CASE PRESENTATION
A 3-week-old full-term female was referred to hepatology for evaluation of acholic stools. Physical examination was notable for scleral icterus, a palpable liver edge with the absence of splenomegaly, and pale-appearing stools. There were no dysmorphic features noted on the exam. The infant had demonstrated adequate weight gain and growth since birth. Mother received routine prenatal care, and maternal serologies (including HIV, syphilis, rubella, hepatitis B, chlamydia, and gonorrhea) were negative. There was no family history of consanguinity, early/unexplained death, or gastrointestinal disease. The infant was exclusively breastfed. Upon review of prior medical records, the infant was found to have a total bilirubin level of 5.9 mg/dL with a conjugated bilirubin of 1 mg/dL obtained at 3 days of life.
Question 1: What is the most appropriate next step in caring for this patient?
Recommend stopping breast feeding and beginning formula supplementation
Check hepatic function panel
Obtain an abdominal ultrasound
Check thyroid function tests
Offer reassurance at this time
Further laboratory testing was significant for a total bilirubin level of 7.1 mg/dL with a conjugated value of 5.9 mg/dL and an elevated gamma-glutamyl transferase of 358 U/L. Matrix metalloproteinase-7 (MMP-7) was also elevated at 119 ng/mL. An abdominal ultrasound with Doppler revealed the presence of a small, contracted gallbladder and patent hepatic vasculature without evidence of hepatosplenomegaly or biliary obstruction. The results of further testing are detailed in Table 1.
TABLE 1.
Summary of patient evaluation and testing results
| Assessment | Result | Interpretation |
|---|---|---|
| Laboratory tests | ||
| Hepatic panel | ||
| AST | 78 U/L | Elevated |
| ALT | 67 U/L | Elevated |
| Total bilirubin | 7.1 mg/dL | Elevated |
| Direct bilirubin | 5.9 mg/dL | Elevated |
| GGT | 358 U/L | Elevated |
| MMP-7 | 119 ng/mL | Elevated |
| INR | 1.0 | Normal |
| Thyroid studies | ||
| TSH | 5.51 mIU/L | Normal |
| Free T4 | 1.3 ng/dL | Normal |
| Newborn screen | Negative | Normal |
| CMV PCR | Undetectable | Negative |
| Alpha-1 antitrypsin | Phenotype MM | Normal |
| Imaging | ||
| Abdominal ultrasound | Small gallbladder, normal hepatic size, contour, and echogenicity. Patent vasculature. No evidence of biliary duct dilation | Nondiagnostic |
| PTCC | Filling of the gallbladder and common bile duct. The common bile duct empties freely into the duodenum. Intrahepatic bile ducts are not visualized despite provocative maneuvers. | Findings consistent with biliary atresia |
| Intraoperative cholangiogram | Patent but small distal common bile duct with filling of the duodenum but no filling of the common hepatic duct | Diagnostic of biliary atresia |
| Histology | ||
| Percutaneous liver biopsy (23 d of life) | Obstructive cholestasis associated with mild bile duct injury, fibrous expansion of the portal tracts, and focal septal formation | Findings consistent with biliary atresia |
| Intraoperative liver biopsy (26 d of life) | Contracted hepatic ducts characterized by single major lumen partially lined by attenuated epithelium with epithelial injury and degenerative changes, surrounded by periductal fibrosis, prominent acute and chronic inflammatory infiltrates, and scattered diminutive biliary structures | Findings consistent with biliary atresia |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; CMV, cytomegalovirus; GGT, gamma-glutamyl transferase; MMP-7, matrix metalloproteinase-7; PTCC, percutaneous transhepatic cholecysto-cholangiography; T4, thyroxine; TSH, thyroid-stimulating hormone.
Question 2: Which of the following is diagnostic of biliary atresia?
Histologic findings of bile duct proliferation
Abdominal ultrasound
Intraoperative cholangiogram
Elevated MMP-7
HIDA scan demonstrating absence of excretion into the small bowel
Given the laboratory findings of cholestasis, with elevated gamma-glutamyl transferase and MMP-7, and acholic stools, a decision was made to proceed with percutaneous transhepatic cholecysto-cholangiography (PTCC). During this procedure, contrast was noted to fill the gallbladder and common bile duct with passage into the duodenum. However, there was no contrast opacification of the intrahepatic bile ducts (Figure 1). A liver biopsy was obtained at the time of PTCC. Histologic findings were consistent with obstructive cholestasis with mild bile duct injury, fibrous expansion of the portal tracts, and focal septal formation (Figure 2). The patient was subsequently referred for intraoperative cholangiogram (IOC) and underwent hepatoportoenterostomy (HPE) or Kasai procedure for a diagnosis of biliary atresia (BA) at 26 days of life.
FIGURE 1.
Percutaneous transhepatic cholecysto-cholangiography (PTCC). [A] Image demonstrates contrast filling the GB and common bile duct (black arrow) with reflux into the PD and free passage into the duodenum (D). Upstream, there is a beaded and truncated appearance of the common hepatic duct (white arrow) without contrast opacification of the intrahepatic bile ducts despite provocative maneuvers. Findings suggest biliary atresia. [B] PTCC is performed in a 1-month-old with normal biliary tree morphology. The needle resides within a normal contrast-opacified GB. A normal common bile duct (black arrow) is shown. Iodinated contrast media flows freely into the duodenum (D). Upstream, there is a normal division of the right and left intrahepatic ducts (white arrows). Abbreviations: D, duodenum; GB, gallbladder; PD, pancreatic duct; PTCC, Percutaneous transhepatic cholecysto-cholangiography.
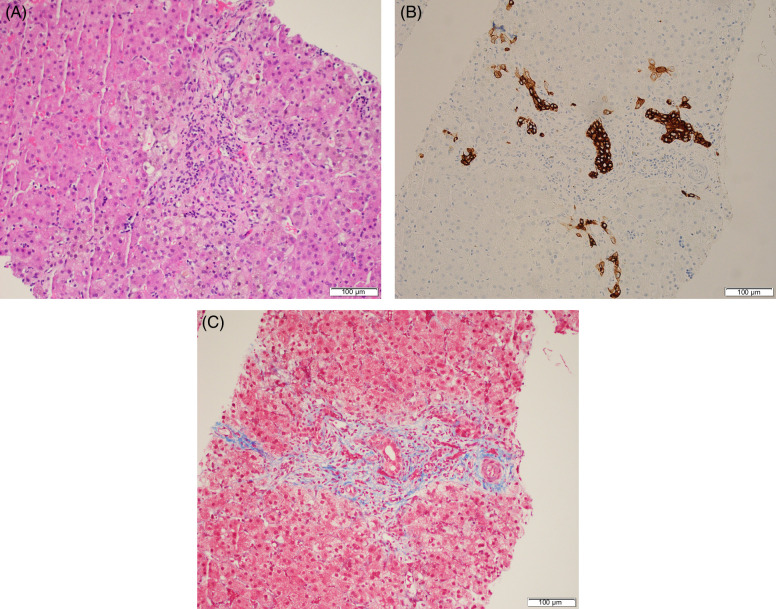
FIGURE 2.
Percutaneous liver biopsy demonstrates bile duct injury with portal edema, fibrous expansion of the portal tracts, and prominent ductular reaction (A). Bile duct proliferation is further highlighted by cytokeratin 7 immunohistochemistry (B). Trichrome stain demonstrates focal septal formation (C).
Question 3: Which of the following are prognostic indicators suggesting a higher likelihood of need for liver transplantation following HPE in patients with biliary atresia?
HPE performed when patients are older than 90 days of age
Total bilirubin level >6mg/dl beyond 3 months from HPE
Evidence of decompensated portal hypertension at the time of HPE
Patient care center with limited experience performing HPE
All of the above
DISCUSSION
Cholestasis refers to reduced bile formation or flow, which results in the accumulation of biliary components in the liver rather than being expelled into the intestine. It is typically identified through serologic testing, highlighting increased levels of conjugated bilirubin and bile acids as key indicators of hepatobiliary dysfunction. Cholestatic jaundice affects 1:2500 term infants and hence may be infrequently seen by primary care providers. Physiologic jaundice typically resolves by 14 days of age in 85% of infants. Any infant presenting with jaundice beyond 2–3 weeks of age should undergo further diagnostic evaluation.1
The first step in the evaluation of cholestasis is to fractionate the total bilirubin level. Cholestasis is defined as a conjugated or direct bilirubin level greater than 1 mg/dL (17.1 μmol/L). Recent studies also suggest that a diagnosis of cholestasis should be considered when the conjugated bilirubin level is > 0.3–0.5 mg/dL and > 10% of the total bilirubin within the first 5 days after birth.2 Once a diagnosis of cholestasis is established, further stepwise evaluation should be performed with a focus on the rapid identification of treatable disorders. It is crucial to identify those disorders that may benefit from early initiation of medical therapy or surgical intervention to optimize outcomes.1,3,4 Conditions such as infection, hypothyroidism, inborn errors of metabolism (ie, galactosemia, tyrosinemia), and panhypopituitarism must be treated promptly to avoid the progression of systemic illness. Biliary obstruction, as seen with choledochal cyst may be identified on imaging such as ultrasound. Common causes of neonatal cholestasis and diagnostic testing are summarized in Table 2.
TABLE 2.
Common etiologies of neonatal cholestasis and diagnostic testing
| Diagnosis | Clinical features and diagnostic clues | |
|---|---|---|
| Anatomic obstruction | ||
| • Biliary atresia • Choledochal cyst • Biliary sludge/inspissated bile • Spontaneous perforation of the common bile duct |
IOC Ultrasound/MRCP Ultrasound/MRCP HIDA/MRCP |
Cardiac defects, splenic malformation, situs inversus, interrupted IVC, preduodenal portal vein Bile peritonitis |
| Infectious | ||
| • Viral • Bacterial • Spirochete • Parasites |
Clinical suspicion Cultures |
Microcephaly, chorioretinitis |
| Drugs/toxins | ||
| • Medications • Total parenteral nutrition-associated cholestasis • Herbal supplements |
History Histology supportive |
|
| Endocrine | ||
| • Hypothyroidism • Panhypothyroidism |
Newborn screen, free T4, TSH TSH, free and total T4, morning cortisol, and brain MRI |
|
| Genetic/inborn errors of metabolism | ||
| • α1-antitrypsin deficiency • Alagille syndrome • Arthrogryposis-renal dysfunction-cholestasis (ARC syndrome) • Congenital hepatic fibrosis • Citrin deficiency (adult citrullinemia type 2) • Cystic fibrosis • Bile acid synthesis defects • Bile acid conjugation defects • Fatty acid oxidation defects • Galactosemia • Glycogen storage disease type IV • Hereditary fructose intolerance • Mitochondrial respiratory chain disorders • Niemann Pick type C disease • Peroxisomal disorders • Progressive familial intrahepatic cholestasis • Tyrosinemia type 1 |
SERPINA1 JAGGED 1, NOTCH2 VPS33B, VIPAR PKHD1 SLC25A13 CFTR AKR1D1, AMACR, CYP7B1, HSD3B7, CYP7A1, CYP27A1 BAAT, SLC27A5 SCAD, LCAD GALT GBE1 ALDOB DGUOK, MPV17, POLG, SUCLG1, Twinkle, TFAM, SERAC1, QIL1, BCS1L, TYMP, SCO1, TRMU, EFG1, EFTu, GFM1, TSFM NPC1, NPC2 PEX1, PEX6, PEX10, PEX11B, PEX12, PEX13, PEX14, PEX16, PEX19, PEX2, PEX26, PEX3, PEX5, PEX7 ATP8B1, ABCB11, ABCB4, TJP2, NR1H4, MYO5B FAH |
Lung disease, panniculitis, family history of early emphysema Cardiac defects, distinctive facies (prominent forehead, pointed chin), posterior embryotoxon, butterfly vertebrae, vascular anomalies, renal dysplasia, RTA, bile duct paucity Multiple joint contractures Polycystic kidneys (ARPKD) Lung disease Low-GGT cholestasis Cataracts, infection Hepatosplenomegaly Hypoglycemia following introduction of fructose Hypotonia, poor feeding, failure to thrive Hepatosplenomegaly Hypotonia, elevated VLCFA Low-GGT cholestasis, intrahepatic cholestasis of pregnancy Renal and neurologic manifestations |
Abbreviations: ARPKD, autosomal recessive polycystic kidney disease; GGT, gamma-glutamyl transferase; HIDA, hepatobiliary iminodiacetic acid; IOC, intraoperative cholangiogram; MRCP, Magnetic resonance cholangiopancreatography; RTA, renal tubular acidosis; T4, thyroxine; TSH, thyroid-stimulating hormone; VLCFA, very long chain fatty acids.
Biliary atresia must also be identified expeditiously, as early surgical intervention is associated with improved long-term outcomes. BA is a progressive, idiopathic obliteration of the bile ducts. It is universally fatal if not treated and can lead to progressive fibrosis/cirrhosis, synthetic dysfunction, and complications of portal hypertension. Although the overall incidence is only 1 in 10,000–20,000 live births, BA is the leading indication for pediatric liver transplantation, accounting for 40%–50% of cases annually.4 Infants with BA typically appear healthy at birth but develop symptoms of jaundice and acholic stools around 3–6 weeks of age. The exact etiology of BA is not fully understood, though causative factors likely include exposure to viral or toxic pathogens, immune dysregulation, and predisposing genetic contributions. BA may occur in isolation (70%) or with laterality malformations known as biliary atresia splenic malformation syndrome. While the work-up often includes a series of laboratory tests, imaging, and histology to exclude other causes of cholestasis, a definitive diagnosis is made with IOC.5
HPE is a surgical attempt to restore bile flow from the liver to the intestine during which a Roux-en-Y loop of the bowel is directly anastomosed to the hilum of the liver. Younger age at the time of HPE, particularly when performed before 30–45 days of life, is associated with improved long-term outcomes with up to an 80% success rate.3 In the United States, the median age for HPE is > 60 days of age, resulting in a higher risk of progressive liver disease. Native liver survival declines when HPE is performed beyond 60 days of life and falls to less than 20% when performed after 90 days of age, thereby emphasizing the importance of early diagnosis. A total serum bilirubin < 2 mg/dL 3 months after HPE predicts a 10-year transplant-free survival rate between 75% and 90%, which decreases to 20% if jaundice persists (total serum bilirubin > 6 mg/dL).6
Serum MMP-7 is highly expressed in the intrahepatic bile ducts and has been explored as a potential noninvasive biomarker of BA. Elevated serum levels of MMP-7 at 1 to 2 months of age have been noted in patients with BA, with positive and negative predictive values exceeding 90 and 95%, respectively. Additionally, MMP-7 levels following HPE may be useful in determining whether infants with BA will require a liver transplant.7
Fasting abdominal ultrasound is a noninvasive diagnostic imaging method employed to assess liver structure, size, position, composition, spleen number and size, ascites, and biliary obstruction (eg, choledochal cyst, mass, gallstone). Certain hepatic sonographic parameters, such as the presence of a triangular cord sign (a cone-shaped fibrotic mass cranial to the bifurcation of the portal vein), interrupted inferior vena cava, preduodenal portal vein, and/or situs inversus, may raise suspicion for a diagnosis of BA. A normal ultrasound does not rule out BA. Hepatobiliary scintigraphy is used to demonstrate patency of the biliary system. The absence of tracer excretion on the hepatobiliary iminodiacetic acid scan is not diagnostic of BA and may be observed in infants with significant cholestasis and severe hepatitis.8 Percutaneous transhepatic cholangiography (PTCC) serves as an effective means of ruling out BA in cholestatic infants with reasonable safety. PTCC may be employed with simultaneous percutaneous liver biopsy to minimize negative laparotomies.9
Liver biopsy may play a role in the diagnostic evaluation for infants with cholestatic jaundice, though noninvasive testing, such as MMP-7 and next-generation sequencing,10 have recently called into question the diagnostic utility of this procedure. The classic histologic features of biliary obstruction are bile duct proliferation, bile plugs, portal or peri-lobular fibrosis, and edema, with preservation of the basic hepatic lobular architecture. In some instances, non-BA causes of cholestasis (including alpha-1 antitrypsin deficiency, Alagille Syndrome, parenteral nutrition-associated cholestasis, and Cystic Fibrosis) can mimic the histologic appearance of BA.11 An IOC is performed when the preliminary evaluation supports a diagnosis of BA, and if BA is confirmed (nonvisualization of a patent biliary tree), HPE is typically performed unless transplant evaluation is deemed more appropriate.
Approximately 50% of children with BA will require a liver transplant before age 2, and 75% by age 20. Those patients who survive into adulthood with their native liver remain at risk for complications of portal hypertension and cirrhosis, including HCC.12 Transition and transfer to an adult care team are essential for ongoing surveillance and close monitoring.
In summary, neonatal cholestasis requires early recognition and a timely approach to diagnostic evaluation aimed at the rapid identification of treatable disorders. Biliary atresia must also be identified promptly, as early surgical intervention with HPE is associated with improved long-term outcomes and native liver survival.
TEACHING POINTS
Any infant presenting with jaundice beyond two to three weeks of life should undergo further evaluation for cholestasis with fractionated bilirubin levels.
A conjugated or direct serum bilirubin level > 1.0 mg/dL warrants further diagnostic evaluation for neonatal cholestasis.
Early diagnosis of biliary atresia is critical as younger age at the time of HPE, specifically when performed before 45 days of life, is associated with improved long-term outcomes and native liver survival.
Serum measurement of MMP-7 is being explored as a potential biomarker of BA that may help guide diagnostic decisions and the pursuit of more invasive testing.
PTCC may offer a less invasive alternative for diagnosis; however, the gold standard for diagnosis of BA remains IOC.
Footnotes
Abbreviations: BA, biliary atresia; HPE, hepatoportoenterostomy; IOC, intraoperative cholangiogram; MMP-7, matrix metalloproteinase-7; PTCC, percutaneous transhepatic cholecysto-cholangiography.
Contributor Information
Shagun Sharma, Email: Shagun.Sharma@downstate.edu.
Kristen Thomas, Email: Kristen.Thomas@nyulangone.org.
Frederic Bertino, Email: Frederic.Bertino@nyulangone.org.
Jennifer Vittorio, Email: Jennifer.Vittorio@nyulangone.org.
CONFLICTS OF INTEREST
Jennifer Vittorio consults for and advises Mirum Pharma. The remaining authors have no conflicts to report
EARN CME FOR THIS ARTICLE
REFERENCES
- 1.Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:154–168. [DOI] [PubMed] [Google Scholar]
- 2.Harpavat S, Ramraj R, Finegold MJ, Brandt ML, Hertel PM, Fallon SC, et al. Newborn direct or conjugated bilirubin measurements as a potential screen for biliary atresia. J Pediatr Gastroenterol Nutr. 2016;62:799–803. [DOI] [PubMed] [Google Scholar]
- 3.Feldman AG, Sokol RJ. Recent developments in diagnostics and treatment of neonatal cholestasis. Semin Pediatr Surg. 2020;29:150945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinz N, Vittorio J. Treatment of cholestasis in infants and young children. Curr Gastroenterol Rep. 2023;25:344–354. [DOI] [PubMed] [Google Scholar]
- 5.Davenport M, Kronfli R, Makin E. Advances in understanding of biliary atresia pathogenesis and progression - A riddle wrapped in a mystery inside an enigma. Expert Rev Gastroenterol Hepatol. 2023;17:343–352. [DOI] [PubMed] [Google Scholar]
- 6.Shneider BL, Magee JC, Karpen SJ, Rand EB, Narkewicz MR, Bass LM, et al. Total serum bilirubin within 3 months of hepatoportoenterostomy predicts short-term outcomes in biliary atresia. J Pediatr. 2016;170:211–217; e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JF, Jeng YM, Chen HL, Ni YH, Hsu HY, Chang MH. Quantification of serum matrix metallopeptide 7 levels may assist in the diagnosis and predict the outcome for patients with biliary atresia. J Pediatr. 2019;208:30–37; e1. [DOI] [PubMed] [Google Scholar]
- 8.Nievelstein RAJ, Robben SGF, Blickman JG. Hepatobiliary and pancreatic imaging in children-techniques and an overview of non-neoplastic disease entities. Pediatr Radiol. 2011;41:55–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MK, Biank VF, Moe DC, Simpson PM, Li SH, Telega GW. HIDA, percutaneous transhepatic cholecysto-cholangiography and liver biopsy in infants with persistent jaundice: Can a combination of PTCC and liver biopsy reduce unnecessary laparotomy? Pediatr Radiol. 2012;42:32–39. [DOI] [PubMed] [Google Scholar]
- 10.Karpen SJ, Kamath BM, Alexander JJ, Ichetovkin I, Rosenthal P, Sokol RJ, et al. Use of a comprehensive 66-gene cholestasis sequencing panel in 2171 cholestatic infants, children, and young adults. J Pediatr Gastroenterol Nutr. 2021;72:654–660. [DOI] [PubMed] [Google Scholar]
- 11.Russo P, Magee JC, Anders RA, Bove KE, Chung C, Cummings OW, et al. Key histopathologic features of liver biopsies that distinguish biliary atresia from other causes of infantile cholestasis and their correlation with outcome: A multicenter study. Am J Surg Pathol. 2016;40:1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148:467–474. [DOI] [PubMed] [Google Scholar]