Abstract
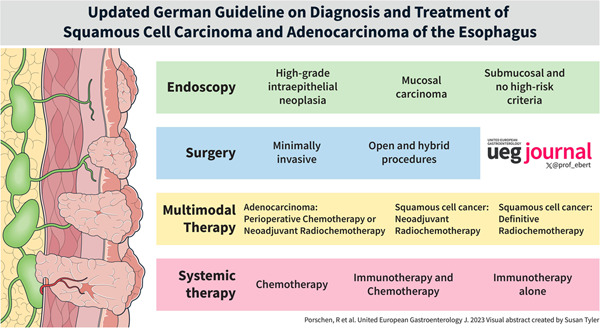
Diagnosis and therapy of esophageal carcinoma is challenging and requires a multidisciplinary approach. The purpose of the updated German guideline “Diagnosis and Treatment of Squamous Cell Carcinoma and Adenocarcinoma of the Esophagus—version 3.1” is to provide practical and evidence‐based advice for the management of patients with esophageal cancer. Recommendations were developed by a multidisciplinary expert panel based on an extensive and systematic evaluation of the published medical literature and the application of well‐established methodologies (e.g. Oxford evidence grading scheme, grading of recommendations). Accurate diagnostic evaluation of the primary tumor as well as lymph node and distant metastases is required in order to guide patients to a stage‐appropriate therapy after the initial diagnosis of esophageal cancer. In high‐grade intraepithelial neoplasia or mucosal carcinoma endoscopic resection shall be performed. Whether endoscopic resection is the definitive therapeutic measure depends on the histopathological evaluation of the resection specimen. Esophagectomy should be performed minimally invasive or in combination with open procedures (hybrid technique). Because the prognosis in locally advanced esophageal carcinoma is poor with surgery alone, multimodality therapy is recommended. In locally advanced adenocarcinomas of the esophagus or esophagogastric junction, perioperative chemotherapy or preoperative radiochemotherapy should be administered. In locally advanced squamous cell carcinomas of the esophagus, preoperative radiochemotherapy followed by complete resection or definitive radiochemotherapy without surgery should be performed. In the case of residual tumor in the resection specimen after neoadjuvant radiochemotherapy and R0 resection of squamous cell carcinoma or adenocarcinoma, adjuvant immunotherapy with nivolumab should be given. Systemic palliative treatment options (chemotherapy, chemotherapy plus immunotherapy, immunotherapy alone) in unresectable or metastastic esophageal cancer depend on histology and are stratified according to PD‐L1 and/or Her2 expression.
Keywords: Barrett's esophagus, endoscopic resection, esophageal carcinoma, immune therapy, multimodal therapy, neoadjuvant radiochemotherapy, perioperative chemotherapy
INTRODUCTION
The diagnosis and therapy of esophageal carcinoma is challenging due to the anatomical location of the esophagus. A further challenge is the fact that patients often have multiple co‐morbidities with respect to accompanying alcohol and tobacco consumption. Therefore, a multidisciplinary approach is required in order to guide patients to a stage‐appropriate therapy.
Although guidelines on therapy for esophageal cancer have been published, 1 , 2 there was a need to develop a German guideline covering all topics of esophageal cancer because the incidence of esophageal cancer in Deutschland was increasing. 3 First published in 2015, 4 the guideline enabled standardization in prevention, surveillance, diagnosis and therapy. The guideline was regularly revised. 5 This review summarizes the recommendations of the most recent version 3.1 of the “German Guideline on Diagnosis and Treatment of Squamous Cell Carcinoma and Adenocarcinoma of the Esophagus.” 6 The purpose of this guideline is to provide practical and evidence‐based advice for the clinicians who care for patients with esophageal cancer.
MATERIAL AND METHODS
It is the aim of this evidence‐ and formally consensus‐based S3‐guideline to assist professional practitioners and patients in decisions about appropriate health care for patients with risk factors for the development of esophageal cancer (e.g. Barrett's esophagus) and for patients with esophageal cancer. The development and the updating process of the guideline is based on the actual AWMF guidance (German Association of the Scientific Medical Societies) (https://www.awmf.org/fileadmin/user_upload/dateien/downloads_regelwerk/AWMF‐Guidance_2013.pdf) which in turn addresses criteria of the AGREE II tool (Appraisal of guidelines for research & evaluation II), as well as criteria of the Guidelines International Network McMaster Guideline Development checklist. 7 This includes the constitution of a representative guideline group including a patient representative, a structured conflict of interest management, systematic literature searches and critical appraisal of the literature as well as a formal consensus process. For this update, the leading professional society (German Society for Gastroenterology, Digestive and Metabolic Diseases; Deutsche Gesellschaft für Gastroenterologie, Verdauungs‐ und Stoffwechselkrankheiten [DGVS]) was in charge to put together experts of all relevant medical societies including a patient representative. All guideline group members had to submit a written declaration of interests. Conflicts of interest were assessed and managed according to the rules of AWMF. For the new version 3.1 of the guideline, topic specific “Patient/Population”, “Intervention”, “Comparison”, “Outcome” items were discussed, and 20 key questions were formulated by the multidisciplinary guideline group. A systematic electronic literature search (search period 06/2016–11/2020) was performed in Medline via PubMed and in the Cochrane Library. The search strategy was performed by methodologists of a third party, the Clinical Guideline Services (CGS) with input from the investigators. Because the immune checkpoint inhibitors pembrolizumab and nivolumab received drug approval in Germany during the final preparation of the guideline, an additional systematic search for studies on nivolumab and pembrolizumab in esophageal cancer was conducted in October 2021. 8
Subsequent evidence assessment was independently conducted by the User‐Group‐Med. Guideline Development e.V./CGS. The methodological quality of each included study was assessed using the 2011 version of the Oxford Center for Evidence‐Based Medicine (OCEBM) Levels of Evidence. 9 The guideline group has been working with the OCEBM Levels of evidence since the first version of the guideline in 2013 and therefore continues to use this evidence classification system.
All included studies were extracted into evidence tables. The methodological quality of the included studies was checked using checklists according to the study design. OCEBM 2011 also allows for considering GRADE criteria in terms of judging inconsistency, indirectness, imprecision or publication bias in addition to the risk of bias assessment. Results of the critical appraisal were recorded in the “Notes” section of the evidence tables. The methodological process for the preparation of the guideline is described in depth in the guideline report. 8 After the completion of the evidence research, the results were primarily reviewed by the steering group and potential topics for changes or additions were identified. Concrete proposals for changes were then formulated in consultation with the working groups.
The methodology of the German Association of the Scientific Medical Societies (AWMF) provides for the award of grades of recommendation (GoR) by the guideline authors within the framework of a formal consensus procedure. 10 , 11 , 12 Accordingly, the guideline group conducted a structured consensus conference with neutral moderation of AWMF certified guideline advisers. Only the recommendations were discussed and formally approved, which did not reach a strong consensus (>95% consent) in the first online‐historical term for decision‐making process to find general consensus voting using Survey Monkey as a digital tool. Within the framework of this process, recommendations were discussed, changed according to arguments and formally voted on by the experts of the multidisciplinary guideline group in alignment with COI rules.
In the guideline, the level of evidence (LoE) of the underlying studies according to the 2009 version of the Oxford Centre for Evidence‐Based Medicine 13 and, in the case of recommendations, the strength of the recommendation (grades of recommendation) are shown for all evidence‐based statements and recommendations. With regard to the strength of the recommendation, this guideline distinguishes between three levels of recommendation, which are also reflected in the wording of the recommendations: A (strong recommendation, “shall/shall not”), B (recommendation, “should/should not”) and 0 (no definite recommendation, “can/cannot”). In principle, the evidence‐based recommendations were based on the strength of the available evidence with regard to their degree of recommendation, that is, a high degree of evidence (e.g. meta‐analyses/systematic reviews of randomized clinical trials or several methodologically high‐quality randomized clinical trials), that is, a high degree of certainty with regard to the results should generally lead to a strong recommendation. Further criteria for determining the strength of recommendation were mostly the perceived benefit‐risk‐ratio of the respective intervention in the light of the quality of the evidence and in some cases also patient preferences, feasibility and acceptability. The background text for each recommendation provides the rationale. Recommendations on which was decided on the basis of an expert consensus of the guideline group without an underlying systematic search and appraisal of the literature are depicted as “expert consensus” (EC). 8
The guideline was sent to all participating professional societies for approval and made available as a consultation version on the DGVS website for comments by the professional public. No recommendation had to be changed due to comments. Eleven relevant and measurable quality indicators were approved or developed based on strong recommendations of this guideline (GoR A). These quality indicators are used in the assessment and auditing of visceral oncology centers.
The development of the guideline was funded by German Cancer Aid (Deutsche Krebshilfe) through the German Guidelines Program in Oncology (GGPO). The guideline was editorially independent of the funding source.
RECOMMENDATIONS
Recommendation 1: Endoscopic therapy
If high‐grade intraepithelial neoplasia (HGIEN) or mucosal carcinoma (L0, V0, no ulceration, grading G1/G2) is detected in Barrett's esophagus after careful pre‐endoscopic evaluation, endoscopic resection shall be performed en‐bloc, as this provides staging of the lesion with the question of infiltration. In patients with superficial submucosal infiltration of adenocarcinoma and no risk criteria (pT1sm1; invasion depth <500 μm, L0, V0, G1/2, <20 mm, no ulceration), endoscopic resection can be regarded as a sufficient alternative to surgery after careful pre‐endoscopic evaluation. After successful resection of neoplasms in Barrett's esophagus, non‐neoplastic Barrett's mucosa shall be thermally ablated to decrease the rate of metachronous neoplasms (EC).
If there is evidence of high‐grade intraepithelial neoplasia or mucosal carcinoma (L0, V0, no ulceration, grading G1/G2, infiltration depth m1/m2) in the squamous epithelium, endoscopic en bloc resection should be attempted after careful pre‐endoscopic evaluation (EC).
The term endoscopic resection (ER) includes both endoscopic mucosal resection (EMR), which is performed using suction and cutting techniques, and endoscopic submucosal dissection (ESD). In Germany, EMR is usually performed using a ligation set or the cap technique. In the meantime, endoscopic resection (ER) in the form of EMR has been established in many Western countries as a standard therapy procedure for HGIEN and mucosal adenocarcinomas. 14 , 15 , 16 Numerous cohort studies have shown ER to be safe and effective, with lower morbidity and mortality than esophageal resection at the same curation rate. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 While the German guidelines provide no preference with regard to the use of EMR versus ESD, current ESGE guidelines recommend EMR for smaller lesions (smaller than 20 mm), whereas larger lesions may be resected by ESD. 26
In patients with superficial submucosal infiltration of adenocarcinoma, endoscopic resection may be a sufficient alternative to surgery in selected cases. 27 Manner et al. treated 66 patients with low‐risk lesions (infiltration sm1, L0,V0, G1/2, no ulceration). Complete remission was achieved in 53 patients. After a median follow‐up of 47 ± 29.1 months, the estimated 5‐year survival rate was 84%. 28 , 29
In a retrospective analysis, the Wiesbaden research group was able to show that ablation by photodynamic therapy or argon plasma coagulation (APC) of the remaining, non‐neoplastic Barrett's mucosa after prior endoscopic therapy of an HGIEN or mucosal carcinoma significantly reduced the rate of metachronous neoplasia. 18 Pouw et al. reported on the successful ablation of non‐neoplastic mucosa using radiofrequency ablation. 24
In analogy to Barrett's adenocarcinoma, endoscopic resection (ER) is the standard procedure for the treatment of mucosal carcinomas in the squamous epithelium (SCC). For SCC, studies have shown ESD to be superior to EMR. 30 Cao et al. confirmed that ESD is superior to EMR with regard to en bloc resection rate, R0‐en bloc resection rate and recurrences. 31 Current ESGE guidelines recommend ESD over EMR for SCC. 26 Risk criteria for recurrence of carcinomas of the squamous epithelium after endoscopic resection were not discussed.
Ideally, ER should be used to remove the neoplastic lesion R0‐en‐bloc to ensure accurate histological staging. Careful histopathological evaluation allows risk stratification so that either ER is the definitive therapeutic measure or the decision for surgical therapy must be made. After successful endoscopic therapy of high‐grade intraepithelial neoplasia or early carcinoma, regular control endoscopies shall be performed after 3 months, then every 6 months for 2 years, and then annually.
Recommendation 2: Surgical technique
Esophagectomy and reconstruction of the esophagus should be performed minimally invasive or in combination with open procedures (hybrid technique) if there are no contraindications against this approach (EC).
Prior to planned esophagectomy, a risk analysis of important organ functions (cardiac, pulmonary and hepatic) of the patient shall be performed. Screening for malnutrition shall be performed as part of the preoperative risk stratification. Patients with severe malnutrition, that is, high metabolic risk, shall receive nutritional therapy before surgery, even if surgery has to be postponed.
The TIME trial (total minimally invasive [MIS] vs. open resection) showed a significantly lower rate of pulmonary complications, a shorter stay in the intensive care unit and a shorter length of hospital stay. The 1‐year quality of life in terms of physical activity, global health and pain was significantly better in the MIS group. The 3‐year long‐term survival was similar in the two groups of the TIME‐trial. 32 , 33
The MIRO trial, 34 , 35 , 36 comparing open esophagectomy (n = 103) versus laparoscopic esophagectomy by thoracotomy (hybrid procedure) (n = 102) showed significantly lower rates regarding postoperative morbidity, postoperative pulmonary complications and postoperative Clavien‐Dindo score II‐IV in favor of the hybrid technique. The 3‐year rates of overall and tumor‐free survival were higher, 67% versus 55% and 57% versus 48%, respectively, for the hybrid group, but without statistical significance. However, the health‐related quality of life remained significantly higher 30 days and 2 years after the hybrid procedure compared to open esophagectomy. 36 The results of the MIRO trial are confirmed by a retrospective study with propensity matching and a meta‐analysis of 2397 patients. 37 , 38
A large meta‐analysis of 55 studies compared the long‐term prognosis of 14,592 patients with esophageal cancer after minimally invasive inclusive hybrid (50.4%) versus open esophagectomy (49.6%). 39 The MIS/hybrid group had 18% lower 5‐year all‐cause mortality. Robotic‐assisted methods were not discussed in the current version of the guideline; however, they will be addressed in the next edition.
Recommendation 3: Multimodal therapy of adenocarcinoma
For localized adenocarcinomas of the esophagus and esophagogastric junction of category cT2, preoperative chemotherapy may be administered and continued postoperatively (GoR 0, LoE 1b).
In patients, who are fit for surgery and present with locally advanced adenocarcinoma of the esophagus or esophagogastric junction (category cT3 or T4 with infiltration of neighboring structures considered resectable or category cN1‐3), perioperative chemotherapy or preoperative radiochemotherapy shall be given (GoR A, LoE 1a).
In the available randomized trials of pre‐ or perioperative chemotherapy for esophageal carcinoma, the proportion of patients with an initial T1/2 category is either not reported, 40 , 41 , 42 or, as far as can be extrapolated from the primary operated patient group, was below 37%. 43 , 44 Moreover, there are no separate data on the benefit of pre‐ or perioperative therapy in this small subgroup of patients. Due to a lower rate of lymph node metastasis as well as occult distant metastases, the T2 category was prognostically more favorable than T3/4 and an expected effect of neoadjuvant therapy is probably lower. Nevertheless, patients with T2 tumors were also part of the study population in whom survival gain could be achieved by perioperative chemotherapy. 43 , 44 However, the strength of recommendation for perioperative chemotherapy in the category T2 is weaker (GoR 0) due to the small number of patients.
The role of radiochemotherapy in the neoadjuvant therapy of esophageal adenocarcinoma is still under debate. Overall survival was significantly prolonged in 2 studies using preoperative RCT compared with surgery alone 45 , 46 : One of them was terminated prematurely due to poor recruitment (23 vs. 19 patients) and in this trial, different histologies and tumor stages were analyzed together. OS was also prolonged in the ChemoRadiotherapy for Oesophageal cancer followed by surgery study (CROSS) study after neoadjuvant radiochemotherapy compared with surgery alone. 47 , 48 However, median survival time with preoperative RCT was lower for adenocarcinomas than for SCC (43.2 vs. 81.6 months). One study showed no significant advantage of neoadjuvant therapy compared to surgery alone 49 (n = 80 vs. 78), and here both chemotherapy (only one course of cisplatin/5‐FU) and radiotherapy (35 Gy) were below the doses usually used. One study reported no survival data. 50 In two studies, recurrence free survival (RFS) was prolonged by trimodal therapy. 44 , 46 RFS was better in the discontinued study after trimodal therapy for the mixed collective of SCC and adenocarcinoma at 1.01 versus 3.47 years. 51 RFS was also prolonged after trimodal therapy in the CROSS study (median 17.7 vs. 29.9 months). 47 , 48 Burmeister et al. showed no benefit in progression‐free survival by trimodal therapy for adenocarcinomas. 49 Zhao et al. did not report RFS. 50
Overall, in locally advanced adenocarcinoma of the esophagus or esophagogastric junction, perioperative chemotherapy or preoperative radiochemotherapy should be given. Meta‐analysis comparing perioperative chemotherapy and neoadjuvant radiochemotherapy in locally advanced adenocarcinoma support equipoise in decision making in clinical practice. 51 , 52 Because the best evidence for the benefit of chemotherapy comes from studies with perioperative application of chemotherapy, postoperative continuation of chemotherapy is recommended. 53 , 54
Recommendation 4: Multimodal therapy of esophageal squamous cell cancer
In patients, who are fit for surgery and present with cT2 squamous cell carcinoma (SCC) of the esophagus, preoperative radiochemotherapy followed by complete resection may be performed (EC).
In patients, who are fit for surgery and present with a locally advanced SCC of the esophagus (category cT3 or T4 with infiltration of neighboring structures considered resectable or category cN1‐3), preoperative radiochemotherapy followed by complete resection shall be performed (GoR A, LoE 1a).
From large meta‐analyses, statistically significant survival advantages for combined preoperative RCT compared to surgery alone were observed. 52 , 55 , 56 , 57 As stated above, many of the underlying papers are of low quality. As a result, the validity of these meta‐analyses also suffers. Individual randomized trials have used cisplatin/5‐FU, sometimes plus a third agent, or carboplatin/paclitaxel simultaneously with radiotherapy at a dose of 40–50.4 Gy in locally advanced tumors in both thoracic SCC and adenocarcinoma. However, the advantages are different in the two tumor entities, so that a separate discussion is indicated although it often remains unclear which patient groups may benefit.
Two studies showed no significant survival benefit with trimodal therapy. In one of these studies, all resectable tumors (category cT1‐3 and cN1‐3) were combined. 49 In the other study, tumors of category >cT1 and/or >cN0 were included. This study was closed early due to an unexpectedly high conversion rate to surgery (31%). 58 One study including 140 patients showed a trend toward better overall survival with RCT in long‐term follow‐up (median OS 56.5 vs. 41.5 months). 59 This benefit was independent of whether RCT was given before or after resection, although only 40% of patients (30/78) were able to receive postoperative therapy according to protocol. Two studies 47 , 48 showed significant median survival advantage after trimodal therapy: 81.6 versus 21.1 months (n = 41 vs. 43) (inclusion of tumors of category >cT1 and/or >cN0) and 100.1 versus 66.5 months (n = 224 vs. 227) (cN+ or cT4 N0 tumors). 60 RFS was prolonged by trimodal therapy in almost all studies including patients with esophageal SCC. RFS, for example, was significantly prolonged by neoadjuvant RCT in the CROSS trial (74.7 vs. 11.6 months), 47 , 48 in a Chinese trial (46.5 vs. 32.5 months) 59 and in the NEOCRTEC5010 trial (100.1 vs. 41.7 months). 60
Overall, a positive effect of preoperative RCT can be demonstrated, especially in squamous cell carcinoma. However, the question of which subgroups of patients benefit from neoadjuvant treatment in clinical reality remains unsolved. This uncertainty arises in particular from the lack of accuracy of preoperative staging, especially with regard to tumor‐involved lymph nodes. For example, a study in early squamous cell carcinoma (stages I to IIb) at predominantly highly experienced French centers 61 showed that 39% of the patients in the group with primary surgery actually had pathological stage III and therefore were “understaged.” Surgical technique and radicality also play a role and are not standardized. In addition, the experience of a center must be considered (hospital volume) because it influences postoperative mortality and long‐term outcomes. It is conceivable that tumors in the cT3N0 category could be treated equally well with primary surgery, that is, that patients would be overtreated by the standard of trimodal therapy. However, we know from older studies that tumors of category cT3 develop at least regional lymph node metastases in more than 80% of cases 62 and patients in this case have a very poor prognosis even after optimal surgery. 63
Few studies precisely differentiated the included stages and only one study investigated tumor stages I and II. The study by Mariette et al. 61 investigated carcinomas of categories T1/2, N0/1, and T3N0 (M0), and included both adenocarcinomas and predominantly squamous cell carcinomas. There was no survival benefit shown by neoadjuvant therapy. A subgroup‐analysis of SCC was previously published from the same group. Here, also, no significant survival benefit was shown by neoadjuvant RCT in tumors of the category T3N0. 64 The center‐specific, often low reliability of preoperative staging with respect to lymph node involvement, the clearly pronounced variability in the radicality of surgery and the associated different R0 resection rates make it difficult in this situation to draw valid recommendations for this particular tumor situation (clinical stage T3 N0 M0).
A recent review reported on all forms of multi‐modality therapy for SCC of the esophagus. 57 In several meta‐analyses, individual therapy strategies were then compared with each other and subsequently calculated in a so‐called rank probability analysis exploring which multimodal therapy may have the highest therapeutic effect compared to primary surgery. A significant advantage over surgery alone was found for preoperative RCT followed by surgery and definitive RCT. In the rank probability analysis, preoperative RCT had the highest probability of improving prognosis than surgery alone. The data of this comprehensive analysis confirm the strategy of preoperative RCT plus surgery as a standard recommendation for (locally advanced) SCC of the esophagus. Therapy algorithms for functionally operable and oncologically resectable adenocarcinomas of the esophagus/esophagogastric junction and squamous cell carcinoma of the thoracic esophagus are shown in Figures 1 and 2.
FIGURE 1.
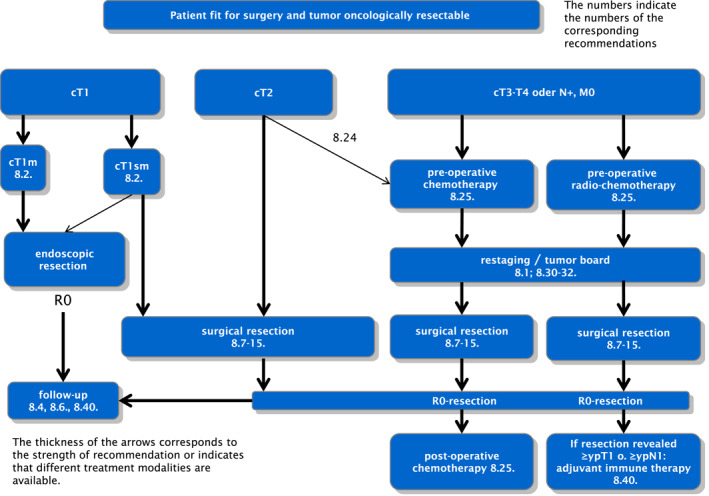
Therapy algorithm for patients fit for surgery with resectable adenocarcinomas of the esophagus and esophagogastric junction. The numbers refer to the corresponding recommendations in the full version of the German guideline on “Diagnosis and Therapy of Squamous Cell carcinoma and Adenocarcinoma of the Esophagus,” please also see section Supporting Information S1. 6 The thickness of the arrows corresponds to the strength of the recommendation or indicates that different treatment modalities are available.
FIGURE 2.
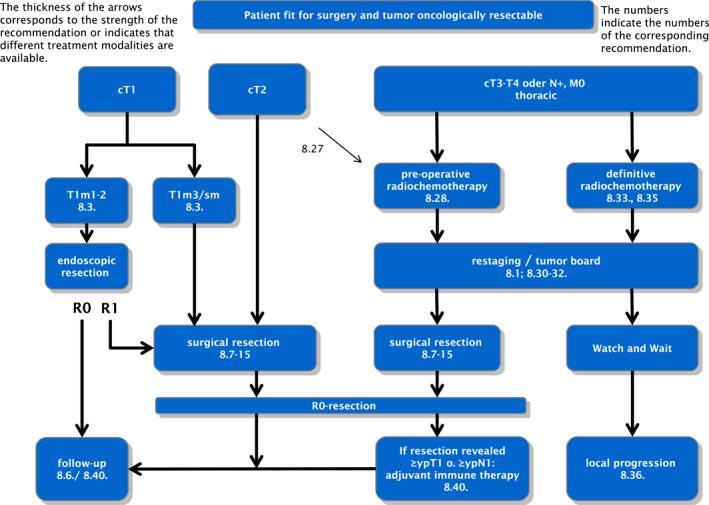
Therapy algorithm for patients fit for surgery with resectable squamous cell carcinoma of the thoracic esophagus. The numbers refer to the corresponding recommendations in the full version of the German guideline on “Diagnosis and Therapy of Squamous Cell carcinoma and Adenocarcinoma of the Esophagus,” please also see section Supporting Information S1. 6 The thickness of the arrows corresponds to the strength of the recommendation or indicates that different treatment modalities are available.
Recommendation 5: Adjuvant immunotherapy
If residual tumor can still be detected histologically in the resected specimen (≥ypT1 or ≥ypN1) after neoadjuvant radiochemotherapy and R0 resection of esophageal squamous cell carcinoma or adenocarcinoma of the esophagus or esophagogastric junction, adjuvant immunotherapy with nivolumab should be performed for 1 year (GoR B, LoE 2).
The international phase III CheckMate 577 trial enrolled patients with stage II/III esophageal cancer or carcinoma of the esophagogastric junction (71% adenocarcinoma, 29% squamous cell carcinoma). 65 If tumor findings ≥ypT1 or ≥ypN1 were still present in the resected specimen after neoadjuvant RCT and surgery, patients were randomized to nivolumab (n = 532) or placebo (n = 262), yp classification categorizes the extent of cancer in the tumor specimen after therapy. The primary endpoint was changed during the study (before evaluation) from the combination of disease‐free and overall survival to disease‐free survival (DFS: events recurrence or death). The analysis showed a significant prolongation of DFS from a median of 11.0 months with placebo to 22.4 months with nivolumab (p < 0.001, HR = 0.69; CI 0.56–0.86). Nivolumab primarily reduced the proportion of distant recurrences (29% vs. 39%). Patients with carcinomas of both histologies benefited significantly (HR = 0.61 for SCC, HR = 0.75 for adenocarcinomas). The outcome did not differ between PD‐L1‐positive (72% of patients) or negative tumors. PD‐L1 expression in tumor cells prior to RCT was considered for this study (TPS score ≥1% or <1%). Tumor proportion score (TPS) is defined as the number of PD‐L1‐positive tumor cells divided by the total number of viable tumor cells multiplied by 100%. Data on overall survival are still lacking.
Recommendation 6: Definitive radiochemotherapy
Definitive radiochemotherapy shall be given irrespective of the histological entity of the esophageal cancer if the tumor is deemed surgically/endoscopically unresectable at an interdisciplinary tumor board or if a patient is functionally inoperable or refuses surgery after detailed information (GoR A, LoE 1b).
For patients who are not fit for surgery or whose esophageal cancer is assessed as unresectable, there is a curative chance with definitive RCT, provided there are no distant metastases. Long‐term survival rates of 10%–35% at 5 years have been observed in prospective studies and in large registry studies 66 , 67 for both stage II‐III SCC and adenocarcinoma. Definitive RCT is more effective than radiotherapy alone; therefore, the combination should always be preferred in patients without contraindications to cisplatin‐, carboplatin‐, or oxaliplatin‐containing chemotherapy. 68 , 69
It is currently unclear whether patients with clinically complete remission (varying definitions in the literature) after curatively intended RCT benefit from surgical resection. 70 A meta‐analysis summarized four retrospective studies (648 patients) that predominantly followed patients with SCC. 71 Patients with surgical resection did have significantly better disease‐free survival (DFS) at 2 years. However, at 5 years, neither DFS (HR 1.78, 95% CI 0.87–3.66) nor overall survival (HR 1.36, 95% CI 0.57–3.24) were significantly different. The problem lies, among others, in the prediction of histologically complete destruction of the tumor by clinical methods including PET‐CT, magnetic resonance imaging, 72 and biopsies in the former tumor region. However, the above‐mentioned data of retrospective studies allow a watch‐and‐wait approach after clinically complete remission in case of patient request for organ preservation or increased surgical risk. Regular follow‐up with endoscopy and computed tomography is useful if evidence of localized tumor progression in the esophagus may lead to delayed surgical resection (so‐called salvage surgery).
In case of tumor persistence or local recurrence without distant metastases after RCT, salvage surgery can be attempted with curative intent. Careful evaluation of operability and resectability should be performed by a treatment team experienced in esophageal surgery. 6 A more recent literature search selected 28 studies in which 1046 patients with persistent or recurrent esophageal cancer underwent salvage esophageal resection after definitive RCT. 73 Pooled 30‐ and 90‐day mortality rates were 2.6% and 8.0%, respectively. The 3‐year and 5‐year overall survival rates were 39% and 19.4%, respectively. The authors concluded that salvage surgery would be a potentially curative treatment option for patients in whom surgery was not initially performed but who are in an operable state.
Recommendation 7: Definitive radiochemotherapy in squamous cell carcinoma
In patients with resectable squamous cell carcinoma of the intrathoracic esophagus of category cT3/cT4, definitive radiochemotherapy can be performed as an alternative to surgical resection (GoR B, LoE 1a).
Randomized trials comparing definitive RCT with surgery were conducted in patients with resectable SCC of the thoracic esophagus category cT3/cT4 without distant metastases. In the majority of studies, surgery was preceded by neoadjuvant RCT in the surgical groups. None of the studies showed a significant survival benefit with surgery. Also, the meta‐analyses showed no differences in survival. 74 On the other hand, loco‐regional recurrences were more frequent after definitive RCT. Following neoadjuvant RCT and surgery, distant metastases were the predominant location of recurrence. 75 Treatment‐related mortality was higher in the surgical groups than after definitive RCT. Thus, there are differences in loco‐regional efficacy and in the incidence of severe side effects, which are important for recommending therapy in individual patients with comparable survival.
Two recent meta‐analyses have been devoted to compare definitive and preoperative RCT. For this purpose, Li et al. 76 evaluated 13 non‐randomized studies and one randomized clinical trials from 2001 to 2018 that included a total of more than 10,000 patients. The heterogeneity of the studies is very large (e.g. number of patients per arm ranged from 23 to 2848). Trimodal therapy showed an advantage in terms of local recurrence rate (HR 0.35; CI 0.22–0.57) and overall survival (HR 0.65; CI 0.56–0.76). However, there is a potential selection bias for the retrospective, non‐randomized studies. Montagnani et al. 57 provided a comprehensive review using 25 studies (1988–2014) with 3866 patients concerning all forms of multimodality therapy for squamous cell carcinoma of the esophagus. This analysis points out the heterogeneity between the studies as well. A significant advantage over surgery alone was found for preoperative RCT followed by surgery and definitive RCT. According to this analysis, the highest risk reduction of 38% was achieved by definitive RCT: HR 0.62 (CI 0.41–0.96). In the rank probability analysis, definitive RCT had the highest probability of improving prognosis compared with surgery alone (82.8% vs. 54.9%) followed by neoadjuvant RCT plus surgery. However, the authors indicate that the data on neoadjuvant RCT had the most robust results concerning survival benefits. The data from this comprehensive analysis confirm the strategy of preoperative RCT plus surgery as a standard recommendation for locally advanced squamous cell carcinoma of the esophagus. Furthermore, definitive RCT is a well‐documented treatment alternative (especially in case of questionable resectability of the tumor, increased risk of surgery for the patient, patient age >70 years, desire for organ preservation).
In patients with localized squamous cell carcinoma of the cervical esophagus, definitive RCT should be preferred over primary surgical resection. Long‐term survival rates of 17%–55% are achieved with definitive RCT in squamous cell carcinoma of the cervical esophagus. 77 , 78 The best results were observed in series with a high proportion of stage I and IIA patients. 79 In cervical carcinomas, the morbidity of surgery with and without pharyngolaryngectomy is higher than in carcinomas of the thoracic esophagus. 78 , 80 , 81 Therefore, surgery should be performed only in specialized centers. The 5 year survival rates after surgery with or without neoadjuvant or adjuvant radiochemotherapy are 14%–47% in the larger series, a range also covered by definitive RCT studies. 78
Recommendation 8: Palliative systemic therapy in adenocarcinoma
If HER2 status is negative and PD‐L1 CPS <5, a platinum (oxaliplatin or cisplatin)/fluoropyrimidine‐containing two‐ (or three‐) drug combination should be used as palliative first‐line therapy in adenocarcinoma of the esophagus and esophagogastric junction (GoR A, LoE 1a).
In case of negative HER2 status and an elevated PD‐L1 CPS (cut‐off for nivolumab PD‐L1 CPS ≥5, for pembrolizumab PD‐L1 CPS ≥10), a platinum (oxaliplatin or cisplatin)/fluoropyrimidine combination should be used together with one of the mentioned immune checkpoint inhibitors (GoR A, LoE 2).
For HER2‐overexpressing tumors (IHC3+ or IHC2+ and ISH+), first‐line cisplatin/fluoropyrimidine‐based chemotherapy should be supplemented with trastuzumab (GoR A, LoE 2).
In many randomized chemotherapy phase III trials for gastric cancer, the subgroup of metastatic adenocarcinomas of the esophagogastric junction and distal adenocarcinomas of the esophagus represented a substantial proportion of the study population. 82 , 83 Thus, platinum‐ and fluoropyrimidine‐based combination chemotherapy with docetaxel or epirubicin demonstrated significant improvement in terms of survival, time to tumor progression, and quality‐of‐life advantage over older chemotherapy protocols. 82 , 83 Patients with negative HER2 status and a PD‐L1 CPS < 5 (combined positive score) should therefore be offered a platinum‐ and fluoropyrimidine‐containing two‐ (or three‐drug) combination. 6 For patients who do not qualify for platinum (oxaliplatin or cisplatin)‐based therapy, infusional 5‐fluorouracil, folinic acid, and irinotecan (FOLFIRI) is an alternative treatment option. 84
In the meantime, phase III trials have been published establishing the value of immunotherapy in the systemic therapy of advanced non‐curable adenocarcinoma of the esophagus, esophagogastric junction, and stomach. In the KEYNOTE‐590 trial, a significant survival benefit was shown in first‐line therapy for the combination of pembrolizumab with cisplatin and 5‐fluorouracil versus chemotherapy alone in tumors with a PD‐L1 CPS ≥10 (HR 0.62; 13.5 vs. 9.4 months, p < 0.0001) for HER‐2‐negative advanced adenocarcinoma of the esophagus and esophagogastric junction (AEG type 1). 85
In the three‐arm CheckMate‐649 trial, patients with advanced HER2‐negative adenocarcinoma of the esophagus, esophagogastric junction, or stomach received either oxaliplatin‐based combination with a fluoropyrimidine (standard chemotherapy) (N = 792) or nivolumab in addition to chemotherapy (N = 789), or immunotherapy with nivolumab and ipilimumab. The majority (70%) of included patients had metastatic adenocarcinoma of the stomach. The initial analysis of the study (chemotherapy +/− nivolumab) showed a significant improvement in survival for the additional administration of nivolumab versus chemotherapy alone for carcinomas with a PD‐L1 CPS ≥5 (median OS 14.4 vs. 11.1 months; HR 0.71 p < 0.0001). 86 However, there was no significant OS improvement with nivolumab plus ipilimumab versus chemotherapy for PD‐L1 CPS ≥5. 87 Based on these data, nivolumab in combination with platinum‐ and fluoropyrimidine‐based chemotherapy was approved for first‐line treatment of advanced HER2‐negative adenocarcinoma of the esophagus, esophagogastric junction, and stomach with a PD‐L1 CPS ≥5. For rapid review of the most recent treatment algorithms, we refer to guidelines from European Society for Medical Oncology. 2
In addition to PD‐L1 status, HER2 status is considered a predictive factor. In the phase III ToGA trial, the HER2 antibody trastuzumab improved OS and PFS in patients with HER2‐positive advanced gastric cancers and adenocarcinomas of the esophagogastric junction whose tumors were either immunohistochemically HER2‐positive (IHC 3+) or had amplification of the HER2 gene on in situ hybridization (FISH+). 88
Recommendation 9: Palliative systemic therapy in squamous cell carcinoma
Patients with metastatic or locally advanced squamous cell carcinoma of the esophagus with a PD‐L1 CPS <10 or TPS <1% that cannot be treated curatively may be offered palliative first‐line systemic chemotherapy (EC).
In patients with metastatic or locally advanced squamous cell carcinoma of the esophagus that cannot be curatively treated and has a PD‐L1 CPS ≥10, platinum/fluoropyrimidine chemotherapy should be used in conjunction with pembrolizumab (GoR B, LoE 2).
Patients with metastatic or locally advanced, non‐curable squamous cell carcinoma of the esophagus may be offered systemic palliative chemotherapy with the goal of maintaining quality of life. A clinically relevant life‐prolonging effect of systemic palliative chemotherapy has not been established for squamous cell carcinoma of the esophagus. Data are very limited regarding randomized clinical trials and often refer only to a subpopulation of patients. 89 , 90 In published clinical trials, combination therapy of cisplatin with a fluoropyrimidine (infusional 5‐fluorouracil or capecitabine) was often used. Other studies, particularly from Asia, have investigated platinum‐based combinations with taxanes and others.
In the meantime, the results of several phase III trials have been reported, establishing the value of immunotherapy also in the first‐line treatment of advanced squamous cell carcinoma. In the KEYNOTE‐590 trial (first‐line therapy of advanced squamous cell carcinoma of the esophagus and HER‐2‐negative adenocarcinoma of the esophagus and gastroesophageal junction [AEG type 1]) a significant overall survival benefit was shown for the combination of pembrolizumab with cisplatin and 5‐fluorouracil compared with chemotherapy alone in squamous cell cancers with a CPS ≥10 [HR 0.57; 13.9 vs. 8.8 months, p < 0.0001] in favor of the additional administration of pembrolizumab. 85
Following the final vote of the recommendations on palliative therapy in the Guideline Committee, the European Medicines Agency (EMA) approved nivolumab in combination with fluoropyrimidine‐ and platinum‐based combination chemotherapy for the first‐line treatment of unresectable advanced, recurrent, or metastatic squamous cell carcinoma of the esophagus with tumor cell PD‐L1 expression ≥1% in adults. In addition, nivolumab in combination with ipilimumab has also been approved for first‐line treatment of unresectable advanced, recurrent, or metastatic squamous cell carcinoma of the esophagus with tumor cell PD‐L1 expression ≥1% in adults due to the positive results of the 3‐arm randomized global CheckMate‐648 trial. 91 The study demonstrated a significant survival benefit for the combination of nivolumab with cisplatin and 5‐fluorouracil versus chemotherapy alone for first‐line treatment of advanced squamous cell carcinoma of the esophagus. The immunotherapy alone arm with nivolumab and ipilimumab also significantly prolonged survival in PD‐L1 positive tumors compared to cisplatin and 5‐fluorouracil (HR 0.64; 13.7 vs. 9.1 months, p = 0.0010), but with survival curves crossing in the first months to the disadvantage of immunotherapy. These positive results will lead to the implementation of nivolumab in combination with fluoropyrimidine‐ and platinum‐based combination chemotherapy and of nivolumab in combination with ipilimumab as new recommendations in the next version 4.0 of the German guideline.
ALL MEMBERS OF THE GERMAN ESOPHAGEAL CANCER GUIDELINE COMMISSION CONTRIBUTED TO THE ESTABLISHMENT OF THE GUIDELINE (IN ALPHABETICAL ORDER)
Gustavo Baretton, Tilmann Bostel, Christian Ell, Ute Goerling, Lars Grenacher, Barbara Kade, Wolfram Trudo Knoefel, Jürgen Körber, Philipp Lenz, Florian Lordick, Dietmar Lorenz, Sylvie Lorenzen, Alexander Meining, Josef Menzel, Helmut Messmann, Hans‐Joachim Meyer, Stefan Paul Mönig, Ute Nöthlings, Heinz Schmidberger, Matthias Schmidt, Thomas Seufferlein, Maria Steingräber, Martin Stuschke, Reina Tholen, Peter Thuss‐Patience, Jörg Trojan, Christoph Wagener, Arved Weimann, Frederick Wenz, Martin Werner.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
Coordination and update of the guideline “Diagnosis and Treatment of Squamous Cell Carcinoma and Adenocarcinoma of the Esophagus” was funded by the “Deutsche Krebshilfe” (70112585) within the framework of the “Leitlinienprogramm Onkologie.” The authors are thankful to Lars Klug, DGVS, Berlin, for excellent organization of the Guidelines Commission and to Dr. M. Nothacker, MPH, AWMF‐Institut für Medizinisches Wissensmanagement, Berlin, Dipl. Soz. Wiss. Th. Langer, Oncology Guidelines Program of the German Cancer Society, Berlin and to Prof. Dr. H.T. Arkenau, Sarah Cannon Research Institute, UK for helpful discussions of the manuscript.
Open Access funding enabled and organized by Projekt DEAL.
Porschen R, Fischbach W, Gockel I, Hollerbach S, Hölscher A, Jansen PL, et al. Updated German guideline on diagnosis and treatment of squamous cell carcinoma and adenocarcinoma of the esophagus. United European Gastroenterol J. 2024;12(3):399–411. 10.1002/ueg2.12523
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.
REFERENCES
- 1. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38(23):2677–2694. 10.1200/jco.20.00866 [DOI] [PubMed] [Google Scholar]
- 2. Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2022;33(10):992–1004. 10.1016/j.annonc.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 3. Robert Koch‐Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. Krebs in Deutschland für 2015/2016. 12th ed. 2019. https://www.rki.de/DE/Content/Gesundheitsmonitoring/Krebsregisterdaten/krebs_node.html
- 4. Porschen R, Buck A, Fischbach W, Gockel I, Görling U, Grenacher L, et al. S3‐Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus (Langversion 1.0 ‐ September 2015, AWMF‐Registernummer: 021/023OL). Z Gastroenterol. 2015;53(11):1288–1347. 10.1055/s-0041-107381 [DOI] [PubMed] [Google Scholar]
- 5. Porschen R, Fischbach W, Gockel I, Hollerbach S, Hölscher A, Jansen PL, et al. S3‐Leitlinie – Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus. Z Gastroenterol. 2019;57(3):336–418. 10.1055/a-0833-5712 [DOI] [PubMed] [Google Scholar]
- 6. German guideline on “diagnosis and treatment of squamous cell carcinoma and adenocarcinoma of the esophagus”, long version 3.1, 2022, AWMF registration number: 021/023OL. German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS). https://www.dgvs.de/wp‐content/uploads/2023/06/ZfG‐LL_OeCA_v3.1_Langversion_19.06.2023_final.pdf [Google Scholar]
- 7. Brouwers MC, Kerkvliet K, Spithoff K; AGREE Next Steps Consortium . The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. 10.1136/bmj.i1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porschen R, Langer T, van Leeuwen P. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3 Leitlinie zur Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus, Leitlinienreport 3.1. Z Gastroenterol. 2022;60:e308–e346. [Google Scholar]
- 9. Howick J, Chalmers I, Glasziou P. OCEBM levels of evidence working group ‘the Oxford 2011 levels of evidence’: Oxford Centre for evidence‐based medicine. Oxford; 2011. [Google Scholar]
- 10. German Association of the Scientific Medical Societies (AWMF)‐Standing Guidelines Commission . AWMF guidance manual and rules for guideline development. English version. 1st ed; 2012. http://www.awmf.org/leitlinien/awmf‐regelwerk.html
- 11. Nothacker MJ, Muche‐Borowski C, Kopp IB. Guidelines in the register of the association of scientific medical societies in Deutschland ‐ a quality improvement campaign. Geburtshilfe Frauenheilkd. 2014;74(3):260–266. 10.1055/s-0034-1368227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ (Can Med Assoc J). 2014;186(3):E123–E142. 10.1503/cmaj.131237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oxford Centre for evidence‐based medicine: levels of evidence; 2009. https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/oxford‐centre‐for‐evidence‐based‐medicine‐levels‐of‐evidence‐march‐2009
- 14. Libânio D, Pimentel‐Nunes P, Bastiaansen B, Bisschops R, Bourke MJ, Deprez PH, et al. Endoscopic submucosal dissection techniques and technology: European society of gastrointestinal endoscopy (ESGE) technical review. Endoscopy. 2023;55(4):361–389. 10.1055/a-2031-0874 [DOI] [PubMed] [Google Scholar]
- 15. Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Holscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high‐volume centers. Ann Surg. 2011;254(1):67–72. 10.1097/sla.0b013e31821d4bf6 [DOI] [PubMed] [Google Scholar]
- 16. Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137(3):815–823. 10.1053/j.gastro.2009.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ell C, May A, Gossner L, Pech O, Gunter E, Mayer G, et al. Endoscopic mucosal resection of early cancer and high‐grade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118(4):670–677. 10.1016/s0016-5085(00)70136-3 [DOI] [PubMed] [Google Scholar]
- 18. Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007;65(1):3–10. 10.1016/j.gie.2006.04.033 [DOI] [PubMed] [Google Scholar]
- 19. Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long‐term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high‐grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57(9):1200–1206. 10.1136/gut.2007.142539 [DOI] [PubMed] [Google Scholar]
- 20. Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, et al. Long‐term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652–660. 10.1053/j.gastro.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 21. Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high‐grade dysplasia and intramucosal carcinoma‐‐an American single‐center experience. Am J Gastroenterol. 2009;104(11):2684–2692. 10.1038/ajg.2009.465 [DOI] [PubMed] [Google Scholar]
- 22. Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, et al. Endoscopic resection for Barrett's high‐grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long‐term therapeutic benefit. Am J Gastroenterol. 2010;105(6):1276–1283. 10.1038/ajg.2010.1 [DOI] [PubMed] [Google Scholar]
- 23. Pouw RE, Seewald S, Gondrie JJ, Deprez PH, Piessevaux H, Pohl H, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59(9):1169–1177. 10.1136/gut.2010.210229 [DOI] [PubMed] [Google Scholar]
- 24. Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8(1):23–29. 10.1016/j.cgh.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 25. van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high‐grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60(6):765–773. 10.1136/gut.2010.229310 [DOI] [PubMed] [Google Scholar]
- 26. Pimentel‐Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European society of gastrointestinal endoscopy (ESGE) guideline ‐ update 2022. Endoscopy. 2022;54(6):591–622. 10.1055/a-1811-7025 [DOI] [PubMed] [Google Scholar]
- 27. Kumarasinghe MP, Bourke MJ, Brown I, Draganov PV, McLeod D, Streutker C, et al. Pathological assessment of endoscopic resections of the gastrointestinal tract: a comprehensive clinicopathologic review. Mod Pathol. 2020;33(6):986–1006. 10.1038/s41379-019-0443-1 [DOI] [PubMed] [Google Scholar]
- 28. Manner H, May A, Pech O, Gossner L, Rabenstein T, Gunter E, et al. Early Barrett's carcinoma with “low‐risk” submucosal invasion: long‐term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103(10):2589–2597. 10.1111/j.1572-0241.2008.02083.x [DOI] [PubMed] [Google Scholar]
- 29. Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, et al. Efficacy, safety, and long‐term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low‐risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11(6):630–635. quiz e45. 10.1016/j.cgh.2012.12.040 [DOI] [PubMed] [Google Scholar]
- 30. Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20(18):5540–5547. 10.3748/wjg.v20.i18.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta‐analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41(9):751–757. 10.1055/s-0029-1215053 [DOI] [PubMed] [Google Scholar]
- 32. Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, et al. Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg. 2015;39(8):1986–1993. 10.1007/s00268-015-3100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, et al. Minimally invasive versus open esophageal resection: three‐year follow‐up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232–236. 10.1097/sla.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 34. Briez N, Piessen G, Claret A, Triboulet J, Mariette C. Is minimally invasive oesophagectomy for cancer decreasing pulmonary complications‐Results from a case‐control study. J Clin Oncol. 2010;28(15):4071. 10.1200/jco.2010.28.15_suppl.4071 [DOI] [Google Scholar]
- 35. Mariette C, Markar SR, Dabakuyo‐Yonli TS, Meunier B, Pezet D, Collet D, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152–162. 10.1056/nejmoa1805101 [DOI] [PubMed] [Google Scholar]
- 36. Mariette C, Markar S, Dabakuyo‐Yonli TS, Meunier B, Pezet D, Collet D, et al. Health‐related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open‐label, randomized phase III controlled trial: the MIRO trial. Ann Surg. 2020;271(6):1023–1029. 10.1097/sla.0000000000003559 [DOI] [PubMed] [Google Scholar]
- 37. Glatz T, Marjanovic G, Kulemann B, Sick O, Hopt UT, Hoeppner J. Hybrid minimally invasive esophagectomy vs open esophagectomy: a matched case analysis in 120 patients. Langenbeck's Arch Surg. 2017;402(2):323–331. 10.1007/s00423-017-1550-4 [DOI] [PubMed] [Google Scholar]
- 38. Yang J, Chen L, Ge K, Yang JL. Efficacy of hybrid minimally invasive esophagectomy vs open esophagectomy for esophageal cancer: a meta‐analysis. World J Gastrointest Oncol. 2019;11(11):1081–1091. 10.4251/wjgo.v11.i11.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gottlieb‐Vedi E, Kauppila JH, Malietzis G, Nilsson M, Markar SR, Lagergren J. Long‐term survival in esophageal cancer after minimally invasive compared to open esophagectomy: a systematic review and meta‐analysis. Ann Surg. 2019;270(6):1005–1017. 10.1097/sla.0000000000003252 [DOI] [PubMed] [Google Scholar]
- 40. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339(27):1979–1984. 10.1056/nejm199812313392704 [DOI] [PubMed] [Google Scholar]
- 41. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. 10.1200/jco.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 42. Medical Research Council Oesophageal Cancer Working Group . Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–1733. [DOI] [PubMed] [Google Scholar]
- 43. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. 10.1200/jco.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 44. Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. 10.1056/nejmoa055531 [DOI] [PubMed] [Google Scholar]
- 45. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. 10.1200/jco.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–313. 10.1200/jco.2001.19.2.305 [DOI] [PubMed] [Google Scholar]
- 47. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. 10.1056/nejmoa1112088 [DOI] [PubMed] [Google Scholar]
- 48. Shapiro J, Lanschot J, Hulshof M, Hagen P, Berge HM, Wijnhoven B, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. 10.1016/s1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 49. Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–668. 10.1016/s1470-2045(05)70288-6 [DOI] [PubMed] [Google Scholar]
- 50. Zhao Q, Li Y, Wang J, Zhang J, Qiao X, Tan B, et al. Concurrent neoadjuvant chemoradiotherapy for Siewert II and III adenocarcinoma at gastroesophageal junction. Am J Med Sci. 2015;349(6):472–476. 10.1097/maj.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. Lancet Oncol. 2011;12(7):681–692. 10.1016/s1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 52. Petrelli F, Ghidini M, Barni S, Sgroi G, Passalacqua R, Tomasello G. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta‐analysis. Gastric Cancer. 2019;22(2):245–254. 10.1007/s10120-018-0901-3 [DOI] [PubMed] [Google Scholar]
- 53. Al‐Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4‐AIO): results from the phase 2 part of a multicentre, open‐label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–1708. 10.1016/s1470-2045(16)30531-9 [DOI] [PubMed] [Google Scholar]
- 54. Al‐Batran S, Homann N, Pauligk C, Goetze T, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. 10.1016/s0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 55. Cai Z, Yin Y, Zhao Z, Xin C, Cai Z, Yin Y, et al. Comparative effectiveness of neoadjuvant treatments for resectable gastroesophageal cancer: a network meta‐analysis. Front Pharmacol. 2018;9:872. 10.3389/fphar.2018.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta‐analysis. Gut. 2004;53(7):925–930. 10.1136/gut.2003.025080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montagnani F, Fornaro L, Frumento P, Vivaldi C, Falcone A, Fioretto L. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: a comprehensive review and network meta‐analysis. Crit Rev Oncol Hematol. 2017;114:24–32. 10.1016/j.critrevonc.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 58. Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15(6):947–954. 10.1093/annonc/mdh219 [DOI] [PubMed] [Google Scholar]
- 59. Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long‐term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16(13):1649–1654. 10.3748/wjg.v16.i13.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. 10.1200/jco.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–2422. 10.1200/jco.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 62. Dresner SM, Lamb PJ, Bennett MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129(1):103–109. 10.1067/msy.2001.110024 [DOI] [PubMed] [Google Scholar]
- 63. Peyre CG, Hagen JA, DeMeester SR, Van Lanschot JJ, Holscher A, Law S, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248(6):979–985. 10.1097/sla.0b013e3181904f3c [DOI] [PubMed] [Google Scholar]
- 64. Mariette C, Piessen G, Lamblin A, Mirabel X, Adenis A, Triboulet JP. Impact of preoperative chemoradiation on postoperative course and survival in patients with locally advanced squamous cell oesophageal carcinoma. Br J Surg. 2006;93(9):1077–1083. 10.1002/bjs.5358 [DOI] [PubMed] [Google Scholar]
- 65. Kelly R, Ajani J, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. 10.1056/nejmoa2032125 [DOI] [PubMed] [Google Scholar]
- 66. Chang DT, Chapman C, Shen J, Su Z, Koong AC. Treatment of esophageal cancer based on histology: a surveillance epidemiology and end results analysis. Am J Clin Oncol. 2009;32(4):405–410. 10.1097/coc.0b013e3181917158 [DOI] [PubMed] [Google Scholar]
- 67. Karran A, Blake P, Chan D, Reid TD, Davies IL, Kelly M, et al. Propensity score analysis of oesophageal cancer treatment with surgery or definitive chemoradiotherapy. Br J Surg. 2014;101(5):502–510. 10.1002/bjs.9437 [DOI] [PubMed] [Google Scholar]
- 68. Herskovic A, Martz K, al‐Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–1598. 10.1056/nejm199206113262403 [DOI] [PubMed] [Google Scholar]
- 69. Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2006;1:Cd002092. [DOI] [PubMed] [Google Scholar]
- 70. Semenkovich TR, Meyers BF. Surveillance versus esophagectomy in esophageal cancer patients with a clinical complete response after induction chemoradiation. Ann Transl Med. 2018;6(4):81. 10.21037/atm.2018.01.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Qin J, Jing S, Liu Q, Cheng Y, Wang Y, et al. Clinical complete response after chemoradiotherapy for carcinoma of thoracic esophagus: is esophagectomy always necessary? A systematic review and meta‐analysis. Thorac Cancer. 2018;9(12):1638–1647. 10.1111/1759-7714.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Gouw D, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Futterer JJ, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta‐analysis. J Thorac Oncol. 2019;14(7):1156–1171. 10.1016/j.jtho.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 73. Faiz Z, Dijksterhuis WPM, Burgerhof JGM, Muijs CT, Mul VEM, Wijnhoven BPL, et al. A meta‐analysis on salvage surgery as a potentially curative procedure in patients with isolated local recurrent or persistent esophageal cancer after chemoradiotherapy. Eur J Surg Oncol. 2019;45(6):931–940. 10.1016/j.ejso.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 74. Pottgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer‐‐a meta‐analysis of the randomized trials. Cancer Treat Rev. 2012;38(6):599–604. 10.1016/j.ctrv.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 75. Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. 10.1200/jco.2005.04.7118 [DOI] [PubMed] [Google Scholar]
- 76. Li F, Ding N, Zhao Y, Yuan L, Mao Y. The current optimal multimodality treatments for oesophageal squamous‐cell carcinoma: a systematic review and meta‐analysis. Int J Surg. 2018;60:88–100. 10.1016/j.ijsu.2018.10.037 [DOI] [PubMed] [Google Scholar]
- 77. Gkika E, Gauler T, Eberhardt W, Stahl M, Stuschke M, Pottgen C. Long‐term results of definitive chemoradiation in locally advanced cancers of the cervical esophagus. Dis Esophagus. 2014;27(7):678–684. 10.1111/dote.12146 [DOI] [PubMed] [Google Scholar]
- 78. Grass GD, Cooper SL, Armeson K, Garrett‐Mayer E, Sharma A. Cervical esophageal cancer: a population‐based study. Head Neck. 2015;37(6):808–814. 10.1002/hed.23678 [DOI] [PubMed] [Google Scholar]
- 79. Burmeister BH, Dickie G, Smithers BM, Hodge R, Morton K. Thirty‐four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg. 2000;126(2):205–208. 10.1001/archotol.126.2.205 [DOI] [PubMed] [Google Scholar]
- 80. Ott K, Lordick F, Molls M, Bartels H, Biemer E, Siewert JR. Limited resection and free jejunal graft interposition for squamous cell carcinoma of the cervical oesophagus. Br J Surg. 2009;96(3):258–266. 10.1002/bjs.6437 [DOI] [PubMed] [Google Scholar]
- 81. Panhofer P, Springer C, Izay B, Grasl M, Burian M, Schoppmann SF, et al. Influence of resection extent on morbidity in surgery for squamous cell cancer at the pharyngoesophageal junction. Langenbeck's Arch Surg. 2013;398(2):221–230. 10.1007/s00423-012-0995-8 [DOI] [PubMed] [Google Scholar]
- 82. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15(1):261–267. 10.1200/jco.1997.15.1.261 [DOI] [PubMed] [Google Scholar]
- 83. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. 10.1200/jco.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 84. Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, André T, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol. 2014;32(31):3520–3526. 10.1200/jco.2013.54.1011 [DOI] [PubMed] [Google Scholar]
- 85. Sun J, Shen L, Shah M, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first‐line treatment of advanced oesophageal cancer (KEYNOTE‐590): a randomised, placebo‐controlled, phase 3 study. Lancet. 2021;398(10302):759–771. 10.1016/s0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 86. Janjigian Y, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First‐line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro‐oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open‐label, phase 3 trial. Lancet. 2021;398(10294):27–40. 10.1016/s0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro‐oesophageal cancer. Nature. 2022;603(7903):942–948. 10.1038/s41586-022-04508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687–697. 10.1016/s0140-6736(10)61121-x [DOI] [PubMed] [Google Scholar]
- 89. Grunberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27(4C):2705–2714. [PubMed] [Google Scholar]
- 90. Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60(11):1449–1472. 10.1136/gut.2010.228254 [DOI] [PubMed] [Google Scholar]
- 91. Doki Y, Ajani J, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous‐cell carcinoma. N Engl J Med. 2022;386(5):449–462. 10.1056/nejmoa2111380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data sharing not applicable—no new data generated.


