Abstract
Transcriptional repression is utilized by human cytomegalovirus to regulate expression of the immediate-early US3 gene. Sequences located 3′ of the US3 TATA box are required for down regulation of expression. Mutagenesis of US3 sequences identified a 10-nucleotide region that is essential for transcriptional repression. In addition to the 10-nucleotide element, an additional region, which includes the US3 initiator element, was needed to confer repression on a heterologous promoter. Thus, a 19-nucleotide element (−18 to +1 relative to the transcription start site) functioned as a transcriptional repressive element (tre). The tre repressed transcription in a position-dependent but orientation-independent manner. In vivo footprinting experiments demonstrated that transcriptional repression is associated with a decrease in protein interactions with the US3 promoter and surrounding sequences. The data presented here suggest that the association of an as yet unidentified repressor protein with the tre represses transcription by inhibiting assembly of the transcription initiation complex on the US3 promoter.
Transcriptional regulation functions as a major control point in determining the levels of gene expression. Although much attention has focused on transcriptional activation, increasingly, transcriptional repression is receiving recognition as an important mechanism for controlling the levels of gene expression. The herpesvirus human cytomegalovirus (HCMV) utilizes multiple mechanisms to regulate viral gene expression, including transcriptional repression.
HCMV is an important opportunistic pathogen in humans and causes disease in immunoincompetent individuals, including transplant recipients, neonates, and people with AIDS (see reference 7 for a review). In these groups of individuals, HCMV can cause a wide variety of diseases ranging from deafness, mental retardation, and death in neonates to pneumonitis in transplant recipients to retinitis, gastroenteritis, and encephalitis in people with AIDS.
In cell culture, viral replication commences with the expression of the immediate-early (IE) genes of the virus followed by early gene and then late gene expression (see reference 29 for a review). IE genes are defined as those that are transcribed following infection in the absence of de novo protein synthesis. A limited number of IE genes have been identified for HCMV; these genes include UL122-123 (the major IE [mIE] gene cluster), US3, UL36-38, and TRS1/IRS1 (nomenclature is according to Chee et al. [9]). Two of the IE genes, the mIE gene and the US3 gene, are subject to complex transcriptional regulation, with expression from these two genes influenced by both transcriptional activation and repression.
The mIE gene encodes two predominant proteins, IE1 and IE2. Expression of the mIE gene is controlled in part through autoregulatory mechanisms. IE1 activates transcription from the mIE promoter, while IE2 is a promiscuous transcriptional activator of other viral and heterologous promoters and an autoregulatory repressor (11, 15, 16, 37). IE2 mediates transcriptional repression of the mIE promoter by interacting with a cis-repressor sequence (crs) located between the TATA box and the transcriptional start site (10, 20, 24, 27, 31). IE2 binds to the crs through minor groove contacts (21). The ability of IE2-crs interactions to repress transcription is dependent on the position and is independent of the orientation of the crs (10, 31). IE2 repression of the mIE promoter appears to occur in part through interference with the function of a cellular protein that binds to the mIE initiator element and increases transcriptional activity (26). In addition, repression of mIE expression is augmented by the expression of another viral protein, pUL84, an early-late protein that associates with IE2 and is involved in lytic-phase replication of viral DNA (13, 34, 35).
The US3 gene encodes three proteins synthesized from alternatively spliced mRNAs (38). The US3 proteins influence cellular gene expression by activating the hsp70 promoter and down regulating expression of the major histocompatibility complex class I heavy chain (1, 12, 18, 39). The US3 gene is not essential for replication in cell culture, but its role in regulating cellular gene expression suggests that it plays a role in pathogenicity of the virus (17, 19).
Expression of the US3 gene, like that of the mIE gene, is regulated both positively and negatively. Silencer and enhancer elements located 5′ of the US3 coding sequences regulate transcription, with the silencer element influencing gene expression in a cell-type-specific manner (8, 40, 41). In addition, sequences located between the TATA box and the transcriptional start site also regulate transcription, repressing expression from the US3 enhancer/promoter element (2). Both viral infection and protein synthesis are required to repress US3 expression mediated through sequences located 3′ of the TATA box. Although the US3 repressive region contains an element with extensive similarity to the mIE crs, IE2 is unable to repress transcription from the US3 promoter (3). I am interested in defining the US3 transcriptional repressive element (tre) and clarifying the role of the US3 sequence element that is similar to the mIE crs. I report here the characterization of the US3 tre.
MATERIALS AND METHODS
Virus and cells.
HCMV (strain Towne) was obtained from Adam Geballe (Fred Hutchinson Cancer Research Center, Seattle, Wash.) and was propagated in primary human fibroblast cultures established from skin tissue samples obtained from O’Bleness Memorial Hospital, Athens, Ohio. Cells were propagated in Dulbecco’s minimal essential medium (DMEM) supplemented with penicillin, streptomycin, glutamine, and 10% NuSerum (Collaborative Research Products, Bedford, Mass.).
Transfections.
Fibroblasts were transfected with plasmid DNA by using DEAE- dextran as described previously (2). Briefly, cells were washed with DMEM containing 25 mM Tris (pH 7) and then incubated with plasmid DNA in a solution of DMEM containing 25 mM Tris and 400 μg of DEAE dextran per ml. After 4 h, the DNA solution was removed and the cells were washed again with DMEM containing 25 mM Tris followed by feeding with complete medium. Cells were infected 40 to 48 h posttransfection at a multiplicity of infection of 10 PFU per cell; 16 to 18 h postinfection (hpi), cells were fed with medium containing 0.44 mM 4-methylumbelliferyl-β-d-galactoside (MUG; Sigma Chemical Co., St. Louis, Mo.). The fluorescence of cell culture medium samples was measured 1 h later as described previously (2). Transfections were repeated a minimum of five times; although there were quantitative differences in values obtained from different transfections, the relative values were consistent among transfections.
Plasmids.
Plasmid DNA was prepared by using alkaline hydrolysis and purified by double banding in cesium chloride gradients. pEQ plasmids were kindly provided by Adam Geballe and have been described previously (6). pEQ3 is a promoterless plasmid that contains the lacZ gene; pEQ235 expresses the lacZ gene under the control of the human immunodeficiency virus type 1 (HIV-1) promoter (sequences from −122 to −20).
The following plasmids were constructed by inserting DNA fragments containing different portions of the US3 repressive region into the XbaI and HindIII sites located 17 nucleotides 3′ of the TATA box present in pEQ235. The DNA fragments containing US3 sequences were prepared by hybridizing pairs of oligonucleotides followed by extension with Klenow polymerase and subsequent digestion with XbaI and HindIII. The oligonucleotides used to construct the various plasmids were as follows: pBJ225, 41 and 48; pBJ256, 70 and 48; pBJ258, 74 and 48; pBJ235, 41 and 9; and pBJ264, 41 and 84. The sequences of the oligonucleotides were as follows: 9, 5′GCCAGCCAAGCTTGTGGACTCAACGGTG3′; 41, 5′AACTCTAGATTCAAAAACACCGTTCAG3′; 48, 5′GCCAGCAAGCTTCGCTGAGAAGTAGCGTGTGGACTGAACGG3′; 70, 5′AACTCTAGAAACACCGTTCAGTCCACA3′; 74, 5′AACTCTAGACACCGTTCAGTCCACA3′; and 84, 5′GCCAGCCAAGCTTGACTGAACGGTGTTTTTGAA3′. PBJ294 contains the US3 sequences from −22 to +2 inserted in the reverse orientation relative to the HIV-1 TATA box in pEQ235 and was constructed by using oligonucleotides 99 (5′ CTAGATGTGCACTGAACGGTGTTTTTGA 3′) and 100 (5′ AGCTTCAAAAACACCGTTCAGTCCACAT 3′).
pBJ171 expresses the lacZ gene under the control of the US3 enhancer, promoter, and sequences to +27 relative to the transcription start site (2); mutations were introduced into pBJ171 by using a Chameleon mutagenesis kit (Stratagene, La Jolla, Calif.). Oligonucleotides for directed mutagenesis of the US3 repressive element were used in combination with oligonucleotide 19, which has the sequence 5′GCAAAAGCCTCCGCGGCCAAAAAAGCC3′ and was designed to convert the unique StuI site present in pBJ171 to a unique SacII site. pBJ171 was mutated with the following oligonucleotides to yield the indicated plasmids as follows: pBJ263, oligonucleotide 80 (5′GCAGTGCTTCGCTGAGTTGAGGCGTGTGGACTGAAC3′); pBJ270, pBJ272, and pBJ273, oligonucleotide 82 (5′ GTAGCGTGTGGACTGGGCAATGGTTTTGAATATATACG3′); pBJ271, oligonucleotide 85 (5′GCGTGTGGACTGGGCAGTGTTTTTGAATATATAGCG3′) pBJ274, oligonucleotide 79 (5′ CTTCGCTGAGAAGTAGATTAGGGACTGAACGGTG3′); pBJ275, oligonucleotide 86 (5′ GGACTGAACGGCGCCTTTGAATATATAGCG3′); and pBJ290, oligonucleotide 81 (5′ GAGAAGT AGCGTGTGTCCCAAACGGTGGTTTTG3′); Plasmid pBJ214 has been described and contains five point mutations in the US3 repressive region (3).
The effect of US3 sequences on another HCMV IE promoter, that of UL122-123 (mIE), was examined by inserting DNA fragments containing US3 sequences 3′ of the mIE promoter and 5′ of the lacZ gene present in pBJ151. pBJ151 contains mIE promoter sequences from −559 to −18 and was constructed from pEQ176 (5) by digestion with SmaI and SpeI, followed by Klenow treatment and religation. pBJ221 was constructed by inserting annealed and extended oligonucleotides 69 and 75 (US3 sequences from −22 to +18) into the XbaI site located 14 nucleotides 3′ of the mIE TATA box in pBJ151. pBJ287 contains the mIE enhancer and TATA box and was constructed by mutating the US3 promoter and surrounding sequences present in pBJ267 to those of the mIE promoter. Oligonucleotide 19 (described above) was used to convert the unique StuI site to a SacII site, and oligonucleotide 89 (5′GGACTGCACGGTGTTTTTGCTTATATAAGGTTTCTTGTCTAGAGG3′) was used to convert the US3 TATA box to the mIE TATA box. pBJ267 was constructed by inserting US3 sequences from −38 to +27 into pBJ242, which contains mIE regulatory sequences from −559 to −45. The pertinent regions of all plasmid constructs were sequenced.
In vivo footprinting.
In vivo footprinting was performed essentially as described by Mueller and Wold (30), using oligonucleotides 104 (5′GCAATCATTTGAGAGATCTGAATTC3′) and 105 (5′GAATTCAGATC3′) as the linker primer pair and oligonucleotides 106 (5′AGTCCCCGGTTTGGAAATC3′), 107 (5′TCCCGGTTTGGAAATCCC3′), and 108 (5′CGGTTTGGAAATCCCAGTACG3′) as the sequential US3-specific primers. For in vivo footprinting, cells were infected with HCMV at multiplicity of infection of 10, with infection and subsequent incubation occurring in the presence of 50 μg of cycloheximide per ml or in the absence of cycloheximide. At 4 hpi, cells were treated with dimethyl sulfate. DNA was extracted, cleaved with piperidine, and subjected to ligation-mediated PCR. Extension products were analyzed on a 6% urea-polyacrylamide gel, using a sequencing ladder for molecular weight markers.
RESULTS
Mutational analysis of the US3 transcriptional repressive region.
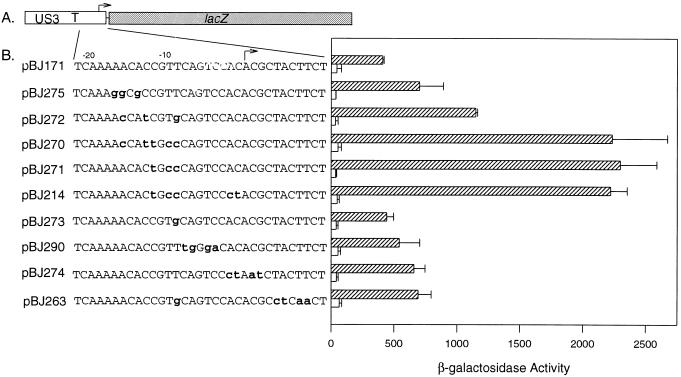
US3 sequences located between the transcription start site and nucleotide −14 (relative to the transcription start site) are essential for transcriptional down regulation of the US3 promoter following infection as defined by deletion analyses (2). However, although this region of the US3 gene contains nucleotides essential for transcriptional repression, the deletion analyses did not delineate the boundaries of the repressive element. To further define the cis-acting repressive element, clustered nucleotide transversions were substituted for nucleotides in the US3 region between the TATA box and nucleotide +10. The plasmid used as the target for mutagenesis was pBJ171, which expresses the lacZ gene under the control of the US3 enhancer, promoter, and sequences 3′ of the TATA box (sequences from −318 to +27 [Fig. 1A, and reference 2]). US3 sequences from −22 to +11 present in pBJ171 are depicted in Fig. 1B, as are the nucleotide substitutions present in the plasmids derived from pBJ171 by using site-directed mutagenesis.
FIG. 1.
Mutational analysis of the US3 repressive region. (A) Diagram of pBJ171, which expresses the lacZ gene (gray rectangle) under the control of the US3 regulatory sequences (open rectangle). Multiple cloning sites (thin line), and transcription start site (bent arrow), are shown. (B) Analysis of β-galactosidase expression from plasmids containing wild-type (pBJ171) or mutated repressive regions. The plasmids used in transient expression assays are listed along with the relevant sequence area of the US3 gene. Mutations in the US3 sequences are indicated by lowercase, bold letters. The reporter gene constructs were transfected into human diploid fibroblasts followed by mock infection (open rectangles) or infection with HCMV (cross-hatched rectangles). β-Galactosidase activity was measured by determining the fluorescence of media containing the cleaved substrate, MUG. The enzyme activities presented are the means of two experiments plus 1 standard deviation. The β-galactosidase values obtained from a promoterless lacZ plasmid, pEQ3, were subtracted from the test values.
The plasmid containing wild-type US3 sequences (pBJ171) and plasmids containing mutations in the US3 repressive region were transfected individually into human diploid fibroblasts by using DEAE-dextran. Promoter activity was analyzed by measuring the levels of β-galactosidase activity following mock infection or infection with HCMV. The results obtained from the transfection experiments are depicted in Fig. 1B. The experiment has been repeated a minimum of five times; the data presented are averages of two experiments. The mutations in the US3 repressive region did not substantially alter the basal levels of transcription for any of the plasmids (levels of β-galactosidase activity seen in the absence of infection [Fig. 1B]).
A low level of β-galactosidase activity was detected for pBJ171 following transfection and infection with HCMV (Fig. 1B and reference 2). Mutagenesis of nucleotides −12, −10, and −9 (pBJ271) was sufficient to alleviate transcriptional repression of the US3 promoter (Fig. 1). Substitutions for nucleotides −16, −13, and −9 also alleviated transcriptional repression, resulting in a threefold increase in β-galactosidase activity (Fig. 1B; compare pBJ272 with pBJ171). Mutagenesis of nucleotides −17, −16, and −14 in pBJ275 had a slight effect on transcriptional repression, with these nucleotide substitutions resulting in increased gene expression compared to pBJ171 (Fig. 1); however, the increase in gene expression was not statistically significant. Thus, analysis of pBJ271 and pBJ272 identified nucleotides from −16 to −9 as playing a critical role in transcriptional repression. A combination of base substitutions at positions −16, −13, −12, −10, and −9 (pBJ270) did not result in greater levels of gene expression compared to pBJ271, suggesting that substitutions at positions −12, −10, and −9 were sufficient for complete elimination of transcriptional repression.
Additional plasmids with other mutations in the US3 repressive region were also examined for an effect on gene expression. A single T-to-G change at nucleotide −9 (pBJ273) had no effect on the activity of the repressive element; substitutions at positions −4, 5, −7, and −8 (pBJ290) also had no significant effect on gene expression. In contrast, base substitutions between nucleotides −2 and +9 (pBJ274 and pBJ263) had a significant and reproducible, albeit slight, effect on gene expression (1.5-fold increase).
These analyses demonstrated that nucleotides −9, −10, and −12 are critical for transcriptional repression of the US3 promoter. Additional sequences, from −16 to −9, also contribute to transcriptional repression, suggesting that this region constitutes the critical DNA element needed for repression of US3 gene expression. The small but reproducible contribution of sequences from −2 to +9 to repression of gene expression suggests that although this region is not essential, it plays a supporting role in transcriptional repression of the US3 promoter.
Transcriptional repression of the HIV-1 promoter by the US3 repressive region.
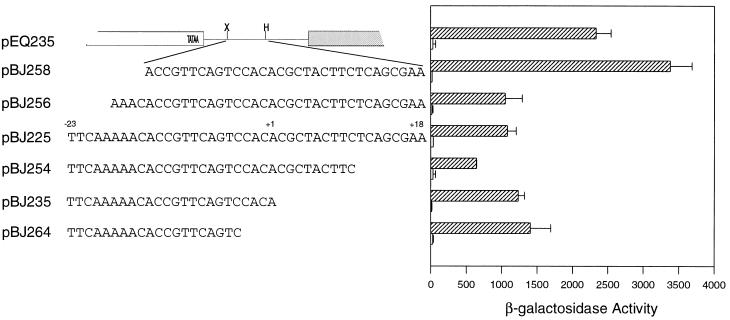
The US3 transcriptional repressive region was also analyzed for the ability to confer transcriptional repression on a heterologous enhancer/promoter, that of HIV-1. Transcription from the HIV-1 promoter is activated by HCMV infection as previously reported (6) and depicted in Fig. 2, where expression from pEQ235 (which contains HIV-1 sequences from −140 to −20 [Fig. 2]) was activated by HCMV infection, resulting in elevated levels of β-galactosidase activity. The effect of US3 sequences on expression from the HIV-1 promoter was examined by inserting portions of the US3 repressive region between the HIV-1 TATA box and the lacZ gene of pEQ235. The resulting plasmids were analyzed for reporter gene expression following transient transfection and mock infection or infection with HCMV.
FIG. 2.
Transcriptional repression of the HIV-1 promoter by the US3 repressive element. The parental reporter gene construct, pEQ235, was drawn to scale. Open rectangle, HIV-1 sequences; gray rectangle, lacZ gene; thin line, multiple cloning region; X, XbaI site; H, HindIII site. US3 sequences were inserted into pEQ235 by using oligonucleotides with XbaI and HindIII ends and containing the depicted US3 sequences. The names of the resulting plasmids are listed. Plasmids were transfected into human diploid fibroblasts and subsequently mock infected (open rectangles) or infected with HCMV (cross-hatched rectangles). β-Galactosidase activity was measured as described in the legend to Fig. 1. The experiment was repeated a minimum of five times; the data presented are averages of duplicate experiments. Background levels of enzyme activity obtained with the promoterless control plasmid were subtracted from the test values.
Initially, US3 sequences from −23 to +18 were inserted into pEQ235, generating pBJ225 (Fig. 2). The insertion of 41 bp of the US3 repressive region 3′ of the HIV-1 TATA box was sufficient to repress expression from the HIV-1 promoter following HCMV infection (Fig. 2; compare pEQ235 and pBJ225 [repression was defined as a 50% or greater reduction in β-galactosidase activity]). This result demonstrated that the US3 sequences repress gene expression in a promoter-nonspecific manner; the presence of the repressive element resulted in decreased expression from the HIV-1 promoter following viral infection.
To identify the minimal element needed to confer transcriptional repression on a heterologous promoter, additional plasmids that contained smaller fragments of the US3 repressive region as depicted in Fig. 2 were constructed. A DNA fragment containing US3 nucleotides from −18 to +18 was also able to repress gene expression (pBJ256 [Fig. 2]); however, removal of an additional four nucleotides (pBJ258, which contains US3 sequences from −14 to +18) resulted in a loss of transcriptional repression (Fig. 2). These constructs demonstrated that the 5′ boundary of the US3 tre is located between nucleotides −17 and −13 and agrees with the mutagenesis data presented in Fig. 1B.
The 3′ boundary of the US3 transcriptional repressive region was identified by analyzing similar plasmid constructions. Insertion of US3 sequences from +10 to −23 (pBJ254) resulted an increase in transcriptional repression compared to pBJ225 (US3 sequences from −23 to +18). This finding suggests that the US3 region from +10 to +18 contains nucleotides that regulate transcription in a positive manner. Further removal of nucleotides from the 3′ end of the US3 repressive region began to alleviate transcriptional repression compared to pBJ254. The insertion of sequences from −23 to +1, creating pBJ235, resulted in transcriptional repression, with a twofold decrease in expression compared to pEQ235 (Fig. 2). Deletion of an additional four nucleotides (pBJ264, containing US3 sequences to from −23 to −4) resulted in β-galactosidase levels that averaged 60% of the activity seen in the absence of US3 sequences (Fig. 2; compare pBJ264 with pEQ235). The less than twofold change in expression defined pBJ264 as not being transcriptionally repressed compared to pEQ235. The gradual increase in gene expression following removal of nucleotides surrounding the US3 transcriptional start site suggests that this region contributes to transcriptional repression and correlates with the mutational analyses performed on the US3 promoter and repressive region (Fig. 1). These experiments defined the 3′ boundary of the repressive element (sequences sufficient to give a twofold decrease in gene expression) as being located between −4 and +1. Thus, a 19-nucleotide element from the US3 gene was sufficient to confer transcriptional repression on a heterologous promoter; the additional sequences present in pBJ254, although not essential, appear to play a supporting role in transcriptional repression.
The 19-nucleotide element encompasses the 13-nucleotide US3 region (−14 to −1) that is similar to the mIE crs; however, the additional 5′ nucleotides (−18 to −15) are critical for conferring transcriptional repression on the HIV-1 promoter. This 19-nucleotide tre contains both regions identified by the mutational analyses in Fig. 1: the essential −16 to −9 region as well as the auxiliary sequences around the US3 transcriptional start site that contribute to transcriptional repression.
Transcriptional repression of the mIE promoter by the US3 tre.
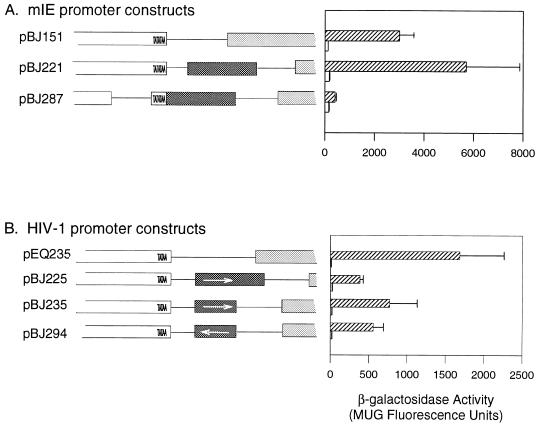
The mIE promoter is subject to complex regulation, including transcriptional repression mediated through the crs, a 13-nucleotide element located at positions −1 to −13. I investigated the ability of the US3 repressor region to substitute for the mIE crs and confer transcriptional repression on the mIE enhancer/promoter. pBJ151 contains the mIE enhancer/promoter region from −18 to −568 and thus lacks the mIE crs (Fig. 3A). To examine the ability of the US3 repressive region to repress expression from the mIE promoter, US3 sequences sufficient to confer transcription regulation on the HIV-1 promoter (nucleotides −23 to +18) were inserted 3′ of the mIE TATA box present in pBJ151, generating pBJ221 (Fig. 3A). The levels of gene expression for the two plasmids, pBJ151 and pBJ221, were compared by using transient expression assays following mock or HCMV infection. Somewhat surprisingly, insertion of the US3 repressive region downstream of the mIE promoter was unable to repress gene expression following infection with HCMV (pBJ221 [Fig. 3]). The inability of these US3 sequences to repress the mIE promoter while repressing the HIV-1 promoter when located in a similar position relative to the TATA box suggested that the strength of the enhancer influences the ability of the tre to regulate transcription. The mIE promoter is regulated through a very strong enhancer element, while expression from the HIV-1 promoter is under the control of a much weaker enhancer element.
FIG. 3.
(A) Analysis of the effect of the US3 tre on the mIE promoter. Reporter gene plasmids that express the lacZ gene under the control of the mIE enhancer/promoter (pBJ151) or the mIE enhancer/promoter and the US3 tre (pBJ221 and pBJ287) were analyzed for β-galactosidase activity following transient transfections of the diagrammed plasmids and mock infection (open rectangles in the graph) or infection (cross-hatched rectangles in the graph). The mIE crs is missing from the parental construct, pBJ151. pBJ287 contains the mIE TATA box and enhancer element; 23 nucleotides of plasmid sequence have been used to replace sequences between the mIE TATA and CAAT boxes. mIE sequences (open rectangles), US3 sequences (dark rectangles), lacZ gene (gray rectangles), and plasmid sequences (thin line) are shown. (B) Orientational effect of the US3 tre on the HIV-1 promoter. Reporter gene plasmids that express the lacZ gene under the control of the HIV-1 enhancer/promoter (pEQ235) or the HIV-1 enhancer/promoter and the US3 tre in the forward (pBJ225 and pBJ235) or reverse (pBJ294) orientation were analyzed for gene expression as depicted in the graph, following mock infection (open rectangles) or infection with HCMV (cross-hatched rectangles). The structures of the plasmids are depicted as follows: HIV-1 promoter, white open-ended rectangle; US3 tre, dark rectangle; lacZ gene, gray rectangle; multiple cloning sequences, thin line. Arrows indicate the orientation of the tre. Diagrams of the regulatory regions in A and B are drawn to scale. Levels of enzyme activity depicted were measured as MUG fluorescence units as described in the legend to Fig. 1; the data presented are averages of two experiments (+1 standard deviation). Background values obtained from a promoterless control plasmid were subtracted from the test values.
Position and orientation of the tre.
The position of a DNA element that regulates transcription, either positively or negatively, can profoundly influence the effect of the element. The position of the tre was also considered a possible contributing factor in determining the efficiency of transcriptional repression. In the US3 gene, the tre is located at nucleotides −18 to +1, while in pBJ221 the tre was inserted an additional 10 nucleotides 3′ of the TATA box (the insertion site was at nucleotide −8 relative to the mIE transcriptional start site). The ability of the US3 tre to repress the mIE promoter was examined when the element was located adjacent to the TATA box. A plasmid, pBJ287, that contains the mIE TATA box and enhancer region with the US3 tre inserted at nucleotide −18 of the mIE gene was constructed and analyzed for β-galactosidase activity following mock or HCMV infection as described above. The level of expression from pBJ287 was markedly repressed compared to that from pBJ151 or pBJ221 (Fig. 3A) or a control plasmid containing a mutant tre (data not shown). This data demonstrated that the US3 repressive region is able to repress the mIE promoter when located in close proximity to the TATA box. Thus, not only is the ability of the tre to repress gene expression influenced by the strength of the relevant enhancer/promoter elements, but it is also influenced by location of the element relative to the TATA box. Appropriate positioning of the tre relative to promoter elements is crucial for its function and influences its ability to act as a transcriptional repressor signal.
The orientational requirement for functionality of the tre was examined by inserting the US3 tre in a reverse orientation relative to the TATA box in pEQ235 generating pBJ294 (Fig. 3B). The ability of a reverse-orientation tre to regulate transcription was analyzed by transient expression assays following mock or HCMV infection. Levels of β-galactosidase obtained from pBJ294 were compared to those from pEQ235, the parent plasmid, and pBJ235, which contains the US3 tre in a forward orientation (Fig. 3B). The presence of the tre in a reverse orientation was able to repress transcription as efficiently as the element inserted in a forward orientation (Fig. 3B; compare pBJ235 with pBJ294). As depicted in Fig. 3B, the tre influences transcription in a position-dependent but orientation-independent manner.
In vivo footprinting.
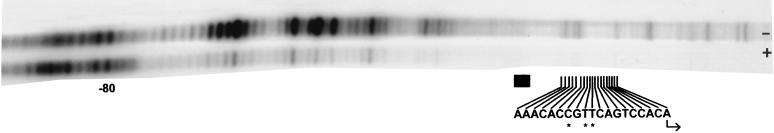
The ability of the tre to influence gene expression negatively suggested that a protein(s) interacts with US3 sequences to regulate the efficiency of transcriptional initiation. The pattern of protein-DNA interactions in the US3 regulatory region was examined by using in vivo footprinting. Human diploid fibroblasts were infected with HCMV, with infection occurring either in the presence of cycloheximide, a protein synthesis inhibitor (an experimental condition that results in abundant US3 transcription) or in the absence of cycloheximide, where US3 transcription is regulated by transcriptional repression (2). Four hours after infection of human diploid fibroblasts with HCMV, infected cells were treated with dimethyl sulfate. DNA was extracted from the infected cells and cleaved with piperidine. Footprinting experiments were performed by using ligation-mediated PCR (30). In cells treated with cycloheximide prior to and during HCMV infection, sequences surrounding the TATA box and the tre were protected from methylation and subsequent piperidine cleavage (Fig. 4), suggesting that under transcriptionally active conditions, a large protein complex interacts with this region of the US3 gene. The protection of a large region including the TATA box and the transcription initiation site is indicative of the presence of the transcriptional initiation complex on the US3 sequences and reflects the high levels of transcription seen in the presence of cycloheximide (2). In contrast, under conditions where US3 is transcribed at very low levels (in the absence of cycloheximide), the transcriptional regulatory region was not protected from methylation and was cleaved at a much higher frequency (Fig. 4), suggesting that conditions of transcriptional repression interfere with the assembly of the transcription initiation complex. The tre was not completely protected under repressive conditions; however, the nucleotides essential for transcriptional repression (−12, −10, and −9 [Fig. 4]) are located in a protected region, suggesting that a repressor protein interacts with this region of the US3 gene. Additionally, a region of protection is located 3′ of the transcription start site and correlates with the involvement of this region in transcriptional repression.
FIG. 4.
In vivo footprinting analysis of the US3 promoter region. Human diploid fibroblasts were infected with HCMV in the presence (+) or absence (−) of cycloheximide treatment. Cells were treated with dimethyl sulfate at 4 hpi; methylated DNA was extracted, cleaved with piperidine, and subjected to ligation-mediated PCR. The PCR products were analyzed on a denaturing 6% polyacrylamide gel; a sequencing ladder (not shown) was used to determine the positions of the PCR products. The location of the TATA box (black rectangle) and the nucleotides involved in transcriptional repression (−18 to +1) are indicated, as is the transcription start site (bent arrow). ∗, nucleotide essential for transcriptional repression.
DISCUSSION
The US3 gene of HCMV is regulated by a complex silencer/enhancer region and sequences located 3′ of the TATA box, which have been termed the tre. Based on the data presented above, the tre consists of a 19-nucleotide element that can be divided into an essential 9-nucleotide region (−18 to −9, termed region A) and an additional region (region B) that encompasses the transcriptional start site and is important for conferring repression on a heterologous promoter (Fig. 2). Region A does not contain a binding site for any known transcription factor, although mutations in region A result in a markedly diminished ability to repress transcription. The ability of mutations in region A to alleviate transcriptional repression suggests that a protein synthesized in virally infected cells interacts with this region to decrease the efficiency of transcriptional initiation. The sequences present in region B contain a consensus initiator element, the sequence of which has been shown to bind a number of cellular proteins (25). In addition to regions A and B, the tre encompasses a predicted IE2 binding site at nucleotides −12 to +2. The relevance of the predicted IE2 binding site is unclear, as IE2 is unable to repress transcription from the US3 promoter in a tre-dependent manner (3).
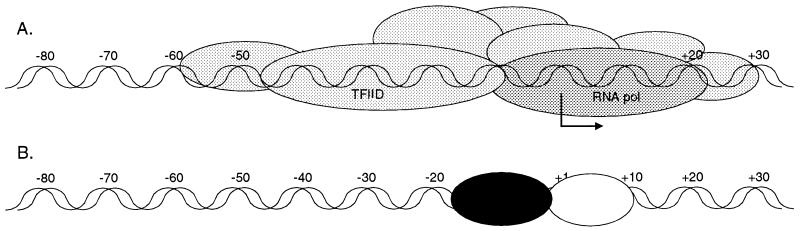
The data presented above has led to the development of a model for transcriptional regulation, where under conditions of active transcription (infection in the presence of cycloheximide), there is very efficient assembly of the transcription initiation complex on the promoter region, leading to abundant levels of US3 RNA. In Fig. 5A, a model depicting the binding of the transcription initiation complex on the promoter is illustrated and is reflective of the in vivo footprinting experiments (Fig. 4). In contrast, under conditions of transcriptional repression (Fig. 5B), proteins interacting with region B facilitate or enhance the interaction of the repressor protein with region A of the tre, accounting for small effects in transcriptional repression with the mutations in region B. The interaction of the repressor protein with the tre is postulated to preclude assembly of the transcription initiation complex.
FIG. 5.
Model of US3 transcriptional regulation. (A) Under conditions of abundant transcription, such as cycloheximide treatment, the transcriptional initiation machinery (depicted as gray ovals) is recruited very efficiently to the US3 promoter. The transcription initiation point is indicated by the bent arrow. (B) Under conditions of transcriptional repression, the transcriptional repressor protein (black oval) interacts with the US3 tre. The binding of the repressor protein to the tre is postulated to be more efficient in the presence of an auxiliary protein (white oval) that binds to sequences around the transcription initiation site. Repressive conditions result in a loss of protein binding of the promoter region (Fig. 4), which is believed to result from the repressor protein interfering with formation of the transcription initiation complex.
The role of the tre is influenced by its position relative to the TATA box and also by the strength of the enhancer region that regulates transcription. The assembly of the transcription initiation complex appears to be a consequence of the balance between transcriptional activators and repressors, with the relative level of transcriptional activity determined by the proximity of the tre to the TATA box and influenced by the efficiency of transcriptional activation.
The utilization of transcriptional repression as a control mechanism expands the repertoire of regulatory pathways that can be utilized by the virus and allows the regulation of US3 expression to be precisely controlled. In other systems, transcription repression occurs through any of several different mechanisms, including competition between activators and repressors for binding of a DNA element, repressor proteins that block the activity of activators, and repressor protein interference with general transcription factors needed for assembly of the transcription initiation complex (14). In addition to the HCMV US3 gene, expression of other herpesvirus genes is also regulated by transcriptional repression. Two of the best-characterized examples are the herpes simplex virus type 1 ICP4 protein and the HCMV IE2 protein. These proteins have a number of properties in common, including the ability to both activate as well as repress transcription, with autoregulation controlling expression of the relevant gene. The interaction of IE2 with the mIE crs blocks the assembly of the transcription initiation complex; specifically, the association of RNA polymerase II with the preinitiation complex is inhibited (22, 42; see reference 36 for a review). The ability of ICP4 to repress gene expression appears to be critical to the life cycle of the virus and determines in part whether the virus will remain latent or become reactivated (23, 28, 32, 33).
The data presented here suggest that the presence of the US3 tre contributes to transcriptional repression by interfering with transcription initiation. The involvement of US3 proteins in modulation of the host immune response suggests that transcriptional repression of the US3 gene is very important in the life cycle of HCMV during infection of the human host.
The question that has yet to be answered is the identification of the proteins that are involved in repression of US3 expression. Transcriptional repression of the US3 gene occurs at 3 to 4 hpi, a time of general transcriptional activity of the HCMV genome. IE2 is unable to repress US3 transcription, and likewise, IE1 either alone or in combination with IE2 is also unable to repress US3 gene expression (3). Examination of the potential roles of other IE proteins in transcriptional repression, either singly or in various combinations, has also failed to identify an IE viral protein that is involved in repression of US3 expression (4). This suggest that a viral protein synthesized at a later stage of infection, i.e., early times postinfection, will contribute to repression of US3 expression.
ACKNOWLEDGMENTS
I thank John Price for expert technical assistance and Frank Horodyski for critical reading of the manuscript.
This work was supported by an Ohio University Baker Award and Council for Tobacco Research grant 4740.
REFERENCES
- 1.Ahn K S, Angulo A, Ghazal P, Peterson P A, Yang Y, Früh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biegalke B J. Regulation of human cytomegalovirus US3 gene transcription by a cis-repressive sequence. J Virol. 1995;69:5362–5367. doi: 10.1128/jvi.69.9.5362-5367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biegalke B J. IE2 protein is insufficient for transcriptional repression of the human cytomegalovirus US3 promoter. J Virol. 1997;71:8056–8060. doi: 10.1128/jvi.71.10.8056-8060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biegalke, B. J. 1998. Unpublished data.
- 5.Biegalke B J, Geballe A P. Translational inhibition by cytomegalovirus transcript leaders. Virology. 1990;177:657–667. doi: 10.1016/0042-6822(90)90531-u. [DOI] [PubMed] [Google Scholar]
- 6.Biegalke B J, Geballe A P. Sequence requirements for activation of the HIV-1 LTR by human cytomegalovirus. Virology. 1991;183:381–385. doi: 10.1016/0042-6822(91)90151-z. [DOI] [PubMed] [Google Scholar]
- 7.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields, virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2493–2524. [Google Scholar]
- 8.Chan Y J, Tseng W P, Hayward G S. Two distinct upstream regulatory domains containing multicopy cellular transcription factor binding sites provide basal repression and inducible enhancer characteristics to the immediate-early IES (US3) promoter from human cytomegalovirus. J Virol. 1996;70:5312–5328. doi: 10.1128/jvi.70.8.5312-5328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, III, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–170. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 10.Cherrington J M, Khoury E L, Mocarski E S. Human cytomegalovirus IE2 negatively regulates αgene expression via a short target sequence near the transcription start site. J Virol. 1991;65:887–896. doi: 10.1128/jvi.65.2.887-896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrington J M, Mocarski E S. Human cytomegalovirus IE1 transactivates the α promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989;63:1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colberg-Poley A M, Santomenna L D, Harlow P P, Benfield P A, Tenney D J. Human cytomegalovirus US3 and UL36-38 immediate-early proteins regulate gene expression. J Virol. 1992;66:95–105. doi: 10.1128/jvi.66.1.95-105.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebert S, Schmolke S, Sorg G, Flöss S, Plachter B, Stamminger T. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J Virol. 1997;71:7048–7060. doi: 10.1128/jvi.71.9.7048-7060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 15.Hermiston T W, Malone C L, Stinski M F. Human cytomegalovirus immediate-early two protein region involved in negative regulation of the major immediate-early promoter. J Virol. 1990;64:3532–3536. doi: 10.1128/jvi.64.7.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermiston T W, Malone C L, Witte P R, Stinski M F. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J Virol. 1987;61:3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones T R, Muzithras V P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J Virol. 1992;66:2541–2546. doi: 10.1128/jvi.66.4.2541-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones T R, Wiertz E J H J, Sun L, Fish K N, Nelson J A, Ploegh H L. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollert-Jons A, Bogner E, Radsak K. A 15-kilobase-pair region of the human cytomegalovirus genome which includes US1 through US13 is dispensable for growth in cell culture. J Virol. 1991;65:5184–5189. doi: 10.1128/jvi.65.10.5184-5189.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang D, Stamminger T. The 86-kilodalton IE-2 protein of human cytomegalovirus is a sequence-specific DNA-binding protein that interacts directly with the negative autoregulatory response element located near the cap site of the IE-1/2 enhancer-promoter. J Virol. 1993;67:323–331. doi: 10.1128/jvi.67.1.323-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang D, Stamminger T. Minor groove contacts are essential for an interaction of the human cytomegalovirus IE2 protein with its DNA target. Nucleic Acids Res. 1996;22:3331–3338. doi: 10.1093/nar/22.16.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Wu J, Luu P, Ghazal P, Flores O. Inhibition of the association of RNA polymerase II with the preinitiation complex by a viral transcriptional repressor. Proc Natl Acad Sci USA. 1998;93:2570–2575. doi: 10.1073/pnas.93.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leopardi R, Michael N, Roizman B. Repression of the herpes simplex virus 1 α4 gene by its gene product (ICP4) within the context of the viral genome is conditioned by the distance and stereoaxial alignment of the ICP4 DNA binding site relative to the TATA box. J Virol. 1995;69:3042–3048. doi: 10.1128/jvi.69.5.3042-3048.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Hermiston T W, Stinski M F. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J Virol. 1991;65:897–903. doi: 10.1128/jvi.65.2.897-903.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo K, Smale S T. Generality of a functional initiator consensus sequence. Gene. 1996;182:13–22. doi: 10.1016/s0378-1119(96)00438-6. [DOI] [PubMed] [Google Scholar]
- 26.Macias M P, Huang L, Lashnit P E, Stinski M F. Cellular or viral protein binding to a cytomegalovirus promoter transcription initiation site: effects on transcription. J Virol. 1996;70:3628–3635. doi: 10.1128/jvi.70.6.3628-3635.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macias M P, Stinski M F. An in vitro system for human cytomegalovirus immediate early 2 protein (IE2)-mediated site-dependent repression of transcription and direct binding of IE2 to the major immediate early promoter. Proc Natl Acad Sci USA. 1993;90:707–711. doi: 10.1073/pnas.90.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael N, Roizman B. Repression of the herpes simplex virus 1 alpha4 gene by its gene product occurs within the context of the viral genome and is associated with all three identified cognate sites. Proc Natl Acad Sci USA. 1993;90:2286–2290. doi: 10.1073/pnas.90.6.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mocarski E S., Jr . Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields, virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 30.Mueller P R, Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989;246:780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- 31.Pizzorno M C, Hayward G S. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major immediate-early promoter through a target sequence located near the cap site. J Virol. 1990;64:6154–6165. doi: 10.1128/jvi.64.12.6154-6165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randall G, Lagunoff M, Roizman B. The product of ORF O located within the domain of herpes simplex virus 1 genome transcribed during latent infection binds to and inhibits in vitro binding of infected cell protein 4 to its cognate DNA site. Proc Natl Acad Sci USA. 1997;94:10379–10384. doi: 10.1073/pnas.94.19.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera-Gonzalez R, Imbalzano A N, Gu B, Deluca N A. The role of ICP4 repressor activity in temporal expression of the IE-3 and latency-associated transcript promoters during HSV-1 infection. Virology. 1994;202:550–564. doi: 10.1006/viro.1994.1377. [DOI] [PubMed] [Google Scholar]
- 34.Sarisky R T, Hayward G S. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J Virol. 1996;70:7398–7413. doi: 10.1128/jvi.70.11.7398-7413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spector D J, Tevethia M J. Protein-protein interactions between human cytomegalovirus IE2-580aa and pUL84 in lyrically infected cells. J Virol. 1994;68:7549–7553. doi: 10.1128/jvi.68.11.7549-7553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenberg R M. The human cytomegalovirus major immediate-early gene. Intervirology. 1996;39:343–349. doi: 10.1159/000150505. [DOI] [PubMed] [Google Scholar]
- 37.Stenberg R M, Stinski M F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985;56:676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenney D J, Colberg-Poley A M. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection. J Virol. 1991;65:6724–6734. doi: 10.1128/jvi.65.12.6724-6734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenney D J, Santomenna L D, Goudie K B, Colberg-Poley A M. The human cytomegalovirus US3 immediate-early protein lacking the putative transmembrane domain regulates gene expression. Nucleic Acids Res. 1993;21:2931–2937. doi: 10.1093/nar/21.12.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thrower A R, Bullock G C, Bissell J E, Stinski M F. Regulation of a human cytomegalovirus immediate-early gene (US3) by a silencer-enhancer combination. J Virol. 1996;70:91–100. doi: 10.1128/jvi.70.1.91-100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988;162:406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, Jupp R, Stenberg R M, Nelson J A, Ghazal P. Site-specific inhibition of RNA polymerase II preinitiation complex assembly by human cytomegalovirus IE86 protein. J Virol. 1993;67:7547–7555. doi: 10.1128/jvi.67.12.7547-7555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]