Abstract
Background & Aims
Hepatic fat content can be non‐invasively estimated by controlled attenuation parameter (CAP) during transient elastography. The aim of this study was to examine the determinants and predictors of CAP values in individuals with metabolic dysfunction.
Methods
We enrolled 1230 consecutive apparently healthy individuals (Liver‐Bible‐2022 cohort) with ≥3 metabolic dysfunction features. CAP was measured by Fibroscan. CAP determinants and predictors were identified using backward stepwise analysis and introduced in generalized linear models.
Results
Participants were predominantly males (82.9%), mean age was 53.8 ± 6.4 years, 600 (48.8%) had steatosis (CAP ≥ 275 dB/m), and 27 had liver stiffness measurement (LSM) ≥ 8 kPa. CAP values correlated with LSM (p < 10−22). In multivariable analysis, fasting insulin and abdominal circumference (AC) were the main determinants of CAP (p < 10−6), together with body mass index (BMI; p < 10−4), age, diabetes, triglycerides, ferritin, and lower HDL and thyroid stimulating hormone (TSH; p < 0.05 for all). In a subset of 592 participants with thyroid hormone measurement, we found an association between higher free triiodothyronine levels, correlating with lower TSH, and CAP values, independent of TSH and of levothyroxine treatment (p = 0.0025). A clinical CAP score based on age, BMI, AC, HbA1c, ALT, and HDL predicted CAP ≥ 275 dB/m with moderate accuracy (AUROC = 0.73), which was better than that of the Fatty Liver Index and of ALT (AUROC = 0.70/0.61, respectively) and validated it in multiple cohorts.
Conclusion
Abdominal adiposity and insulin resistance severity were the main determinants of CAP in individuals with metabolic dysfunction and may improve steatotic liver disease risk stratification. CAP values were modulated by the hypophysis‐thyroid axis.
Keywords: abdominal circumference, CAP, controlled attenuation parameter, fasting insulin, fibroscan, liver stiffness measurement, LSM, metabolic syndrome, steatosis, thyroid, transient elastography

Key summary.
Summarize the established knowledge on this subject
Adiposity, insulin resistance and dyslipidemia drive steatotic liver disease in high‐risk individuals.
What are the significant and/or new findings of this study?
Metabolic‐dysfunction associated steatotic liver disease (MASLD) is associated with an impairment of the hypophysis‐thyroid axis.
A non‐invasive score (clinical CAP score) is useful in identifying MASLD in high‐risk individuals.
Modulation of thyroid hormone signaling shows potential for MASLD treatment.
INTRODUCTION
The prevalence of non‐alcoholic fatty liver disease (NAFLD), recently defined as metabolic‐dysfunction associated steatotic liver disease (MASLD) when it is associated with metabolic alterations and insulin resistance (IR) 1 , has progressively risen in the last decades alongside that of obesity and type 2 diabetes (T2D), becoming the most common chronic liver disease in the population. 2
Although hepatic fat accumulation was previously considered an innocent bystander in the natural history of liver diseases, recent evidence from experimental and human genetics suggests that it represents a key driver in the development of liver disease and its complications. 3 , 4 Because of the prognostic implications related to the risk of liver fibrosis and hepatocellular carcinoma, the early detection of MASLD in individuals with metabolic dysfunction has clinical relevance. 5 MASLD is mainly diagnosed by imaging, and the most commonly used approach is still conventional ultrasound since it is relatively cheap, non‐invasive and widely available. 6 A more accurate quantification of hepatic fat content is provided by magnetic resonance proton density fat fraction (MRI‐PDFF), 7 but its use in clinical practice is limited because of the costs and limited availability.
In the last decade, the “Controlled Attenuation Parameter” (CAP) has been implemented in vibration‐controlled elastography (VCTE) devices (“FibroScan”®). CAP is a measure of hepatic viscoelasticity, correlating with the number of lipid droplets accumulated in hepatocytes. 8 CAP is a promising non‐invasive tool to detect hepatic steatosis during screening for fibrosis by VCTE in individuals with metabolic dysfunction, 9 as recommended by the major scientific societies, 10 , 11 , 12 showing low failure and high reliability rates. 13 Therefore, CAP measurement can allow the identification of individuals with MASLD without advanced fibrosis but at risk of progression. However, while the prioritization for fibrosis screening is guided by non‐invasive scores (e.g. FIB‐4), little is known about the predictive accuracy of fatty liver indices.
Within this context, the aim of this study was to examine the independent determinants (that is, causes of hepatic fat accumulation) and clinical predictors (including biomarkers of liver damage) of CAP values in a cohort of apparently healthy individuals at risk of MASLD because of metabolic dysfunction. The overall goal was to better characterize the main drivers of disease at an early stage of development and to improve disease risk stratification.
PATIENTS AND METHODS
Study cohort
Liver‐bible‐2022 cohort
We considered 1230 individuals, who were consecutively enrolled from June 2019 to June 2022 in a primary prevention program conducted among candidate blood donors in Milan, Italy, and aimed at the early diagnosis and prevention of hepatic and extra‐hepatic complications of fatty liver disease (the Liver‐Bible‐2022 Biobank cohort). Part of this cohort has previously been described. 14 , 15 The Liver‐Bible‐2022 cohort included apparently healthy blood donors, aged 40–65, who underwent non‐invasive screening of liver and cardiovascular damage due to the presence of at least three criteria of metabolic dysfunction (detailed in the Supporting Information S1: Methods). None reported use of alcohol ≥ 60/40 g/day in M/F. All markers for HCV, HBV, and HIV infections were negative. Laboratory assessment is reported in the Supporting Information S1: Methods; blood samples were collected after overnight fasting.
The presence of hepatic steatosis and liver fibrosis was non‐invasively evaluated with liver stiffness measurement (LSM) and CAP by VCTE with FibroScan® 530 Compact (Echosens, Paris, France). The examination was conducted as recommended by the manufacturer. 16 Patients were in overnight fasting condition and placed on supine position with the right arm behind the head; the probe transducer was place between the rib bones at the level of the right hepatic lobe on the median axillary line. The device was equipped with an automatic probe selection tool. Each exam was performed using the M or XL probe as prompted. Each examination was considered valid when: (a) at least 10 successful measurements were acquired; and (b) the variability of LSM, evaluated as LSM interquartile range (IQR)/LSM ratio, was ≤0.30 or >0.30 and LSM median < 7.1 kPa. 17 CAP ≥ 275 dB/m and LSM ≥ 8 kPa were used, respectively, to identify steatotic liver disease (SLD) and the possible presence of advanced fibrosis according to the EASL Clinical Practice Guidelines. 10
The study was approved by the Ethical committee of the Fondazione IRCCS Ca’ Granda, and each patient gave written informed consent to the study (ID 1650, revision 23 June 2020).
The MAFALDA, NHANES, and UK Biobank (UKBB) validation cohorts are described in the Supporting Information S1: Methods.
Statistical analysis
For descriptive statistics, continuous variables are shown as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate; categorical variables are shown as number and proportion. Not normally distributed variables (e.g. ferritin) were log‐transformed before entering the analysis.
Observational associations were performed by fitting data to generalized linear models (GLM). Binary outcomes were examined by fitting logistic regression models. GLM and logistic regression models were adjusted for sex, age, ethnicity and clinical or lifestyle factors that were selected by a backward stepwise selection procedure (p to leave <0.1). Additional approaches were undertaken in sensitivity analyses to confirm the robustness of covariate selection for the predictive models.
Performance of non‐invasive fatty liver markers was assessed by the area under the receiver operating characteristic curve (AUROC) in the derivation and validation cohorts. AUROCs were compared using the DeLong test.
Statistical analyses were carried out using the JMP Pro 16.0 Statistical Analysis Software (SAS Institute, Cary, NC) and the software R, version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). p values < 0.05 (two tailed) were considered statistically significant.
RESULTS
Study cohort
The clinical features of the 1230 individuals included in the Liver‐Bible‐2022 cohort are presented in Table 1, left panel.
TABLE 1.
Clinical features of the overall liver‐bible‐2022 cohort (left panel) and after stratification by CAP values (CAP < or ≥275 dB/m, right panel).
| Overall (n = 1230) | CAP < 275 dB/m (n = 630, 51.2%) | CAP ≥ 275 dB/m (n = 600, 48.8%) | p‐value b | |
|---|---|---|---|---|
| Age, years | 53.8 ± 6.4 | 53.7 ± 6.4 | 54.0 ± 6.4 | 0.34 |
| Sex, F | 210 (17.1) | 121 (19.2) | 89 (14.8) | 0.042 |
| BMI, kg/m2 | 28.6 ± 3.2 | 27.6 ± 2.8 | 29.6 ± 3.2 | 4 × 10 − 32 |
| Obesity, yes | 406 (33.0) | 132 (20.9) | 274 (45.7) | 2 × 10 − 19 |
| Abdominal circumference, cm | 102.6 ± 9.0 | 99.5 ± 7.8 | 105.8 ± 9.0 | 4 × 10 − 30 |
| Glucose, mg/dL | 96.7 ± 15.5 | 96.2 ± 14.6 | 97.3 ± 16.4 | 0.20 |
| Insulin, mIU/L | 14.7 ± 9.1 | 12.7 ± 7.3 | 16.8 ± 10.3 | 6 × 10 − 14 |
| HOMA‐IR, units | 3.5 ± 2.4 | 3.0 ± 1.9 | 4.1 ± 2.7 | 2 × 10 − 13 |
| HbA1c, mM | 35.7 ± 4.8 | 35.2 ± 3.8 | 36.2 ± 5.7 | 0.0002 |
| T2D, yes | 18 (1.5) | 2 (0.32) | 16 (2.7) | 0.0042 |
| Hypertension, yes | 1055 (85.8) | 529 (84.0) | 526 (87.7) | 0.064 |
| LDL‐C, mg/dL | 125.7 ± 31.4 | 126.0 ± 31.2 | 125.5 ± 31.7 | 0.79 |
| HDL‐C, mg/dL | 45.4 ± 10.1 | 46.4 ± 10.9 | 44.3 ± 9.1 | 0.0003 |
| TG, mg/dL | 160.3 ± 83.9 | 158.7 ± 91.8 | 162.1 ± 74.6 | 0.48 |
| Hypercholesterolemia, yes | 609 (49.5) | 318 (50.5) | 291 (48.5) | 0.49 |
| ALT, IU/L | 26 [21–35] | 25 [20–33] | 29 [22–39] | 2 × 10 − 9 |
| AST, IU/L | 23 [19–27] | 22 [19–25] | 23 [20–28] | 0.0004 |
| GGT, IU/L | 23 [17–32] | 22 [15–30] | 24 [18–34] | 0.057 |
| Ferritin, ng/mL | 78 [43–139] | 75 [42–133] | 79 [45–153] | 0.0058 |
| CRP, md/dL | 0.15 [0.09–0.26] | 0.13 [0.08–0.23] | 0.16 [0.10–0.29] | 0.63 |
| TSH, mU/L | 1.71 ± 1.46 | 1.78 ± 1.86 | 1.64 ± 0.85 | 0.13 |
| fT3, ng/L a | 3.34 ± 0.39 | 3.31 ± 0.43 | 3.39 ± 0.33 | 0.013 |
| fT4, ng/L a | 11.87 ± 1.48 | 11.91 ± 1.57 | 11.84 ± 1.38 | 0.61 |
| CAP, dB/m | 274.6 ± 41.1 | 242.3 ± 23.1 | 308.6 ± 25.5 | ‐ |
| IQR CAP, dB/m | 30 [23–39] | 31 [24–40] | 30 [23–39] | 0.32 |
| Levothyroxin replacement therapy, yes | 21 (1.7) | 11 (1.7) | 10 (1.7) | 0.91 |
| LSM, kPa | 4.9 ± 1.3 | 4.6 ± 1.1 | 5.2 ± 1.3 | 2 × 10 − 15 |
| LSM IQR/LSM, % | 15 [12–20] | 15 [11–20] | 16 [12–20] | 0.051 |
| Advanced fibrosis, LSM ≥ 8 kPa | 27 (2.2) | 5 (0.8) | 22 (3.7) | 0.002 |
Note: Values are reported as mean ± SD, number (%) or median [IQR] as appropriate. Statistically significant values are highlighted in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CRP, C‐reactive protein; fT3, free triiodothyronine; fT4, free thyroxine; GGT, gamma‐glutamyl transferase; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; LSM, liver stiffness measurement; LSM IQR/LSM, LSM interquartile range/LSM ratio; T2D, type 2 diabetes; TG, triglycerides; TSH, thyroid‐stimulating hormone.
Available in 592.
At logistic regression model.
The majority of Fibroscan examination could be performed using the M probe (n = 1127, 91.6%), whereas for the remaining, we used the XL probe. MASLD was detected in about half of the participants, whereas only 27 individuals (2.2%) had LSM ≥ 8.0 kPa, consistent with the presence of advanced liver fibrosis. As expected, although the cohort was characterized on average by mild liver damage, LSM was associated with CAP values (estimate +0.008, SE 0.001, p = 6.9 × 10−23).
Independent determinants of CAP values
The independent determinants of CAP values at GLM considering as covariates those selected by backward stepwise analysis are shown in Table 2. At univariate analysis (Table 2, left panel) CAP values were associated with older age (p = 0.021) and alterations in metabolic profile, in particular adiposity (abdominal circumference (AC), p = 1 × 10−56; BMI, p = 9 × 10−49), glucose metabolism (fasting insulin, p = 3 × 10−27; hyperglycemia, p = 0.0095; and T2D, p = 0.0002), lower high‐density lipoprotein cholesterol (HDL‐C) (p = 0.0004), higher levels of ferritin (p = 6 × 10−5) and nearly with higher TG (p = 0.057).
TABLE 2.
Independent determinants of CAP values in 1230 individuals from the liver‐bible‐2022 cohort.
| Estimate a | SE a | p‐value a | Estimate b | SE b | p‐value b | Estimate c | SE c | p‐value c | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 0.42 | 0.18 | 0.021 | 0.59 | 0.17 | 0.0005 | 0.59 | 0.17 | 0.0005 |
| BMI, kg/m2 | 4.98 | 0.34 | 9 × 10 − 49 | 2.22 | 0.55 | 5 × 10 − 5 | 2.23 | 0.55 | 5 × 10 − 5 |
| AC, cm | 1.89 | 0.12 | 1 × 10 − 56 | 1.07 | 0.20 | 9 × 10 − 8 | 1.06 | 0.20 | 1 × 10 − 7 |
| Glucose, mg/dL | 0.19 | 0.07 | 0.0095 | 0.14 | 0.07 | 0.063 | 0.14 | 0.07 | 0.060 |
| Insulin, mIU/L | 1.32 | 0.12 | 3 × 10 − 27 | 0.68 | 0.12 | 2 × 10 − 8 | 0.68 | 0.12 | 1 × 10 − 8 |
| T2D, yes | 18.05 | 4.85 | 0.0002 | 10.48 | 4.46 | 0.019 | 10.31 | 4.46 | 0.021 |
| HDL‐C, mg/dL | −0.41 | 0.11 | 0.0004 | −0.28 | 0.11 | 0.015 | −0.28 | 0.11 | 0.015 |
| TG, mg/dL | 0.03 | 0.01 | 0.057 | 0.03 | 0.01 | 0.038 | 0.03 | 0.01 | 0.037 |
| Ferritin, log ng/mL | 5.48 | 1.37 | 6 × 10 − 5 | 3.63 | 1.27 | 0.0042 | 3.60 | 1.27 | 0.0045 |
| TSH, mU/L | −0.97 | 0.80 | 0.23 | −1.46 | 0.72 | 0.044 | −1.57 | 0.74 | 0.032 |
Note: Statistically significant values are highlighted in bold.
Abbreviations: AC, abdominal circumference; BMI, body mass index; CAP, controlled attenuation parameter; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; MDS, Mediterranean diet score; T2D, type 2 diabetes; TG, triglycerides; TSH, thyroid‐stimulating hormone.
At GLM (unadjusted). Variables were selected at Backward Stepwise Regression analysis: p‐value to leave = 0.10. Variables fitted in Backward Stepwise Regression analysis: age, sex, ethnicity, BMI, obesity, abdominal circumference, glucose, insulin, HOMA‐IR, HbA1c, T2D, hypertension, LDL‐C, HDL‐C, TG, hypercholesterolemia, ferritin, TSH, MDS, MDS >7, number of alcohol drinks/week, number of soft drinks/week, hours of physical activity/week.
At GLM, adjusted for sex, ethnicity and reported variables.
At GLM, further adjustment for Levothyroxine replacement.
At multivariable analysis (Table 2, middle panel), CAP values were associated with older age (0.59 ± 0.17, p = 0.0005), larger AC (estimate 1.07 ± 0.20 per 1 cm increase, p = 9 × 10−8) and higher BMI (estimate 2.22 ± 0.55, p = 5 × 10−5), fasting insulin (estimate 0.68 ± 0.12, p = 2 × 10−8) and T2D diagnosis (estimate 10.48 ± 4.46, p = 0.019), higher ferritin (estimate 3.63 ± 1.27, p = 0.0042) and higher TG (estimate 0.03 ± 0.01, p = 0.038), whereas HDL‐C maintained a protective association (estimate −0.28 ± 0.11, p = 0.015). In addition, an independent protective association between thyroid‐stimulating hormone(TSH) levels and CAP values emerged (estimate −1.46 ± 0.72, p = 0.044), and was maintained even after adjustment for levothyroxine replacement therapy (estimate −1.75 ± 0.74, p = 0.032; Table 2, right panel).
Similar results, confirming the robustness of the independent determinants identified, were obtained using the forward stepwise analysis to select the independent determinants (Table S1), except that glucose and insulin levels were substituted by HOMA‐IR (an index of IR directly proportional to glucose and insulin).
The aforementioned associations were not modified when CAP values were also adjusted for variability of CAP measurement (IQR) and the type of probe (not shown in details).
Independent determinants of steatosis
The clinical characteristics of the Liver‐Bible‐2022 cohort stratified by the presence of steatosis as non‐invasively estimated by CAP ≥ 275 dB/m are presented in Table 1, right panel, whereas its independent determinants are shown in Table 3. Steatosis was associated with indices of adiposity (AC, p = 4.0 × 10−30; BMI, p = 2.0 × 10−26), fasting insulinemia (p = 6.0 × 10−14), T2D (p = 0.0042) and lower HDL (p = 0.0003). Moreover, steatosis tended to be associated with higher alcohol intake (drinks per week; p = 0.071). No significant association was detected between steatosis and other lifestyle and dietary factors.
TABLE 3.
Independent determinants of CAP ≥ 275 dB/m in 1230 individuals from the liver‐bible‐2022 cohort.
| OR a | 95% c.i. a | p‐value a | OR b | 95% c.i. b | p‐value b | OR c | 95% c.i. c | p‐value c | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 1.01 | 0.99–1.03 | 0.34 | 1.02 | 0.99–1.04 | 0.094 | 1.02 | 1.00–1.04 | 0.094 |
| BMI, kg/m2 | 1.27 | 1.21–1.32 | 2 × 10 − 26 | 1.11 | 1.04–1.19 | 0.0019 | 1.11 | 1.04–1.19 | 0.0019 |
| AC, cm | 1.09 | 1.08–1.11 | 4 × 10 − 30 | 1.06 | 1.03–1.09 | 8 × 10 − 6 | 1.06 | 1.03–1.09 | 8 × 10 − 6 |
| Insulin, mU/mL | 1.06 | 1.05–1.08 | 6 × 10 − 14 | 1.03 | 1.01–1.05 | 0.0002 | 1.03 | 1.01–1.05 | 0.0002 |
| T2D, yes | 8.60 | 1.97–37.58 | 0.0042 | 6.89 | 1.49–31.78 | 0.013 | 6.87 | 1.49–31.79 | 0.014 |
| HDL‐C, mg/dL | 0.98 | 0.97–0.99 | 0.0003 | 0.98 | 0.97–0.99 | 0.0026 | 0.98 | 0.97–0.99 | 0.0026 |
| TSH, mU/L | 0.92 | 0.81–1.03 | 0.13 | 0.87 | 0.76–0.99 | 0.046 | 0.87 | 0.76–0.99 | 0.046 |
| Alcohol drinks, n/week | 1.02 | 1.00–1.04 | 0.071 | 1.02 | 1.00–1.04 | 0.056 | 1.02 | 1.00–1.04 | 0.056 |
Note: Statistically significant values are highlighted in bold.
Abbreviations: 95% c.i., 95% confidence interval; AC, abdominal circumference; BMI, body mass index; CAP, controlled attenuation parameter; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; MDS, Mediterranean diet score; OR, odds ratio; T2D, type 2 diabetes; TG, triglycerides; TSH, thyroid‐stimulating hormone.
At logistic regression (unadjusted). Variables were selected at Backward Stepwise Regression analysis: p‐value to leave = 0.10. Variables fitted in backward stepwise regression analysis were age, sex, ethnicity, BMI, obesity, abdominal circumference, glucose, insulin, HOMA‐IR, T2D, hypertension, LDL‐C, HDL‐C, TG, hypercholesterolemia, ferritin, TSH, MDS, MDS >7, number of alcohol drinks/week, number of soft drinks/week, hours of physical activity/week.
At logistic regression, adjusted for sex, ethnicity and reported variables.
At logistic regression, further adjustment for Levothyroxine replacement.
At multivariable analysis (Table 3, right panel), independent determinants of steatosis were adiposity (AC, OR 1.06, 95% CI 1.03–1.09, p = 8 × 10−6; BMI, OR 1.11, 95% CI 1.04–1.19, p = 0.0019), fasting insulin levels (odds ratio [OR] 1.03, 95% CI 1.01–1.05, p = 0.0002), T2D (OR 6.89, 95% CI 1.49–31.78, p = 0.013), lower HDL‐C (OR 0.98, 95% CI 0.97–0.99, p = 0.0026), and nearly alcohol consumption (OR 1.02 per drink/week, 95% CI 1.00–1.04, p = 0.056). TSH levels displayed a protective association against steatosis (OR 0.87, 95% CI 0.76–0.99, p = 0.046), independent of levothyroxine replacement (OR 0.87, 95% CI 0.76–0.99, p = 0.046).
The selection of independent determinants of steatosis using the forward stepwise analysis led to similar results, except that HOMA‐IR substituted fasting insulinemia (Table S2), confirming the robustness of the independent determinants identified.
The relationship between the activation status of the hypophysis‐thyroid axis and steatosis in a subset of 592 individuals for whom complete hormone levels were available is reported in the Supporting Information S1: Results.
Independent predictors of steatosis
The independent predictors of CAP ≥ 275 dB/m in the Liver‐Bible‐2022 cohort, selected by backward stepwise analysis, are shown in Table 4. Higher ALT levels and indices of adiposity (AC and BMI) were the parameters most robustly associated with the presence of steatosis, together with older age, higher HbA1c and lower HDL‐C.
TABLE 4.
Independent predictors of CAP ≥ 275 dB/m in the liver‐bible‐2022 cohort.
| OR | 95% c.i. | p‐value a | |
|---|---|---|---|
| Age, years | 1.02 | 1.01–1.04 | 0.031 |
| BMI, kg/m2 | 1.12 | 1.05–1.20 | 0.0003 |
| Abdominal circumference, cm | 1.05 | 1.03–1.08 | 3.4 × 10 − 6 |
| HbA1c, mM | 1.04 | 1.01–1.07 | 0.0091 |
| HDL‐C, mg/dL | 0.98 | 0.97–0.99 | 0.0031 |
| ALT, IU/L | 1.02 | 1.01–1.03 | 5.2 × 10 − 7 |
Note: Statistically significant values are highlighted in bold.
Abbreviations: 95%, c.i., 95% confidence interval ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CRP, C‐reactive protein; GGT, gamma‐glutamyl transferase; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; TG, triglycerides; TSH, thyroid‐stimulating hormone.
At logistic regression, adjusted for the covariates specified in the table. Variables were selected at Backward Stepwise Regression analysis: p‐value to leave = 0.05. Variables fitted in Backward Stepwise Regression analysis: age, sex, BMI, abdominal circumference, AST, ALT, GGT, platelet count, glucose, HbA1c, LDL‐C, HDL‐C, TG, ferritin, TSH, and CRP.
Based on the aforementioned variables, we developed a clinical predictive score (the CAP‐score, CAPS; Table S5) that can be calculated as follows: (0.0225 × age) + (0.1173 × BMI) + (0.0537 × AC) + (0.0371 × HbA1c) + (0.0249 × ALT) − (0.0192 * HDL‐C). In the Liver‐Bible‐2022 cohort, CAPS had an AUROC of 0.734 for predicting CAP ≥275dB/m, and it was associated with a higher risk of steatosis (OR 2.72 per 1 point increase, 95% 2.33–3.16, p = 5 × 10−38). A value of ≥10.982 (hereinafter defined as “positive” test) was identified as the best single cut‐off for CAPS, with 76.0% sensitivity and 61.0% specificity. A positive CAPS was associated with an almost 5‐fold increased risk of steatosis in the Liver‐Bible‐2022 cohort (OR 4.85, 95% CI 3.79–6.20, p = 3 × 10−36).
Inclusion of insulin and even TSH in this predictive model led to only marginal improvement in the AUROC of steatosis (from 0.734 to 0.738 and 0.740, respectively, p = 0.92 and p = 0.97) at the price of limiting its applicability in clinical practice.
We next compared the diagnostic performance of CAPS with other non‐invasive fatty liver markers currently used in clinical practice, namely ALT levels, Fatty Liver Index (FLI) and Hepatic Steatosis Index (HSI). Positive CAPS predicted the presence of steatosis more accurately than the other non‐invasive scores, although the accuracy was moderate (CAPS vs. ALT levels, p = 2 × 10−13; CAPS vs. FLI, p = 3 × 10−4; and CAPS vs. HSI, p = 5 × 10−5; Table S6 and Figure 1).
FIGURE 1.
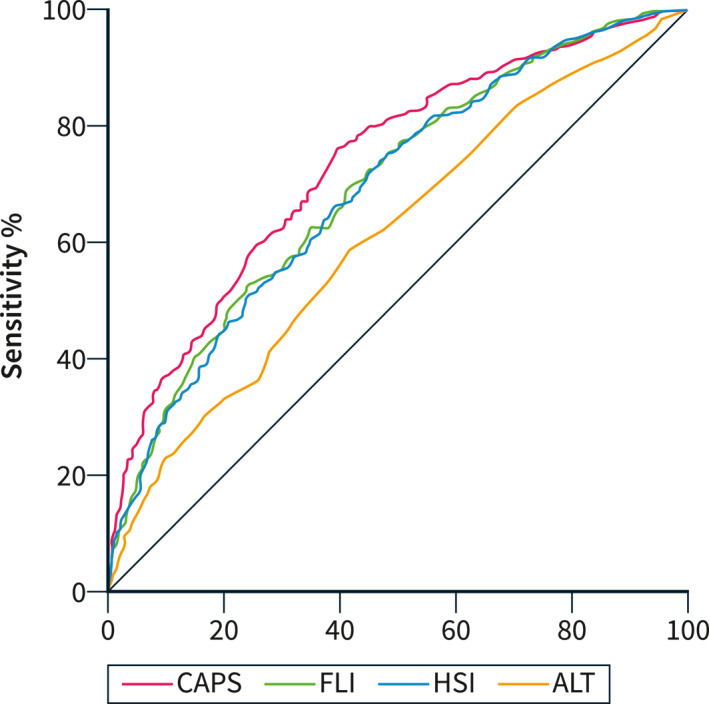
Comparison of the diagnostic accuracy of non‐invasive markers of SLD in the liver‐bible‐2022 cohort.
We also tested the performance of non‐invasive fatty liver markers in three independent external validation cohorts (MAFALDA cohort, NHANES 2017–2018 cohort and UKBB cohort; Table 5). In all three cohorts, CAPS showed a satisfactory performance for detecting steatosis (AUROCs 0.76–0.84). In MAFALDA, CAPS had a higher performance than FLI and HSI (p = 0.017 and 0.011, respectively), whereas no difference was found compared with ALT levels (p = 0.34). In NHANES 2017–2018 and UKBB, CAPS had a higher performance than HSI (p = 6 × 10−5 and 8 × 10−5, respectively) and ALT levels (p = 7 × 10−9 and 2 × 10−89, respectively), and a similar performance to FLI (p = 0.82 for both).
TABLE 5.
Diagnostic accuracy of non‐invasive markers for SLD diagnosis in the external validation cohorts.
| MAFALDA (n = 264) | NHANES 2017–2018 (n = 936) | UK biobank (n = 21,042) | ||||
|---|---|---|---|---|---|---|
| AUROC (95% c.i.) | p‐value | AUROC (95% c.i.) | p‐value | AUROC (95% c.i.) | p‐value | |
| CAPS | 0.76 (0.70–0.82) | ‐ | 0.78 (0.75–0.81) | ‐ | 0.84 (0.83–0.85) | ‐ |
| ALT | 0.79 (0.74–0.85) | 0.34 | 0.66 (0.62–0.69) | 7 × 10−09 | 0.71 (0.70–0.72) | 2 × 10−89 |
| FLI | 0.73 (0.66–0.79) | 0.017 | 0.78 (0.75–0.81) | 0.82 | 0.84 (0.83–0.85) | 0.82 |
| HSI | 0.70 (0.64–0.77) | 0.011 | 0.74 (0.70–0.77) | 6 × 10−05 | 0.82 (0.81–0.83) | 8 × 10−05 |
Abbreviations: 95% c.i., 95% confidence interval; ALT, alanine aminotransferase; AUROC, area under the receiver operating characteristic curve; CAPS, CAP‐score; FLI, fatty liver index; HSI, hepatic steatosis index.
DISCUSSION
In this study, we examined the clinical determinants and predictors of hepatic fat content and steatosis, as estimated by CAP values, in a biobank cohort of 1230 apparently healthy middle‐aged blood donors at risk of liver disease due to metabolic dysfunction.
We were prompted by two main considerations. First, converging data from epidemiological, clinical, human genetics and experimental studies suggest that steatosis is a key driver in the development and progression of MASLD. 3 , 4 Secondly, measurement of CAP values is cheap, easy to perform and highly reproducible, and it is progressively being implemented in clinical practice to non‐invasively assess hepatic fat accumulation during screening for advanced liver fibrosis in high‐risk individuals with metabolic dysfunction and abnormal fibrosis scores. 10 , 12 In fact, evaluation of LSM alone may miss patients with less advanced but rapidly progressive disease due to the presence of high hepatic fat and lipotoxicity. In clinical settings where the MASLD prevention or early treatment is the priority, it may therefore be useful to identify by simple non‐invasive scores, among individuals with metabolic dysfunction, also those with a high likelihood of MASLD irrespective of advanced fibrosis. Furthermore, available non‐invasive scores of steatosis still lack validation in a large cohort of patients with metabolic dysfunction with direct quantification of hepatic fat by CAP.
The main findings were that: (a) adiposity and the severity of IR remain the main determinants of CAP even among individuals with metabolic dysfunction; (b) other endocrine alterations, and in particular modulation of the hypophysis‐thyroid axis as captured by TSH levels influence CAP levels; c) a clinical score taking into consideration measurements of overall and abdominal adiposity, IR and liver enzymes outperforms currently available scores in identifying patients with high values of CAP consistent with a diagnosis of early stages of MASLD.
Indeed, in the Liver‐Bible‐2022 cohort the main determinants of both CAP values and MASLD were AC, BMI, and T2D. Importantly, AC, BMI and T2D were associated with CAP and steatosis independently of each other, with a larger effect of AC. AC is a better predictor of visceral fat than BMI, 18 in line with the notion that the distribution and quality of fat, besides the total amount, is the main driver of MASLD. Indeed, visceral fat is directly linked with the development and the progression of MASLD, 19 , 20 and it is a predictor of liver‐specific mortality in patients with MASLD. 20 These data are in line with previous evidence indicating that CAP values are influenced by BMI, 9 , 21 , 22 T2D 9 , 22 and metabolic comorbidities. 9 , 21
Notably, among the other determinants of CAP and steatosis, we identified hyperinsulinemia, which may be secondary to IR due to a suboptimal cellular response to circulating insulin, or a consequence of downregulation of hepatic insulin clearance due to excess hepatic fat accumulation. 23 On one hand, proinflammatory cytokines derived from adipose tissue and the increase in free fatty acids worsen IR in patients with MASLD. 24 On the other hand, during metabolic dysfunction in the liver, there is a paradoxical dissociation between the lack of suppression of hepatic glucose output and increased de novo lipogenesis in response to insulin. This apparent conundrum is linked to a different response in the insulin signaling pathways that drive cholesterol and glucose homeostasis, resulting in the classic T2D triad of hyperinsulinemia‐hyperglycemia‐hypertriglyceridemia. 25 , 26 Unsurprisingly, another typical manifestation of hepatic IR, namely reduced HDL levels, 27 was the last independent determinant of steatosis we identified in the cohort. In keeping, we showed that there is a causal association between genetically determined hepatic fat accumulation and the development of IR and diabetes, with a borderline impact on HDL levels. 28 The present data are not helpful to dissect causality in this association but support the notion that an early detection and treatment of metabolic alterations could prevent MASLD onset, the development of T2D and cardiovascular complications.
An intriguing finding was the evidence of a protective association between higher TSH levels and lower CAP values. This association was independent of several indices of IR and the other clinical risk factors. In addition, it was confirmed in sensitivity analyses in participants with TSH levels within the normal range and not taking levothyroxine supplementation. Previous observational studies identified a robust relationship between impaired thyroid function and the presence and severity of MASLD, 29 and a causal association emerged from Mendelian randomization approaches. 30 The association was also observed in euthyroid individuals. 31 In addition, previous studies reported increased conversion of T4 to reverse‐T3 in patients with chronic liver injury, which may be involved in disease predisposition due to the detrimental impact on intracellular fat oxidation. 32 In the present cohort, however, lower TSH, and in a subset higher free triiodothyronine (fT3) and fT3/T4 ratios were linked with higher CAP and risk of steatosis. It could therefore be speculated that the increased T4 conversion to fT3 in participants with steatosis, possibly due to enhanced expression of deiodinase 1 in the liver, may represent a feedback loop to contrast hepatic/visceral fat accumulation. However, this attempt was not completely effective due to the tight regulation of fT3 levels, as highlighted by the reduced TSH levels in individuals with steatosis, in order to avoid systemic side effects of excess activation of thyroid hormone signaling. In keeping with the present results, Chung et al. showed that subjects with lower baseline TSH levels and higher increase during a 4‐year follow‐up were at increased risk of developing MASLD. 33 However, the impact of thyroid axis activation in protection against steatosis can be exploited by specific agonists of thyroid receptor β, which is selectively expressed by hepatocytes, whose administration improved steatosis and liver damage in randomized controlled studies in patients with fibrotic MASH. 34 , 35
Finally, we developed a new simple non‐invasive score, named CAPS, to predict the presence of steatosis in individuals with multiple metabolic abnormalities. CAPS was significantly superior not only to ALT, but also to the HSI and FLI, in predicting SLD. CAPS was based on evaluation of BMI and AC, ALT levels, plus HDL‐C and HBA1c capturing both hepatic IR and altered hepatic lipid metabolism. Interestingly, HDL‐C and HBA1c were not included in previous scores, but included in the fibrotic NASH index, recently developed to predict fibrotic NASH and demonstrating higher accuracy than classical non‐invasive scores. 36 Moreover, the ability of CAPS to predict steatosis was validated in three external cohorts. CAPS showed a better performance than ALT and HSI in detecting steatosis in general population‐based cohorts (NHANES and UKBB), whereas the improvement in the stratification of the risk of MASLD was marginal compared to FLI in this setting. 37 However, CAPS was more accurate in predicting histological steatosis than FLI in severely obese individuals (MAFALDA cohort). Thus, CAPS may be useful in epidemiological studies to estimate the prevalence of steatosis when direct assessment is not available, and in clinical settings to predict the presence of early stage MASLD in young individuals with metabolic dysfunction to prioritize them for intervention aimed at preventing the hepatic and cardiometabolic complications of excess hepatic fat accumulation.
Limitations of the present study include the lack of assessment of hepatic fat content by gold‐standard imaging approaches, such as H1‐magnetic resonance spectroscopy or MRI‐PDFF. Future studies should examine the predictive accuracy of CAPS on histological liver damage and the ability to predict liver‐related events in long‐term follow‐up. Furthermore, results may not be applicable in non‐European ethnicity with different average body compositions.
In conclusion, the severity of IR and abdominal adiposity were the main independent determinants of CAP in individuals with metabolic dysfunction and may improve the risk stratification of early SLD. CAP values were modulated by the hypophysis‐thyroid axis activity. We developed a new simple CAPS score to predict the presence of steatosis in individuals with metabolic dysfunction.
AUTHOR CONTRIBUTIONS
Conceptualization: Luca Valenti, Serena Pelusi; Data curation: Cristiana Bianco, Serena Pelusi, Sara Margarita, Federica Tavaglione, Oveis Jamialahmadi, Francesco Malvestiti, Giulia Periti, Jessica Rondena, Melissa Tomasi, Rossana Carpani, Luisa Ronzoni, Matteo Vidali, Ferruccio Ceriotti, Mirella Fraquelli, Umberto Vespasiani‐Gentilucci, Stefano Romeo, Daniele Prati, Luca Valenti; Formal analysis: Cristiana Bianco, Federica Tavaglione, Oveis Jamialahmadi, Luca Valenti; Funding acquisition: Luca Valenti, Daniele Prati; Investigation: Cristiana Bianco, Serena Pelusi, Sara Margarita, Federica Tavaglione, Oveis Jamialahmadi, Francesco Malvestiti, Giulia Periti, Jessica Rondena, Melissa Tomasi, Rossana Carpani, Luisa Ronzoni, Matteo Vidali, Ferruccio Ceriotti, Mirella Fraquelli, Umberto Vespasiani‐Gentilucci, Stefano Romeo, Daniele Prati, Luca Valenti; Methodology: Luca Valenti, Serena Pelusi; Project administration: Luca Valenti; Supervision: Luca Valenti, Daniele Prati, Stefano Romeo, Umberto Vespasiani‐Gentilucci; Visualization: Cristiana Bianco; Writing – original draft: Cristiana Bianco, Luca Valenti; Writing – review and editing: Cristiana Bianco, Luca Valenti, Federica Tavaglione, Oveis Jamialahmadi, Stefano Romeo, Umberto Vespasiani‐Gentilucci, Mirella Fraquelli.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest relevant to the present study. LV has received speaking fees from MSD, Gilead, AlfaSigma and AbbVie, served as a consultant for Gilead, Pfizer, AstraZeneca, Novo Nordisk, Intercept, Diatech Pharmacogenetics, Ionis Pharmaceuticals, Boeringher Ingelheim, Resalis and received research grants from Gilead. DP served as a consultant for and has received speaking fees, travel grants, and research grants from Macopharma, Ortho Clinical Diagnostics, Grifols, Terumo, Immucor, Diamed, and Diatech Pharmacogenetics.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
We thank the staff and the participants of the Liver Bible, MAFALDA, NHANES and UK Biobank studies. This research has been conducted using the UK Biobank resource (application #37142). Italian Ministry of Health (Ministero della Salute), Ricerca Finalizzata RF‐2016‐02364358 (“Impact of whole exome sequencing on the clinical management of patients with advanced nonalcoholic fatty liver and cryptogenic liver disease”) (LV); Italian Ministry of Health (Ministero della Salute), Rete Cardiologica “CV‐PREVITAL” (DP, LV); Italian Ministry of Health (Ministero della Salute), Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Ricerca corrente (LV, DP); Fondazione Patrimonio Ca’ Granda, “Liver BIBLE” (PR‐0391) (LV); Innovative Medicines Initiative 2 joint undertaking of European Union's Horizon 2020 research and innovation program and EFPIA European Union (EU) Program Horizon 2020 (under grant agreement No. 777377) for the project LITMUS (LV); The European Union, H2020‐ICT‐2018‐20/H2020‐ICT‐2020‐2 program “Photonics” under grant agreement No. 101016726 ‐ REVEAL (LV); Gilead_IN‐IT‐989‐5790 (LV); The European Union, HORIZON‐MISS‐2021‐CANCER‐02‐03 under grant agreement No. 101096312 ‐ GENIAL (LV). The Swedish Research Council (Vetenskapsradet, VR 2021–005208) (SR); The Swedish state under the Agreement between the Swedish government and the county councils (the ALF agreement, SU 2018–04276) (SR); The Swedish Diabetes Foundation (DIA2020‐518) (SR); The Swedish Heart Lung Foundation (20200191) (SR); The Wallenberg Academy Fellows from the Knut and Alice Wallenberg Foundation (KAW 2017.0203) (SR); The Novonordisk Project grants in Endocrinology and Metabolism (NNF20OC0063883) (SR); Astra Zeneca Agreement for Research (SR); Grant SSF ITM17‐0384, Swedish Foundation for Strategic Research (SR).
Open access funding provided by BIBLIOSAN.
Bianco C, Pelusi S, Margarita S, Tavaglione F, Jamialahmadi O, Malvestiti F, et al. Predictors of controlled attenuation parameter in metabolic dysfunction. United European Gastroenterol J. 2024;12(3):364–373. 10.1002/ueg2.12513
DATA AVAILABILITY STATEMENT
Individual participant‐sensitive data cannot be rendered public based on the terms of the informed consent of the Liver‐Bible cohort and regulations by the Italian Guarantor for Privacy. The 2017–2018 NHANES data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. The UK Biobank data are publicly available through a procedure described at "http://www.ukbiobank.ac.uk/using‐the‐resource/". All other data, code, and materials used in the analysis are available upon reasonable request for collaborative studies regulated by materials/data transfer agreements (MTA/DTAs) to the corresponding author.
REFERENCES
- 1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi‐society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556. 10.1016/j.jhep.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. https://gut.bmj.com/lookup/doi/10.1136/gutjnl‐2019‐318813 [DOI] [PubMed] [Google Scholar]
- 3. Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non‐invasive stratification of hepatocellular carcinoma risk in non‐alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74(4):775–782. 10.1016/j.jhep.2020.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valenti L, Pelusi S. Redefining fatty liver disease classification in 2020. Liver Int. 2020;40(5):1016–1017. https://onlinelibrary.wiley.com/doi/abs/10.1111/liv.14430 [DOI] [PubMed] [Google Scholar]
- 5. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428. 10.1038/s41575-019-0145-7 [DOI] [PubMed] [Google Scholar]
- 6. EASL–EASD–EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 7. Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, et al. Diagnostic value of MRI‐PDFF for hepatic steatosis in patients with non‐alcoholic fatty liver disease: a meta‐analysis. Eur Radiol. 2019;29(7):3564–3573. 10.1007/s00330-019-06072-4 [DOI] [PubMed] [Google Scholar]
- 8. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36(11):1825–1835. 10.1016/j.ultrasmedbio.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 9. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. 10.1016/j.jhep.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 10. Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich‐Rust M, et al. EASL Clinical Practice Guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659–689. 10.1016/j.jhep.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 11. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai‐Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–1669. 10.1053/j.gastro.2021.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rinella ME, Neuschwander‐Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. 10.1097/hep.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration‐controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67(1):134–144. 10.1002/hep.29489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valenti L, Pelusi S, Bianco C, Ceriotti F, Berzuini A, Iogna Prat L, et al. Definition of healthy ranges for alanine aminotransferase levels: a 2021 update. Hepatol Commun. 2021;5(11):1824–1832. 10.1002/hep4.1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valenti L, Tripodi A, La Mura V, Pelusi S, Bianco C, Scalambrino E, et al. Clinical and genetic determinants of the fatty liver–coagulation balance interplay in individuals with metabolic dysfunction. JHEP Rep. 2022;4(12):100598. 10.1016/j.jhepr.2022.100598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castera L, Forns X, Alberti A. Non‐invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847. 10.1016/j.jhep.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 17. Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–1191. 10.1002/hep.25993 [DOI] [PubMed] [Google Scholar]
- 18. Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75(4):683–688. 10.1093/ajcn/75.4.683 [DOI] [PubMed] [Google Scholar]
- 19. van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48(2):449–457. https://onlinelibrary.wiley.com/doi/10.1002/hep.22350 [DOI] [PubMed] [Google Scholar]
- 20. Otgonsuren M, Stepanova M, Gerber L, Younossi ZM. Anthropometric and clinical factors associated with mortality in Subjects with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(4):1132–1140. 10.1007/s10620-012-2446-3 [DOI] [PubMed] [Google Scholar]
- 21. De Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026–1031. 10.1016/j.jhep.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 22. Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta‐analysis. Lancet Gastroenterol Hepatol. 2021;6(3):185–198. 10.1016/s2468-1253(20)30357-5 [DOI] [PubMed] [Google Scholar]
- 23. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki‐Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–130. 10.1053/j.gastro.2008.03.021 [DOI] [PubMed] [Google Scholar]
- 24. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019;70(2):711–724. 10.1002/hep.30429 [DOI] [PubMed] [Google Scholar]
- 25. Chao HW, Chao SW, Lin H, Ku HC, Cheng CF. Homeostasis of glucose and lipid in non‐alcoholic fatty liver disease. Int J Mol Sci. 2019;20(2):298. 10.3390/ijms20020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biddinger SB, Hernandez‐Ono A, Rask‐Madsen C, Haas JT, Alemán JO, Suzuki R, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–134. 10.1016/j.cmet.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. 2020;42(October):101092. 10.1016/j.molmet.2020.101092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–370. 10.1111/joim.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mantovani A, Nascimbeni F, Lonardo A, Zoppini G, Bonora E, Mantzoros CS, et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Thyroid. 2018;28(10):1270–1284. https://www.liebertpub.com/doi/10.1089/thy.2018.0257 [DOI] [PubMed] [Google Scholar]
- 30. Qiu S, Cao P, Guo Y, Lu H, Hu Y. Exploring the causality between hypothyroidism and non‐alcoholic fatty liver: a Mendelian randomization study. Front Cell Dev Biol. 2021;9(March):1–7. 10.3389/fcell.2021.643582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bano A, Chaker L, Plompen EPC, Hofman A, Dehghan A, Franco OH, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam study. J Clin Endocrinol Metab. 2016;101(8):3204–3211. 10.1210/jc.2016-1300 [DOI] [PubMed] [Google Scholar]
- 32. Bohinc BN, Michelotti G, Xie G, Pang H, Suzuki A, Guy CD, et al. Repair‐related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology. 2014;155(11):4591–4601. 10.1210/en.2014-1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung GE, Kim D, Kwak MS, Yim JY, Ahmed A, Kim JS. Longitudinal change in thyroid‐stimulating hormone and risk of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19(4):848.e1–849.e1. 10.1016/j.cgh.2020.02.039 [DOI] [PubMed] [Google Scholar]
- 34. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL‐3196) for the treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2019;394(10213):2012–2024. 10.1016/s0140-6736(19)32517-6 [DOI] [PubMed] [Google Scholar]
- 35. Harrison S, Bedossa P, Guy C, Schattenberg J, Loomba R, Taub R, et al. Primary results from MAESTRO‐NASH a pivotal phase 3 52‐week serial liver biopsy study in 966 patients with NASH and fibrosis. J Hepatol. 2023;78(Suppl 1):S1. 10.1016/s0168-8278(23)00440-3 [DOI] [Google Scholar]
- 36. Tavaglione F, Jamialahmadi O, De Vincentis A, Qadri S, Mowlaei ME, Mancina RM, et al. Development and validation of a score for fibrotic nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2022;21(6):1523.e1–1532.e1. 10.1016/j.cgh.2022.03.044 [DOI] [PubMed] [Google Scholar]
- 37. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Individual participant‐sensitive data cannot be rendered public based on the terms of the informed consent of the Liver‐Bible cohort and regulations by the Italian Guarantor for Privacy. The 2017–2018 NHANES data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. The UK Biobank data are publicly available through a procedure described at "http://www.ukbiobank.ac.uk/using‐the‐resource/". All other data, code, and materials used in the analysis are available upon reasonable request for collaborative studies regulated by materials/data transfer agreements (MTA/DTAs) to the corresponding author.


