Abstract
BACKGROUND:
The notion that gas-bloat syndrome (GBS) after magnetic sphincter augmentation (MSA) is less detrimental has not been substantiated by data. This study aimed to identify the incidence, natural history, risk factors, and impact on outcomes of GBS after MSA.
STUDY DESIGN:
Records of patients who underwent MSA at our institution were reviewed. GBS was defined as a score of 4 or more on the gas bloat–specific item within the GERD health-related quality-of-life (GERD-HRQL) questionnaire. Preoperative clinical and objective testing data were compared between those with and without GBS at 1 year using univariate followed by multivariable analysis. GBS evolution over time and its impact on outcomes were assessed in those with 1- and 2-year follow-up.
RESULTS:
A total of 489 patients underwent MSA. At a mean (SD) follow-up of 12.8 (2.1) months, patient satisfaction was 88.8%, 91.2% discontinued antisecretory medications, and 74.2% achieved DeMeester score normalization.
At 1 year, 13.3% of patients developed GBS, and had worse GERD-HRQL scores and antisecretory medication use and satisfaction (p < 0.0001). DeMeester score normalization was comparable (p = 0.856). Independent predictors of GBS were bloating (odds ratio [OR] 1.8, p = 0.043), GERD-HRQL score greater than 30 (OR 3, p = 0.0010), and MSA size 14 or less beads (OR 2.5, p = 0.004). In a subgroup of 239 patients with 2-year follow-up, 70.4% of patients with GBS at 1 year had resolution by 2 years. The GERD-HRQL total score improved when GBS resolved from 11 (7 to 19) to 7 (4 to 10), p = 0.016. Patients with persistent GBS at 2 years had worse 2-year GERD-HRQL total scores (20 [5 to 31] vs 5 [3 to 12], p = 0.019).
CONCLUSIONS:
GBS affects 13.3% of patients at 1 year after MSA and substantially diminishes outcomes. However, GBS resolves spontaneously with quality-of-life improvement. Patients with preoperative bloating, high GERD-HRQL scores, or small MSA devices are at greatest risk of this complication.
Gas-bloat syndrome (GBS) is thought to be less detrimental after magnetic sphincter augmentation (MSA) due to the preserved ability to belch. We tested this hypothesis and found that GBS affects 13% of patients after MSA and substantially diminishes outcomes. Fortunately, most GBS resolves by 2 years with accompanied improved outcomes.
Antireflux surgery (ARS) is a highly effective treatment for GERD yet is often reserved for patients with refractory symptoms despite medical optimization.1 This reluctance in referrals for ARS is rooted in fear of postoperative complications.2 Gas-bloat syndrome (GBS) is a commonly reported yet subjectively defined complication of ARS. The syndrome is characterized by bloating but can variably include a constellation of symptoms such as abdominal pain, early satiety, nausea, and inability to belch or vomit.3 Fortunately, these symptoms largely resolve spontaneously after 2 years.4 The etiology of GBS is thought to result from an inability to vent gas through a surgically restored lower esophageal sphincter (LES). The exact mechanism of GBS is not well understood, but proposed mechanisms include failure of the LES to relax in response to gastric distention, frequent aerophagia, impaired gastric accommodation with rapid emptying, and iatrogenic vagus nerve injury.3 GBS has a marked impact on postoperative quality of life and, in addition to dysphagia, is the most common complaint reported by patients dissatisfied with fundoplication.2
Magnetic sphincter augmentation (MSA) was introduced as a technically standardized outpatient alternative to fundoplication, designed to expand the appeal and availability of ARS. The unique use of magnetic forces enables augmentation of the LES while preserving the ability to vent gas from the stomach. Select studies comparing MSA to fundoplication have demonstrated equivalently excellent symptomatic and objective reflux control with an arguably superior side-effect profile.5-8 Specifically, these studies have demonstrated a lower rate of GBS. However, there is a paucity of data on the impact of GBS on outcomes after MSA.
The reported lower rates of GBS and the preserved ability to belch have led to the notion that GBS is less detrimental after MSA. However, this relationship has never been substantiated with data. No studies have examined the impact of GBS on reflux symptom control, freedom from antisecretory medications, normalization of distal esophageal acid exposure, or other outcomes after MSA. Although GBS typically resolves spontaneously after fundoplication, there is a paucity of data on the natural course of GBS after the distinct physiologic changes that follow MSA. Therefore, we designed this study to identify the incidence, natural history, risk factors, and impact of GBS on outcomes after MSA over time.
METHODS
Study population
This is a retrospective review of prospectively collected data from patients who underwent MSA at Allegheny Health Network hospitals (Pittsburgh, PA) between 2014 and 2021. Inclusion criteria were patients 18 years or older who completed standardized preoperative and at least the 1-year postoperative questionnaires after MSA. This study was evaluated and approved by the IRB of the Allegheny Health Network (IRB number 2021-259).
Symptomatic and quality-of-life assessment
All patients were asked to complete the GERD health-related quality-of-life (GERD-HRQL) questionnaire preoperatively, at 6 months, at 12 months, and annually after surgery. The GERD-HRQL consists of 16 questions that specifically address GERD symptoms. Each question has a score ranging from 0 to 5, indicating “No symptoms,” “Noticeable but not bothersome symptoms,” “Occasionally bothersome symptoms,” “Daily bothersome symptoms,” “Symptoms that affect daily activities,” and “Symptoms that are incapacitating to daily activities,” respectively. Within the GERD-HRQL are specific items pertaining to the symptoms of heartburn, regurgitation, dysphagia, and gas bloat. A GERD-HRQL total score greater than 30 was considered as severe reflux symptoms.
Preoperative assessment
All patients underwent a comprehensive clinical evaluation with a focus on their foregut symptoms and antisecretory medication use. Patients also underwent a routine preoperative objective esophageal physiology assessment, including the following tests:
Videoesophagram was done to evaluate gross pharyngeal and esophageal motility; to delineate anatomy; to assess for any masses, mucosal lesions, or diverticulum; and to evaluate for hiatal hernia (HH) and esophageal stricture.
Esophagogastroduodenoscopy with biopsy was used to assess the presence of esophagitis, Barrett’s esophagus, and the presence and size of HH. The size of HH was measured from the gastroesophageal junction to the crural impression.
High-resolution impedance manometry was used to assess esophageal body peristalsis (organization and pressure) as well as LES competency. A structurally competent sphincter was defined as LES total length 2.7 cm or more, LES abdominal length 1.7 cm or more, and LES resting pressure 13 mmHg or greater. A sphincter failing to meet all 3 criteria was considered incompetent. Patients with severe esophageal motility dysfunction were preferentially offered alternatives to MSA.
Esophageal pH monitoring was performed selectively using either Bravo pH monitoring (Medtronic, Minneapolis, MN) or pH impedance (Diversatek, Milwaukee, WI). Proton pump inhibitors were discontinued for 10 days before pH testing. Abnormal distal esophageal acid exposure was defined as a DeMeester score more than 14.7.
Gastric emptying scintigraphy (GES) was performed in select patients with nonspecific symptoms common in GERD but potentially suggestive of gastroparesis (eg nausea, vomiting, and bloating). GES was performed at 1-hour intervals for 4 hours after ingestion of a standardized solid meal. A percent meal retention at 4 hours of >10% was considered delayed gastric emptying.
Surgical technique
The LINX device (Ethicon, Johnson & Johnson, Shoreview, MN) consists of a series of magnetic titanium beads. The beads are interlinked with independent titanium wires to form a flexible and expandable ring with a “Roman arch” configuration. Each bead can move independently of the adjacent beads, creating a dynamic implant without limiting the esophageal range of motion. The device is manufactured in different sizes, ranging from 13 to 17 beads, and is capable of nearly doubling its diameter when all beads are separated. MSA is performed laparoscopically and consists of complete posterior mediastinal esophageal mobilization with restoration of intra-abdominal esophageal length (≥3 cm), interrupted posterior crural closure (without pledgets or meshes), and device placement at the level of the gastroesophageal junction with the posterior vagus nerve trunk located on the outside of the magnetic ring.
Postoperative assessment
Postoperative follow-up visits were routinely scheduled at 2 weeks, 6 weeks, 6 months, and annually. Patients were empirically placed on simethicone for the first 6 weeks after surgery. Patients were assessed for clinical resolution of their symptoms and freedom from antisecretory medications at each visit. GERD-HRQL questionnaires were completed at 6 months, 1 year, and annually. At 1 year, patients were also approached to repeat their preoperative esophageal physiology testing. A score of 4 or more on the heartburn-, dysphagia-, or regurgitation-specific items was considered clinically significant.
Outcomes assessment and statistical analysis
Patients were divided into groups based on the presence of GBS at 1-year follow-up. GBS was defined as a score of 4 or more on the GERD-HRQL “gas bloat”–specific item. Outcomes were compared between patients with and without GBS. Preoperative demographic, clinical, and objective testing data were assessed for impact on the development of GBS on univariate followed by multivariable analysis. Subanalyses were performed on patients who also completed 6-month questionnaires to compare 6- and 12-month outcomes based on whether GBS was present or absent at each time point. Patients were divided into groups based on whether GBS was present at 6 months and was persistent at 1 year, present at 6 months and resolved at 1-year, and absent at 6 months, but had a delayed onset at 1 year or never developed. An additional subanalysis was performed in patients who completed preoperative, 6-month, 1-year, and 2-year questionnaires, focusing on the patterns and prevalence of GBS over time.
Data are expressed as median (interquartile range) or mean (SD). Values for categorical variables are presented as frequency and percentage. Statistical analysis was performed by means of nonparametric tests, including Mann-Whitney U test, Wilcoxon signed rank test, chi-square test, and Fisher’s exact test where appropriate. An α level of 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
A total of 489 patients underwent MSA and completed preoperative and 1-year postoperative GERD-HRQL questionnaires during the study period. Demographic and preoperative clinical characteristics are shown in Table 1. At a mean (SD) follow-up of 12.8 (2.1) months, the median GERD-HRQL score decreased from 31 (18 to 48) to 5 (2 to 12), p < 0.0001, with 81% of patients achieving at least a 50% improvement in their total score. The gas-bloat score decreased from 2 (1 to 4) to 1 (0 to 3), p < 0.0001. Additionally, 88.8% of patients reported that they were satisfied with their surgical outcomes, 91.2% were free from antisecretory medications, and 74.2% achieved normalization of their distal esophageal acid exposure (DeMeester score <14.7) at 1 year after MSA.
Table 1.
Baseline Demographics and Clinical Characteristics
| Characteristic | Data, N = 489 |
|---|---|
| Sex, n (%) | |
| Male | 176 (36) |
| Female | 313 (64) |
| Age, y, median (IQR) | 57.7 (48–65) |
| BMI, kg/m2, median (IQR) | 29 (26.2–32.1) |
| Bloating, n (%) | 130 (28.3) |
| Simethicone use, n (%) | 35 (7.2) |
| GERD health-related quality of life total score, median (IQR) | 31 (18–48) |
| DeMeester score, median (IQR) | 30.5 (18–44.4) |
| LA grade C/D esophagitis, n (%) | 51 (10.4) |
| Hiatal hernia >3 cm, n (%) | 107 (21.9) |
IQR, interquartile range; LA, Los Angeles.
Gas-bloat syndrome and outcomes at 1 year
GBS was reported by 65 (13.3%) patients at 1-year follow-up. Patients with GBS were more likely to be dissatisfied with their surgical outcomes and to report higher GERD-HRQL total scores, with less improvement in all GERD symptoms compared with patients without GBS. The incidence of heartburn, regurgitation, and dysphagia was compared between patients with and without GBS (Fig. 1). Despite comparable rates of normalization of distal esophageal acid, patients with GBS were more likely to take antisecretory and gas-reducing medications after MSA (Table 2). A subset of 30 patients with GBS and 88 patients without GBS underwent postoperative GES and was found to have comparable rates of postoperative delayed gastric emptying (p = 0.353). Preoperative sphincter characteristics were compared between patients with and without GBS (Table 3). There was no difference in any preoperative sphincter characteristics or the percentage of patients with a structurally competent LES.
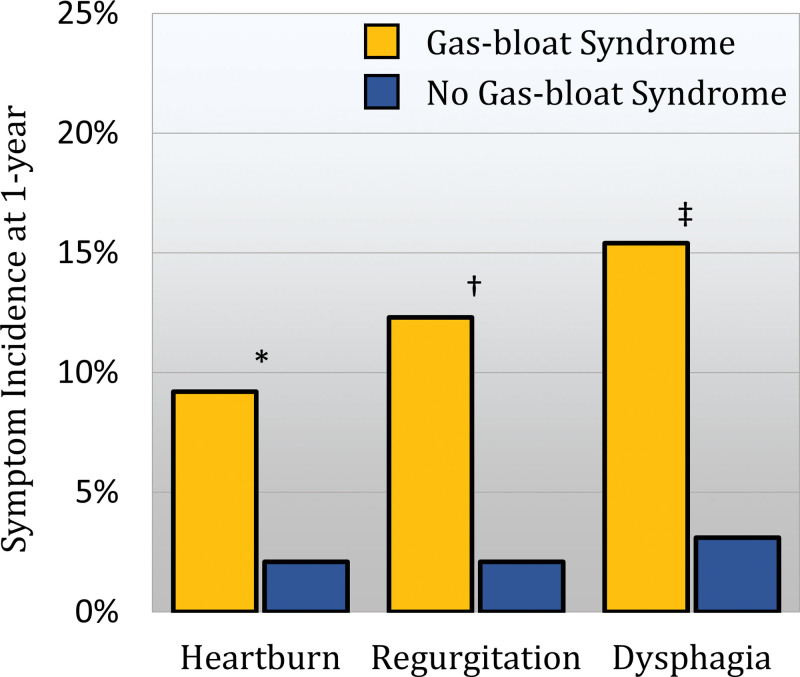
Figure 1.
Comparison of the incidence of 1-year postoperative typical reflux symptoms between patients with and without gas-bloat syndrome (GBS). Patients with GBS were significantly more likely to score 4 or more on their 1-year postoperative GERD health-related quality-of-life heartburn item (*, p = 0.008), regurgitation item (†, p = 0.0006), and dysphagia item (‡, p = 0.0002).
Table 2.
Comparison of 1-Year Outcomes Based On Status of Gas-Bloat Syndrome
| Variable | GBS, N = 65 (13.3%) | No GBS, N = 424 (86.7%) | p Value |
|---|---|---|---|
| GERD health-related quality of life | |||
| Total score, median (IQR) | 18.5 (7–31) | 5 (2–10) | <0.0001 |
| 50% reduction from baseline, % | 66.1 | 83.3 | 0.003 |
| Heartburn item, median (IQR) | 1 (0–2) | 0 (0–1) | <0.0001 |
| Regurgitation item, median (IQR) | 1 (0–2) | 0 (0–1) | 0.0002 |
| Dysphagia item, median (IQR) | 2 (0–3) | 0 (0–2) | <0.0001 |
| Gas-bloat item, median (IQR) | 4 (4–5) | 1 (0–2) | <0.0001 |
| Freedom from proton pump inhibitor use, % | 75.8 | 93.7 | <0.0001 |
| Simethicone use, % | 49.2 | 27.6 | 0.0008 |
| Patient satisfaction, % | 72.1 | 91.2 | <0.0001 |
| pH normalization, % | 73.3 | 74.4 | 0.856 |
GBS, gas-bloat syndrome; IQR, interquartile range.
Table 3.
Preoperative Manometric Lower Esophageal Sphincter Characteristics and Gas-Bloat Syndrome
| Variable | GBS, N = 65 (13.3%) | No GBS, N = 424 (86.7%) | p Value |
|---|---|---|---|
| LES total length, cm, median (IQR) | 2.7 (2–4) | 2.9 (2–4) | 0.342 |
| LES total length <2.7 cm, % | 20.9 | 37.8 | 0.209 |
| LES abdominal length, cm, median (IQR) | 0.8 (0–2) | 1.1 (0–2) | 0.850 |
| LES abdominal length <1.7 cm, % | 68.2 | 64.1 | 0.573 |
| LES resting pressure, cm, median (IQR) | 21.9 (13–33) | 20.6 (13–31) | 0.795 |
| LES resting pressure <13 mmHg, % | 25.4 | 24.6 | 0.876 |
| Integrated relaxation pressure, median (IQR) | 7.4 (4–11) | 6.0 (2–10) | 0.434 |
| Structurally competent LES*, % | 23.1 | 30.4 | 0.246 |
Defined as sphincter with total length ≥2.7 cm, abdominal length ≥1.7 cm, and resting pressure ≥13 mmHg.
GBS, gas-bloat syndrome; IQR, interquartile range; LES, lower esophageal sphincter.
Factors contributing to gas-bloat syndrome 1 year after magnetic sphincter augmentation
The results of the univariate analysis of demographic and preoperative clinical parameters with potential association with GBS are shown in Table 4. Patients who developed GBS were significantly younger, but all other demographic characteristics were similar between groups. Preoperative GERD-HRQL scores were significantly worse in patients who developed GBS, both in terms of the total score and across all individual symptoms. However, there was no difference in any preoperative objective testing data, including degree and severity of reflux, HH size, manometric characteristics, or delayed gastric emptying rate. Patients who developed GBS were significantly more likely to have required a small MSA device size (≤14 beads). Significant and borderline significant potential predictors of GBS were used to construct a multivariable logistic model. Preoperative bloating, GERD-HRQL total score of more than 30, and small MSA device size were found to be the 3 independent predictors of GBS after MSA (Table 5).
Table 4.
Univariate Comparison of Potential Preoperative Predictors of Gas-Bloat Syndrome 1 Year After Magnetic Sphincter Augmentation
| Characteristic | GBS, N = 65 (13.3%) | No GBS, N = 424 (86.7%) | Odds ratio (95% CI) | p Value |
|---|---|---|---|---|
| Age, y, median (IQR) | 55.4 (42–61) | 58.1 (48–65) | 0.98 (0.96–1.00) | 0.043 |
| Female sex, % | 72.3 | 62.7 | 1.53 (0.68–2.71) | 0.165 |
| BMI, kg/m2, median (IQR) | 28.2 (26–33) | 29 (26–32) | 0.98 (0.92–1.04) | 0.675 |
| Obesity (BMI ≥ 30 kg/m2), % | 43.1 | 41.5 | 1.07 (0.63–1.81) | 0.893 |
| GERD health-related quality of life score, median (IQR) | 46 (31–58) | 30 (17–49) | 1.04 (1.02–1.05) | <0.0001 |
| Total score (>30), % | 77.4 | 47.3 | 3.73 (2.01–6.92) | <0.0001 |
| Heartburn (≥4), % | 45.2 | 28.2 | 2.10 (1.22–3.61) | 0.008 |
| Regurgitation (≥4), % | 43.5 | 26.8 | 2.11 (1.22–3.63) | 0.008 |
| Dysphagia (≥4), % | 30.6 | 12.6 | 3.09 (1.68–5.71) | 0.0003 |
| Bloating (≥4), % | 54.2 | 24.2 | 3.63 (2.08–6.34) | <0.0001 |
| Simethicone use | 12.3 | 6.4 | 2.14 (0.93–4.89) | 0.115 |
| DeMeester score, median (IQR) | 29.6 (15–45) | 30.5 (18–44) | 0.61 (0.33–1.12) | 0.522 |
| LA grade C/D esophagitis, % | 12.3 | 10.1 | 1.30 (0.89–2.86) | 0.662 |
| Presence of hiatal hernia, % | 87.7 | 92.5 | 0.56 (0.25–1.26) | 0.221 |
| Large or paraesophageal hernia, % | 13.8 | 23.1 | 0.56 (0.27–1.15) | 0.108 |
| Delayed gastric emptying, % | 8.0 | 14.7 | 0.60 (0.14–2.57) | 0.519 |
| Device size (≤14 beads), % | 73.8 | 54.2 | 2.35 (1.31–4.20) | 0.003 |
GBS, gas-bloat syndrome; IQR, interquartile range; LA, Los Angeles.
Table 5.
Independent Predictors of Gas-Bloat Syndrome 1 Year After Magnetic Sphincter Augmentation on Multivariable Analysis
| Predictor | Estimate (SE) | Odds ratio (95% CI) | p Value |
|---|---|---|---|
| Bloating | 0.58 (0.29) | 1.78 (1.02–3.11) | 0.043 |
| GERD health-related quality-of-life score >30 | 1.10 (0.34) | 3.01 (1.56–5.90) | 0.0010 |
| Small device size (13/14 beads) | 0.91 (0.32) | 2.49 (1.34–4.63) | 0.004 |
Persistent and resolved gas-bloat syndrome
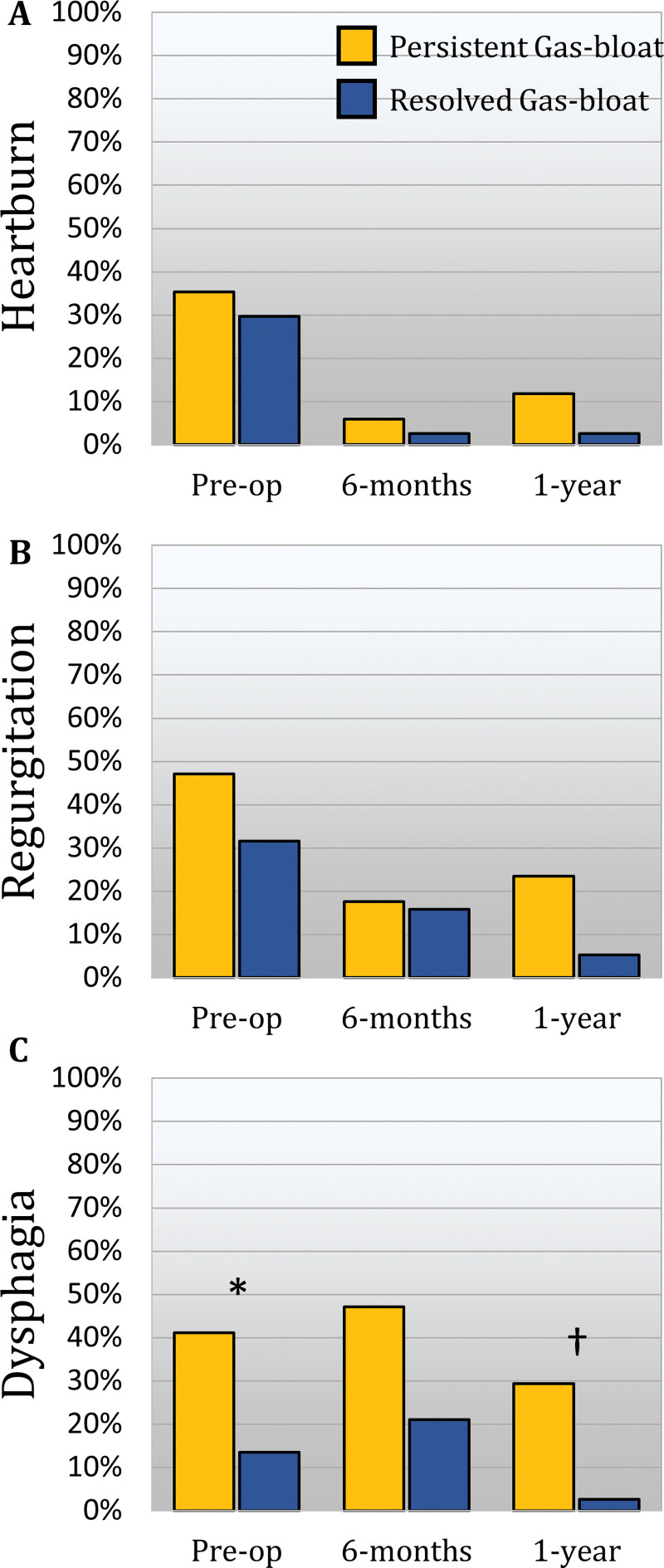
There were 348 patients who completed both 6-month and 1-year follow-up. Of these, 55 (15.8%) patients had GBS at 6 months. In 38 (69.1%) of these patients, GBS resolved by 1 year. The GERD-HRQL score in these patients in whom GBS resolved significantly decreased from 32.5 (19 to 50) at preoperative to 11 (8 to 22) at 6 months (p < 0.0001), and further decreased to 7 (4 to 17) by 1 year (p = 0.0009). By contrast, in the 17 (30.9%) patients with persistent GBS from 6 months to 1 year, the GERD-HRQL score only decreased from 48 (35 to 58) at preoperative to 29 (14 to 31) at 6 months (p = 0.0008) but not from 6 months to the 1-year score of 26 (15 to 36, p = 0.084). Compared with those with resolved GBS, patients with persistent GBS had worse GERD-HRQL and dysphagia scores and were less satisfied with their outcomes at both 6- and 12-month follow-up (Table 6). The prevalence of heartburn, regurgitation, and dysphagia was compared at preoperative, 6 months, and 1 year between those with persistent vs resolved GBS (Fig. 2). Antisecretory medication use was comparable at 6 months but was higher in the persistent GBS group at 1 year. Normalization of the distal esophageal acid exposure (p = 0.681) was similar between groups.
Table 6.
Comparison of Outcomes Based On the Status of Persistent or Resolved Gas-Bloat Syndrome from 6 Months to 1 Year
| Variable | 6-mo GBS persistent at 1 y, N = 17 (30.9%) | 6-mo GBS resolved by 1 y, N = 38 (69.1%) | p Value |
|---|---|---|---|
| GERD-HRQL at 6 mo | |||
| Total score, median (IQR) | 29 (14–31) | 11 (8–21) | 0.033 |
| Heartburn item, median (IQR) | 0 (1–2) | 0 (0–2) | 0.653 |
| Regurgitation item, median (IQR) | 2 (0–3) | 0 (0–2) | 0.226 |
| Dysphagia item, median (IQR) | 3 (2–5) | 2 (0–3) | 0.008 |
| Gas-bloat item, median (IQR) | 5 (4–5) | 4 (4–5) | 0.211 |
| Freedom from proton pump inhibitor use, % | 80 | 91.7 | 0.343 |
| Patient satisfaction, % | 53.3 | 84.2 | 0.032 |
| GERD-HRQL at 1 y | |||
| Total score, median (IQR) | 26 (15–36) | 7 (4–17) | 0.0007 |
| Heartburn item, median (IQR) | 1 (0–2) | 0 (0–2) | 0.168 |
| Regurgitation item, median (IQR) | 2 (0–4) | 0 (0–1) | 0.026 |
| Dysphagia item, median (IQR) | 3 (2–4) | 1 (0–2) | 0.002 |
| Gas-bloat item, median (IQR) | 4 (4–5) | 2 (1–3) | <0.0001 |
| Freedom from PPI use, % | 69.2 | 96.9 | 0.020 |
| Patient satisfaction, % | 56.3 | 80 | 0.047 |
| Simethicone use, % | 52.9 | 39.5 | 0.367 |
| pH normalization, % | 75 | 80 | 0.681 |
GBS, gas-bloat syndrome; GERD-HRQL, GERD health-related quality of life; IQR, interquartile range.
Figure 2.
Comparison of the prevalence of pre- and postoperative typical reflux symptoms. (A) Heartburn, (B) regurgitation, and (C) dysphagia between patients who had gas-bloat syndrome (GBS) at 6 months that either resolved (blue) or was persistent (yellow) at 1 year. Patients with persistent GBS had worse dysphagia preoperatively (*, p = 0.036), at 6-month follow-up (p = 0.066) and at 1-year follow-up (†, p = 0.009). All other symptoms were comparable over time.
Delayed-onset gas-bloat syndrome
There were 293 (84.2%) patients who did not develop GBS at 6 months. However, 25 (8.5%) of these patients met GBS criteria at 1 year. Patients who developed this delayed-onset GBS had similar GERD-HRQL scores at 6 months to patients who did not develop delayed GBS (p = 0.465). They did, however, have a significantly higher gas-bloat score but were comparable across all other measures at 6 months (Table 7). However, by 1 year, their GERD-HRQL scores worsened significantly, increasing from 4 (2 to 9) to 10 (5 to 25), p < 0.0001, which was significantly higher than patients without delayed GBS [10 (5 to 25) vs 4 (1 to 9), p = 0.0010]. Delayed GBS was also associated with lower patient satisfaction. Antisecretory medication use and normalization of the distal esophageal acid exposure were similar between groups. Nevertheless, delayed GBS was associated with a significant decrease in the GERD-HRQL score from 29 (14 to 31) at preoperative to 10 (5 to 25) at 1 year (p < 0.0001).
Table 7.
Comparison of Outcomes Based On Status of Delayed Gas-Bloat Syndrome
| Variable | Delayed GBS, N = 25 (8.5%) | No GBS, N = 268 (91.5%) | p Value |
|---|---|---|---|
| GERD-HRQL at 6 mo | |||
| Total score, median (IQR) | 4 (2–9) | 4 (1–8) | 0.476 |
| Heartburn item, median (IQR) | 0 (0–1) | 0 (0–0) | 0.156 |
| Regurgitation item, median (IQR) | 0 (0–0) | 0 (0–1) | 0.617 |
| Dysphagia item, median (IQR) | 0 (0–1) | 0 (0–2) | 0.407 |
| Gas-bloat item, median (IQR) | 2 (1–3) | 1 (0–2) | 0.031 |
| Freedom from PPI use | 84 | 94.6 | 0.062 |
| Patient satisfaction | 91.7 | 93.6 | 0.664 |
| GERD-HRQL at 1 y | |||
| Total score, median (IQR) | 10 (5–25) | 4 (1–9) | <0.0001 |
| Heartburn item, median (IQR) | 1 (0–2) | 0 (0–1) | 0.073 |
| Regurgitation item, median (IQR) | 1 (0–1) | 0 (0–1) | 0.112 |
| Dysphagia item, median (IQR) | 1 (0–1) | 0 (0–2) | 0.569 |
| Gas-bloat item, median (IQR) | 4 (4–4) | 1 (0–2) | <0.0001 |
| Freedom from PPI use, % | 86.4 | 94.4 | 0.149 |
| Patient satisfaction, % | 79.2 | 92.8 | 0.037 |
| Simethicone use, % | 40 | 23.9 | 0.073 |
| pH normalization, % | 66.7 | 72.5 | 0.544 |
GBS, gas-bloat syndrome; GERD-HRQL, GERD health-related quality of life; IQR, interquartile range; PPI, proton pump inhibitor.
Gas-bloat syndrome over time
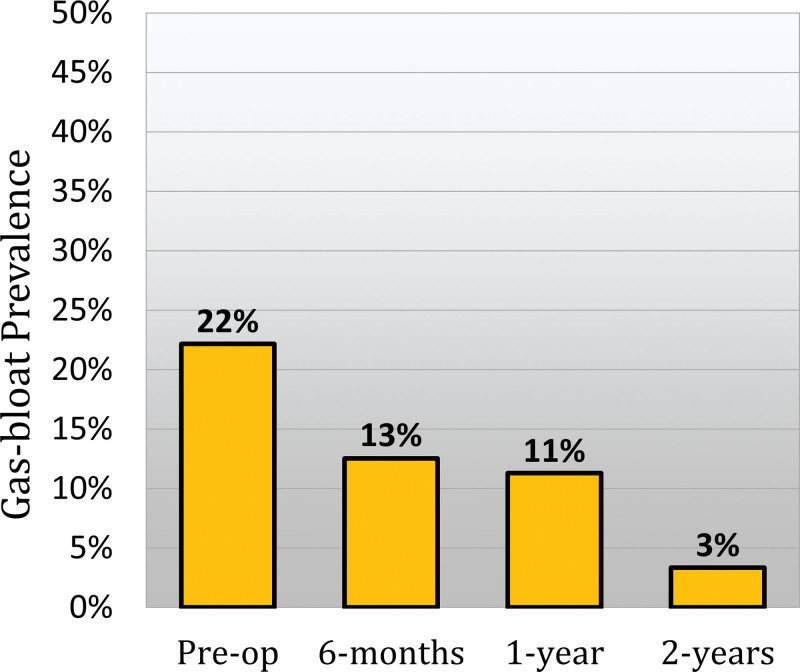
A subset of 239 patients completed 6-month, 1-year, and 2-year follow-up. Within this cohort, 30 (12.6%) patients reported GBS at 6 months. However, 24 (80%) of these patients had resolved GBS by 1 year and 27 (90%) had resolved it by 2 years. Delayed GBS was found in 21 (8.8%) patients. Of these patients, 16 (76.2%) had resolved GBS by 2 years. Therefore, the total rate of resolution for GBS at 1 year was 19 (70.4%). Resolution of GBS was associated with an improvement in GERD-HRQL total score from 11 (7 to 19) to 7 (4 to 10), p = 0.016. The overall prevalence of persistent GBS at 2 years was 3.3%. Figure 3 shows the prevalence of gas bloat over time. Patients with persistent GBS were also associated with a higher 2-year GERD-HRQL score compared with those without GBS at 2 years [20 (5 to 31) vs 5 (3 to 12), p = 0.019].
Figure 3.
Bar graph showing the prevalence of preoperative gas bloat (GERD health-related quality-of-life gas-bloat score ≥4) and gas-bloat syndrome at 6-month, 1-year, and 2-year follow-up after magnetic sphincter augmentation.
Of the patients with GBS at 1 year after surgery, 55.6% had a preoperative GERD-HRQL gas-bloat score of 4 or more. By 2 years, 86.7% of these patients with new-onset GBS achieved resolution of their gas-bloat symptoms.
DISCUSSION
Since the advent of the Nissen fundoplication in 1956, ARS has been a highly successful treatment for GERD, eliminating reflux symptoms in 91% of patients at 10-year follow-up.9 However, excellent reflux control has been marred by new postoperative symptoms such as GBS, which diminish patient satisfaction and outcomes. The goal of the developments in ARS technique for the past 70 years has been eliminating these new postoperative symptoms without compromising the efficacy of reflux control. MSA is one such development, which has demonstrated equivalent reflux control to Nissen fundoplication, with some studies demonstrating lower rates of GBS, owing to its preservation of the ability to belch. As a result, it has been suggested that MSA may be the procedure of choice to minimize the impact of gastrointestinal symptoms after ARS and address the treatment gap between those refractory to medical management yet unwilling to accept the risks of new symptoms after surgery.10,11 To understand the role of MSA in the treatment of GERD, it is imperative to understand its complications. Therefore, in this study, we assessed the incidence, natural course, risk factors, and impact of GBS after MSA.
The relationship between GBS and patient dissatisfaction with their surgical outcomes is well established. A study of 1,063 patients undergoing ARS found that 59% of the 101 dissatisfied patients reported that their primary complaint was new-onset symptoms.2 Postoperative gas bloat was rated as the most bothersome symptom, even more so than postoperative dysphagia or preoperative heartburn.2 A major finding of the current study is that the presence of GBS had a substantial detrimental effect on more than patient satisfaction. Patients’ quality-of-life measures were also markedly worse, with higher postoperative heartburn, regurgitation, and dysphagia rates, and diminished overall symptom improvement. This effect was independent of pH normalization, which was excellent for patients with or without GBS, suggesting that persistent reflux symptoms were not due to a failure to restore competency to the LES. Although the majority of reports of MSA outcomes acknowledge GBS as an adverse outcome, the association of GBS with worse reflux symptoms has not been previously reported in the MSA literature. However, it is consistent with a study from our center assessing outcomes after ARS, which found that patients who were unable to achieve ≥50% improvement in their GERD-HRQL total score and freedom from proton pump inhibitor were more likely to complain of daily bothersome to incapacitating GBS symptoms (p = 0.0008).12 Clinicians should be aware that although studies suggest lower rates of GBS after MSA, unfavorable outcomes after MSA may be related to GBS.
The exact etiology of GBS is poorly understood; however, a number of hypotheses have been proposed, including aerophagia, impaired receptive relaxation and accommodation, and postsurgical gastroparesis. Aerophagia, repetitive swallowing of air and saliva, is often seen in patients with GERD and is thought to be a self-soothing behavior for heartburn. However, the unintended side effect of swallowing air in addition to saliva is bloating. Studies have demonstrated that the aerophagia habit often persists after ARS and can negatively impact outcomes. A study comparing ARS outcomes of 112 patients with and without aerophagia found that both groups improved with ARS, but patients with aerophagia had diminished improvement with some subjective mild-to-moderate heartburn and dysphagia without objective correlation, consistent with our findings with MSA.13 Over time, as the inciting pathological reflux has resolved and patients with postoperative bloating are counseled to avoid aerophagia, the habit, along with its associated symptoms, may also resolve. This is consistent with the finding that GBS resolves over time. Aerophagia is also consistent with our finding that preoperative bloating was an independent predictor of GBS. However, bloating is a nonspecific symptom. Another potential explanation for the relationship between pre- and postoperative bloating is colonic motility. Previous studies have demonstrated that symptoms of irritable bowel syndrome, such as bloating and constipation, are associated with suboptimal ARS outcomes.14 A study from our center assessing subjective and objective colonic and whole gut motility data in relation to ARS outcomes found that bloating, constipation, and a longer objective colonic transit time were associated with suboptimal ARS outcomes. Additionally, colonic transit was significantly longer in patients with bloating.12 These findings suggest that preoperative bloating and colonic motility should be considered during risk-stratification and preoperative planning for ARS.
Previous hypotheses of the etiology of GBS were born out of the assessment of fundoplication. However, there are a number of differences between MSA and fundoplication related to the proposed etiologies of GBS. One hypothesis is that GBS is a result of impaired receptive relaxation and accommodation secondary to mobilization of the fundus.3 However, in MSA the gastric anatomy is largely undisturbed, meaning this explanation for GBS after MSA is unlikely. Another unique factor of the MSA is that it does not relax. Normally, belching corresponds with a transient LES relaxation to allow venting.15 Although fundoplication retains some inherent ability to relax, the MSA must be forced open by the buildup of pressure. Therefore, although MSA preserves the ability to belch, a higher degree of gastric distension may be necessary before venting is possible. Variable patient tolerance to this increased distension may contribute to variable reports of subjective gas-bloat symptoms. This is further supported by our finding that patients who received a smaller-sized MSA device were more likely to develop GBS. Previous studies have demonstrated that smaller devices were associated with higher integrated relaxation pressure and distal contractile integral, suggesting that the esophagus must contract more forcefully to overcome the higher threshold outflow resistance.16 Similarly, this suggests that this higher resistance must be overcome to vent.
The final proposed etiology of GBS is that it is a manifestation of delayed gastric emptying secondary to gastroparesis. A study from our center assessed the impact of delayed gastric emptying on MSA outcomes over time and found that heartburn, bloating, and GERD-HRQL were worse at 6 months but improved over time and were comparable by 1 year and durable at 2 years.17 The improvement in bloating over time is consistent with the current study’s findings. However, at 1-year GBS, outcomes remained significantly worse. Additionally, we found that both pre- and postoperative delayed gastric emptying were similar between patients with and without GBS. In fact, all objective testing data were similar between groups, whereas GBS was associated with severe preoperative subjective reflux and diminished subjective postoperative outcomes. This finding suggests that GBS after MSA is a distinct clinical entity characterized by bloating and associated with worse reflux symptom improvement without any objective evidence of anatomical or physiological pathology.
A challenge with the assessment of the incidence of GBS is the lack of a consensus definition. The most commonly reported rates of GBS after MSA rely on the 1-year GERD-HRQL gas-bloat item. Nevertheless, there remains some heterogeneity in definitions and rates of GBS in the MSA literature. Using the GBS definition of gas-bloat symptoms that affect or are incapacitating to daily activities, we found a GBS rate of 13.3% at 1-year follow-up. A multicenter study of 202 patients who underwent MSA similarly found a GBS rate of 10% using the same definition.18 Asti and colleagues19 found a similar rate of 11% using an equivalent definition in their cohort of 130 patients who underwent MSA. However, other centers have reported 1-year GBS rates as high as 23%, without defining a specific cutoff in the GERD-HRQL.20 Another study of 169 patients who underwent MSA reported that 47% of patients had some degree of GBS; however, they subcategorized GBS as mild (27%), moderate (14%), or severe (5%).21 A multicenter study of 200 patients used the definition of daily bothersome bloating symptoms and found that GBS decreased from 56% before surgery to 16.2% 1 year after MSA. The heterogeneity in the literature highlights the need for increased consensus and attention to this topic.
In this study, we found that the vast majority of GBS spontaneously resolved, with only 3% of patients reporting persistent GBS at 2 years. This finding is consistent with reported rates in the literature ranging from 2% to 6% among studies with at least 2-year follow-up after MSA.22-24 This suggests that although GBS is prevalent early on after MSA, the natural history is spontaneous resolution for 1 to 2 years. This course is similar to reports from studies on Nissen fundoplication. Following fundoplication GBS is very common with studies showing up to 39% of patients reporting bothersome to incapacitating symptoms and 81% reporting some degree of GBS during the first year after surgery.18,25 However, longer-term studies have demonstrated that the majority of these symptoms are transient, spontaneously resolving within the first 6 months to 2 years. Several studies comparing outcomes between MSA and Nissen fundoplication have found lower rates of GBS early on after MSA.10,18,20 However, a propensity matched study of 135 and 103 patients who underwent MSA and Nissen fundoplication, respectively, assessed gas-bloat symptoms for a 1- to 7-year period and found no difference between MSA and Nissen over time (p = 0.532).26 These findings suggest that although GBS may be less common after MSA early on, over time symptoms improve and the prevalence of GBS becomes equivalently low. Therefore, patients can be reassured that the development of even severe GBS symptoms after surgery will likely resolve without further intervention.
We acknowledge a number of important limitations in this study, including its retrospective nature and variable follow-up. It is possible that patients without postoperative symptoms, mild GBS symptoms, or resolved GBS were less likely to present for follow-up visits. This potential is somewhat mitigated by scheduling routine annual follow-up, and the finding that the vast majority of patients had reflux control without GBS symptoms at 2 years, suggesting that this dataset is representative. Additionally, although the GERD-HRQL gas-bloat item has been used consistently in the literature to define GBS after MSA, clinically the condition is much more ill-defined. Patients can present with a constellation of symptoms attributed to GBS, which are not assessed by the GERD-HRQL. The addition of patient-reported quality-of-life measures specifically assessing GBS-related symptoms may be able to better define the diagnosis.
The findings of the current study have a number of implications for clinical practice. Despite studies demonstrating a lower rate compared with fundoplication, the incidence and impact of GBS on MSA outcomes are not negligible. Patients should be informed of these risks before MSA. However, given that the vast majority of patients had resolution of GBS within 1 year with associated improvement in outcomes, we recommend that these findings be used for risk-stratification and expectation management, not surgical candidacy. There are a number of useful strategies for managing postoperative gas-bloat symptoms. Given that gas bloat is most prevalent early on, all of our patients are empirically started on simethicone for the first 6 weeks after surgery. If bloating symptoms persist, this course is extended. It is also important to consider alternative causes of bloating symptoms, such as gastroparesis. GES can be useful in determining whether a pyloric drainage procedure may be warranted. Finally, MSA is removable if necessary. However, we previously published our experience with MSA removal and found that the indications for removal were persistent dysphagia (78%) followed by inadequate control (22%).27 In our experience, no patients have required device removal for GBS. Therefore, we recommend watchful waiting with lifestyle modification and medications for the management of GBS.
CONCLUSIONS
GBS is a common complaint after traditional ARS, but studies have demonstrated lower rates of GBS after MSA owing to a preserved ability to belch, suggesting GBS may only be mildly disruptive after MSA. In this study, we tested this hypothesis by assessing the incidence, natural history, risk factors, and impact on outcomes of GBS after MSA. The incidence of GBS at 1 year after MSA was 13.3%, but by 2 years, only 3% of patients had persistent GBS. Patients with GBS had substantially diminished symptomatic improvement with worse postoperative symptom control. However, as GBS resolved, symptoms and quality-of-life measures improved. Although patients with preoperative bloating, a GERD-HRQL score of more than 30, or a small (13 or 14 beads) MSA device are at higher risk of developing GBS, no objective testing data were associated with GBS. Therefore, patients considering ARS should be counseled that although some studies suggest that GBS is less likely following MSA, it is still a risk with a substantial impact on outcomes. Additionally, patients who develop GBS with recurrent reflux symptoms despite normal physiology testing data should be reassured and managed conservatively with medications such as simethicone and avoidance of behaviors such as aerophagia.
Author Contributions
Conceptualization: Eriksson, Ayazi, Sarici
Data curation: Eriksson, Zheng, Sarici, Hannan
Formal analysis: Eriksson, Ayazi, Zheng, Sarici
Investigation: Eriksson, Ayazi, Hannan
Methodology: Eriksson, Ayazi, Zheng, Jobe
Visualization: Eriksson, Ayazi, Sarici
Writing – original draft: Eriksson, Zheng, Sarici
Writing – review & editing: Eriksson, Ayazi, Zheng, Sarici, Jobe
Project administration: Ayazi
Supervision: Ayazi, Jobe
Abbreviations and Acronyms
- ARS
- antireflux surgery
- GBS
- gas-bloat syndrome
- GERD-HRQL
- GERD health-related quality of life
- GES
- gastric emptying scintigraphy
- HH
- hiatal hernia
- LES
- lower esophageal sphincter
- MSA
- magnetic sphincter augmentation
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 108th Annual Clinical Congress, Scientific Forum, San Diego, CA, October 2022.
REFERENCES
- 1.Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc. 2006;20:1698–1701. [DOI] [PubMed] [Google Scholar]
- 2.Humphries LA, Hernandez JM, Clark W, et al. Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc. 2013;27:1537–1545. [DOI] [PubMed] [Google Scholar]
- 3.Spechler SJ. The management of patients who have “failed” antireflux surgery. Am J Gastroenterol. 2004;99:552–561. [DOI] [PubMed] [Google Scholar]
- 4.Anvari M, Allen C. Postprandial bloating after laparoscopic Nissen fundoplication. Can J Surg. 2001;44:440–444. [PMC free article] [PubMed] [Google Scholar]
- 5.Aiolfi A, Asti E, Bernardi D, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and meta-analysis. Int J Surg. 2018;52:82–88. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JL, Zehetner J, Wu P, et al. Laparoscopic magnetic sphincter augmentation vs laparoscopic Nissen fundoplication: a matched-pair analysis of 100 patients. J Am Coll Surg. 2015;221:123–128. [DOI] [PubMed] [Google Scholar]
- 7.Skubleny D, Switzer NJ, Dang J, et al. LINX((R)) magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. 2017;31:3078–3084. [DOI] [PubMed] [Google Scholar]
- 8.Louie BE, Farivar AS, Shultz D, et al. Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg. 2014;98:498–504; discussion 504. [DOI] [PubMed] [Google Scholar]
- 9.DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease Evaluation of primary repair in 100 consecutive patients. Ann Surg. 1986;204:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheu EG, Nau P, Nath B, et al. A comparative trial of laparoscopic magnetic sphincter augmentation and Nissen fundoplication. Surg Endosc. 2015;29:505–509. [DOI] [PubMed] [Google Scholar]
- 11.Triadafilopoulos G, Azagury D. How can we deal with the GERD treatment gap? Ann N Y Acad Sci. 2016;1381:14–20. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson SE, Maurer N, Zheng P, et al. Impact of objective colonic and whole gut motility data as measured by wireless motility capsule on outcomes of antireflux surgery. J Am Coll Surg. 2023;236:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamolz T, Granderath F, Bammer T, et al. Dysphagia and quality of life after laparoscopic Nissen fundoplication in patients with and without prosthetic reinforcement of the hiatal crura. Surg Endosc. 2002;16:572–577. [DOI] [PubMed] [Google Scholar]
- 14.Axelrod DA, Divi V, Ajluni MM, et al. Influence of functional bowel disease on outcome of surgical antireflux procedures. J Gastrointest Surg. 2002;6:632–637. [DOI] [PubMed] [Google Scholar]
- 15.Straathof J, Ringers J, Lamers C, Masclee A. Provocation of transient lower esophageal sphincter relaxations by gastric distension with air. Am J Gastroenterol. 2001;96:2317–2323. [DOI] [PubMed] [Google Scholar]
- 16.Ayazi S, Schwameis K, Zheng P, et al. The impact of magnetic sphincter augmentation (MSA) on esophagogastric junction (EGJ) and esophageal body physiology and manometric characteristics. Ann Surg. 2023;277:e545–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson SE, Zheng P, Sarici IS, et al. The impact of delayed gastric emptying as measured by gastric emptying scintigraphy on the outcome of magnetic sphincter augmentation. Surg Endosc. 2023;37:7144–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riegler M, Schoppman SF, Bonavina L, et al. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc. 2015;29:1123–1129. [DOI] [PubMed] [Google Scholar]
- 19.Asti E, Milito P, Froiio C, et al. Comparative outcomes of Toupet fundoplication and magnetic sphincter augmentation. Dis Esophagus. 2023;36(Suppl 1):doac090. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds JL, Zehetner J, Nieh A, et al. Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc. 2016;30:3225–3230. [DOI] [PubMed] [Google Scholar]
- 21.Warren HF, Reynolds JL, Lipham JC, et al. Multi-institutional outcomes using magnetic sphincter augmentation versus Nissen fundoplication for chronic gastroesophageal reflux disease. Surg Endosc. 2016;30:3289–3296. [DOI] [PubMed] [Google Scholar]
- 22.Nikolic M, Matic A, Feka J, et al. Expanded indication for magnetic sphincter augmentation: outcomes in weakly acidic reflux compared to standard GERD patients. J Gastrointest Surg. 2022;26:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonavina L, Saino G, Bona D, et al. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg. 2013;217:577–585. [DOI] [PubMed] [Google Scholar]
- 24.Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:719–727. [DOI] [PubMed] [Google Scholar]
- 25.Spechler SJ; Group* DoVAGRDS. Comparison of medical and surgical therapy for complicated gastroesophageal reflux disease in veterans. N Engl J Med. 1992;326:786–792. [DOI] [PubMed] [Google Scholar]
- 26.Asti E, Bonitta G, Lovece A, et al. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: observational cohort study with propensity score analysis. Medicine (Baltimore). 2016;95:e4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson S, Schwameis K, Ayazi S, et al. Removal of the magnetic sphincter augmentation device: an assessment of etiology, clinical presentation, and management. Surg Endosc. 2023;37:3769–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]