Abstract
Rationale
Proinflammatory processes have been implicated in alcohol addiction, craving, and relapse, while studies in experimental animals have suggested that activation of peroxisome proliferator-activated receptor gamma (PPARγ) inhibits proinflammatory signaling. Accordingly, it is hypothesized that medications with PPARγ activity may have therapeutic potential in alcohol dependence.
Objectives
We conducted a double-blind, placebo-controlled mechanistic proof of principle study in alcohol-dependent inpatients to investigate the effect of pioglitazone on alcohol craving.
Methods
Participants were treated for withdrawal, if needed, and then randomized to pioglitazone (target dose 45 mg/day) or placebo. Once at target dose, they completed two experimental manipulations: guided imagery, which used personalized auditory scripts to induce alcohol cravings, and a low-dose challenge with i.v. lipopolysaccharide (LPS; 0.8 ng/kg) or placebo, on two separate sessions, in counterbalanced order. Behavioral and endocrine responses as well as CSF levels of proinflammatory cytokines were evaluated.
Results
The study was prematurely terminated after randomization of 16 subjects, following an independent review that established a high risk of myopathy in the active treatment group. Analysis of those who completed the study indicated that pioglitazone was associated with elevated, rather than suppressed alcohol cravings in response to alcohol-associated stimuli. LPS did not induce cravings for alcohol and thus did not lend itself to evaluating pioglitazone effects; however, pioglitazone increased the neuroendocrine stress response to LPS. CSF levels of IL-6, TNF-α, or MCP-1 were unaffected by pioglitazone treatment.
Conclusions
Both safety and efficacy biomarker data suggest that pioglitazone lacks potential as a medication for the treatment of alcohol dependence.
Clinical trial registration
Keywords: Alcohol dependence, Proinflammatory cytokines, Alcohol craving, Pioglitazone
Introduction
Innate immune signaling has emerged as a mechanism of potential importance for both the development and maintenance of alcohol use disorder (hereafter equated with alcohol addiction, or simply alcoholism (Coleman and Crews 2018; Crews et al. 2011)). Proinflammatory cytokines, such as IL1β, IL6, and TNFα, are produced by activated peripheral macrophages and Kupffer cells, and can access the central nervous system (CNS) through multiple routes, including vagal afferents or signaling pathways across the blood–brain barrier. This in turn can result in local production of proinflammatory mediators by microglia within the CNS, as well as an activation of the hypothalamic-pituitary-adrenal (HPA) axis (Dantzer and Kelley 2007; Engblom et al. 2002; Fritz et al. 2016).
Relapse is a core clinical feature of alcohol addiction, and relapse prevention is a key treatment objective (Brandon et al. 2007; Hendershot et al. 2011). Craving, both during and following treatment, is thought to promote relapse and has been shown to predict relapse rates (Flannery et al. 2003; Schneekloth et al. 2012; Sinha et al. 2011; Stohs et al. 2019). Correlations between plasma cytokine levels and alcohol craving have been reported (Heberlein et al. 2014; Leclercq et al. 2014), but whether these correlations reflect a causal relationship remains unknown. Addressing whether this is the case is critical in order to assess the therapeutic potential of anti-inflammatory interventions in alcohol addiction. If proinflammatory signaling causally contributes to craving, then interventions that attenuate the former should also reduce the latter. Experimental methods that induce and assess craving in a controlled setting allow this hypothesis to be evaluated under conditions that allow safety to be closely monitored, and offer a tool for early stage medication development (Kwako et al. 2015b; Umhau et al. 2011).
The peroxisome proliferator-activated receptor gamma (PPARγ) receptor is a ligand-activated transcription factor that belongs to the superfamily of nuclear hormone receptors, and whose activation can under certain conditions counteract pro-inflammatory signals (Berger and Moller 2002; Chang et al. 2007; Tontonoz and Spiegelman 2008). PPARγ are expressed in fat cells, where they are involved in regulation of sugar and fat metabolism; macrophages, where they are involved in the control of inflammatory responses (Berger and Moller 2002; Kapadia et al. 2008; Landreth and Heneka 2001); and in both neurons and glial cells of the CNS (Gofflot et al. 2007; Moreno et al. 2004; Sarruf et al. 2009). PPARγ are activated as part of neuroprotective responses to excitotoxic processes and inflammatory damage, and their activation is associated with improvement of cognitive performance, slower progression of Alzheimer’s disease, and potentially with protection against epileptic tissue damage (Landreth et al. 2008; Sastre et al. 2006). Based on results in animal models, activation of PPARγ has recently also been proposed as a candidate mechanism for pharmacotherapy of stimulant (Daynes and Jones 2002), opioid (de Guglielmo et al. 2017; de Guglielmo et al. 2015), and alcohol (Stopponi et al. 2011) addiction.
Here, we therefore carried out a proof of principle study to evaluate the ability of the PPARγ agonist pioglitazone to suppress experimentally induced alcohol cravings in treatment-seeking alcohol-addicted patients. Pioglitazone (Actos) is an approved medication for diabetes that produces its effects via activation of PPARγ. In non-diabetic populations, pioglitazone has been shown to reduce inflammatory brain damage and improve cognition in Alzheimer’s patients (Feinstein 2003; Kapadia et al. 2008; Landreth and Heneka 2001). Compared with rosiglitazone (Avandia), another PPARγ agonist approved for diabetes, pioglitazone crosses the blood–brain barrier more easily (Breidert et al. 2002; Young et al. 1998) and appears to be more effective in activating brain PPARγ (Maeda et al. 2007).
Two procedures were used to experimentally induce craving in the laboratory. The first of these was guided imagery, an established paradigm involving stress- and alcohol cue–associated auditory scripts that have been shown to induce behavioral and physiological reactions, including craving (Kwako et al. 2015a, c; Schwandt et al. 2016; Sinha et al. 2011). The second method was low-dose lipopolysaccharide (LPS) administration, which has been used as an experimental stimulus to study psychological responses to activation of innate immunity (Eisenberger et al. 2010a, b). These studies have shown a robust effect of LPS administration to induce depressed mood, reward deficit, and social withdrawal, but to our knowledge it is unknown whether low-dose LPS challenge also elicits craving for alcohol. These two challenge paradigms were carried out in the context of a double-blind, placebo-controlled experimental medicine study of pioglitazone. We hypothesized that individuals receiving pioglitazone would show a reduced craving response compared with those receiving placebo. Secondary aims included investigation of the effects of pioglitazone on physiological and subjective measures of stress and mood during these experimental procedures, as well as on spontaneous measures of craving and negative affect collected throughout the 5-week study. These exploratory outcomes were assessed because there is considerable interest in the role of proinflammatory cytokines in mood–anxiety disorders (Dantzer et al. 2008), and because these are frequently co-morbid with alcohol use disorders.
Methods
Subjects
Participants were recruited through advertisements and referrals between August 2012 and March 2015. Potential subjects were screened over the phone and admitted to the NIH Clinical Center in Bethesda, MD, where they underwent medically managed withdrawal if needed. Only when subjects had an undetectable breath alcohol concentration, and did not require treatment for alcohol withdrawal, were they evaluated for eligibility. Detailed eligibility criteria are available at https://www.clinicaltrials.gov/ct2/show/NCT01631630. All participants met DSM-IV criteria for current alcohol dependence according to the Structured Clinical Interview for DSM Diagnosis (SCID-IV) (First et al. 1995). DSM-IV criteria were used because the majority of participants were enrolled prior to the availability of the SCID 5. Subjects were excluded if they had complicated medical or psychiatric problems or were unable to participate in study procedures. All participants provided written informed consent. Participant demographics are presented in Table 1.
Table 1.
Subject characteristics. Variables are means (SD), or n (percentage) as appropriate
| Pioglitazone (n = 6) | Placebo (n = 8) | Total (n = 14a) | p valueb | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 49.7 (6.6) | 43.3 (6.0) | 46.0 (6.8) | 0.08 |
| Male | 6 (100%) | 7 (87.5%) | 13 (92.9%) | 0.37 |
| Black/African Americanc | 5 (83.3%) | 4 (50.0%) | 9 (64.3%) | 0.40 |
| Education (years) | 13.3 (2.4) | 12.5 (4.0) | 12.9 (3.3) | 0.66 |
| Smoker | 5 (83.3%) | 5 (62.5%) | 10 (71.4%) | 0.39 |
| Fagerstrom Score | 3.2 (3.1) | 3.0 (1.7) | 3.1 (2.4) | 0.90 |
| Family history densityd | 0.2 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.27 |
| Alcohol-related measures | ||||
| Avg. drinks/drinking day | 10.4 (4.3) | 14.3 (4.0) | 12.6 (4.4) | 0.11 |
| Heavy drinking days | 67.5 (27.3) | 76.4 (15.0) | 72.6 (20.7) | 0.45 |
| ADSe score | 19.2 (9.0) | 12.9 (6.4) | 15.6 (8.0) | 0.15 |
| PACSf craving | 16.3 (8.6) | 7.3 (3.5) | 11.1 (7.5) | 0.05 |
| Psychological characteristics | ||||
| Current anxiety disorder | 1 (16.7%) | 2 (25.0%) | 3 (21.4%) | 0.71 |
| Current mood disorder | 0 (0.0%) | 1 (12.5%) | 1 (7.1%) | 0.37 |
| Current substance use disorderg | 1 (16.7%) | 0 (0.0%) | 1 (7.1%) | 0.23 |
| CTQh total score | 41.5 (20.4) | 41.6 (9.2) | 41.6 (14.3) | 0.98 |
| Neuroticism score | 53.3 (7.2) | 54 (6.8) | 53.7 (6.7) | 0.86 |
| CPRSi anxiety | 12.5 (7.9) | 9.9 (6.6) | 11 (7.0) | 0.51 |
| CPRS depression | 13.7 (8.3) | 13.1 (7.2) | 13.4 (7.4) | 0.90 |
Of the 14 subjects, 13 completed the entire protocol while 1 subject completed the scripts challenge but not the LPS challenge
Based on a chi-square test for categorical measures, and a t test for continuous measures
The remaining subjects were White/Caucasian (4) and Asian (1)
Calculated as the proportion of first- and second-degree relatives known to have been treated for alcohol abuse or have alcohol use–related problems, as measured by the Family Tree Questionnaire (FTQ)
Alcohol Dependence Scale
Penn Alcohol Craving Scale
Substances other than alcohol
Childhood Trauma Questionnaire
Comprehensive Psychopathological Rating Scale
Based on a priori power analysis, the original recruitment target for the study was 40 participants. A total of 17 subjects signed the consent for the protocol before the study was terminated for safety reasons (see below); however, 1 subject was subsequently found to be ineligible and thus was not randomized. Two subjects were withdrawn from the protocol before performing any study procedures, and 1 subject underwent the guided imagery procedure before being withdrawn (see Fig. 1a for CONSORT graph). Subjects were randomized to pioglitazone or placebo using a double-blind parallel group design with a 1:1 allocation. Subjects receiving pioglitazone were titrated over the course of 6 days (15 mg/day for 3 days; 30 mg day for 3 days) and then given 45 mg daily, while subjects randomized to placebo received an identical looking placebo. The specific dosing schedule was as follows—day 1: one capsule of pioglitazone 15 mg or placebo at 19:00; days 2–3: one capsule of pioglitazone 15 mg or placebo at 8:00 (once daily); days 4–6: two capsules of pioglitazone 15 mg or placebo at 8:00 (once daily); days 7–34: three capsule of pioglitazone 15 mg or placebo at 8:00 (once daily). All subjects remained hospitalized throughout the study and participated in standard-of-care behavioral treatment for alcohol dependence.
Fig. 1.

a CONSORT graph for the clinical study. b Study timeline
In addition to the SCID interview, subjects were assessed for AD severity using the AD Scale (ADS; (Skinner and Horn 1984)), for family history of alcohol problems using the Family Tree Questionnaire (FTQ; (Mann et al. 1985)), for addiction severity phenotypes using the Addiction Severity Index (ASI; (McLellan et al. 1980)), for alcohol consumption in the past 90 days using the Timeline Follow-Back (TLFB; (Sobell and Sobell 1996)), for alcohol craving using the Penn Alcohol Craving Scale (PACS; (Flannery et al. 1999)), for nicotine dependence severity using the Fagerstrom Test for Nicotine Dependence (FTND; (Heatherton et al. 1991), for depression and anxiety symptoms using the Comprehensive Psychopathological Rating Scale (CPRS; (Asberg and Schalling 1979)), for personality traits using the NEO Personality Inventory Revised (NEO; (Costa and McCrae 2002)), and for early life adversity using the Childhood Trauma Questionnaire (CTQ; (Bernstein et al. 2003)).
Study procedures
The overall study timeline is presented in Fig. 1b. During week 1 participants underwent detoxification, with monitoring of withdrawal symptoms using the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) and treatment with benzodiazepines when indicated. By the end of week 1 withdrawal symptoms were negligible or absent for all participants, and no benzodiazepine treatment was required for any participant beyond day 4 of the inpatient stay. Although an MRI scan was carried out as indicated, the data were insufficient for a meaningful analysis due to the low sample size and were not further processed.
Lumbar puncture
To evaluate the effects of pioglitazone on CSF levels of proinflammatory cytokines, a lumbar puncture (LP) was performed on day 15 of the study timeline. LPs were done in the L3/L4 or L4/L5 interspace following administration of local anesthetic. A volume of less than or equal to 20 mL of CSF was collected in silicone-coated tubes. Tubes were gently mixed to avoid gradient effects. CSF samples were centri-fuged at 2000g, 8 °C, for 10 min to remove cells and other insoluble material, aliquoted into 1-mL tubes to eliminate the need for repeated freezing and thawing, and stored at – 80 °C. In addition to the CSF, a collection of less than or equal to 25 mL of blood at the time of the lumbar punctures was obtained for analysis of cytokines. Details of the analysis of CSF samples are presented in the Supplement.
Behavioral challenge sessions
Alcohol cravings and emotional responses were assessed using personalized auditory guided imagery scripts (hereafter referred to as scripts). Script development and challenge presentation followed the procedures first described by Sinha et al. (Sinha et al. 2011), and were conducted by the same staff and in the same manner as detailed in three previous medication studies published by our research group (Kwako et al. 2015a; Kwako et al. 2015c; Schwandt et al. 2016). Briefly, subjects underwent three sessions on days 21–23 of the study timeline that used personalized scripts, each approximately 5 min in duration, to present alcohol cue–, stress-associated, or neutral stimuli. The order of the script types was counterbalanced across subjects. During each session, craving for alcohol was rated using the Alcohol Urges Questionnaire (AUQ; (Bohn et al. 1995)). The Spielberger State Trait Anxiety Inventory-State Version (STAI; (Spielberger et al. 1970)) and the Subjective Units of Distress Scale (SUDS; (Wolpe 1969)), a visual analog scale ranging 1–100, were used to assess anxiety and emotional responses. Vital signs (temperature, pulse, blood pressure) and blood samples for ACTH and cortisol were also collected throughout each session (eFigure 1), which began at 3 pm to minimize differences in circulating endocrine measures.
Lipopolysaccharide challenge sessions
Subjects underwent two sessions, in counterbalanced order, on days 25 and 32 of the study timeline, during which they were administered either saline or lipopolysaccharide (LPS; Clinical Center Reference Endotoxin E. coli O113:H10:K-). The LPS challenge sessions were carried out as described in previous studies (Eisenberger et al. 2010a, b). Following a standardized light meal and the opportunity to smoke, IV catheters were inserted into the dominant forearm for hourly blood draws, and into the non-dominant forearm for a continuous saline flush and for drug administration, respectively. Ninety minutes after insertion of the lines, each participant received either LPS (0.8 ng/kg of body weight) or saline (same volume of 0.9% saline), administered as an intravenous bolus. Throughout the procedure, vital signs were assessed every half hour. At baseline, and then approximately every hour for the next 6 h, blood samples were obtained for measures of endocrine response (ACTH, cortisol). In addition, hourly measures of alcohol craving (AUQ) and emotional responses (STAI and SUDS) were collected, as well as symptoms of physical sickness (muscle pain, shivering, nausea, breathing difficulties, fatigue) (eFigure 1).
Biweekly measures
In addition to experimentally induced outcomes, spontaneous measures of alcohol craving and negative affect were also collected twice a week during the inpatient stay. Craving was measured using the Penn Alcohol Craving Scale (PACS), while anxiety and depression symptom ratings were measured using the Comprehensive Psychopathological Rating Scale (CPRS).
Statistical analysis
Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). CSF concentrations of proinflammatory cytokines were analyzed using general linear models with treatment (pioglitazone vs placebo) as the between-subjects factor. Behavioral, neuroendocrine, and vital signs data collected during the scripts and LPS challenges were analyzed using PROC MIXED for mixed-effect modeling, with treatment (pioglitazone vs placebo) as the between-subjects factor. Repeated-measures, within-subjects factors included script condition (neutral, alcohol cue, or stress) and time point for the guided imagery challenge, and challenge condition (LPS vs saline) and time point for the LPS challenge. Biweekly behavioral measures were also analyzed with PROC MIXED, with study day as the within-subjects factor. Significance was set at p < 0.05 for all tests, and all post hoc comparisons were conducted using Tukey’s honestly significant difference test. The Kenward–Roger correction (Kenward and Roger 1997) was used in all models, as the use of this correction is highly recommended in repeated-measures models with more complex covariance structures, especially when there is an unbalanced design (Littell et al. 2006). We note that this correction often results in atypical denominator degrees of freedom compared with traditional repeated-measures models (e.g., denominator degrees of freedom higher than the number of subjects, or with a decimal fraction rather than an integer). Potential covariates were evaluated on a model-by-model basis such that covariates that significantly predicted the outcome measure were retained in the model. Covariates that were evaluated included age, race, years of education, smoking status, ADS score, family history density from the FTQ, the average number of drinks per drinking day from the TLFB, alcohol craving at screening from the PACS, total score from the CTQ, neuroticism score from the NEO, and anxiety and depression ratings at screening from the CPRS. For models testing the effect of pioglitazone, PACS alcohol craving at screening was always included as a covariate due to a significant difference between treatment groups (see below). Model-specific covariates are noted in the relevant figure legends.
Results
Baseline characteristics of participants are summarized in Table 1. Treatment groups did not differ significantly on the majority of measures listed, the exception being PACS alcohol craving. Subjects receiving pioglitazone reported higher craving at screening compared with subjects receiving placebo.
Safety and tolerability
Adverse events reported during the study are summarized in Table 2. Six subjects (5 receiving pioglitazone, 1 receiving placebo) had elevated creatine kinase (CK) levels, and for one subject receiving pioglitazone, this was rated as a serious adverse event. Following an independent Data Safety and Monitoring Board review that identified a high risk of myopathy in the active treatment group, the study was prematurely terminated after inclusion and randomization of 16 subjects.
Table 2.
Adverse events: number of enrolled subjects experiencing each event
| Event | Pioglitazone (n = 8) | Placebo (n = 8) |
|---|---|---|
| Abdominal pain | 0 | 1 |
| Abnormal glucose | 1 | 0 |
| Abnormal platelet count | 1 | 0 |
| Acne | 0 | 1 |
| Anxiety | 0 | 1 |
| Asthma exacerbation by viral infection | 1a | 0 |
| Bloating | 1 | 0 |
| Blood in stool | 0 | 1 |
| Blurred vision | 0 | 1 |
| Buzzed | 1 | 0 |
| Chills | 0 | 1 |
| Cold-like symptoms | 0 | 3 |
| Dark urine | 0 | 1 |
| Deep vein thrombosis | 1a | 0 |
| Elevated aspartate aminotransferase | 1 | 0 |
| Elevated creatine kinase | 5b | 1 |
| Fatigue | 0 | 1 |
| Fever | 1 | 0 |
| Frequent urination | 0 | 1 |
| Headache | 2 | 4 |
| Irritability | 0 | 1 |
| Itching | 1 | 1 |
| Lightheaded | 1 | 1 |
| Loss of appetite | 2 | 0 |
| Low red blood count/hemoglobin A1C | 1 | 0 |
| Muscle aches | 1 | 3 |
| Muscle tightness | 0 | 1 |
| Nausea | 1 | 2 |
| Neck pain | 1 | 0 |
| Pain | 2 | 0 |
| Pain in extremity | 1 | 0 |
| Phlebitis | 0 | 1 |
| Sinus pain | 0 | 2 |
| Sleepiness | 1 | 1 |
| Sore throat | 2 | 2 |
| Stomach pain | 1 | 1 |
| Tiredness | 5 | 5 |
| Toothache | 1 | 3 |
| Trouble sleeping | 1 | 0 |
Reported as a serious adverse event
One occurrence reported as a serious adverse event
Guided imagery (scripts)
Craving response
Unlike previous studies, exposure to guided imagery scripts alone did not significantly induce alcohol craving (F[2,28.9] = 1.36, p = 0.27; eFigure 2). There was, however, a main effect of pioglitazone treatment on craving during the alcohol script (F[1,12.4] = 13.82, p = 0.03; Fig. 2a), such that subjects receiving pioglitazone showed higher (rather than the predicted lower) craving throughout the challenge. There was no effect of treatment on craving response to the stress script (F[1,13.6] = 0.93, p = 0.35; Fig. 2b).
Fig. 2.
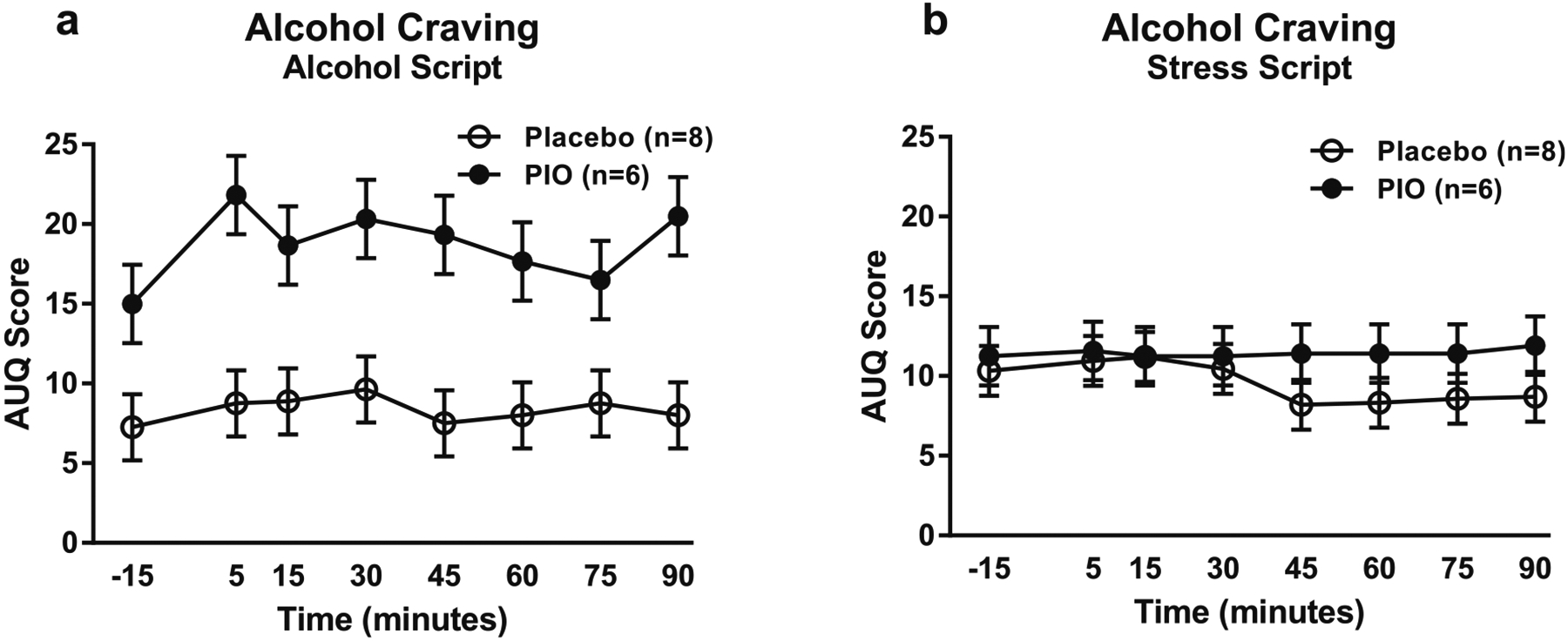
Alcohol craving responses to the guided imagery challenge session. Data points represent mean ± standard error of the mean (SEM). a Effect of pioglitazone on craving response to the alcohol script (pioglitazone: n = 6; placebo: n = 8). Covariates in the model included age, CTQ total score, and baseline PACS craving score. b Effect of pioglitazone on craving response to the stress script (pioglitazone: n = 6; placebo: n = 8). Baseline PACS craving score was the only covariate in the model. For detailed statistics, see “Results”
Anxiety and stress responses
Exposure to guided imagery scripts did not significantly induce anxiety (F[2,26.1] = 0.65, p = 0.53) or stress ratings (F[2,27.3] = 0.34, p = 0.72) (eFigure 2). Furthermore, there were no significant effects of pioglitazone treatment on either anxiety or stress in response to the alcohol (F[1,11.4] = 0.56, p = 0.47 and F[1,12.2] = 2.15, p = 0.17, respectively) and stress scripts (F[1,11.3] = 0.65, p = 0.44 and F[1,11.5] = 0.09, p = 0.77, respectively) (eFigure 3).
Neuroendocrine responses
Consistent with previous studies from our laboratory using guided imagery, exposure to the scripts did not significantly elevate ACTH (F[2,19.1] = 0.95, p = 0.40) or cortisol (F[2,16.5] = 3.29, p = 0.06) (eFigure 4). There was a trend for a main effect of script type on cortisol; however, this was reflected in overall reduced cortisol levels during the alcohol script. There were no significant effects of pioglitazone treatment on either ACTH or cortisol in response to the alcohol (F[1,6.6] = 0.41, p = 0.54 and F[1,7.3] = 0.13, p = 0.73, respectively) and stress scripts (F[1,6] = 0.49, p = 0.51 and F[1,6.4] = 0.84, p = 0.39, respectively) (eFigure 4).
Blood pressure and heart rate
Exposure to guided imagery scripts did not significantly increase blood pressure (systolic: F[2,31.7] = 1.13, p = 0.34; diastolic: F[2,25.6] = 0.10, p = 0.90) (eFigure 5). There was a trend towards elevated diastolic blood pressure among subjects receiving pioglitazone in response to the alcohol script (F[1,10.8] = 4.35, p = 0.06), but no elevation of systolic blood pressure (F[1,12.6] = 0.81, p = 0.38; eFigure 5). There were no significant effects of pioglitazone on either systolic (F[1,12.6] = 0.85, p = 0.37) or diastolic (F[1,12.5] = 0.18, p = 0.67) blood pressure during the stress script.
Exposure to guided imagery scripts did not significantly increase heart rate (F[2,25.1] = 0.43, p = 0.66) eFigure 6). There were no significant effects of pioglitazone on heart rate during either the alcohol script (F[1,11.7] = 0.09, p = 0.77) or stress script (F[1,10.4] = 0.26, p = 0.62) (eFigure 6).
Lipopolysaccharide challenge
Craving response
Administration of lipopolysaccharide (LPS) did not significantly induce alcohol craving (F[1,29.3] = 0.02, p = 0.88; Fig. 3a) compared with saline, nor was there an effect of pioglitazone treatment on the craving response to just the LPS challenge (F[1,11.7] = 0.05, p = 0.83; Fig. 3b).
Fig. 3.

Alcohol craving responses to the LPS challenge session. Data are mean ± SEM. a Effect of the LPS challenge on alcohol craving, compared with saline (n = 13). Covariates in the model included smoking status (yes/no) and average drinks per drinking day. b Effect of pioglitazone on craving response to the LPS challenge (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included average drinks per drinking day and baseline PACS craving score. For detailed statistics, see “Results”
Anxiety and stress responses
Administration of LPS did not induce anxiety (F[1,17.7] = 0.84, p = 0.37; Fig. 4a) or stress (F[1,22.9] = 0.47, p = 0.50; Fig. 4c) compared with saline. However, there was a trend for a main effect of pioglitazone treatment on anxiety (F[1,10.5] = 3.31, p = 0.09; Fig. 4b), and a significant main effect of pioglitazone treatment on stress (F[1,12] = 8.49, p = 0.01; Fig. 4d) during just the LPS challenge. In both cases, subjects receiving pioglitazone reported higher anxiety and stress ratings than subjects receiving placebo.
Fig. 4.
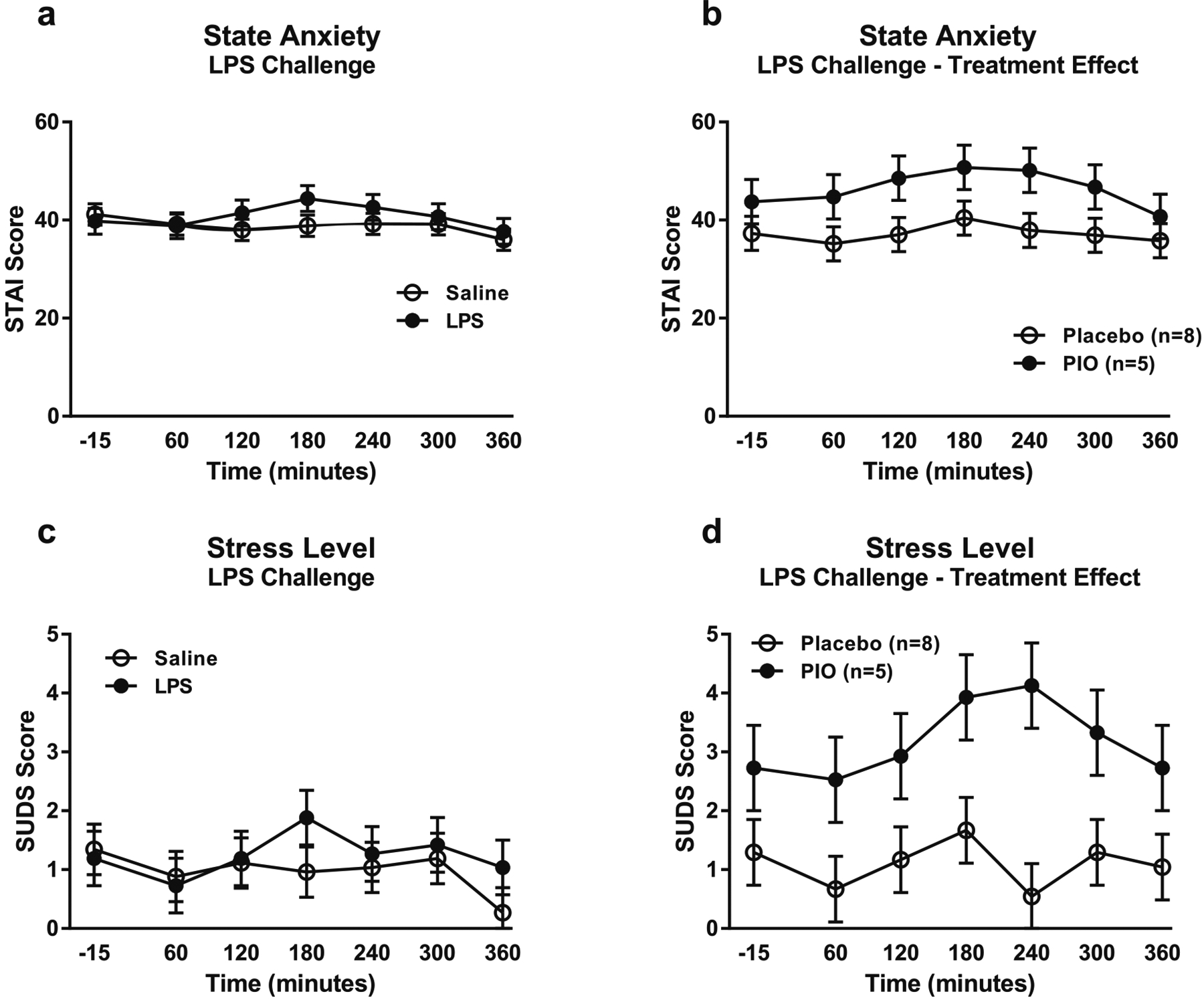
Anxiety and stress responses to the LPS challenge session. Data are mean ± SEM. a Effect of the LPS challenge on state anxiety, compared with saline (n = 13). Covariates in the model included alcohol dependence severity (ADS) score and baseline anxiety measured by the CPRS. b Effect of pioglitazone on anxiety response to the LPS challenge (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included ADS score and baseline PACS craving score. c Effect of the LPS challenge on subjective stress level, compared with saline (n = 13). Covariates in the model included race, smoking status, ADS score, neuroticism score, years of education, and baseline CPRS anxiety. d Effect of pioglitazone on subjective stress response to the LPS challenge (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included ADS score, neuroticism score, years of education, and baseline PACS craving score. For detailed statistics, see “Results”
Neuroendocrine responses
Administration of LPS significantly increased both ACTH (F[1,32.7] = 10.46, p < 0.0001; Fig. 5a) and cortisol (F[1,28.5] = 43.33, p < 0.0001; Fig. 5c) compared with saline. Furthermore, there were significant main effects of pioglitazone treatment for both ACTH (F[1,21.9] = 7.02, p = 0.01; Fig. 5b) and cortisol (F[1,15.8] = 5.67, p = 0.03; Fig. 5d) during just the LPS challenge. In both cases, subjects receiving pioglitazone showed higher ACTH and cortisol levels than subjects receiving placebo.
Fig. 5.
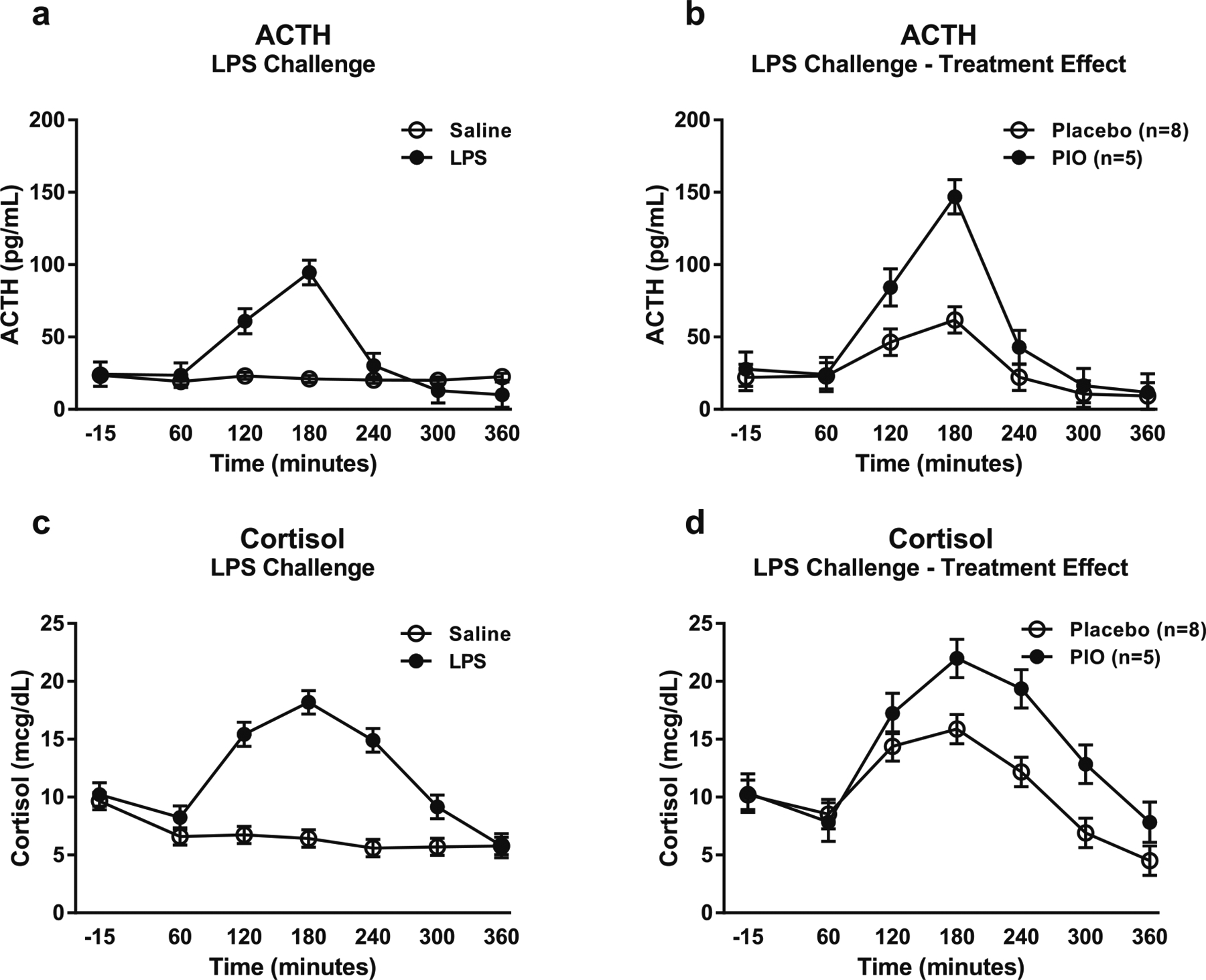
ACTH and cortisol responses to the LPS challenge session. Data are mean ± SEM. a Effect of the LPS challenge on ACTH, compared with saline (n = 13). There were no significant covariates in the model. b Effect of pioglitazone on ACTH response to the LPS challenge (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included family history of alcohol problems and baseline PACS craving score. c Effect of the LPS challenge on cortisol, compared with saline (n = 13). There were no significant covariates in the model. d Effect of pioglitazone on cortisol response to the LPS challenge (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included baseline depression rating from the CPRS and baseline PACS craving score. For detailed statistics, see “Results”
Blood pressure, heart rate, and temperature
Administration of LPS was not found to significantly increase blood pressure (systolic: F[1,36.3] = 2.82, p = 0.20; diastolic: F[1,44.6] = 0.60, p = 0.44) (eFigure 7) compared with saline. There was a significant main effect of pioglitazone treatment on diastolic blood pressure (F[1,25.8] = 37.62, p < 0.0001), but not on systolic blood pressure (F[1,23.5] = 2.09, p = 0.15; eFigure 7) during just the LPS challenge. Diastolic blood pressure was reduced in subjects receiving pioglitazone during the LPS challenge.
Administration of LPS was found to significantly increase heart rate (F[1,28.6] = 18.97, p = 0.0002) and temperature (F[1,41.5] = 62.80, p < 0.0001; eFigure 8) compared with saline. There was a trend for a main effect of pioglitazone treatment on heart rate (F[1,19.2] = 3.89, p = 0.06), and a significant main effect of pioglitazone treatment on temperature (F[1,18.9] = 5.2, p = 0.03; eFigure 8d) during just the LPS challenge. Both heart rate and temperature were increased in subjects receiving pioglitazone during the LPS challenge.
Symptom ratings
Administration of LPS was found to increase a number of physical symptoms, including muscle pain, shivering, nausea, fatigue, fever, weakness, aching joints, and backache (eFigure 9). During the LPS infusion, there were effects of pioglitazone on several psychological symptoms, including sadness, feeling overly sensitive around others, wanting to be alone, and feeling disconnected from others, all of which were higher in subjects receiving pioglitazone compared with placebo (eFigure 10).
Biweekly craving, anxiety, and depression ratings
There was no significant effect of pioglitazone treatment on biweekly ratings of alcohol craving (F[1,10.9] = 0.75, p = 0.49; eFigure 11). There were, however, significant main effects of pioglitazone treatment on biweekly ratings of both anxiety (F[1,14.2] = 21.70, p = 0.0004; Fig. 6a) and depression (F[1,12.6] = 38.44, p < 0.0001; Fig. 6b). Subjects receiving pioglitazone reported higher anxiety and depression symptom ratings during the duration of the study compared with subjects receiving placebo.
Fig. 6.
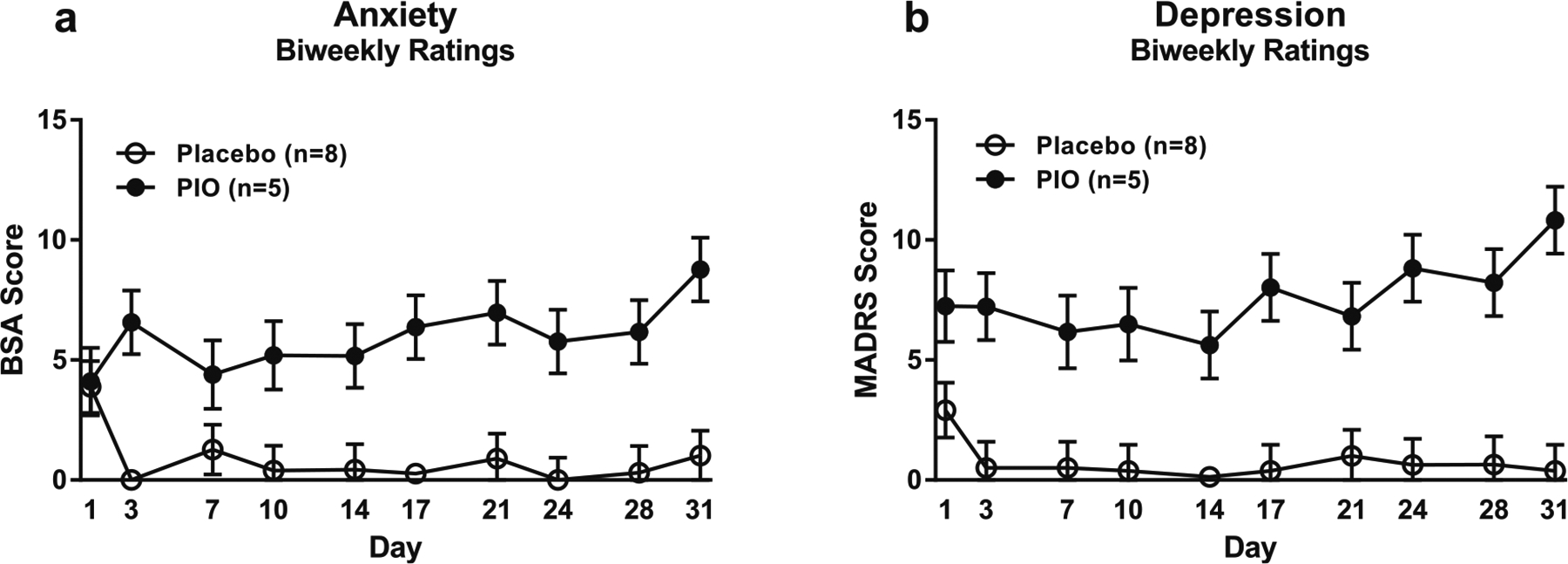
Biweekly ratings of anxiety and depression. Data are mean ± SEM. a Effect of pioglitazone on biweekly anxiety ratings (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included baseline CPRS anxiety and baseline PACS craving score. b Effect of pioglitazone on biweekly depression ratings (pioglitazone: n = 5; placebo: n = 8). Covariates in the model included ADS score, baseline CPRS depression, and baseline PACS craving score. For detailed statistics, see “Results”
CSF proinflammatory cytokines
There were no significant differences between treatment groups in MCP-1 (F[1,11] = 0.67, p = 0.43; mean = 353.6 pg/mL, standard error (SEM) = 32.46 for placebo group; mean = 399.4 pg/mL, SEM = 38.8 for pioglitazone group), IL-6 (F[1,11] = 0.08, p = 0.78; mean = 1.5 pg/mL, SEM = 0.4 for placebo group; mean = 1.3 pg/mL, SEM = 0.5 for pioglitazone group), or TNF-α (F[1,10] = 0.66, p = 0.44; mean = 0.16 pg/mL, SEM = 0.02 for placebo group; mean = 0.13 pg/mL, SEM = 0.03 for pioglitazone group).
Discussion
The main finding of our study is that pioglitazone, administered at doses clinically approved for diabetes treatment, does not have acceptable safety and tolerability in treatment-seeking alcohol-addicted patients, where it is associated with a high rate of skeletal muscle (SKM) toxicity as evidenced by elevated creatine kinase levels. This finding led to early termination of the study, resulting in low power and a limited ability to carry out analyses of the a priori study objectives. While the safety results are important to disseminate, any additional results must therefore be considered highly preliminary. However, analyses of the primary and secondary outcome measures from participants who did complete the study unexpectedly suggest that, in addition to the safety signal observed, pioglitazone is also associated with a profile of worsened, rather than attenuated craving, negative mood and anxiety symptoms, as well as an increased reactivity of the HPA-axis. Together, these data are important to inform considerations regarding the potential of pioglitazone to be repurposed for the treatment of alcohol addiction.
Our experimental medicine paradigm allowed rapid detection of safety signals through frequent collection of multiple safety monitoring parameters. In this case, the signal was detected from multiple cases of creatine kinase (CK) elevations, which were reviewed by an independent data safety and monitoring board (DSMB) that found them to be associated with active treatment allocation. CK elevations reflect damage to SKM cells, and are a precursor of rhabdomyolysis, a serious condition that can result in renal failure and death. CK elevations and rhabdomyolysis are a known but uncommon side effect of pioglitazone (Peraza et al. 2006). It is unlikely that the increased frequency of CK elevations in alcohol addiction results from non-specific ill health of patients with addictive disorders, since no such elevation was found in our recent study of pioglitazone in opioid addiction (Schroeder et al. 2018).
A key question prompted by our safety findings is therefore whether the SKM toxicity observed in alcohol-addicted patients is mechanism-related, or represents off-target effects that are unique to pioglitazone and therefore could be engineered out while retaining PPARγ activity. In the former scenario, a path to successful clinical development of any PPARγ agonist seems unlikely. Whether SKM toxicity of PPARγ agonists in general is mechanism-related has long remained unknown (Peraza et al. 2006). However, recent work in a mouse model suggests that this toxicity may be related to oxidative stress rather than PPARγ activity (Akai et al. 2017). Heavy alcohol use is associated with a risk of myopathy per se, and oxidative stress is thought to be a key pathophysiological mechanism behind this risk (Simon et al. 2017). It therefore seems plausible that the high frequency of CK elevations following pioglitazone administration to alcohol-addicted patients reflects additive or synergistic effects to cause mitochondrial damage and oxidative stress. Establishing whether this is the case and whether effects on oxidative stress can be engineered out while retaining PPARγ activity will be critical to determine the clinical potential of this mechanism in alcohol addiction from a safety standpoint.
Multiple studies from our and other laboratories have previously used guided imagery script procedures to induce cravings, and modulation of this response as a biomarker of potential medication activity (for review, see e.g. (Kwako et al. 2015b)). In the present study, which used the same script procedures and development staff as our previous studies using this method, we did not observe significant increases in alcohol craving, nor in subjective measures of anxiety or stress, in response to either the alcohol or stress cues. The reason for this lack of response is not immediately clear, although it may have been due to the small number of participants evaluated. Another factor may have been the fact that, despite all participants being heavy drinkers, the severity of alcohol dependence was lower in this sample (average ADS score of 15.6) compared with that seen in our previous studies using guided imagery (average ADS score of around 20–23). The lack of response to the scripts notwithstanding, we did observe that participants receiving pioglitazone reported significantly elevated, rather than attenuated, cravings throughout the alcohol script. This observation was accompanied by markedly elevated spontaneous ratings of anxiety and depressed mood in pioglitazone allocated patients throughout the duration of the inpatient study. While limited by the small number of participants, these findings do call into question the efficacy of pioglitazone in the treatment of clinical alcohol addiction.
Our findings stand in contrast to promising results reported from animal models of addiction-related behaviors, which provided the rationale for the present study. It has been reported that activation of PPARγ reduces the expression of methamphetamine-induced locomotor sensitization in mice (Daynes and Jones 2002), an effect attributed to the ability of the activated receptor to reduce glia-mediated inflammatory responses in the brain by inhibiting the expression of proinflammatory genes (Dasu et al. 2009; Maeda et al. 2007). Glia-derived proinflammatory mediators, such as IL1ß, IL6, and TNFα, increase locomotor sensitization and the neurotoxic effects of psychostimulants, and are also involved in development of tolerance to opioids (Ladenheim et al. 2000; Lin et al. 2010; Mika 2008; Zalcman et al. 1999). Inhibition of proinflammatory cytokine production has also been associated with reduction of opioid tolerance, reduction of morphine reward, and prevention of opioid withdrawal (Bland et al. 2009; Hutchinson et al. 2007; Hutchinson et al. 2009; Watkins et al. 2009). Human studies with pioglitazone in opioid use and addiction have yielded mixed results, with some support for a suppression of craving (Jones et al. 2018, 2016; Schroeder et al. 2018). Our findings do not support a similar activity in alcohol addiction.
It is unclear why suppression of relapse to alcohol seeking observed with pioglitazone in preclinical experiments (Stopponi et al. 2011) did not translate into reduced alcohol craving, but considerable caution needs to be exercised in interpreting their failure to do so. Aside from the small number of subjects who could be evaluated, it is possible that combined effects on oxidative stress from heavy alcohol exposure and pioglitazone act to overshadow any beneficial effects on motivational processes due to PPARγ activation.
In mice, LPS administration has been reported to result in a prolonged increase in alcohol intake (Blednov et al. 2011), a finding that supports a role for proinflammatory signaling in the motivation to consume alcohol. In the present study, we therefore also examined whether a low-dose LPS challenge would offer a useful experimental medicine paradigm to evaluate alcohol cravings. While LPS administration did not result in any significant increase in alcohol craving, we did observe fairly robust physiological effects, including elevated neuroendocrine measures, heart rate and body temperature, and other physical symptoms such as pain, nausea, fatigue, fever, and weakness. Furthermore, subjects receiving pioglitazone exhibited a greater neuroendocrine response compared with subjects receiving placebo, as well as higher ratings of psychological symptoms including sadness and feeling disconnected from others. Taken together, these findings indicate that LPS administration may not be a useful model to evaluate the efficacy of potential anti-craving medications, and provide even further evidence that pioglitazone may result in adverse rather than beneficial effects in treatment-seeking alcoholics.
In summary, we report data indicating that the PPARγ agonist pioglitazone is unlikely to have potential as an alcoholism medication due to safety, and potentially also efficacy issues. This conclusion may be preliminary in nature, however, due to the small number of subjects evaluated in the current study.
Supplementary Material
Acknowledgments
We would like to thank Anthony Suffredini of the NIH Clinical Center for his overview and assistance in administration of the LPS endotoxin. This study was supported by the NIH/NIAAA intramural program.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Compliance with ethical standards
All participants provided written informed consent.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00213-020-05540-w) contains supplementary material, which is available to authorized users.
References
- Akai S, Oda S, Yokoi T (2017) Establishment of a novel mouse model for pioglitazone-induced skeletal muscle injury. Toxicology 382:1–9 [DOI] [PubMed] [Google Scholar]
- Asberg M, Schalling D (1979) Construction of a new psychiatric rating instrument, the Comprehensive Psychopathological Rating Scale (CPRS). Prog Neuropsychopharmacol 3:405–412 [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27:169–190 [DOI] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW (2009) The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun 23:492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25(Suppl 1):S92–S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606 [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB (2007) Relapse and relapse prevention. Annu Rev Clin Psychol 3:257–284 [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC (2002) Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem 82:615–624 [DOI] [PubMed] [Google Scholar]
- Chang F, Jaber LA, Berlie HD, O’Connell MB (2007) Evolution of peroxisome proliferator-activated receptor agonists. Ann Pharmacother 41:973–983 [DOI] [PubMed] [Google Scholar]
- Coleman LG, Crews FT (2018) Innate Immune Signaling and Alcohol Use Disorders. In: Grant KA, Lovinger DM (eds) The neuropharmacology of alcohol. Springer International Publishing, Cham, pp 369–396 [Google Scholar]
- Costa PT, McCrae RR (2002) NEO Personality Inventory-Revised (NEO PI-R). APA, Washington, DC [Google Scholar]
- Crews FT, Zou J, Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25(Suppl 1):S4–S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Park S, Devaraj S, Jialal I (2009) Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 150:3457–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes RA, Jones DC (2002) Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2:748–759 [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, de Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, Gaitanaris G, Demopulos G, Damadzic R, Tapocik J, Heilig M, Ciccocioppo R (2015) PPARgamma activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology 40:927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R (2017) Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology 234:223–234 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010a) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR (2010b) Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 24:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A (2002) Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med (Berl) 80:5–15 [DOI] [PubMed] [Google Scholar]
- Feinstein DL (2003) Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol Ther 5:67–73 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (1995) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). Biometrics Research: New York State Psychiatric Institute, New York [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM (1999) Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res 23:1289–1295 [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli JR (2003) Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol 64:120–126 [DOI] [PubMed] [Google Scholar]
- Fritz M, Klawonn AM, Nilsson A, Singh AK, Zajdel J, Wilhelms DB, Lazarus M, Löfberg A, Jaarola M, Kugelberg UÖ, Billiar TR, Hackam DJ, Sodhi CP, Breyer MD, Jakobsson J, Schwaninger M, Schütz G, Parkitna JR, Saper CB, Blomqvist A, Engblom D (2016) Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J Clin Invest 126:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J (2007) Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell 131: 405–418 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127 [DOI] [PubMed] [Google Scholar]
- Heberlein A, Käser M, Lichtinghagen R, Rhein M, Lenz B, Kornhuber J, Bleich S, Hillemacher T (2014) TNF-alpha and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol 48:671–676 [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Witkiewitz K, George WH, Marlatt GA (2011) Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 7:98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW (2009) Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23:240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay JM, Mogali S, Metz VE, Ciccocioppo R, Comer SD (2016) The effects of pioglitazone, a PPARgamma receptor agonist, on the abuse liability of oxycodone among nondependent opioid users. Physiol Behav 159:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Bisaga A, Metz VE, Manubay JM, Mogali S, Ciccocioppo R, Madera G, Doernberg M, Comer SD (2018) The PPARgamma agonist pioglitazone fails to alter the abuse potential of heroin, but does reduce heroin craving and anxiety. J Psychoactive Drugs 50:390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci 13:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997 [PubMed] [Google Scholar]
- Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M (2015a) The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology 232:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M (2015b) Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: behavioral and physiological outcomes. Addict Biol 20:733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M (2015c) The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a random-ized controlled experimental medicine study. Neuropsychopharmacology 40:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL (2000) Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol 58:1247–1256 [DOI] [PubMed] [Google Scholar]
- Landreth GE, Heneka MT (2001) Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging 22:937–944 [DOI] [PubMed] [Google Scholar]
- Landreth G, Jiang Q, Mandrekar S, Heneka M (2008) PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics 5:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P (2014) Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry 76:725–733 [DOI] [PubMed] [Google Scholar]
- Lin SL, Tsai RY, Tai YH, Cherng CH, Wu CT, Yeh CC, Wong CS (2010) Ultra-low dose naloxone upregulates interleukin-10 expression and suppresses neuroinflammation in morphine-tolerant rat spinal cords. Behav Brain Res 207:30–36 [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberge O (2006) SAS for Mixed Models, Second edn. SAS Institute Inc., Cary [Google Scholar]
- Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S (2007) Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology 32:1133–1140 [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D (1985) Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend 15:61–67 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP (1980) An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis 168:26–33 [DOI] [PubMed] [Google Scholar]
- Mika J (2008) Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 60: 297–307 [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145 [DOI] [PubMed] [Google Scholar]
- Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM (2006) The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicol Sci 90:269–295 [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW (2009) Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 150: 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT (2006) Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A 103:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneekloth TD, Biernacka JM, Hall-Flavin DK, Karpyak VM, Frye MA, Loukianova LL, Stevens SR, Drews MS, Geske JR, Mrazek DA (2012) Alcohol craving as a predictor of relapse. Am J Addict 21(Suppl 1):S20–S26 [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Phillips KA, Epstein DH, Jobes ML, Furnari MA, Kennedy AP, Heilig M, Preston KL (2018) Assessment of pioglitazone and proinflammatory cytokines during buprenorphine taper in patients with opioid use disorder. Psychopharmacology 235:2957–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M (2016) The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology 41:2818–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Jolley SE, Molina PE (2017) Alcoholic myopathy: pathophysiologic mechanisms and clinical implications. Alcohol Res 38:207–217 [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ (2011) Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 68:942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H, Horn J (1984) Alcohol Dependence Scale: user’s guide. Addiction Research Foundation, Toronto [Google Scholar]
- Sobell LC, Sobell MB (1996) Timeline Followback user’s guide: a calendar method for assessing alcohol and drug use. Addiction Reseaerch Foundation, Toronto [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE (1970) The State-Trait Anxiety Inventory. Consulting Psychologists Press, Palo Alto [Google Scholar]
- Stohs ME, Schneekloth TD, Geske JR, Biernacka JM, Karpyak VM (2019) Alcohol craving predicts relapse after residential addiction treatment. Alcohol Alcohol 54:167–172 [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R (2011) Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry 69:642–649 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M (2011) Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology 36:1178–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30:581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J (1969) The practice of behavior therapy, 1st edn. Pergamon Press, New York [Google Scholar]
- Young PW et al. (1998) Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther 284: 751–759 [PubMed] [Google Scholar]
- Zalcman S, Savina I, Wise RA (1999) Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res 847:276–283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


