Abstract
Acetaminophen is currently the only analgesic considered safe for use throughout pregnancy, but recent studies indicate that prenatal exposure to acetaminophen may be related to poorer neurodevelopmental outcomes. Multiple studies have suggested that it may be associated with attention problems, but few have examined this association by trimester of exposure. The Illinois Kids Development Study is a prospective birth cohort located in east-central Illinois. Exposure data were collected between December 2013 and March 2020, and 535 newborns were enrolled during that period. Mothers reported the number of times they took acetaminophen at six time points across pregnancy. When children were 2, 3, and 4 years of age, caregivers completed the Child Behavior Checklist for ages 1.5-5 years (CBCL). Associations of acetaminophen use during pregnancy with scores on the Attention Problems and ADHD Problems syndrome scales, the Internalizing and Externalizing Behavior composite scales, and the Total Problems score were evaluated. Higher acetaminophen exposure during the second trimester of fetal development was associated with higher Attention Problems, ADHD Problems, Externalizing Behavior, and Total Problems scores at ages 2 and 3. Higher second trimester exposure was only associated with higher Externalizing Behavior and Total Problems scores at 4 years. Higher cumulative exposure across pregnancy was associated with higher Attention Problems and ADHD Problems scores at ages 2 and 3. Findings suggest that prenatal acetaminophen exposure, especially during the second trimester, may be related to problems with attention in early childhood.
Keywords: acetaminophen, paracetamol, early childhood, attention, prenatal, CBCL
1. Introduction
Acetaminophen (paracetamol) is the most common drug ingredient in the United States and currently the only analgesic considered safe to use throughout pregnancy (Servey and Chang, 2014). Previous research has found that 50-65% of pregnant women in North America and Europe report taking acetaminophen, or a medication containing it, at least once during pregnancy (Brandlistuen et al., 2013; Ji et al., 2018; Servey and Chang, 2014; Thorpe et al., 2013a). Due to the lack of clinical trials in pregnant women and the very few reported cases of adverse effects in the developing child, little is known about the safety of acetaminophen use during pregnancy for the developing fetus (Black and Hill, 2003; Cendejas-Hernandez et al., 2022). Acetaminophen is known to cross the placenta (Levy et al., 1975; Nitsche et al., 2017; Thiele et al., 2013), and while some studies have found no relationship of maternal acetaminophen use during pregnancy with neurodevelopmental outcomes (Castro et al., 2022; De Castro et al., 2022; Rebordosa et al., 2009; Smarr et al., 2019; Streissguth et al., 1987; Tovo-Rodrigues et al., 2020), many others suggest there is a relationship (Baker et al., 2022; Blecharz-Klin et al., 2018, 2017, 2016, 2015a, 2015b, 2013; Cendejas-Hernandez et al., 2022; Duesman, 2015; Herrington et al., 2022; Khan et al., 2022; Kwok et al., 2022; Labba et al., 2022; E. Patel et al., 2022; R. Patel et al., 2022; Philippot et al., 2017). Several studies utilizing data from large birth cohorts indicate that acetaminophen use during pregnancy is associated with increases in internalizing and externalizing behaviors (Brandlistuen et al., 2013; Inoue et al., 2020; Trønnes et al., 2020), and in particular, attention-deficit/hyperactivity disorder (ADHD)-related behaviors (Avella-Garcia et al., 2016; Golding et al., 2019; Ji et al., 2020, 2018; Liew et al., 2019, 2016, 2014; Stergiakouli et al., 2016; Thompson et al., 2014; Tovo-Rodrigues et al., 2018; Ystrom et al., 2017). Previous work suggests that prenatal acetaminophen exposure is related to more attention problems (Avella-Garcia et al., 2016; Golding et al., 2019; Liew et al., 2016), impulsive behavior (Avella-Garcia et al., 2016), and activity or hyperactivity (Avella-Garcia et al., 2016; Brandlistuen et al., 2013; Golding et al., 2019; Stergiakouli et al., 2016; Tovo-Rodrigues et al., 2018) in early childhood, and greater risk of ADHD diagnosis (Ji et al., 2020, 2018; Liew et al., 2019, 2014; Ystrom et al., 2017). However, most previous research has relied on maternal self-report of acetaminophen use via interview once or twice during pregnancy and sometimes only after giving birth, requiring mothers to recall acetaminophen use over long periods of time during pregnancy, likely resulting in inaccuracies in reported acetaminophen use. This makes accurately assessing whether prenatal acetaminophen exposure is related to neurodevelopmental problems difficult, as well as whether the timing of exposure is important in the relationship.
The Illinois Kids Development Study (IKIDS) cohort recruited pregnant people during the first trimester of pregnancy, and participants reported acetaminophen use across six discrete periods of pregnancy, allowing for a more accurate evaluation of prenatal acetaminophen exposure and allowing for a closer examination of whether the timing of acetaminophen exposure is important. In this study, the relationship of acetaminophen use during pregnancy with behavior in early childhood was assessed using the Child Behavior Checklist – 1.5-5 years (CBCL)(Achenbach and Rescorla, 2000) when children were 2, 3, and 4 years of age. Children with higher prenatal exposure to acetaminophen were expected to show deficits in attention, and male children were expected to be disproportionately affected. It was also expected that greater exposure during the second trimester would be associated with greater deficits, particularly in male children. The second trimester was expected to be particularly susceptible to impacts of prenatal acetaminophen exposure because acetaminophen has been suggested to act via antiandrogenic action mechanisms(Golding et al., 2019; Hay-Schmidt et al., 2017; Holm et al., 2016; Interrante et al., 2017; Kristensen et al., 2012, 2011; Lind et al., 2017; Mazaud-Guittot et al., 2013), and the second trimester is thought to be the time of peak androgen production by the male fetus (Auyeung et al., 2013).
2. Methods
2.1. Study cohort
IKIDS is a prospective birth cohort located in east-central Illinois, United States. Participants were recruited between December 2013 and March 2020 at two local obstetric clinics and gave birth at two local hospitals. Clinics provided brochures regarding the study to pregnant patients at their first prenatal visit, and patients were asked to complete a reply card indicating their interest in being contacted about participation. Those who indicated interest were contacted by telephone to receive additional information about participation and determine eligibility. Individuals were eligible to participate if they had not yet reached 15 weeks of gestation; did not have a child already participating in IKIDS; resided within a 30-minute drive of the University of Illinois campus; were fluent in English; were between 18 and 40 years of age; not carrying multiples; were willing to provide a fasting blood sample and five urine samples throughout pregnancy; their doctor had not told them they had a high-risk pregnancy; and they planned to remain in the geographical area until the child’s first birthday. Those who opted to participate were enrolled between 8 and 14 weeks of gestation. Demographics, pregnancy and health history, pregnancy symptoms, medication use, and lifestyle factors were collected via interview shortly after enrollment and updated throughout the pregnancy.
2.2. Acetaminophen Use During Pregnancy
At approximately 10-14, 16-18, 22-24, 28-30, and 34-36 weeks of gestation, and within 24 hours of the child’s birth, participants were interviewed about their use of medications during pregnancy. At the first interview (10-14 weeks), participants were asked to list all medications they had used beginning at their estimated conception date through the time of the interview. They were also asked to report their reason for use (indication), the dates they started and stopped taking the medication, and frequency of use. Participants were asked to recall the same information for the period between their last interview and the current one at each subsequent interview. Medications containing acetaminophen as an active ingredient, the temporal period in which they were used and the indication for use were identified from this information. Reported frequency and dates of use were used to calculate the number of times participants took acetaminophen during the first, second, and third trimesters, and cumulative use was the total number of times acetaminophen was taken across all three trimesters.
2.3. Child behavior measures
The CBCL consists of 100 questions divided into two composite scales (internalizing behaviors and externalizing behaviors), both of which are further divided into syndrome subscales. Parents rate each question on a 3-point Likert scale (“not true” = 0 points, “somewhat true” = 1 point, and “very true or often true” = 2 points), and point values are summed to provide scores for both the internalizing and externalizing behavior composite scales and their corresponding syndrome subscales. Higher scores correspond with more problems in the area of behavior addressed by each question, subscale, and composite scale. In these analyses, raw scores on the Attention Problems subscale, the ADHD Problems subscale, and the Externalizing Behaviors scale were used to examine attentional problems in the target age ranges of 26.5-28.5, 36-38, and 46-48 months, or approximately 2, 3, and 4 years, respectively. Raw scores for the Internalizing Behaviors and Total Problems scales were also examined.
The Attention Problems subscale has five questions, and scores can range from 0 to 10. The ADHD Problems subscale has six questions regarding behaviors consistent with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnostic criteria for ADHD (Achenbach and Rescorla, 2000), three of which (items 5, 6, and 59) are also part of the Attention Problems subscale. The Externalizing Behaviors composite score is composed of the sum of the scores on the Attention Problems and Aggressive Behavior subscales. The Aggressive Behavior subscale includes items 8 and 16 which are also part of the ADHD Problems subscale. Externalizing Behavior scores can range from 0 to 48. Similarly, The Internalizing Behavior composite score is composed of the sum of the Emotionally Reactive, Anxious/Depressed, Somatic Complaints, and Withdrawn subscale scores and can range from 0 to 72. The Total Problems score is composed of the sum of all items on the CBCL and ranges from 0 to 200 (Supplemental Figure S1). The Externalizing Behavior, Internalizing Behavior, and Total Problems scores can be converted to a standardized T-score where scores above 70 are considered potentially clinically relevant (Achenbach and Rescorla, 2000). For these analyses, the raw unstandardized scores were used. Raw unstandardized scores were selected over the standardized T-scores in order to examine potential sex-specific associations and because the CBCL manual suggests that using the raw scores is more appropriate for research purposes (Achenbach and Rescorla, 2000). Because the Attention Problems, ADHD Problems, and Externalizing Behavior scales include some of the same items, these measures were not independent of one another, but in the present analysis, they were examined as separate outcomes.
2.4. Covariates
The following sociodemographic factors were considered for inclusion as covariates based on a priori knowledge and using directed acyclic graphs: acetaminophen formulation and indication (i.e., reason for taking acetaminophen); maternal age, parity, education, tobacco smoking and alcohol use during pregnancy; annual household income, whether the mother had ever been diagnosed with ADHD, child gestational age at birth, delivery type, and age at the time of the assessment in months (Supplemental Figure S2). Additionally, mean maternal perceived stress score (PSS) (Cohen et al., 1983) and Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987) scores averaged across pregnancy, infancy, and early childhood were considered, as well as individual scores at the time of each assessment. Maternal verbal IQ and birth weight were not considered due to the high amount of missingness (Tables 1 and 2). Correlations of potential covariates with both exposure and outcome variables were explored, and covariates were selected for inclusion when they were correlated with exposure and at least one CBCL outcome. Child sex was included as a potential modifier in all models. The following covariates were included in all models: child age at the time of assessment in months, gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), annual household income (<$50,000, $50,000-99,999, ≥$100,000), mean PSS and EPDS scores during pregnancy, and indication (pain, illness, or other; for detailed information as to how indications provided by participants were categorized, see Supplemental Table S1).
Table 1.
Parental demographics for all IKIDS participants with an infant enrolled at birth and each subsample who provided CBCL data when children were 26.5-28.5, 36-38, and 46-48 months.
| Parental Demographics | Participants with an infant enrolled in IKIDS (n = 535) |
Participants who completed the CBCL when their child was 26.5-28.5 months (n = 268) |
Participants who completed the CBCL when their child was 36-38 months (n = 240) |
Participants who completed the CBCL when their child was 46-48 months (n = 180) |
p-value† |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Maternal race & ethnicity | 0.99 | ||||
| White, Non-Hispanic | 446 (83.4) | 229 (85.5) | 208 (86.7) | 156 (86.7) | |
| Other | 87 (16.3) | 39 (14.5) | 31 (12.9) | 23 (12.8) | |
| Unknown/Missing | 2 (0.4) | 0 (0,0) | 1 (0.4) | 1 (0.6) | |
| Paternal race & ethnicity | 0.90 | ||||
| White, Non-Hispanic | 437 (81.7) | 233 (86.9) | 207 (86.3) | 156 (86.7) | |
| Other | 96 (17.9) | 35 (13.1) | 32 (13.3) | 23 (12.8) | |
| Unknown/Missing | 2 (0.4) | 0 (0.0) | 1 (0.4) | 1 (0.6) | |
| Maternal marital status | 0.91 | ||||
| Married/Living as married | 496 (92.7) | 252 (94.0) | 229 (95.4) | 170 (94.4) | |
| Separated/Divorced/Widowed/Single | 39 (7.3) | 15 (5.6) | 10 (4.2) | 10 (5.6) | |
| Unknown/Missing | 0 (0.0) | 1 (0.4) | 1 (0.4) | 0 (0.0) | |
| Maternal education | 0.95 | ||||
| < Bachelor's degree | 103 (19.3) | 33 (12.3) | 28 (11.7) | 23 (12.8) | |
| ≥ Bachelor's degree | 432 (80.7) | 235 (87.7) | 212 (88.3) | 157 (87.2) | |
| Paternal education | 0.92 | ||||
| < Bachelor's degree | 158 (29.5) | 58 (21.6) | 55 (22.9) | 42 (23.3) | |
| ≥ Bachelor's degree | 374 (69.9) | 210 (78.4) | 185 (77.1) | 138 (76.7) | |
| Unknown/Missing | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Maternal parity | 0.99 | ||||
| 0 | 275 (51.4) | 152 (56.7) | 127 (52.9) | 97 (53.9) | |
| ≥1 | 259 (48.4) | 116 (43.3) | 112 (46.7) | 82 (45.6) | |
| Missing | 1 (0.2) | 0 (0.0) | 1 (0.4) | 1 (0.6) | |
| Household income | 0.84 | ||||
| $0-$49,999 | 103 (19.3) | 44 (16.4) | 35 (14.6) | 27 (15.0) | |
| $50,000-$99,999 | 256 (47.9) | 124 (46.3) | 120 (50.0) | 91 (50.6) | |
| ≥$100,000 | 172 (32.1) | 100 (37.3) | 84 (35.0) | 62 (34.4) | |
| Unknown/Missing | 4 (0.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| Maternal health insurance | 0.80 | ||||
| Insured | 532 (99.4) | 268 (100.0) | 240 (100.0) | 180 (100.0) | |
| Uninsured | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Mother previously diagnosed with ADHD | 0.93 | ||||
| Yes | 16 (3.0) | 7 (2.6) | 6 (2.5) | 5 (2.8) | |
| No | 519 (97.0) | 261 (97.4) | 234 (97.5) | 175 (97.2) | |
| Maternal tobacco smoking during pregnancy | 0.74 | ||||
| Yes | 25 (4.7) | 7 (2.6) | 10 (4.2) | 7 (3.9) | |
| No | 475 (88.8) | 261 (97.4) | 230 (95.8) | 173 (96.1) | |
| Unknown/Missing | 35 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Maternal alcohol consumption during entire pregnancy | 0.65 | ||||
| Yes | 401 (74.9) | 200 (74.6) | 182 (75.8) | 131 (72.8) | |
| No | 134 (25.1) | 68 (25.4) | 58 (24.2) | 49 (27.2) | |
| Maternal alcohol consumption during 1st trimester | |||||
| Gestational weeks 0-4 | 0.96 | ||||
| None | 320 (59.8) | 163 (60.8) | 144 (60.0) | 114 (63.3) | |
| < 4 drinks/week | 171 (32.0) | 84 (31.3) | 71 (29.6) | 50 (27.8) | |
| 4-10 drinks/week | 37 (6.9) | 17 (6.3) | 21 (8.8) | 15 (8.3) | |
| ≥11 drinks/week | 6 (1.1) | 4 (1.5) | 4 (1.7) | 1 (0.6) | |
| Unknown/Missing | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gestational weeks 5-8 | 0.77 | ||||
| None | 518 (96.8) | 259 (96.6) | 230 (95.8) | 174 (96.7) | |
| < 4 drinks/week | 9 (1.7) | 7 (2.6) | 5 (2.1) | 3 (1.7) | |
| Unknown/Missing | 8 (1.5) | 2 (0.7) | 5 (2.1) | 3 (1.7) | |
| Gestational weeks 9-14 | 0.77 | ||||
| None | 525 (98.1) | 264 (98.5) | 238 (99.2) | 178 (98.9) | |
| < 4 drinks/week | 6 (1.1) | 4 (1.5) | 2 (0.8) | 2 (1.1) | |
| Unknown/Missing | 4 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Maternal age (years) at baseline | 30.34 (4.11) | 30.73 (3.91) | 30.73 (3.78) | 30.58 (3.78) | 0.45 |
| Maternal verbal IQ (PPVTa standardized score) | 107.88 (11.34) | 108.23 (11.35) | 109.27 (11.06) | 109.24 (10.80) | 0.91 |
| Mean maternal stress (PSS-10 b ) score during pregnancy | 11.15 (5.68) | 10.54 (5.46) | 10.37 (5.57) | 10.14 (5.31) | 0.98 |
| Mean maternal stress (PSS-10 c ) score during child's infancy | 10.34 (6.10) | 10.20 (6.03) | 10.04 (6.08) | 10.00 (6.00) | 0.88 |
| Maternal stress (PSS-10) score at time CBCL was completed | -- | 11.48 (6.20) | 11.95 (6.30) | 13.15 (6.35) | 0.01 |
| Mean maternal depression (EPDS d ) score during pregnancy | 4.23 (3.31) | 3.96 (3.11) | 3.87 (3.19) | 3.82 (3.18) | 0.78 |
| Maternal depression (EPDS e ) score during child's infancy | 3.86 (3.47) | 3.83 (3.34) | 3.64 (3.35) | 3.67 (3.25) | 0.99 |
| Mean maternal depression (EPDS) score at time CBCL was completed | -- | 4.34 (3.54) | 4.72 (3.89) | 5.21 (4.11) | 0.13 |
PPVT-IV: Peabody Picture Vocabulary Test - Fourth Edition
PPVT was collected when children were infants. We were unable to collect this for n = 170 participants due to participant missing or not completing the infancy visits at 4-5 months and 7-8 months in person.
Participants completed the Perceived Stress Scale (PSS-10) 1-3 times during pregnancy.
Participants completed the Perceived Stress Scale (PSS-10) 1-3 times while their child was an infant.
Participants completed the Edinburgh Postnatal Depression Scale (EPDS) 1-3 times during pregnancy.
Participants completed the Edinburgh Postnatal Depression Scale (EPDS) 1-3 while their child was an infant.
p-values for categorical variables estimated using Chi-square tests and Wilcoxon-sum rank tests for continuous variables.
Table 2.
Child demographics for children enrolled in IKIDS and each subsample with CBCL data collected when children were 26.5-28.5, 36-38, and 46-48 months.
| Child Demographics | All enrolled infants | Children with CBCL data at 26.5-28.5 months |
Children with CBCL data at 36-38 months |
Children with CBCL data at 36-38 months |
|
|---|---|---|---|---|---|
| (n = 535) | (n = 268) | (n = 240) | (n = 180) | p-value† | |
| N (%) | N (%) | N (%) | N (%) | ||
| Child sex | 0.26 | ||||
| Male | 260 (48.6) | 128 (47.8) | 118 (48.2) | 91 (50.6) | |
| Female | 275 (51.4) | 140 (52.2) | 122 (50.8) | 89 (49.4) | |
| Child race & ethnicity | 0.95 | ||||
| White, Non-Hispanic | 404 (75.5) | 214 (79.9) | 193 (80.4) | 144 (80.0) | |
| Other | 129 (24.1) | 54 (20.1) | 46 (19.2) | 35 (19.4) | |
| Unknown/Missing | 2 (0.4) | 0 (0.0) | 1 (0.4) | 1 (0.6) | |
| Delivery type | 0.86 | ||||
| Vaginal | 365 (68.2) | 191 (71.3) | 169 (70.4) | 124 (68.9) | |
| Cesarean section | 141 (26.4) | 69 (25.7) | 63 (26.3) | 49 (27.2) | |
| Unknown/Missing | 29 (5.4) | 8 (3.0) | 8 (3.3) | 7 (3.9) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Child gestational age at birth (weeks) | 39.29 (1.48) | 39.37 (1.53) | 39.39 (1.53) | 39.42 (1.48) | 0.98 |
| Child weight at birth (kg) | 3.48 (0.43)a | 3.49 (0.43)b | 3.51 (0.44)c | 3.53 (0.44)d | 0.69 |
| Child age at time CBCL was completed (months) | -- | 27.95 (1.02) | 37.52 (0.93) | 48.09 (1.11) | -- |
Child weight at birth missing for n = 92
Child weight at birth missing for n = 46
Child weight at birth missing for n = 39
Child weight at birth missing for n = 29
p-values for categorical variables estimated using Chi-square tests and Wilcoxon-sum rank tests for continuous variables. There were no differences in pairwise comparisons.
2.5. Statistical approach
Multivariate generalized linear regression models were used to evaluate the relationship between the number of times acetaminophen was taken during the first, second, and third trimester, and throughout pregnancy, and raw scores for each continuous outcome at each age. These associations were also investigated using the standardized T-scores as a supplementary analysis. Standardizing the CBCL scores accounts for both child sex and age at the time of the assessment; thus, these were excluded from models in this supplementary analysis. Sensitivity analyses were used to evaluate the impact of several other variables on the associations, specifically: acetaminophen formulation, maternal alcohol use and smoking during pregnancy, maternal verbal IQ, whether the mother had ever been diagnosed with ADHD, and child weight and gestational age at birth. Models that excluded potential leverage points (Cook’s D >0.06) were also examined. Sensitivity analyses including only children who had data available at all time points (n = 149) were also conducted. All statistical analyses were conducted using SAS software, Version 9.4 of the SAS System for Windows (Copyright © 2013, SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Participation and Demographics
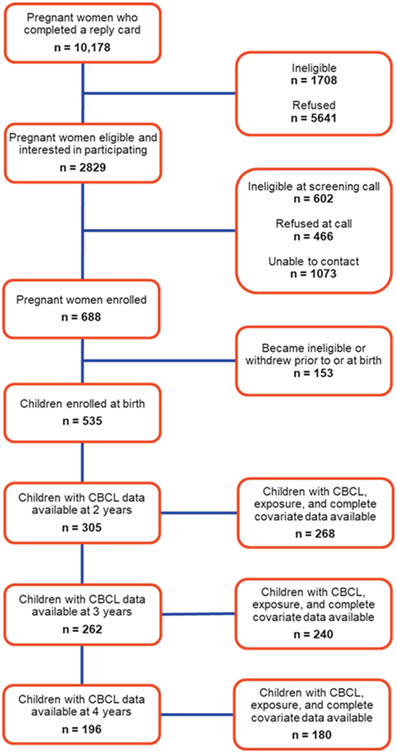
By January 2023, 688 pregnant women had been enrolled in the IKIDS cohort. Of those 688, 153 withdrew or became ineligible before or at the time of birth, resulting in 535 infants born and enrolled in the study. Cohort recruitment and retention is outlined in Figure 1. Demographic information for the 535 mothers with an infant enrolled in IKIDS was largely similar to the demographic information of those who completed the CBCL at 2 (n = 268), 3 (n = 240), or 4 years of age (n = 180). In general, while more parents were white, non-Hispanic, married or living as married, did not use cigarettes during pregnancy, and had attained at least a bachelor’s degree for each subsample compared to the full cohort, the subsamples did not significantly differ from the full cohort or each other (Table 1). Each subsample also had higher maternal verbal IQ scores and lower mean PSS and EPDS scores during pregnancy and infancy, but these were also not significantly different. However, there was an apparent increasing trend in PSS and EPDS scores in the subsample with data available at each age that CBCL data were collected, although this was only significant for the PSS scores. Specifically, the PSS scores collected when children were 2 and 3 years of age did not differ from one another, but both were lower compared to the scores collected when children were 4 (2 vs. 4 years p = 0.01; 3 vs. 4 years p = 0.05). Demographic information for children enrolled in the study is shown in Table 2, and the subsets with data at each age were largely similar to those of the full cohort, although more children in each subset were white, non-Hispanic (Table 2).
Figure 1.
Flowchart of recruitment and retention to IKIDS study visits at 2, 3, and 4 years as of January 2023.
3.2. Acetaminophen Use During Pregnancy
In IKIDS, 70.5% of participants used a medication containing acetaminophen at least once during pregnancyClick or tap here to enter text.. While 58.3% reported use during the first trimester, fewer participants reported use in subsequent trimesters, with 52.9% reporting use in the second trimester, and 36.3% reporting use in the third trimester (Table 3). Of participants reporting any use of medications containing acetaminophen, most reported using acetaminophen itself (76.1%) rather than other medications containing acetaminophen, such as cold medications and multi-drug pain medications (e.g., acetaminophen + aspirin + caffeine and acetaminophen + butalbital), and most took acetaminophen for pain (62.3%). There was no difference in acetaminophen use between the subsets with CBCL data available and the full sample of children enrolled in IKIDS for use during the first (Kruskal-Wallis Test, p=0.99), second (p=0.89), or third trimesters (p=0.96), or throughout pregnancy (p=0.99; Supplemental Figure S3). There were also no differences between the full sample and each subset in acetaminophen formulation used or indication (Supplemental Table S2).
Table 3.
Maternal report of use of medications containing acetaminophen during pregnancy in the IKIDS cohort for all participants with a child enrolled at birth and each subsample with CBCL data available at 26.5-28.5, 36-38, and 46-48 months.
| Participants with an infant enrolled in study (n = 535) |
Participants who completed the CBCL when their child was 26.5-28.5 months (n = 268) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1st Trimester N (%) |
2nd Trimester N (%) |
3rd Trimester N (%) |
Entire Pregnancy N (%) |
1st Trimester N (%) |
2nd Trimester N (%) |
3rd Trimester N (%) |
Entire Pregnancy N (%) |
|
| None | 220 (41.1) | 250 (46.7) | 340 (63.6) | 155 (29.0) | 125 (46.6) | 139 (51.9) | 181 (67.5) | 87 (32.5) |
| Any | 312 (58.3) | 283 (52.9) | 194 (36.3) | 377 (70.5) | 143 (53.4) | 129 (48.1) | 87 (32.5) | 181 (67.5) |
| Missing | 3 (0.6) | 2 (0.4) | 1 (0.2) | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 17.9 (39.7) | 22.0 (44.1) | 15.1 (25.6) | 39.1 (75.1) | 13.7 (26.3) | 20.6 (47.1) | 12.6 (21.9) | 31.6 (67.5) |
| Median (IQR) | 5.0 (14.0) | 7.5 (15.0) | 5.0 (12.0) | 14.0 (31.8) | 5.8 (14.0) | 7.0 (13.0) | 4.0 (10.8) | 12.0 (27.0) |
| Range | 0.5 - 314.0 | 0.5 - 350.0 | 0.5 - 153.0 | 1.0 - 641.0 | 1.0 - 248.5 | 0.5 - 339 | 0.5 - 119.5 | 1.0 - 550.5 |
| Formulation | ||||||||
| Paracetamol alone | 258 (82.7) | 229 (80.9) | 158 (81.4) | 287 (76.1) | 118 (82.5) | 104 (80.6) | 72 (82.8) | 138 (76.2) |
| Acetaminophen in combination | 25 (8.0) | 19 (6.7) | 17 (8.8) | 26 (6.9) | 14 (9.8) | 13 (10.1) | 8 (9.2) | 18 (9.9) |
| with other active ingredients | ||||||||
| Multiple formulations | 29 (9.3) | 35 (12.4) | 19 (9.8) | 64 (17.0) | 11 (7.7) | 12 (9.3) | 7 (8.0) | 25 (13.8) |
| Indication | ||||||||
| Pain | 214 (68.6) | 202 (71.4) | 134 (69.1) | 235 (62.3) | 106 (74.1) | 96 (74.4) | 64 (73.6) | 114 (63.0) |
| Other | 39 (12.5) | 31 (11.0) | 26 (13.4) | 54 (14.3) | 23 (16.1) | 19 (14.7) | 13 (14.9) | 35 (19.3) |
| Multiple reasons given | 27 (8.7) | 28 (9.9) | 19 (9.8) | 61 (16.2) | 14 (9.8) | 11 (8.5) | 9 (10.3) | 31 (17.1) |
| Missing | 32 (10.3) | 22 (7.8) | 15 (7.7) | 27 (7.2) | 0 (0.0) | 3 (2.3) | 1 (1.1) | 1 (0.6) |
| Participants who completed the CBCL when their child was 36-38 months (n = 240) |
Participants who completed the CBCL when their child was 46-48 months (n = 180) |
|||||||
| 1st Trimester N (%) |
2nd Trimester N (%) |
3rd Trimester N (%) |
Entire Pregnancy N (%) |
1st Trimester N (%) |
2nd Trimester N (%) |
3rd Trimester N (%) |
Entire Pregnancy N (%) |
|
| None | 111 (46.3) | 119 (49.6) | 164 (68.3) | 82 (34.2) | 85 (47.2) | 98 (54.4) | 126 (70.0) | 64 (35.6) |
| Any | 129 (53.8) | 121 (50.4) | 76 (31.7) | 158 (65.8) | 95 (52.8) | 82 (45.6) | 54 (30.0) | 116 (64.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 14.3 (29.1) | 23.1 (49.3) | 14.5 (24.9) | 36.3 (73.5) | 16.4 (32.2) | 24.9 (47.2) | 14.3 (23.6) | 37.7 (71.4) |
| Median (IQR) | 4.5 (11.0) | 9.0 (14.0) | 4.0 (11.6) | 13.8 (31.5) | 5.8 (16.5) | 9.0 (16.0) | 5.3 (10.0) | 13.0 (34.5) |
| Range | 1.0 - 248.5 | 0.5 - 339.0 | 0.5 - 119.5 | 1.0 - 550.5 | 1.0 - 248.5 | 0.5 - 302.0 | 0.5 - 119.5 | 1.0 - 550.5 |
| Formulation | ||||||||
| Paracetamol alone | 105 (81.4) | 97 (80.2) | 63 (82.9) | 120 (75.9) | 77 (81.1) | 62 (75.6) | 43 (79.6) | 87 (75.0) |
| Paracetamol in combination | 14 (10.9) | 12 (9.9) | 7 (9.2) | 15 (9.5) | 12 (12.6) | 11 (13.4) | 6 (11.1) | 14 (12.1) |
| with other active ingredients | ||||||||
| Multiple formulations | 10 (7.8) | 12 (9.9) | 6 (7.9) | 23 (14.6) | 6 (6.3) | 9 (11.0) | 5 (9.3) | 15 (12.9) |
| Indication | ||||||||
| Pain | 96 (74.4) | 90 (74.4) | 57 (75.0) | 100 (63.3) | 67 (70.5) | 58 (70.7) | 38 (70.4) | 71 (61.2) |
| Other | 21 (16.3) | 16 (13.2) | 11 (14.5) | 28 (17.7) | 18 (18.9) | 13 (15.9) | 11 (20.4) | 22 (19.0) |
| Multiple reasons given | 12 (9.3) | 12 (9.9) | 6 (7.9) | 29 (18.4) | 10 (10.5) | 9 (11.0) | 4 (7.4) | 22 (19.0) |
| Missing | 0 (0.0) | 3 (2.5) | 2 (2.6) | 1 (0.6) | 0 (0.0) | 2 (2.4) | 1 (1.9) | 1 (0.9) |
3.3. CBCL scores at 2, 3, and 4 years of age
As of April 2023, 305 children had CBCL data available at age 2, 262 at age 3, and 196 at age 4; however, not all parents provided answers to all questions, thus not all children with CBCL data had scores for the outcomes examined in this study (Supplemental Table S3). At the time of the 2-year assessment, children with data ranged in age from 25.67 to 30.90 months with an average age of 27.94 (±0.06) months (Table 2), and there were no differences in scores on any outcomes between males and females (Supplemental Figures S4-S8). Children with data available at the 3-year assessment were between 36.03 and 41.27 months, and on average 37.52 (±0.06) months of age, and there were some differences in scores between male and female children. Male children had higher Attention Problems (p=0.02), ADHD Problems (p=0.01), Externalizing Behavior (p=0.03), and Total Problems scores (p=0.03) compared to female children (Supplemental Figures S4-S6, S8). At the 4-year time point, children were between 46.37 and 58.10 months of age and 48.09 (±0.08) months on average. Male children again had higher Attention Problems (p=0.003), ADHD Problems (p=0.002), and Externalizing Behavior scores (p=0.003), but not Total Problems scores (p=0.18), compared to female children (Supplemental Figures S43-S6). Within each age range, the Attention Problems, ADHD Problems, Externalizing Behavior, and Total Problems scores were strongly correlated (0.60 ≤ ρ ≤ 1.00) with one another, and the Internalizing Behavior score was strongly correlated with only the Total Problems score (Supplemental Table S4). Scores for each outcome across age ranges tended to be moderately-to-strongly correlated (0.40 ≤ ρ ≤ 1.00) with one another (Swinscow, 1997).
3.4. Associations of prenatal acetaminophen exposure with CBCL outcomes
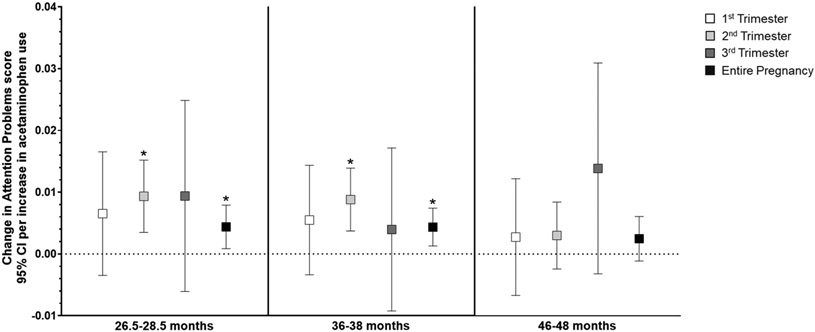
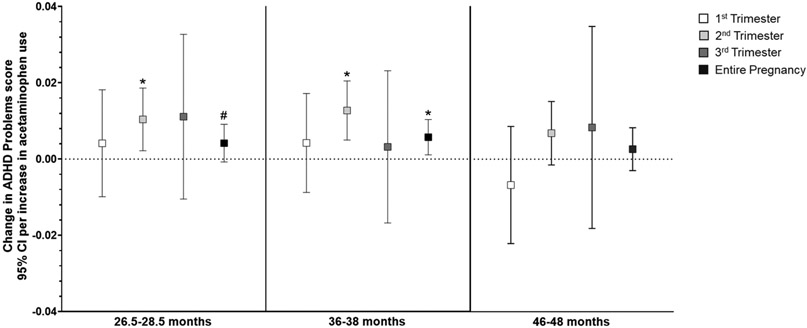
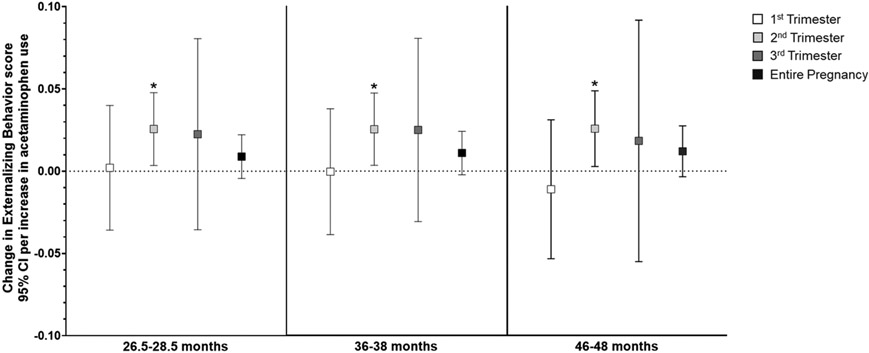
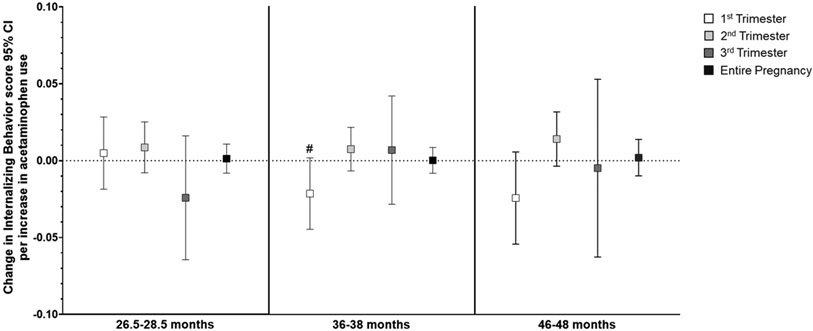
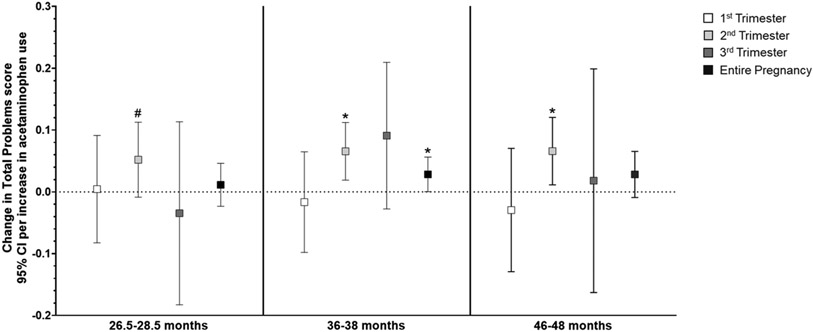
In general, higher acetaminophen exposure during prenatal development was associated with higher scores on attention-related outcomes across all three ages (Supplemental Table S5), and the relationship did not differ by child sex. Per unit increase in acetaminophen use (i.e., report of number of times taking acetaminophen) during the second trimester, there was a small but highly significant increase in Attention Problems scores (range 0-10, Supplemental Table S5) at both 2 (β=0.009, 95% CI: 0.003, 0.015; p=0.002) and 3 years of age (β=0.009, 95% CI: 0.004, 0.014; p=0.001). Increased use throughout pregnancy was also related to slightly higher Attention Problems scores at 2 (β=0.004, 95% CI: 0.001, 0.008; p=0.01) and 3 years (β=0.004, 95% CI: 0.001, 0.007; p=0.006; Figure 2). Similarly, as shown in Figure 3, more acetaminophen use during the second trimester was associated with small increases in ADHD Problems scores (range 0-12) at 2 (β=0.0.010, 95% CI: 0.002, 0.019; p=0.01) and 3 years of age (β=0.013, 95% CI: 0.005, 0.021; p=0.001), as was greater cumulative exposure (2 years: β=0.004, 95% CI: −0.001, 0.01; p=0.09; 3 years: β=0.006, 95% CI: 0.001, 0.010; p=0.01). Higher exposure during the second trimester was associated with higher Externalizing Behavior scores (range 0-48) at 2 (β=0.026, 95% CI: 0.004, 0.048; p=0.02), 3 (β=0.025, 95% CI: 0.003, 0.047; p=0.02), and 4 years of age (β=0.026, 95% CI: 0.003, 0.049; p=0.03; Figure 4). Prenatal acetaminophen exposure was generally not related to Internalizing Problems scores (range 0-72, Figure 5), except for a weak association of higher exposure during the first trimester with lower scores at 3 years (β=−0.021, 95% CI: −0.045, 0.002; p=0.07). There were also increases in Total Problems scores (range 0-200) at 2 (β=0.052, 95% CI: −0.009, 0.113; p=0.09), 3 (β=0.066, 95% CI: 0.019, 0.112; p=0.006), and 4 years (β=0.066, 95% CI: 0.011, 0.121; p=0.02) associated with greater exposure to acetaminophen during the second trimester (Figure 6). Higher cumulative exposure was only significantly associated with an increase in Total Problems scores at 3 years (β=0.028, 95% CI: 0.0002, 0.056; p=0.04). Associations were largely similar when standardized CBCL scores were used instead of raw CBCL scores (Supplemental Figures S9-13), and associations were also generally unchanged in sensitivity analyses (Supplemental Table S6).
Figure 2.
Associations of acetaminophen use during pregnancy and Attention Problems scores on the CBCL at 2 (left panel), 3 (middle panel), and 4 years of age (right panel).a At 2 years of age, higher acetaminophen exposure during the second trimester (β=0.009, 95% CI: 0.003, 0.015) and throughout pregnancy (β=0.004, 95% CI: 0.001, 0.008) were related to higher Attention Problems scores. Higher acetaminophen exposure during the second trimester (β=0.009, 95% CI: 0.004, 0.014) and throughout pregnancy (β=0.004, 95% CI: 0.001, 0.007) were also associated with higher Attention Problems scores at 3 years of age.
aAll models were adjusted for child sex, age at assessment, and gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), mean perceived stress score during pregnancy, mean Edinburgh Postnatal Depression Scale score during pregnancy, and indication (N/A, pain, other, or multiple).
*p < 0.05, **p < 0.01
Figure 3.
Associations of acetaminophen use during pregnancy and ADHD Problems scores on the CBCL at 2 (left panel), 3 (middle panel), and 4 years of age (right panel).a Per unit increase in prenatal acetaminophen exposure during the second trimester (i.e., report of number of times taking acetaminophen), children had a 0.010-point increase (95% CI: 0.002, 0.019) in ADHD Problems scores at age 2. Increasing use throughout pregnancy was also associated with an increase in scores (β=0.004, 95% CI: −0.001, 0.009) at 2 years of age. Similarly, more acetaminophen use during the second trimester (β=0.013, 95% CI: 0.005, 0.021) and throughout pregnancy (β=0.006, 95% CI: 0.001, 0.010) were related to higher ADHD Problems scores at age 3. While the trend was present at 46-48 months as well, neither was p <0.10.
aAll models were adjusted for child sex, age at assessment, and gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), mean perceived stress score during pregnancy, mean Edinburgh Postnatal Depression Scale score during pregnancy, and indication (N/A, pain, other, or multiple).
# p < 0.10, *p < 0.05
Figure 4.
Associations of acetaminophen use during pregnancy and Externalizing Behavior scores on the CBCL at 2 (left panel), 3 (middle panel), and 4 years of age (right panel).a Higher acetaminophen exposure during the second trimester was associated with higher scores at ages 2 (β=0.026, 95% CI: 0.004, 0.048), 3 (β=0.025, 95% CI: 0.003, 0.047), and 4 (β=0.026, 95% CI: 0.003, 0.049).
aAll models were adjusted for child sex, age at assessment, and gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), mean perceived stress score during pregnancy, mean Edinburgh Postnatal Depression Scale score during pregnancy, and indication (N/A, pain, other, or multiple).
* p < 0.05
Figure 5.
Associations of acetaminophen use during pregnancy and Internalizing Behavior scores on the CBCL at ages 2 (left panel), 3 (middle panel), and 4 (right panel).a Higher acetaminophen exposure during the first trimester was associated with lower Internalizing Behavior scores (β=−0.021, 95% CI: −0.045, 0.002).
aAll models were adjusted for child sex, age at assessment, and gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), mean perceived stress score during pregnancy, mean Edinburgh Postnatal Depression Scale score during pregnancy, and indication (N/A, pain, other, or multiple).
# p < 0.10
Figure 6.
Associations of acetaminophen use during pregnancy and Total Problems scores on the CBCL at 2 (left panel), 3 (middle panel), and 4 years of age (right panel).a Higher acetaminophen exposure during the second trimester was related to higher scores at 2 (β=0.052, 95% CI: −0.009, 0.113), 3 (β=0.066, 95% CI: 0.019, 0.112), and 4 years (β=0.066, 95% CI: 0.011, 0.121). Increasing cumulative exposure throughout pregnancy was associated with higher scores only at age 3 (β=0.028, 95% CI: 0.0002, 0.056).
aAll models were adjusted for child sex, age at assessment, and gestational age, maternal age, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), mean perceived stress score during pregnancy, mean Edinburgh Postnatal Depression Scale score during pregnancy, and indication (N/A, pain, other, or multiple).
# p < 0.10, *p < 0.05, **p < 0.01
4. Discussion
In this this largely white, non-Hispanic, highly educated, and high-income cohort, acetaminophen use during pregnancy was higher than previous studies have reported (Brandlistuen et al., 2013; Ji et al., 2018; Servey and Chang, 2014; Thorpe et al., 2013b). In the present analyses, higher prenatal exposure to acetaminophen, particularly during the second trimester of development, was consistently associated with increased (poorer) scores on CBCL outcomes related to attention across early childhood. Specifically, increased exposure during the second trimester was associated with increases in Attention Problems, ADHD Problems, Externalizing Behavior, and Total Problems scores at ages 2 and 3, and Externalizing Behavior and Total Problems scores at age 4. Higher cumulative exposure was also associated with increases in Attention Problems and ADHD Problems scores at 2 and 3 years of age, and Total Problems scores only at age 3. Higher exposure during the first trimester was associated with a small decrease in Internalizing Behavior scores at 3 years, but there was no clear pattern of the timing of exposure or direction of associations across ages for internalizing behaviors. While male children in this cohort had higher scores on the Attention Problems, ADHD Problems, and Externalizing Behavior scores than female children, especially at ages 3 and 4 years, no sex-specific associations with acetaminophen use were observed.
Some previous studies have examined the potential effect of prenatal exposure to acetaminophen on child behavior using the CBCL, but only two have found that acetaminophen was associated with an increase in attention-related outcomes. Similar to the results of the present study, Brandlistuen et al. (2013) found that both short- and long-term acetaminophen use during pregnancy was associated with an increase in Externalizing Behavior scores at 3 years in the Norwegian Mother and Child Cohort (MoBa) Study. When these children were 5 years old, Trønnes et al. (2020) observed that acetaminophen exposure during all three trimesters of prenatal development was related to higher Externalizing Behavior scores. However, Tovo-Rodrigues et al. (2020) found no relationship between any use of acetaminophen during pregnancy and any scores on the CBCL at 48 months in the Pelotas Birth Cohort. Unlike in Tovo-Rodrigues et al. (2020), while there was no relationship of prenatal acetaminophen exposure with Attention Problems, ADHD Problems, or Internalizing Behavior scores in IKIDS at age 4, more use during the second trimester was associated with higher Externalizing Behavior and Total Problems scores. Several other previous studies have found associations of prenatal acetaminophen exposure and attention using other assessment instruments. Other studies have observed an association between prenatal exposure to acetaminophen and increased externalizing behavior at 11 years (Inoue et al., 2020), poorer attention in early childhood (Avella-Garcia et al., 2016; Golding et al., 2019; Liew et al., 2016), and ADHD diagnosis or related behaviors (Ji et al., 2020, 2018; Liew et al., 2019, 2014; Thompson et al., 2014; Ystrom et al., 2017) using other scales and methods, such as the Strengths and Difficulties Questionnaire (SDQ) and medical records.
Previous studies have rarely been able to evaluate the relationship of prenatal acetaminophen exposure with behavioral outcomes by trimester because participants were surveyed only one to three times during pregnancy. In the present study, participants were surveyed about medication use six times during pregnancy; thus, prenatal acetaminophen exposure was likely more accurately measured than previous studies have been able to, and it was possible to examine use by trimester. Despite potential concerns with self-report measures of acetaminophen use during pregnancy leading to recall bias, and the small sample size of this study compared to previous studies, these findings indicate that higher prenatal exposure to acetaminophen during the second trimester as well as cumulative exposure throughout pregnancy was consistently associated with increases in attention-related problems. The results of this study align with the results of several previous studies which observed associations of prenatal exposure to acetaminophen with attention problems and further suggest that the second trimester may be a time period of neurodevelopment that is particularly sensitive to acetaminophen exposure.
Recent evidence indicates the analgesic effect of acetaminophen occurs via the endocannabinoid system. The endocannabinoid system has been shown to play an important role in brain development, playing roles in cell differentiation, migration, and synaptogenesis, in addition to immune regulation via microglia (Bauer et al., 2018). Cannabinoid receptor 1 (CB1R) has been implicated in both inattention and hyperactivity in animal models (Navarrete et al., 2020; Philippot et al., 2017). CB1Rs are expressed early in neurodevelopment with evidence indicating that they may be present in neural tissue as early as approximately gestational week 5 in human fetal development (Buckley et al., 1997; Jutras-Aswad et al., 2009) based on rodent models (Wang et al., 2003), and are functional by gestational week 9 in humans (Bara et al., 2021; Biegon and Kerman, 2001; Jutras-Aswad et al., 2009). Given that CB1Rs are involved in neuronal differentiation, proliferation, and migration (Bara et al., 2021; Levy et al., 1975; Nitsche et al., 2017; Thiele et al., 2013), it may be that prenatal exposure to acetaminophen during this period interferes with the development of the endocannabinoid system in the cerebral cortex, resulting in attentional deficits, such as those observed in the present study. However, further investigation of this potential relationship is needed.
This study has several strengths. First, it uses data from an ongoing prospective birth cohort study, the most robust of the observational epidemiologic study designs; these children continue to be followed, allowing for future studies to determine whether the problems detected in early childhood persist as children age. Second, acetaminophen use was collected multiple times across pregnancy, which both reduced the likelihood of recall bias and allowed for examination of acetaminophen use by trimester. This study also evaluated behavioral outcomes utilizing the same measure across early childhood. Finally, these results add to the limited research on the relationship of prenatal exposures to acetaminophen and developmental outcomes in early childhood, providing direction for future and follow-up studies.
This study also has some limitations. First, many analyses were conducted without correction for multiple comparisons. It has been argued that the focus should be on looking for trends in results to better inform future research in epidemiology (Rothman, 1990), which is the approach that was taken for this study, and findings were consistent across all three ages. Second, this cohort is fairly homogenous, and the participants who continue to participate in the study after the assessments in infancy tend to be more biased towards being white, non-Hispanic, high income and highly educated, potentially limiting the applicability of these findings to the general population. While participants reported medication use several times during pregnancy, it is likely these reports were not entirely accurate due to recall bias. Furthermore, dosage information was not collected, and therefore could not be evaluated in this study. Additionally, because most participants reported taking acetaminophen for pain, this cannot be ruled out as a potential cause for the associations observed in this study. While a few studies have attempted utilizing biomarkers of acetaminophen exposure in maternal urine, maternal plasma, umbilical cord blood, and meconium, these have their own limitations due to the short half-life and rapid elimination of acetaminophen from the human body (Baker et al., 2020; Bauer et al., 2018; Ji et al., 2020, 2018; Laue et al., 2019). A promising potential biomarker that has not yet been investigated is shed teeth. Teeth begin to grow during the second trimester of fetal development, and, due to their growth pattern, have growth rings like those of trees resulting in a timeline of exposures from the time they begin to develop in the prenatal period through when they are shed (Andra et al., 2016, 2015; Arora and Austin, 2013; Petrick et al., 2020; Yu et al., 2021). Temporal exposure to heavy metals in teeth has been examined for decades (Arora and Austin, 2013), but recent technological advances have allowed for measurement of other compounds, including perfluoroalkyl substances, pesticides, phthalates, and lipids (Andra et al., 2016; Petrick et al., 2020; Yu et al., 2021). One previous study found that acetaminophen use during infancy can also be measured in shed teeth (Camann et al., 2012); thus, future studies should investigate deciduous teeth as a biomarker of acetaminophen exposure across prenatal development. In spite of these limitations, the results of the present study indicate that the impact of prenatal acetaminophen exposure on neurodevelopment should be further investigated.
5. Conclusions
Acetaminophen is widely used and generally recognized as safe for use in pregnancy because use at the recommended dosage has not been associated with birth defects seen with maternal use of other classes of medications used for pain relief, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids. However, a growing body of literature suggests use of acetaminophen during pregnancy may be related to poorer neurodevelopmental outcomes, and while the associations in this study were small in magnitude, these results add to the evidence indicating it may be related to deficits in attention. Furthermore, this study suggests that the second trimester of fetal development may be a sensitive window of exposure for acetaminophen exposure. These results can inform future research design to investigate potential mechanisms and examine whether similar results are observed in larger and more diverse cohorts in order to establish whether there is strong causal evidence.
Supplementary Material
Highlights.
Prenatal acetaminophen exposure was related to higher attention-related CBCL scores
More use in especially the second trimester was related to more attention problems
Increases in CBCL scores were small but apparent at 2, 3, and 4 years of age
Acetaminophen use in pregnancy may be related to worse attention in early childhood
Acknowledgements
We would like to thank all IKIDS participants and their families, as well as the study staff for their continued dedication to this study.
Funding
This work was supported by the Children’s Environmental Health and Disease Prevention Research Center funded by the National Institute of Environmental Health Sciences (grant number P01 ES022848) and the U.S. Environmental Protection Agency (grant number RD83543401), and the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) Program (grant number OD023272).
Footnotes
Consent statement
Written informed consent was obtained from participants during pregnancy and at each time of assessment (when children were 2, 3, and 4 years old).
Ethics approval
University of Illinois at Urbana-Champaign Institutional Review Board (IRB) protocol code 09498
References
- Achenbach T, Rescorla L, 2000. Child Behavior Checklist for Ages 1 ½ - 5. University of Vermont, Burlington, VT. [Google Scholar]
- Andra SS, Austin C, Arora M, 2016. The tooth exposome in children’s health research. Curr Opin Pediatr 28, 221–227. 10.1097/MOP.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra SS, Austin C, Arora M, 2015. Tooth matrix analysis for biomonitoring of organic chemical exposure: Current status, challenges, and opportunities. Environ Res 142, 387–406. 10.1016/J.ENVRES.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Austin C, 2013. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr 25, 261–267. 10.1097/MOP.0B013E32835E9084 [DOI] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Baron-Cohen S, 2013. Prenatal and postnatal hormone effects on the human brain and cognition. European Journal of Physiology 465, 557–571. 10.1007/s00424-013-1268-2 [DOI] [PubMed] [Google Scholar]
- Avella-Garcia CB, Julvez J, Fortuny J, Rebordosa C, García-Esteban R, Riañ O Galá I, Tardó A, Rodríguez-Bernal CL, Iñ C, Andiarena A, Santa-Marina L, Sunyer J, Galán IR, Tardón A, Rodríguez-Bernal CL, Iñiguez C, Andiarena A, Santa-Marina L, Sunyer J, 2016. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol 45, dyw115. 10.1093/ije/dyw115 [DOI] [PubMed] [Google Scholar]
- Baker BH, Lugo-Candelas C, Wu H, Laue HE, Boivin A, Gillet V, Aw N, Rahman T, Lepage JF, Whittingstall K, Bellenger JP, Posner J, Takser L, Baccarelli AA, 2020. Association of prenatal acetaminophen exposure measured in meconium with risk of attention-deficit/hyperactivity disorder mediated by frontoparietal network brain connectivity. JAMA Pediatr 174, 1073–1081. 10.1001/jamapediatrics.2020.3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BH, Rafikian EE, Hamblin PB, Strait MD, Yang M, Pearson BL, 2022. Sex-specific neurobehavioral and prefrontal cortex gene expression alterations following developmental acetaminophen exposure in mice. Neurobiol Dis 177. 10.1016/J.NBD.2022.105970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara A, Ferland JMN, Rompala G, Szutorisz H, Hurd YL, 2021. Cannabis and synaptic reprogramming of the developing brain. Nature Reviews Neuroscience 2021 22:7 22, 423–438. 10.1038/S41583-021-00465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AZ, Kriebel D, Herbert MR, Bornehag C-G, Swan SH, 2018. Prenatal paracetamol exposure and child neurodevelopment: A review. Horm Behav 101, 125–147. 10.1016/J.YHBEH.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Biegon A, Kerman IA, 2001. Autoradiographic Study of Pre- and Postnatal Distribution of Cannabinoid Receptors in Human Brain. Neuroimage 14, 1463–1468. 10.1006/NIMG.2001.0939 [DOI] [PubMed] [Google Scholar]
- Black RA, Hill DA, 2003. Over-the-counter medications in pregnancy. Am Fam Physician 67, 2517–2524. [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna K, Pyrzanowska J, Piechal A, Wawer A, Widy-Tyszkiewicz E, 2015a. Effect of prenatal and early life paracetamol exposure on the level of neurotransmitters in rats—Focus on the spinal cord. International Journal of Developmental Neuroscience 47, 133–139. 10.1016/j.ijdevneu.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna K, Pyrzanowska J, Piechal A, Wawer A, Widy-Tyszkiewicz E, 2015b. Developmental exposure to paracetamol causes biochemical alterations in medulla oblongata. Environ Toxicol Pharmacol 40, 369–374. 10.1016/J.ETAP.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Joniec-Maciejak I, Jawna-Zboińska K, Pyrzanowska J, Piechal A, Wawer A, Widy-Tyszkiewicz E, Jawna-Zboi Nska K, Pyrzanowska J, Piechal A, Wawer A, Widy-Tyszkiewicz E, 2016. Cerebellar level of neurotransmitters in rats exposed to paracetamol during development. Pharmacological Reports 68, 1159–1164. 10.1016/j.pharep.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Jawna-Zboińska K, Pyrzanowska J, Wawer A, Joniec-Maciejak I, Widy-Tyszkiewicz E, Jawna-Zboí Nska K, Pyrzanowska J, Wawer A, Joniec-Maciejak I, Widy-Tyszkiewicz E, Jawna-Zboińska K, Pyrzanowska J, Wawer A, Joniec-Maciejak I, Widy-Tyszkiewicz E, 2017. Paracetamol – Effect of early exposure on neurotransmission, spatial memory and motor performance in rats. Behavioural Brain Research 323, 162–171. 10.1016/j.bbr.2017.01.051 [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Pyrzanowska J, Joniec-Maciejak I, Kiliszek P, Widy-Tyszkiewicz E, 2013. Paracetamol—The outcome on neurotransmission and spatial learning in rats. Behavioural Brain Research 253, 157–164. 10.1016/J.BBR.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Wawer A, Jawna-Zboińska K, Pyrzanowska J, Piechal A, Mirowska-Guzel D, Widy-Tyszkiewicz E, 2018. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats. Pharmacol Biochem Behav 168, 25–32. 10.1016/j.pbb.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H, 2013. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol 42, 1702–1713. 10.1093/ije/dyt183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, Hansson S, Harta G, Mezey É, 1997. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 82, 1131–1149. 10.1016/S0306-4522(97)00348-5 [DOI] [PubMed] [Google Scholar]
- Camann DE, Schultz ST, Yau AY, Heilbrun LP, Zuniga MM, Palmer RF, Miller CS, 2012. Acetaminophen, pesticide, and diethylhexyl phthalate metabolites, anandamide, and fatty acids in deciduous molars: potential biomarkers of perinatal exposure. Journal of Exposure Science & Environmental Epidemiology 2013 23:2 23, 190–196. 10.1038/JES.2012.71 [DOI] [PubMed] [Google Scholar]
- Castro CT, Gama RS, Pereira M, Oliveira MG, Dal-Pizzol TS, Barreto ML, Santos DB, 2022. Effect of Acetaminophen use during pregnancy on adverse pregnancy outcomes: a systematic review and meta-analysis. Expert Opin Drug Saf 21, 241–251. 10.1080/14740338.2022.2020246 [DOI] [PubMed] [Google Scholar]
- Cendejas-Hernandez J, Sarafian JT, Lawton VG, Palkar A, Anderson LG, Larivière V, Parker W, Lariviere V, Parker W, 2022. Paracetamol (acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: A systematic review with citation tracking. Eur J Pediatr. 10.1007/S00431-022-04407-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression scale. British Journal of Psychiatry 150, 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- De Castro CT, Pereira M, Dos Santos DB, 2022. Association between paracetamol use during pregnancy and perinatal outcomes: Prospective NISAMI cohort. PLoS One 17, e0267270. 10.1371/JOURNAL.PONE.0267270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesman SJ, 2015. The effects of maternal acetaminophen exposure on the development of ADHD-like behaviors in rat offspring. Western Illinois University. [Google Scholar]
- Golding J, Gregory S, Clark R, Ellis G, Iles-Caven Y, Northstone K, Iles-Caven Y, Northstone K, Iles-Caven Y, Northstone K, 2019. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: A longitudinal cohort study. Paediatr Perinat Epidemiol 1–10. 10.1111/ppe.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A, Finkielman OTEE, Jensen BAHH, Høgsbro CF, Bak Holm J, Johansen KH, Jensen TK, Andrade AM, Swan SH, Bornehag C-GG, Brunak S, Jegou B, Kristiansen K, Kristensen DM, Ejlstrup Finkielman OT, Jensen BAHH, Høgsbro CF, Holm JB, Johansen KH, Jensen TK, Andrade AM, Swan SH, Bornehag C-GG, Brunak S, Jegou B, Kristiansen K, Kristensen DM, Finkielman OTEE, Jensen BAHH, Høgsbro CF, Holm JB, Johansen KH, Jensen TK, Andrade AM, Swan SH, Bornehag C-GG, Brunak S, Jegou B, Kristiansen K, Kristensen DM, 2017. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction 154, 145–152. 10.1530/REP-17-0165 [DOI] [PubMed] [Google Scholar]
- Herrington JA, Guss Darwich J, Harshaw C, Brigande AM, Leif EB, Currie PJ, 2022. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev Psychobiol 64. 10.1002/DEV.22252 [DOI] [PubMed] [Google Scholar]
- Holm JB, Mazaud-Guittot S, Danneskiold-Samsøe NB, Chalmey C, Jensen B, Nørregård MM, Hansen CH, Styrishave B, Svingen T, Vinggaard AM, Koch HM, Bowles J, Koopman P, Jégou B, Kristiansen K, Kristensen DM, 2016. Intrauterine Exposure to Paracetamol and Aniline Impairs Female Reproductive Development by Reducing Follicle Reserves and Fertility. Toxicological Sciences 150, 178–189. 10.1093/toxsci/kfv332 [DOI] [PubMed] [Google Scholar]
- Inoue K, Ritz B, Ernst A, Tseng W-L, Yuan Y, Meng Q, Ramlau-Hansen CH, Strandberg-Larsen K, Arah OA, Obel C, Li J, Olsen J, Liew Z, 2020. Behavioral problems at age 11 years after prenatal and postnatal exposure to acetaminophen: Parent-reported and self-reported outcomes. Am J Epidemiol 190, 1009–1020. 10.1093/aje/kwaa257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interrante JD, Ailes EC, Lind JN, Anderka M, Feldkamp ML, Werler MM, Taylor LG, Trinidad J, Gilboa SM, Broussard CS, Epidemiol Author manuscript A, 2017. Risk comparison for prenatal use of analgesics and selected birth defects, National Birth Defects Prevention Study 1997-2011 HHS Public Access Author manuscript. Ann Epidemiol 27, 645–653. 10.1016/j.annepidem.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, Riley A, Pearson C, Zuckerman B, Wang X, 2020. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry 77, 180–189. 10.1001/jamapsychiatry.2019.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Riley AW, Lee L-C, Hong X, Wang G, Tsai H-J, Mueller NT, Pearson C, Thermitus J, Panjwani A, Ji H, Bartell TR, Burd I, Fallin MD, Wang X, 2018. Maternal biomarkers of acetaminophen use and offspring attention deficit hyperactivity disorder. Brain Sci 8. 10.3390/brainsci8070127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL, 2009. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. European Archives of Psychiatry and Clinical Neuroscience 2009 259:7 259, 395–412. 10.1007/S00406-009-0027-Z [DOI] [PubMed] [Google Scholar]
- Khan FY, Kabiraj G, Ahmed MA, Adam M, Mannuru SP, Ramesh V, Shahzad A, Chaduvula P, Khan S, 2022. A Systematic Review of the Link Between Autism Spectrum Disorder and Acetaminophen: A Mystery to Resolve. Cureus 14. 10.7759/CUREUS.26995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Hass U, Lesné L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, Boberg J, Petersen JH, Toppari J, Jensen TK, Brunak S, Skakkebæk NE, Nellemann C, Main KM, Jégou B, Leffers H, 2011. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Human Reproduction 26, 235–244. 10.1093/humrep/deq323 [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Lesné L, Le Fol V, Desdoits-Lethimonier C, Dejucq-Rainsford N, Leffers H, Jégou B, 2012. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl 35, 377–384. 10.1111/j.1365-2605.2012.01282.x [DOI] [PubMed] [Google Scholar]
- Kwok J, Luedecke E, Hall HA, Murray AL, Auyeung B, 2022. Analgesic drug use in pregnancy and neurodevelopment outcomes: an umbrella review. Neurosci Biobehav Rev 136. 10.1016/J.NEUBIOREV.2022.104607 [DOI] [PubMed] [Google Scholar]
- Labba NA, Wæhler HA, Houdaifi N, Zosen D, Haugen F, Paulsen RE, Hadera MG, Eskeland R, 2022. Paracetamol perturbs neuronal arborization and disrupts the cytoskeletal proteins SPTBN1 and TUBB3 in both human and chicken in vitro models. Toxicol Appl Pharmacol 449. 10.1016/J.TAAP.2022.116130 [DOI] [PubMed] [Google Scholar]
- Laue HE, Cassoulet R, Abdelouahab N, Serme-Gbedo YK, Desautels A-S, Brennan KJM, Bellenger J-P, Burris HH, Coull BA, Weisskopf MG, Takser L, Baccarelli AA, 2019. Association between meconium acetaminophen and childhood neurocognitive development in GESTE, a Canadian cohort study. Toxicological Sciences 167, 138–144. 10.1093/toxsci/kfy222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Garrettson LK, Soda DM, 1975. Letter: Evidence of placental transfer of acetaminophen. Pediatrics 55, 895. [PubMed] [Google Scholar]
- Liew Z, Bach CC, Asarnow RF, Ritz B, Olsen J, 2016. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int J Epidemiol 45, dyw296. 10.1093/ije/dyw296 [DOI] [PubMed] [Google Scholar]
- Liew Z, Kioumourtzoglou M-A, Roberts AL, O’Reilly ÉJ, Ascherio A, Weisskopf MG, 2019. Use of negative control exposure analysis to evaluate confounding: An example of acetaminophen exposure and attention-deficit/hyperactivity disorder in Nurses’ Health Study II. Am J Epidemiol 188, 768–775. 10.1093/aje/kwy288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J, 2014. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr 168, 313. 10.1001/jamapediatrics.2013.4914 [DOI] [PubMed] [Google Scholar]
- Lind DV, Main KM, Kyhl HB, Kristensen DM, Toppari J, Andersen HR, Andersen MS, Skakkebæk NE, Jensen TK, 2017. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: A cohort study of 1027 mother-child pairs. Human Reproduction 32, 223–231. 10.1093/humrep/dew285 [DOI] [PubMed] [Google Scholar]
- Mazaud-Guittot S, Nicolaz CN, Desdoits-Lethimonier C, Coiffec I, Maamar M. Ben, Balaguer P, Kristensen DM, Chevrier C, Lavoué V, Poulain P, Dejucq-Rainsford N, Jégou B, 2013. Paracetamol, Aspirin, and Indomethacin Induce Endocrine Disturbances in the Human Fetal Testis Capable of Interfering With Testicular Descent. J Clin Endocrinol Metab 98, E1757–E1767. 10.1210/jc.2013-2531 [DOI] [PubMed] [Google Scholar]
- Navarrete F, García-Gutiérrez MS, Jurado-Barba R, Rubio G, Gasparyan A, Austrich-Olivares A, Manzanares J, 2020. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front Psychiatry 0, 315. 10.3389/FPSYT.2020.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche JF, Patil AS, Langman LJ, Penn HJ, Derleth D, Watson WJ, Brost BC, 2017. Transplacental passage of acetaminophen in term pregnancy. Am J Perinatol 34, 541–543. 10.1055/s-0036-1593845 [DOI] [PubMed] [Google Scholar]
- Patel E, Jones III JP, Bno-Lunn D, Kuchibhatla M, Palkar A, Cendejas-Hernandez J, Sarafian JT, Lawton VG, Anderson LG, Konoula Z, Reissner KJ, Parker W, 2022. The safety of pediatric use of paracetamol (acetaminophen): a narrative review of direct and indirect evidence. Minerva pediatrics 74. 10.23736/S2724-5276.22.06932-4 [DOI] [PubMed] [Google Scholar]
- Patel R, Sushko K, van den Anker J, Samiee-zafarghandy S, 2022. Long-Term Safety of Prenatal and Neonatal Exposure to Paracetamol: A Systematic Review. Int J Environ Res Public Health 19. 10.3390/IJERPH19042128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick LM, Arora M, Niedzwiecki MM, 2020. Minimally Invasive Biospecimen Collection for Exposome Research in Children’s Health. Curr Environ Health Rep 7, 198–210. 10.1007/S40572-020-00277-2/TABLES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot G, Gordh T, Fredriksson A, Viberg H, 2017. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. Journal of Applied Toxicology 37, 1174–1181. 10.1002/jat.3473 [DOI] [PubMed] [Google Scholar]
- Rebordosa C, Kogevinas M, Bech BH, Sørensen HT, Olsen J, Sorensen HT, Olsen J, Sørensen HT, Olsen J, 2009. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes 38, 706–714. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
- Servey J, Chang J, 2014. Over-the-counter medications in pregnancy. Am Fam Physician 90, 548–555. [PubMed] [Google Scholar]
- Smarr MM, Bible J, Gerlanc N, Buck Louis GM, Bever A, Grantz KL, 2019. Comparison of fetal growth by maternal prenatal acetaminophen use. Pediatr Res 86, 261–268. 10.1038/S41390-019-0379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Thapar A, Smith GD, Davey Smith G, Smith GD, 2016. Association of acetaminophen use during pregnancy with behavioral problems in childhood: Evidence against confounding. JAMA Pediatr 170, 964–970. 10.1001/jamapediatrics.2016.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Treder RP, Barr HM, Shepard TH, Bleyer WA, Sampson PD, Martin DC, 1987. Aspirin and acetaminophen use by pregnant women and subsequent child IQ and attention decrements. Teratology 35, 211–219. 10.1002/tera.1420350207 [DOI] [PubMed] [Google Scholar]
- Swinscow TDV, 1997. Correlation and regression, in: Campbell MJ (Ed.), Statistics at Square One. BMJ Publishing Group, London, United Kingdom, pp. 75–85. [Google Scholar]
- Thiele K, Kessler T, Arck P, Erhardt A, Tiegs G, 2013. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. J Reprod Immunol 97, 128–139. 10.1016/J.JRI.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Thompson JMD, Waldie KE, Wall CR, Murphy R, Mitchell EA, Group, the A. study, Mitchell EA, Thompson JMD, 2014. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One 9, e108210. 10.1371/journal.pone.0108210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA, 2013a. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf 22, 1013–1018. 10.1002/pds.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA, 2013b. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf 22, 1013–1018. 10.1002/pds.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovo-Rodrigues L, Carpena MX, Martins-Silva T, Santos IS, Anselmi L, Barros AJD, Barros FC, Bertoldi AD, Matijasevich A, 2020. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development. Paediatr Perinat Epidemiol 34, 278–286. 10.1111/ppe.12649 [DOI] [PubMed] [Google Scholar]
- Tovo-Rodrigues L, Schneider BC, Martins-Silva T, Del-Ponte B, Loret de Mola C, Schuler-Faccini L, Sales Luiz Vianna F, Munhoz TN, Entiauspe L, Silveira MF, Santos IS, Matijasevich A, Barros AJD, Rohde LA, Dâmaso Bertoldi A, Vianna FSL, Munhoz TN, Entiauspe L, Silveira MF, Santos IS, Matijasevich A, Barros AJD, Rohde LA, Bertoldi AD, 2018. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas Birth Cohort. BMC Psychiatry 18, 368. 10.1186/s12888-018-1942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trønnes JN, Wood M, Lupattelli A, Ystrom E, Nordeng H, 2020. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool-aged children. Paediatr Perinat Epidemiol 34, 247–256. 10.1111/ppe.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL, 2003. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694. 10.1016/S0306-4522(03)00020-4 [DOI] [PubMed] [Google Scholar]
- Ystrom E, Gustavson K, Brandlistuen RE, Knudsen GP, Magnus P, Susser E, Smith GD, Stoltenberg C, Surén P, Håberg SE, Hornig M, Lipkin WI, Nordeng H, Reichborn-Kjennerud T, 2017. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics 140, 20163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Tu P, Dolios G, Dassanayake PS, Volk H, Newschaffer C, Fallin MD, Croen L, Lyall K, Schmidt R, Hertz-Piccioto I, Austin C, Arora M, Petrick LM, 2021. Tooth biomarkers to characterize the temporal dynamics of the fetal and early-life exposome. Environ Int 157, 106849. 10.1016/J.ENVINT.2021.106849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.