Abstract
Background
Delayed cholecystectomy in patients with symptomatic gallstone disease is associated with recurrence. Limited data on the recurrence patterns and the factors that determine them are available.
Objective
We aimed to determine the pattern of relapse in each symptomatic gallstone disease (acute pancreatitis, cholecystitis, cholangitis, symptomatic choledocholithiasis, and biliary colic) and determine the associated factors.
Methods
RELAPSTONE was an international multicenter retrospective cohort study. Patients (n = 3016) from 18 tertiary centers who suffered a first episode of symptomatic gallstone disease from 2018 to 2020 and had not undergone cholecystectomy during admission were included. The main outcome was relapse‐free survival. Kaplan–Meier curves were used in the bivariate analysis. Multivariable Cox regression models were used to identify prognostic factors associated with relapses.
Results
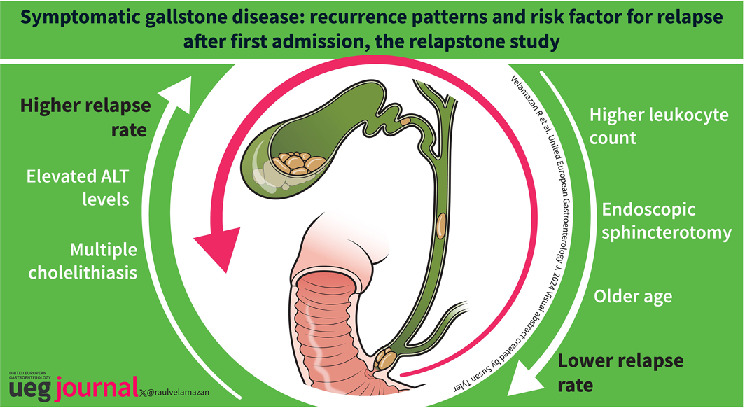
Mean age was 76.6 [IQR: 59.7–84.1], and 51% were male. The median follow‐up was 5.3 months [IQR 2.1–12.4]. Relapse‐free survival was 0.79 (95% CI: 0.77–0.80) at 3 months, 0.71 (95% CI: 0.69–0.73) at 6 months, and 0.63 (95% CI: 0.61–0.65) at 12 months. In multivariable analysis, older age (HR = 0.57; 95% CI: 0.49–0.66), sphincterotomy (HR = 0.58, 95% CI: 0.49–0.68) and higher leukocyte count (HR = 0.79; 95% CI: 0.70–0.90) were independently associated with lower risk of relapse, whereas higher levels of alanine aminotransferase (HR = 1.22; 95% CI: 1.02–1.46) and multiple cholelithiasis (HR = 1.19, 95% CI: 1.05–1.34) were associated with higher relapse rates.
Conclusion
The relapse rate is high and different in each symptomatic gallstone disease. Our independent predictors could be useful for prioritizing patients on the waiting list for cholecystectomies.
Keywords: biliary colic, biliary pain, cholangitis, cholecystectomy, cholecystitis, cholelithiasis, gallstones, pancreatitis, recurrences, relapse
Key summary.
Summarize the established knowledge on this subject:
The incidence of symptomatic gallstone disease is gradually increasing.
The recommended treatment to prevent relapses is early cholecystectomy.
Early cholecystectomy is not always possible, particularly in under‐resourced healthcare systems. A delay in cholecystectomy leads to a high relapse rate, economic costs, and morbimortality.
Limited knowledge exists regarding relapse patterns and predictors of relapse.
What are the significant and/or new findings of this study:
Each symptomatic gallstone disease's relapse pattern (recurrence rate, recurrence form, time elapsed, and severity) differs.
Age, endoscopic sphincterotomy, presence of multiple cholelithiasis, blood leukocyte count, and blood ALT levels during admission are predictors of relapse and could be useful tools to prioritize patients for cholecystectomy.
INTRODUCTION
The incidence of symptomatic gallstone disease (SGD) is gradually increasing, 1 , 2 with around 10%–20% of the global population having gallstones, and the risk of developing symptoms exceeding 20% within 10 years. 3 , 4 In the United States, SGD entailed more than 600,000 admissions and 3600 deaths which generated a cost higher than $17,000 million in 2021. 5
The recommended treatment of SGDs is early cholecystectomy. Most guidelines recommend same‐admission cholecystectomy as the ideal timing, as delayed cholecystectomy is associated with high relapse rates. 6 , 7 , 8 , 9 In patients with acute pancreatitis (AP), delayed cholecystectomy is associated with a 17%–20% relapse rate, compared to only 5% in patients undergoing early cholecystectomy. 10 , 11 In acute cholecystitis (ACC), the relapse rate is 14% after 6 weeks if cholecystectomy is not performed. 12
Of particular concern is that despite these recommendations, a significant proportion of patients undergo delayed cholecystectomy. 13 According to a systematic review, only 48% of patients with AP underwent same‐admission cholecystectomy; for the rest, the median time from discharge to cholecystectomy was 40 days. 14 The large volume of SGD makes it difficult for healthcare systems to manage the performance of early cholecystectomy, resulting in a high relapse frequency, with significant resource consumption and potentially avoidable suffering for the patient. 15 , 16 In addition, the COVID‐19 pandemic has dramatically increased the waiting lists. 17
It is well established that timing for cholecystectomy is the most important factor associated with relapses, 10 , 11 , 18 , 19 but limited knowledge exists regarding additional factors that may help to identify patients with a higher risk of relapse so they can be prioritized in the waiting list. Previous studies are small in size or with highly selected populations and usually focus on a single specific complication of gallstones.
To our knowledge, this is the first international multicenter study that compares the relapse patterns in the different types of SGDs in a large cohort of patients.
The aims of RELAPSTONE were to describe the recurrence patterns after each specific gallstone complication and to investigate the predictors of relapse in patients hospitalized for the first time due to SGD.
METHODS
Study design
RELAPSTONE (REcurrence patterns and risk factors for reLAPse after the first symptomatic gallSTONE admission) is an international multicenter retrospective cohort study endorsed by the Spanish Association of Gastroenterology (AEG). The project was developed by AEG's young talent group (grupo joven AEG). The study design was approved by the central Institutional Review Board (IRB) (CEiM, Dr. Balmis General University Hospital, reference 2020‐257, 18 January 2021) and by the local IRBs of collaborating centers. Given the retrospective nature of the study and its independence from commercial interests, the IRBs waived the need for informed consent in accordance with Spanish law.
Study subjects and recruitment
Sixteen Spanish and two Mexican centers (see Supplementary Material) reviewed consecutive cases of patients with a first episode of SGD requiring admission between 1 January 2018 and 30 April 2020.
The inclusion criteria were as follows: patients ≥18 years old; hospital admission due to SGD, that is biliary AP, 20 calculous ACC, 9 , 21 acute calculous cholangitis (ACL), 22 symptomatic choledocholithiasis (SC), biliary colic (BC) or any combination of them (see Supplementary Material for definitions). Patients were excluded when the following conditions were satisfied: previous admission or visit to the emergency room for SGD, previous or same‐admission cholecystectomy, previous biliary sphincterotomy, same admission death, previous biliary‐pancreatic surgery, biliary, pancreatic, or duodenal cancer, and benign biliary stricture.
Data collection and measures
For case identification, the Minimum Basic Data Set registry was consulted upon hospital discharge from each center. The following data were collected using the electronic medical record: demographics, smoking and alcohol consumption habits, and Charlson comorbidity index 23 for each patient at admission. First SGD episode characteristics: clinical, imaging test, and laboratory parameters (at three different moments during hospital admission). Moreover, if the patient had a relapse, its characteristics were gathered (clinical, imaging tests and laboratory parameters). All collected variables are detailed in Supplementary Methods. The end of follow‐up was considered the date of cholecystectomy or death or the last clinical visit if any of the previous conditions were not satisfied until 30 April 2020. All data were anonymized.
Primary and secondary outcomes
The primary outcome of the study was the relapse‐free survival rate. Relapse was defined as AP, ACC, ACL, SC, BC or a combination of them that required hospital admission or outpatient care.
Exploratory secondary outcomes were the pattern of relapse according to each SGD and factors associated with an increased risk of relapse.
Sample size
Based on Peery et al., 5 AP (n = 259,000) and ACC (n = 287,000) were the most common causes of hospitalization due to cholelithiasis in the United States during 2021. Considering that they did not include ACL, SC, or BC, we conservatively estimate that AP can account for at least 20% of admissions related to SGDs.
Assuming a relapse rate in AP of 17%, 10 the estimated sample size, with a confidence level of 95% and a precision of ±3%, was 603 AP. Given the assumption that AP accounts for at least 20% of admissions due to SGD, a total of 3015 patients would be required.
This sample size would allow modeling approximately 50 parameters in the multivariable model equation (i.e., each HR in the regression model), meeting the established rule of having at least 10 events (relapses) for each parameter considered for inclusion. When developing prediction models for time‐to‐event outcomes, an established rule of thumb for the required sample size is to ensure at least 10 events (relapses) for each parameter being considered for inclusion in the multivariable model equation. 24
Statistical analysis
Categorical variables are presented as absolute values and relative frequencies. Continuous variables are summarized as means and standard deviation for normal distribution and by the median and interquartile range (IQR: first and third quartiles) for non‐normal distributions.
Bivariate and multivariable analyses were conducted to determine the factors influencing the likelihood of relapse. Missing data were imputed (median values for continuous variables and mode values for categorical variables) for independent variables with less than 5% missing data. Relapse or censored times (relapse‐free survival, cholecystectomy, or death) were calculated from the hospital discharge date until 30 April 2020, including patients lost to follow‐up, at which point follow‐up was censored.
Using the log‐rank test, Kaplan‐Meier survival curves were used for the bivariate analysis, comparing the probability of relapse‐free survival during the 24 months after the first episode of SGD.
Crude and adjusted hazard ratios and 95% confidence intervals were calculated using simple or multivariable Cox proportional regression models. Covariates with a p‐value ≤0.20 in the bivariate analysis or with established associations in the literature were included in the multivariable model using a backward exclusion strategy. The proportionality of hazards was assessed using Schoenfeld residual plots.
The level of statistical significance was two‐sided 5% (p < 0.05). IBM SPSS Statistics v.28 (IBM Corporation®, Armonk, New York, USA) and Stata v.14 (StataCorpLP®, College Station, Texas, USA) were used for statistical analysis.
RESULTS
Participants
Of the initial cohort of 6163 patients, 3089 (50.1%) were excluded for meeting one or more exclusion criteria, and 58 (1.9%) due to the loss of follow‐up. Finally, 3016 (48.0%) patients were included in the study sample (Supplementary Methods: Supplementary Figure 1).
Baseline patient characteristics
The most frequent SGD was AP (31.2%), followed by ACC (27.6%). Fifty‐one percent of patients were women, median age was 74.6 years (59.7–84.1) and median Charlson comorbidity index was 1 (0–2). The median follow‐up was 5.3 months (2.1–12.4) (Table 1). Demographic, clinical, and analytical parameters for all patients and according to the different SGDs are shown in Supplementary Tables 1 and 2.
TABLE 1.
Baseline sample characteristics and relapse at 3, 6, and 12 months according to demographic and clinical variables.
| N valid | N (%) | S3 | S6 | S12 | p‐value | Unadjusted HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| All patients | 3016 (100) | 0.79 | 0.71 | 0.63 | ||||
| Type of gallstone disease | 3016 | |||||||
| Acute cholangitis | 379 (12.6) | 0.85 | 0.79 | 0.71 | <0.001 | 1 | ||
| Acute pancreatitis | 941 (31.2) | 0.77 | 0.68 | 0.59 | 1.55 | 1.24–1.92 | ||
| Acute cholecystitis | 831 (27.6) | 0.79 | 0.71 | 0.62 | 1.38 | 1.10–1.72 | ||
| Symptomatic choledocholithiasis | 302 (10.0) | 0.79 | 0.75 | 0.69 | 1.19 | 0.89–1.58 | ||
| Biliary colic | 314 (10.4) | 0.75 | 0.64 | 0.53 | 1.70 | 1.31–2.21 | ||
| Any combination | 249 (8.3) | 0.81 | 0.74 | 0.70 | 1.26 | 0.94–1.68 | ||
| Age (years) | 3016 | 74.6 [59.7–84.1] | ||||||
| ≤54 | 557 (18.5) | 0.68 | 0.60 | 0.46 | <0.001 | 1 | ||
| >54 | 2459 (81.5) | 0.81 | 0.74 | 0.66 | 0.53 | 0.46–0.62 | ||
| Gender | 3016 | |||||||
| Women | 1538 (51.0) | 0.78 | 0.71 | 0.61 | 0.102 | 1 | ||
| Men | 1478 (49.0) | 0.80 | 0.72 | 0.64 | 0.90 | 0.80–1.02 | ||
| Charlson comorbidity index | 3014 | 1 [0–2] | ||||||
| Low (0–1) | 1825 (60.5) | 0.77 | 0.70 | 0.62 | 0.021 | 1 | ||
| Medium comorbidity 2 | 515 (17.1) | 0.81 | 0.72 | 0.59 | 0.97 | 0.83–1.15 | ||
| High comorbidity 3 | 676 (22.4) | 0.82 | 0.74 | 0.68 | 0.80 | 0.69–0.94 | ||
| Diabetes | 3014 | |||||||
| No | 2336 (77.5) | 0.79 | 0.71 | 0.63 | 0.680 | 1 | ||
| Without complications | 589 (19.5) | 0.78 | 0.70 | 0.62 | 1.00 | 0.89–1.21 | ||
| With complications | 91 (3.0) | 0.83 | 0.76 | 0.63 | 0.88 | 0.61–1.27 | ||
| Chronic renal disease | 3014 | |||||||
| No | 2684 (89.0) | 0.78 | 0.70 | 0.62 | 0.003 | 1 | ||
| Yes | 332 (11.0) | 0.86 | 0.80 | 0.71 | 0.67 | 0.54–0.83 | ||
| Acute renal dysfunction | 3014 | |||||||
| No | 2821 (93.5) | 0.79 | 0.71 | 0.63 | 0.325 | 1 | ||
| Yes | 193 (6.4) | 0.82 | 0.76 | 0.65 | 0.88 | 0.68–1.14 | ||
| Acute respiratory dysfunction | 3015 | |||||||
| No | 2959 (98.1) | 0.79 | 0.71 | 0.63 | 0.264 | 1 | ||
| Yes | 56 (1.9) | 0.78 | 0.73 | 0.59 | 1.26 | 0.84–1.89 | ||
| Acute cardiovascular dysfunction | 3016 | |||||||
| No | 2946 (97.7) | 0.79 | 0.71 | 0.63 | 0.833 | 1 | ||
| Yes | 70 (2.3) | 0.77 | 0.75 | 0.65 | 0.96 | 0.64–1.44 | ||
| ICU admission | 3014 | |||||||
| No | 2961 (98.2) | 0.79 | 0.71 | 0.63 | 0.888 | 1 | ||
| Yes | 53 (1.8) | 0.75 | 0.71 | 0.62 | 1.03 | 0.66–1.63 | ||
| Biliary tract | 3012 | |||||||
| Not dilated | 1973 (65.4) | 0.77 | 0.69 | 0.59 | <0.001 | 1 | ||
| Dilated | 1039 (34.6) | 0.82 | 0.76 | 0.69 | 0.76 | 0.66–0.87 | ||
| Cholelithiasis | 3015 | |||||||
| Single cholelithiasis | 1504 (49.9) | 0.80 | 0.74 | 0.66 | <0.001 | 1 | ||
| Multiple cholelithiasis | 1511 (50.1) | 0.77 | 0.69 | 0.60 | 1.19 | 1.05–1.34 | ||
| Duodenal diverticula a | 2144 | |||||||
| No | 1922 (89.6) | 0.80 | 0.73 | 0.65 | 0.550 | 1 | ||
| Yes | 222 (10.4) | 0.82 | 0.75 | 0.68 | 0.92 | 0.73–1.18 | ||
| Pancreas divisum a | 2138 | |||||||
| No | 2107 (98.6) | 0.80 | 0.73 | 0.65 | 0.970 | 1 | ||
| Yes | 31 (1.4) | 0.90 | 0.82 | 0.69 | 1.01 | 0.56–1.84 | ||
| Sphincterotomy | 3016 | |||||||
| No | 2237 (74.2) | 0.77 | 0.68 | 0.59 | <0.001 | 1 | ||
| Yes | 779 (25.8) | 0.85 | 0.79 | 0.74 | 0.60 | 0.51–0.70 | ||
| Cholecystostomy | 3013 | |||||||
| No | 2770 (91.9) | 0.79 | 0.71 | 0.63 | 0.080 | 1 | ||
| Yes | 243 (8.1) | 0.80 | 0.71 | 0.63 | 0.97 | 0.77–1.22 | ||
| Median follow‐up time (months) | 5.3 [2.1–12.4] |
Note: N = 3016. Data presented as: n (%); median [25th percentile–75th percentile].
Abbreviations: CI, Confidence Interval; HR, Hazard Ratio; ICU, Intensive care unit.
Duodenal diverticula and pancreas divisum were considered only when computed axial tomography, magnetic resonance imaging, endoscopic ultrasound, or ERCP was performed.
Relapse‐free survival and prognostic factors
Relapse‐free survival was 0.79 (95% CI: 0.77–0.80) at 3 months, 0.71 (95% CI: 0.69–0.73) at 6 months, and 0.63 (95% CI: 0.61–0.65) at 12 months (see details according to demographic, clinical, and analytical factors in Table 1). Figure 1 shows the Kaplan‐Meier relapse‐free survival graphics overall and according to each SGD.
FIGURE 1.
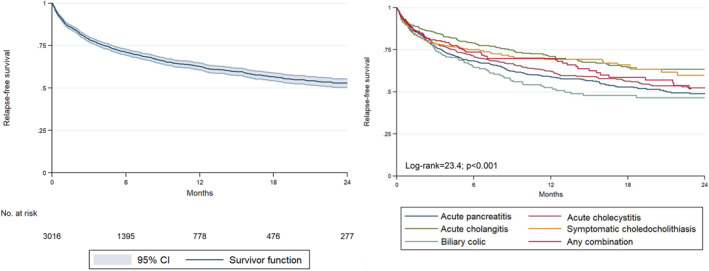
Kaplan–Meier survival curves for the probability of relapse during 24 months after the first episode of gallstone‐related disease in the overall sample and according to the different types of gallstone disease.
In the bivariate analysis, patients who developed relapses were younger, had a lower Charlson comorbidity index, multiple cholelithiasis, and did not have a dilated biliary tract or sphincterotomy. The diseases with the highest relapse rates were bc (HR = 1.70, 95% CI: 1.31–2.21) and AP (HR = 1.55, 95% CI: 1.10–1.72). Higher blood leukocyte count, neutrophil count and urea levels in the first 24/48 h of admission, as well as their highest level during the entire index admission, higher blood level of C‐reactive protein in the first 24/48 h of admission and higher urea level at discharge were statistically associated with lower relapse rates. A higher hematocrit level at discharge was statistically associated with higher relapse rates (Table 2).
TABLE 2.
Relapse‐free survival at 3, 6, and 12 months according to analytical parameters.
| Biomarker | Time | Units | N valid | N (%) | S3 | S6 | S12 | p‐value | Unadjusted HR | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| AST (U/L) | At first 24/48h of admission | ≤35 | 3016 | 712 (23.6) | 0.79 | 0.72 | 0.61 | 0.445 | 1 | |
| >35 | 2304 (76.4) | 0.79 | 0.71 | 0.63 | 0.94 | 0.82–1.09 | ||||
| Highest value | ≤35 | 3016 | 528 (17.5) | 0.79 | 0.73 | 0.64 | 0.779 | 1 | ||
| >35 | 2488 (82.5) | 0.79 | 0.71 | 0.63 | 1.02 | 0.87–1.20 | ||||
| At discharge | ≤35 | 2852 | 1517 (53.2) | 0.79 | 0.72 | 0.63 | 0.434 | 1 | ||
| >35 | 1335 (46.8) | 0.78 | 0.70 | 0.63 | 1.05 | 0.93–1.19 | ||||
| ALT (U/L) | At first 24/48h of admission | ≤35 | 3016 | 685 (22.7) | 0.80 | 0.73 | 0.65 | 0.494 | 1 | |
| >35 | 2331 (77.3) | 0.78 | 0.71 | 0.62 | 1.05 | 0.91–1.22 | ||||
| Highest value | ≤35 | 2812 | 481 (15.9) | 0.80 | 0.75 | 0.67 | 0.147 | 1 | ||
| >35 | 2535 (84.1) | 0.79 | 0.71 | 0.62 | 1.13 | 0.96–1.35 | ||||
| At discharge | ≤35 | 3016 | 1077 (35.7) | 0.81 | 0.73 | 0.64 | 0.278 | 1 | ||
| >35 | 1939 (64.3) | 0.78 | 0.70 | 0.62 | 1.07 | 0.94–1.22 | ||||
| GGT (U/L) | At first 24/48h of admission | ≤78 | 3016 | 616 (20.4) | 0.81 | 0.73 | 0.64 | 0.373 | 1 | |
| >78 | 2400 (79.6) | 0.78 | 0.71 | 0.62 | 1.07 | 0.92–1.25 | ||||
| Highest value | ≤78 | 3016 | 408 (13.5) | 0.82 | 0.73 | 0.66 | 0.309 | 1 | ||
| >78 | 2608 (86.5) | 0.78 | 0.71 | 0.62 | 1.10 | 0.92–1.32 | ||||
| At discharge | ≤78 | 3016 | 607 (20.1) | 0.81 | 0.72 | 0.64 | 0.499 | 1 | ||
| >78 | 2409 (79.9) | 0.78 | 0.71 | 0.62 | 1.05 | 0.90–1.23 | ||||
| ALP (U/L) | At first 24/48h of admission | ≤150 | 3016 | 1663 (55.1) | 0.78 | 0.71 | 0.63 | 0.943 | 1 | |
| >150 | 1353 (44.9) | 0.78 | 0.71 | 0.63 | 1.00 | 0.80–1.13 | ||||
| Highest value | ≤150 | 3016 | 1186 (39.3) | 0.78 | 0.71 | 0.63 | 0.493 | 1 | ||
| >150 | 1830 (60.7) | 0.79 | 0.72 | 0.63 | 0.96 | 0.84–1.09 | ||||
| At discharge | ≤150 | 3016 | 1797 (59.6) | 0.79 | 0.71 | 0.63 | 0.845 | 1 | ||
| >150 | 1219 (40.4) | 0.79 | 0.71 | 0.62 | 1.01 | 0.89–1.15 | ||||
| Bilirubin (mg/dl) | At first 24/48h of admission | ≤1.2 | 3016 | 1144 (37.9) | 0.79 | 0.70 | 0.61 | 0.113 | 1 | |
| >1.2 | 1872 (62.1) | 0.79 | 0.72 | 0.64 | 0.90 | 0.80–1.02 | ||||
| Highest value | ≤1.2 | 3016 | 1012 (33.6) | 0.79 | 0.71 | 0.61 | 0.454 | 1 | ||
| >1.2 | 2004 (66.4) | 0.79 | 0.71 | 0.64 | 0.95 | 0.84–1.08 | ||||
| At discharge | ≤1.2 | 3016 | 2300 (76.3) | 0.79 | 0.72 | 0.63 | 0.956 | 1 | ||
| >1.2 | 716 (23.7) | 0.78 | 0.69 | 0.63 | 1.00 | 0.86–1.15 | ||||
| Leukocytes (count/mm3) | At first 24/48h of admission | ≤11,000 | 3016 | 1535 (50.9) | 0.77 | 0.69 | 0.61 | 0.018 | 1 | |
| >11,000 | 1481 (49.1) | 0.81 | 0.74 | 0.65 | 0.86 | 0.76–0.98 | ||||
| Highest value | ≤11,000 | 3016 | 1288 (42.7) | 0.76 | 0.67 | 0.59 | <0.001 | 1 | ||
| >11,000 | 1728 (57.3) | 0.81 | 0.75 | 0.66 | 0.78 | 0.69–0.88 | ||||
| At discharge | ≤11,000 | 3016 | 2668 (88.5) | 0.79 | 0.71 | 0.62 | 0.655 | 1 | ||
| >11,000 | 348 (11.5) | 0.79 | 0.75 | 0.68 | 0.97 | 0.79–1.16 | ||||
| Neutrophils (count/mm3) | At first 24/48h of admission | ≤8500 | 3016 | 1421 (47.1) | 0.77 | 0.69 | 0.61 | 0.004 | 1 | |
| >8500 | 1595 (52.9) | 0.80 | 0.73 | 0.65 | 0.88 | 0.78–0.99 | ||||
| Highest value | ≤8500 | 3016 | 1193 (39.6) | 0.76 | 0.67 | 0.59 | <0.001 | 1 | ||
| >8500 | 1823 (60.4) | 0.81 | 0.74 | 0.66 | 0.78 | 0.69–0.89 | ||||
| At discharge | ≤8500 | 3016 | 2744 (91.0) | 0.79 | 0.71 | 0.63 | 0.911 | 1 | ||
| >8500 | 272 (9.0) | 0.78 | 0.73 | 0.66 | 0.98 | 0.80–1.23 | ||||
| Lymphocytes (count/mm3) | At first 24/48h of admission | ≤4500 | 3016 | 2989 (99.1) | 0.79 | 0.71 | 0.63 | 0.865 | 1 | |
| >4500 | 27 (0.9) | 0.76 | 0.69 | 0.69 | 0.94 | 0.45–1.98 | ||||
| Highest value | ≤4500 | 3016 | 2984 (98.9) | 0.79 | 0.71 | 0.63 | 0.492 | 1 | ||
| >4500 | 32 (1.1) | 0.84 | 0.74 | 0.67 | 0.78 | 0.39–1.57 | ||||
| At discharge | ≤4500 | 3016 | 3001 (99.5) | 0.79 | 0.71 | 0.63 | 0.964 | 1 | ||
| >4500 | 15 (0.5) | 0.86 | 0.66 | 0.55 | 1.02 | 0.42–2.46 | ||||
| Hematocrit (%) | At first 24/48h of admission | ≤47 F/51 M | 3016 | 2920 (96.8) | 0.79 | 0.71 | 0.63 | 0.392 | 1 | |
| ≤47 F/51 M | 96 (3.2) | 0.74 | 0.66 | 0.58 | 1.16 | 0.83–1.61 | ||||
| Highest value | ≤47 F/51 M | 3016 | 2898 (96.1) | 0.79 | 0.71 | 0.63 | 0.361 | 1 | ||
| ≤47 F/51 M | 118 (3.9) | 0.74 | 0.66 | 0.60 | 1.15 | 0.85–1.55 | ||||
| At discharge | ≤47 F/51 M | 3016 | 2993 (99.2) | 0.79 | 0.71 | 0.63 | 0.023 | 1 | ||
| ≤47 F/51 M | 23 (0.8) | 0.53 | 0.47 | 0.47 | 1.96 | 1.08–3.55 | ||||
| Urea (mg/L) | At first 24/48h of admission | ≤54 | 3016 | 2319 (76.9) | 0.78 | 0.70 | 0.62 | 0.008 | 1 | |
| >54 | 697 (23.1) | 0.82 | 0.76 | 0.65 | 0.82 | 0.70–0.95 | ||||
| Highest value | ≤54 | 3016 | 2089 (69.3) | 0.77 | 0.69 | 0.61 | <0.001 | 1 | ||
| >54 | 927 (30.7) | 0.83 | 0.77 | 0.68 | 0.75 | 0.65–0.85 | ||||
| At discharge | ≤54 | 3016 | 2602 (86.3) | 0.78 | 0.71 | 0.62 | 0.060 | 1 | ||
| >54 | 414 (13.7) | 0.83 | 0.78 | 0.67 | 0.82 | 0.67–1.01 | ||||
| CRP (mg/L) | At first 24/48h of admission | ≤5 | 3016 | 537 (17.8) | 0.75 | 0.68 | 0.58 | 0.019 | 1 | |
| >5 | 2479 (82.2) | 0.80 | 0.72 | 0.64 | 0.83 | 0.71–0.97 | ||||
| Highest value | ≤5 | 3016 | 144 (4.8) | 0.75 | 0.70 | 0.59 | 0.266 | 1 | ||
| >5 | 2872 (95.2) | 0.79 | 0.71 | 0.63 | 0.85 | 0.64–1.13 | ||||
| At discharge | ≤5 | 3016 | 361 (12.0) | 0.76 | 0.67 | 0.59 | 0.122 | 1 | ||
| >5 | 2655 (88.0) | 0.79 | 0.72 | 0.63 | 0.87 | 0.72–1.04 |
Note: N = 3016. Data presented as: n (%).
Abbreviations: ALP, Alkaline Phosphatase; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CI, Confidence Interval; CRP, C‐Reactive protein; F, female; GGT, Gamma Glutamyl Transferase; HR, Hazard Ratio; M, male.
Multivariable analysis (Table 3) showed that the prognostic factors independently associated with a lower risk of relapse were older age (HR = 0.57, 95% CI: 0.49–0.66), sphincterotomy (HR = 0.58, 95% CI: 0.49–0.68) and a higher leukocyte count during admission (HR = 0.79, 95% CI: 0.70–0.90). In contrast, multiple cholelithiasis (HR = 1.19, 95% CI: 1.05–1.34) and the highest level of ALT during admission (HR = 1.22, 95% CI: 1.02–1.46) were associated with an increased risk. Figure 2 shows the Kaplan‐Meier curves for each prognostic factor independently associated with relapse.
TABLE 3.
Independent prognostic factors for the probability of relapse after the first episode of symptomatic gallstone disease.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | p‐value | Adjusted HR | 95% CI | p‐value | |
| Age (years) | ||||||
| ≤54 | 1 | 1 | ||||
| >54 | 0.56 | 0.48–0.64 | <0.001 | 0.57 | 0.49–0.66 | <0.001 |
| Cholelithiasis | ||||||
| No multiple cholelithiasis | 1 | 1 | ||||
| Multiple cholelithiasis | 1.20 | 1.06–1.36 | 0.004 | 1.19 | 1.05–1.34 | 0.006 |
| Sphincterotomy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.60 | 0.52–0.71 | <0.001 | 0.58 | 0.49–0.68 | <0.001 |
| ALT highest value (U/L) | ‐ | ‐ | ‐ | |||
| ≤35 | 1 | |||||
| >35 | 1.22 | 1.02–1.46 | 0.027 | |||
| Leukocytes highest value (count/mm3) | ‐ | ‐ | ‐ | |||
| ≤11,000 | 1 | |||||
| >11,000 | 0.79 | 0.70–0.90 | <0.001 | |||
Note: Adjusted hazard ratio and corresponding statistical significance according to multivariable regression models. Variable not introduced in the model.
Abbreviations: ALT, Alanine Aminotransferase; CI, Confidence Interval; HR, Hazard Ratio.
FIGURE 2.
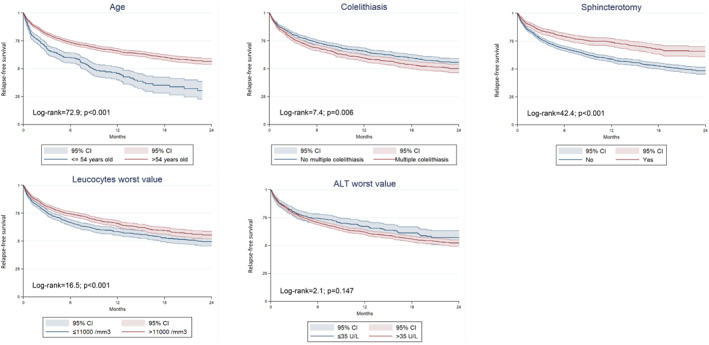
Kaplan‐Meier survival curves for the probability of relapse during follow‐up after discharge according to independent prognostic factors of multivariable analysis.
Supplementary Tables 3 to 7 show relapse‐free survival rates at 3, 6, and 12 months according to demographic, clinical, and analytical parameters according to specific types of SGDs.
Characteristics of the relapses
Table 4 summarizes the relapse rates, median time, and type of relapse according to each specific SGD at index admission. The median time from the index admission to the first recurrence was 2.1 months [IQR: 0.7–5.5]. There were significant differences between the specific diseases in the time elapsed from discharge from the index admission to the development of the first relapse. Considering the entire sample, bc (29.8%) was the most frequent form of relapse. However, considering each disease separately, the most frequent form of relapse was presenting the same SGD that justified index admission, followed by BC.
TABLE 4.
Median time to first relapse and type of relapse according to each gallstone disease at index admission.
| Relapses | Type of relapse | |||||||
|---|---|---|---|---|---|---|---|---|
| Index admission cause | N (%) | Median time to first relapse (months) a | AP | ACC | ACL | SC | BC | Comb |
| Acute pancreatitis | 346 (33.9) | 2.27 [0.79–5.09] | 197 (56.9) | 25 (7.2) | 13 (3.8) | 19 (5.5) | 69 (19.9) | 23 (6.6) |
| Acute cholecystitis | 284 (27.8) | 2.23 [0.69–5.77] | 17 (6.0) | 110 (38.7) | 28 (9.9) | 15 (5.3) | 97 (34.2) | 17 (6.0) |
| Acute cholangitis | 107 (10.5) | 2.92 [0.66–7.85] | 6 (5.6) | 27 (25.2) | 31 (29.0) | 11 (10.3) | 27 (25.2) | 5 (4.7) |
| Symptomatic choledocholithiasis | 85 (8.3) | 1.15 [0.46–4.20] | 10 (11.8) | 16 (18.8) | 11 (12.9) | 23 (27.1) | 24 (28.2) | 1 (1.2) |
| Biliary colic | 118 (11.6) | 2.62 [0.57–5.26] | 18 (15.3) | 21 (17.8) | 4 (3.4) | 5 (4.2) | 68 (57.6) | 2 (1.6) |
| Any combination | 81 (7.9) | 2.10 [0.66–6.59] | 16 (19.8) | 19 (23.5) | 11 (13.6) | 3 (3.7) | 20 (24.7) | 12 (14.7) |
| All | 1021 | 2.17 [0.68–5.49] | 264 (25.9) | 218 (21.4) | 98 (9.6) | 76 (7.4) | 305 (29.9) | 60 (5.8) |
Note: Data presented as: n (%); median [25th percentile–75th percentile].
Abbreviations: AP, Acute pancreatitis; ACC, Acute cholecystitis; ACL, Acute cholangitis; SC, Symptomatic choledocholithiasis; Comb, any combination of different symptomatic gallstone diseases.
p‐value<0.001.
Supplementary Table 8 compares the severity between the index admission and the relapse episode. Regarding AP, the relapses exhibited less severity than the index admission; there were no differences in severity in the case of ACC and ACL.
There were 1764 episodes of relapse in 1021 patients. Supplementary Table 9 presents the number of relapse episodes by patients during the follow‐up period.
Cholecystectomy
As shown in Supplementary Table 10, the total number of cholecystectomies was 1572 (52.1%), with a mean waiting time of 4.27 (2.17–6.90) months. Five hundred and fifty‐one (35.5%) were performed in <3 months, 477 (30.7%) in 3–6 months, and 525 (33.8%) in more than 6 months.
DISCUSSION
RELAPSTONE is an international multicenter retrospective study that provides a global description of the pattern of SGD recurrence and its predictors in patients in whom cholecystectomy is not performed at the index admission, involving over 3000 patients. Relapse‐free survival was 0.79 at 3 months, 0.71 at 6 months, and 0.63 at 12 months, highlighting the need to promote early cholecystectomy. The prognostic factors independently associated with relapse were age, endoscopic sphincterotomy, presence of multiple cholelithiasis, leukocyte count, and blood ALT levels during admission.
Regarding age, the actual evidence is controversial. Some studies showed no increased risk with age in patients with AP. 25 , 26 However, these studies were single‐center, retrospective, with few patients, and in the case of Zhang et al. 26 took into account different etiologies of AP. Our study showed that older patients have a lower risk of relapse (older than 54 years HR = 0.57, 95% CI: 0.49–0.66). Similar findings were reported by Park et al. 27 in non‐cholecystectomized patients who had undergone endoscopic common bile duct stone removal. Additionally, Yadav et al. 18 observed that younger patients had a higher risk of recurrent AP, and de Mestral et al. 12 showed similar results in patients with ACC discharged without cholecystectomy.
Sphincterotomy reduced the overall SGD recurrence rate (HR = 0.58, 95% CI: 0.49–0.68). This finding aligns with other studies. In a systematic review, van Geenen et al. 28 found a 5% recurrence rate for biliary AP with sphincterotomy versus 48%–57% in the conservative group. However, sphincterotomy had limited impact in preventing ACC or BC. McAlister et al. 29 show that 16% of patients with gallbladders left in situ after endoscopic sphincterotomy developed ACC or BC. Prior studies have noted that the number and size of gallstones may influence the type of SGD. Moreover, biliary sludge and small gallstones (<5 mm) could be risk factors for AP. 30 In this study, we compared multiple versus any other type of gallstone (absent, sludge, or simple) as a recurrence factor. Multiple cholelithiasis was found to carry a slightly higher risk of recurrence (HR = 1.19; 95% CI: 1.05–1.34), contradicting the findings by Kim et al. 30 who found no association between the number of gallstones and the risk of AP relapse.
Higher blood levels of ALT and lower blood leukocyte count during admission were associated with a greater risk of relapses; ALT>35U/L (HR = 1.22; 95% CI: 1.02–1.46), leukocytes >11.000/mm3 (HR = 0.79; 95% CI: 0.70–0.90) contradicting previous studies which showed no association between analytical values and relapses. 25 , 26 , 30 Elevated ALT might be linked to gallstone passage through bile ducts, as observed in AP, where ALT >150U/L suggests gallstone etiology. 31
As for leukocytosis, there is no previous evidence. It could be hypothesized that the increased leukocyte count may be a sign of significant gallbladder inflammation, which could subsequently be associated with some degree of fibrosis of the gallbladder wall with subsequent hypomotility and decreased risk of further gallstones leading to symptoms or complications, or, alternatively, it may be a manifestation of significant pancreatic inflammation leading subsequently to pancreatic scarring, decreasing the risk of further episodes of AP.
No other demographic or analytical variables were associated with recurrences. Necrotizing pancreatitis, local complications, pancreas divisum, or duodenal diverticula, previously suggested as risk factors, showed no association either. 26 , 32 , 33 , 34
An interesting finding was that bivariate analysis revealed an association between post‐ERCP biliary stenting and increased recurrences. However, multivariate analysis did not corroborate this. This aligns with Sasani A et al. 35 recent randomized clinical trial, demonstrating higher recurrence (20.6% vs. 9.4%, p = 0.306) and complications (14.7% vs. 0%, p = 0.024) in stented patients waiting for cholecystectomy after complete choledochal cleaning by ERCP.
When analyzing each SGD independently, age and leukocyte count influenced recurrences in all SGDs. Endoscopic sphincterotomy had no impact on preventing ACC relapses, suggesting that local factors at the gallbladder and cystic duct are more relevant than the possibility of future common bile duct stones in such patients. Multiple cholelithiasis did not influence relapse in ACL and bc patients. Finally, higher blood levels of ALT influenced relapses in AP and bc, probably as a marker of common bile duct stones (Supplementary Tables 3‐7).
Regarding relapse‐free survival, our study, with a median follow‐up time of 5.3 months, showed a higher relapse rate (0.79, 0.71, and 0.63 at 3, 6, and 12 months, respectively) compared with other reports (0.82–0.89) 10 , 18 , 19 with median follow‐ups ranging from 6 to 48 months. Differences may be attributed to our lower cholecystectomy rate (52.1%) and longer waiting time for cholecystectomy (4.2 months). Results from a Randomized Controlled Study by Da costa et al. 10 showed a 17% relapse rate in AP patients undergoing delayed cholecystectomy with a 6‐month follow‐up. However, all patients underwent cholecystectomy within a median of 27 days, with a corresponding decrease in the recurrence rate.
With regard to the recurrence pattern in the different SGDs, ACL and SC showed lower relapse rates compared with AP, ACC, or BC. However, these differences in the bivariate but not in the multivariable analysis were probably due to the influence of older age and higher sphincterotomy rates in ACL and SC.
The median time to first relapse was 2.2 months; it varied among different SGDs; SC had the shortest (1.2 months), while ACL had the longest (2.9 months). Previous studies reported contradictory results. Our findings were similar to those of Yadav et al. 18 and de Mestral et al. 12 (in both studies the median time to relapse was 2.6 months since first recurrence) but differed from Da Costa et al. 10 (median time 15 days (IQR 8–21)) possibly due to their shorter waiting time for cholecystectomy and shorter follow‐up time.
In general, the most common relapse pattern was bc (29.8%), followed by AP (25.8%). Interestingly, the most common presentation of relapse in each SGD was consistent with the same disease that caused the index episode, except for SC where bc emerged as the most common relapse pattern, probably due to endoscopic sphincterotomy that prevented new SC episodes.
What stands out in Supplementary Table 3 is that there were no differences between the severity of the index admission and the first relapse episode except for AP. This also accords with earlier studies, 25 which showed that the severity of AP relapses was milder than the index episode.
Another important finding was that the majority of patients who suffered relapses only had one episode (56.5%); however, 43.5% suffered two (27.3%) or more (16.2%) episodes of recurrences until the end of follow‐up.
Our study has several limitations inherent to its retrospective design. Only hospitalized cases as the first episodes of SGDs were included, resulting in a smaller number of recruited bc patients due to its predominantly outpatient nature. Nevertheless, this study exhibits significant strengths, including a large representative sample size (n = 3016) and the ability to compare the relapse patterns and determining factors in the five types of SGDs.
In conclusion, we have comprehensively characterized the various recurrence patterns observed in different SGDs, providing patients and healthcare providers with valuable information on the timing, presentation, and likelihood of recurrence. Age, endoscopic sphincterotomy, presence of multiple cholelithiasis, leukocyte count and ALT during admission are predictors of relapse. These variables could be a useful tool to prioritize selected patients for cholecystectomy. Furthermore, they can be a valuable tool to determine which patients with a high surgical risk may benefit from less aggressive therapies such as endoscopic sphincterotomy.
CONFLICT OF INTEREST STATEMENT
All the authors confirm no conflicts of interest to disclose.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors would like to express their gratitude for the grant awarded by the Spanish Association of Gastroenterology (AEG grupo joven 2021). We also wish to thank Sylva‐Astrik Torossian for her assistance in language editing. The authors did not preregister the research with the analysis plan in an independent institutional registry.
Velamazán R, López‐Guillén P, Martínez‐Domínguez SJ, Abad Baroja D, Oyón D, Arnau A, et al. Symptomatic gallstone disease: recurrence patterns and risk factors for relapse after first admission, the RELAPSTONE study. United European Gastroenterol J. 2024;12(3):286–298. 10.1002/ueg2.12544
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta‐analysis. Gastroenterology. 2021;162(1):122–134. 10.1053/j.gastro.2021.09.043 [DOI] [PubMed] [Google Scholar]
- 2. Wadhwa V, Jobanputra Y, Garg SK, Patwardhan S, Mehta D, Sanaka MR. Nationwide trends of hospital admissions for acute cholecystitis in the United States. Gastroenterol Rep. 2017;5(1):36–42. 10.1093/gastro/gow015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez‐Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Prim. 2016;28(2):16024. 10.1038/nrdp.2016.24 [DOI] [PubMed] [Google Scholar]
- 4. Morris‐Stiff G, Sarvepalli S, Hu B, Gupta N, Lal P, Burke CA, et al. The natural history of asymptomatic gallstones: a longitudinal study and prediction model. Clin Gastroenterol Hepatol. 2023;21(2):319–327. 10.1016/j.cgh.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 5. Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology. 2022;162(2):621–644. 10.1053/j.gastro.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 2019;89(6):1075–1105. 10.1016/j.gie.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crockett SD, Wani S, Gardner TB, Falck‐Ytter Y, Barkun AN. American gastroenterological association institute clinical guidelines committee. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096–1101. 10.1053/j.gastro.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 8. Mayumi T, Okamoto K, Takada T, Strasberg SM, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):96–100. 10.1002/jhbp.519 [DOI] [PubMed] [Google Scholar]
- 9. Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):55–72. 10.1002/jhbp.516 [DOI] [PubMed] [Google Scholar]
- 10. Da Costa DW, Bouwense SA, Schepers NJ, Besselink MG, van Santvoort HC, van Brunschot S, et al. Same‐admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet. 2015;386(10000):1261–1268. 10.1016/s0140-6736(15)00274-3 [DOI] [PubMed] [Google Scholar]
- 11. Moody N, Adiamah A, Yanni F, Gomez D. Meta‐analysis of randomized clinical trials of early versus delayed cholecystectomy for mild gallstone pancreatitis. Br J Surg. 2019;106(11):1442–1451. 10.1002/bjs.11221 [DOI] [PubMed] [Google Scholar]
- 12. De Mestral C, Rotstein OD, Laupacis A, Hoch JS, Zagorski B, Nathens AB. A population‐based analysis of the clinical course of 10,304 patients with acute cholecystitis, discharged without cholecystectomy. J Trauma Acute Care Surg. 2013;74(1):26–31. 10.1097/ta.0b013e3182788e4d [DOI] [PubMed] [Google Scholar]
- 13. Garg SK, Bazerbachi F, Sarvepalli S, Majumder S, Vege SS. Why are we performing fewer cholecystectomies for mild acute biliary pancreatitis? Trends and predictors of cholecystectomy from the National Readmissions Database (2010‐2014). Gastroenterol Rep. 2019;7(5):331–337. 10.1093/gastro/goz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Baal MC, Besselink MG, Bakker OJ, van Santvoort HC, Schaapherder AF, Nieuwenhuijs VB, et al. Timing of cholecystectomy after mild biliary pancreatitis: a systematic review. Ann Surg. 2012;255(5):860–866. 10.1097/sla.0b013e3182507646 [DOI] [PubMed] [Google Scholar]
- 15. Somasekar K, Shankar PJ, Foster MHL, Lewis MH. Costs of waiting for gall bladder surgery. Postgr Med J. 2002;78(925):668–669. 10.1136/pmj.78.925.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmisano S, Benvenuto C, Casagranda B, Dobrinja C, Piccinni G, de Manzini N. Long waiting lists and health care spending the example of cholecystectomy. Ann Ital Chir. 2015;86:327–332. [PubMed] [Google Scholar]
- 17. Ruhi‐Williams P, Manasa M, Fazl Alizadeh R, Sullivan B, Kirby KA, Amin A, et al. Impact of COVID‐19 pandemic on management and outcomes of acute cholecystitis at US academic centers. J Am Coll Surg. 2023;237(1):87–93. 10.1097/xcs.0000000000000668 [DOI] [PubMed] [Google Scholar]
- 18. Yadav D, Connell MO, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096–1103. 10.1038/ajg.2012.126 [DOI] [PubMed] [Google Scholar]
- 19. Magnusdottir BA, Baldursdottir MB, Kalaitzakis E, Björnsson ES. Risk factors for chronic and recurrent pancreatitis after first attack of acute pancreatitis. Scand J Gastroenterol. 2019;54(1):87–94. 10.1080/00365521.2018.1550670 [DOI] [PubMed] [Google Scholar]
- 20. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis — 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 21. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;2013(1):41–54. 10.1002/jhbp.515 [DOI] [PubMed] [Google Scholar]
- 22. Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):17–30. 10.1002/jhbp.512 [DOI] [PubMed] [Google Scholar]
- 23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 24. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 25. Bejarano‐González N, Romaguera‐Monzonís A, García‐Borobia FJ, García‐Monforte N, Serra‐Pla S, Rebasa‐Cladera P, et al. Cómo afecta el retraso de la colecistectomía tras la pancreatitis aguda litiásica en la aparición de recidivas. Consecuencias de la falta de recursos. Rev Esp Enferm Dig. 2016;108:117–122. [DOI] [PubMed] [Google Scholar]
- 26. Zhang W, Shan HC, Gu Y. Recurrent acute pancreatitis and its relative factors. World J Gastroenterol. 2005;11(19):3002–3004. 10.3748/wjg.v11.i19.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park BK, Seo JH, Jeon HH, Choi JW, Won SY, Cho YS, et al. A nationwide population‐based study of common bile duct stone recurrence after endoscopic stone removal in Korea. J Gastroenterol. 2018;53(5):670–678. 10.1007/s00535-017-1419-x [DOI] [PubMed] [Google Scholar]
- 28. Van Geenen EJM, Van Der Peet DL, Mulder CJJ, Cuesta MA, Bruno MJ. Recurrent acute biliary pancreatitis: the protective role of cholecystectomy and endoscopic sphincterotomy. Surg Endosc. 2009;23(5):950–956. 10.1007/s00464-009-0339-0 [DOI] [PubMed] [Google Scholar]
- 29. McAlister VC, Davenport E, Renouf E. Cholecystectomy deferral in patients with endoscopic sphincterotomy. Cochrane Database Syst Rev. 2007;4(1):CD006233. 10.1002/14651858.cd006233.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SB, Kim TN, Chung HH, Kim KH. Small gallstone size and delayed cholecystectomy increase the risk of recurrent pancreatobiliary complications after resolved acute biliary pancreatitis. Dig Dis Sci. 2017;62(3):777–783. 10.1007/s10620-016-4428-3 [DOI] [PubMed] [Google Scholar]
- 31. Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: a meta‐analysis. Am J Gastroenterol. 1994;89:1863–1866. [PubMed] [Google Scholar]
- 32. Ali UA, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, et al. Dutch pancreatitis study group. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. 2016;14(5):738–746. [DOI] [PubMed] [Google Scholar]
- 33. Zakko L, Gardner TB. Endoscopic management of recurrent acute pancreatitis. Clin Gastroenterol Hepatol. 2019;17(11):2167–2170. 10.1016/j.cgh.2019.04.069 [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Xia L, Lu Y, Bie L, Gong B. Influence of periampullary diverticulum on the occurrence of pancreaticobiliary diseases and outcomes of endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. 2017;29(1):105–111. 10.1097/meg.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 35. Sasani A, Mandavdhare HS, Sharma V, Shah J, Patil A, Gupta P, et al. Role of biliary stent in recurrence of biliary stones and complications after stone clearance in patients awaiting cholecystectomy: a randomized trial. Am J Gastroenterol. 2023;1(10):1864–1870. 10.14309/ajg.0000000000002471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


