Abstract
Background:
This study aims to evaluate the efficacy and safety of a blunt cannula technique using hyaluronic acid fillers for achieving the Bratz doll lip aesthetic, characterized by enhanced volume and sharp definition.
Methods:
Thirty volunteers, 22–40 years of age, were selected based on specific inclusion criteria at Albany Cosmetic and Laser Center for 6 months. The technique involved precise filler injections using a Steriglide blunt cannula. Pre- and postprocedure measurements of lip dimensions and angular changes were meticulously documented and analyzed using IBM SPSS Statistics software.
Results:
The procedure significantly increased the average height of both the upper and lower vermilion zones, with notable changes in angular measurements and the distance between the midline and oral commissure, aligning with the Bratz doll aesthetic. Importantly, none of the participants experienced bruising, a common side effect in traditional needle-based methods. The overall satisfaction rate was high, with an average score of 8.5 out of 10, reflecting the procedure’s success in meeting aesthetic goals and ensuring participant comfort.
Conclusions:
The blunt cannula technique for lip augmentation presents a safe and effective alternative to traditional needle-based methods. The absence of bruising and high satisfaction rates underscore the technique’s precision and alignment with patient safety and comfort. This study contributes to the field of cosmetic lip enhancement, offering a novel approach that balances aesthetic aspirations with health considerations, potentially influencing future practices in cosmetic procedures.
Takeaways
Question: Can a blunt cannula technique using hyaluronic acid fillers effectively achieve the Bratz doll lip aesthetic while ensuring safety and patient comfort?
Findings: In this study, 30 volunteers underwent lip augmentation with a blunt cannula technique, resulting in significant increases in lip height and changes in angular measurements, aligning with the Bratz doll aesthetic. Notably, there were no instances of bruising, and the satisfaction rate was high at an average of 8.5 of 10.
Meaning: This study demonstrates that a blunt cannula technique for lip fillers is a safe and effective alternative to needle-based methods, offering a balance between aesthetic enhancement and patient comfort.
INTRODUCTION
The “Bratz doll” (BD) or “Russian lip” look has emerged as a highly sought-after outcome in recent times.1,2 This lip style is inspired by the exaggerated features of the popular BDs. It is characterized by distinctively enhanced volume, sharp definition, and a pronounced Cupid bow.3
Hashtags such as #bratzdolllips and #bratzlips have garnered significant attention, with usage reaching 1.7 and 58.8 million on TikTok, respectively.4,5 According to an online report, the BD aesthetic, as a part of a broader movement towards bold cosmetic choices, has paralleled the rise in accessibility, safety, and affordability of hyaluronic acid (HA) fillers.6,7 This trend highlights the impact of social media in shaping aesthetic preferences and underscores the need for academic research to stay attuned to these evolving trends.8
Traditionally, achieving this look has predominantly involved the use of sharp needles for filler injections, a method that, while effective in volumizing and reshaping, comes with certain limitations and risks.9–11 These include uneven filler distribution, increased pain, and higher chances of bruising and vascular complications, often diverging from the more conservative approaches of traditional lip aesthetics, which focus on subtlety and natural enhancement.12,13
In this study, we introduce a novel approach to lip augmentation aimed at achieving the BD aesthetic using a blunt cannula technique. This method aligns with the current trend in aesthetic medicine that favors minimally invasive procedures with reduced recovery time and lower-risk profiles.14,15 By analyzing the efficacy and safety of this approach, we hope to contribute valuable insights to the evolving landscape of cosmetic lip enhancement and offer a viable alternative to traditional needle-based methods.
MATERIALS AND METHODS
A prospective observational study was conducted at Albany Cosmetic and Laser Centre, recruiting 30 volunteers during 6 months. Participants, between 22 and 40 years of age, demonstrated a specific interest in the BD lip aesthetic. This group included both individuals with and without previous lip augmentation procedures, representing a diverse client base. Inclusion was also based on good general health status, nonpregnancy, nonlactation, and willingness to comply with postprocedure care instructions. Consent for data inclusion was obtained separately from the treatment decision, according to our privacy policy.16 Participants with any active infection in the treatment area or history of severe reactions to HA were also excluded to prevent complications and ensure accurate assessment of the technique’s results.
Pretreatment documentation of the lips was conducted using an Apple iPad (Apple Inc., Cupertino, Calif.) coupled with the RXphoto clinical photography application (RXphoto, Boston, Mass.). EMLA Cream (lidocaine 2.5% and prilocaine 2.5%; Aspen Pharma, Dublin, Ireland) was applied directly on the lips without the use of any occlusion membrane and left for 10 minutes.
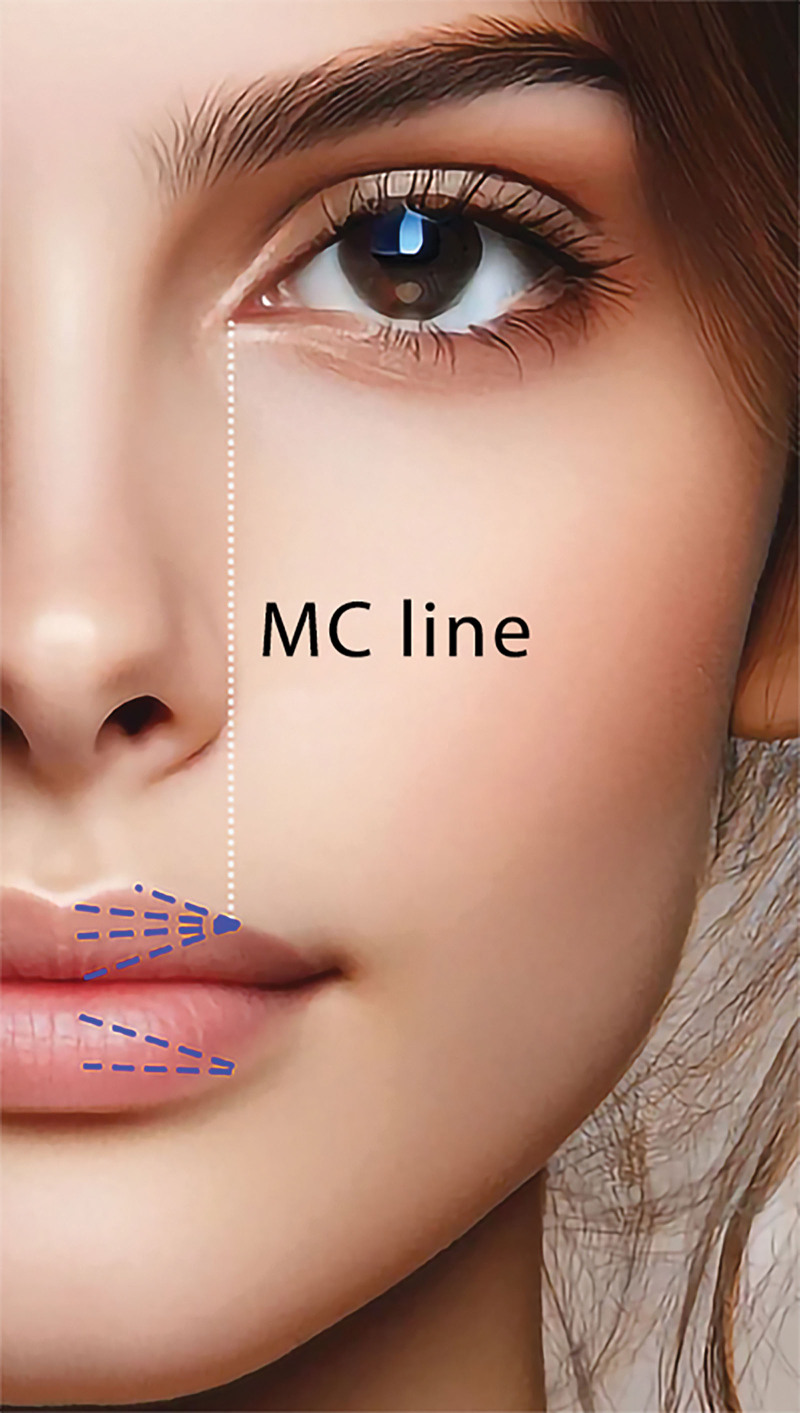
A Steriglide blunt cannula (TSK Laboratory, Tochigi-Kan, Japan) was inserted to align with the medial canthus line, as shown in Figure 1. The cannula was kept at a superficial level, with the hole mark facing outward.
Fig. 1.
Photograph of the anatomical landmarks used for the precise placement of the blunt cannula. The entry point for the cannula is marked in alignment with the medial canthus. The location of the entry point is determined to be approximately one-third of the distance from the oral commissure to the Glogau-Klein points to maintain facial symmetry. MC, medial canthus.
An amount of 0.4 mL of Revanesse Kiss+ HA filler (Prollenium Medical Technologies, Aurora, Ontario, Canada) was injected on each side of the upper lip, divided into four aliquots. Each aliquot involved repositioning the Steriglide cannula. The injections targeted the vermilion border, upper, central, and lower levels of the vermilion zone to achieve the desired volumization and contour, as shown in Figure 1. [See Video (online), which demonstrates the application of 1.2 mL of Revanesse Kiss+ HA filler, using a 30-G cannula.]
Video 1. This video demonstrates the application of 1.2 ml of Revanesse Kiss+ Hyaluronic acid filler, using a 30-G cannula.
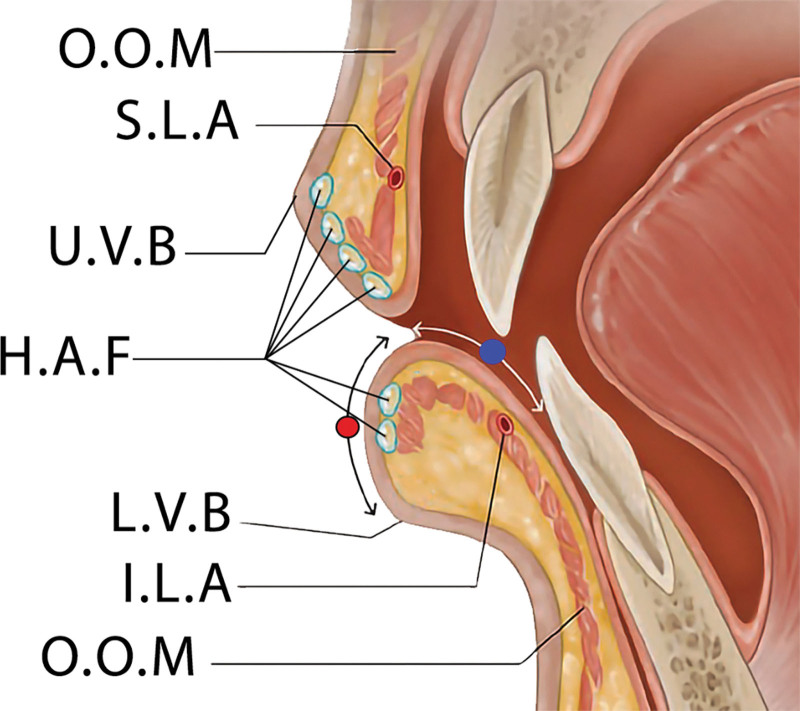
An amount of 0.2 mL was injected on the left and 0.2 mL on the right side of the lower lip, each in two aliquots. The first 0.1 mL aliquot was injected into the lower part of the vermilion zone, followed by the second aliquot targeting the central part to achieve the desired fullness and contour. The HA filler is intended to be placed superficially directly behind the dry vermilion mucosa, at the level of lamina propria as shown in Figure 2.
Fig. 2.
Sagittal section illustration of the lip demonstrates the ideal superficial placement for hyaluronic acid fillers. Key anatomical landmarks are labeled to guide the precise administration of the filler. “O.O.M.” denotes the orbicularis oris muscle, which encircles the mouth and is fundamental for lip movements. “S.L.A” indicates the superior labial artery, which supplies blood to the upper lip. “H.A.F” represents the hyaluronic acid fillers, depicted at the preferred superficial injection sites to enhance lip volume and contour. “U.V.B” is marked as the upper vermilion border, the transitional zone between the lip tissue and the normal skin above the lip. “I.L.A” refers to the inferior labial artery, the blood supply to the lower lip. The illustration emphasizes the importance of anatomical knowledge for aesthetic procedures to ensure both efficacy and safety. The red circle indicates the dry vermilion area, and the blue circle indicates the wet vermilion area.
After the procedure, the lips were cleaned and treated with cold compresses to minimize swelling and discomfort. Thirty minutes later, follow-up photographs were taken. Both the photographs and the subsequent measurements were conducted by the same trained assistant to ensure consistency.
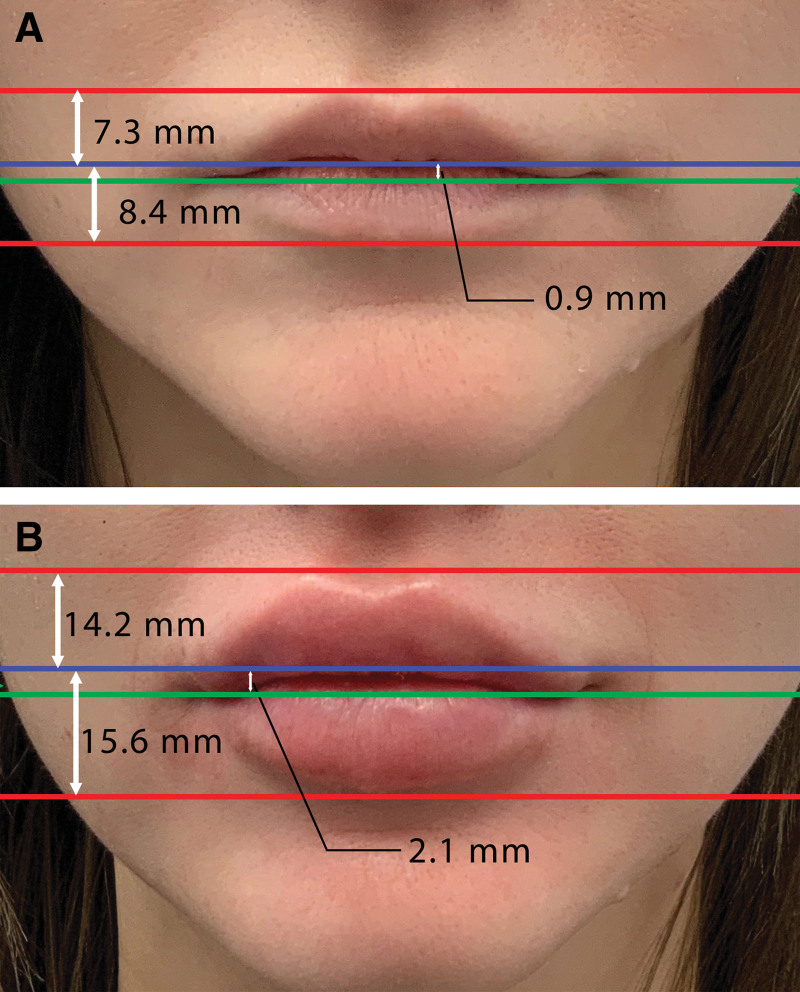
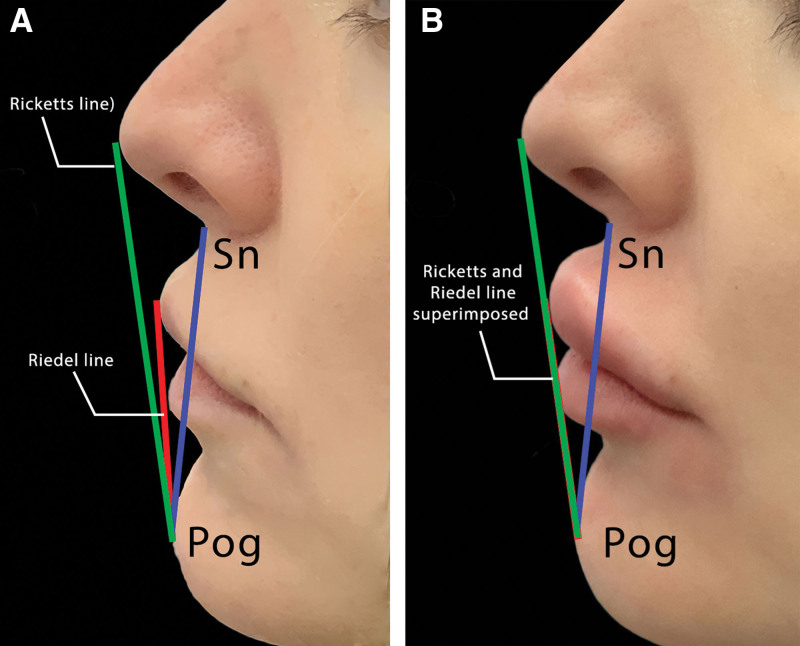
The upper lip line, lower lip line, and the middle line on the frontal view were identified and analyzed as shown in Figure 3. Measurements were taken both before and after the lip filler procedure to assess changes in lip volume and shape including the difference in the vermilion zone height, the angular measurements, and the midline to oral commissures distance as shown in Figure 4.
Fig. 3.
A frontal view comparison of facial lines before (A) and after (B) lip augmentation with improved facial features. The numbers displayed are average changes in lip dimensions as per our study, not the actual measurements for this specific individual (A) before and (B) after the lip injection. The red lines indicate the vermilion borders of the upper and lower lips. The blue lines mark the area where the vermilion zones of the upper and lower lips touch, typically the point of fullest pout. The green line delineates the oral commissure, the corner of the mouth where the lips meet.
Fig. 4.
A lateral view comparison of facial lines before (A) and after (B) lip augmentation with imporved facial profile featurs. The blue line represents the ‘Sn-Pog’ line, extending from the subnasale (Sn) to the soft tissue pogonion (Pog’), which lies on a tangent to the upper and lower lips (Riedel line). The red line is the Riedel line, and the green line is the Ricketts line, which should ideally connect the Pog’ and the pronasale (Prn), with the lips positioned behind it. The post-augmentation image (B) illustrates the alignment of the Riedel and Ricketts lines, showing that the angle between them has diminished to the point where the lines are superimposable, indicating a change in the facial profile congruent with the desired aesthetic outcome of lip augmentation.
IBM SPSS Statistics software (SPSS Inc., Chicago, Ill.) was used to perform statistical analysis using paired t tests at a 0.05 significance level.
Follow-up with the participants was conducted over the phone to evaluate their satisfaction and monitor any side effects postprocedure. Each participant received a phone call for a follow-up assessment 1 week after undergoing the lip augmentation procedure. This timing was chosen to allow the initial swelling to subside and for the filler to settle, providing a more accurate reflection of the outcome.
Participants were asked to rate their satisfaction with the results on a scale from 1 to 10, where one represented complete dissatisfaction and 10 represented complete satisfaction. Specific attention was given to identifying any side effects experienced by the participants. Participants were asked whether they experienced any bruising, its extent and duration, and any other discomfort or complications they might have encountered.
RESULTS
The results from our study showed notable changes in lip dimensions and facial geometry. Before the procedure, the average upper lip height across participants was measured at 7.3 mm, with an SD of 1.2 mm. In comparison, after the procedure, the average upper vermilion one height significantly increased to 11.2 mm, maintaining a similar SD of 1.2 mm. The lower lip also exhibited a substantial increase in height, moving from an average of 8.4 mm (SD 1.5 mm) before the procedure to 15.6 mm (SD 1.6 mm) postprocedure.
In terms of angular measurements, there was a remarkable change observed. Initially recorded at 7 degrees with a minimal SD of 0.2, the average angle shifted to an average of 0 degrees postprocedure, albeit with a slightly higher SD of 0.3. This change indicates a significant alteration in the lip’s angle relative to the facial profile, aligning with the desired aesthetic goals of the procedure, as shown in Figure 4.
The distance between the midline and oral commissure also showed a notable increase. Before the lip fillers were applied, the average distance was 0.9 mm with an SD of 0.15 mm. After the procedure, this average expanded to 2.1 mm, with an SD of 0.21 mm. All statistical analyses conducted to compare pre- and postprocedure average measurements were found to be significant.
Our study also reported favorable participant experience and satisfaction outcomes. Notably, none of the participants reported experiencing bruising as a side effect of the procedure. This absence of bruising is particularly noteworthy, as it suggests a high level of safety and precision in the technique used for the lip filler application, minimizing common adverse effects such as vascular complications or tissue trauma.
Furthermore, the overall satisfaction rate among participants was remarkably high. On a scale from 1 to 10, where 10 represents complete satisfaction, the average satisfaction rate reported was 8.5. This high level of satisfaction underscores the procedure’s success in meeting the participants’ aesthetic expectations, as shown in Figure 5.
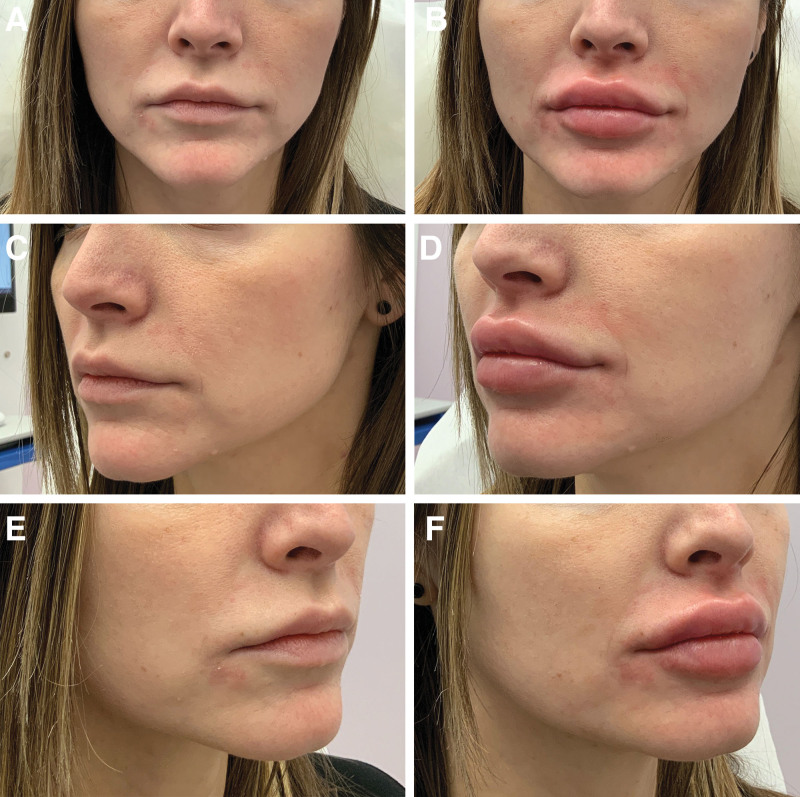
Fig. 5.
A series of photographs that illustrates the outcomes of lip augmentation from multiple viewing angles. A–B, Frontal view, capturing the overall impact on lip fullness and balance from the direct anterior perspective. C–D, View from the left at a 45-degree angle, detailing the profile changes, volumetric enhancement, and the new silhouette of the lips. E–F, Right side at a 45-degree angle, offering a comparative view of the augmentation’s symmetry and consistency.
DISCUSSION
Lip injections with HA fillers have become popular for achieving different aesthetic goals. However, with the increasing popularity of these procedures, there has also been a corresponding rise in awareness of potential side effects, with bruising being one of the most common concerns.17
The anatomy of the human lip is a study in complexity and function, seamlessly integrating diverse structures that cater to sensitivity, expression, and survival.18–20 The superior and inferior labial arteries are significant blood vessels that provide vascular supply to the upper and lower lips, respectively. These arteries are branches of the facial artery, which supplies blood to various face structures. Similarly, the inferior labial artery supplies the lower lip and requires the same careful consideration during filler placement.
In-depth anatomical studies reveal that the superior and inferior labial arteries are predominantly in a submucosal position (78.1%) within the lips, making them susceptible to damage during injections. The position of these arteries can vary significantly, with 29% variability in the upper lip and 32% in the lower lip, particularly in the midline region.21 This highlights the importance of choosing safer locations for injections, such as the subcutaneous plane in paramedian locations of both the upper and lower lips, to minimize risks of vascular complications.
Bruising, as a side effect of lip injections, occurs due to the disruption of blood vessels during the procedure. The incidence of such complications can vary depending on the technique used and the anatomical understanding of the injector.22 A key factor in minimizing these risks is a thorough knowledge of facial anatomy, particularly the position and course of the labial arteries.23
The BD look is characterized by a significant increase in the vermilion zone height, decrease in the angular measurement, and increase in the distance between the midline and oral commissure. These changes contribute to pronounced fullness and a distinctive contour.
Traditionally, achieving the BD look with lip fillers involves several vertical injections using sharp hypodermic needles of a large amount of HA filler. These injections usually start from the vermilion border and are directed perpendicular to the path of the labial arteries.2,9,10 Although this method can be effective in creating the desired aesthetic, it also carries a higher risk of bruising and other vascular-related complications due to the potential for arterial puncture or compression.
The risk of vascular occlusion during lip filler procedures has been significantly lower when using cannulas than needles.24–26 A large-scale study involving 1.7 million syringe injections reported an occlusion risk of one per 6410 needles and one per 40,882 with cannulas. This suggests that the choice of instrument can greatly impact the procedure’s safety. Moreover, injectors with more than 5 years of experience showed a 70.7% lower odds of occlusion, indicating the importance of experience in reducing procedural risks.27
In this study, we introduce a blunt cannula technique that achieves substantial lip enhancement with a minimal volume of HA filler, specifically just 0.4 mL per side of the upper lip. Furthermore, the overall satisfaction rate among participants was remarkably high. On a scale from 1 to 10, where 10 represents complete satisfaction, the average satisfaction rate reported was 8.5. This high level of satisfaction underscores the success of the technique.
This unique approach is in contrast to traditional methods that generally require multiple injections of large volumes of HA filler. The core principle lies in the injection method by administering the filler in a thin, superficial plane just beneath the skin of the lips. This approach significantly differs from deeper injections, where the filler tends to create a more rounded appearance.28 Our approach enables the filler to spread out in a flat, even layer, tracing the natural contours of the lips.
This outcome is largely facilitated by the rheological properties of the filler material, which exhibits shear-thinning behavior.29,30 This means the filler becomes more fluid and spreadable under the mechanical stress of injection, allowing for a thin, uniform distribution when applied superficially.31 When applied superficially, the shear forces effectively distribute the filler in a thin, uniform layer. This distribution is facilitated by the large surface area and the minimal resistance from the surrounding lip tissues.
Furthermore, the interaction between the filler and the lip tissue, particularly in terms of surface tension, influences the final appearance.32 In a superficial plane, the filler may adhere closely to the surface characteristics of the lips, resulting in a smooth, flat appearance that enhances the vermilion border and creates a sharp delineation.
In addition to the significant changes observed in lip dimensions and facial geometry, our study also reported favorable participant experience and satisfaction outcomes. Notably, none of the participants reported experiencing bruising as a side effect of the procedure. This absence of bruising is particularly noteworthy, as it suggests a high level of safety and precision in the technique used for the lip filler application, minimizing common adverse effects such as vascular complications or tissue trauma.
Previous studies have utilized microcannulas for lip enhancement.10,33–35 However, our technique differentiates itself by focusing on the superficial implementation with a finer gauge cannula tailored for the BD look. This represents a unique contribution to the repertoire of lip augmentation techniques, blending artistic vision with precise medical practice.
In considering the limitations of this study, it is important to acknowledge areas where potential biases and imprecision may exist. The study was conducted with a relatively small sample of 30 volunteers, primarily within the age range of 22–40 years. Although this allowed for a detailed analysis of individual responses, the limited sample size and specific age focus may reduce the generalizability of our findings to a broader population, potentially introducing a demographic bias. The absence of a control group, such as individuals undergoing traditional needle-based lip augmentation, limits our ability to make direct comparative assessments, posing a challenge in objectively evaluating the advantages of our blunt cannula technique over conventional methods. This could lead to a comparative bias in assessing efficacy and safety. Additionally, the short-term follow-up of only 1-week postprocedure, while sufficient for immediate outcome assessment, does not capture long-term effects or late-onset complications, potentially introducing a bias in evaluating the durability and longevity of the results. Moreover, the measurements and photography conducted by the same assistant may introduce a consistency bias, despite ensuring uniformity. Finally, ethical considerations and study design constraints, such as the exclusion of detailed individual demographic data for ethical compliance, may limit the depth of demographic analysis, potentially influencing the direction and magnitude of the study’s applicability and outcomes. These limitations highlight the need for further research with larger, more diverse samples, longer follow-up periods, and comprehensive control comparisons to fully ascertain the efficacy, safety, and applicability of the BD lip aesthetic technique in cosmetic lip augmentation.
It is noteworthy that most of these limitations are linked to the study’s design, which was predefined to acquire exemption from the lengthy process of ethical approval. This decision was substantially influenced by constraints in funding, support, and personnel typical of the private sector setting in which this study was conducted. The primary aim was to provide an expedient exploration of an alternative technique for practitioners, offering a potential avenue for enhancement in practice should they feel the need. Although this approach facilitated a more expedient commencement of the study, it inherently imposed certain constraints that shaped the scope and depth of the investigation, reflecting a pragmatic balance between available resources and the pursuit of innovative clinical techniques.
The highlighted limitations underscore the necessity for further research, requiring larger and more diverse samples, extended follow-up durations, and the inclusion of comprehensive control comparisons. Such expansive studies are essential to fully ascertain and validate the efficacy, safety, and broad applicability of this technique.
CONCLUSIONS
Our study on the BD lip aesthetic using blunt cannula and HA fillers shows promising aesthetic and safety outcomes. Significant enhancement in lip dimensions, aligning with the desired aesthetic, confirms the technique’s potential in the lip augmentation field. The absence of bruising, a common issue with traditional needle-based fillers, highlights the safety of our approach, offering an alternative for vertical fencing techniques of needle-based methods. Participants’ high satisfaction, with an average score of 8.5 of 10, indicates the technique’s success in meeting aesthetic goals. Although hypodermic needle is the most common tool to achieve the BD lip look, our findings position this advanced blunt cannula technique as an alternative for practitioners in the aesthetic field.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 15 April 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Hernandez J. The art and politics of Black and Latina embodiment. In Hernandez J. (ed.), Aesthetics of Excess. Durham, NC: Duke University Press; 2020:63–72. [Google Scholar]
- 2.Harris S. Minimally invasive lip treatments: from alienisation to normalisation. J Aesthet Nurs. 2023;12:S18–S20. [Google Scholar]
- 3.Vitosyte M, Malinauskaite D, Chalas R, et al. Lip morphometry and morphologic pattern variation by ethnicity. Anthropol Anz. 2023;80:13–21. [DOI] [PubMed] [Google Scholar]
- 4.#bratzlips on TikTok. Tiktok.com. Available at https://www.tiktok.com/tag/bratzlips. Published 2024. Accessed on January 18, 2024.
- 5.#bratzdolllips on TikTok. Tiktok.com. Available at https://www.tiktok.com/tag/bratzdolllips. Published 2024. Accessed on January 18, 2024.
- 6.Peach N. New “Bratz doll” lip trend explodes: here’s what you need to know. Available at https://closeronline.co.uk/beauty/makeup/bratz-doll-lips/. Accessed December 6, 2023.
- 7.Omran D, Tomi S, Abdulhafid A, et al. Expert opinion on non-surgical eyebrow lifting and shaping procedures. Cosmetics 2022;9:116. [Google Scholar]
- 8.Hopkins ZH, Moreno C, Secrest AM. Influence of social media on cosmetic procedure interest. J Clin Aesthet Dermatol. 2020;13:28–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Ghasemi S, Akbari Z. Lip augmentation. Dent Clin North Am. 2022;66:431–442. [DOI] [PubMed] [Google Scholar]
- 10.Adel N. A new approach for lip filler injection using an inverted Mercedes Benz sign. Plast Reconstr Surg Global Open. 2021;9:e3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wick EH, Ostby E, Grunebaum LD. Lip rejuvenation and filler complications in the perioral region. Plast Aesthet Res. 2022;9:413–419. [Google Scholar]
- 12.Diwan Z, Trikha S, Etemad-Shahidi S, et al. Evaluation of current literature on complications secondary to lip augmentation following dermal filler injection. J Clin Aesthet Dermatol. 2023;16:26–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Trinh LN, McGuigan KC, Gupta A. Delayed granulomas as a complication secondary to lip augmentation with dermal fillers: a systematic review. Surg J (NY) 2022;8:e69–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hage J. The needle versus cannula debate in soft tissue augmentation. In Galadari H. (ed.), Hot Topics in Cosmetic Dermatology, An Issue of Dermatologic Clinics [E-Book]. 2023;42:69–77. [DOI] [PubMed] [Google Scholar]
- 15.Mehta P, Kaplan JB, Zhang-Nunes S. Ischemic complications of dermal fillers. Plast Aesthetic Res. 2022;9:57. [Google Scholar]
- 16.Al Hallak K. Privacy policy. Available at https://albanylaser.ca/privacy-policy/. Accessed January 18, 2024.
- 17.Kroumpouzos G, Harris S, Bhargava S, et al. Complications of fillers in the lips and perioral area: prevention, assessment, and management focusing on ultrasound guidance. J Plast Reconstr Aesthet Surg. 2023;84:656–669. [DOI] [PubMed] [Google Scholar]
- 18.Gomi T, Imamura T. Age-related changes in the vasculature of the dermis of the upper lip vermilion. Aging (Albany NY). 2019;11:3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe K, Saga T. Anatomy and variations of the lip. In Watanabe K. (ed.), Anatomical Variations in Clinical Dentistry. New York: Springer; 2019:177–184. [Google Scholar]
- 20.Alhallak K, Abdulhafid A, Tomi S, et al. Hair removal. In The Ultimate Guide for Laser and IPL in the Aesthetic Field. New York: Springer; 2023:101–151. [Google Scholar]
- 21.Cotofana S, Pretterklieber B, Lucius R, et al. Distribution pattern of the superior and inferior labial arteries: impact for safe upper and lower lip augmentation procedures. Plast Reconstr Surg. 2017;139:1075–1082. [DOI] [PubMed] [Google Scholar]
- 22.Wollina U, Goldman A. Filler migration after facial injection—a narrative review. Cosmetics. 2023;10:115. [Google Scholar]
- 23.Moorefield AK, Rose-Reneau Z, Wright BW, et al. Venous tributaries of the lip: implications for lip filler injection. Plast Reconstr Surg. 2023;152:257e–263e. [DOI] [PubMed] [Google Scholar]
- 24.Jones D, Palm M, Cox SE, et al. Safety and effectiveness of hyaluronic acid filler, VYC-20L, via cannula for cheek augmentation: a randomized, single-blind, controlled study. Dermatolog Surg. 2021;47:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Hage J, Galadari HI. The needle versus cannula debate in soft tissue augmentation. Dermatol Clin. 2024;42:69–77. [DOI] [PubMed] [Google Scholar]
- 26.Drone N. Implementation of the blunt tip cannula for dermal fillers to decrease adverse events intra and post treatment [doctoral manuscript]. San Diego, CA: University of San Diego; 2022. [Google Scholar]
- 27.Alam M, Kakar R, Dover JS, et al. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021;157:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.sami AlSogair S. Rheology of hyaluronic acid dermal fillers: understanding the science to improve results in clinical practice. Inter J Health Med Res. 2023;2:96–103. [Google Scholar]
- 29.Molliard SG, Bétemps JB, Hadjab B, et al. Key rheological properties of hyaluronic acid fillers: from tissue integration to product degradation. Plast Aesthet Res. 2018;5:17. [Google Scholar]
- 30.Fundarò SP, Salti G, Malgapo DMH, et al. The rheology and physicochemical characteristics of hyaluronic acid fillers: their clinical implications. Int J Mol Sci . 2022;23:10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hema Sundaram M, Jason Michaels M. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974–980. [PubMed] [Google Scholar]
- 32.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41:S120–S126. [DOI] [PubMed] [Google Scholar]
- 33.Niamtu J, III. Filler injection with micro-cannula instead of needles. Dermatol Surg. 2009;35:2005–2008. [DOI] [PubMed] [Google Scholar]
- 34.de Lima AS. Understanding the anatomy of the lips and its relationship with needle and cannula filling procedures. Acta Scientific MEDICAL SCIENCES (ISSN: 2582-0931). 2023;7:54–57. [Google Scholar]
- 35.Beer K, Biesman B, Cox SE, et al. Efficacy and safety of resilient hyaluronic acid fillers injected with a cannula: a randomized, evaluator-blinded, split-face controlled study. Clin Cosmet Investigational Dermatol. 2023;16:959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]