Abstract
Purpose
This study describes the emergence of Candida auris in Hong Kong, focusing on the incidence and trends of different Candida species over time. Additionally, the study analyzes the relationship between C. auris and antifungal prescription, as well as the impact of outbreaks caused by C. auris.
Patients and Methods
Data were collected from 43 public hospitals across seven healthcare networks (A to G) in Hong Kong, including Candida species culture and antifungal prescription information. Among 150,267 patients with 206,405 hospitalization episodes, 371,653 specimens tested positive for Candida species. Trends in Candida species and antifungal prescription were analyzed before (period 1: 2015 1Q to 2019 1Q) and after (period 2: 2019 2Q to 2023 2Q) the emergence of C. auris in Hong Kong.
Results
Candida albicans was the most prevalent species, accounting for 57.1% (212,163/371,653) of isolations, followed by Candida glabrata (13.1%, 48,666), Candida tropicalis (9.2%, 34,261), and Candida parapsilosis (5.3%, 19,688). C. auris represented 2.0% of all Candida species isolations. Comparing period 2 to period 1, the trend of C. albicans remained stable, while C. glabrata, C. tropicalis, and C. parapsilosis demonstrated a slower increasing trend in period 2 than in period 1. Other species, including C. auris, exhibited a 1.1% faster increase in trend during period 2 compared to period 1. Network A, with the highest antifungal prescription, did not experience any outbreaks, while networks F and G had 40 hospital outbreaks due to C. auris in period 2. Throughout the study period, healthcare networks B to G had significantly lower antifungal prescription compared to network A, ranging from 54% to 78% less than that of network A.
Conclusion
There is no evidence showing correlation between the emergence of C. auris and antifungal prescription in Hong Kong. Proactive infection control measures should be implemented to prevent nosocomial transmission and outbreak of C. auris.
Keywords: epidemiology, outbreak, antifungal prescription, infection control measure
Introduction
Fungal infections have a significant global impact, causing over 1.5 million fatalities annually and affecting billions of individuals each year. Recent global assessments indicate approximately 3,000,000 instances of chronic pulmonary aspergillosis, around 223,100 cases of cryptococcal meningitis in people living with HIV/AIDS, roughly 700,000 cases of invasive candidiasis, about 500,000 cases of Pneumocystis jirovecii pneumonia, and approximately 250,000 cases of invasive aspergillosis occur annually.1 Although the incidence of invasive fungal infections is high, these infections primarily affect immunocompromised patients with limited person-to-person transmission. Therefore, infection control professionals may direct more attention towards hospital-acquired pathogens that possess greater epidemiological significance, such as pathogens that can be transmitted through person-to-person contact, in addition to contaminated environments, or the air. These pathogens may include multidrug-resistant organisms, respiratory viruses, or gastrointestinal viruses within healthcare settings.2–8
However, the incidence of fungal infections among hospitalized patients has shown a marked increase during the COVID-19 pandemic, as reported in a study conducted by Centers for Disease Control and Prevention in the United States. The study revealed an annual increase of 8.5% in hospitalizations involving fungal infections between 2019 and 2021. Additionally, during the period of 2020–2021, patients hospitalized with COVID-19–associated fungal infections had a higher in-hospital mortality rate (48.5%) compared to those with non–COVID-19–associated fungal infections (12.3%).9 Fluconazole-resistant Candida parapsilosis has emerged during the COVID-19 era, emphasizing the increased incidence of candidemia in COVID-19 patients, as well as causing hospital outbreaks.10,11 The emergence of Candida auris, a multidrug-resistant Candida species, also had a notable increase during the COVID-19 pandemic. Prolonged hospitalization resulting from severe illness caused by COVID-19 and the utilization of antimicrobial agents may have played a role in the development of C. auris infections.12,13 Unlike other invasive fungal infections, C. auris has a high potential for hospital outbreaks due to person-to-person transmission, as demonstrated by the multicenter outbreak in Colombia from 2015 to 2016.14 In Israel, C. auris outbreaks were observed in multiple healthcare facilities between May 2014 and May 2022, affecting a total of 209 patients with C. auris infection or colonization. The incidence rate of C. auris experienced a 30-fold increase in 2021, coinciding with surges in COVID-19-related hospitalizations. These outbreaks primarily began among mechanically ventilated patients in specialized COVID-19 units, spreading sequentially to non-COVID-19 ventilated patients and non-ventilated patients.15
In Hong Kong, C. auris has emerged since the first imported case in June 2019 and has subsequently led to ongoing outbreaks since 2020. The objective of our study is to analyze the quarterly incidence of Candida species before and during the C. auris outbreak, as well as the prescription of antifungal agents. The findings from this analysis could provide valuable insights for developing infection control policies to effectively manage the spread of C. auris within hospital settings.
Materials and Methods
Setting
This is a retrospective study to investigate the epidemiology of Candida infection, including C. auris in the public hospitals in Hong Kong from January 1, 2015, to June 30, 2023. In Hong Kong, majority of the public healthcare service is managed by 43 hospitals which include primary, secondary and tertiary care services. The hospitals are geographically divided into seven healthcare networks, which are under the governance of the Hospital Authority. For the purpose of this study, these healthcare networks are anonymously named as network A, B, C, D, E, F, and G. The total number of hospital beds in network A to G increased from 27,895 in 2015 to 30,568 in 2023.16,17
Data Collection
The clinical records of hospitalized patients from public hospitals can be obtained from an electronic platform known as the Clinical Data Analysis and Reporting System (CDARS). This platform allows access to the records for the purpose of service planning and medical research, as previously described.18–22 A specific hospital number is assigned to each episode of hospitalization per patient. During data retrieval, a reference number is generated to replace the patient’s personal identity number in order to protect patient confidentiality. Using either the specific hospital number or the reference number, clinical information, laboratory data, prescription records, and radiological images can be retrieved for an individual. Alternatively, specific requests can be made to the system to collect data from a specific category, such as patient days or patient admission, within a certain period for the purpose of data analysis.
Epidemiology of Candida Species in Hong Kong
Episodes of each hospitalization involving a positive culture of Candida species from clinical specimens between January 1, 2015, and June 30, 2023 (2015 1Q to 2023 3Q), in networks A to G were retrieved from CDARS. The total number of Candida isolates, number of specific Candida species, as well as the types of clinical specimen during the study period, were analyzed. If an episode of hospitalization had multiple clinical specimens growing Candida species, only the first isolate was considered as the non-duplicate isolate for further analysis. The quarterly incidence of Candida isolation was expressed as the number of Candida species isolations per 1000 patient days in the seven healthcare networks.
Emerging Candida Auris in Hong Kong
The analysis of C. auris was performed from 2019 2Q to 2023 2Q, as it was first isolated in Hong Kong in June 2019.23 Since C. auris was predominantly detected in two healthcare networks, the quarterly incidence of C. auris, expressed as the number of Candida species isolations per 1000 patient days, was only reported specifically for these two healthcare networks. The number of outbreaks due to C. auris was compared with that of other epidemiologically important multidrug-resistant organisms, respiratory viruses, and gastrointestinal viruses reported in Hong Kong during the study period. The definition of outbreak reporting was described previously.24
Trend of Candida Species Before and After Emergence of Candida Auris in Hong Kong
The entire study period was further divided into two periods: period 1 (from 2015 1Q to 2019 1Q, 17 quarters before the emergence of C. auris) and period 2 (from 2019 2Q to 2023 2Q, 17 quarters after the emergence of C. auris). The changes in the trends of Candida species in periods 1 and 2 were analyzed.
Prescription of Antifungal Agents in Hong Kong
The prescription of antifungal agents in each healthcare network was also retrieved from CDARS. Three major classes of antifungal agents available for hospitalized patients in the public hospitals were included in the analysis. These antifungal agents include amphotericin B (parenteral), echinocandins (anidulafungin, caspofungin, micafungin – all parenteral), azoles (fluconazole – oral and parenteral, isavuconazole – oral and parenteral, itraconazole – oral and parenteral, posaconazole – oral and parenteral, voriconazole – oral and parenteral), and others [5-flucytosine (oral and parenteral), griseofulvin (oral), ketoconazole (oral), miconazole (parenteral), nystatin (oral), terbinafine (oral)]. The quarterly prescription of all antifungal agents was expressed as the defined daily dose (DDD) per 1000 patient days in the seven healthcare networks.
The correlation between antifungal prescription in Hong Kong and the quarterly incidence of all Candida species, including the most commonly identified Candida species, Candida albicans, was analyzed. This analysis was conducted both overall and within each of the seven healthcare networks. Additionally, the change in the trend of prescription of antifungal agents before (period 1) and after (period 2) the emergence of C. auris in the healthcare networks in Hong Kong was also analyzed.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster (UW23-393). Written consent from individual patients was waived by the Institutional Review Board because the study is a retrospective review of clinical and laboratory information from a database where the patients’ identities were anonymized.
Statistical Analysis
The emergence of C. auris in Hong Kong in 2019 2Q prompted an investigation into the change in slope by including the corresponding term in the regression. Differences in the trends of Candida species and antifungal prescription were evaluated between period 1 (2015 1Q to 2019 1Q) and period 2 (2019 2Q to 2023 2Q) using interrupted time series analysis, specifically segmented Poisson regression. Since healthcare network A had the highest level of antifungal prescription, the prescription of antifungal agents in the other healthcare networks (B to G) was compared to that of healthcare network A using Poisson regression. The chi-square test was used to compare the proportional of C. auris outbreaks in different time periods. Pearson’s correlation was used to compare the prescription of antifungal agents, expressed as DDD per 1000 patient days, with the incidence of Candida species, expressed as isolations per 1000 patient days in Hong Kong, as well as within each of the seven healthcare networks. All statistical analyses were performed using IBM SPSS Statistics (version 28). A two-sided p-value of <0.05 was considered statistically significant.
Results
Epidemiology of Candida Species in Hong Kong
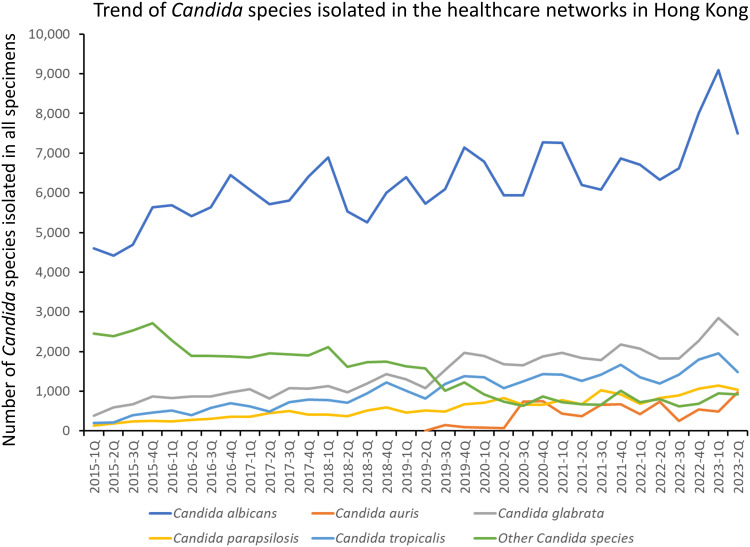
During the study period (2015 1Q to 2023 2Q), Candida species were isolated in a total of 371,653 clinical specimens, which were collected from 150,267 patients who experienced 206,405 episodes of hospitalization across seven healthcare networks in Hong Kong. Among the Candida species identified, C. albicans was the most predominant isolate (57.1%, 212,163/371,653), followed by Candida glabrata (13.1%, 48,666/371,653), Candida tropicalis (9.2%, 34,261/371,653), and Candida parapsilosis (5.3%, 19,688/371,653). C. auris accounted for 2.0% (7,456/371,653) of all Candida species. Other Candida species, such as Candida lusitaniae and Candida dubliniensis, each constituted 0.4%, Candida guilliermondii constituted 0.2%, while the remaining 29 different Candida species represented 0.1% or less of the total Candida isolations. The trend of Candida species isolated in the healthcare networks in Hong Kong per quarter is shown in Figure 1. Out of 371,653 clinical specimens with Candida species isolations, urinary specimens accounted for 48.1% (178,811), followed by respiratory specimens (22.5%, 83,545), swab specimens (17.7%, 65,739), specimens from sterile body sites other than blood (3.9%, 14,447), and blood culture (1.6%, 6039).
Figure 1.
Trend of Candida species isolated in the healthcare networks in Hong Kong.
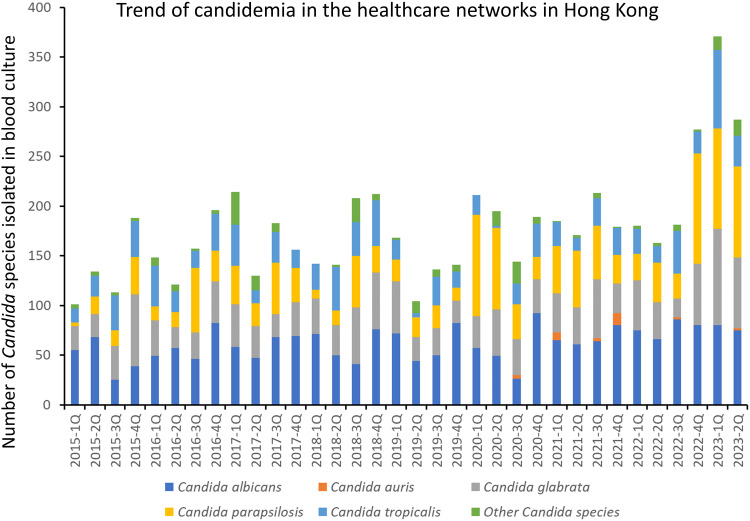
Of these 6039 blood culture isolations of Candida species, they were collected from 2471 patients during 2605 episodes of hospitalization over the study period. C. albicans remained the commonest Candida species, constituting 34.9% (2105/6039) of the isolations, followed by C. glabrata (22.6%, 1367/6039), C. parapsilosis (22.5%, 1357/6039), and C. tropicalis (15.4%, 929/6039). The trend of candidemia in the healthcare networks in Hong Kong is illustrated in Figure 2.
Figure 2.
Trend of candidemia in the healthcare networks in Hong Kong.
The non-duplicate isolates of Candida species per each episode of hospitalization were selected to analyze the trends of Candida species during the study period. The overall incidence of Candida isolation was 3.05 and 1.90 per 1000 patient days for all Candida species and C. albicans, respectively. Similarly, the incidence of candidemia was 0.09 per 1000 patient days during the study period.
Emerging Candida Auris in Hong Kong
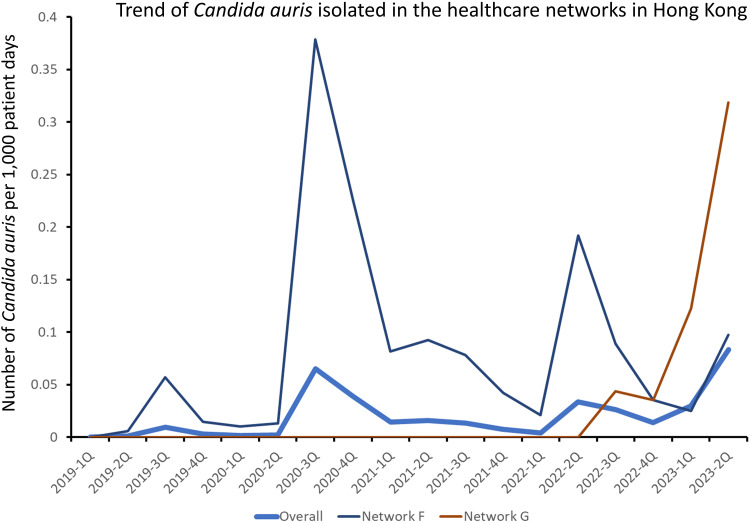
The first case of C. auris was identified in a patient with a history of travel to Switzerland in June 2019, and outbreaks of C. auris have been reported in hospitals since July 2020. Between 2019 1Q and 2023 2Q, a total of 7456 specimens grew C. auris, collected from 487 patients. Among these 487 patients, 457 (93.4%) were diagnosed through active surveillance using a combination of axilla, groin, and nasal swabs upon admission or during outbreak investigations. Furthermore, 482 (99.0%) patients with C. auris colonization or infections were detected in two healthcare networks in Hong Kong (Figure 3).
Figure 3.
Trend of Candida auris isolated in the healthcare networks in Hong Kong.
Notes: Healthcare network F and G contributed to 482 (99.0%) of the patients with C. auris colonization or infections in Hong Kong. Other healthcare networks with sporadic cases of C. auris are not shown.
During the study period (2015 1Q to 2023 2Q), a total of 456 reported outbreaks were recorded across seven healthcare networks. Among these outbreaks, 40 (8.8%) of 456 hospital outbreaks were attributed to C. auris (Supplementary Figure 1). Since the emergence of C. auris, it accounted for 28 (13.1%) of 214 hospital outbreaks between 2019 and 2022. In the most recent two-quarters (2023 1Q to 2023 2Q), the proportion of outbreaks caused by C. auris was significantly higher than those reported from 2019 to 2022 (32.4%, 12/37 vs 13.1%, 28/214, p = 0.003).
Trend of Candida Species Before and After Emergence of Candida Auris in Hong Kong
For all Candida species, the number of isolates per 1000 patient days increased by 0.5% per quarter in period 1 (relative risk, RR: 1.005, 95% confidence interval, CI: 1.002–1.008, p = 0.001). In period 2, an increase of 1.2% per quarter was observed (RR: 1.012, 95% CI: 1.009–1.015, p < 0.001). Period 2 exhibited a 0.7% faster increase compared to the trend in period 1 (RR: 1.007, 95% CI: 1.001–1.013, p = 0.026). For C. albicans, the number of isolates per 1000 patient days increased by 0.7% per quarter (RR: 1.007, 95% CI: 1.006–1.009, p < 0.001) in both period 1 and period 2. There were no significant changes in the trend of C. albicans isolates between period 1 and period 2 (p = 0.416). While C. glabrata, C. tropicalis, and C. parapsilosis demonstrated a slower increasing trend in period 2 than in period 1, other Candida species, including C. auris, showed a 1.1% faster increase in trend during period 2 compared to that in period 1 (RR: 1.011, 95% CI: 1.003–1.019, p < 0.001) (Supplementary Table 1).
Prescription of Antifungal Agents in Hong Kong
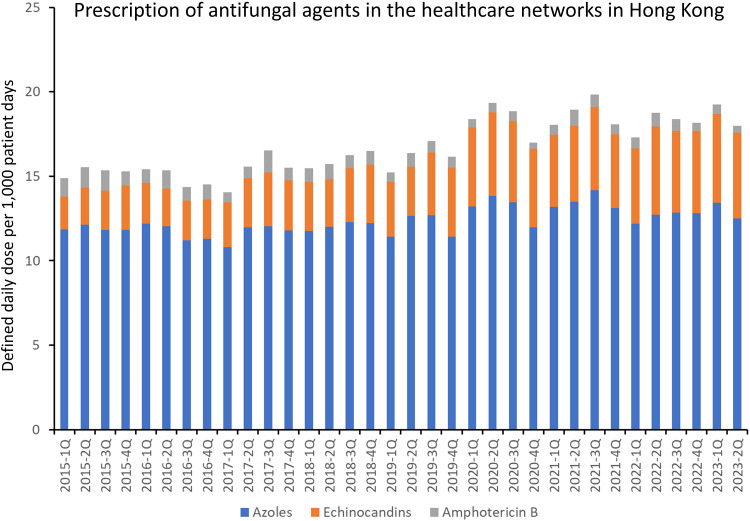
The trend of the prescription of antifungal agents, expressed as DDD per 1000 patient days, is illustrated in Figure 4. The overall prescription of antifungal agent per 1000 patient days increased by 1.3% per quarter in period 1 (RR: 1.013, 95% CI: 1.009–1.017, p < 0.001). In period 2, an increase of 2.1% per quarter was observed (RR: 1.021, 95% CI: 1.017–1.026, p < 0.001). Period 2 exhibited a 0.8% faster increase compared to the trend in period 1 (RR: 1.008, 95% CI: 1.001–1.015, p = 0.023). The prescription of antifungal agents in healthcare network A was significantly higher than that of the other networks (Table 1).
Figure 4.
Prescription of antifungal agents in the healthcare networks in Hong Kong.
Notes: The antifungal agents include amphotericin B (parenteral), echinocandins [anidulafungin (parenteral), caspofungin (parenteral), micafungin (parenteral)], azoles [fluconazole (oral and parenteral), isavuconazole (oral and parenteral), itraconazole (oral and parenteral), posaconazole (oral and parenteral), voriconazole (oral and parenteral)]. The other antifungal agents, including 5-flucytosine (oral and parenteral), griseofulvin (oral), ketoconazole (oral), miconazole (parenteral), nystatin (oral), and terbinafine (oral), are not shown in this figure due to the small amount of prescription.
Table 1.
Comparison of Antifungal Agent Prescription in Relation to Healthcare Network A in Hong Konga
| Healthcare Network | Relative Risk and 95% Confidence Interval | p value | Remark |
|---|---|---|---|
| Network A | 1b | NA | NA |
| Network B | 0.299 (0.272–0.329) | <0.001 | 70.1% less than network A |
| Network C | 0.225 (0.200–0.253) | <0.001 | 77.5% less than network A |
| Network D | 0.289 (0.262–0.318) | <0.001 | 71.1% less than network A |
| Network E | 0.354 (0.325–0.386) | <0.001 | 64.6% less than network A |
| Network Fc | 0.347 (0.317–0.380) | <0.001 | 65.3% less than network A |
| Network Gc | 0.460 (0.417–0.508) | <0.001 | 54.0% less than network A |
Notes: aThe antifungal agents includes amphotericin B (parenteral), echinocandin [anidulafungin (parenteral), caspofungin (parenteral), micafungin (parenteral)], triazole [fluconazole (oral and parenteral), isavuconazole (oral and parenteral), itraconazole (oral and parenteral), posaconazole (oral and parenteral), voriconazole (oral and parenteral)], and others [5-flucytosine (oral and parenteral), griseofulvin (oral), ketoconazole (oral), miconazole (parenteral), nystatin (oral), terbinafine (oral)]. bRelative risk of antifungal agent prescription in network A is set as 1 for calculation. cHealthcare network with nosocomial outbreaks of C. auris.
Abbreviation: NA, not applicable.
The Pearson correlation between the prescription of antifungal agents (DDD per 1000 patient days) and isolation of Candida species (per 1000 patient days) within the same quarter during the study period in the healthcare networks in Hong Kong is shown in Table 2. Overall, a significant positive correlation was observed between the prescription of antifungal agents and all Candida species (correlation coefficient 0.886, 95% CI: 0.782–0.942, p < 0.001), as well as C. albicans (correlation coefficient 0.760, 95% CI: 0.568–0.874, p < 0.001). However, network E did not demonstrate a significant correlation between antifungal prescription and the isolation of Candida species and C. albicans, while network F revealed a significant negative correlation between antifungal prescription and the isolation of C. albicans (correlation coefficient −0.602, 95% CI: −0.781 – −0.331, p < 0.001).
Table 2.
Pearson Correlation Between Prescription of Antifungal Agents (Defined Daily Dose per 1000 Patient Days) and Isolation of Candida Species (per 1000 Patient Days) Within the Same Quarter in the Healthcare Network in Hong Kong.a,b
| Healthcare Network | Pearson Correlation for all Candida Species (95% Confidence Interval) | p value | Pearson Correlation for Candida Albicans (95% Confidence Interval) | p value |
|---|---|---|---|---|
| Network A | 0.699 (0.473–0.839) | <0.001 | 0.634 (0.376–0.800) | <0.001 |
| Network B | 0.503 (0.199–0.719) | 0.002 | 0.678 (0.441–0.827) | <0.001 |
| Network C | 0.869 (0.752–0.933) | <0.001 | 0.600 (0.329–0.780) | <0.001 |
| Network D | 0.791 (0.619–0.891) | <0.001 | 0.637 (0.380–0.802) | <0.001 |
| Network E | 0.308 (−0.033–0.586) | 0.076 | −0.125 (−0.444–0.223) | 0.482 |
| Network Fc | −0.128 (−0.447–0.220) | 0.471 | −0.602 (−0.781 – −0.331) | <0.001 |
| Network Gc | 0.850 (0.718–0.923) | 0.001 | 0.871 (0.756–0.934) | <0.001 |
| Overall (Network A to G) | 0.886 (0.782–0.942) | <0.001 | 0.760 (0.568–0.874) | <0.001 |
Notes: aThe isolation of Candida species is expressed as the number of unique Candida species per 1000 patient days. A unique Candida species refers to a non-duplicated isolate of the first Candida species identified during the same episode of hospitalization throughout the study period from 2015 1Q to 2023 2Q. bThe antifungal agents includes amphotericin B (parenteral), echinocandin [anidulafungin (parenteral), caspofungin (parenteral), micafungin (parenteral)], triazole [fluconazole (oral and parenteral), isavuconazole (oral and parenteral), itraconazole (oral and parenteral), posaconazole (oral and parenteral), voriconazole (oral and parenteral)], and others [5-flucytosine (oral and parenteral), griseofulvin (oral), ketoconazole (oral), miconazole (parenteral), nystatin (oral), terbinafine (oral)]. cHealthcare network with nosocomial outbreaks of C. auris.
Discussion
This is the first epidemiological study to reveal the incidence of Candida isolation among hospitalized patients in Hong Kong from 2015 to the second quarter of 2023, where the burden of all Candida species and candidemia was 3.05 and 0.09 per 1000 patient days, respectively. Our incidence of candidemia was lower than that of an early study conducted in Singapore, where the incidence of candidemia was 0.14 per 1000 patient days from 2012 to 2015.25 Regarding the distribution of Candida species, our findings align with a laboratory-based surveillance study on the species distribution of candidemia in Asia, which reported that C. albicans was the most frequently isolated species (41.3%), followed by C. tropicalis (25.4%), C. glabrata (13.9%), and C. parapsilosis (12.1%).26 Likewise, our study showed a similar pattern in candidemia, with C. albicans (34.9%) being the most predominant Candida species, followed by C. glabrata (22.6%), C. parapsilosis (22.5%), and C. tropicalis (15.4%).
The incidence of Candida species isolation has shown an increasing trend in Hong Kong even prior to the onset of the COVID-19 pandemic. Similarly, an analysis of an electronic medical record database spanning from 2009 to 2017 in the United States revealed a rise in non-bloodstream invasive candidiasis, along with a 7.2% annual increase in the incidence of Candida species other than C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis.27
Among these non-albicans Candida species, C. auris has emerged as a prevalent pathogen in healthcare facilities worldwide, capable of colonizing the skin and causing hospital outbreaks before and during the COVID-19 pandemic.28,29 Since its emergence in June 2019, C. auris has been responsible for persistent nosocomial outbreaks in two out of seven healthcare networks in Hong Kong, contributing to 13% of reported hospital outbreaks involving epidemiologically important multidrug-resistant organisms and viruses. We analyzed the trends of all Candida species, including C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis, before (period 1) and after the emergence of C. auris (period 2), using the second quarter of 2019 as the cutoff. While there was a significant and faster increase in all Candida species during period 2, no significant increase was observed in C. albicans. Interestingly, a paradoxically slower increase was noted in C. glabrata, C. tropicalis, and C. parapsilosis during period 2. In contrast, other Candida species demonstrated a significant and faster increase during this period. As C. auris becomes the predominant species among Candida species other than C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis, the significant and faster increase observed in period 2 could be attributed to the emergence of C. auris in Hong Kong. There is no specific publication suggesting the replacement of C. auris with other Candida species. However, the unique characteristics and adaptations of C. auris, particularly its drug resistance and ability to form biofilms, enable it to outcompete other Candida species in specific environments.30–32
The risk factors for C. auris infections are not different from the risk factors associated with infections caused by other Candida species, including the prescription of antifungal agents.33 The global prescription of systemic antifungal agents showed a notable increase from 0.50 to 0.92 DDD per 1000 inhabitants per day between 2008 and 2018, reflecting a compound annual growth rate of 6.2%.34 The increasing prescription of antifungal agents was also observed in Hong Kong. The mean quarterly prescription of antifungal agents, expressed as 1000 patient days, increased by 35% from period 1 (21.46 ± 1.51) to period 2 (29.00 ± 3.25) within our healthcare system. It is interesting to note that healthcare network A had the highest prescription of antifungal agents, while the other healthcare networks had significantly lower prescription ranging from 54% to 78% compared to network A during the entire study period. The relatively high level of antifungal prescription in network A may be attributed to its nature as a tertiary referral center in Hong Kong, which specializes in providing services such as blood and marrow transplant, heart-lung transplant, and liver transplant. However, C. auris outbreaks were reported in two other healthcare networks instead of network A. There seems to be no direct relationship between antifungal prescription and the emergence of C. auris in our healthcare networks. Therefore, infection control continues to play a critical role in preventing the nosocomial transmission of C. auris.35,36
Proactive infection control measures have been adopted in healthcare network A, leading to successful control of nosocomial transmission of epidemiologically important multidrug-resistant organisms and viruses.37–39 Furthermore, healthcare network A has achieved the lowest number of hospital outbreaks in Hong Kong.24 Directly observed hand hygiene remains a key component of these proactive infection control measures, involving the direct delivery of alcohol-based hand rub to conscious hospitalized patients before meals and medication rounds to mitigate the ingestion of pathogens.40–43 While the controversy regarding the air dispersal of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) pathogens has been settled,44,45 with a consensus that SARS-CoV-2 is an airborne pathogen,6,46,47 there is increasing evidence to show that respiratory viruses and bacteria, such as multidrug-resistant Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus, have been found to have the potential for air dispersal.3,5,48 An early study also demonstrated that C. auris could be detected in air samples collected using settle plates around colonized patients during turbulent activities.49 Therefore, as a new component of our proactive infection control measures against nosocomial transmission of C. auris, we have incorporated the use of portable air purifiers in rooms where patients colonized or infected with C. auris are cared for.50 Subsequently, long-range air dispersal of C. auris was documented in one of the two healthcare networks experiencing ongoing outbreaks due to C. auris,51 further emphasizing the importance of improving indoor air quality to mitigate the risk of C. auris transmission.
Recently, C. auris was identified in apples, with approximately 13% of the apples exhibiting colonies of this pathogen on their surfaces. The strains of C. auris found in apples were found to be closely related to strains found in other sources in India, including patients, hospitals, marine environments, and clinical strains from other parts of the world.52 Additionally, C. auris has been isolated from the coastal wetlands of Andaman Islands, India.53 These studies highlight the significance of comprehending how pathogens like C. auris can exist in natural environments and agricultural settings, ultimately affecting human health. It emphasizes the need for a broader perspective on disease transmission and prevention strategies. If the food and environment serve as important sources of this multidrug-resistant organism, it could pose significant challenges in terms of control and containment.
There are several limitations in this study. First, we did not analyze the clinical information of individual cases, which prevented us from assessing the risk factors of patients infected with different Candida species. However, the risk factors for Candida infection have been well documented in various clinical settings.54,55 Second, we did not analyze the trends of antifungal resistance among different Candida species because routine antifungal susceptibility tests were not performed across different healthcare networks in Hong Kong. Consequently, we were unable to study the temporal relationships between antifungal use and the development of antifungal resistance. However, the goal of this study was to analyze the trends of Candida species before and after the emergence of C. auris in Hong Kong, as well as their correlation with the trends of antifungal prescription.
Conclusion
Our findings indicate that despite the increasing trend in Candida species isolation and the prescription of antifungal agents during the study period, there is no direct relationship between the prescription of antifungal agents and outbreaks of C. auris in our healthcare networks. Further investigation is necessary to understand the impact of antifungal stewardship in preventing the emergence of C. auris. Nevertheless, infection control measures are crucial in controlling the nosocomial transmission of C. auris.
Acknowledgments
We are grateful for the contributions of our frontline and laboratory staff in implementing infection control measures and conducting the laboratory work, respectively, at the Hospital Authority.
Funding Statement
This study was partially supported by the Health and Medical Research Fund (HMRF), Commissioned Research on Control of Infectious Disease (Phase IV), CID-HKU1-16, Health Bureau, Hong Kong SAR Government.
Disclosure
Dr Celine Chui reports grants from Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, Amgen; personal fees from Primevigilance, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3:57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong SC, Chan VWM, Lam GK, et al. The use of multi-pronged screening strategy to understand the epidemiology of carbapenemase-producing Enterobacteriaceae in Hong Kong: transition from epidemic to endemic setting. Eur J Clin Microbiol Infect Dis. 2021;40:2017–2022. doi: 10.1007/s10096-021-04173-x [DOI] [PubMed] [Google Scholar]
- 3.Wong SC, Lam GK, Chen JH, et al. Air dispersal of multidrug-resistant Acinetobacter baumannii: implications for nosocomial transmission during the COVID-19 pandemic. J Hosp Infect. 2021;116:78–86. doi: 10.1016/j.jhin.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SC, Chen JH, So SY, Ho PL, Yuen KY, Cheng VC. Gastrointestinal colonization of meticillin-resistant Staphylococcus aureus: an unrecognized burden upon hospital infection control. J Hosp Infect. 2022;121:65–74. doi: 10.1016/j.jhin.2021.12.016 [DOI] [PubMed] [Google Scholar]
- 5.Wong SC, Chan VW, AuYeung CH, et al. Air dispersal of respiratory viruses other than severe acute respiratory coronavirus virus 2 (SARS-CoV-2) and the implication on hospital infection control. Infect Control Hosp Epidemiol. 2023;44:768–773. doi: 10.1017/ice.2022.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SC, Chan VW, Yuen LL, et al. Air dispersal of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): implications for hospital infection control during the fifth wave of coronavirus disease 2019 (COVID-19) due to the SARS-CoV-2 omicron variant in Hong Kong. Infect Control Hosp Epidemiol. 2023;44:1321–1324. doi: 10.1017/ice.2022.258 [DOI] [PubMed] [Google Scholar]
- 7.Cheng VC, Wong LM, Tai JW, et al. Prevention of nosocomial transmission of norovirus by strategic infection control measures. Infect Control Hosp Epidemiol. 2011;32:229–237. doi: 10.1086/658330 [DOI] [PubMed] [Google Scholar]
- 8.Cheng VCC, Wong SC, Chiu KHY, Yip CCY, Wong SCY, Yuen KY. Detection of norovirus in air samples in a non-vomiting patient: implications of testing saliva for norovirus in an immunocompromised host. J Hosp Infect. 2019;103:357–358. doi: 10.1016/j.jhin.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Gold JAW, Adjei S, Gundlapalli AV, et al. Increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020-December 2021. Emerg Infect Dis. 2023;29:1433–1437. doi: 10.3201/eid2907.221771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escribano P, Guinea J. Fluconazole-resistant Candida parapsilosis: a new emerging threat in the fungi arena. Front Fungal Biol. 2022;3:1010782. doi: 10.3389/ffunb.2022.1010782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koulenti D, Karvouniaris M, Paramythiotou E, et al. Severe Candida infections in critically ill patients with COVID-19. J Intensive Med. 2023;3:291–297. doi: 10.1016/j.jointm.2023.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson BM, Dinh AQ, Tran TT, et al. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob Agents Chemother. 2021;65:e0114621. doi: 10.1128/AAC.01146-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khojasteh S, Jafarzdeh J, Hosseini SA, et al. Candida auris and COVID-19: a health threatening combination. Curr Med Mycol. 2022;8:44–50. doi: 10.18502/cmm.8.3.11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong PA, Rivera SM, Escandon P, et al. Hospital-associated multicenter outbreak of emerging fungus candida auris, Colombia, 2016. Emerg Infect Dis. 2019;25:1339–1346. doi: 10.3201/eid2507.180491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biran R, Cohen R, Finn T, et al. Nationwide outbreak of candida auris infections driven by COVID-19 hospitalizations, Israel, 2021-2022. Emerg Infect Dis. 2023;29:1297–1301. doi: 10.3201/eid2907.221888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hospital authority annual report 2015-2016. Available from: https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=235572&Lang=ENG. Accessed October 15, 2023.
- 17.Hospital authority major statistics. Available from: https://www3.ha.org.hk/Data/HAStatistics/MajorReport. Accessed October 15, 2023.
- 18.Cheng VC, Wong SC, So SY, et al. Decreased antibiotic consumption coincided with reduction in bacteremia caused by bacterial species with respiratory transmission potential during the COVID-19 pandemic. Antibiotics. 2022;11:746. doi: 10.3390/antibiotics11060746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong SC, Chau PH, So SY, et al. Control of healthcare-associated carbapenem-resistant Acinetobacter baumannii by enhancement of infection control measures. Antibiotics. 2022;11:1076. doi: 10.3390/antibiotics11081076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SC, Chen JH, Chau PH, et al. Gastrointestinal colonization of carbapenem-resistant Acinetobacter baumannii: what is the implication for infection control? Antibiotics. 2022;11:1297. doi: 10.3390/antibiotics11101297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SC, Chau PH, So SY, et al. Epidemiology of multidrug-resistant organisms before and during COVID-19 in Hong Kong. Infect Prev Pract. 2023;5:100286. doi: 10.1016/j.infpip.2023.100286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan M, Wong SC, Yu Q, et al. Epidemiology and risk factors for carbapenemase-producing Enterobacteriaceae carriage in the hospital: a population-based nested case-control study. J Glob Antimicrob Resist. 2023;33:242–248. doi: 10.1016/j.jgar.2023.03.013 [DOI] [PubMed] [Google Scholar]
- 23.Centre for Health Protection. Alert on the rise in Candida auris colonisation in Hong Kong; 2020. Available from: https://www.chp.gov.hk/files/pdf/lti_c_auris_20201015_eng.pdf. Accessed November 16, 2023.
- 24.Cheng VC, Tai JW, Wong LM, et al. Effect of proactive infection control measures on benchmarked rate of hospital outbreaks: an analysis of public hospitals in Hong Kong over 5 years. Am J Infect Control. 2015;43:965–970. doi: 10.1016/j.ajic.2015.04.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teo JQ, Candra SR, Lee SJ, et al. Candidemia in a major regional tertiary referral hospital - epidemiology, practice patterns and outcomes. Antimicrob Resist Infect Control. 2017;6:27. doi: 10.1186/s13756-017-0184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan BH, Chakrabarti A, Li RY; Asia Fungal Working Group (AFWG), et al. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 21;2015:946–953. doi: 10.1016/j.cmi.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 27.Ricotta EE, Lai YL, Babiker A, et al. Invasive candidiasis species distribution and trends, United States, 2009-2017. J Infect Dis. 2021;223:1295–1302. doi: 10.1093/infdis/jiaa502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes J, Fisher MC. Global epidemiology of emerging Candida auris. Curr Opin Microbiol. 2019;52:84–89. doi: 10.1016/j.mib.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. doi: 10.1186/s13756-016-0132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prestel C, Anderson E, Forsberg K, et al. Candida auris Outbreak in a COVID-19 specialty care unit - Florida, July-august 2020. MMWR Morb Mortal Wkly Rep. 2021;70:56–57. doi: 10.15585/mmwr.mm7002e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen. J Clin Microbiol. 2018;56:e01588–17. doi: 10.1128/JCM.01588-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakrabarti A, Sood P. On the emergence, spread and resistance of Candida auris: host, pathogen and environmental tipping points. J Med Microbiol. 2021;70:001318. doi: 10.1099/jmm.0.001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarma S, Upadhyay S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resist. 2017;10:155–165. doi: 10.2147/IDR.S116229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathadka S, Yan VKC, Neoh CF, et al. Global consumption trend of antifungal agents in humans from 2008 to 2018: data from 65 middle- and high-income countries. Drugs. 2022;82:1193–1205. doi: 10.1007/s40265-022-01751-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabino R, Veríssimo C, Pereira ÁA, Antunes F. Candida auris, an agent of hospital-associated outbreaks: which challenging issues do we need to have in mind? Microorganisms. 2020;8:181. doi: 10.3390/microorganisms8020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad S, Asadzadeh M. Strategies to prevent transmission of candida auris in healthcare settings. Curr Fungal Infect Rep. 2023;17:36–48. doi: 10.1007/s12281-023-00451-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng VC, Chan JF, Wong SC, et al. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J. 2013;126:4504–4509. doi: 10.3760/cma.j.issn.0366-6999.20130741 [DOI] [PubMed] [Google Scholar]
- 38.Cheng VC, Tai JW, Chen JH, et al. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc. 2014;113:734–741. doi: 10.1016/j.jfma.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 39.Cheng VCC, Wong SC, KKW T, Ho PL, Yuen KY. Preparedness and proactive infection control measures against the emerging novel coronavirus in China. J Hosp Infect. 2020;104:254–255. doi: 10.1016/j.jhin.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng VC, Chen JH, Poon RW, et al. Control of hospital endemicity of multiple-drug-resistant Acinetobacter baumannii ST457 with directly observed hand hygiene. Eur J Clin Microbiol Infect Dis. 2015;34:713–718. doi: 10.1007/s10096-014-2281-x [DOI] [PubMed] [Google Scholar]
- 41.Cheng VC, Tai JW, Li WS, et al. Implementation of directly observed patient hand hygiene for hospitalized patients by hand hygiene ambassadors in Hong Kong. Am J Infect Control. 2016;44:621–624. doi: 10.1016/j.ajic.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 42.Cheng VC, Tai JW, Chau PH, et al. Successful control of emerging vancomycin-resistant enterococci by territory-wide implementation of directly observed hand hygiene in patients in Hong Kong. Am J Infect Control. 2016;44:1168–1171. doi: 10.1016/j.ajic.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 43.Cheng VCC, Wong SC, Wong SCY, Yuen KY. Directly observed hand hygiene - from healthcare workers to patients. J Hosp Infect. 2019;101:380–382. doi: 10.1016/j.jhin.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 44.Cheng VCC, Wong SC, Chen JHK, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng VC, Wong SC, Chan VW, et al. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2020;41:1258–1265. doi: 10.1017/ice.2020.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang JW, Marr LC, Tellier R, Dancer SJ. Airborne transmission of respiratory viruses including severe acute respiratory syndrome coronavirus 2. Curr Opin Pulm Med. 2023;29:191–196. doi: 10.1097/MCP.0000000000000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong SC, Yuen LL, Chan VW, et al. Airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): what is the implication of hospital infection control? Infect Control Hosp Epidemiol. 2022;43:1522–1523. doi: 10.1017/ice.2021.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong SC, Chen JH, Yuen LL, et al. Air dispersal of meticillin-resistant Staphylococcus aureus in residential care homes for the elderly: implications for transmission during the COVID-19 pandemic. J Hosp Infect. 2022;123:52–60. doi: 10.1016/j.jhin.2022.02.012 [DOI] [PubMed] [Google Scholar]
- 49.Taori SK, Rhodes J, Khonyongwa K, et al. First experience of implementing Candida auris real-time PCR for surveillance in the UK: detection of multiple introductions with two international clades and improved patient outcomes. J Hosp Infect. 2022;127:111–120. doi: 10.1016/j.jhin.2022.06.009 [DOI] [PubMed] [Google Scholar]
- 50.Wong SC, Yuen LL, Li CK, Kwok MO, Chen JH, Cheng VC. Proactive infection control measures to prevent nosocomial transmission of Candida auris in Hong Kong. J Hosp Infect. 2023;134:166–168. doi: 10.1016/j.jhin.2022.12.020 [DOI] [PubMed] [Google Scholar]
- 51.Didik T, Yau AP, Cheung HL, et al. Long-range air dispersion of Candida auris in a cardiothoracic unit outbreak in Hong Kong. J Hosp Infect. 2023;142:105–114. doi: 10.1016/j.jhin.2023.09.019 [DOI] [PubMed] [Google Scholar]
- 52.Yadav A, Jain K, Wang Y, et al. Candida auris on apples: diversity and clinical significance. mBio. 2022;13:e0051822. doi: 10.1128/mbio.00518-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arora P, Singh P, Wang Y, et al. Environmental isolation of candida auris from the coastal Wetlands of Andaman Islands, India. mBio. 2021;12:e03181–20. doi: 10.1128/mBio.03181-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Zhu R, Luan Z, Ma X. Risk of invasive candidiasis with prolonged duration of ICU stay: a systematic review and meta-analysis. BMJ Open. 2020; 10:e036452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas-Rüddel DO, Schlattmann P, Pletz M, Kurzai O, Bloos F. Risk factors for invasive candida infection in critically Ill patients: a systematic review and meta-analysis. Chest. 2022;161:345–355. doi: 10.1016/j.chest.2021.08.081 [DOI] [PMC free article] [PubMed] [Google Scholar]