Abstract
Background:
Tyrosine kinase inhibitors (TKIs) have been approved for treating patients with clinically advanced metastatic thyroid cancer. However among the many TKIs, it remains unknown which regimen is the best choice for these patients.
Methods:
We conducted a systematic review and network meta-analysis to compare the survival benefits and efficacy of the available first-line regimens. We conducted an active search for phase II, III, or IV randomized controlled trials (RCTs) in the PubMed, Embase, and Cochrane databases to compare the effects of at least 2 drugs in the systemic treatment of advanced or metastatic thyroid cancer up to May 2023. The network meta-analysis model was adjusted using Bayesian Network model. Twelve trials with 2535 patients were included in our meta-analysis. The overall survival (OS), progression-free survival (PFS), and serious adverse events (SAEs) were taken as reference indicators. We also performed subgroup analyses of OS and PFS in medullary thyroid cancer (MTC) and radioiodine-refractory differentiated thyroid cancer (RR-DTC) to explore the variations of TKIs in different groups.
Results:
As a result, apatinib had the best effect on overall survival (OS) (hazards ratio [HR] = 0.42, 95% confidence interval [CI] = 0.18–0.98), lenvatinib 18 mg/d has the best effect on progression-free survival (PFS) (HR = 0.13, 95% CI = 0.064–0.27), and cabozantinib 60 mg/d has the best safety profile.
Conclusions:
Our network meta-analysis showed that we believe that cabozantinib has the potential to become a widely used drug in clinical practice.
Keywords: Bayesian Network model, cabozantinib, medullary thyroid cancer, radioiodine-refractory differentiated thyroid cancer, randomized controlled trials, serious adverse events
1. Introduction
Thyroid cancer is the most common malignancy among endocrine malignant diseases. Its global incidence has increased approximately 3-fold over the past 30 years. In the past 10 years, the incidence of thyroid cancer in China has increased by approximately 5 times.[1] Thyroid cancer is divided into 4 main types: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid cancer (MTC), and anaplastic thyroid cancer (ATC). The first two are differentiated thyroid cancers that account for 90% of all thyroid cancers, and the vast majority of patients with differentiated thyroid cancer (DTC) can achieve satisfactory efficacy with surgery, radioactive iodine and thyroid-stimulating hormone (TSH) suppression.[2] However, during radioactive iodine-131 (I131) treatment, some metastases may be dedifferentiated and lose iodine uptake ability, resulting in radioiodine-refractory DTC (RR-DTC) with a 10-year survival rate of only 10%.[3] In addition, MTC and ATC are insensitive to I131 treatment. MTC, ATC, and RR-DTC are broadly referred to as radioiodine-refractory thyroid cancer (RR-TC).[3]
With deepening of the research on the occurrence and development of thyroid cancer, molecular targeted drugs represented by kinase inhibitors have emerged in recent years. These have the advantages of strong specificity, reliable efficacy, and small adverse reactions.[4] Tyrosine kinase inhibitors (TKIs), mainly targeting vascular endothelial growth factor receptor, fibroblast growth factor receptor, and stem cell growth factor receptor, have been widely used in the clinical treatment of lung, colorectal, breast, liver, and other cancers.[5] Different TKIs can effectively improve the progression-free survival (PFS) and overall survival (OS) of patients with RRTC. Among them, lenvatinib, sorafenib, cabozantinib, and vandetanib have been approved by the Food and Drug Administration (FDA) for the treatment of thyroid cancer.[6] Nevertheless, side effects, especially adverse events (AEs) grade 3 or higher such as hypertension, diarrhea, and hand-foot syndrome, cannot be ignored.[7,8]
Previous meta-analyses have shown that TKI targeted therapy has a promising advantage over placebo in terms of disease control rate (DCR), overall response rate (ORR), and PFS for patients with RRTC,[9–11] but the OS could not be synthesized due to immature follow-up data. Furthermore, other emerging tyrosine kinase inhibitors, such as apatinib and anlotinib, are in clinical trials. Of the many treatment options, it is unclear which one is most effective for patients. In this study, we conducted a systematic review and network meta-analysis of approved and published TKIs and some promising TKIs in clinical trials to assist in future treatment methods. Thus, to further improve the treatment strategy and management of RR-TC, we performed this updated meta-analysis to summarize the efficacy and safety of TKI target therapy for these patients.
2. Materials and methods
2.1. Search strategy
We searched PubMed, Cochrane, and Embase databases for phase II, III, and IV randomized controlled trials (RCTs) to compare the effects of multiple drugs in systemic therapies for advanced or metastatic thyroid cancer through May 2023. We screened all eligible articles. Our search process used the following search formula: (tyrosine kinase inhibitors OR multikinase inhibitors OR lenvatinib OR sorafenib OR pazopanib OR cabozantinib OR vandetanib OR apatinib OR anlotinib OR dovitinib) AND (radioiodine-refractory thyroid cancer OR radioiodine-refractory thyroid carcinoma OR medullary carcinoma OR medullary cancer OR advanced or metastatic thyroid cancer OR advanced or metastatic thyroid carcinoma). We extracted data related to efficacy and safety, including PFS, OS and serious adverse events (SAEs). The preliminary screening was mainly completed by 2 researchers independently, and the screening was mainly based on the title and abstract of the paper, excluding the documents that did not meet the requirements, and extracting data from the documents that met the requirements.
2.2. Inclusion and exclusion criteria
All studies included in the network meta-analysis were RCTs. The target patients had to have advanced metastatic DTC or advanced MTC that could not be treated with I131 and had received at least one tyrosine inhibitor. Non-randomized controlled clinical trials, repeated tests, simple subgroup analysis experiments, no-control experiments, reviews, and editorials were excluded. After a full review of the remaining studies, twelve eligible RCTs were included in our studies and analyzed further. The primary outcome measures included the measurements of PFS and OS, and the secondary outcome measure was the evaluation of grade 3 or more SAEs. Two investigators searched and reviewed titles and abstracts for potential studies. When reading the abstract alone does not determine the eligibility of a trial, we will read the full text. When there was a disagreement between the 2 investigators, the third investigator resolved it.
2.3. Data extraction
The following information and data was extracted independently from the studies by 2 investigators: the first author’s name, year of publication, trial phase, number of patients, treatment dose, age, sex percentage, characteristics of thyroid carcinoma, and initial treatment scheme. In addition, hazard ratios (HRs), odds ratios (ORs), and 95% confidence intervals (CIs) related to PFS, OS, and rates of grade ≥ 3 SAEs were retrieved. When 2 researchers disagreed, the problem was resolved through consultation or consultation with a third investigator. After the data is extracted, the 2 investigators jointly checked the accuracy of the data.
2.4. Risk of bias assessment
The risk of bias of the studies was assessed by 2 assessors using the Cochrane risk-of-bias tool. The quality of the studies was assessed mainly as follows, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, data integrity, selective reporting, and other biases. Each item was categorized as low, unclear, or high risk.
2.5. Statistical analyses
Based on the Bayesian Network structure recommended by the United Kingdom’s National Institute for Health and Care Excellence (NICE), we performed a network indirect comparison.[12] For survival outcomes, including OS and PFS, HRs were used to estimate the overall HRs and corresponding 95% confidence interval (CI). To obtain safety results, we estimated the overall ORs and 95% CIs using the incidence of SAEs in each treatment group. We fitted a consistency model and assessed heterogeneity using the I2 statistic. If I2 > 50%, a random-effect model was used. The Markov Chain Monte Carlo (MCMC) algorithm was applied to estimate the processing effect after aging 100,000 samples. The probability of processing the rank was assessed using the surface under the cumulative ranking curve (SUCRA). The SUCRA ranges from 0 to 1, with 1 being the best.[13] Subgroup analyses were performed according to the type of clinical trial evaluated and were divided into MTC and RR-DTC groups. All analyses were performed using R version 3.6.1 with the “gemtc” and “rjags” package.
2.6. Ethics statement
Because the data for our analyses were obtained from public databases, this study did not require ethical approval or patient consent.
3. Results
3.1. Literature search and data extraction
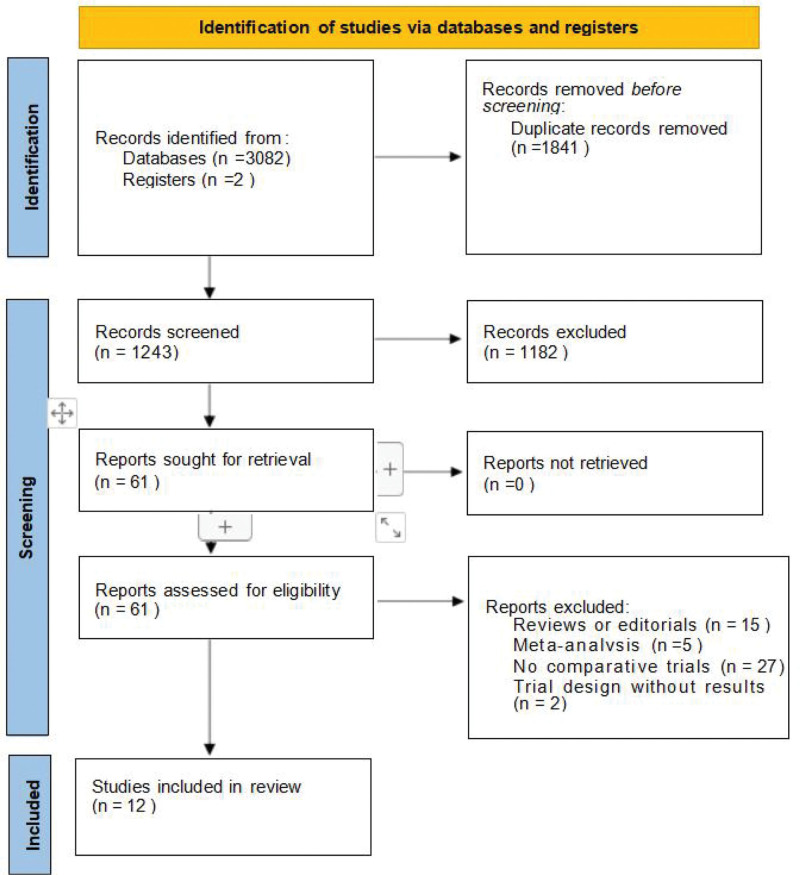
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 3084 studies were searched, and 12 trials were selected for network meta-analysis,[14–25] with a total sample size of 2535. The screening process is shown in Figure 1. Of the 12 studies, 7[14–20] were RR-DTC and 5[21–25] were MTC. The baseline data for all the trials are presented in Table 1. It’s important to note that we specifically label the dosage of the drug. Lenvatinib 24 and 18 mg/d represent the same drug of different dosages. Cabozantinib 60 mg/d is a tablet, while Cabozantinib 140 mg/d is a capsule. We extracted OS, PFS, and SAEs data for the network meta-analysis. PFS data were counted in all 12 trials, OS data were counted in 11 trials except one trial,[20] and SAE data were statistically recorded in 8 trials (all but 4 trials[19,21,23,24]). We also performed a subgroup analysis of OS and PFS according to disease type, divided into RR-DTC[14–20] and MTC[21–25] groups. Finally, the network plot was based on PFS, OS, and SAEs data from all trials.
Figure 1.
Literature retrieval and selection.
Table 1.
Main characteristics of included in network meta-analysis.
| Authors (year) | Cancer type | Registration ID | Median follow-up duration, months | Experimental/control group | Trial phase | Number of patients | Number of patients, Experimental/control | Inclusion criteria | Age | Sex (%male) | Intervention, experimental/control | OS HR (95% CI) | PFS HR (95% CI) | Grade 3 or more AE rates (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brose 2021 | RAIR-DTC | NCT03690388 | C:6.2 P:6.2 |
Cabozantinib/placebo | phase 3 trial | 187 | 125/62 | 1. age: ≥16 2. radioiodine-refractory DTC |
C:65 (56–72) P:66 (56–72) |
46/45 | C: Cabozantinib 60 mg qd P: Placebo |
0.54 (0.27–1.11) | 0.22 (0.13–0.36) | C:64 P:37 |
| Leboulleux 2012 | RAIR-DTC | NCT00537095 | V:18.9 P:19.5 |
Vandetanib/placebo | phase 2 trial | 145 | 72/73 | 1. age: ≥18 2. locally advanced(surgically unresectable) or metastatic differentiated thyroid carcinoma |
V:63 (29–81) P:64 (23–87) |
54/53 | V: Vandetanib 300 mg qd P: Placebo |
0.83 (0.52–1.33) | 0.63 (0.43–0.92) | V:53 P:19 |
| Li 2021 | MTC | NCT02586350 | A:25.8 P:24.8 |
Anlotinib/placebo | phase 2 trial | 91 | 62/29 | 1. age: 18–70 2. unresectable locally advanced or metastatic MTC |
A:51.8 ± 10.6 P:50.9 ± 11.1 |
68/62 | A: Anlotinib 12 mg qd P: Placebo |
0.92 (0.43–1.97) | 0.53 (0.3–0.95) | A:58.1 P:NA |
| Lin 2022 | RAIR-DTC | NCT03048877 | A:18.1 P:18.1 |
Apatinib/placebo | phase 3 trial | 92 | 46/46 | 1. age: ≥18 2. locally advanced or metastatic RAIR-DTC |
A:56 (31–75) P:59.5 (18–79) |
41.3/37.0 | A: Apatinib 500 mg qd P: Placebo |
0.42 (0.18–0.97) | 0.26 (0.14–0.47) | A:78.3 P:17.4 |
| Elisei 2013 | MTC | NCT00704730 | C:13.9 P:13.9 | Cabozantinib/placebo | phase 3 trial | 330 | 219/111 | 1. age: ≥18 2. histologically confirmed, unresectable, locally advanced, or metastatic MTC. |
C:55 (20–86) P:55 (21–79) |
68.9/63.1 | C: Cabozantinib 140 mg qd P: Placebo |
0.98 (0.63–1.52) | 0.28 (0.19–0.40) | C:69 P:33 |
| Schlumberger 2017 | MTC | NCT00704730 | NA | Cabozantinib/placebo | phase 3 trial | 330 | 219/111 | 1. age: ≥18 2.unresectable locally advanced or metastatic MTC |
C:55 (20–86) P:55 (21–79) |
68.9/63.1 | C: Cabozantinib 140 mg qd P: Placebo |
0.85 (0.64–1.12) | 0.28 (0.19–0.40) | NA |
| Schlumberger 2015 | RAIR-DTC | NCT01321554 | L:17.1 P:17.4 |
Lenvatinib/placebo | phase 3 trial | 392 | 261/131 | 1. age: ≥18 2. measurable, pathologically confirmed differentiated thyroid cancer, evidence of iodine-131–refractory disease |
L:64 P:61 |
47.9/57.3 | L: Lenvatinib 24mg qd P: Placebo |
0.62 (0.40–1.00) | 0.21 (0.14–0.31) | L:75.9 P:9.9 |
| Zheng 2021 | RAIR-DTC | NCT02966093 | L:14.8 P:15.6 |
Lenvatinib/placebo | phase 3 trial | 151 | 103/48 | 1.age: ≥18 2.measurable, pathologically confirmed differentiated thyroid cancer, evidence of iodine-131–refractory disease |
L:61 (28–80) P:60 (22–80) |
55.3/43.8 | L: Lenvatinib 24 mg qd P: Placebo |
0.84 (0.39–1.83) | 0.16 (0.10–0.26) | L:87.4 P:45.8 |
| Brose 2014 | RAIR-DTC | NCT00984282 | S:16.2 P:16.2 | Sorafenib/placebo | phase 3 trial | 417 | 207/209 | 1. age: ≥18 2. locally advanced or metastatic RAIR-DTC |
S:63 (24–82) P:63 (30–87) |
50.2/45.2 | S: Sorafenib 400mg bid P: Placebo |
0.8 (0.54–1.19) | 0.59 (0.45–0.76) | NA |
| Wells 2012 | MTC | NCT00410761 | V:24 P:24 |
Vandetanib/placebo | phase 3 trial | 331 | 231/100 | 1. age: ≥18 2. measurable, unresectable locally advanced or metastatic, hereditary or sporadic MTC |
V:50.7 P:53.4 |
58/56 | V: Vandetanib 300 mg qd P: Placebo |
0.89 (0.48–1.65) | 0.46 (0.31–0.69) | NA |
| Capdevila 2022 | MTC | NCT01896479 | C:30 C:30 |
Cabozantinib 60 mg/Cabozantinib 140 mg | phase 4 trial | 247 | 123/124 | 1.age: ≥18 2.histologically confirmed progressive metastatic MTC |
C:59.0 (20–81) C:61.0 (20–82) |
73/60 | C: Cabozantinib 60 mg qd C: Cabozantinib 140 mg qd |
1.12 (0.77–1.63) | 1.24 (0.9–1.7) | C:63 C:72 |
| Brose 2022 | RAIR-DTC | NCT02702388 | L:12.8 L:11.2 |
Lenvatinib 24 mg/Lenvatinib 18 mg | phase 2 trial | 152 | 75/77 | 1. age: ≥18 2. a histologically or cytologically confirmed diagnosis of RR-DTC |
L:65 (36–92) L:66 (21-89) |
54.7/48.1 | L: Lenvatinib 24 mg qd L: Lenvatinib 18 mg qd |
无 | 1.44 (0.76–2.74) | L:61.3 L:57.1 |
3.2. Risk of bias assessment results
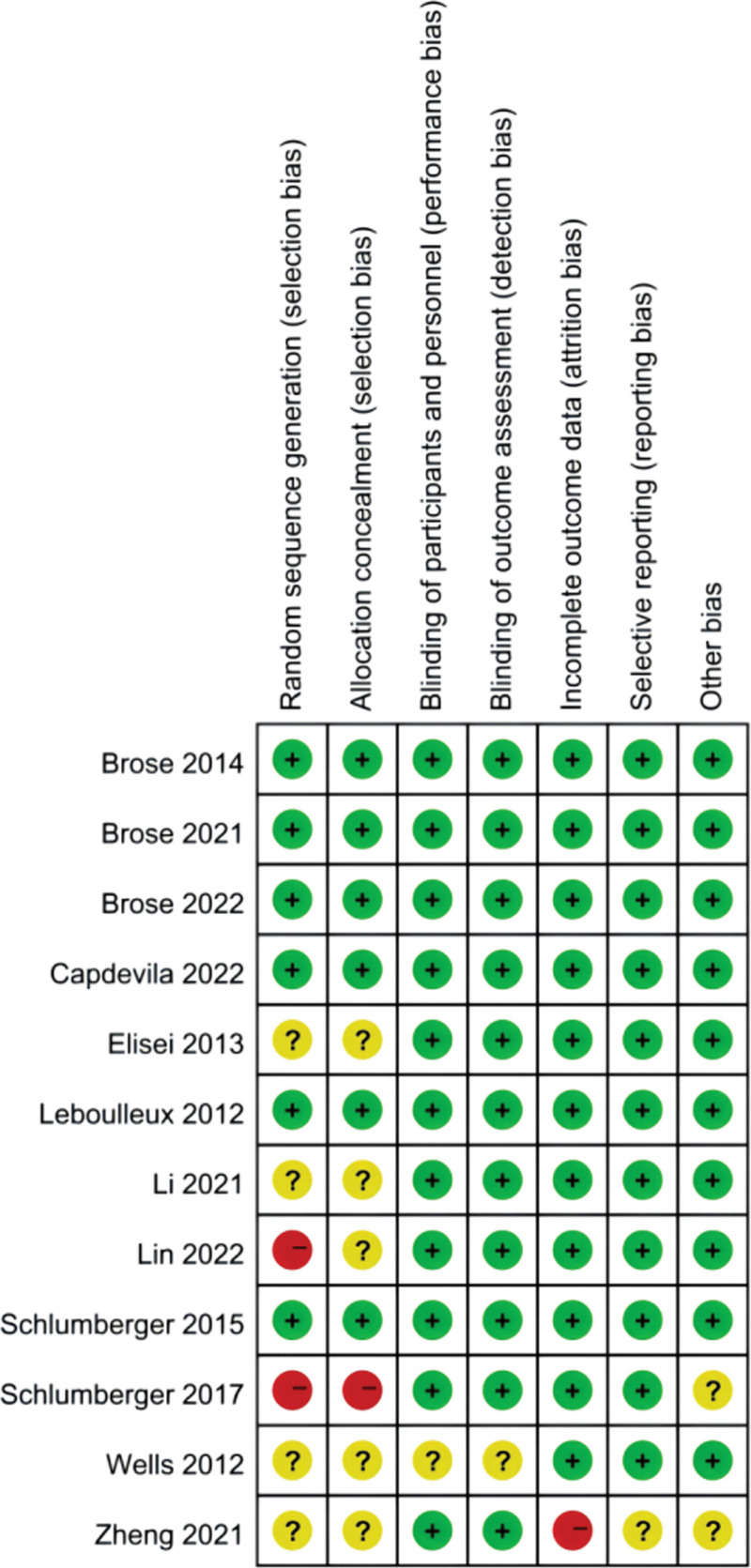
The risk of bias for each study was assessed independently by 2 investigators using the Cochrane Collaboration tool.[26] Disagreements were resolved by consulting a third investigator. The study’s overall risk of bias was judged to be low when more than 4 items were considered low risk, moderate when only 2 to 3 items were considered low risk, and high when fewer than 2 items were considered low risk or more than one high risk of bias. Schlumberger’s study[23] was considered to be of high risk. Wells’ and Zheng’s studies[18,24] were considered to be of moderate risk. The other included studies were well designed and at low risk of bias (Fig. 2).
Figure 2.
Risk of bias summary of the included studies for network meta-analysis.
3.3. Network meta-analysis
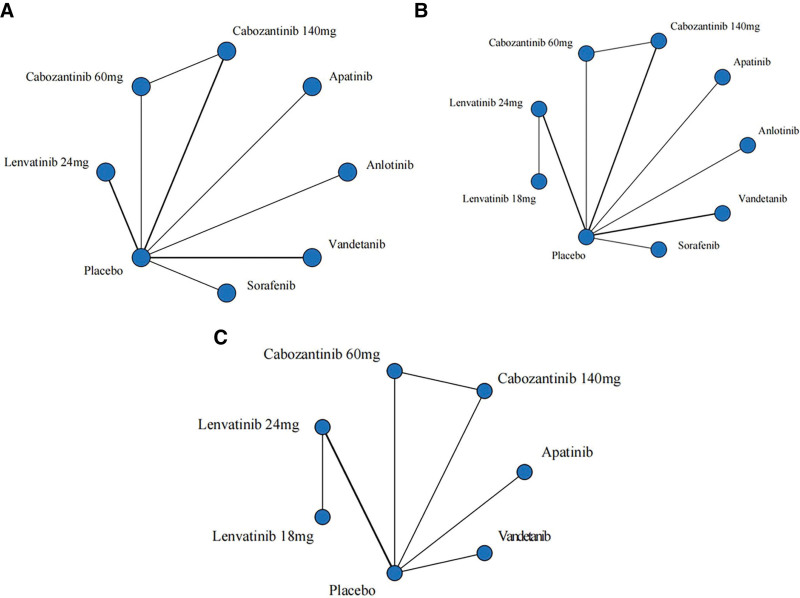
Network contact diagrams about OS, PFS, and SAEs were placed below in the form of pictures (Fig. 3).
Figure 3.
Network of comparative interventions. The thickness of the line indicates the degree of correlation between the 2 scenarios. (A) overall survival; (B) Progression-free survival; (C) adverse event.
3.4. Overall survival
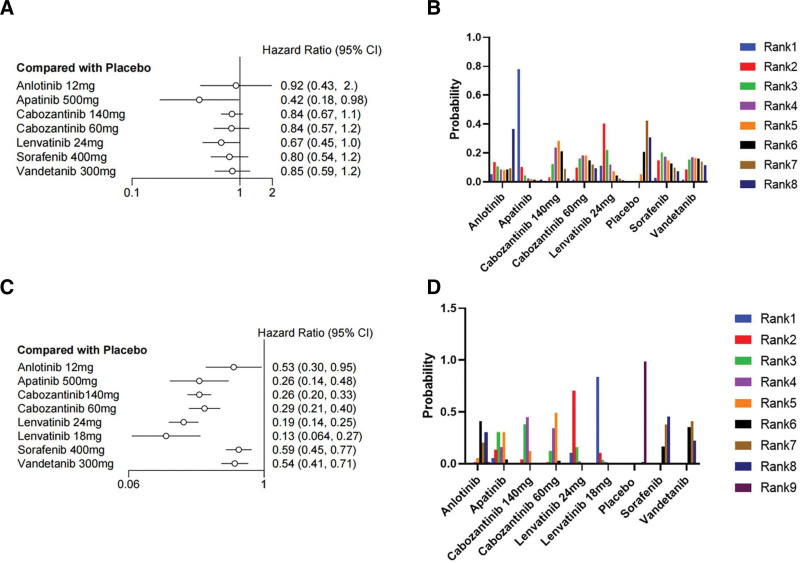
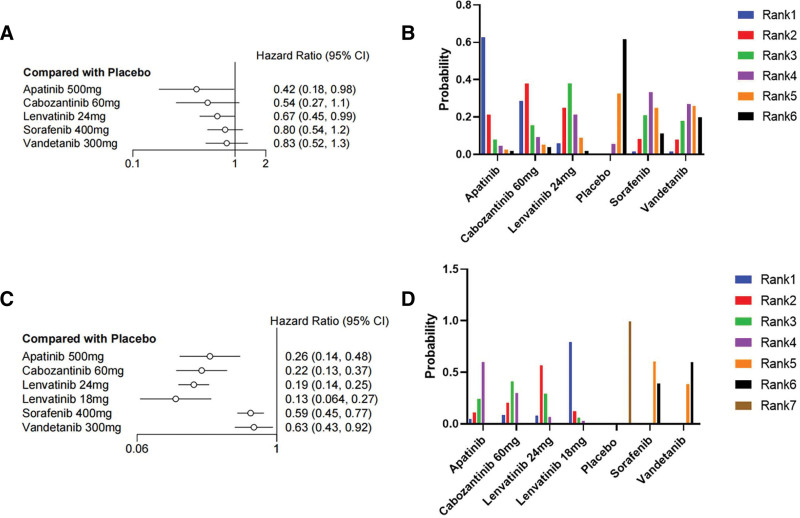
We first made an indirect comparison of OS. Seven drugs were included in the network meta-analysis for indirect comparison. As shown in Figure 4A, lenvatinib 24 mg/d (HR = 0.67, 95% CI 0.45–1.0) and apatinib (HR = 0.42, 95% CI 0.18–0.98) achieved significant clinical survival benefits compared with placebo, exhibiting significantly reduced mortality rates of 33% and 58%, respectively. Of these 2 drugs, apatinib showed the best probability ranking (SURCA = 0.9203957), meaning there is more than 90% probability that it is the best choice, followed by lenvatinib 24 mg/d (SUCRA = 0.7237686). The other 5 drugs (anlotinib, cabozantinib 140 mg/d, cabozantinib 60 mg/d, sorafenib, and vandetanib) did not show a significant survival benefit compared to placebo. I2 = 0% indicated no significant heterogeneity was found in the statistical results. The same trend can be seen in the SUCRA value (Table S1, Supplemental Digital Content, http://links.lww.com/MD/M151) and the rank probability plot (Fig. 4B).
Figure 4.
Forest plot and ranking plot of all drugs. (A) The forest diagram of the OS; (B) The ranking graph of the OS; (C) The forest plot of PFS; d the ranking plot of PFS. CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.5. Progression free survival
We subsequently included 8 drugs in the network meta-analysis of PFS. As shown in Figure 4C, all 8 drugs achieved significant clinical benefits compared to placebo, showing a significant reduction in the risk of progression. Among them, lenvatinib 18 mg/d (HR = 0.13, 95% CI 0.064–0.27) and lenvatinib 24 mg/d (HR = 0.19, 95% CI 0.14–0.25) reduced the risk of progression by more than 87% and 81%, respectively. SUCRA = 0.96699813 indicated that lenvatinib 18 mg/d has more than 90% probability of being the optimal choice. This was followed by lenvatinib 24 mg/d with a SUCRA of 0.85990063, indicating the probability of it being the choice was more than 80%. I2 = 7% indicated that no significant heterogeneity was found. The SUCRA value (Table S1, Supplemental Digital Content, http://links.lww.com/MD/M151) and grade probability plot (Fig. 4D) showed the same treatment trends.
3.6. Subgroup analysis
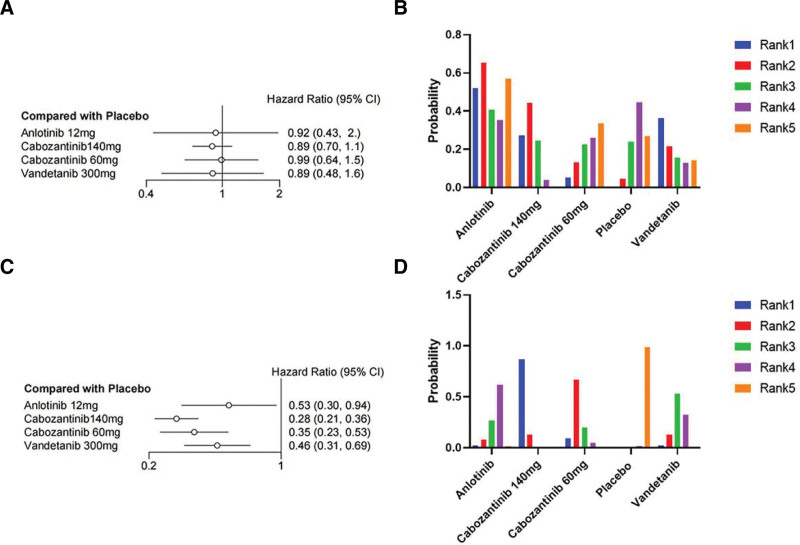
Based on the included clinical trials that assessed differences in thyroid cancer disease types, we performed subgroup analyses of OS and PFS in MTC and RR-DTC. For the RR-DTC group, only apatinib (HR = 0.42, 95% CI 0.18–0.98) and lenvatinib 24 mg/d (HR = 0.67, 95% CI 0.45–0.99) significantly reduced mortality by 58% and 33%, respectively (Fig. 5A). Apatinib was most likely the optimal choice (SUCRA = 0.863248), followed by lenvatinib 24mg/day (SUCRA = 0.729741), with I2 = 8%, indicating no significant heterogeneity (Table S2, Supplemental Digital Content, http://links.lww.com/MD/M152). The PFS results showed all drugs significantly reduced the risk of progression (Fig. 5C); lenvatinib 18 mg/d (HR = 0.13, 95% CI 0.064–0.27) and lenvatinib 24 mg/d (HR = 0.19, 95% CI 0.14–0.25) reduced the risk of progression by more than 87% and 81%, respectively. Lenvatinib 18 mg/d had more than 90% probability of being the optimal choice (SUCRA = 0.945234167), followed by lenvatinib 24 mg/d (SUCRA = 0.776472500). I2 = 11% indicated no significant heterogeneity was found. For the MTC group, the OS results showed that none of the 4 drugs [anlotinib, cabozantinib 140 mg/d, cabozantinib 60 mg/d, and vandetanib] showed a significant reduction in mortality compared to placebo (Fig. 6A), and I2 = 6% indicated that no significant heterogeneity was found. However, the encouraging PFS results showed that all 4 drugs significantly reduced the risk of progression. Among them (Fig. 6C), cabozantinib 140 mg/d (HR = 0.28, 95% CI 0.21–0.36) could reduce the risk of progression by 72% with more than 90% probability of being the choice for patients with MTC (SUCRA = 0.96556750), followed by cabozantinib 60 mg/d (HR = 0.35, 95% CI 0.23–0.53), vandetanib (HR = 0.46, 95% CI 0.31–0.69), and anlotinib (HR = 0.53, 95% CI 0.30–0.94). I2 = 0% indicated that no significant heterogeneity was found. The SUCRA value (Table S2, Supplemental Digital Content, http://links.lww.com/MD/M152) and grade probability plots (Figs. 5B, 5D, 6B, and 6D) showed the same treatment trends.
Figure 5.
Forest plot and ranking plot of drugs treating RR-DTC. (A) The forest diagram of the OS; (B) The ranking graph of the OS; (C) The forest plot of PFS; (D) The ranking plot of PFS. CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
Figure 6.
Forest plot and ranking plot of drugs treating MTC. (A) The forest diagram of the OS; (B) The ranking graph of the OS; (C) The forest plot of PFS; (D) The ranking plot of PFS. CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
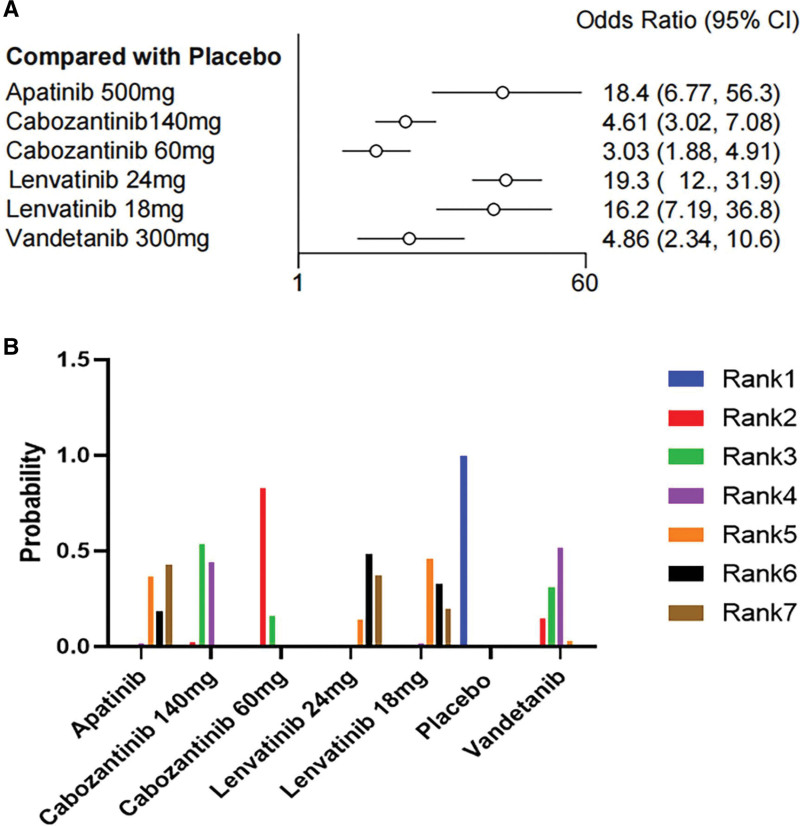
3.7. Serious adverse events
Eight clinical trials included and evaluated SAEs data. As shown, all 6 drugs significantly increased the rates of grade ≥ 3 SAEs compared with placebo. Among the drugs included in the evaluation (Fig. 7A), cabozantinib 60 mg/d (OR = 3.03, 95% CI 1.88–4.91, SUCRA = 0.8031287) demonstrated the highest safety profile, followed by cabozantinib 140 mg/d (OR = 4.61, 95% CI 3.02–7.08, SUCRA = 0.5953671) and vandetanib (OR = 4.86, 95% CI 2.34–10.6, SUCRA = 0.5932262). Lenvatinib 24 mg/d had the highest risk of SAEs (OR = 19.3, 95% CI 12–31.9, SUCRA = 0.1278858) and demonstrated the lowest safety profile. I2 = 24% indicated no significant heterogeneity was found. Network meta-analysis comparison of 7 regimens of high-grade AEs is shown in Table 2. The SUCRA value (Table S3, Supplemental Digital Content, http://links.lww.com/MD/M153) and hierarchical probability plot (Fig. 7B) showed similar security trends.
Figure 7.
Forest plot and ranking plot of the probability of grade 3 or more adverse events in patients treated with tyrosine kinase inhibitor group versus placebo group. (A) The forest diagram of AEs; (B) The ranking graph of the AEs. AEs = adverse events, CI= confidence interval, OR = odds ratio.
Table 2.
Network meta-analysis comparison of 7 regimens of high-grade AEs. AEs adverse events.
| Apatinib | 0.25 (0.08, 0.75) | 0.16 (0.05, 0.5) | 1.05 (0.31, 3.22) | 0.88 (0.22, 3.23) | 0.05 (0.02, 0.15) | 0.26 (0.07, 0.94) |
| 3.99 (1.34, 13.17) | Cabozantinib 140 mg | 0.66 (0.42, 1.03) | 4.19 (2.2, 8.08) | 3.51 (1.4, 8.86) | 0.22 (0.14, 0.33) | 1.06 (0.45, 2.56) |
| 6.08 (1.99, 20.45) | 1.52 (0.97, 2.37) | Cabozantinib 60 mg | 6.37 (3.23, 12.7) | 5.34 (2.07, 13.8) | 0.33 (0.2, 0.53) | 1.61 (0.67, 4) |
| 0.95 (0.31, 3.2) | 0.24 (0.12, 0.45) | 0.16 (0.08, 0.31) | Lenvatinib 24 mg | 0.84 (0.44, 1.6) | 0.05 (0.03, 0.08) | 0.25 (0.1, 0.63) |
| 1.14 (0.31, 4.51) | 0.28 (0.11, 0.71) | 0.19 (0.07, 0.48) | 1.19 (0.62, 2.29) | Lenvatinib 18 mg | 0.06 (0.03, 0.14) | 0.3 (0.1, 0.92) |
| 18.38 (6.77, 56.29) | 4.61 (3.02, 7.08) | 3.03 (1.88, 4.91) | 19.26 (12, 31.95) | 16.18 (7.19, 36.83) | Placebo | 4.86 (2.34, 10.62) |
| 3.79 (1.06, 14.38) | 0.95 (0.39, 2.22) | 0.62 (0.25, 1.5) | 3.97 (1.59, 9.65) | 3.32 (1.09, 10.01) | 0.21 (0.09, 0.43) | Vandetanib |
4. Discussion
This network meta-analysis indirectly compared the treatment options for RR-TC based on 12 RCTs. This study yielded several major findings. First, apatinib worked best in terms of OS. Second, lenvatinib improved PFS better than other treatment regimens. In addition, in the subgroup analysis, apatinib and lenvatinib 24 mg/d had positive effects on patients with RR-DTC; cabozantinib 140 mg/d had a good effect on MTC patients. Finally, in terms of SAEs, cabozantinib 60 mg/d had the highest safety profile and lenvatinib had the highest risk of developing SAEs among the 8 drugs.
Through our indirect comparison, in terms of OS, the indirect comparison of 6 drugs and placebo showed apatinib had a relatively better OS improvement effect than other drugs, perhaps because apatinib is a novel vascular endothelial cell growth factor receptor inhibitor made in China,[27] which can effectively inhibit the kinase activity of vascular endothelial growth factor receptor2 (VEGFR-2), KIT proto-oncogene receptor tyrosine kinase (KIT), and sarcoma gene (SRC) and inhibit the phosphorylation of VEGFR-2, KIT and platelet growth factor receptor b (PDGFRb).[28] In previous studies, apatinib was shown to have an effect on human patients who had received chemotherapy for thyroid cancer and other advanced cancers, such as gastric cancer (6.5 vs 4.7 months, P = .0149), liver cancer, colorectal cancer, and breast cancer.[29–32] It can effectively exert anti-tumor effects. Previous clinical studies have shown that apatinib is a viable treatment option for advanced thyroid cancer with significant efficacy in ORR, PFS, and OS, and a good safety profile.[27] Secondly, lenvatinib had a certain improvement effect on OS, while the other 5 drugs did not show a good improvement effect.
Lenvatinib is the most favorable PFS regimen for treatment of advanced thyroid cancer. Indirect comparisons of placebo, anlotinib, apatinib, sorafenib, cabozantinib, vandetanib, and lenvatinib showed the best PFS benefits. Lenvatinib target the vascular endothelial growth factor receptor (VEGFR1-3), fibroblast growth factor receptor (FGFR1-4), platelet growth factor receptor α (PDGFRα), platelet growth factor receptor (RET), and KIT, inhibiting the vascular and lymphatic vessel growth of tumors, thereby exerting anti-tumor effects.[33–35] In the Brose’s study,[20] the PFS benefits of lenvatinib 18 and 24 mg/d were consistent in the treatment of RR-DTC. Lower starting doses of lenvatinib may affect therapeutic efficacy, but the safety profile of the 2 regimens was similar. In summary, the results of our study support the continued use of approved lenvatinib 24 mg/d in patients with DTC, and a dose of 18 mg/d can be adjusted when tolerated for maximum clinical benefit.
We performed subgroup analyses to determine the best drug regimen choice in RR-DTC and MTC and found that Cabozantinib has some remission effect on progression in patients with MTC, consistent with the results of the included EXAM trial.[22] Cabozantinib has been approved in the United States and Europe for the treatment of progressive metastatic MTC and has shown significant PFS benefit and good tolerability.[36] This finding is consistent with the trend observed in our analysis. Michael’s research shows that Inhibiting MET and VEGFR2 with cabozatinib effectively blocks the progression of MET-driven tumor resistance from drugs that target the VEGF pathway alone, thereby providing a more sustained anti-tumor effect.[37] For RR-DTC, apatinib and lenvatinib have different degrees of remission, and lenvatinib can significantly reduce the risk of progression in these patients.
In terms of drug safety, network analysis revealed that cabozantinib showed the best tolerability. The grade ≥ 3 SAEs were diarrhea, hand-foot syndrome, nausea, and decreased appetite.[14,22,23,25] Cabozantinib 60 mg/d tablet had fewer patients with SAEs than Cabozantinib 140 mg/d capsule. Cabozantinib 60 mg/d tablet may be easier to tolerate, and timely dose adjustment is an important strategy for managing SAEs and improving cabozantinib tolerance. The risk of drug-related SAEs was the highest with lenvatinib, and the SAEs associated with lenvatinib were mainly hand, foot, and mouth disease; hypertension; proteinuria; diarrhea; and fatigue. Most toxic effects were managed by dose adjustment and drug therapy.[17,18,20] Our indirect comparisons showed a close association between lenvatinib and drug-related grade ≥ 3 SAEs. Since the approval of lenvatinib, clinicians have improved their experience with lenvatinib and their ability to predict SAEs and quickly manage toxicity,[20] which has mitigated the effects of drug toxicity to some extent.
From our analysis results, it can be observed that cabozantinib has a statistically better improvement in PFS compared to other drugs. Although there was no statistical difference in OS, possibly due to the fact that some of the included studies only conducted an interim analysis of OS during a limited follow-up time. Based on the existing analysis, the overall trend showed cabozantinib was more likely to benefit OS compared to other groups. Cabozantinib is currently being studied in clinical trials for a variety of tumor types, including medullary and differentiated thyroid cancer, prostate cancer, hepatocellular carcinoma, renal cell carcinoma, and other diseases.[38–42] It is generally well tolerated, does not easily develop drug resistance, and has good effects in many different types of tumors.[38–42] At the same time, cabozantinib has better safety in clinical use because of the lower probability of SAEs. The effectiveness and safety of comprehensive drugs make cabozantinib a better choice than other drugs for the treatment of RR-TC.
Our study is the first analysis in which a network meta-method has been used to compare drugs for the treatment of RR-TC, and our findings may contribute to the best treatment plan being selected more systematically. Secondly, we used subgroup analyses to discuss the 2 most common advanced thyroid cancers: medullary cancer and radioiodine-refractory differentiated thyroid cancer. The most effective treatment options were comprehensively evaluated for these 2 diseases. Our study also included new drugs that had little or no previous inclusion in meta-analysis, such as anlotinib, apatinib, and regimens of lenvatinib and cabozantinib. Our network analysis comprehensively evaluate the efficacy and safety of these drugs that have been proven to be effective in RR-TC. We also indirectly link several drugs that were not directly compared to seek the best treatment plan, and provide treatment rankings of drugs according to indicators such as OS, PFS, SAEs. Furthermore, the heterogeneity in our study was very small, which ensured the accuracy of the study results.
Our study had some drawbacks. The indirect comparison of RCTs had certain limitations when compared to direct comparisons. The differences in the quality of the included articles may have affected the accuracy of the final results. In addition, some trials did not reach the final analysis time for OS. Therefore, the analysis of OS may not be accurate, and more studies are needed to further validate the effects of the drugs on OS.
5. Conclusions
This network meta-analysis indirectly compared the treatment options for RR-TC based on 12 randomized controlled studies and yielded several important findings. First, lenvatinib 18 mg/d was statistically more effective than the other treatments in improving PFS. Secondly, in terms of OS, apatinib resulted in a more pronounced improvement than other drugs. Of all the drugs evaluated, the risk of SAEs was highest with lenvatinib 24 mg/d, while cabozantinib 60 mg/d was considered to have the best safety profile among the included drugs. For RR-DTC, apatinib and lenvatinib have good efficacy, and lowering the dose may help reduce the side effects of lenvatinib to improve PFS. Cabozantinib is the most suitable for MTC and cabozantinib 60mg/day has lower side effects. After combining the results of PFS, OS, and SAEs, we believe that cabozantinib has the potential to become a widely used drug in clinical practice.
Author contributions
Conceptualization: Ruowen Li, Jinghui Lu.
Data curation: Ruowen Li, Mingjian Zhao, Chengxu Miao.
Formal analysis: Ruowen Li, Mingjian Zhao.
Funding acquisition: Jinghui Lu.
Investigation: Ruowen Li, Mingjian Zhao, Zhimin Song, Chengxu Miao.
Methodology: Ruowen Li, Jinghui Lu.
Project administration: Ruowen Li.
Resources: Ruowen Li.
Software: Ruowen Li, Chengxu Miao.
Supervision: Ruowen Li, Mingjian Zhao.
Validation: Mingjian Zhao.
Visualization: Ruowen Li, Mingjian Zhao.
Writing – original draft: Ruowen Li, Zhimin Song.
Writing – review & editing: Jinghui Lu.
Supplementary Material
Abbreviations:
- ATC
- anaplastic thyroid cancer
- CI
- confidence interval
- MTC
- medullary thyroid cancer
- OS
- overall survival
- PFS
- progression-free survival
- RCTs
- randomized controlled trials
- RR-TC
- radioiodine-refractory thyroid cancer
- SAEs
- serious adverse events
- SUCRA
- surface under the cumulative ranking curve
- TKIs
- tyrosine kinase inhibitors
This work was supported by the Natural Science Foundation of Shandong Province (no. ZR2021LZL003).
Ethical approval are not required for the current study due to the nature of the current study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Zhao M, Li R, Song Z, Miao C, Lu J. Efficacy and safety of tyrosine kinase inhibitors for advanced metastatic thyroid cancer: A systematic review and network meta-analysis of randomized controlled trials. Medicine 2024;103:15(e37655).
Contributor Information
Mingjian Zhao, Email: zhaomingjian2022@163.com.
Ruowen Li, Email: 18254887757@163.com.
Zhimin Song, Email: zhiminsong1128@163.com.
Chengxu Miao, Email: mcx133@163.com.
References
- [1].Deng Y, Li H, Wang M, et al. Global burden of thyroid cancer From 1990 to 2017. JAMA Network Open. 2020;3:e208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cooper DS, Doherty GM, Haugen BR, et al.; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. [DOI] [PubMed] [Google Scholar]
- [3].Haugen BR. American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2015;123:372–81. [DOI] [PubMed] [Google Scholar]
- [4].Renouf DJ, Velazquez-Martin JP, Simpson R, et al. Ocular toxicity of targeted therapies. J Clin Oncol. 2012;30:3277–86. [DOI] [PubMed] [Google Scholar]
- [5].Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J Hematol Oncol. 2020;13:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gild ML, Tsang VHM, Clifton-Bligh RJ, et al. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol. 2021;17:225–34. [DOI] [PubMed] [Google Scholar]
- [7].Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80:1271–7. [DOI] [PubMed] [Google Scholar]
- [8].Li J, Gu J. Hand-foot skin reaction with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;119:50–8. [DOI] [PubMed] [Google Scholar]
- [9].Liu JW, Chen C, Loh EW, et al. Tyrosine kinase inhibitors for advanced or metastatic thyroid cancer: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2018;34:795–803. [DOI] [PubMed] [Google Scholar]
- [10].Oba T, Chino T, Soma A, et al. Comparative efficacy and safety of tyrosine kinase inhibitors for thyroid cancer: a systematic review and meta-analysis. Endocr J. 2020;67:1215–26. [DOI] [PubMed] [Google Scholar]
- [11].Tsoli M, Alexandraki KI, Spei ME, et al. Anti-Tumor activity and safety of multikinase inhibitors in advanced and/or metastatic thyroid cancer: a systematic review and network meta-analysis of randomized controlled trials. Horm Metab Res. 2020;52:25–31. [DOI] [PubMed] [Google Scholar]
- [12].Shim SR, Kim SJ, Lee J, et al. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brose MS, Robinson B, Sherman SI, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1126–38. [DOI] [PubMed] [Google Scholar]
- [15].Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. [DOI] [PubMed] [Google Scholar]
- [16].Lin Y, Qin S, Li Z, et al. Apatinib vs Placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY Randomized Clinical Trial. JAMA Oncol. 2022;8:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- [18].Zheng X, Xu Z, Ji Q, et al. A Randomized, Phase III Study of Lenvatinib in Chinese patients with radioiodine-refractory differentiated thyroid cancer. Clin Cancer Res. 2021;27:5502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brose MS, Nutting CM, Jarzab B, et al.; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brose MS, Panaseykin Y, Konda B, et al. A Randomized Study of Lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid cancer. J Clin Endocrinol Metab. 2022;107:776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li D, Chi Y, Chen X, et al. Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB Trial. Clin Cancer Res. 2021;27:3567–75. [DOI] [PubMed] [Google Scholar]
- [22].Elisei R, Schlumberger MJ, Muller SP, et al.Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schlumberger M, Elisei R, Muller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28:2813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wells SA, Jr, Dralle H, Fagin JA, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Capdevila J, Klochikhin A, Leboulleux S, et al. A Randomized, double-blind noninferiority study to evaluate the efficacy of the cabozantinib tablet at 60 mg Per Day compared with the cabozantinib capsule at 140 mg Per Day in patients with progressive, metastatic medullary thyroid cancer. Thyroid. 2022;32:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Du W, Shi X, Fang Q, et al. Feasibility of apatinib in radioiodine-refractory differentiated thyroid carcinoma. Front Endocrinol. 7680;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li H, Huang H, Zhang T, et al. Apatinib: a novel antiangiogenic drug in monotherapy or combination immunotherapy for digestive system malignancies. Front Immunol. 9373;13:07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled Phase III Trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [31].Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [32].Rong X, Liu H, Yu H, et al. Efficacy of apatinib combined with FOLFIRI in the first-line treatment of patients with metastatic colorectal cancer. Invest New Drugs. 2022;40:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–65. [DOI] [PubMed] [Google Scholar]
- [34].Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–71. [DOI] [PubMed] [Google Scholar]
- [35].Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Su J, Wang M, Fu Y, et al. Efficacy and safety of multi-kinase inhibitors in patients with radioiodine-refractory differentiated thyroid cancer: a systematic review and meta-analysis of clinical trials. Expert Rev Anticancer Ther. 2022;22:999–1008. [DOI] [PubMed] [Google Scholar]
- [37].Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–308. [DOI] [PubMed] [Google Scholar]
- [38].Choueiri TK, Escudier B, Powles T, et al.; METEOR investigators. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–27. [DOI] [PubMed] [Google Scholar]
- [39].Koehler VF, Adam P, Fuss CT, et al.; German Study Group for Rare Malignant Tumors of the Thyroid and Parathyroid Glands. German Study Group for rare malignant tumors of the, G. Parathyroid, Treatment of RET-Positive advanced medullary thyroid cancer with multi-tyrosine kinase inhibitors-A Retrospective Multi-Center Registry Analysis. Cancers. 2022;14:3405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cabanillas ME, Brose MS, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24:1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.