Abstract
The BCL2 inhibitor venetoclax has established therapeutic roles in chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). As BCL2 is an important determinant of survival of both myeloid progenitor and B cells, we investigated whether clinical and molecular abnormalities arise in the myeloid compartment during long-term continuous venetoclax treatment of CLL in 89 patients (87 with relapsed/refractory CLL). Over a median follow-up of 75 (range 21-98) months, persistent cytopenias (≥1 of neutropenia, thrombocytopenia, anemia) lasting ≥4 months and unrelated to CLL occurred in 25 patients (28%). Of these patients, 20 (80%) displayed clonal hematopoiesis, including 10 with therapy-related myeloid neoplasms (t-MNs). t-MNs occurred exclusively in patients previously exposed to fludarabine-alkylator combination therapy with a cumulative 5-year incidence of 10.4% after venetoclax initiation, consistent with rates reported for patients exposed to fludarabine-alkylator combination therapy without venetoclax. To determine whether the altered myelopoiesis reflected the acquisition of mutations, we analyzed samples from patients with no or minimal bone marrow CLL burden (n = 41). Mutations in the apoptosis effector BAX were identified in 32% (13/41). In cellular assays, C-terminal BAX mutants abrogated outer mitochondrial membrane localization of BAX and engendered resistance to venetoclax killing. BAX-mutated clonal hematopoiesis occurred independently of prior fludarabine-alkylator combination therapy exposure and was not associated with t-MNs. Single-cell sequencing revealed clonal co-occurrence of mutations in BAX with DNMT3A or ASXL1. We also observed simultaneous BCL2 mutations within CLL cells and BAX mutations in the myeloid compartment of the same patients, indicating lineage-specific adaptation to venetoclax therapy.
Key points
-
•
Cytopenias and clonal hematopoiesis occur frequently during long-term continuous venetoclax therapy for patients with relapsed CLL.
-
•
Loss-of-function BAX mutations emerge in the myeloid compartment as an adaptation to ongoing venetoclax therapy.
Introduction
The anti-apoptotic protein BCL2 is highly expressed in chronic lymphocytic leukemia (CLL), and its selective targeting by venetoclax induces high response rates and durable disease control in the first-line1 and relapsed/refractory settings.2 Normally, BCL2 and the closely related proteins MCL1 and BCL-xL play key roles in regulating cell survival at specific developmental points in various lineages during hematopoiesis.3, 4, 5 Consistent with this, both B cell lymphopenia and mild neutropenia are common in patients without hematological disease receiving venetoclax,6 and marked thrombocytopenia/mild neutropenia are common with navitoclax, which inhibits BCL-xL in addition to BCL2.7 Approximately 40% of patients treated with venetoclax for relapsed/refractory CLL develop neutropenia which, when requiring intervention, typically responds to growth factor support or dose reduction.8
Given the potential impact of venetoclax throughout normal and malignant hematopoiesis, we aimed to investigate and characterize any clinical and molecular abnormalities arising outside the target tumor compartment in patients with CLL receiving long-term continuous BCL2 inhibition with venetoclax. In this predominantly heavily pre-treated patient cohort, we document that both persistent cytopenias and clonal hematopoiesis (with or without coexisting cytopenias) are common. We present the novel observation that BAX-mutated clonal hematopoiesis emerges in the myeloid compartment during venetoclax therapy and uncover a phenomenon of simultaneous hematopoietic adaptation to venetoclax therapy occurring in both the on-target (CLL) and off-target (myeloid) hematopoietic compartments.
Methods
Clinical cohort
The cohort studied consisted of 92 patients treated across 4 clinical trials of venetoclax as monotherapy or in combination with rituximab (M12-175 phase-1 monotherapy [NCT01328626; n = 42],9 M13-365 phase-1b venetoclax plus rituximab [NCT01682616; n = 16],10 M13-982 phase-2 venetoclax monotherapy [NCT01889186; n = 12]11 and M15-889 phase-3b venetoclax monotherapy [NCT02980731; n = 22]) at the Peter MacCallum Cancer Centre and Royal Melbourne Hospital. All patients provided written informed consent, and the studies were conducted in accordance with the Declaration of Helsinki and after Human Research Ethics Committee approval. Patients were enrolled from June 2011 to February 2020 and were reviewed at a minimum every 3 months until progression, death, or discontinuation from trial.
Persistent cytopenias
To identify patients with persistent cytopenias following initiation of venetoclax-based therapy, we searched patient records, applying the same threshold values as used to define myeloid neoplasms according to 2017 World Health Organization (WHO) criteria12 and myeloid lesions of undetermined significance.13, 14, 15 “Persistent cytopenia” was defined as the presence of one or more of the following continuously for ≥4 months: hemoglobin (Hb) <110 g/L, absolute neutrophil count (ANC) <1.8 × 109/L, or platelet count <100 × 109/L. Potential causes of persistent cytopenias were identified or excluded by bone marrow assessment and examination of patient records for the presence of hematinic deficiencies, myelotoxic medication, or persisting splenomegaly. Causality was attributed to underlying CLL in the absence of clear alternatives if contemporaneous bone marrow assessment showed morphological involvement with CLL combined with a sufficient reduction in normal hematopoiesis. The remainder of patients with persistent cytopenias were categorized as therapy-related myeloid neoplasm (t-MN) and myeloid abnormalities of undetermined significance (adapted from a framework established for patients without hematologic disorders [CHIP, ICUS, CCUS] and hereafter referred to as clonal hematopoiesis [CH], idiopathic cytopenias [IC] and clonal cytopenias [CC], respectively) based on clinical and laboratory findings, including full blood examination, peripheral blood film, bone marrow aspirate and trephine assessment, cytogenetic analysis, and molecular characterization by next-generation sequencing (NGS; described below), in accordance with WHO criteria12 and 2017 Working Conference on MDS and Pre-MDS Conditions criteria.15 Assignment of myeloid disorder descriptors followed a hierarchy whereby patients were classified according to the most clinically significant category (t-MN > CC > IC > CH) attributable, and therefore no patient was assigned to more than one group. Date of diagnosis was recorded as the first date that requisite diagnostic criteria were met for the assigned category. Cumulative exposure to prior lines of myelotoxic therapy was quantified using an adapted scoring system (supplemental Table 116). Outcome data for all cohorts were updated to 29 February 2020.
Genomic analyses
Unique molecular index (UMI)-based NGS targeting BH3-family genes and genes recurrently mutated in hematological malignancy (supplemental Table 2) was performed as previously described.17 In addition, hybridization-based NGS17 and Sanger sequencing were performed on patients CLL43 and CLL16, respectively. Further details are described in the supplemental material.
Targeted amplicon single-cell sequencing
Single-cell DNA sequencing and analysis were performed as previously described.18 Briefly, single-cell library preparation used the Tapestri platform according to the manufacturer's instructions (Mission Bio, CA). Further details are described in the supplemental material.
Cell culture, death assays, and retroviral infection
MOLM-13 BAX/BAK double knockout (DKO) cells were created using CRISPR/Cas9 technology as previously described19 and passaged in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) at 37°C and 10% CO2. BAX mutant constructs were cloned into the retroviral expression vector pMSCV-IRES-GFP and transduced into MOLM-13 BAX/BAK DKO cells using the HEK293T packaging cell line. All cell lines were routinely tested for mycoplasma using the MycoAlert™ kit (Lonza, Switzerland). Cell death induction in MOLM-13 BAX/BAK DKO cells expressing different BAX variants was assessed by Annexin V-APC (WEHI antibody facility) and DAPI (Sigma, Australia) staining and flow cytometry according to standard protocols.
Assessing BAX integration into membranes by carbonate extraction
Cells were pre-treated (1 h) with 25 µM Q-VD (MP Biomedicals Australasia, New South Wales, Australia) and then incubated with 500 nM venetoclax (Cat. No. A-1231, ActiveBiochem, Hong Kong, China). After 5 hours, cells were harvested and carbonate extraction of BAX was performed as previously described.20 Further details are described in the supplemental material.
Results
Cohort characteristics
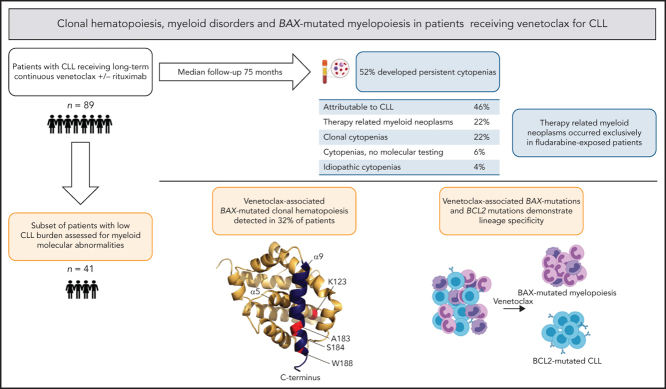
Of the 92 patients treated with venetoclax (±rituximab) at our institutions on prospective trials, three patients were excluded as they received <30 days of venetoclax (n = 2) or had t-MN prior to commencing venetoclax (n = 1; retrospectively diagnosed after study entry). The characteristics of the study cohort (n = 89) are shown in Table 1. This was a heavily pre-treated cohort (median 3 lines of therapy) with 72 patients (81%) having previous fludarabine-alkylator combination chemotherapy exposure (FCR, n = 68; FC, n = 4). Twenty-seven patients (30%) remain on venetoclax after a median survivor follow-up of 75 (range 21-98) months. The predominant reasons for venetoclax cessation were progressive disease (n = 41, 46%) or elective cessation in deep response (n = 5, 6%). Other reasons (n = 16, 18%) included unrelated death (n = 3), intercurrent solid organ malignancy (n = 3), t-MN (n = 4), allogeneic SCT (n = 3), poor performance status (n = 2) or withdrawal of consent (n = 1).
Table 1.
Clinical characteristics of patient cohort with relapsed/refractory CLL treated with venetoclax (n = 89)
| Clinical characteristic | Cohort (n = 89) |
|---|---|
| Median age at venetoclax initiation, years (range) | 67 (45-87) |
| Male:Female | 71:18 |
| Median number of prior therapies (range) | 3 (0-12) |
| Prior fludarabine-combination therapy exposure | 72 (81%) |
| Del(17p) and/or TP53 mutation prior to venetoclax initiation (n/N, %) | 46/81 (57%) |
| Median duration on venetoclax, months (range) | 29 (1-98+) |
| Median survivor follow-up from venetoclax initiation, months (range) | 75 (21-98+) |
Clinical characterization of persistent cytopenias and therapy-related myeloid neoplasms emerging during venetoclax treatment
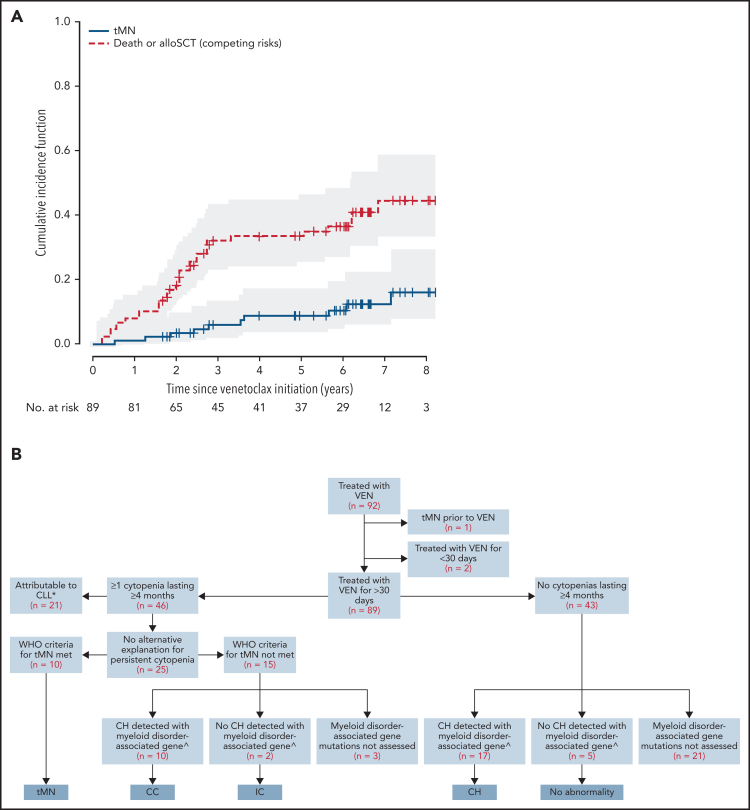
Persistent cytopenias of ≥4 months occurred in 46/89 patients (52%) during venetoclax therapy. After clinicopathological review, 21 (46%) instances were attributed to underlying CLL. Among the remaining 25 patients with persistent cytopenias, 10 patients met WHO criteria for diagnosis of t-MN, and 15 did not. Among these 25 patients with persistent cytopenia, bi- or tri-lineage cytopenia were significantly associated with t-MN diagnosis (≥2 lineages 8/12 (75%) t-MN v <2 lineages 2/13 (15%) t-MN; P = 0.015). From initiation of venetoclax, the 5-year cumulative incidence of t-MN in the 89-patient cohort was 8.8% (95% CI 2.5% to 15.0%; Figure 1A).
Figure 1.
Cytopenias and clonal hematopoiesis in patients with relapsed/refractory CLL treated with venetoclax. (A) Kaplan-Meier curve of cumulative incidence of therapy-associated myeloid neoplasm (t-MN) (solid line) across a cohort of 89 patients. The dashed line represents competing risks of death and allo-SCT. The numbers at risk for a given time point refer to at-risk for developing t-MN. (B) Categorization of a cohort of 89 patients into clonal hematopoiesis (CH), idiopathic cytopenias (IC), clonal cytopenias (CC), and t-MNs *CH detected with myeloid disorder-associated gene in 3 cases—not assessed in remaining 18. ^Refers to established myeloid-disorder associated genes, excluding BAX.
The characteristics of patients diagnosed with t-MN after venetoclax exposure are shown in supplemental Table 3. On univariate analysis of putative risk factors for t-MN, there was an association between previous fludarabine-alkylator combination therapy and development of t-MN of borderline significance (P = .050; supplemental Table 4). All cases of t-MN occurred in the context of previous fludarabine-alkylator combination chemotherapy at a median of 9 (range 5-16) years from first exposure. Among the 72 patients with previous fludarabine-alkylator combination chemotherapy exposure, the cumulative incidence of t-MN was 10.4% (95% CI 5.2% to 21.1%) at 5 years after venetoclax initiation. In contrast, no t-MN was observed in the 17 patients unexposed to fludarabine-alkylator combination chemotherapy after a median survivor follow-up of 8.8 years from first-line treatment and 6.3 years (range 1.8-7.3) from initiation of venetoclax.
The categories of t-MNs observed were myelodysplastic syndrome (MDS) (n = 8), acute myeloid leukemia (AML) (n = 1) and myeloproliferative/MDS overlap syndrome (n = 1). The median overall survival was 17 (range 2-73) months from diagnosis of t-MN. After the diagnosis of t-MN, five patients ceased venetoclax (four because of the t-MN and one because of intercurrent Richter transformation). Four patients had ceased venetoclax prior to t-MN diagnosis due to progressive CLL (n = 2), Richter transformation (n = 1), and intercurrent lung cancer (n = 1). One patient (CLL7) continued venetoclax after diagnosis of t-MN characterized by mild cytopenias only, which then spontaneously resolved on follow-up (patient alive without further myeloid manifestations 73 months post-t-MN diagnosis). Of the five patients that ceased venetoclax at the time of t-MN diagnosis, cytopenias did not resolve in four cases (CLL80, CLL45, CLL9, CLL69), including one patient for whom azacitidine was initiated simultaneously (CLL80). Complete resolution of cytopenias and excess blasts occurred in one patient who commenced azacitidine while concomitantly ceasing venetoclax and who remains alive at 14 months post-t-MN diagnosis (CLL16).
Molecular characterization of clonal hematopoiesis in venetoclax-treated patients
To further investigate molecular abnormalities in the non-CLL compartment of these 89 patients, we identified a subset of 41 patients that had both adequate follow-up (>6 months) and available samples from timepoints when the stored peripheral blood/bone marrow aspirate had either a low CLL burden (typically <5% involvement by flow cytometry) or had no detectable disease by flow cytometry (sensitivity 10−4). The clinical characteristics of these 41 patients were comparable to the overall cohort of 89 patients (supplemental Table 5). Using UMI-based error-corrected NGS (sensitivity 0.5% to 1%) we detected mutations in the non-CLL compartment in 34 (83%) of these 41 patients (supplemental Table 6). The spectrum of the genes mutated in these samples overlapped with that observed in age-related clonal hematopoiesis (ARCH) and myeloid neoplasms (herein referred to as ‘myeloid-disorder associated mutations'), with the most frequently mutated genes being DNMT3A (n = 41 mutations detected across cohort), TET2 (n = 15), TP53 (n = 12), U2AF1 (n = 7), ZRSR2 (n = 6), and ASXL1 (n = 4). Integrating these molecular data with clinical cytopenia/t-MN occurrence, the 15 patients with cytopenias not meeting WHO t-MN criteria were subclassified as either CC (n = 10), IC without myeloid-disorder associated mutations (n = 2), and not assessed for myeloid-disorder associated mutations (n = 3). Among 22 patients without persistent cytopenias tested for myeloid-disorder associated mutations, 17 (77%) had detectable mutations and were subclassified as CH (Figure 1B).
Loss-of-function BAX mutations are detectable in the non-CLL compartment of patients with CLL treated with long-term venetoclax
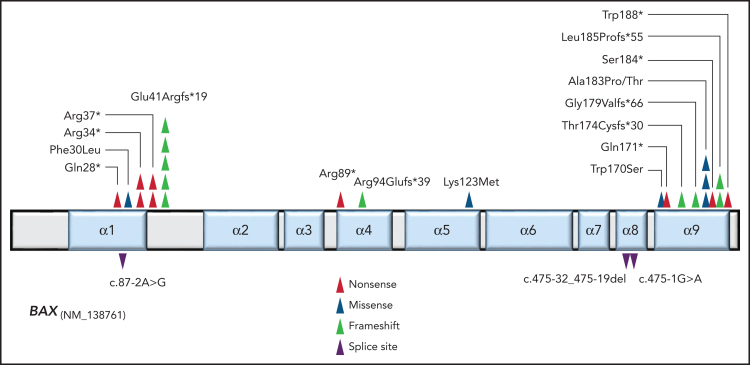
To address the possibility that the changes in the non-CLL compartments reflected altered regulation of apoptosis, we performed UMI-based error-corrected NGS analysis of BCL2-family genes (BCL2, MCL1, BCL2L1, BAK1, and BAX) on stored, non-enriched peripheral blood/bone marrow aspirate at time points where there was minimal (<5%) CLL involvement. We detected 20 different BAX mutations in bone marrow or blood samples from 13 patients (32%) from this subgroup of 41 patients (including in five samples that were MRD-negative for CLL by flow cytometry) (Figure 2, supplemental Table 7). No candidate mutations were detected in other BCL2-family genes. More than one BAX mutation was detected in samples from seven of these patients. The BAX variants included a combination of frameshift, canonical splice acceptor/donor site, and nonsense mutations observed throughout the gene as well as missense mutations in α-helices, a pattern indicative of loss of protein function. A recurrent G-homopolymer single base pair deletion leading to the frameshift mutation Glu41Argfs*19 was observed in 5/13 patients (confirmed by Sanger sequencing in patient CLL16), a mutation also recurrently observed in colorectal carcinoma.21 Missense mutations were observed in different patients affecting residues in the α1, α5, and α9 helices (Figure 2). Missense and frameshift/nonsense mutations were observed near the BAX C-terminus, affecting the critical α9-helix which targets BAX to the mitochondrial outer membrane (MOM). Whereas the nonsense and frameshift mutations observed upstream in the gene (eg, Glu41Argfs*19) are predicted to be null alleles as a result of nonsense-mediated decay (NMD)22 of the BAX mRNA, these C-terminal mutations (eg, Ser184* and Trp188*) are predicted to avoid NMD and yield a translated, truncated BAX protein lacking the critical four terminal amino acids (LysLysMetGly) in addition to variable amounts of the α9 helix due to the presence of a premature stop codon.
Figure 2.
BAX mutations detected in the non-CLL compartment of patients on long-term venetoclax therapy for relapsed/refractory CLL. Location and type of variants are indicated by color-coded triangles on linear protein structure of BAX. Splice site variants are indicated below the protein at positions relative to their nucleotide position.
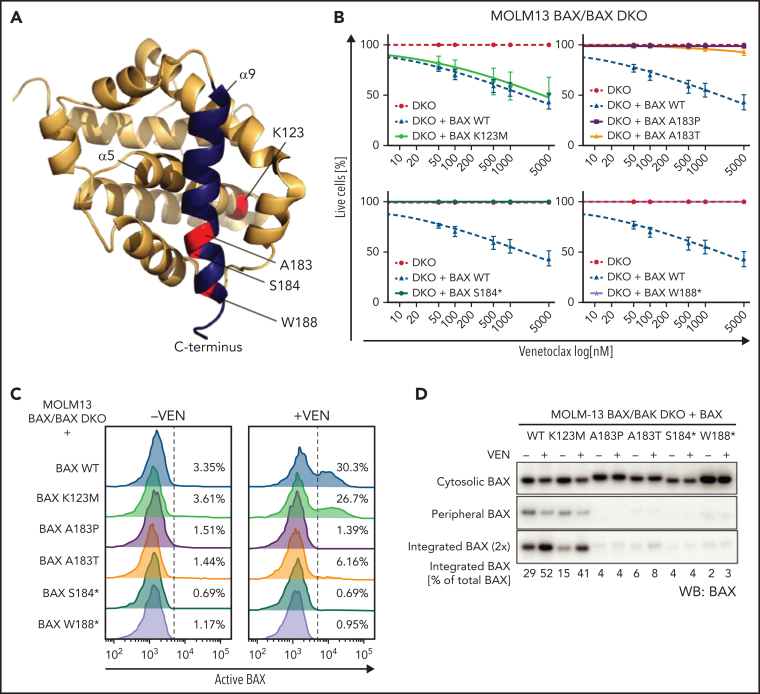
To define the functional effects of the BAX α9-helix mutations observed in this cohort, we re-expressed both missense (Lys123Met, Ala183Pro, and Ala183Thr) and truncating (Ser184* and Trp188*) mutations (Figure 3A) in the AML cell line MOLM-13 in which the endogenous BAX and BAK genes were deleted (BAX/BAK double knockout [DKO] MOLM-13). In order to investigate the role of BAX specifically, DKO MOLM-13 cells re-expressing wild-type BAX (but not BAK) were treated with venetoclax and were found to be very sensitive with cell death observed at relatively low doses (from 50 nM) of venetoclax. In contrast, cell lines expressing both BAX Ala183 codon missense mutations and BAX truncating mutants demonstrated significant resistance to venetoclax killing (Figure 3B) with no increase in cell death observed even at the highest concentrations of venetoclax (5000 nM). The BAX Lys123Met (α5-helix) expressing cells did not demonstrate resistance to venetoclax killing in vitro (Figure 3B).
Figure 3.
BAX α9 mutants show reduced activity when expressed in cells. (A) BAX structure (PDB: 1F16) showing the positions of the BAX missense mutations tested in this study (red). BAX helix α9 is highlighted in blue. (B) Test of activity of BAX mutants K123M, A183P, A183T, S184*, W188* in cells. BAX/BAK DKO MOLM-13 cells expressing the indicated BAX variants were treated with increasing concentration of venetoclax (50-5000 nM) for 24 hours, and cell viability was determined by Annexin V/DAPI staining and flow cytometry. Data are means ± SEM of 3 independent experiments. (C) Upon venetoclax treatment, BAX α9, but not BAX K123M, show reduced exposure for conformation-specific epitope 6A7. MOLM-13 DKO cells expressing the different BAX variants were pre-incubated with 25 μM Q-VD oph for 1 hour and then treated with 500 nM venetoclax. After 5 hours, cells were permeabilized with 0.025% digitonin prior to staining with conformation-specific anti-BAX antibody 6A7. After several washes, samples were stained with a PE-conjugated secondary antibody and analyzed by flow cytometry. The results are representative of at least two independent experiments. (D) Mutations in BAX α9 reduce MOM translocation and integration of BAX. Cells were treated as described in (C), subjected to carbonate extraction and fractions run on SDS-PAGE and immunoblotted for BAX. The results are representative of three independent experiments.
Inactive BAX is primarily cytosolic in healthy cells, and upon activation, it translocates to and integrates into the MOM via its transmembrane domain located in α9 helix.23 To further understand the mechanism for the observed resistance to venetoclax killing, the 6A7 BAX antibody (which binds to a BAX conformer early in its activation upon interaction with the MOM24, 25) was used. When compared with wild-type BAX, both BAX Ala183 codon and truncating mutants (but not the Lys123Met mutant) demonstrated reduced binding of the 6A7 BAX antibody, indicating that these BAX variants remain in an inactive conformation even after venetoclax treatment (Figure 3C). Furthermore, these results indicate that the BAX α9 helix variants studied have defects in MOM translocation and/or integration. We tested this directly by carbonate extraction (which reveals if proteins are stably inserted into the lipid bilayer) on the DKO MOLM-13 cells expressing the BAX mutants, with or without venetoclax treatment. Indeed, all α9-helix BAX mutants tested showed a marked reduction in both mitochondrial membrane association (peripheral) and membrane integration in response to venetoclax treatment (Figure 3D).
The variant allele frequency (VAF) of BAX mutations varied markedly across the cohort. In general, each patient had a “dominant” BAX mutation (ie, one mutation present at relatively high VAF [mean VAF of dominant clone 15.6%]) and further BAX mutation(s) detected at relatively low VAF (supplementary Table 7). All patients had at least one BAX mutation detected with a VAF consistent with its presence in the non-CLL compartment.
Baseline samples before venetoclax exposure were available for assessment in 6 of 13 patients. In 1 of the 6 patients (CLL43), the BAX mutation that was detectable in the non-CLL compartment during venetoclax therapy was also detectable in the sample taken directly before venetoclax exposure. BAX mutations were not detectable in the pre-venetoclax samples from the other five patients; however high CLL burdens reduced sensitivity to detect mutations in the non-CLL cells at that timepoint. We therefore also investigated the incidence of BAX mutations in peripheral blood/bone marrow aspirate samples from a cohort of venetoclax-naïve patients with relapsed CLL treated with BTK inhibitor therapy after a similar extent of preceding chemotherapy (n = 15). Samples from these 15 patients were again selected for timepoints where there was minimal CLL involvement (9/15 samples with no lymphocytosis/<5% disease burden). No BAX mutations were detected in the non-CLL compartment from these patients. We also sequenced tumor samples from 111 venetoclax-naïve patients with either myeloid (n = 77) or lymphoid (n = 34) malignancies and did not detect BAX mutations in any samples. Notably, unlike the acquired origin of the BAX variants in our cohort, the germline population database gnomAD (v2.1.1, Broad Institute, USA) showed rare (frequency <0.005%) loss-of-function variants in BAX present, including the Arg89*, Ala183Thr, and the c.475-1G>A splice site mutations observed in our cohort.
Longitudinal testing for BAX mutations in the non-CLL compartment was possible in 9/13 patients ranging over 21-93 months of follow-up. In four patients (CLL3, CLL16, CLL21, and CLL43), a significant and progressive increase in VAF of at least one BAX mutation over time was observed (supplemental Figure 1). In addition to BAX mutations with progressively increasing VAFs, multiple patients had BAX mutations that either remained stable, fluctuated, or decreased in VAF during follow-up (supplemental Figure 1). One patient (CLL43) had a BAX mutation (Ser184*) detectable at a VAF of 72.3% during longitudinal testing. Copy number/B-allele frequency analysis from targeted sequencing data in this patient was consistent with copy neutral loss of heterozygosity involving the BAX locus.
We then analyzed the BAX mutations in the context of clinical cytopenias, CH, and t-MN. BAX mutations were detected in samples from 1/5 (20%) patients with t-MN, 4/12 (33%) patients with persistent cytopenias (IC/CC), and 8/24 (33%) patients without persistent cytopenias (P = .835) (supplemental Figure 2). There was no association between t-MN and the presence of BAX mutations (1/13 [8%] with BAX mutations, 4/28 [14%] without BAX mutations P > .999, Fisher's exact test) (supplemental Table 8). Furthermore, there was no association between prior fludarabine-alkylator combination chemotherapy exposure and incidence of BAX mutations (previously exposed, 9/29 (31%); unexposed 4/12 (33%); P > .999, Fisher's exact test). Indeed, we identified a BAX homopolymer deletion (Glu41Argfs*19) in samples from a patient receiving single-agent venetoclax as first-line therapy.
BAX mutations are present in the myeloid compartment in patients with CLL on venetoclax harboring BCL2 mutations in the CLL compartment
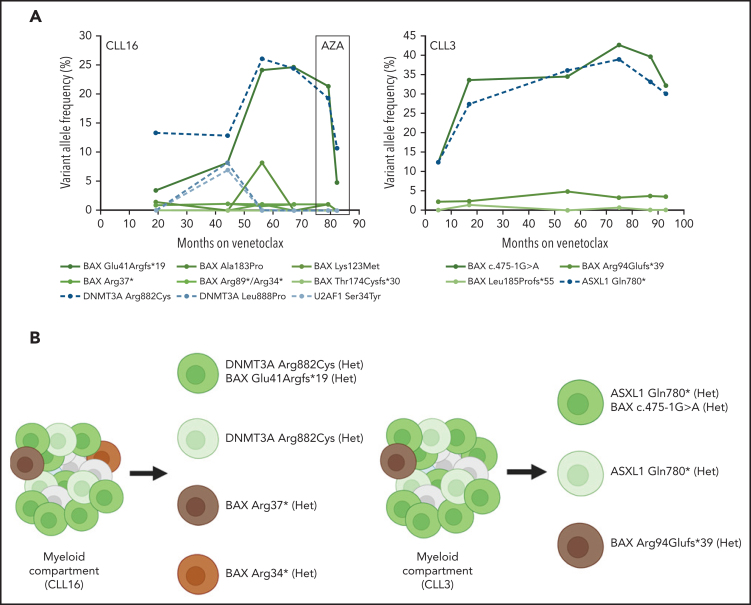
To further characterize the non-CLL compartments that harbored these BAX mutations, we analyzed DNA from flow cytometry sorted T cells (CD3+), B cells (CD19+), and myeloid compartment cells (CD3-/CD19-) from peripheral blood samples from three patients (CLL3, CLL16, and CLL81). From these three patients, we observed that BAX mutations were dominantly present within the myeloid (CD3-/CD19-) compartment (supplemental Table 9). In one patient, the same BAX mutation was detected in the myeloid compartment at high VAF (45.5%) and also the T cell compartment (CD3+/CD19-) at a relatively low level (VAF 1.4%). In order to understand the clonal relationship between BAX and other mutations in the non-CLL compartment, we identified two patients with BAX mutations (CLL3 and CLL16) that had multiple serial samples collected over approximately 7 years of continuous venetoclax treatment. Both of these patients had progressively increasing BAX mutations over time as well as other BAX mutations that remained stable in VAF over the same time period (Figure 4A). In both of these patients, the progressively increasing BAX mutations were observed to increase in parallel with mutations in DNMT3A and ASXL1. Ultimately, patient CLL16 was diagnosed with a t-MN after approximately 68 months on venetoclax therapy. Treatment with azacitidine was temporally associated with a decrease in the observed VAF of both BAX/DNMT3A clones (Figure 4A). Targeted amplicon single-cell sequencing performed on samples from these patients confirmed the presence of the DNMT3A Arg882Cys and ASXL1 Gln780* mutations to be in the same clone as the dominant progressive BAX mutation (Glu41Argfs*19 and c.475-1G>A) in patients CLL16 and CLL3, respectively (Figure 4B). Moreover, both of these patients had multiple BAX mutations that were confirmed to be in mutually exclusive clones by single-cell sequencing without co-existing myeloid-disorder associated mutations in genes targeted. BAX mutations were observed to be present in the heterozygous state in both CLL3 and CLL16 from single-cell sequencing.
Figure 4.
(A) Longitudinal changes in variant allele frequency (VAF) of BAX mutations and other mutations in the non-CLL compartment with time in two patients treated with continuous venetoclax. Box indicates a period of azacitidine (AZA) treatment of therapy-related myeloid neoplasm in patient CLL16. Three mutations in DNMT3A (Lys382*, Gly308Alafs*8, and Trp753Arg) detected at <1.5% VAF in patient CLL3 are not shown. Two BAX mutations, Arg34* and Arg89*, were detected in CLL16 at one time point (month 56), both at VAF of 1.1%, and are therefore depicted together. (B) Schematic diagram of targeted amplicon single-cell sequencing for patient CLL16 and CLL3. Het, heterozygous.
All three patients (CLL3, CLL16, and CLL81) harboring confirmed myeloid compartment BAX mutations had concurrent low-level detectable CLL present in the peripheral blood at the time of sampling. NGS performed on DNA extracted from the CLL compartment (CD3-/CD19+) from these patients revealed multiple BCL2 resistance mutations, including the BCL2 Gly101Val,26 in all three patients at varying VAF (Table 2). Despite multiple BAX mutations being present in the myeloid compartment of these patients, no BAX mutations were detected in the coexisting CLL compartment. Conversely, no BCL2 mutations were detected in the myeloid compartment of these patients despite the presence of multiple BCL2 mutations present within the CLL compartment (Table 2).
Table 2.
Variants in BAX/BCL2 detected in flow sorted myeloid compartment (CD3-/CD19-) and CLL compartment (CD3-/CD19+) cells in patients on long-term venetoclax
| Patient | Myeloid compartment (%) | CLL compartment (%) |
|---|---|---|
| CLL3 | BAX c.475-1G>A (45.5) BAX Arg94Glufs*39 (4.6) | BCL2 Gly101Val (27.3) BCL2 Arg107_Arg110dup† (8.4) BCL2 Arg107_Arg110dup‡ (6.4) |
| CLL16 | BAX Glu41Argfs*19 (24.0) BAX Arg37* (2.6) | BCL2 Ala113Gly (10.0) BCL2 Gly101Val (1.3) |
| CLL81 | BAX Glu41Argfs*19 (15.3) BAX Arg37* (0.9) | BCL2 Asp103Glu (20.0) |
NCBI RefSeq transcripts: NM_138761.3 (BAX), NM_000633.2 (BCL2).
c.326_327insGCGCCGCTACCG.
c.319_330dup.
Discussion
We report the clinical and molecular abnormalities arising within the myeloid compartment of a heavily pre-treated cohort of patients with CLL receiving long-term continuous venetoclax. Myeloid disorder-associated mutations were detectable in the majority of patients, including 77% of those without clinically evident myeloid abnormalities. Persistent cytopenias not attributable to other causes occurred in 28% of patients during continuous venetoclax therapy and were frequently associated with underlying clonal hematopoiesis. Nevertheless, the majority of these instances did not meet diagnostic criteria for t-MN. Despite the high prevalence of myeloid disorder-associated mutations within the cohort, t-MNs occurred exclusively in the context of prior fludarabine-alkylator combination chemotherapy exposure, a well-established cause of t-MN. Among exposed patients, the cumulative incidence of t-MNs during venetoclax therapy (10.4% at 5 years) was similar to that observed previously in fludarabine-alkylator combination chemotherapy-treated but venetoclax-naïve patients (10.8%).27 These data should inform the clinical evaluation of myeloid abnormalities emergent on venetoclax, demonstrating that although clonal hematopoiesis is common among chemotherapy-exposed patients, t-MNs are infrequent, occur exclusively in the context of prior fludarabine-alkylator exposure, and typically manifest with bi- or tri-lineage cytopenias.
In addition to myeloid disorder-associated mutations observed in the non-CLL compartment, we have also observed either the incidence, emergence, or longitudinal clonal expansion over time of loss-of-function mutations in the apoptosis effector BAX in 32% of patients. Our data demonstrate that C-terminal BAX mutations result in resistance to cell killing by venetoclax through impaired ability to translocate to the MOM. In contrast to BCL2 mutations, which are not observed in CLL outside the context of venetoclax therapy, BAX mutations were present in the non-CLL compartment in one patient in our cohort prior to venetoclax therapy. In addition, while not identified in 111 other samples with myeloid or lymphoid malignancy in our cohort, BAX mutations have been observed rarely in other cohorts, including in myelodysplastic/myeloproliferative neoplasms.28 Further studies are therefore required to understand the prevalence and time-course of acquisition of BAX mutations and any potential relationship to previous chemotherapy, venetoclax therapy, germline variation, and other co-existing myeloid disorder-associated mutations.
We did not observe an increase in the incidence of t-MN in patients with BAX-mutated myelopoiesis compared with those without. Therefore, even when occurring in the context of preexisting clonal hematopoiesis, the biological consequences of BAX mutations in the myeloid compartment may be limited to imparting a survival advantage during continuous venetoclax exposure without necessarily increasing the likelihood of malignant transformation. Notably, not all BAX mutations were observed to increase in VAF over time, and multiple mutations remained at low VAF over many years of venetoclax exposure. The contributors to this observed differential fitness cannot be definitively determined from our data; however, we observed progressive BAX-mutated clones with clonally cooccurring mutations in genes that have established roles in increasing myeloid cell fitness (DNMT3A and ASXL1). Importantly, while our functional data proves a survival advantage for biallelic loss of BAX function in vitro in the presence of venetoclax, the progressive dominant clones observed in two patients in our cohort had BAX-mutations present in the heterozygous state. Further modeling is required to fully understand the interaction of partial loss of BAX and the effects of other clonally cooccurring mutations which may alter cell fitness and survival in the context of prolonged venetoclax exposure.
Our data also suggest that mechanisms of venetoclax adaptation differ between lymphoid and myeloid lineages. Despite the prevalence of BCL2 resistance mutations observed in patients with CLL progressing on venetoclax, we did not observe this specific mechanism within the myeloid compartment. One possible explanation for this finding may include the relative importance of the alternative prosurvival proteins MCL1/BCL-xL in myeloid cells compared with CLL leading to a greater growth advantage of cells harboring adaptation mechanisms that involve downstream pathways that are common to multiple upstream prosurvival proteins. Another contributing factor may be a difference in extrinsic or intrinsic mutational mechanisms within CLL cells compared with myeloid cells leading to a predilection for BCL2 mutations in CLL and BAX in myeloid cells. Although the most common BAX mutation in our cohort (Glu41Argfs*19) is observed in colorectal carcinoma due to defective mismatch repair,21, 29 this phenomenon is thought to be very rarely observed in myeloid malignancy (including t-MN).30 Finally, the presence of a BAX mutation in at least one patient in our cohort preceding venetoclax exposure suggests that the role of previous therapies merits further investigation.
In summary, cellular adaptation mechanisms to venetoclax may occur in a relatively lineage-specific manner throughout the hematopoietic compartment in patients with CLL treated with long-term continuous therapy. Adaptive BAX-mutated myelopoiesis emerged during long-term venetoclax, but we did not find an increased prevalence of clinically significant cytopenias or t-MN in patients with these variants. Finally, our findings provide further evidence for the central nature of the BCL2 family of proteins throughout hematopoiesis and suggest that acquired BAX mutations should be further investigated as a resistance mechanism in the context of myeloid malignancies such as AML.
Acknowledgments
The authors are grateful to the patients who enrolled in the venetoclax clinical trials, Naomi Sprigg for collection and curation of samples, Angela Georgiou for flow cytometric sorting of hematopoietic cells, and Chris Riffkin and Leonie Gibson for engineering the MOLM-13 cells. This research was supported by grants from the Snowdome Foundation (P.B. and M.A.A.), Vision Super and the Wilson Centre for Lymphoma Genomics (P.B. and D.A.W.), Jock Brockhoff Foundation (M.A.A.), the Leukemia and Lymphoma Society (USA) (Special Center of Research [7015-18] to J.M.A., A.H.W., D.C.S.H., and A.W.R.), the National Health and Medical Research Council (NHMRC) of Australia (D.C.S.H. [1156024, 1113133], A.W.R. [1113577, 1174902], M.A.A. [APP1177718]). Statistical support was provided by Michael Fahey and Mathias Bressel from the Centre for Biostatistics and Clinical Trials (BaCT), Melbourne, Australia. Illustrations created with BioRender.com. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Footnotes
The online version contains data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Blombery et al report on the clonal dynamics of myelopoiesis in patients with relapsed chronic lymphocytic leukemia who received the BCL2 inhibitor venetoclax after DNA-damaging prior chemotherapy. They identify emergence of late-onset clonal hematopoiesis and lossof-function mutations in the apoptotic activator BAX in myeloid clones. This study shows that a targeted therapy can select for resistant mutations not only in malignant populations but also within normal tissue displaying clonal mosaicism.
Contributor Information
Piers Blombery, Email: Piers.Blombery@petermac.org.
Mary Ann Anderson, Email: manderson@wehi.edu.au.
Authorship
Contribution: P.B., T.E.L, A.H.W., A.W.R., and M.A.A. conceived of the project and designed the study; J.F.S., A.W.R., C.S.T., D.A.C., M.A.A., and S.M.H. were responsible for patient care; P.B., T.E.L., M.A.D., E.R.T., V.S.L., A.P., and T.N. collected the data; D.A.W. contributed flow cytometry and molecular data; P.B., E.R.T., T.N., and X.C. performed and analyzed genomic data; T.E.L. analyzed the clinical data; M.A.D., D.C.S.H., J.M.A., X.C., and T.N. performed and evaluated the laboratory experiments; P.B., T.E.L., and M.A.D. wrote the first version of the manuscript; all authors reviewed the data and contributed to critical revision of the manuscript.
Conflict-of-interest disclosure: A.W.R., M.A.A., T.E.L., M.A.D., J.M.A., and V.S.L. are employees of the Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax. A.W.R., M.A.A., J.M.A., and T.E.L. are recipients of a share in royalty payments paid to the Walter and Eliza Hall Institute of Medical Research. S.M.H. has received honoraria from Gilead and nonfinancial assistance from AbbVie. C.S.T. has received honoraria and research funding from AbbVie and Janssen and honoraria from BeiGene. A.W.R. has received research funding from AbbVie, Genentech, Servier, Janssen, and BeiGene. J.F.S. receives research funding from AbbVie, Genentech, Celgene, and Janssen, and is an advisory board member for and has received honoraria from AbbVie, Acerta, Celgene, Genentech, Janssen, Roche, Sunesis, and Takeda. M.A.A. has received honoraria from AbbVie, Janssen, AstraZeneca, Novartis, and CSL Behring. T.E.L. has received honoraria from AbbVie. The remaining authors declare no competing financial interests.
Supplementary Material
REFERENCES
- 1.Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. doi: 10.1056/NEJMoa1900574. [DOI] [PubMed] [Google Scholar]
- 2.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 3.Kollek M, Müller A, Egle A, Erlacher M. Bcl-2 proteins in development, health, and disease of the hematopoietic system. FEBS J. 2016;283(15):2779–2810. doi: 10.1111/febs.13683. [DOI] [PubMed] [Google Scholar]
- 4.Vo TT, Ryan J, Carrasco R, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151(2):344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 6.Lok SW, Whittle JR, Vaillant F, et al. A phase Ib dose-escalation and expansion study of the BCL2 inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 2018;9(3):354–369. doi: 10.1158/2159-8290.CD-18-1151. [DOI] [PubMed] [Google Scholar]
- 7.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davids MS, Hallek M, Wierda W, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24(18):4371–4379. doi: 10.1158/1078-0432.CCR-17-3761. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–240. doi: 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–1980. doi: 10.1200/JCO.2017.76.6840. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol. 2. Revised 4th ed. International Agency for Research on Cancer; Lyon, France: 2017. [Google Scholar]
- 13.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371–3378. doi: 10.1182/blood-2017-01-763425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valent P, Horny HP, Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leuk Res. 2007;31(6):727–736. doi: 10.1016/j.leukres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Valent P, Orazi A, Steensma DP, et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes (MDS) and potential pre-MDS conditions. Oncotarget. 2017;8(43):73483–73500. doi: 10.18632/oncotarget.19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake M, Ranaghan L, Morris TC, et al. Analysis of the effect of prior therapy on progenitor cell yield: use of a chemotherapy scoring system. Br J Haematol. 2003;98(3):745–749. doi: 10.1046/j.1365-2141.1997.2743091.x. [DOI] [PubMed] [Google Scholar]
- 17.Blombery P, Thompson ER, Nguyen T, et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135(10):773–777. doi: 10.1182/blood.2019004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson ER, Nguyen T, Kankanige Y, et al. Clonal independence of JAK2 and CALR or MPL mutations in comutated myeloproliferative neoplasms demonstrated by single cell DNA sequencing. Haematologica. 2021;106(1):313–315. doi: 10.3324/haematol.2020.260448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubrey BJ, Kelly GL, Kueh AJ, et al. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10(8):1422–1432. doi: 10.1016/j.celrep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Dengler MA, Robin AY, Gibson L, et al. BAX activation: mutations near its proposed non-canonical BH3 binding site reveal allosteric changes controlling mitochondrial association. Cell Rep. 2019;27(2):359–373.e6. doi: 10.1016/j.celrep.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Miquel C, Borrini F, Grandjouan S, et al. Role of bax mutations in apoptosis in colorectal cancers with microsatellite instability. Am J Clin Pathol. 2005;123(4):562–570. doi: 10.1309/JQ2X-3RV3-L8F9-TGYW. [DOI] [PubMed] [Google Scholar]
- 22.Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 2015;16(11):665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 23.Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2013;21(2):196–205. doi: 10.1038/cdd.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272(21):13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 25.Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6(6):e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blombery P, Anderson MA, Gong JN, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2018;9(3):342–353. doi: 10.1158/2159-8290.CD-18-1119. [DOI] [PubMed] [Google Scholar]
- 27.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24(12):2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 28.Palomo L, Meggendorfer M, Hutter S, et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood. 2020;136(16):1851–1862. doi: 10.1182/blood.2019004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 30.Walker CJ, Eisfeld AK, Genutis LK, et al. No evidence for microsatellite instability in acute myeloid leukemia. Leukemia. 2017;31(6):1474–1476. doi: 10.1038/leu.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.