadequate bowel cleansing, some studies have shown that patients with suboptimal bowel preparation present with up to 27% missed AAs in early repeat colonoscopy. 27 , 28 On the other hand, large polyps with piecemeal excision are known to be associated with high rates of incomplete resection and adenoma recurrence. 29 , 30 However, another factor classically linked with PCCRC, incomplete colonoscopy, 26 was not significantly associated in our population. We think this could be explained by our current clinical practice: patients with incomplete procedures undergo second examinations or CT‐colonoscopy in the first 6 months, turning these lesions into diagnosed CRC. To the best of our knowledge, only two other studies 11 , 31 have compared PCCRC with a cohort of non‐CRC individuals to assess index colonoscopy‐related factors. However, its multivariate analysis was not adjusted by important cofounders (e.g., endoscopist or indication), its analysis did not include important potential predictive factors for PCCRC, such as serrated or flat lesions, or piecemeal resection, and in one of them 31 the nomenclature was not actualized according to WEO recommendations (CRC diagnosed up to 1 year after index colonoscopy considered as detected CRC).
Despite our low PCCRC rates, there is still room for improvement. Our results emphasize the need to adhere to post‐polypectomy surveillance guidelines 32 , 33 and ensure optimal follow‐up of patients after index colonoscopy, especially for large polyps with piecemeal excision for which early review of the polypectomy scar is advised due to the high rates of incomplete resection. 32 Furthermore, early repeat colonoscopy or non‐colonoscopy imaging should be performed in the first 6 months after an incomplete index colonoscopy or with suboptimal bowel preparation. 27 Previous studies have proposed the use of FIT in high‐risk populations in the intervals between surveillance colonoscopy to minimize the risk of PCCRC, with a reported detection rate of 2% of CRCs in FIT‐positive patients despite having a colonoscopy in the previous 3 years. 34 Finally, several studies have reported an important association between PCCRC and endoscopist quality indicators 9 , 35 ; therefore, these indicators should be routinely measured and reported in endoscopy units.
Our study has several strengths. This was a large population‐based study performed in academic and non‐academic medical centers, encompassing more than 100,000 colonoscopies with a large number of PCCRC cases. Our PCCRC rate calculation methods are actualized and adapted to the WEO recommendations. Also, as suggested in the WEO consensus, we provide PCCRC rates for various time cutoffs (1, 3, 5, and 10 years) to develop an evidence‐base for different timeframes and how they evolve in time. In our endoscopy services, all colonoscopies are performed by a gastroenterologist, with high‐quality index procedures and endoscopy reports with actualized nomenclature, and bowel cleansing reported using a validated scale. Moreover, we used non‐cancer controls from the same underlying population and adjusted for age, sex, center, date, indication of index colonoscopy, and endoscopist to minimize selection bias and provide an adjustment of OR estimates for important potential confounders. Finally, most of the previous studies regarding PCCRC use administrative databases, whereas in this population‐based study, we used chart review to verify diagnoses of PCCRC and index colonoscopy‐related risk factors.
Our study also has some limitations. First, it is an observational retrospective study with an inherent risk of bias. Second, we were unable to account for important clinical factors (e.g., family history of CRC, obesity, diverticulosis), endoscopist‐related factors (ADR, withdrawal time), or molecular characteristics of the PCCRCs. Finally, some PCCRCs may have been misclassified as detected if patients underwent a colonoscopy performed 6–120 months before the diagnosis at a different center from those included in our study.
In conclusion, following the WEO methodology, we estimated a 3‐year PCCRC rate of 2.2% over a 4‐year period, attributed mostly to missed lesions despite an adequate index colonoscopy. In addition, in our population of 107 PCCRC cases and 428 matched non‐CRC patients, inadequate bowel preparation and piecemeal polypectomy were independently associated with PCCRC, indicating the high‐risk populations where we must target our efforts to try to prevent these lesions. We think our findings may help identify opportunities for improved colonoscopy performance and help other services initiate monitoring and review of their PCCRC cases and rates.
AUTHOR CONTRIBUTIONS
Sandra Baile‐Maxía and Rodrigo Jover conceptualized and designed the study and drafted the manuscript. All authors acquired the data from their respective centers. Sandra Baile‐Maxía, Pedro Zapater, and Rodrigo Jover analyzed and interpreted the data and performed the statistical analysis. All authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version.
CONFLICT OF INTEREST STATEMENT
R.J. has received research grants from MSD and participated as an advisor to MSD, Norgine, Alpha‐Sigma, and GISupply. The rest of the authors have nothing to disclose.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This work was supported by the Instituto de Salud Carlos III (PI17/01756, PI20/01527), the Fundación de Investigación Biomédica de la Comunidad Valenciana–Instituto de Investigación Sanitaria y Biomédica de Alicante Foundation (UGP‐190276), and a grant from the Sociedad Valenciana de Patología Digestiva (SVPD) in 2019. Asociación para la Investigación en Gastroenterología de la Provincia de Alicante (AIGPA), a private association that promotes research in gastrointestinal diseases in Alicante, also supported the logistical aspects of the study.
Baile‐Maxía S, Mangas‐Sanjuan C, Sala‐Miquel N, Barquero C, Belda G, García‐del‐Castillo G, et al. Incidence, characteristics, and predictive factors of post‐colonoscopy colorectal cancer. United European Gastroenterol J. 2024;12(3):309–318. 10.1002/ueg2.12512
DATA AVAILABILITY STATEMENT
Data, analytical methods, and study materials will be made available to other researchers from the corresponding author, [R.J.], upon reasonable request.
REFERENCES
- 1. Winawer SJ, Zauber AG, Ho MN, O'brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(24):1753–1759. [DOI] [PubMed] [Google Scholar]
- 2. Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Bretthauer effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547–1556. 10.1056/nejmoa2208375 [DOI] [PubMed] [Google Scholar]
- 3. Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population‐based study. Clin Gastroenterol Hepatol. 2008;6(10):1117–1121. quiz1064. 10.1016/j.cgh.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 4. Rutter MD, Beintaris I, Valori R, Chiu HM, Corley DA, Cuatrecasas M, et al. World Endoscopy Organization consensus statements on postcolonoscopy and post‐imaging colorectal cancer. Gastroenterology. 2018;155(3):909–925. 10.1053/j.gastro.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 5. Le Clerq CMC, Bouwens MWE, Rondagh EJA, Bakker CM, Keulen ETP, de Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: a population‐based study. Gut. 2014;63(6):957–963. 10.1136/gutjnl-2013-304880 [DOI] [PubMed] [Google Scholar]
- 6. Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population‐based study. Am J Gastroenterol. 2010;105(12):2588–2596. 10.1038/ajg.2010.390 [DOI] [PubMed] [Google Scholar]
- 7. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population‐based analysis. Gastroenterology. 2007;132(1):96–102. 10.1053/j.gastro.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 8. Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8(3):275–279. 10.1016/j.cgh.2009.10.022 [DOI] [PubMed] [Google Scholar]
- 9. Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology. 2017;153(1):98–105. 10.1053/j.gastro.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 10. Lambert R. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–S43. [DOI] [PubMed] [Google Scholar]
- 11. Samadder NJ, Curtin K, Tuohy TMF, Pappas L, Boucher K, Provenzale D, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population‐based study. Gastroenterology. 2014;146(4):950–960. 10.1053/j.gastro.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 12. Muñoz García‐Borruel M, Hervás‐Molina AJ, Rodríguez Perálvarez ML, Moreno Rincón E, Pérez Medrano I, Serrano Ruiz FJ, et al. Post‐colonoscopy colorectal cancer: characteristics and predictive factors. Med Clin. 2018;150(1):1–7. 10.1016/j.medcle.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 13. Anderson R, Burr NE, Valori R. Causes of post‐colonoscopy colorectal cancers based on World Endoscopy Organization System of analysis. Gastroenterology. 2020;158(5):1287–1299.e2. 10.1053/j.gastro.2019.12.031 [DOI] [PubMed] [Google Scholar]
- 14. Forsberg A, Widman L, Bottai M, Ekbom A, Hultcrantz R. Postcolonoscopy colorectal cancer in Sweden from 2003 to 2012: survival, tumor characteristics, and risk factors. Clin Gastroenterol Hepatol. 2020;18(12):2724–2733.e3. 10.1016/j.cgh.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 15. Macken E, Van Dongen S, De Brabander I, Francque S, Driessen A, Van Hal G. Post‐colonoscopy colorectal cancer in Belgium: characteristics and influencing factors. Endosc Int Open. 2019;7(5):E717–E727. 10.1055/a-0751-2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris EJ, Rutter MD, Finan PJ, Thomas JD, Valori R. Post‐colonoscopy colorectal cancer (PCCRC) rates vary considerably depending on the method used to calculate them: a retrospective observational population‐based study of PCCRC in the English National Health Service. Gut. 2015;64(8):1248–1256. 10.1136/gutjnl-2014-308362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tadros M, Anderson JC. Serrated polyps: clinical implications and future directions. Curr Gastroenterol Rep. 2013;15(9):342. 10.1007/s11894-013-0342-4 [DOI] [PubMed] [Google Scholar]
- 18. Sawhney MS, Farrar WD, Gudiseva S, Nelson DB, Lederle FA, Rector TS, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131(6):1700–1705. 10.1053/j.gastro.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 19. Stoffel EM, Erichsen R, Froslev T, Pedersen L, Vyberg M, Koeppe E, et al. Clinical and molecular characteristics of post‐colonoscopy colorectal cancer: a population‐based study. Gastroenterology. 2016;151(5):870–878.e3. 10.1053/j.gastro.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samadder NJ, Neklason D, Snow A, Samowitz W, Cessna MH, Rowe K, et al. Clinical and molecular features of post‐colonoscopy colorectal cancers. Clin Gastroenterol Hepatol. 2019;17(13):2731–2739.e2. 10.1016/j.cgh.2019.02.040 [DOI] [PubMed] [Google Scholar]
- 21. Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129(1):34–41. 10.1053/j.gastro.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 22. Beaton D, Beintaris J, Rutter MD. Utilization and reproducibility of World Endoscopy Organization post‐colonoscopy colorectal cancer algorithms: retrospective analysis. Endoscopy. 2022;54(3):270–277. 10.1055/a-1409-5531 [DOI] [PubMed] [Google Scholar]
- 23. Leung LJ, Lee JK, Merchant SA, Jensen CD, Alam A, Corley DA. Post‐colonoscopy colorectal cancer etiologies in a large integrated US Health Care Setting. Gastroenterology. 2023;164(3):470–472.e3. 10.1053/j.gastro.2022.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh S, Singh PP, Murad MH, Samadder JN. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta‐analysis. Am J Gastroenterol. 2014;109(9):1375–1389. 10.1038/ajg.2014.171 [DOI] [PubMed] [Google Scholar]
- 25. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140(1):65–72. 10.1053/j.gastro.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 26. Dossa F, Sutradhar R, Saskin R, Hsieh E, Henry P, Richardson DP, et al. Clinical and endoscopist factors associated with post‐colonoscopy colorectal cancer in a population‐based sample. Colorectal Dis. 2021;23(3):635–645. 10.1111/codi.15400 [DOI] [PubMed] [Google Scholar]
- 27. Baile‐Maxía S, Mangas‐Sanjuan C, Medina‐Prado L, Martínez‐Sempere J, Murcia O, Ruíz‐Gómez F, et al. Diagnostic yield of early repeat colonoscopy after suboptimal bowel preparation in a fecal immunochemical test‐based screening program. Endoscopy. 2020;52(12):1093–1100. 10.1055/a-1191-3011 [DOI] [PubMed] [Google Scholar]
- 28. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;14(73):1207–1214. 10.1016/j.gie.2011.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, et al. Incomplete polyp resection during colonoscopy‐results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144(1):74–80.e71. 10.1053/j.gastro.2012.09.043 [DOI] [PubMed] [Google Scholar]
- 30. Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49(6):731–735. 10.1016/s0016-5107(99)70291-9 [DOI] [PubMed] [Google Scholar]
- 31. Tollivoro TA, Jensen CD, Marks AR, Zhao WK, Schottinger JE, Quinn VP, et al. Index colonoscopy‐related risk factors for postcolonoscopy colorectal cancers. Gastrointest Endosc. 2019;89(1):168–176.e3. 10.1016/j.gie.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, et al. Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – update 2020. Endoscopy. 2020;52(8):687–700. 10.1055/a-1185-3109 [DOI] [PubMed] [Google Scholar]
- 33. Mangas‐Sanjuan C, Jover R, Cubiella J, Marzo‐Castillejo M, Balaguer F, Bessa X, et al. Endoscopic surveillance after colonic polyps and colorrectal cancer resection. 2018 update. Gastroenterol Hepatol. 2019;42(3):188–201. 10.1016/j.gastre.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 34. Kim NH, Jung YS, Lim JW, Park JH, Park DI, Sohn CI. Yield of repeat colonoscopy in asymptomatic individuals with a positive fecal immunochemical test and recent colonoscopy. Gastrointest Endosc. 2019;89(5):1037–1043. 10.1016/j.gie.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 35. Corley DA, Christopher DJ, Amy RM, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298–1306. 10.1056/nejmoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data, analytical methods, and study materials will be made available to other researchers from the corresponding author, [R.J.], upon reasonable request.


