Abstract
Malaria vaccines that disrupt the Plasmodium life cycle in mosquitoes and reduce parasite transmission in endemic areas are termed transmission-blocking vaccines (TBVs). Despite decades of research, there are only a few Plasmodium falciparum antigens that indisputably and reproducibly demonstrate transmission-blocking immunity. So far, only two TBV candidates have advanced to phase 1/2 clinical testing with limited success. By applying an unbiased transcriptomics-based approach, we have identified Pf77 and male development gene 1 (PfMDV-1) as two P. falciparum TBV antigens that, upon immunization, induced antibodies that caused reductions in oocyst counts in Anopheles mosquito midguts in a standard membrane feeding assay. In-depth studies were performed to characterize the genetic diversity of, stage-specific expression by, and natural immunity to these two molecules to evaluate their suitability as TBV candidates. Pf77 and PfMDV-1 display limited antigenic polymorphism, are pan-developmentally expressed within the parasite, and induce naturally occurring antibodies in Ghanaian adults, which raises the prospect of natural boosting of vaccine-induced immune response in endemic regions. Together, these biological properties suggest that Pf77 and PfMDV-1 may warrant further investigation as TBV candidates.
INTRODUCTION
The past 20 years have seen unprecedented progress in reducing global malaria mortality and morbidity. According to the World Health Organization, 1.5 billion cases and 7.6 million deaths have been averted since 2000. In 2019, 229 million cases and 409,000 deaths were reported (1). However, several factors such as environmental changes (2), along with emerging resistance against effective drugs (3) and insecticides (4, 5), are affecting intervention efforts. As a result, progress on elimination and eradication has slowed, with the number of cases per capita remaining stable from 2014 to 2019 and some countries experiencing an increase in malaria incidence rate. The current coronavirus disease 2019 pandemic is further compromising the underresourced health care systems in endemic countries and is potentially leading to a more adverse malaria situation in the coming years. According to one modeling study, malaria-related mortality in 2020 could double that reported in 2019 (6). In conjunction with other interventions, a successful malaria vaccine would be an important tool in the fight against malaria, particularly in the face of current and future global pandemics.
Malaria vaccines targeting gametocytes and early stages of parasite development within the mosquitoes can potentially block or reduce transmission. Such vaccines are called transmission-blocking vaccines (TBVs) and depend primarily on the ingestion of human antibodies by Anopheline mosquitoes as part of a blood meal containing sexual-stage malaria parasites.
The feasibility of malaria TBV was first established with avian Plasmodium parasites (7, 8). Several lines of evidence indicate the feasibility of the TBV approach to prevent the transmission of human Plasmodium parasites. Naturally exposed individuals living in endemic regions develop antibodies that reduce oocyst development in mosquitoes as measured in the standard membrane feeding assay (SMFA) (9–14). In addition, monoclonal and polyclonal antibodies directed against sexual-stage Plasmodium falciparum (9, 15) and Plasmodium vivax (16) antigens also effectively block oocyst development. Lastly, multiple candidate TBV constructs induced strong transmission-blocking antibodies in preclinical studies (17–19). If delivered in conjunction with an effective pre-erythrocytic or erythrocytic stage vaccine, TBVs could be an important tool to further curtail malaria transmission.
Because of their complex expression pattern, TBV antigens have characteristics that are different from those seen with asexual pre-erythrocytic and erythrocytic stage antigens. Whereas intraerythrocytic gametocyte antigens are expressed in the human host and induce naturally occurring antibodies that are boosted by repeated infections (13, 20), parasite surface antigens expressed during the mosquito stage of the parasite life cycle are not exposed to the host immune system and do not induce naturally occurring antibodies (21, 22). Compared to asexual blood-stage parasites, gametocyte-stage antigens exhibit a lower degree of genetic diversity (20, 23) as mosquito stages are not subjected to immune pressure and display minimal sequence variations.
Progress in TBV development has been hindered by challenges in producing biologically functional antigens because of multiple cysteine-rich domains, reactogenicity of some vaccine formulations, and limited ability to sustain high concentrations of functional antibodies (17). In addition, despite decades of research, only five malarial antigens (Pfs230, Pfs48/45, HAP2, Pfs25, and Pfs28) reproducibly demonstrate transmission-blocking immunity and efficacy (24). Among these, only two antigens—Pfs25 (25–27) and Pfs230 (28)—have progressed into clinical testing, although other antigens are under different stages of preclinical development (29). Additional challenges in TBV approaches include a difficult clinical development pathway and a need for community-level coverage for any direct benefit to vaccine recipients.
The limited clinical success of the current TBV candidates has intensified the need to identify and evaluate antigens that are relatively antigenically conserved, can be produced as a biologically active immunogen, and induce effective and sustained transmission-blocking antibodies in clinical studies in endemic areas. By applying an unbiased transcriptomics approach, we have identified 56 genes that are abundantly expressed in the P. falciparum gametocyte stage of the parasite’s life cycle. Thirteen of the genes from this dataset, plus one additional gametocyte gene (PF3D7_120400), were expressed as recombinant proteins in Escherichia coli and used in immunization studies in mice. The transmission-reducing activity (TRA) of the elicited antibodies was then determined in the SMFA in two independent laboratories. We identified two TBV candidates, Pf77 and male development gene 1 (PfMDV-1), that displayed transmission-reducing antibody (TRA) activity in the SMFA. Pf77 and PfMDV-1 are relatively invariant and recognized by naturally occurring antibodies in Ghanaian adults, raising the possibility that natural exposure would maintain durable antibody responses after vaccination.
RESULTS
A transcriptomics-based identification of TBV candidates identified Pf77 and PfMDV-1
We have identified gametocyte-enriched biomarkers by performing microarray analysis comparing RNA isolated from P. falciparum stage V gametocytes versus asynchronous asexual blood-stage parasites. A total of 56 genes were identified to be transcriptionally over-expressed by >50-fold in gametocytes compared to asynchronous asexual blood-stage parasites. Previously, from this dataset, we discovered a gametocyte biomarker (Pfg17) that is superior to Pfs25 in detecting asymptomatic infectious reservoirs (30). Here, we used this same dataset of 56 gametocyte-enriched genes to screen for TBV candidates (data file S1). To select genes that were both differentially expressed in gametocyte versus asexual blood-stage parasites and transcriptionally abundant in gametocytes, we plotted the fold change and the cyanine 5-cytidine triphosphate (cy5) signal, which represents the number of labeled transcripts of an expressed gene that hybridized to the corresponding oligonucleotide on the microarray (Fig. 1). In this dataset, we searched for antigens that were not previously studied as a TBV, were highly expressed on gametocytes, and preferably were expressed on the surface of or secreted from the parasite. Using these criteria, we selected 13 highly expressed gametocyte genes, plus one additional conserved gene (PF3D7_1204200) of unknown function as an internal control, for production as purified recombinant proteins to evaluate their ability to induce antibodies that have TRA after vaccination in mice.
Fig. 1. Gene expression profiling of Plasmodium falciparum gametocytes by microarray identified Pf77 and PfMDV-1 as candidate transmission-blocking vaccine antigens.
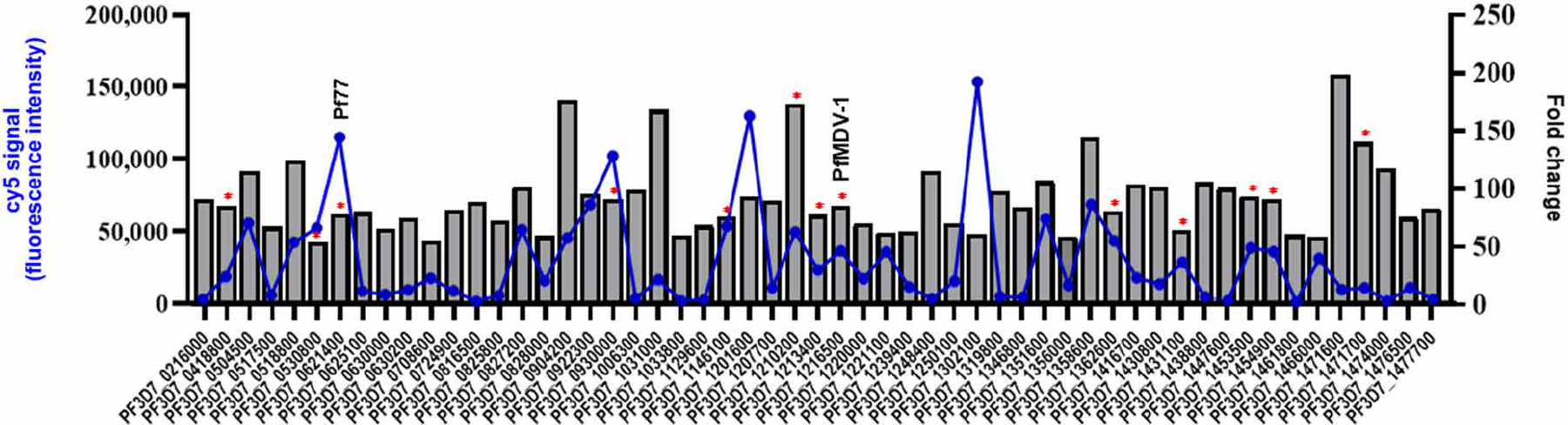
Identification of gametocyte-enriched transcripts by gene expression profiling was used as a first screen for selection of transmission-blocking vaccine candidates. cRNA microarray was used to compare cRNA prepared from stage V gametocytes versus asynchronous blood-stage parasites. A total of 56 genes were transcriptionally up-regulated by >50-fold across four replicates (P < 0.01) in the P. falciparum gametocyte and were plotted by differential gene expression (fold change) and transcriptional abundance within the gametocyte (cy5 signal). Selection of a subset of these 56 genes for further evaluation as transmission-blocking vaccine candidates was guided by two-layered criteria of strong fold up-regulation and high cy5 intensity to ensure that selected transmission-blocking vaccine candidates were not only highly differentially expressed (increased fold change) by the gametocyte but also transcriptionally abundant (increased cy5 signal) within the gametocyte. Asterisk (*) denotes genes selected for recombinant expression and efficacy studies to determine transmission-reducing activity. One additional P. falciparum gametocyte gene, PF3D7_120400 (not in this dataset), was also selected for recombinant expression.
Antigenic analysis of P. falciparum recombinant proteins
We constructed 16 recombinant plasmids encoding for protein domains from the 14 P. falciparum gametocyte genes. Of these 16 constructs, 12 encoded a single protein domain and 2 genes were expressed as 2 different protein domains encoded by separate constructs. Table S1 contains details pertaining to the PlasmoDB ID, protein domain, and primers used for polymerase chain reaction (PCR) amplification. Recombinant proteins were expressed in E. coli with a hexahistidine tag at the N or C terminus and purified. We were able to successfully express and purify 16 recombinant proteins, which were further evaluated for TRA. The mobility and purity of each protein were determined using a Coomassie blue–stained polyacrylamide gel (fig. S1). We also confirmed the identity of the two most potent TRA antigens by mass spectrometry (table S2).
Antibodies targeting TBV candidates exhibit transmission-reducing activity in a SMFA
During this study, we immunized a total of 195 BALB/c mice four times at 4-week intervals with 16 recombinant antigens delivered in one of two different adjuvants—complete Freund’s adjuvant (CFA) or Montanide/CpG. The TRA efficacy of antibodies was determined in serum samples collected 2 weeks after the fourth immunization.
Total immunoglobulin G (IgG) from antisera generated in mice against each recombinant protein was purified on protein G columns and tested at the concentration of 750 μg/ml, with human complement, in SMFA. In each SMFA, IgG from naïve mice was used as a negative control and 4B7 monoclonal antibody (anti-Pfs25) was used as a positive control. Primary screening of all 32 TRA immune sera (16 CFA and 16 Montanide/CpG) in SMFA was performed at the Johns Hopkins Malaria Research Institute (JHMRI). Purified IgGs from the 10 serum samples showing the highest TRA are indicated in table S3. Further analysis of the SMFA data revealed that anti-Pf77 and anti–PfMDV-1 antibodies were capable of completely blocking oocyst development in a large proportion of mosquitoes that had received an infectious blood meal. Results showed that in the trial of the experiment evaluating the TRA of anti-Pf77 IgG, 0 of 27 (0%) mosquitoes in the control IgG group and 10 of 32 (31.25%) mosquitoes in the anti-Pf77 IgG group had no oocysts in the midgut. Likewise, in the experiment evaluating the TRA of anti–PfMDV-1 IgG, 3 of 47 (6.38%) mosquitoes in the control IgG group and 6 of 40 (15%) in the anti–PfMDV-1 IgG group had no developing midgut oocysts (fig. S2).
To obtain independent verification, a secondary screening was performed at the Laboratory of Malaria and Vector Research (LMVR) at the National Institute of Allergy and Infectious Diseases (NIAID). Overall, vaccination with Montanide/CpG adjuvant induced antibodies with superior TRA (table S4) compared to vaccination with CFA; 7 of the 10 TRA candidates selected for secondary screening were delivered in Montanide/CpG.
The 10 IgG samples selected for secondary screening were tested at 750 μg/ml with complement by SMFA at LMVR, NIAID. Each test antibody was examined in two independent assays, and the results of each assay are shown in table S4. Figure 2 shows the estimates of percent inhibition (%TRA) from two feeds. A total of seven sera showed reduction in oocysts, and two of these, Pf77 (Pf3D7_0621400) and PfMDV-1 (Pf3D7_1216500), displayed substantial (>80%) inhibition of transmission. In two independent experiments, Pf77 and PfMDV-1 exhibited an average of 93 and 84% inhibition, respectively. Although one cannot mathematically determine the best estimate of percent inhibition in infected mosquitoes from the two independent assays, the number of infected mosquitoes out of 20 mosquitoes dissected for each IgG sample in each feed is presented in table S4.
Fig. 2. Serum from mice immunized with Pf77 and PfMDV-1 blocks transmission in a standard membrane feeding assay.
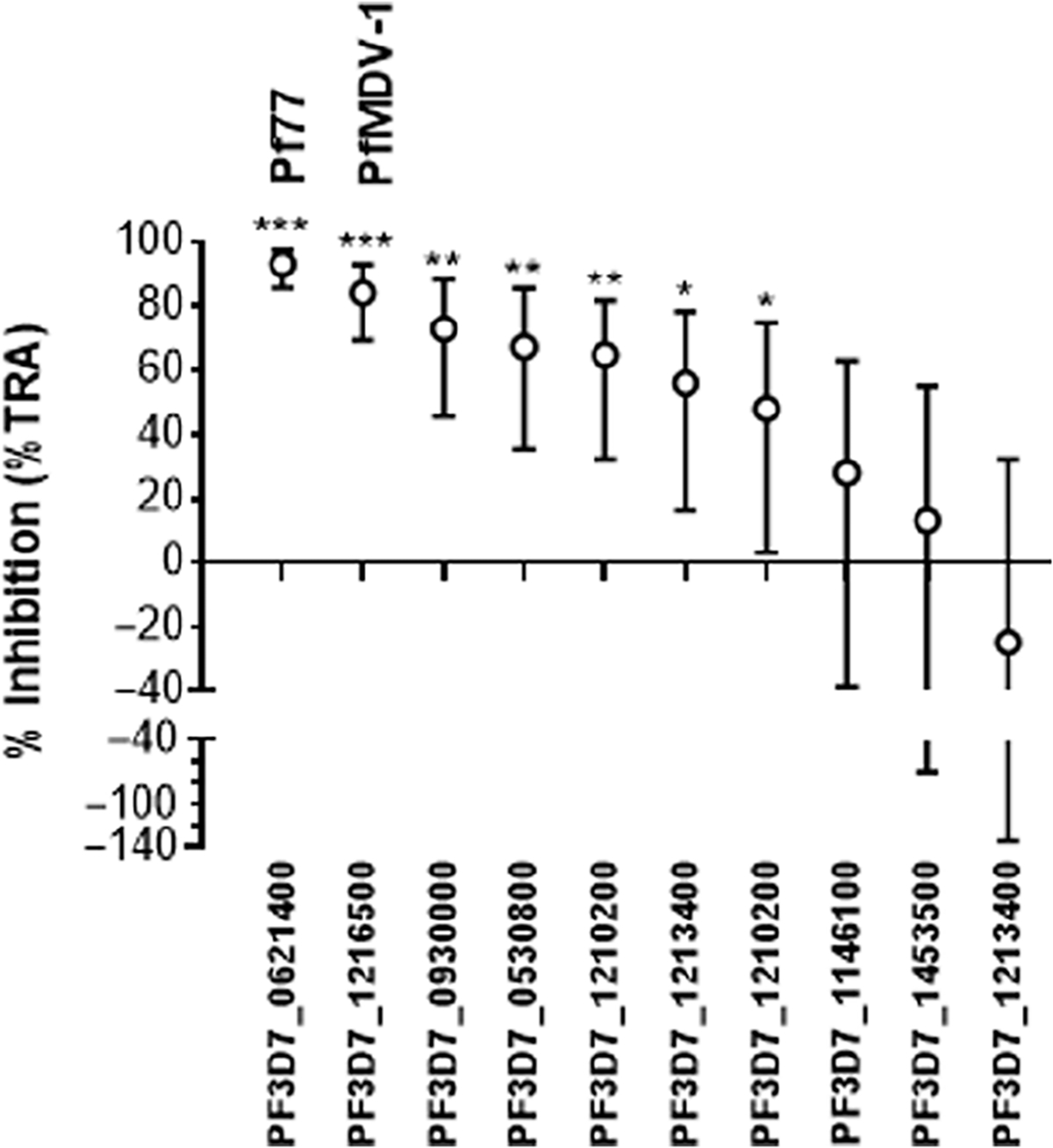
Seven IgG antibody samples display functional activity by the standard membrane feeding assay (SMFA). Ten total IgG samples were tested at 750 μg/ml with complement in two independent SMFAs. The best estimates of percent reduction of oocyst density [% transmission-reducing activity (TRA), open circles] and the 95% confidence interval (error bars) from two independent feeds are shown. *P < 0.05, **P < 0.005, and ***P < 0.001.
The specificity of SMFA in our laboratory has been evaluated during the qualification of the assay (31). In addition, in our hands, the same concentration of mouse IgGs purified from noninhibitory antisera, such as adjuvant control or antisera against non-TBV antigens, did not show any inhibition (32–35). Therefore, the substantial TRA observed in this study is specific to antibodies derived from immunization against Pf77 and PfMDV-1 antigens.
Pf77 and PfMDV-1 are expressed throughout the P. falciparum life cycle
To determine the expression profile of Pf77 and PfMDV-1, transcript and protein levels were measured in stage V gametocytes, developing oocysts, sporozoites, and asexual blood stages.
Using quantitative PCR (qPCR), we found that Pf77 is transcriptionally expressed throughout the developmental cycle. Although expression peaked during the gametocyte stage (2,627,397 copies), Pf77 mRNA was also abundantly detected in asexual blood forms (123,297 copies), sporozoites (8680 copies), and developing oocysts (day 2: 2785 copies; day 6: 107,714 copies) (Fig. 3A). Likewise, for PfMDV-1, although peak expression occurred during the gametocyte stage (2,136,614 copies), PfMDV-1 mRNA was also clearly detected in asexual blood forms (104,057 copies), sporozoites (11,315 copies), and developing oocysts (day 2: 543 copies; day 6: 70,695 copies) (Fig. 3A).
Fig. 3. Measurement of stage-specific expression of Pf77 and PfMDV-1.
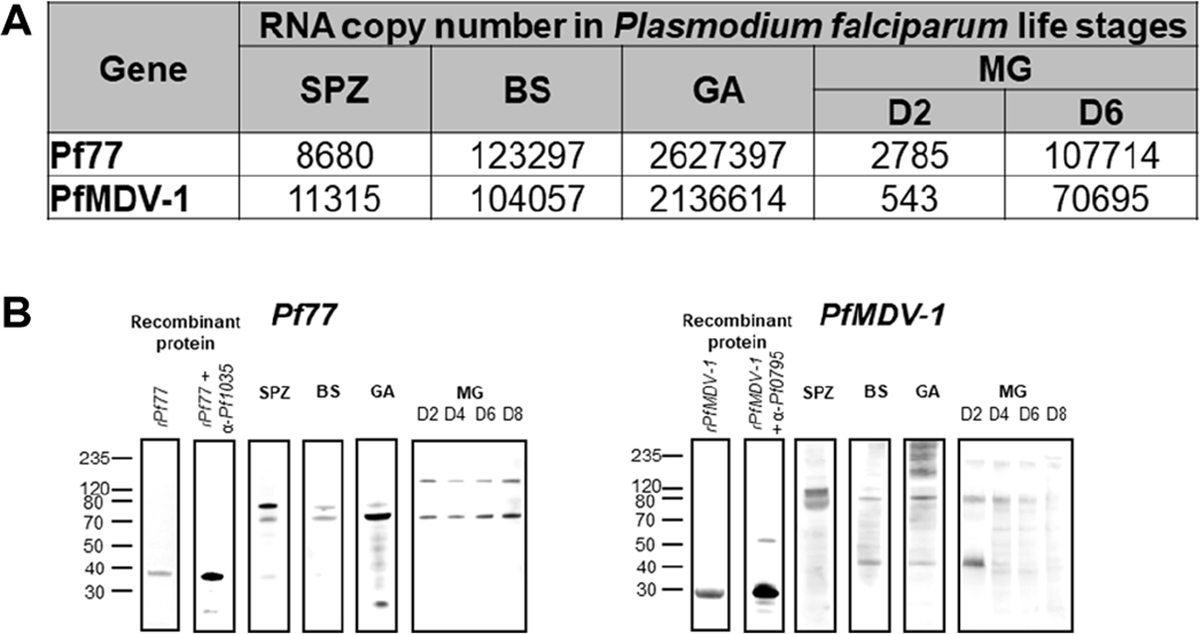
(A) Quantitative real-time PCR during the sporozoite (SPZ), intraerythrocytic (BS), gametocyte (GA), and mosquito midgut (MG) oocyst stages of the P. falciparum parasite. Values shown represent mRNA copy number per 50 ng of template RNA. (B) Analysis of stage-specific expression of Pf77 and PfMDV-1 protein in P. falciparum parasites by Western blot. For Pf77, 100 ng of Pf77RR315–664RR was loaded and an appropriately sized band (about 40 kDa) was observed by both Coomassie blue staining and Western blot analysis using anti-Pf77 IgG purified from mouse polyclonal serum. In addition, 4.5 × 105 sporozoites, 1.875 × 106 parasitized erythrocytes, 1.2 × 106 gametocytes, and 10 oocysts were loaded per lane and a 77-kDa band was detected in the sporozoite, intraerythrocytic, gametocyte, and mosquito midgut oocyst (days 2, 4, 6, and 8) stages of the parasite by Western blot. For PfMDV-1, 4.5 × 105 sporozoites, 1.875 × 106 parasitized erythrocytes, 1.2 × 106 gametocytes, and 10 oocysts were loaded per lane and a 43-kDa band was detected in the intraerythrocytic, gametocyte, and mosquito midgut oocyst (day 2) stages of the parasite by Western blot.
We further investigated the multistage expression of Pf77 and PfMDV-1 in P. falciparum parasites. Because we used asynchronous asexual-stage P. falciparum parasite cultures for mRNA isolation, we were concerned that the transcriptional expression observed in blood-stage parasites could be an artifact of residual gametocytes in asexual-stage P. falciparum parasite cultures. To address this concern, expression of Pf77 and PfMDV-1 was compared to six other genes in qPCR that displayed a similar degree of transcription in gametocytes (table S5). We found that transcription of Pf77 (Pf3D7_0621400) and PfMDV-1 (Pf3D7_1216500) was more than 10-fold higher in blood-stage culture than other genes tested in our dataset (PF3D7_1453500, PF3D7_0930000, and PF3D7_1213400) despite exhibiting similar abundance of gametocyte-specific transcripts. These results confirm transcriptional expression of Pf77 and PfMDV-1 in asexual blood forms.
Stage-specific expression of Pf77 and PfMDV-1 at the level of translation was measured by Western blot analysis after reactivity with mouse antibodies generated against recombinant Pf77 and PfMDV-1 (Fig. 3B). Consistent with qPCR results, although peak protein expression was observed in gametocytes, Pf77 is pan-developmentally expressed in oocysts, sporozoites, and asexual blood stages of the parasite. Whereas asexual blood stages exhibited low expression, protein concentrations increased in oocysts as they progressed from days 2 to 8 with detectable abundance in mature salivary gland sporozoites. For PfMDV-1, we observed a protein band on the gametocyte, asexual blood form, and oocyst stages (day 2) of the parasite by Western blot. In Western blot, normal mouse serum did not exhibit reactivity to parasite lysates from P. falciparum sporozoites, schizonts, gametocytes, and recombinantly expressed Pf77 or PfMDV-1 (fig. S3).
Antigen specificity of polyclonal serum derived from immunization of mice with recombinant Pf77 or PfMDV-1 was further demonstrated by enzyme-linked immunosorbent assay (ELISA). Compared to pooled normal mouse serum from nonimmunized mice, pooled sera from immunized mice demonstrated significantly higher ELISA IgG reactivity against parasite lysates from P. falciparum asexual schizonts (Pf77: P = 0.0242; PfMDV-1: P = 0.00053) and stage V gametocytes (Pf77: P = 0.00107; PfMDV-1: P = 0.000006) (fig. S4). These results demonstrated that anti-Pf77 and anti-PfMDV-1 antibodies raised against recombinantly produced antigens recognized native antigenic epitopes expressed by P. falciparum asexual-stage schizonts and gametocytes.
Confocal microscopy images reveal multistage expression of Pf77 and PfMDV-1 in P. falciparum
We also performed confocal microscopy using anti-Pf77 and anti-PfMDV-1 antibodies raised in mice by immunization with corresponding recombinant proteins to determine the expression of these molecules in P. falciparum during different developmental stages in the human host. Anti-Pf77 antibody showed a distinct dense and punctate pattern of expression on the surface of P. falciparum sporozoites (Fig. 4A). For asexual early- and late-stage schizonts, a light scattered pattern of expression was observed, which was mostly localized on the parasite surface, but some cytoplasmic expression was also observed (Fig. 4, B and C). In comparison, Pf77 expression on mature gametocytes was intense and uniformly distributed on the surface as well as in the cytoplasm (Fig. 4D). For PfMDV-1, a patchy and punctate expression was detected on sporozoites (Fig. 4E). Curiously, in the sporozoite stage, PfMDV-1 protein was not detected by Western blot (Fig. 3), but it could be clearly seen in confocal microscopy (Fig. 4E), indicating either very low expression or that PfMDV-1 recognition may be conformation dependent. Expression in asexual early- and late-stage schizonts was both on the surface and in the cytoplasm (Fig. 4, F and G). For late gametocyte stages, PfMDV-1 expression was strong and punctate and distributed throughout the parasite surface and distributed internally in a spiral-shaped expression pattern surrounding the parasite nucleus (Fig. 4H). Previously, by immunoelectron microscopy, Furuya et al. (36) have shown PfMDV-1 expression on the gametocyte plasma membrane, parasitophorous membrane (PVM), and cleft-like structures that appear with extensions of PVM. Overall, our confocal microscopy studies have further confirmed that Pf77 and PfMDV-1 are multistage antigens expressed in stage V gametocytes as well as sporozoites and asexual early- and late-stage schizonts.
Fig. 4. Pf77 and PfMDV-1 expression in P. falciparum by confocal microscopy.
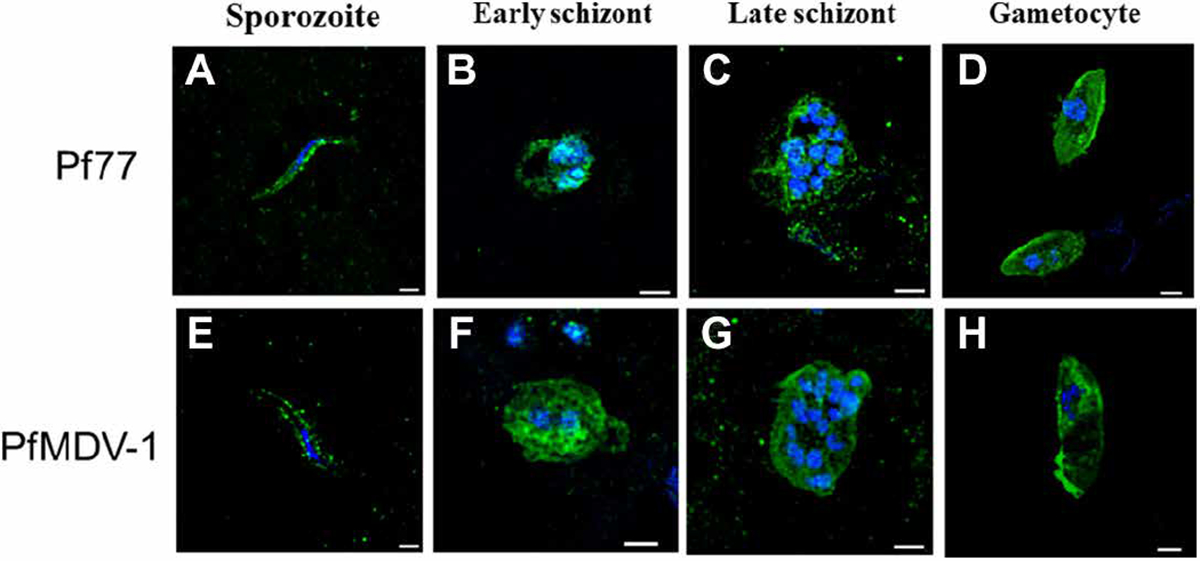
Expression of Pf77 was assessed in (A) sporozoites, (B) early schizonts, (C) late schizonts, and (D) gametocytes. Expression of PfMDV-1 was also evaluated in (E) sporozoites, (F) early schizonts, (G) late schizonts, and (H) gametocytes. Parasites were stained with mouse anti-Pf77 or mouse anti–PfMDV-1 primary antibody followed by Alexa Fluor 488 AffiniPure Alpaca Anti-Mouse IgG (H+L) secondary antibody and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 1 μm.
Orthologs of Pf77 and PfMDV-1 are present in other human Plasmodium species
We performed a search using the National Center for Biotechnology Information Protein Basic Local Alignment Search Tool (NCBI Protein BLAST) based on the amino acid sequences for Pf77 and PfMDV-1 from the P. falciparum 3D7 strain available at PlasmoDB.org (37). Of the available protein sequences from the other four human Plasmodium species—P. vivax, P. malariae, P. ovale, and P. knowlesi—the sequences with the highest identity to the P. falciparum were aligned using the NCBI Constrain-Based Alignment Tool (COBALT) for multiple protein sequences (38). Sequence alignment was further visualized with the Jalview multiple sequence alignment software, which determines a consensus sequence across all isolates and calculates conservation of the proteins at the level of each amino acid (39). Our analyses showed that orthologs of both PfMDV-1 and Pf77 exist in each of the other four species of Plasmodium that infect humans. Pf77 exhibits the following sequence identity: P. vivax, 51.92%; P. malariae, 49.93%; P. ovale, 57.64%; and P. knowlesi, 51.29% relative to the P. falciparum reference protein. The PfMDV-1 ortholog sequence identity is slightly lower: P. vivax, 35%; P. malariae, 32.83%; P. ovale, 43.39%; and P. knowlesi, 39.22% sequence identity relative to the P. falciparum reference protein (fig. S5). These results raise the possibility that the Pf77- and PfMDV-1–based vaccines might also induce a partial immunity against all Plasmodium species circulating in the areas where these vaccines are administered.
Sera from humans who have been naturally exposed to P. falciparum contain antibodies recognizing Pf77 and PfMDV-1
To further evaluate their potential suitability as TBV candidates, we determined antibody responses against Pf77 and PfMDV-1 in sera from residents from a malaria-endemic region on the eastern coast of Ghana. Serum samples were collected between January 2003 and November 2007 from malaria asymptomatic Ghanaian adults who visited the Kpone Katamanso District Hospital, located in the Greater Accra Region of Ghana. In this area, malaria transmission is considered high during rainy season (April to October) and low during dry season (November to March). Study participants included 100 adults between the ages of 30 and 85 (median = 43.5, interquartile range = 37.75 to 55, 73% female; 84 samples had age and sex data).
ELISA was performed using recombinant Pf77 and PfMDV-1 antigens. Results showed that 90% of serum samples were reactive to PfMDV-1 and 52% of serum samples were reactive to Pf77. Among reactive samples, the mean optical density (OD) measurement was 1.86 ± 0.66 for PfMDV-1 and 2.19 ± 0.34 for Pf77 (Table 1). These results demonstrate that both Pf77 and PfMDV-1 are immunogenic and induce antibodies in individuals naturally exposed to P. falciparum parasites. Although a larger proportion of samples were reactive to PfMDV-1 compared to Pf77, among reactive samples, the mean OD was significantly higher (P = 0.001, t test; Table 1) for Pf77 compared to MDV-1, indicating that the degree of recognition and breadth of immune response for these antigens varies in a relatively human leukocyte antigen (HLA)–compatible population.
Table 1. ELISA IgG reactivity against recombinant Pf77 and PfMDV-1 in serum samples collected from Ghanaian adults.
ELISA IgG reactivity was determined in serum samples (n = 100) collected from adults living in a malaria endemic area in the Greater Accra Region of Ghana. Serum samples (n = 5) from U.S. residents who had never been exposed to malaria served as negative controls. Reactivity was determined by comparison of the average optical density (OD)RR410RR of each sample, run in triplicate, and the ODRR410RR of the negative control wells. All serum samples were tested at 1:100 dilution. The threshold for positivity was set at the mean ODRR410RR of the negative control wells + 2 SDs.
| Pf77 (n = 100) | PfMDV-1 (n = 100) | |
|---|---|---|
| Proportion reactive | 52% | 90% |
| Mean OD410 of reactive samples | 2.19 ± 0.34* | 1.86 ± 0.66* |
The mean OD410 among samples reactive to recombinant Pf77 was significantly higher than that of samples reactive to recombinant PfMDV-1 (P = 0.001).
Although this study included only adults, we analyzed the data for a correlation of magnitude of antibody response against Pf77 and PfMDV-1 with increasing age (fig. S6, A and B). We also investigated whether older individuals were more likely to be seropositive, irrespective of the intensity of antibody titer. Our data did not demonstrate a clear positive correlation between age and seropositivity (Pf77: P = 0.028; PfMDV-1: P = 0.0631) among the adult population in the study area (fig. S6C). Given the multistage expression and induction of naturally occurring antibodies, we hypothesize that, apart from the sexual stages, Pf77- and PfMDV-1–based vaccines may target all stages of parasite development and antibody concentrations may remain boosted by natural exposures in high endemic settings.
Pf77 and PfMDV-1 antigens show limited genetic diversity
We next analyzed antigenic variation in Pf77 and PfMDV-1 in 218 P. falciparum isolates from the geographically diverse regions available in PlasmoDB.org. Analyses showed that Pf77 exhibited 37 nonsynonymous and 10 synonymous substitutions, resulting in a nonsynonymous/synonymous single-nucleotide polymorphism (SNP) ratio (dN/dS) of 3.7 for 1995 nucleotides. PfMDV-1 exhibited 15 nonsynonymous and 3 synonymous substitutions, resulting in a dN/dS ratio of 5 for 666 nucleotides. The dN/dS ratio is a reliable measure of evolutionary pressure on the protein-coding regions. Among the nonsynonymous substitutions, minor allele frequencies are generally lower than 5%, except for four positions in Pf77 (5.7, 6.4, 7.3, and 33.0%) and one position in PfMDV-1 (5.6%) proteins (table S6). These results suggest limited antigenic diversity in a large dataset of naturally circulating parasites, indicating the prospect of inducing strain-transcending immunity after vaccination by these antigens.
DISCUSSION
In this study, we applied a transcriptomics-based approach to select P. falciparum genes that are abundantly expressed in the gametocyte stage of the parasite. By using a variety of criteria described earlier, we selected 13 of the 56 genes in our dataset, plus one more gametocyte-stage gene, for further study as TBV. Our studies have led to discovery of two TBV candidates, Pf77 and PfMDV-1, that displayed potent TRA activity of 93 and 84%, respectively, at an IgG concentration of 750 μg/ml.
In a previous report, Pf77 was identified as a P. falciparum sexual stage–specific antigen that is expressed exclusively in female gametocytes (40). Subsequent expression profiling studies in P. falciparum gametocyte cultures revealed that Pf77 expression starts to increase around day 10 and continues up to day 20, demonstrating transcription specific for late gametocyte-stage development (41). Pf77 is located in the inner membrane pellicle complex and has an unknown function. PfMDV-1 was first described as a P. falciparum male development gene with expression peaking in stage III to IV intraerythrocytic gametocytes (36). PfMDV-1 plays a key role in gametocyte membrane formation and integrity (36), and the P. berghei ortholog of P. falciparum MDV-1 mediates egress of both male and female gametocytes from the host erythrocyte (42). Disruption of PfMDV-1 results in a 20-fold decrease in gametocyte production, arrest of gametocytes at stage I, an exceptionally low ratio of male to female gametocytes, and diminished mosquito infectivity (36, 43), indicating its importance in the mosquito-stage developmental cycle.
Despite more than two decades of efforts, only three TBV candidate antigens—Pfs25 (P. falciparum), Pvs25 (P. vivax), and Pfs230 (P. falciparum)—have undergone clinical testing in humans. Of these, Pfs25 has undergone testing in several clinical studies and, when delivered in a variety of adjuvants, induced only modest TRA antibody for a short duration in phase 1 clinical studies (17, 25–27, 44). Pfs25 has stage-specific expression on the surface of zygotes and ookinetes in the mosquito vector (45) and appears not to induce naturally occurring antibodies in endemic areas (21, 22). The other major sexual-stage vaccine antigens, Pfs230 and Pfs48/45, are expressed in prefertilization stages (46, 47) and induce naturally occurring antibodies in individuals living in malaria-endemic regions (48, 49). In addition, in endemic regions, antibodies against Pfs230 and Pfs48/45 have been associated with TRA in many, but not all, seroepidemiological studies (11, 50). Preclinical studies using distinct vaccine constructs and adjuvant formulations (19, 51) and delivery systems (29, 52) have demonstrated the potential of these antigens as vaccine targets. Pfs230 formulated with AS01 is in an age de-escalation phase 2 study in Mali (28), and a monoclonal antibody against Pfs48/45 (53) is in a phase 1 study in the Netherlands.
We conducted additional studies to assess the biological characteristics of Pf77 and PfMDV-1 as potential TRA antigens. We quantified stage-specific expression of Pf77 and PfMDV-1 to determine whether these immunogens could potentially also target other stages of the parasite life cycle, measured naturally occurring antibody response to Pf77 and PfMDV-1 to evaluate whether vaccine-induced immunity could be boosted or maintained by natural infections, and evaluated the antigenic conservation of Pf77 and PfMDV-1 in 218 naturally circulating P. falciparum isolates in Africa, Southeast Asia, and Central and South America to predict whether a vaccine against these targets could induce strain-transcending immunity against P. falciparum.
We determined stage-specific expression of Pf77 and PfMDV-1 through the P. falciparum developmental cycle at the mRNA, protein, and cellular level. Remarkably, although a previous study reported that Pf77 is expressed exclusively in female gametocytes by in situ hybridization (40), our studies indicate that Pf77 is pan-developmentally expressed in the gametocyte, oocyst, sporozoite, and asexual blood stages of the parasite by qPCR, Western blot, and confocal microscopy. PfMDV-1 transcripts were observed by qPCR in the gametocytes, day 6 oocysts, sporozoites, and asexual blood stages of the parasite. Western blots using an antibody probe directed against recombinant PfMDV-1 encoding amino acids 30 to 221 showed protein expression in gametocytes and asexual blood stages. In addition, in oocysts, we observed protein expression on day 2 after blood meal. The presence of PfMDV-1 protein in asexual blood-stage parasites by Western blot is supported by confocal microscopy, demonstrating expression of PfMDV-1 in early and late schizonts. These results differ from findings by Furuya et al. (36), which report the absence of protein in the lysate from asexual blood-stage parasites by immunoblot. This difference in results could be reconciled by the fact that the antibody probe used in our study was generated against a 191–amino acid–long PfMDV-1 domain (amino acids 30 to 221), whereas Furuya et al. used an antibody probe against a 21-mer peptide (amino acids 201 to 220), which may have failed to detect low expression, or a differently processed or conformationally distinct form of PfMDV-1 in the asexual blood stage.
Malaria transmission-blocking immunity is predominantly antibody-mediated (13). We found that 90 and 52% of Ghanaian adults had IgG antibodies specific to PfMDV-1 and Pf77, respectively. Whether these naturally occurring antibodies have TRA properties remains to be determined. Nonetheless, these findings indicate that antibodies induced by PfMDV-1– or Pf77-based vaccines may be boosted in areas of intense parasite transmission.
The degree of polymorphism in any vaccine target is an important aspect to be analyzed early in development. A dN/dS ratio of <1 is predictive of a protein under selection constraint, whereas a ratio of >1 indicates a protein under positive selection. Analyses of nucleotide sequences of Pf77 and PfMDV-1 gleaned from 218 P. falciparum isolates collected from endemic populations where diversity is driven by immune pressure revealed a Pf77 dN/dS ratio of 3.7 for 1995 nucleotides and a PfMDV-1 dN/dS ratio of 5 for 666 nucleotides. In addition, the minor allele frequencies for those nonsynonymous mutations are very low, except one position in Pf77. These data indicate relatively low degrees of antigenic diversity in these antigens in P. falciparum isolates circulating globally. Furthermore, the presence of orthologs of Pf77 and PfMDV-1 in P. vivax, P. malariae, P. ovale, and P. knowlesi parasites raises the prospect of cross-species immunity after vaccinations in areas where multiple Plasmodium species are transmitted.
Our study has several limitations. The major challenges facing the development of TBVs are production of conformationally correct recombinant antigens and availability of adjuvant formulations that are safe and tolerogenic and would generate long-lasting, transmission-blocking antibodies in HLA-diverse populations. In this study, we have only evaluated E. coli–expressed Pf77 and PfMDV-1 and did not address the question of conformational folding on transmission-reducing efficacy of these antigens. Multistage expression and stage-specific biological function(s) of Pf77 and PfMDV-1 also remain to be carefully examined. In addition, prevalence of anti-Pf77 and anti–PfMDV-1 antibodies in young children and low-transmission areas should be determined. Future studies to evaluate the TBV potential of Pf77 and PfMDV-1 should meet these challenges during the preclinical development pathway. Antigen expression platforms, including expression as virus-like particles that may also perform adjuvant function, should be explored to produce these molecules as immunogens (54). In addition, protein domain(s) on Pf77 and PfMDV-1 that are targets of transmission-blocking antibodies should be identified for recombinant expression and evaluation in the SMFA.
In summary, we report the identification and biological characterization of two P. falciparum multistage proteins, Pf77 and PfMDV-1, that are relatively antigenically invariant and induced naturally occurring antibodies in individuals living in an endemic area in Africa. Our observations suggest that Pf77 and PfMDV-1 have all the classical characteristics necessary to generate highly potent TRA antibodies, which can be sustained by natural boosting in endemic regions. On the basis of our observations, these antigens warrant further evaluation as candidate TBV antigens.
MATERIALS AND METHODS
Study design
The objective of this study was to screen our P. falciparum transcriptome database to identify candidate TBV antigens that would induce effective TRA antibodies in mice. A total of 13 highly expressed gametocyte genes in the dataset, plus one additional gametocyte gene, were expressed as recombinant proteins in E. coli. Purified recombinant proteins were emulsified in two different adjuvants and used for immunizations in BALB/c mice. At least five mice were used per group. IgG fractions were purified from serum samples collected from immunized mice and tested in the SMFA for their ability to block P. falciparum oocyst development in the mosquito midgut in two sequential screens conducted by JHMRI and LMVR, NIAID. The IgG samples that exhibited the highest TRA activity in the first screening at JHMRI were independently verified at LMVR, NIAID. In both laboratories, at least 20 blood meal–fed mosquito midguts were dissected to enumerate mean oocyst counts.
Two P. falciparum antigens, Pf77 and PfMDV-1, exhibited the highest TRA in the SMFA when tested as blinded samples in two independent experiments. To better assess their potential as candidate TBVs, additional studies were performed to measure stage-specific expression of, genetic diversity of, and natural immunity to Pf77 and PfMDV-1. Stage-specific expression of Pf77 and PfMDV-1 was measured by qPCR, Western blot, and confocal microscopy throughout the P. falciparum developmental cycle. Genetic diversity by computational analyses was measured to determine genetic polymorphism among P. falciparum field isolates and the presence of orthologs in the other human Plasmodium species. Natural immunity to Pf77 and PfMDV-1 was determined by measuring antibodies induced after natural exposure in serum samples from 100 adults living in an endemic area in Ghana by ELISA.
P. falciparum gametocyte culture and human samples
Human malaria parasite P. falciparum NF54 was cultured as described earlier (55). Parasites were cultured using O+ human erythrocytes at 4% hematocrit in parasite culture medium (RPMI 1640 supplemented with 25 mM Hepes, 10 mM glutamine, 0.074 mM hypoxanthine, and 10% O+ human serum). Gametocyte cultures were initiated at 0.5% parasitemia from low-passage stock and were maintained up to day 18 with daily medium changes. Culture plates were incubated at 37°C in a microaerophilic environment inside a candle jar. Use of human erythrocytes to support the growth of P. falciparum was approved by the Internal Review Board (IRB) of the Johns Hopkins University Bloomberg School of Public Health (#NA 00019050). The Ghanaian samples used in the ELISA were collected under the IRB approved by the University of Ghana (GHS-ERC-3), and informed consent was obtained from the study participants. These retrospectively collected samples were acquired at the Food and Drug Administration (FDA) as anonymous and coded under FDA IRB #09–122.
Mosquito rearing
Anopheles stephensi mosquitoes were maintained in the insectary at 27°C and 80% humidity with a 14-hour day/10-hour night cycle. Mosquito larvae were reared on cat food pellets and ground fish food supplement; adult mosquitoes were maintained on 10% sucrose and fed on mice anesthetized with ketamine for egg production.
Microarray studies
P. falciparum gametocyte–enriched genes were identified by microarray analysis of cultured gametocytes versus cultured asynchronous intraerythrocytic parasites in two independent experiments as follows. Gametocyte and asynchronous blood-stage parasites were homogenized in TRIzol reagent, snap-frozen by immersion in dry ice, and then stored at −80°C until use. Microarray was then performed in quadruplicate on isolated RNA using the Low Input QuickAmp Labeling Kit (Agilent Technologies) as previously described (30, 56). Briefly, complementary DNA (cDNA) was synthesized from 200 ng of RNA template for 2 hours at 40°C in a reaction mixture containing AffinityScript Reverse Transcriptase, Oligo dT-Promoter Primer, dithiothreitol (DTT), and deoxynucleoside triphosphates (dNTPs). Complementary RNA (cRNA) was then amplified and labeled from cDNA for 2 hours at 40°C in a reaction mixture containing T7 RNA polymerase, DTT, NTPs, and either cyanine 3-cytidine triphosphate (cy3) (asynchronous blood-stage parasites) or cyanine 5-cytidine triphosphate (cy5) (gametocytes) label (Agilent Technologies). Using the RNeasy Mini Kit (Qiagen) per the manufacturer’s instructions, cRNA transcripts were next washed twice to eliminate the unincorporated label and resuspended in ribonuclease (RNase)–free water. Amplified labeled cRNA products were quantified using NanoDrop 2000, and equivalent amounts (70 pmol) of cy3 (asynchronous blood-stage parasites)– and cy5 (gametocytes)–labeled cRNAs were combined in a single tube and concentrated using a Vivaspin 500 column (Sartorius Stedim Biotech, Goettingen, Germany) to a loading volume (20 μl) appropriate for hybridization to a custom-designed Agilent SurePrint oligonucleotide array containing 44,000 features (including 5254 60-mer probes specific for Pf. 3D7 transcripts and spotted eight times). After hybridization, the microarray chip was scanned at a 5-μm resolution and image-analyzed using Feature Extraction software version 9. Data were then filtered using NIAID microarray database tools as previously described (57–60).
Protein expression and purification
Genes encoding nicotinamide-adenine dinucleotide (phosphate) transhydrogenase (PF3D7_1453500), conserved Plasmodium protein, unknown function (PF3D7_1471700), Pf77 protein (PF3D7_0621400), procollagen lysine 5-dioxygenase (PF3D7_0930000), zinc finger protein (PF3D7_1210200), PhiL1 interacting protein PIP2 (PF3D7_1431100), male development gene 1 (PF3D7_1216500), Kelch domain-containing protein (PF3D7_1213400), CPW-WPC family protein (PF3D7_0530800), and four conserved proteins, unknown function (PF3D7_1362600, PF3D7_1146100, PF3D7_1204200, and PF3D7_1454900) were expressed as recombinant proteins as follows. First, putative signal and transmembrane sequences were identified using SignalP 4.1 Server and TMHMM Server v. 2.0, respectively, and excised, and the in-domain region was selected for recombinant expression. The antigenic domain of each gene was next amplified using primers listed in table S1 and cloned into the Not I and Asc I (New England Biolabs) restriction sites of a pET11a or pET11b vector (Merck), which was modified to include an N- or C-terminal hexahistidine tag to facilitate purification. Recombinant protein was then expressed in E. coli (λDE3) cells with isopropyl-β-d-thiogalactopyranoside (IPTG) induction, and induced E. coli cells were lysed with BugBuster Protein Extraction Reagent (EMD Millipore). Soluble proteins were purified from the supernatant on a HisTrap column (GE Healthcare Life Sciences), and insoluble proteins were purified by lysing the cell pellet with a combination of lysozyme and sonication, washing four to six times with buffer [50 mM tris (pH 8.0) and 20 mM EDTA] to obtain pure inclusion bodies, and denaturing in solubilization buffer [0.1 M tris (pH 8.0), 2 mM EDTA, and 6 M guanidine HCl]. Insoluble proteins were refolded under controlled redox condition in renaturation buffer [0.1 M tris (pH 8.0), 2 mM EDTA, 0.5 M l-arginine HCl, and 0.9 mM oxidized glutathione], and refolded proteins were then dialyzed against a gradient of urea in 20 mM tris (pH 8.0) buffer and purified on a HisTrap column. Purified recombinant proteins were quantified using Bradford’s reagent (Sigma-Aldrich), and the purity and quality were assessed by Coomassie blue staining of an SDS–polyacrylamide gel electrophoresis (PAGE) gel, which allows to determine protein identity based on migration pattern by molecular weight and its degradation products and additional bands indicative of host cell contaminants.
Mice and immunizations
Female BALB/c mice (6 to 8 weeks old) were purchased from The Jackson Laboratory and maintained at the Center for Biologics Evaluation and Research (CBER) animal care facility. All studies in mice were performed in accordance and under the guidelines of animal study protocol 2009–21, which was reviewed and approved by the CBER Animal Care and Use Committee at FDA. Mice (n = 5 per group) were immunized with an emulsion of 20 μg of recombinant protein and 25 μg of CpG ODN 1826 (Coley Pharmaceuticals) in 100 μl of phosphate-buffered saline (PBS) and 100 μl of Montanide ISA51 (Seppic Inc.) adjuvant. Each mouse received four immunizations with 200 μl of vaccine delivered in CFA or Montanide ISA 51 adjuvant by the subcutaneous route at 4-week intervals. Two weeks after the third and fourth immunization, a blood sample was collected from each mouse by bleeding the lateral tail vein. Serum samples were separated from the collected blood samples and stored at −20°C for later use.
Standard membrane feeding assay
The biological activity of TBV candidates was initially evaluated at JHMRI and LMVR using standardized SMFA as described previously (31, 61). Day 16 to day 18 mature gametocyte cultures were adjusted to 0.02% gametocytemia and 50% hematocrit by adding fresh red blood cells (RBCs) and non–heat-inactivated normal human serum. Blood feed was prepared by mixing 60 ml of PBS containing test antibodies or controls (protein G–purified normal mouse total IgG or 4B7, anti-Pfs25 monoclonal antibody) with 200 ml of gametocyte culture. Female A. stephensi mosquitoes, 3 to 7 days after emergence, were starved overnight before feeding, and cups were prepared with about 50 female mosquitoes in each. Mosquitoes were fed for ~30 min using glass membrane feeders. Unfed mosquitoes were then removed, and cups were kept for another 8 days at 27°C. Eight days after blood feed, at least 20 mosquitoes were dissected to enumerate the oocysts in the midgut. Only midguts from mosquitoes with any eggs at the time of dissection were analyzed. The human serum and RBCs used for the gametocyte cultures and SMFA were purchased from Interstate Blood Bank.
Quantitative real-time PCR
Transcript abundance of Pf77 (PF3D7_0621400) and PfMDV-1 (PF3D7_1216500) was measured in RNA extracted from the sporozoite, asynchronous blood stage, gametocyte, and oocyst (2, 4, 6, and 8 days after infection of A. stephensi mosquitoes) stages of P. falciparum NF54 by quantitative real-time PCR using the iTaq Universal SYBR Green One-Step Kit (Bio-Rad Laboratories) and primers specific for Pf77 (forward: 5′-TTGGGCAAGTACCAGAAGGA-3′, reverse: 5′-TTGAAGGTGCATTTATACGAGGAG-3′) and PfMDV-1 (forward: 5′-AATATATAGGAGCAAAAGCAGGTGA-3′, reverse: 5′-TCCACAAAATTTTCCGTTTCTTCA-3′). A 20-μl reaction mixture containing 20 ng (sporozoite) or 50 ng (asynchronous blood stage, gametocyte, and oocyst) of template RNA, 3.2 nmol each of forward and reverse primer, 10 μl of iTaq universal SYBR green reaction mix (2×), 0.25 μl of iScript reverse transcriptase, and nuclease-free water was first prepared. Next, quantitative real-time PCR was performed using the CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) with the following cycle profile: 1 cycle of reverse transcription at 50°C for 10 min, 1 cycle of polymerase activation and DNA denaturation at 95°C for 2 min, and 40 cycles of amplification with 1 cycle consisting of 15 s of denaturation at 95°C, 30 s of annealing at 57.5°C, and 1 min of extension at 72°C. Relative RNA concentrations were established using a standard curve derived from the PCR products of a 10-fold serially diluted plasmid containing a P. falciparum β-actin gene fragment.
Western blot analysis
Stage-specific expression of Pf77 and PfMDV-1 protein was determined by Western blot analysis. To prepare protein samples for Western blot, sporozoite, asynchronous blood stage, gametocyte, and oocyst (2, 4, 6, and 8 days after infection of A. stephensi mosquitoes) samples were pulse-homogenized in PBS containing 1% sarkosyl and centrifuged for 2 min at 13,000 rpm, and supernatant was then diluted to the appropriate concentration in SDS lysis buffer and boiled for 5 min before gel loading. Two micrograms of recombinant protein (positive control) and P. falciparum lysates equivalent to 4.5 × 105 sporozoites, 1.875 × 106 asynchronous asexual blood-stage parasites, 1.2 × 106 gametocytes, and 10 oocysts were loaded per well, and Pf77 and PfMDV-1 proteins were detected in each stage using mouse IgG specific for each protein and a chemiluminescence-linked Western blotting kit (Western Light Tropix). After incubation with enhanced chemiluminescence detection reagents, protein bands were observed and the integrated optical densities (IODs) for each lane were quantitated using ImageJ software. Raw SDS-PAGE and Western blot data are provided in fig. S7.
Confocal microscopy
Twenty-two mm2 poly-l-lysine–coated coverslips containing P. falciparum sporozoites, blood-stage schizonts, or gametocytes were fixed for 30 min at room temperature with 3% paraformaldehyde, 10 mM piperazine-N,N′-bis (2-ethanesulfonic acid) buffer (Pipes) (pH 6.4), and PBS, permeabilized for 10 min with 0.25% Triton X-100 and PBS, washed three times with PBS, and then blocked for 1 hour with 3% bovine serum albumin/PBS. Samples were then stained for 1 hour with mouse polyclonal anti-Pf77 or mouse anti–MDV-1 primary antibodies (generated in-house by immunizations with respective recombinant proteins in mice) diluted 1:50 or 1:100, respectively, in 1 ml of blocking buffer, washed three times with PBS, stained for 30 min in the dark with Alexa Fluor 488 AffiniPure Alpaca Anti-Mouse IgG (H+L) secondary antibody (Jackson ImmunoResearch Laboratories Inc.) diluted 1:1000 in 1 ml of blocking buffer, and washed three times with PBS. Samples were next postfixed for 15 min in 3% paraformaldehyde, washed two times with PBS, and stained for 10 min in 4′,6-diamidino-2-phenylindole (DAPI) (2 μg/ml). Coverslips were then washed two times with PBS, rinsed briefly with distilled water, mounted onto slides with ProLong antifade mountants, and sealed with nail polish.
Enzyme-linked immunosorbent assay
ELISA was used to measure anti-Pf77 and anti–MDV-1 IgG in human serum samples from Ghana. Briefly, 96-well ELISA plates were coated with 0.5 μg of Pf77 or 0.25 μg of MDV-1 antigen in a 50-μl volume by overnight incubation at 4°C. Plates were then washed three times with PBS–0.1% Tween using a microplate washer and stacker (BioTek) and blocked with PBS–5% Blotting Grade Blocker (Bio-Rad Laboratories) plus 0.5% Tween for 1 hour at 37°C. The supernatant was discarded, and 50 μl of serum samples at 1:100 dilution in blocking buffer was added to triplicate wells and incubated for 2 hours at 37°C. After three washes with PBS–0.1% Tween, 50 μl of anti-human IgG (H+L) peroxidase-labeled antibody (SeraCare Life Sciences Inc.) diluted 1:4000 in blocking buffer was added to each well and incubated for 1 hour at 37°C. After three washes with PBS–0.1% Tween, 100 μl of KPL ABTS (2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) Peroxidase Substrate System (2-component) (SeraCare Life Sciences Inc.) prewarmed to room temperature was added to each well and incubated for 30 min at room temperature in the dark. After the addition of 100 μl of ABTS Peroxidase Stop Solution, OD values were read at 410 nm with the SpectraMax M5 multidetection microplate reader system.
Statistical analysis
Analysis of microarray transcript expression data was performed as described earlier (30). Student’s t test was applied to measure significance of the 56 genes with ≥50-fold transcriptional up-regulation in P. falciparum gametocytes versus asynchronous blood-stage parasites.
For the SMFA, percent inhibition in mean oocyst intensity (percent TRA) of a test IgG was calculated against a control normal mouse IgG examined in the same feeding experiment. The best estimate and the 95% confidence intervals (CIs) of percent TRA and P values (whether the inhibition is different from 0% inhibition) from single or two assays were calculated using a negative binomial model with zero inflation model, as described previously (62). The statistical analysis was performed in R (version 3.4.1), and P values <0.05 were considered significant.
For comparison of OD410 values obtained by ELISA, we performed t tests using the Holm-Sidak method. The threshold for positivity by ELISA was set based on the mean + 2 SDs of the negative control wells. Correlation of ELISA reactivity with age was assessed by linear regression based on minimizing the residual sum of squares; the R2 value reported indicates the goodness-of-fit of the line to the data, whereas the P value represents the probability of the slope of the regression line being nonzero.
Supplementary Material
Acknowledgments:
We thank Y. Wu and A. Birkett for critically reading the manuscript. We thank the Veterinary Staff of CBER Animal Care and Use Facility. We also thank the parasitology and insectary core facilities at the JHMRI and Bloomberg Philanthropies for their support of these facilities.
Funding:
This research was supported by PATH’s Malaria Vaccine Initiative (S.K.) and intramural funding from the Food and Drug Administration (S.K.) as well as an intramural program of the National Institute of Allergy and Infectious Diseases/National Institutes of Health (C.A.L.). A.K.T. and G.M. were supported by Bloomberg Philanthropies and Johns Hopkins Malaria Research Institute.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.World Health Organization, World Malaria Report 2020—20 Years of Global Progress and Changes (World Health Organization, Geneva, 2020); https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/9789240015791-eng.pdf?sfvrsn=d7a8ec53_3&download=true. [Google Scholar]
- 2.Rossati A, Bargiacchi O, Kroumova V, Zaramella M, Caputo A, Garavelli PL, Climate, environment and transmission of malaria. Infez. Med. 24, 93–104 (2016). [PubMed] [Google Scholar]
- 3.Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, Rahim-Awab G, Barnadas C, Berry A, Boum Y, Bustos MD, Cao J, Chen J-H, Collet L, Cui L, Thakur G-D, Dieye A, Djallé D, Dorkenoo MA, Eboumbou-Moukoko CE, Espino F-E-CJ, Fandeur T, Ferreira-da-Cruz M-F, Fola AA, Fuehrer H-P, Hassan AM, Herrera S, Hongvanthong B, Houzé S, Ibrahim ML, Jahirul-Karim M, Jiang L, Kano S, Ali-Khan W, Khanthavong M, Kremsner PG, Lacerda M, Leang R, Leelawong M, Li M, Lin K, Mazarati J-B, Ménard S, Morlais I, Muhindo-Mavoko H, Musset L, Na-Bangchang K, Nambozi M, Niaré K, Noedl H, Ouédraogo J-B, Pillai DR, Pradines B, Quang-Phuc B, Ramharter M, Randrianarivelojosia M, Sattabongkot J, Sheikh-Omar A, Silué KD, Sirima SB, Sutherland C, Syafruddin D, Tahar R, Tang L-H, Touré OA, Tshibangu-wa-Tshibangu P, Vigan-Womas I, Warsame M, Wini L, Zakeri S, Kim S, Eam R, Berne L, Khean C, Chy S, Ken M, Loch K, Canier L, Duru V, Legrand E, Barale J-C, Stokes B, Straimer J, Witkowski B, Fidock DA, Rogier C, Ringwald P, Ariey F, Mercereau-Puijalon O; for the KARMA Consortium, A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N. Engl. J. Med. 374, 2453–2464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killeen GF, Ranson H, Insecticide-resistant malaria vectors must be tackled. Lancet 391, 1551–1552 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Minetti C, Ingham VA, Ranson H, Effects of insecticide resistance and exposure on Plasmodium development in Anopheles mosquitoes. Curr. Opin. Insect Sci. 39, 42–49 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Sherrard-Smith E, Hogan AB, Hamlet A, Watson OJ, Whittaker C, Winskill P, Ali F, Mohammad AB, Uhomoibhi P, Maikore I, Ogbulafor N, Nikau J, Kont MD, Challenger JD, Verity R, Lambert B, Cairns M, Rao B, Baguelin M, Whittles LK, Lees JA, Bhatia S, Knock ES, Okell L, Slater HC, Ghani AC, Walker PGT, Okoko OO, Churcher TS, The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 9, 1411–1416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huff CG, Marchbank DF, Shiroishi T, Changes in infectiousness of malarial gametocytes. II. Analysis of the possible causative factors. Exp. Parasitol. 7, 399–417 (1958). [DOI] [PubMed] [Google Scholar]
- 8.Gwadz RW, Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 193, 1150–1151 (1976). [DOI] [PubMed] [Google Scholar]
- 9.Carter R, Graves PM, Keister DB, Quakyi IA, Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12, 587–603 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Graves PM, Carter R, Burkot TR, Quakyi IA, Kumar N, Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 10, 209–218 (1988). [DOI] [PubMed] [Google Scholar]
- 11.Healer J, McGuinness D, Carter R, Riley E, Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119 (Pt. 5), 425–433 (1999). [DOI] [PubMed] [Google Scholar]
- 12.van der Kolk M, de Vlas SJ, Sauerwein RW, Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int. J. Parasitol. 36, 1091–1095 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Stone W, Bousema T, Sauerwein R, Drakeley C, Two-faced immunity? The evidence for antibody enhancement of malaria transmission. Trends Parasitol. 35, 140–153 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Stone WJR, Campo JJ, Ouédraogo AL, Meerstein-Kessel L, Morlais I, da D, Cohuet A, Nsango S, Sutherland CJ, van de Vegte-Bolmer M, Siebelink-Stoter R, van Gemert GJ, Graumans W, Lanke K, Shandling AD, Pablo JV, Teng AA, Jones S, de Jong RM, Fabra-García A, Bradley J, Roeffen W, Lasonder E, Gremo G, Schwarzer E, Janse CJ, Singh SK, Theisen M, Felgner P, Marti M, Drakeley C, Sauerwein R, Bousema T, Jore MM, Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat. Commun. 9, 558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH, The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139, 4213–4217 (1987). [PubMed] [Google Scholar]
- 16.Blagborough AM, Musiychuk K, Bi H, Jones RM, Chichester JA, Streatfield S, Sala KA, Zakutansky SE, Upton LM, Sinden RE, Brian I, Biswas S, Sattabonkot J, Yusibov V, Transmission-blocking potency and immunogenicity of a plant-produced Pvs25-based subunit vaccine against Plasmodium vivax. Vaccine 34, 3252–3259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spångberg M, Juraska M, Wille-Reece U, Weiner A, Howard RF, Long CA, Duffy PE, Johnston L, O’Neil CP, Loré K, TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 3, e120692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaria PV, Rowe CG, Chen BB, Muratova OV, Fischer ER, Barnafo EK, Anderson CF, Zaidi IU, Lambert LE, Lucas BJ, Nahas DD, Narum DL, Duffy PE, Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines 4, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theisen M, Jore MM, Sauerwein R, Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert Rev. Vaccines 16, 329–336 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Dantzler KW, Ma S, Ngotho P, Stone WJR, Tao D, Rijpma S, De Niz M, Nilsson Bark SK, Jore MM, Raaijmakers TK, Early AM, Ubaida-Mohien C, Lemgruber L, Campo JJ, Teng AA, Le TQ, Walker CL, Hermand P, Deterre P, Huw Davies D, Felgner P, Morlais I, Wirth DF, Neafsey DE, Dinglasan RR, Laufer M, Huttenhower C, Seydel K, Taylor T, Bousema T, Marti M, Naturally acquired immunity against immature Plasmodium falciparum gametocytes. Sci. Transl. Med. 11, eaav3963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner J, Huang C-Y, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, Kayentao K, Traore B, Crompton PD, Williamson KC, Plasmodium falciparum gametocyte-specific antibody profiling reveals boosting through natural infection and identifies potential markers of gametocyte exposure. Infect. Immun. 83, 4229–4236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T, Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 81, 4377–4382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niederwieser I, Felger I, Beck HP, Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am. J. Trop. Med. Hyg. 64, 9–11 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Delves MJ, Angrisano F, Blagborough AM, Antimalarial transmission-blocking interventions: Past, present, and future. Trends Parasitol. 34, 735–746 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP, Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLOS ONE 3, e2636 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, Rausch KM, Zhu D, Muratova O, Herrera R, Anderson C, Jones D, Aebig J, Brockley S, MacDonald NJ, Wang X, Fay MP, Healy SA, Durbin AP, Narum DL, Wu Y, Duffy PE, Safety and immunogenicity of Pfs25-EPA/Alhydrogel, a transmission blocking vaccine against Plasmodium falciparum: An open-label study in malaria naïve adults. PLOS ONE 11, e0163144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, Lee CK, Ockenhouse CF, Morin MJ, Streatfield SJ, Yusibov V, Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A phase 1 dose-escalation study in healthy adults. Vaccine 36, 5865–5871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healy SA, Anderson C, Swihart BJ, Mwakingwe A, Gabriel EE, Decederfelt H, Hobbs CV, Rausch KM, Zhu D, Muratova O, Herrera R, Scaria PV, MacDonald NJ, Lambert LE, Zaidi I, Coelho CH, Renn JP, Wu Y, Narum DL, Duffy PE, Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Invest. 131, e146221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy PE, Patrick Gorres J, Malaria vaccines since 2000: Progress, priorities, products. NPJ Vaccines 5, 48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essuman E, Grabias B, Verma N, Chorazeczewski JK, Tripathi AK, Mlambo G, Addison EA, Amoah AGB, Quakyi I, Oakley MS, Kumar S, A novel gametocyte biomarker for superior molecular detection of the Plasmodium falciparum infectious reservoirs. J. Infect. Dis. 216, 1264–1272 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Miura K, Deng B, Tullo G, Diouf A, Moretz SE, Locke E, Morin M, Fay MP, Long CA, Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLOS ONE 8, e57909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Othman AS, Lin J-W, Franke-Fayard BM, Kroeze H, van Pul FJA, Chevalley-Maurel S, Ramesar J, Marin-Mogollon C, Jore MM, Morin MJ, Long CA, Sauerwein R, Birkett A, Miura K, Janse CJ, Khan SM, Expression of full-length Plasmodium falciparum P48/45 in P. berghei blood stages: A method to express and evaluate vaccine antigens. Mol. Biochem. Parasitol. 224, 44–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brod F, Miura K, Taylor I, Li Y, Marini A, Salman AM, Spencer AJ, Long CA, Biswas S, Combination of RTS S and Pfs25-IMX313 induces a functional antibody response against malaria infection and transmission in mice. Front. Immunol. 9, 2780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini A, Zhou Y, Li Y, Taylor IJ, Leneghan DB, Jin J, Zaric M, Mekhaiel D, Long CA, Miura K, Biswas S, A universal plug-and-display vaccine carrier based on HBsAg VLP to maximize effective antibody response. Front. Immunol. 10, 2931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SM, Hickey JM, Miura K, Joshi SB, Volkin DB, King CR, Plieskatt JL, A C-terminal Pfs48/45 malaria transmission-blocking vaccine candidate produced in the baculovirus expression system. Sci. Rep. 10, 395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuya T, Mu J, Hayton K, Liu A, Duan J, Nkrumah L, Joy DA, Fidock DA, Fujioka H, Vaidya AB, Wellems TE, Su XZ, Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 16813–16818 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ, Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadopoulos JS, Agarwala R, COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 23, 1073–1079 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ, Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker DA, Thompson J, Daramola OO, Carlton JMR, Targett GAT, Sexual-stage-specific RNA expression of a new Plasmodium falciparum gene detected by in situ hybridisation. Mol. Biochem. Parasitol. 72, 193–201 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Tadesse FG, Meerstein-Kessel L, Gonçalves BP, Drakeley C, Ranford-Cartwright L, Bousema T, Gametocyte sex ratio: The key to understanding Plasmodium falciparum transmission? Trends Parasitol. 35, 226–238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponzi M, Sidén-Kiamos I, Bertuccini L, Currà C, Kroeze H, Camarda G, Pace T, Franke-Fayard B, Laurentino EC, Louis C, Waters AP, Janse CJ, Alano P, Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cell. Microbiol. 11, 1272–1288 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Josling GA, Llinas M, Sexual development in Plasmodium parasites: Knowing when it’s time to commit. Nat. Rev. Microbiol. 13, 573–587 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, Tembine I, Guindo MA, Doucoure M’B, Niaré K, Dolo A, Rausch KM, Narum DL, Jones DL, MacDonald NJ, Zhu D, Mohan R, Muratova O, Baber I, Coulibaly MB, Fay MP, Anderson C, Wu Y, Traore SF, Doumbo OK, Duffy PE, Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: A randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect. Dis. 18, 969–982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH, A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74–76 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH, Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162, 1460–1476 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N, Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 9, 321–335 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Ouedraogo AL, Roeffen W, Luty AJF, de Vlas SJ, Nebie I, Ilboudo-Sanogo E, Cuzin-Ouattara N, Teleen K, Tiono AB, Sirima SB, Verhave J-P, Bousema T, Sauerwein R, Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect. Immun. 79, 4957–4964 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones S, Grignard L, Nebie I, Chilongola J, Dodoo D, Sauerwein R, Theisen M, Roeffen W, Singh SK, Singh RK, Singh S, Kyei-Baafour E, Tetteh K, Drakeley C, Bousema T, Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J. Infect. 71, 117–127 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, Rijpma SR, Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology 143, 187–198 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Singh SK, Thrane S, Chourasia BK, Teelen K, Graumans W, Stoter R, van Gemert G-J, van de Vegte-Bolmer MG, Nielsen MA, Salanti A, Sander AF, Sauerwein RW, Jore MM, Theisen M, Pfs230 and Pfs48/45 fusion proteins elicit strong transmission-blocking antibody responses against Plasmodium falciparum. Front. Immunol. 10, 1256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datta D, Bansal GP, Gerloff DL, Ellefsen B, Hannaman D, Kumar N, Immunogenicity and malaria transmission reducing potency of Pfs48/45 and Pfs25 encoded by DNA vaccines administered by intramuscular electroporation. Vaccine 35, 264–272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radbound University, The PATH Malaria Vaccine Initiative (2020), https://ClinicalTrials.gov/show/NCT04238689. [Google Scholar]
- 54.Naskalska A, Pyrc K, Virus like particles as immunogens and universal nanocarriers. Pol. J. Microbiol. 64, 3–13 (2015). [PubMed] [Google Scholar]
- 55.Trager W, Jensen JB, Human malaria parasites in continuous culture. Science 193, 673–675 (1976). [DOI] [PubMed] [Google Scholar]
- 56.Oakley MS, Verma N, Myers TG, Zheng H, Locke E, Morin MJ, Tripathi AK, Mlambo G, Kumar S, Transcriptome analysis based detection of Plasmodium falciparum development in Anopheles stephensi mosquitoes. Sci. Rep. 8, 11568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oakley MS, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, Moch JK, Fairhurst R, McCutchan TF, Aravind L, Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect. Immun. 75, 2012–2025 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oakley MS, McCutchan TF, Anantharaman V, Ward JM, Faucette L, Erexson C, Mahajan B, Zheng H, Majam V, Aravind L, Kumar S, Host biomarkers and biological pathways that are associated with the expression of experimental cerebral malaria in mice. Infect. Immun. 76, 4518–4529 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oakley MS, Anantharaman V, Venancio TM, Zheng H, Mahajan B, Majam V, McCutchan TF, Myers TG, Aravind L, Kumar S, Molecular correlates of experimental cerebral malaria detectable in whole blood. Infect. Immun. 79, 1244–1253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oakley MS, Gerald N, Anantharaman V, Gao Y, Majam V, Mahajan B, Pham PT, Lotspeich-Cole L, Myers TG, McCutchan TF, Morris SL, Aravind L, Kumar S, Radiation-induced cellular and molecular alterations in asexual intraerythrocytic Plasmodium falciparum. J. Infect. Dis. 207, 164–174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripathi AK, Mlambo G, Kanatani S, Sinnis P, Dimopoulos G, Plasmodium falciparum gametocyte culture and mosquito infection through artificial membrane feeding. J. Vis. Exp. e61426 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, Burton T, Fay MP, Long CA, Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 34, 4145–4151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


