Summary:
This case exhibits a presentation of multiple primary malignancies in a patient with Li-Fraumeni syndrome, necessitating surgical excision and multistaged reconstruction. Due to Li-Fraumeni syndrome patients’ predisposition to developing malignancies, management includes lifelong surveillance and aggressive treatment of cancers. Plastic surgeons can minimize damage to patient’s quality of life by carrying out reconstruction in a thoughtful manner that maximizes function and considers a potential lifetime of future reconstructive needs.
Li-Fraumeni syndrome (LFS) is a relatively rare (one in 5000 prevalence) autosomal dominant genetic disease that causes predisposition to multiple cancers.1 The risk of individuals with LFS developing cancer over a lifetime is 75% for men and nearly 100% for women.2 Patients have a germline mutation in their TP53 gene, which causes aberrant activation of cell growth, division, and angiogenesis, as well as suppression of normal DNA repair and apoptosis.1
Due to risk of secondary radiation-induced malignancies, LFS is a relative contraindication to radiation therapy.3 Many cancers in LFS patients require surgical treatment, often involving resection with wide margins.3 This often leads to need for multiple reconstructive procedures for both primary and recurrent cancers.2,3
In this case, we present a 34-year-old woman with newly diagnosed LFS who presented with concurrent invasive ductal breast carcinoma and undifferentiated pleomorphic sarcoma. These primary cancers were both treated surgically with bilateral mastectomy and resection with wide margins, respectively.
CASE
A 34-year-old woman with no known medical history presented in 2020 with a 3-month history of painful right breast lump with foul smelling nipple discharge and nipple inversion (Fig. 1). Physical examination revealed a 5-cm right retroareolar breast mass. The patient underwent chest MRI and radiography to exclude contralateral breast involvement, and after biopsy of right breast mass, she was diagnosed with ER+PR+HER2+ grade 2 invasive ductal carcinoma with lobular features. The patient was subsequently worked up for genetic abnormalities given her young age at breast cancer diagnosis. Genetic screen revealed LFS with deleterious TP53 transmutation. Of note, her family history is negative for any instances of breast or ovarian cancer, indicating a likely idiopathic de novo mutation. Patient history was negative for significant ionizing radiation or trauma.
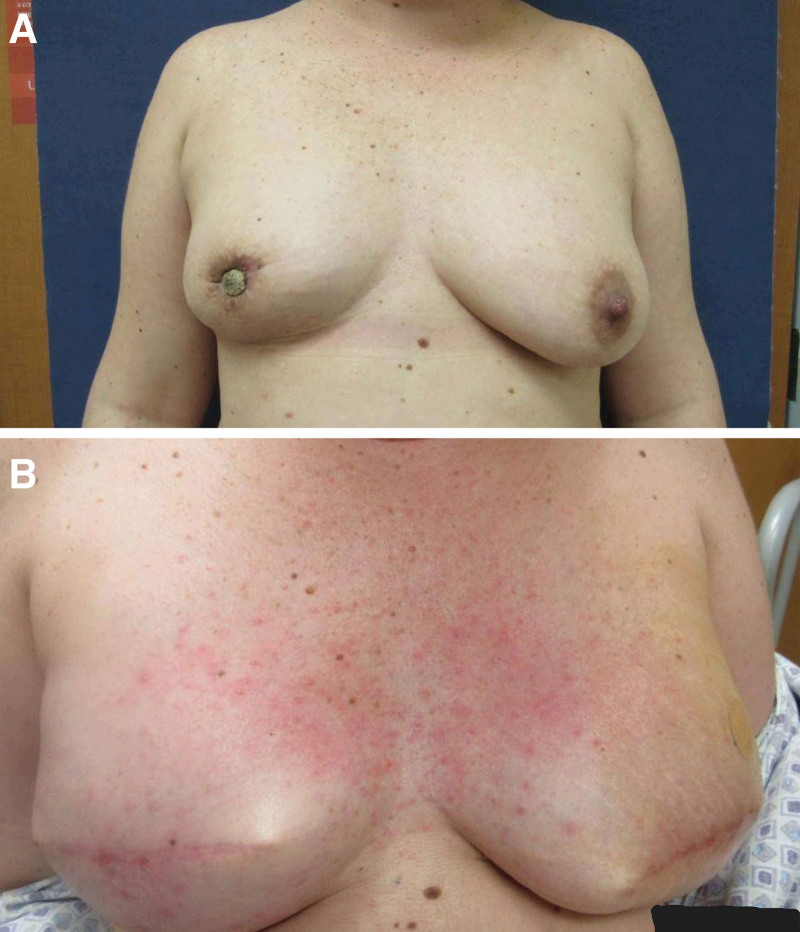
Fig. 1.
Before and after bilateral mastectomy and breast reconstruction in patient with invasive ductal carcinoma. A, Patient presented with painful breast lump, nipple discharge, and nipple inversion. B, Eleven weeks postoperative breast reconstruction using Alloderm slings and tissue expanders.
The patient had concurrent complaints of a painful, chronic soft tissue mass of the anterior lower right extremity, which had been previously diagnosed as a lipoma. Given her new diagnosis of LFS, she elected to undergo excisional biopsy as opposed to core needle biopsy.
Three months after initial consult, she underwent surgical resection of the mass; it was superficial without invasion to the dermis or muscle fascia. A 6 cm × 6.6 cm × 3.1 cm necrotic specimen was collected, and primary wound closure was performed. A diagnosis of undifferentiated pleomorphic sarcoma (UPS) grade 2 was made. The specimen was found to have multiple positive margins, necessitating reoperation to excise malignant epidermal invasion and obtain clear margins. The postoperative course was complicated by skin dehiscence, hematoma, and seroma. The patient underwent whole-body MRI, which was negative for metastatic disease.
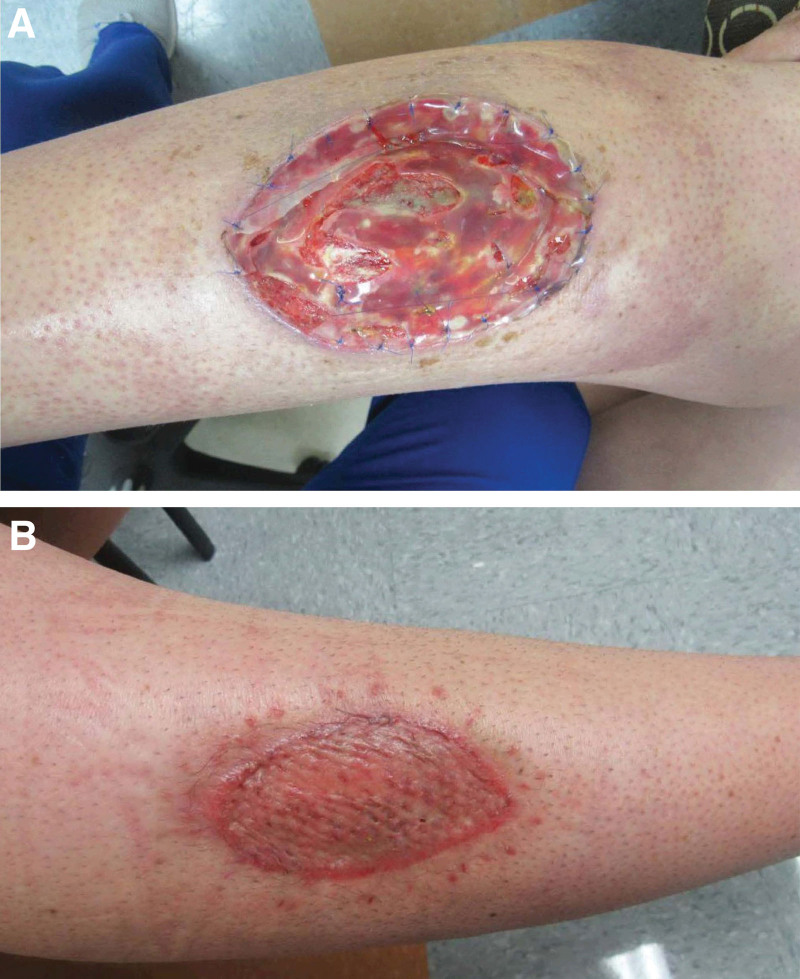
Due to the impact of coronavirus disease 2019 on scheduling and operational limitations, the second resection took place 3 months after the first. A 10 × 2 × 0.5 cm specimen was taken, and clear margins were achieved, leaving a 12 × 10 × 2 cm defect. Reconstruction of the defect was performed by the plastic surgery team using Integra matrix (artificial skin substitute). Artificial skin substitute was used in this case as indicated in similar operations.4 During the follow-up visit 20 days after placement, it was found that the artificial skin substitute had not taken well due to infection, and the patient required reoperation to place a new artificial skin substitute once signs of active infection had cleared. The replacement was scheduled at the same time (May 2021) as the bilateral mastectomy and primary breast reconstruction using Alloderm slings and tissue expanders (Fig. 1), as well as performing a right axillary sentinel lymph node biopsy, which returned negative. The patient completed neoadjuvant chemotherapy and underwent a technetium 99-m uptake study before the scheduled bilateral mastectomy. A month after the combined surgery, the patient was taken back to the operation room for the leg site to be covered using an autologous split thickness skin graft from a right thigh donor site (Fig. 2). No major complications were noted, and a diffuse maculopapular rash was observed, spreading across the patient’s chest and axilla 11 weeks postoperative (Fig. 1B), which resolved later on.
Fig. 2.
Reconstruction of anterior leg after wide resection of UPS. A, Wound 3 weeks after initial Integra placement. B, Wound 1 month after skin grafting.
DISCUSSION
Our patient presented at the time of her LFS diagnosis, already having developed two primary cancers: invasive ductal carcinoma of the breast and undifferentiated pleomorphic sarcoma. It is notable that she was the first in her family to be diagnosed with LFS and has no family history of cancer or significant radiation exposure, indicating a likely de novo mutation. Before LFS diagnosis, her sarcoma was previously missed and believed to be a benign lipoma.
UPS is a rare, aggressive soft tissue cancer.5 In our patient, the course was further complicated by positive margins after initial resection. Each reconstruction after resection also involved multistage components, necessitating multiple operations.
Patients with LFS have significant contraindication to radiation therapy due to the risk of new cancers forming due to their defective DNA repair mechanisms.3 Mastectomy, rather than lumpectomy plus radiation therapy, is generally recommended because of the risks of a second breast primary or a radiation-induced second cancer.6 Although 50% of patients with LFS and unilateral breast cancer develop contralateral breast cancer at some point, there is still ambiguity in indications to undergo prophylactic mastectomy.7 Our patient was consulted on the risk and elected to proceed with the operation. After the mastectomy, we placed Alloderm slings and tissue expanders, rather than direct-to-implant, to allow time for further evaluation of malignancy before adding implants, after the MD Anderson delayed-immediate reconstruction protocol.8 Tissue expanders can be followed with implants for patients willing to undergo further surgery; however, our patient was lost to follow-up and, despite numerous attempts to contact the patient for completion of reconstruction, she did not respond.
After surgery, annual whole-body MRI is typically standard, but for survivors of UPS such as our patient, surveillance is required every 6 months.9 MRI is the preferred modality due to lack of ionizing radiation while still maintaining high sensitivity and specificity. Screening protocol dramatically increases survival, with one study demonstrating 3-year survival increase from 21% to 100%.1 Screening also confers positive psychological benefits, including increased sense of control and security.3,10
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 15 April 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Consul N, Amini B, Ibarra-Rovira JJ, et al. Li-Fraumeni syndrome and whole-body MRI screening: screening guidelines, imaging features, and impact on patient management. AJR Am J Roentgenol. 2021;216:252–263. [DOI] [PubMed] [Google Scholar]
- 2.Guha T, David M. Inherited TP53 mutations and the Li-Fraumeni syndrome. Cold Spring Harbor Perspect Med. 2017;7:a026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langan RC, Lagisetty KH, Atay S, et al. Surgery for Li Fraumeni syndrome: pushing the limits of surgical oncology. Am J Clin Oncol. 2015;38:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers RL, Smock E, Geh JLC. Experience of Integra in cancer reconstructive surgery. J Plast Reconstr Aesthet Surg. 2010;63:2081–2090. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, Antonia S, Benjamin RS, et al. ; National Comprehensive Cancer Network Soft Tissue Sarcoma Panel. Soft tissue sarcoma. J Natl Compr Canc Netw. 2010;8:630–674. [DOI] [PubMed] [Google Scholar]
- 6.Weiss A, Garber JE, King T. Breast cancer surgical risk reduction for patients with inherited mutations in moderate penetrance genes. JAMA Surg. 2018;153:1145–1146. [DOI] [PubMed] [Google Scholar]
- 7.Siegel A, Bremer RC, Klein WMP, et al. Uptake and timing of bilateral and contralateral risk-reducing mastectomy in women with Li-Fraumeni syndrome. Breast Cancer Res Treat. 2022;191:159–167. [DOI] [PubMed] [Google Scholar]
- 8.Kronowitz SJ. Delayed-immediate breast reconstruction: technical and timing considerations. Plast Reconstr Surg. 2010;125:463–474. [DOI] [PubMed] [Google Scholar]
- 9.Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. [DOI] [PubMed] [Google Scholar]
- 10.Bancroft EK, Saya S, Brown E, et al. Psychosocial effects of whole-body MRI screening in adult high-risk pathogenic TP53 mutation carriers: a case-controlled study (SIGNIFY). J Med Genet. 2020;57:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]