Abstract
Background
Paroxysmal nocturnal hemoglobinuria is a rare, acquired disease characterized by hemolytic episodes and associated with significant clinical burden. The introduction of C5 inhibitory monoclonal antibodies (C5i) represented a major breakthrough in PNH treatment, effectively reducing intravascular hemolysis (IVH) but showing limited impact on extravascular hemolysis (EVH). In 2021, the C3 inhibitor pegcetacoplan was approved by EMA and recently reimbursed in Italy, which also has the advantages in the reduction of both IVH and EVH, increasing hemoglobin values and simultaneously improving the quality of life and fatigue of patients. A cost-utility analysis was developed to compare pegcetacoplan to C5i (eculizumab and ravulizumab) in the PNH population who remain anemic after treatment with C5i for at least 3 months.
Materials and Methods
The analysis employed a Markov model with a 5-year time horizon whereby patients can transition among 3 PNH health states, adopting the perspective of the Italian NHS. Efficacy data were sourced from the PEGASUS study, with drug prices reflecting ex-factory costs. Additionally, costs associated with resource utilization, adverse events, and complications were estimated based on outpatient and hospital care rates, excluding indirect expenses. Utility and disutility values related to transfusions were also considered, with pegcetacoplan allowing for dose escalation.
Results
The cumulative cost of treatment per individual patient at 5 years was estimated to be €1,483,454 for pegcetacoplan, €1,585,763 for eculizumab, and €1,574,826 for ravulizumab. Pegcetacoplan demonstrated a superior increase in quality-adjusted life years (QALYs) compared to both eculizumab (0.51 increase) and ravulizumab (0.27 increase). Furthermore, pegcetacoplan showed a reduction in complication management costs (€22,891 less compared to eculizumab and €22,611 less compared to ravulizumab) and lower transfusion-related expenses (€14,147 less than both C5i treatments).
Conclusion
Pegcetacoplan emerged as the dominant strategy in this analysis, being more effective, less expensive and improves quality of life in the analyzed population affected by PNH.
Keywords: PNH, pegcetacoplan, eculizumab, ravulizumab, IVH, EVH, cost-utility analysis, pharmacoeconomics
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and life-threatening hematological disorder resulting from a somatic mutation in hematopoietic stem cells, causing episodes of hemolysis.1
Clinically, PNH is characterized by intravascular hemolysis, hemoglobinuria, bone marrow failure, leukopenia, thrombocytopenia, and episodic crises.2–4 Complications may include renal failure, pulmonary hypertension, and a high risk of progressing to myelodysplastic syndromes.2,3 It is estimated that fatigue and shortness of breath affect approximately 81% and 45% of PNH patients, respectively.2 The international PNH registry has reported that approximately 23% of patients experience PNH-related hospitalizations, mainly associated with red blood cell (RBC) transfusions due to breakthrough haemolysis (BTH).4 Diagnosis is typically achieved through flow cytometry.2
PNH, though uncommon in children, predominantly manifests in individuals between 30 and 42 years of age, affecting both genders equally.5 The global prevalence of the disease is currently unknown.4 PNH incidence is estimated at 1–1.5 cases per million individuals worldwide.3 In the United States of America (USA), the prevalence of PNH is estimated to be approximately 15.9 cases per 1,000,000 persons.5 In the UK, the prevalence is estimated to be 1 in 62,500, whereas incidence 1 in 770,000/year.4,6 The disease occurs more frequently in Asian countries, such as Japan, Korea and China, than in Western ones.3
The median survival of PNH patients increased from approximately 10 years in the 1990s to more than 20 years in the early 2000s.3 If left untreated, the 10-year mortality rate is approximately 24–29%.6,7 Although spontaneous remission is observed, true remission is uncommon and typically occurs after decades of living with the disease. Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potential cure; however, its high-risk nature makes it unsuitable for most classical PNH patients.
The current standard of care for symptomatic patients with classical haemolytic PNH in Italy is monoclonal antibody C5is like eculizumab and ravulizumab. This class of drugs target and inhibit the C5 component of the complement pathway.4 C5i therapies effectively reduce intravascular haemolysis (IVH) and thrombotic risk among most treated patients, which has changed the PNH treatment landscape considerably through improvement of IVH-associated clinical outcomes, overall survival and HRQoL.
Although C5i drugs are very effective, they present some clinical problems including breakthrough IVH and most recipients experience C3-mediated extravascular haemolysis (EVH).6 Proximal complement inhibitors, such as the C3 inhibitor pegcetacoplan, have been developed to address this remaining clinical gap.4 Pegcetacoplan targets C3, the central component in the complement cascade involved in both complement-mediated EVH and IVH, thereby controlling IVH without subsequent increases in EVH. The product is self-administered twice weekly. Pegcetacoplan was approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2021, based on evidence from PEGASUS (NCT03500549), the pivotal Phase 3, prospective, randomized, multicenter, open-label, active-comparator, controlled clinical trial of pegcetacoplan versus eculizumab in adults with PNH and hemoglobin (Hb) levels lower than 10.5 g/dl despite eculizumab therapy.8 In PEGASUS, pegcetacoplan has demonstrated significant improvement compared to eculizumab in the primary endpoint and change in serum Hb levels. In addition, 85% of the patients receiving pegcetacoplan no longer required transfusions compared to 15% of those treated with eculizumab. Moreover, pegcetacoplan was non-inferior to eculizumab in reducing reticulocyte counts, with comparable safety to eculizumab.9 The EMA indication for pegcetacoplan concerns patients who continue to have anemia despite treatment with a C5i for at least 3 months.10,11.
A cost-utility analysis (CUA), based on a Markov model, was developed to compare pegcetacoplan with anti-C5 monoclonal antibodies, ie, eculizumab and ravulizumab, in the treatment of PNH, from the perspective of the Italian National Health Service (in Italian known as Servizio Sanitario Nazionale, SSN).
Materials and Methods
The decision problem in this cost-utility analysis was whether pegcetacoplan plus supportive treatments would be cost-effective in treating adult patients with PNH who are anemic despite treatment with C5i compared to anti-C5 treatments plus supportive treatments.
The model was based on a Markov cohort framework whereby patients transitioned among 3 PNH health states representing Hb level and red blood cell transfusion requirements during the previous 4 weeks (28 days cycle). The model consisted of three health states: Transfusion Avoidance and Hb <10.5 g/dL, Transfusion Avoidance and Hb ≥10.5 g/dL, and Transfusion Required. Hb cut-off at 10.5 g/dL is consistent with inclusion criteria in the PEGASUS clinical trial.
The modelled patient population was the licensed population for pegcetacoplan in the treatment of PNH: adults who continue to have Hb levels <10.5 g/dL despite treatment with eculizumab. The patient characteristics (eg, baseline age, percentage of patients who were female, mean weight, and time since diagnosis) were based on the patients included in the PEGASUS trial.
The model was developed with a 5-year time horizon and with the perspective of the Italian National Health Service (SSN). Discounting rates for both costs and outcomes were set at 3%.
The effectiveness data vs eculizumab were obtained directly from PEGASUS, and the longer term data were extrapolated using parametric curves (beta and gamma distributions). The relative effectiveness data for pegcetacoplan vs ravulizumab were derived from a Matching-Adjusted Indirect Comparison (MAIC), which considered direct evidence from PEGASUS and the 302 study (a non-inferiority trial for eculizumab and ravulizumab)7,12 in order to derive pegcetacoplan-ravulizumab indirect evidence. In this project, we utilized a research methodology based on the development of a simulation model using cost and efficacy data identified from published international literature. As such, there was no need to seek institutional/ethical review and approval for this study.
The prices of the three drugs analyzed in the model were provided by Tunnel®, the software developed by Farmadati Italia. Best supportive care treatments (BSC) included anticoagulant (warfarin), ciclosporin, and androgens. The drugs listed as “supportive treatments” were corticosteroids/immunosuppressants and prophylactic antibiotics. Costs for managing complications, adverse events, blood transfusions and other health costs (general practitioner [GP] visit, hematologist, oncologist, blood test) were included based on NHS (outpatient and inpatient national tariffs) and from the scientific literature. This was a direct cost-only analysis, thus not considering indirect costs (also known as social costs, ie transportation costs, loss of productivity costs due to blood transfusion and intravenous (IV) Infusion, and loss of productivity costs due to fatigue).
Drug cost of supportive treatments was expected to differ by health states, which was estimated through a basket of non-active treatments and drug utilization per health state. Transfusion-related severe acute reactions and related morbidity were analyzed, based on the Italian scenario.
The utility values as a function of the healthy states of the model were obtained from the PEGASUS trial. Since there is no ad hoc scale for PNH, the coefficients were calculated using the EORTC QLQ-30 scale using the Longworth et al algorithm used by the British National Institute for Health and Care Excellence (NICE).13 Disutility of complications that were observed in the trial were not included based on the assumption that such disutility would already be accounted for within mapped utility data from the trial. Probabilities of developing complications were assumed to be the same for all treatments; the included complications were breakthrough hemolysis, thrombosis, acute kidney damage, chronic kidney disease, pulmonary hypertension, and iron overload. Disutility values associated with IV infusion were considered and assumed based on Lloyd et al 201914 and Stoner et al 2015.15 IV infusion costs were also considered. Transfusion-related severe acute reactions morbidity and costs were included.
Sensitivity analysis helps researchers and decision-makers understand the impact of uncertainty on the findings. It allows for a more in-depth exploration of the model’s assumptions and the implications of potential variations in data or parameters. The Cost-Effectiveness Acceptability Curve is a graphical representation that serves as a crucial tool in evaluating the cost-effectiveness of medical interventions graphically. By plotting the probability that a strategy is cost-effective across a range of willingness-to-pay threshold (WPT) values on the graph.
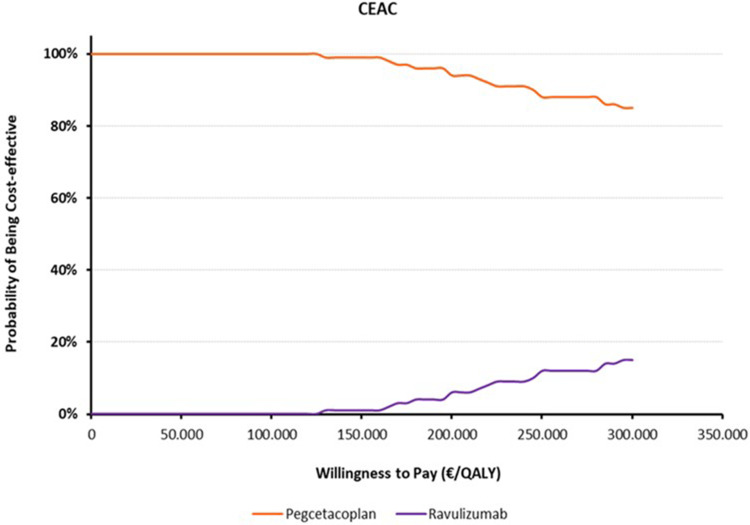
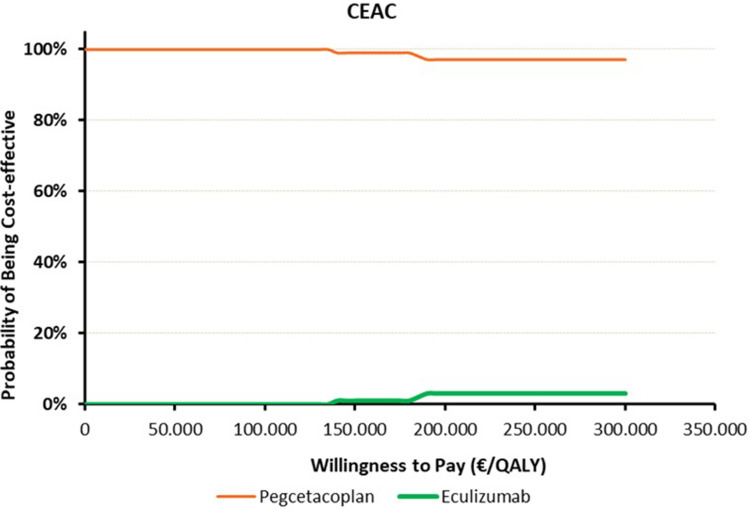
To assess the probability that pegcetacoplan is considered cost-effective compared to ravulizumab (Figure 1) or eculizumab (Figure 2), based on a specific threshold of cost per QALY, different thresholds of cost per QALY gained are represented on the x-axis, indicating the Willingness to Pay. On the y-axis, the probability that pegcetacoplan is considered cost-effective compared to ravulizumab or eculizumab.
Figure 1.
Cost effectiveness acceptability curve (CEAC) – Pegcetacoplan vs Ravulizumab.
Figure 2.
Cost effectiveness acceptability curve (CEAC) – Pegcetacoplan vs Eculizumab.
The ex-factory prices of the included drugs were € 2802.96 for pegcetacoplan, € 4151.50 for eculizumab, and € 4528.91 for ravulizumab; the details were listed in Table S1, whereas Table S2 reported the related dosing regimens. The BSC drugs included (see Table S3) were warfarin (anticoagulant), ciclosporin (immunosuppressive), and danazol (androgens), with prices of € 2.42, € 26.27, and € 3.08, respectively. As far as supportive care is concerned, the drugs considered were prednisone (corticosteroid/immunosuppressant) and ciproxin (antibiotic), with ex-factory prices equal to € 1.36 and € 4.58, respectively. Pack sizes and dosing regimens were listed in Table S4.
The administration cost for the IV infusion of eculizumab and ravulizumab was estimated at € 9.71, as SSN tariffs reported. As for pegcetacoplan, being a self-administered subcutaneous drug, no administration cost was included.
The costs of managing six complications are mentioned prior.
As regards blood transfusions, the cost per transfusion of two blood units was made as follows: cost of two blood units (average cost equal to € 245.88, based on the Italian State-Regions conference tariffs) plus the cost of a single blood transfusion (administration cost), equal to € 25.82 as stated in the SSN tariffs. Thus, the whole cost of a two-unit blood transfusion resulted in € 517.58.
The CUA also considered transfusion-related severe acute reactions and related morbidity, based on the 2022 ISTISAN 22/25 Report “Italian Blood System 2021: activity data, hemovigilance and epidemiological surveillance” by the Italian National Institute of Health (Istituto Superiore di Sanità - ISS).16 (Catalano et al, s.d.)
The costs related to the other resources included in the model are reported in Table S5. Blood test cost included the cost of a complete blood test, consulting a fellow clinician and summing all the test elements provided by SSN tariffs. As a result, the cost for a complete blood test resulted in € 91.29.
As regards the cost of AEs, each one of them was estimated considering SSN tariffs (see Table S6).
Results
As a result, the 5-year total per-patient discounted cost resulted in € 1,483,454 for pegcetacoplan, € 1,585,763 for eculizumab, and € 1,574,826 for ravulizumab (see Table 1).
Table 1.
Final Results – Summary
| Results Summary | Pegcetacoplan | Eculizumab | Ravulizumab | Incremental Costs/QALYs | |
|---|---|---|---|---|---|
| Pegcetacoplan vs Eculizumab | Pegcetacoplan vs Ravulizumab | ||||
| Total discounted costs (€) | 1,483,454 € | 1,585,763 € | 1,574,826 € | −102,309 € | −91,372 € |
| Total discounted QALYs | 3.313 | 2.803 | 3.045 | 0.510 | 0.268 |
| Incremental cost per QALY gained (€/QALY gained) | Dominant | Dominant | |||
Note: Bold text refers to main titles and important results.
As it can be seen in Table 1, the total discounted quality-adjusted life-years (QALYs) was higher with pegcetacoplan, resulting in 3.31 QALYs vs 2.80 with eculizumab, 3.05 with ravulizumab, leading to an increase in QALYs, +0.51 and +0.26 compared to eculizumab and ravulizumab, respectively. Treatment with pegcetacoplan would lead to savings equal to € 102,309 and € 91,372 vs eculizumab and ravulizumab, respectively, in 5 years.
The disaggregate cost of the 5-year analysis are shown in Table 2. The main cost drivers for the significant difference between pegcetacoplan and its competitors were treatment costs, health state costs and AEs costs. The difference was largely due to treatment costs of pegcetacoplan and eculizumab (- € 62,500). Moreover, a reduction in management costs for complications (- € 22,891 vs eculizumab, and - € 22,611 vs ravulizumab) and in blood transfusions (- € 14,147 vs both eculizumab and ravulizumab) was reported. The QALY gains with pegcetacoplan were mainly linked to transfusion avoidance with fewer AEs and no requirement for IV infusion (see Table 3).
Table 2.
Final Results – Details
| Disaggregated Costs (Discounted) | Costs | Incremental Costs | |||
|---|---|---|---|---|---|
| Pegcetacoplan | Eculizumab | Ravulizumab | Pegcetacoplan vs Eculizumab | Pegcetacoplan vs Ravulizumab | |
| Drug costs | 1,456,569 € | 1,518,947 € | 1,509,183 € | −62,378 € | −52,614 € |
| Complement inhibitor (total) | 1,455,724 € | 1,518,223 € | 1,508,460 € | −62,500 € | −52,736 € |
| Supportive treatments | 846 € | 723 € | 723 € | 122 € | 122 € |
| Administration costs | 578 € | 1874 € | 981 € | −1296 € | −403 € |
| Vaccination costs | 0 € | 0 € | 0 € | 0 € | 0 € |
| Health state costs | 22,570 € | 59,608 € | 59,328 € | −37,038 € | −36,758 € |
| Costs of managing complications | 10,452 € | 33,343 € | 33,063 € | −22,891 € | −22,611 € |
| Costs of blood transfusion | 5973 € | 20,120 € | 20,120 € | −14,147 € | −14,147 € |
| Other resource use costs | 6145 € | 6145 € | 6145 € | 0 € | 0 € |
| AE costs | 3737 € | 5334 € | 5334 € | −1597 € | −1597 € |
| Total costs | 1,483,454 € | 1,585,763 € | 1,574,826 € | −102,309 € | −91,372 € |
Note: Bold text refers to main titles and important results.
Table 3.
Utility – Details
| Disaggregated Outcomes (Discounted) | Outcomes | Incremental Outcomes | |||
|---|---|---|---|---|---|
| Pegcetacoplan | Eculizumab | Ravulizumab | Pegcetacoplan vs Eculizumab | Pegcetacoplan vs Ravulizumab | |
| Transfusion avoidance AND Hb <10.5 g/dL | 0.706 | 1.812 | 1.812 | −1.106 | −1.106 |
| Transfusion avoidance AND Hb ≥10.5 g/dL | 2.443 | 0.003 | 0.003 | 2.440 | 2.440 |
| Transfusion required | 0.446 | 1.501 | 1.501 | −1.055 | −1.055 |
| Spontaneous remission | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Disutility associated with complications | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Disutility associated with AEs | −0.202 | −0.246 | −0.246 | 0.044 | 0.044 |
| Disutility associated with IV infusion | −0.080 | −0.267 | −0.025 | 0.187 | −0.055 |
| Total QALYs | 3.313 | 2.803 | 3.045 | 0.510 | 0.268 |
Note: Bold text refers to main titles and important results.
When comparing pegcetacoplan with ravulizumab on the CEAC graph, the data reveals that pegcetacoplan achieves more than 80% of superiority at 300k/QALY. This finding indicates that for each additional QALY gained, pegcetacoplan proves to be considerably more cost-effective than ravulizumab and dominant in 56% of the iterations (Figure 1).
The dominance of pegcetacoplan on the CEAC graph, is even more pronounced when compared to eculizumab than it was with ravulizumab (Figure 2). There is a 100% dominance of pegcetacoplan over eculizumab in 47% of cases, up to a superiority percentage of 97%, even at 300k/QALY.
Discussion
The C3 inhibitor pegcetacoplan was developed and constitute today a significant breakthrough in the therapy of PNH.7,12 The advantages of pegcetacoplan are significant in those patients who present with EVH despite treatment with C5i. The CUA presented here revealed a notable variance in total discounted costs between eculizumab, ravulizumab, and pegcetacoplan. Indeed, pegcetacoplan resulted as the most cost-effective drug, leading to 5-year savings of € 102,309 and € 91,372 vs eculizumab and ravulizumab, respectively. The significant difference in administration costs of the three drugs is noteworthy: as expected, eculizumab resulted as the most expensive treatment due to the required frequency of intravenous infusion. Other relevant advantages of pegcetacoplan come from the reduction in health state costs, in particular concerning costs of complications’ management, and blood transfusion costs. In fact, a significant reduction in blood transfusion costs was reported due to the increase in Hb levels (average Hb level equal to 11.5 g/dL at week-16 in PEGASUS) and transfusion avoidance linked to pegcetacoplan treatment in comparison to eculizumab and ravulizumab, as demonstrated in PEGASUS trial.9 Moreover, the lower occurrence of complications reported with pegcetacoplan, in the study, lead to cost savings contributing to its better cost-effectiveness profile. It should be noted that pegcetacoplan dominates both eculizumab and ravulizumab with greater QALY gain and a reduction in total health care cost.
In other words, pegcetacoplan leads to a better quality of life for patients in comparison to those administered with C5i. The results of the model were confirmed by sensitivity analyses performed, and therefore, were considered robust. The graph of the One-Way Sensitivity Analysis (OWSA) has not been included as pegcetacoplan was found to be dominant so the analysis cannot show bar results.
In the CEAC graph, regardless of the parameter variations in the sensitivity analysis, pegcetacoplan continued to demonstrate a significant superiority over ravulizumab and eculizumab, both in terms of clinical effectiveness and cost. This finding substantiates the significance of pegcetacoplan as the favored therapeutic choice for patients experiencing anemia despite undergoing treatment with a C5 inhibitor.
Following the expiration of the patent for eculizumab, the scenario is also being evaluated with the possible introduction of the same active ingredient on the market at a lower price of 40% compared to the original. Consequently, according to the predictions regarding the entry of the product at the lower price into the market, it is expected to cover the entire portion of the diseased population newly diagnosed cases (incidents) which represents 8% of the entire population. In the conservative case where all the incident patients receive the biosimilar of eculizumab, the dominance of pegcetacoplan is maintained (Table S7).
As a result, pegcetacoplan proved to be cost-effective in comparison to C5i in a cohort of patients affected by PNH who were sub-optimally treated with eculizumab. One of the most significant features of pegcetacoplan lies in its capability to significantly reduce both IVH and EVH, leading to its potential breakthrough role in the treatment of PNH.
These considerations must be properly interpreted as PNH is a rare disease, and each patient is an individual case to be analyzed and studied. PNH requires a treatment personalization approach.
Limitations
This study was based on PEGASUS trial, which included a cohort of patients who were anemic on eculizumab treatment. Therefore, the results of the present analysis refer only to a pre-treated population, excluding the newly diagnosed patients for which C5i are the only therapeutic alternatives currently approved.
In the event of hemolysis in patients who had previously received transfusions, it may be necessary to better phenotyping the presence of secondary antigens and antibodies; thus, the blood unit costs could have been underestimated in this evaluation.
Conclusion
After the approval of the C3 inhibitor pegcetacoplan by the FDA and EMA in 2021, a new era has emerged in the treatment of PNH, thanks to its effectiveness in targeting the complement cascade, thereby controlling IVH without associated increases in EVH. Furthermore, the results of the base-case analysis of the CUA, along with all scenario analyses and sensitivity analyses, revealed gains in QALY and lifetime cost savings for pegcetacoplan compared to ravulizumab and eculizumab. Therefore, by providing improved control of anemia, greater enhancements in HRQoL, and reduced healthcare costs, pegcetacoplan may represent significant clinical and economic value within the PNH treatment pathway. This underscores its potential role in PNH patients in the years to come.
Funding Statement
Sobi, Milan, Italy, provided financial support to cover the cost of this project.
Disclosure
G. L. Colombo, S. Di Matteo, G. M. Bruno, A. Ciccarone and S. Moumene are employees of S.A.V.E. Studi Analisi Valutazioni Economiche Srl that received funding from Sobi, Milan, Italy for this research. N. Martone and C. Teruzzi are employees of Sobi, Milan, Italy. R. Freilone and R. Notaro report consultancy fees from Sobi, Milan, Italy, for contributing to this research. R. Notaro also reports personal fees from Novartis and Alexion, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Colden MA, Kumar S, Munkhbileg B, et al. Insights into the emergence of paroxysmal nocturnal hemoglobinuria. Front Immunol. 2022;12(gennaio):830172. doi: 10.3389/fimmu.2021.830172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merck & Co. MSD manuals professional edition. s.d. Available from: https://www.msdmanuals.com/it-it/professionale. Accessed August 28, 2023.
- 3.Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Prim. 2017;3(1):17028. doi: 10.1038/nrdp.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Fontbrune S, Flore PB, Piggin M, et al. The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors: clinical outcomes and medical encounters from the patient perspective. Hematology. 2022;27(1):1140–1151. doi: 10.1080/16078454.2022.2127630 [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Bhatt EH. Paroxysmal nocturnal hemoglobinuria. National Library of Medicine; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562292/. Accessed March 27, 2024.
- 6.Hakimi Z, Wilson K, McAughey E, et al. The cost–effectiveness, of pegcetacoplan compared with ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria, in a UK setting. J Comp Eff Res. 2022;11(13):969–985. doi: 10.2217/cer-2022-0076 [DOI] [PubMed] [Google Scholar]
- 7.Bhak RH, Mody-Patel N, Baver SB, et al. Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison. Curr Med Res Opin. 2021;37(11):1913–1923. doi: 10.1080/03007995.2021.1971182 [DOI] [PubMed] [Google Scholar]
- 8.FDA approves new treatment for adults with serious rare blood disease | FDA. s.d. Consultato 4 agosto 2023. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-treatment-adults-serious-rare-blood-disease. Accessed March 27, 2024.
- 9.Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi: 10.1056/NEJMoa2029073 [DOI] [PubMed] [Google Scholar]
- 10.Aspaveli | European medicines agency. s.d. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/aspaveli. Accessed August 4th, 2023.
- 11.Drabo EF, Padula WV. Introduction to Markov modeling. In: David B, Logan B, William VP, editors. Handbook of Applied Health Economics in Vaccines. Oxford University Press. © Oxford University Press;2023. doi: 10.1093/oso/9780192896087.003.0022 [DOI] [Google Scholar]
- 12.Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor–experienced adult patients with PNH: the 302 Study. Blood. 2019;133(6):540–549. doi: 10.1182/blood-2018-09-876805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longworth L, Yang Y, Young T, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18(9). doi: 10.3310/hta18090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd AJ, Gallop K, Ali S. PSY9 preference weights for quality-adjusted life-years estimation for treatments of paroxysmal nocturnal hemoglobinuria in the United Kingdom. Value Health. 2019;22(November):S902. doi: 10.1016/j.jval.2019.09.2637 [DOI] [Google Scholar]
- 15.Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2015;8(2):145–153. doi: 10.1007/s40271-014-0075-y [DOI] [PubMed] [Google Scholar]
- 16.Catalano L. Italian blood system 2021: activity data, haemovigilance and epidemiological surveillance. ISTISAN Reports, Available from: www.iss.it/documents/20126/6682486/22-25+web.pdf/192d899e-0afe-c3f7-c52df373a89097e2?t=1667829051240. Accessed March 27, 2024.