Abstract
Many microorganisms encode proteins that interact with molecules involved in host immunity; however, few of these molecules have been proven to promote immune evasion in vivo. Herpes simplex virus type 1 (HSV-1) glycoprotein C (gC) binds complement component C3 and inhibits complement-mediated virus neutralization and lysis of infected cells in vitro. To investigate the importance of the interaction between gC and C3 in vivo, we studied the virulence of a gC-null strain in complement-intact and C3-deficient animals. Using a vaginal infection model in complement-intact guinea pigs, we showed that gC-null virus grows to lower titers and produces less severe vaginitis than wild-type or gC rescued virus, indicating a role for gC in virulence. To determine the importance of complement, studies were performed with C3-deficient guinea pigs; the results demonstrated significant increases in vaginal titers of gC-null virus, while wild-type and gC rescued viruses showed nonsignificant changes in titers. Similar findings were observed for mice where gC null virus produced significantly less disease than gC rescued virus at the skin inoculation site. Proof that C3 is important was provided by studies of C3 knockout mice, where disease scores of gC-null virus were significantly higher than in complement-intact mice. The results indicate that gC-null virus is approximately 100-fold (2 log10) less virulent that wild-type virus in animals and that gC-C3 interactions are involved in pathogenesis.
Viruses encode receptors and secrete molecules that interfere with host immune mediators (35); however, the importance of viral immune evasion molecules in vivo remains relatively unexplored. Herpes simplex virus type 1 (HSV-1) encodes several immune modulators, including glycoprotein C (gC), a C3 binding protein (14, 33), gE/gI, an Fc receptor for immunoglobulin G (1, 8, 32), and ICP47, an inhibitor of HSV peptide presentation within major histocompatibility complex class I molecules (18, 26). gC inhibits activation of the complement cascade by binding complement component C3b (14, 17) and by blocking binding of properdin and C5 to C3b (17, 28, 33). These processes interfere with amplification of both the classical and alternative complement pathways as well as generation of the membrane attack complex. In vitro, gC protects HSV-infected cells from complement-mediated lysis (21) and cell-free virus from complement-mediated neutralization (16, 19, 24); however, a role for gC in vivo as an inhibitor of complement has not been established.
HSV-1 is among a growing number of viruses that interact with molecules of the complement system. Interactions between viruses and complement can be considered in two broad categories: (i) viruses that use complement receptors on cells for entry (e.g., Epstein-Barr virus, measles virus, and echoviruses) (2, 11, 31) and (ii) viruses that inhibit complement activation (e.g., HSV-1, HSV-2, Epstein-Barr virus, herpesvirus saimiri, pseudorabies virus, bovine herpesvirus 1, and vaccinia virus) (10, 12, 21, 27, 30, 36, 38, 40). The widespread presence of complement-modifying proteins in pathogenic viruses suggests that they play an important role in disease progression and indicate that they may be appropriate targets for prevention or treatment strategies.
Numerous studies have examined the role of gC in animal models; however, none were designed specifically to assess the interaction between gC and complement. For example, studies performed with HSV-1 strain KOS and gC mutants of KOS (45) are difficult to evaluate for gC-complement interactions because KOS is unusual among HSV-1 strains in that it exhibits much less C3b binding than most isolates (15). Studies that inoculate virus directly into the brain or footpad use routes that bypass complement defense mechanisms present at mucosal surfaces (5, 7, 45). Studies that infect the cornea (4, 22, 41) evaluate a tissue that is relatively avascular and expected to have low complement levels (25). We chose to infect by a mucosal route in guinea pigs (44) and by dermal scratch inoculation in mice (43). Both routes of infection permit interactions between virus and complement. The gC mutant strain used was known to be susceptible to complement inactivation in vitro (16), and a marker-rescued revertant was included as control. The results establish the importance of gC in pathogenesis and indicate that gC is a virulence factor because it interacts with C3.
MATERIALS AND METHODS
Cell culture and virus strains.
Vero cells were grown at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 20 μg of gentamicin per ml, and 20 mM HEPES (pH 7.3). To prepare purified virus pools, Vero cells were infected at a multiplicity of infection (MOI) of 5; supernatant fluids were harvested for cell-free virus and centrifuged on a 5 to 65% sucrose gradient as previously described (16). The visible virus band was collected and dialyzed against 500 volumes of phosphate-buffered saline (PBS) at 4°C, and titers were determined by plaque assay on Vero cells.
NS is a wild-type, low-passage-number HSV-1 clinical isolate that is the parent strain for the mutants used in these studies (14). NS-gCnull is a gC-negative virus constructed by replacing the entire gC-1 protein coding sequence with the ICP6::LacZ cassette (16). It does not express gC or bind C3b. rNS-gCnull is a rescue of the gC-null virus back to wild-type phenotype by using gC from NS (16). Southern blotting, Western blotting, and susceptibility to complement-mediated neutralization of the virus strains have been previously described (16). The gC mutant NS-gCnull is rapidly neutralized by human complement and shows 50- to 100-fold-greater susceptibility to complement-mediated neutralization than wild-type or rescued virus, both of which are almost totally resistant to complement (16). All mutant and rescued strains were plaque purified three times prior to preparation of purified virus pools for in vivo studies.
Determining the replication phenotypes of wild-type, gC mutant, and rescued viruses.
Single-step growth curves were performed by infecting Vero cells at an MOI of 5; at 1, 4, 8, 12, 20, and 24 h postinfection, the cells plus supernatant fluids were harvested and titers were determined by plaque assay (16).
Determining the attachment phenotypes of wild-type and gC mutant viruses. (i) Measuring particle-to-infectivity ratios.
The particle-to-infectivity ratios were determined by loading equivalent PFU of purified viruses onto sodium dodecyl sulfate–7.5% polyacrylamide gels and probing by Western blot analysis for the viral capsid antigen VP5, using rabbit polyclonal antibody NC-1 (6, 23). The intensities of the VP5 bands were compared by densitometry analysis.
(ii) Virus attachment assays.
35S-labeled virus was prepared by infecting Vero cells at an MOI of 5. At 4 h postinfection, cells were rinsed in Hanks balanced salt solution and overlaid with DMEM with 0.1× methionine, 0.1× cysteine, 2% dialyzed FBS, and 100 μCi of Tran35S-label (ICN) per ml. At 24 h postinfection, the cell-free virus was purified (16). Virus attachment to cells was measured as previously described (23), with modifications. Briefly, HEp-2 or Vero cells were grown in 24-well tissue culture plates until confluent and washed with PBS, and then 1 ml of PBS–1% bovine serum albumin (BSA) was added for 1 h at 37°C. Plates were cooled to 4°C and washed with PBS–0.1% BSA, and 35S-labeled virus was added in 200 μl of PBS–1% BSA. Plates were rocked at 4°C and processed for bound virus at time zero and at 1, 2, 3, 4, 6, 8, and 20 h by washing with PBS, then adding 0.5 ml of 10 mM Tris–10 mM EDTA–0.25% Triton X-100 at room temperature for 30 min, and transferring the solution to a scintillation vial (Ecolume; ICN). Radioactivity was determined in a scintillation counter. To plot attachment kinetics, radioactive counts were converted to PFU by determining the number of 35S counts per PFU.
Determining the phenotypes of gC mutant viruses in MDCK cells.
Assays were performed to determine if gC mutant viruses are defective in binding to the apical surface of MDCK cells (42). Serial 10-fold dilutions of virus were added for 1 h at 37°C to 5- to 8-day-old confluent monolayers of MDCK cells, and then monolayers were washed and overlaid with 0.6% agarose and DMEM–5% FBS. After 48 h, MDCK cells were fixed with cold methanol-acetone and washed, and foci were stained by immunoperoxidase assay using rabbit anti-gB polyclonal antibody R69 (42). The ratio of plaques on Vero cells to plaques on MDCK cells was calculated to indicate whether gC mutants were defective in apical attachment. To measure basolateral infection, Vero cells were grown on collagen-coated Transwell insert membranes (Costar; Fisher Scientific) containing 3.0-μm holes, infected at the basal surface, and stained for gB at 48 h as described above.
In vivo studies. (i) Inoculation of guinea pigs.
Female Hartley strain guinea pigs weighing 175 to 225 g were infected as previously described (44). Briefly, vaginal membranes were broken with PBS-moistened calcium alginate swabs (Calgiswab 3; Spectrum Labs, Houston, Tex.), proteinaceous material was removed, and 50 μl of virus was introduced via a soft catheter. Vaginal titers were measured at 4 h and at 1, 3, 5, 7, and 10 days postinoculation by inserting a moistened swab into the vagina, rotating the swab five times, and then placing the swab in 1 ml of DMEM–10% FBS. Samples were stored at −70°C until titers were determined by plaque assay.
(ii) Scoring for vaginitis.
Animals were observed daily, and disease severity was scored as follows: 0.5 point for mild or moderate erythema, 1.0 point for severe erythema, 0.5 point for vaginal discharge, and 1.0 point for vaginal vesicles. The maximum daily score assigned to an animal was 1.5. Mean vaginitis scores were calculated by summing the daily scores of all animals over the first 10 days postinfection and dividing by the number of animals in the treatment group. Statistical analysis to compare groups was performed with the Student t test.
(iii) C3D guinea pigs.
Experiments were performed with a unique colony of C3-deficient (C3D) inbred guinea pigs that have an inherited defect in complement component C3 (3). These animals were originally identified within a colony of inbred strain 2 animals. They have serum C3 levels that are 5.7% of normal. This amount of C3 is sufficient to support hemolysis of antibody-coated erythrocytes and results in 50% total hemolytic complement activity (16) of 1:16 to 1:32, which represents 12.5 to 25% of the activity present in normal guinea pig serum.
(iv) Dermal inoculation of C57BL/6 and C3 knockout mice.
C3-null mice were generated by target deletion of the C3 gene (48). Briefly, the 2.4-kb segment flanking the C3 gene including its promoter and exon 1 was replaced by the neomycin resistance gene by homologous recombination in embryonic stem cells. The stem cells were injected into C57BL/6 blastocysts, chimeric offspring bred with C57BL/6 females, and homozygous C3−/− animals bred from heterozygous C3-null founders. These mice lack complement activity and C3 protein assessed by an enzyme-linked immunosorbent assay capable of detecting C3 at 1 ng/ml.
The C3-null mice and the parental strain C57BL/6 were infected by scratch inoculation as previously described (39, 43). Infection was initiated by shaving the right flank and denuding the fur with a depilatory cream. Twenty-four hours later, 5 × 105 PFU in 10 μl of sterile DMEM was applied to the denuded flank several millimeters from the spinal column by scratching gently 30 times with a 27-gauge needle in an area of approximately 3 by 3 mm. Disease scores were expressed as the sum of the scores from days 3 to 8 postinfection (39). Disease at the inoculation site was scored as follows: 0 points for no disease, 0.5 point for swelling without vesicles, and 1.0 point for each vesicle or scab, to a maximum daily score of 5 points. If vesicles or scabs became confluent, points were assigned based on the size of the confluent lesion. Swelling and lesions at locations separate from the site of inoculation were considered dermatomal or zosteriform lesions. Scoring of these lesions was the same as at the inoculation site except that a maximum daily score of 10 was used since a larger number of lesions could be counted over the greater skin area involved.
Calculation of the mean AUC and statistical methods.
Vaginal titers were plotted as the mean log10 ± standard error of the mean (SEM), and the area under the curve (AUC) was determined by using a geometric mean for the treatment group at each time point. The AUC was calculated by using pcnonlin 4.0 (SCI Software, Lexington, Ky.), and statistical significance between treatment groups was evaluated by analysis of variance (34).
RESULTS
Attachment phenotypes of wild-type and gC mutant viruses.
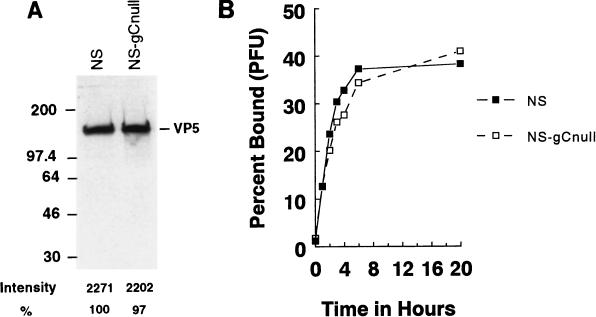
Some strains of gC-null virus are defective in virus attachment to cells (49), whereas others are normal (20); therefore, we characterized the attachment phenotype of NS-gCnull virus. Wild-type and gC-null viruses were standardized according to their content of VP5 capsid (23) since measurement based on PFU could give misleading values for mutants defective in attachment. By Western blotting, equivalent PFU of NS and NS-gCnull yielded VP5 bands of similar intensities (Figure 1A), which indicates that the plaquing efficiencies of the two viruses are comparable. Binding kinetics of 35S-labeled NS and NS-gCnull were similar when the viruses were added to HEp-2 cells (Fig. 1B) or Vero cells (not shown). We conclude that the attachment phenotype of NS-gCnull is comparable to that of the wild-type virus.
FIG. 1.
(A) Particle-to-infectivity ratios of wild-type and gC mutant viruses. NS or NS-gCnull virus (106 PFU of each) was tested by Western blotting for viral capsid antigen VP5. Bands were quantified by densitometry. The two viruses had similar PFU/VP5 ratios, which indicates comparable plaquing efficiency. Sizes are indicated in kilodaltons. (B) Attachment kinetics of NS or NS-gCnull virus. [35S]methionine- and [35S]cysteine-labeled cell-free virus was added to HEp-2 cells at 4°C, and at various times bound counts were determined. NS and NS-gCnull have similar binding kinetics.
Infection of polarized MDCK cells.
A gC-null strain of HSV-1(F) was reported to be >10,000-fold (4 log10) less effective than wild-type virus in its ability to infect the apical surface of polarized cells, which suggests that gC may be important for interaction with apical cell surface receptor(s) (42). However, gC mutant strains of HSV-1 SC16 and HFEM do not show this phenotype and differ less than twofold from wild-type virus (20). To determine the phenotype of NS-gCnull virus, we evaluated its ability to infect polarized MDCK cells when added to the apical surface. NS-gCnull produced 12.6-fold (1.1 log10) fewer plaques than NS (Table 1). As reported by others, no differences were noted when viruses were added to the basolateral surfaces (not shown) (20, 42). These results indicate that NS-gCnull differs from wild-type virus in its ability to infect polarized cells; however, differences were small compared with those reported for a gC null mutant of HSV-1(F) (42).
TABLE 1.
Infection of MDCK cells by wild-type or gC null mutant virus
| Virus | Mean titer ± SEM (n = 4)
|
Titer on Vero cells/titer on MDCK cells | Fold increase in PFU needed to form plaques on MDCK cells | |
|---|---|---|---|---|
| Vero cell | MDCK cell apical surface | |||
| NS | 108.0±0.6 | 105.1±0.6 | 794:1 | 1 |
| NS-gCnull | 108.0±0.2 | 104.0±0.1 | 10,000:1a | 12.6 |
Results comparing NS-gCnull and NS plaquing efficiency on MDCK cells are statistically different, P = 0.003.
HSV-1 infection of guinea pigs.
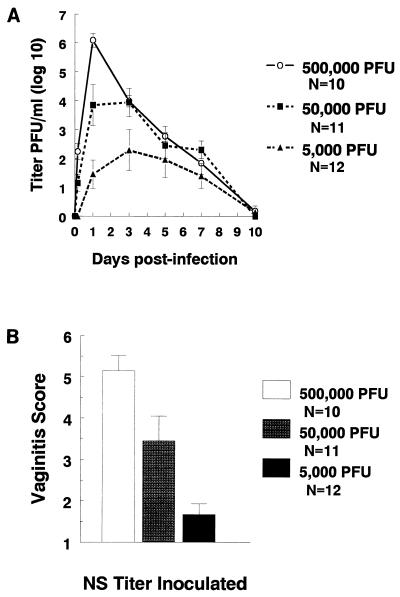
Before infecting animals, we performed one-step growth curves; the results demonstrated comparable replication kinetics for mutant, rescued, and wild-type viruses (not shown). Wild-type HSV-1 was inoculated intravaginally into Hartley strain guinea pigs at three doses over a 100-fold titer range (5 × 103 to 5 × 105) to determine the dose-response kinetics of viral replication and vaginal disease scores. At the highest inoculum, vaginal titers peaked at day 1 postinoculation, while at the two lower doses, titers peaked between days 1 and 3 (Fig. 2A). Each 10-fold increase in inoculum caused an approximately 2 log10 increase in peak titers. Vaginitis scores correlated with vaginal titers, since animals infected at 5 × 105 PFU had the most severe vaginitis, those infected with 5 × 104 PFU had intermediate scores, and those inoculated with 5 × 103 PFU had the lowest scores (Fig. 2B).
FIG. 2.
Dose-response studies with wild-type virus NS. (A) NS was inoculated intravaginally at 5 × 105, 5 × 104, or 5 × 103 PFU. Vaginal swabs were taken 4 h and at 1, 3, 5, 7, and 10 days postinfection, and titers were determined. AUC was calculated as a measure of viral load. AUCs were 28.3 at 5 × 105 PFU, 24.6 at 5 × 104 PFU, and 14.3 at 5 × 103. AUC at 5 × 105 PFU is significantly greater than AUC at 5 × 103 PFU (P < 0.01), and AUC at 5 × 104 PFU is significantly greater than AUC at 5 × 103 PFU (P = 0.05). (B) Vaginitis scores at 5 × 105, 5 × 104, and 5 × 103 PFU. Scores at 5 × 105 PFU are significantly greater than scores at 5 × 104 PFU (P = 0.03), and scores at 5 × 104 are significantly greater than scores at 5 × 103 PFU (P < 0.01). Error bars represent ± SEM.
Virulence of gC-null virus in complement-intact guinea pigs.
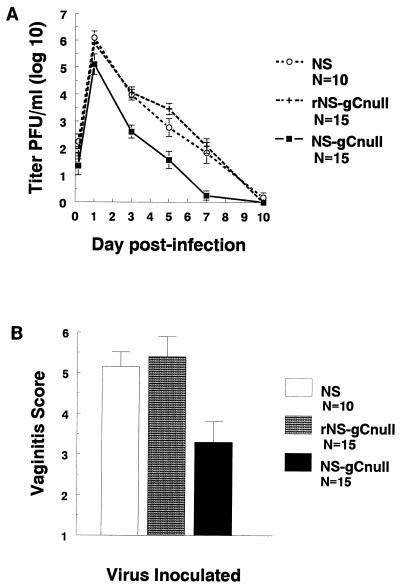
We examined the role of gC in virulence by comparing disease caused by the gC-null virus with that caused by the wild-type or rescued virus. Hartley strain guinea pigs were inoculated intravaginally with 5 × 105 PFU. The gC-null strain replicated to titers 10- to 100-fold (1 to 2 log10) lower than those for wild-type or rescued virus over the 10-day course of infection (P < 0.01, comparing the AUC of gC-null virus with that of wild-type or rescued virus) (Fig. 3A). Severity of vaginitis was also significantly reduced in gC-null virus-infected animals (P < 0.01, comparing NS-gCnull with NS or rNS-gCnull) (Fig. 3B), indicating that gC-null virus grows to lower titers and produces less severe vaginitis than wild-type or gC-rescued virus. These results indicate that gC is important in virulence.
FIG. 3.
Vaginal titers and vaginitis scores are lower for gC-null virus than for rescued and wild-type strains. (A) Guinea pigs were inoculated with 5 × 105 PFU of NS, rNS-gCnull, or NS-gCnull virus. Vaginal swabs were taken at the indicated times, and titers were measured. AUCs are 28.3 for NS, 30.3 for rNS-gCnull, and 16.9 for NS-gCnull. NS and rNS-gCnull are significantly different from NS-gCnull (P < 0.01). (B) Vaginitis scores for the three viruses. NS-gCnull is significantly different from rNS-gCnull (P < 0.01) and NS (P = 0.02). Error bars represent ± SEM.
Virulence of gC-null virus in C3D guinea pigs.
Experiments were performed with C3D guinea pigs to determine whether complement accounts for differences in virulence between gC-null and wild-type or rescued virus. We postulated that if gC-complement interactions are important, titers of gC-null virus should be significantly higher in C3D guinea pigs and that titers of gC-null virus should increase more than those of wild-type or rescued virus. An inoculum of 5 × 103 PFU was selected for infection because this was the lowest dose to yield detectable vaginal titers in complement-intact animals; therefore, we could detect substantial increases in titers. Vaginal titers of gC-null virus were significantly higher in C3D guinea pigs than in complement-intact animals (Fig. 4A). The AUC of NS-gCnull in C3D guinea pigs was 12.7, compared with 3.6 in complement-intact animals (P < 0.02). Peak viral titers on day 1 postinfection were over 300-fold (2.5 log10) higher in C3D animals than in complement-intact controls (P = 0.003). In contrast, vaginal titers of rescued virus were only slightly higher in C3D animals (AUC of 24.1) than in complement-intact controls (AUC of 16, P = nonsignificant [Fig. 4B]). Vaginal titers of wild-type virus NS were also only slightly higher in C3D animals (AUC of 17.4) than in complement-intact controls (AUC of 14.3, P = nonsignificant [Fig. 4C]). The results indicate that titers of NS-gCnull virus are significantly affected by the presence of C3 in the animals, while titers of wild-type and rescued viruses are not. Therefore, we conclude that C3 accounts at least in part for the decreased vaginal titers of gC null virus in complement-intact animals.
FIG. 4.
The interaction between gC and complement is important for HSV-1 virulence in guinea pigs. C3D or complement-intact guinea pigs were inoculated with 5 × 103 PFU of NS-gCnull (A), rNS-gCnull (B), or NS (C). Vaginal swabs were taken at the indicated times, and titers were measured. AUC of NS-gCnull in complement-intact animals (3.6) is significantly different from that of C3D animals (12.7) (P = 0.02). AUCs of rescued or wild-type virus are not significantly different in complement-intact and C3D guinea pigs. Error bars represent ± SEM.
Vaginitis scores were low in complement-intact animals infected with gC-null, rescued, or wild-type virus at 5 × 103 PFU (range of scores, 1.1 to 1.7), while scores were higher for all three viruses in C3D guinea pigs (range, 4.5 to 5.2) (result not shown). Thus, in C3D guinea pigs, NS-gCnull virus caused vaginitis scores that were comparable to those caused by rescued and wild-type viruses.
We compared the vaginal titers of gC-null, rescued, and wild-type viruses in complement-intact guinea pigs inoculated with 5 × 103 PFU. The mean AUC of NS-gCnull (3.6) was significantly lower than that of rescued virus (15) or wild-type virus (14.3) (compare the bottom curves in each panel of Fig. 4; NS-gCnull versus rNS-gCnull or NS, P < 0.01), which supports results shown in Fig. 3A at a 100-fold-higher inoculum. Of interest, in C3D guinea pigs the AUC of NS-gCnull virus (12.7) was lower than that of rescued virus (24.1) (compare the top curves of Fig. 4A and B; P = 0.045), although the AUC was not significantly different from that of wild-type virus (17.4) (top curve of Fig. 4C). Since C3D guinea pigs are not totally deficient in C3, it is possible that gC null virus grows to lower titers than rescued virus because of residual C3 activity. Alternatively, this result raises the possibility that gC mediates additional functions in vivo, such as attachment to cells (19, 23, 46, 49) or infection of the apical surface of polarized cells (20, 42), leading to lower titers of gC-null virus. Since C3 knockout guinea pigs are not available, we chose to perform additional studies in C3 knockout mice.
Virulence of gC-null, rescued, and wild-type viruses in C3 knockout mice.
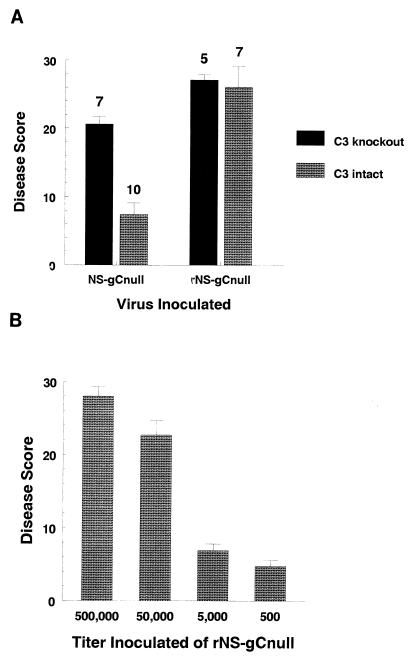
The availability of C57BL/6 transgenic C3 knockout mice enabled us to test the role of gC-C3 interactions in another animal model. The murine flank model (43) was used to evaluate disease at the inoculation and zosteriform spread sites (39). In this model, infection is initiated by scratch inoculation, virus replicates locally, spreads by axonal transport to ganglia, where additional replication occurs, and then returns to skin by axonal transport and produces lesions in a dermatomal (zosteriform) distribution (39, 43). In complement-intact C57BL/6 mice, highly significant differences were found between disease scores of gC-null (7.4) and rescued (26.1) viruses (P < 0.0001) (Fig. 5A), which indicates that gC is a virulence factor and confirms results for guinea pigs. To evaluate the role of C3, disease scores of gC-null virus were compared in C3 knockout and complement-intact mice (Fig. 5A). Scores in C3 knockout mice (20.6) were significantly higher than in complement-intact mice (7.4) (P < 0.0001). In contrast, disease scores of rescued virus were not different in C3 knockout and complement-intact mice (Fig. 5A). These results provide strong evidence that gC protects against the effects of complement. Of note, disease scores with gC-null virus remained lower than rescued virus in C3 knockout mice (Fig. 5A; NS-gCnull disease score of 20.6 compared with rNS-gCnull score of 27.1, P < 0.01). This result suggests that gC may mediate functions in addition to inhibiting C3. Zosteriform disease scores provide further support for another function of gC since rescued virus caused dermatomal disease in C3 knockout mice whereas NS-gCnull virus did not (result not shown).
FIG. 5.
The interaction between gC and C3 is important for HSV-1 virulence in mice. (A) C57BL/6 C3 knockout or C57BL/6 parental strain complement-intact mice were inoculated intradermally with 5 × 105 PFU of NS-gCnull or rNS-gCnull virus. Disease scores were determined at the inoculation site by counting the number of lesions. Disease scores for NS-gCnull were 20.6 in C3 knockout mice and 7.4 in complement-intact mice (P < 0.0001). Disease scores for rNS-gCnull were 27.1 in C3 knockout mice and 26.1 in complement-intact mice (P = nonsignificant). The number of animals studied is indicated above each bar. (B) Dose-response studies of rNS-gCnull in C57BL/6 complement-intact mice inoculated with 5 × 105, 5 × 104, 5 × 103, or 5 × 102 PFU. Disease scores plotted represent the mean ± SEM of six mice per group (five mice in the 5 × 105 PFU group). The disease score with 5 × 103 PFU of rNS-gCnull was 6.9, which is comparable to the score of 7.4 shown in panel A for animals inoculated with 5 × 105 PFU of NS-gCnull virus. Error bars represent ± SEM.
To estimate the magnitude of the gC effect, a dose-response experiment was performed with complement-intact mice. Animals were inoculated with serial 10-fold dilutions of rNS-gCnull virus at doses ranging from 500,000 to 500 PFU (Fig. 5B). A disease score of 6.9 was recorded when animals were inoculated with 5,000 PFU, which is comparable to the disease score of 7.4 noted when animals were inoculated with 500,000 PFU of gC-null virus (Fig. 5A). Thus, 100-fold more gC null virus is required to produce disease comparable to that produced by gC rescued virus in complement-intact mice.
DISCUSSION
This study addresses in vivo activities mediated by HSV-1 gC, with a focus on its role in immune evasion. HSV-1 gC has been shown to mediate several activities in vitro, including inhibition of complement-mediated neutralization of cell-free virus and complement-mediated lysis of infected cells (16, 21). Studies have reported that HSV-1 gC also mediates virus attachment to cell surface heparan sulfate (23, 49) and is required for apical infection of polarized cells (42). However, other reports using different HSV-1 gC-null strains and different cell lines noted that gC is not required for virus attachment or infection of polarized epithelial cells (20). Our previous results demonstrated that NS-gCnull is 50- to 100-fold more susceptible to complement-mediated neutralization than wild-type or rescued virus (16). We now define the attachment phenotype of NS-gCnull virus and report that this mutant is similar to wild-type virus in attachment to HEp-2 and Vero cells. However, NS-gCnull differs slightly from wild-type virus in that it produces 12.6-fold (1.1 log10) fewer plaques on the apical surface of polarized MDCK cells. This deviation is considerably less than the >10,000-fold (4 log10) difference reported for HSV-1(F) gC-null virus on MDCK cells (42) but is comparable to the 2-fold difference noted for gC mutants of HSV-1 strains SC16 and HFEM on Caco-2 cells (20). Thus, in vitro studies indicate that the NS-gCnull virus is normal for cell attachment and slightly defective in apical infection of polarized MDCK cells. However, the most striking abnormality is the marked susceptibility to complement-mediated neutralization.
Results shown in Fig. 3 and 5 for complement-intact guinea pigs and mice clearly demonstrate that gC is a virulence factor. We hypothesized that if gC-complement interactions were important in virulence, gC null virus should be more virulent in C3D than in complement-intact animals. In addition, if the only function of gC is to inhibit the activities of C3, gC-null virus should be as virulent as wild-type and rescued viruses in C3D animals. We demonstrated that in C3D guinea pigs, gC-null virus grew to significantly higher titers than in complement-intact animals, while titers of wild-type or rescued virus did not change significantly. As further support for the importance of gC-C3 interactions, we showed that in C3 knockout mice disease scores of gC-null virus were significantly higher than in complement-intact mice, while scores of rescued virus remained unchanged. The results in C3D guinea pigs and C3 knockout mice provide strong support for the importance of gC as an immune evasion molecule in vivo.
The experiments also suggest that gC may have functions in addition to interaction with C3. In C3D guinea pigs, infection with gC-null virus did not result in vaginal titers comparable to those for rescued virus. In C3D guinea pigs, C3 levels are reduced but not absent; therefore, residual C3 could account for the lower gC-null virus titers. However, residual C3 cannot explain the observation that C3 knockout mice showed significant differences between gC-null and rescued viruses. Therefore, the results indicate that in addition to binding C3 and C3 fragments iC3b and C3c (14, 33, 47), gC likely mediates other activities in vivo. This may reflect a role for gC in apical infection of polarized epithelial cells or perhaps in modifying other steps in the complement cascade, since our previous results indicate that gC blocks C5 and properdin involvement in complement activation (17, 28, 33). Another possible explanation for reduced virulence of NS-gCnull in C3D animals is that silent mutations had been introduced outside the gC locus. However, this seems unlikely since a rescue strain of gC-null virus is as virulent as the wild-type strain. Studies are planned to address whether attachment to polarized cells contributes to virulence by constructing gC mutant viruses that disrupt complement binding domains (29) but leave adjacent regions of the molecule intact, while the possible role of gC-C5 interaction in virulence can be evaluated in C5D mice (37).
The dose-response studies in guinea pigs and mice enable us to assess the magnitude of the gC effect on virulence. When NS-gCnull virus was inoculated into guinea pigs at 5 × 105 PFU, the AUC for vaginal titers was 16.9, which is lower than the AUC when NS was inoculated at 5 × 104 PFU (22.9) (P = 0.01) and comparable to the AUC at 5 × 103 PFU (14.3). Therefore, in the absence of gC, approximately 100-fold more virus is required to produce comparable vaginal titers as when gC is present. A similar result was obtained for mice, since 5 × 105 PFU of gC-null virus produced disease scores comparable to those produced by 5 × 103 PFU of rescued virus. A somewhat lower estimate of the magnitude of the gC effect comes from comparisons of vaginitis scores in guinea pigs. NS-gCnull virus at 5 × 105 PFU resulted in a vaginitis score of 3.3, which is similar to the score of 3.5 when NS was inoculated at a 10-fold (1 log10)-lower dose. We conclude that gC has a 10- to 100-fold (1 to 2 log10) effect on virulence.
How effective is gC on wild-type virus in preventing complement-mediated virus inactivation? Studies in C3 knockout mice show no differences between wild-type virus in complement-intact or C3D animals. In C3D guinea pigs, wild-type titers are only slightly higher than in complement-intact animals. These results are consistent with in vitro results that demonstrate little effect of complement on neutralization of wild-type virus or on lysis of infected cells (16, 21). We conclude that gC provides wild-type virus near-total protection against complement attack.
Vaginal titers of gC null virus were significantly higher in C3D guinea pigs than in complement-intact animals by day 1 postinfection (P < 0.005), which is before antibodies develop. This finding suggests that gC protects against antibody-independent complement activation, which is consistent with in vitro results (16, 21, 24). Whether this protection is most important for cell-free virus or for virus-infected cells remains to be determined, as does defining which of the complement pathways modifies HSV infection in vivo.
The information from this study can be used to develop strategies to modify gC-mediated immune evasion. gC is present on the virus and expressed at the infected cell surface. This makes gC an attractive target for vaccines with the goal of inducing antibodies to block gC functions, thereby rendering the virus or infected cell more susceptible to complement. One strategy would be to add gC and perhaps other immune evasion molecules, such as gE and gI (9, 13), to a gD vaccine and determine if the multivalent vaccine improves vaccine efficacy by blocking immune evasion.
ACKNOWLEDGMENTS
This work was supported by grants HL 28220 and AI 25011 from the National Institutes of Health.
REFERENCES
- 1.Baucke R B, Spear P G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979;32:779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Chan M, Solomon K R, St. John N F, Lin H, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger R, Gordon J, Stevenson G, Ramadori G, Zanker B, Hadding U, Bitter-Suermann D. An inherited deficiency of the third component of complement, C3, in guinea pigs. Eur J Immunol. 1986;16:7–11. doi: 10.1002/eji.1830160103. [DOI] [PubMed] [Google Scholar]
- 4.Centifanto-Fitzgerald Y M, Yamaguchi T, Kaufman H E, Tognon M, Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982;155:475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrisp C E, Sunstrum J C, Averill D R, Jr, Levine M, Glorioso J C. Characterization of encephalitis in adult mice induced by intracerebral inoculation of herpes simplex virus type 1 (KOS) and comparison with mutants showing decreased virulence. Lab Investig. 1989;60:822–830. [PubMed] [Google Scholar]
- 6.Cohen G H, Katze M, Hydrean-Stern C, Eisenberg R J. Type common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope protein. J Virol. 1978;27:172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dix R D, McKendall R R, Baringer J R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983;40:103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin G, Frank I, Friedman H M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990;64:2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubin G, Socolof E, Frank I, Friedman H M. The herpes simplex virus Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991;65:7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg R J, de Leon M P, Friedman H M, Fries L F, Frank M M, Hastings J C, Cohen G H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987;3:423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- 11.Fingeroth J D, Weiss J J, Tedder T F, Strominger J L, Biro P A, Fearon D T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci USA. 1984;81:4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fodor W L, Rollins S A, Bianco-Caron S, Rother R P, Guilmette E R, Burton W V, Albrecht J-C, Fleckenstein B, Squinto S P. The complement control protein homolog of herpesvirus saimiri regulates serum complement by inhibiting C3 convertase activity. J Virol. 1995;69:3889–3892. doi: 10.1128/jvi.69.6.3889-3892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank I, Friedman H M. A novel function of the herpes simplex virus type 1 Fc receptor, participation in antibody bipolar bridging of antiviral immunoglobulin G. J Virol. 1989;63:4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman H M, Cohen G H, Eisenberg R J, Seidel C A, Cines D B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 15.Friedman H M, Glorioso J C, Cohen G H, Hastings J C, Harris S L, Eisenberg R J. Binding of complement component C3b to glycoprotein gC of herpes simplex virus type 1: mapping of gC-binding sites and demonstration of conserved C3b binding in low-passage clinical isolates. J Virol. 1986;60:470–475. doi: 10.1128/jvi.60.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman H M, Wang L, Fishman N O, Lambris J D, Eisenberg R J, Cohen G H, Lubinski J. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70:4253–4260. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries L F, Friedman H M, Cohen G H, Eisenberg R J, Hammer C H, Frank M M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986;137:1636–1641. [PubMed] [Google Scholar]
- 18.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 19.Gerber S I, Belval B J, Herold B C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology. 1995;214:29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths A, Renfrey S, Minson T. Glycoprotein C-deficient mutants of two strains of herpes simplex virus type 1 exhibit unaltered adsorption characteristics on polarized or non-polarized cells. J Gen Virol. 1998;79:807–812. doi: 10.1099/0022-1317-79-4-807. [DOI] [PubMed] [Google Scholar]
- 21.Harris S L, Frank I, Yee A, Cohen G H, Eisenberg R J, Friedman H M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990;162:331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Hendricks R L, Epstein R J, Tumpey T. The effects of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Investig Ophthalmol Visual Sci. 1989;30:105–115. [PubMed] [Google Scholar]
- 23.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hidaka Y, Sakai Y, Yasushi T, Mori R. Glycoprotein gC of herpes simplex virus type 1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of virus-infected cells. J Gen Virol. 1991;72:915–921. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- 25.Hidaka Y, Sakuma S, Kumano Y, Minagawa H, Mori R. Characterization of glycoprotein C-negative mutants of herpes simplex virus type 1 isolated from a patient with keratitis. Arch Virol. 1990;113:195–207. doi: 10.1007/BF01316673. [DOI] [PubMed] [Google Scholar]
- 26.Hill A, Jugovic P, York I, Russ G, Bennick J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 27.Huemer H P, Larcher C, van Drunen Littel-van den Hurk S, Babiuk L A. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch Virol. 1993;130:353–364. doi: 10.1007/BF01309666. [DOI] [PubMed] [Google Scholar]
- 28.Hung S-L, Peng C, Kostavasili I, Friedman H M, Lambris J D, Eisenberg R J, Cohen G H. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology. 1994;203:299–312. doi: 10.1006/viro.1994.1488. [DOI] [PubMed] [Google Scholar]
- 29.Hung S-L, Srinivasan S, Friedman H M, Eisenberg R J, Cohen G H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992;66:4013–4027. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs S N, Kotwal G J, Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci USA. 1992;89:628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata K, Seya T, Yanagi Y, Pesando J M, Johnson P, Okabe M, Ueda S, Ariga H, Nagasawa S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J Biol Chem. 1995;270:15148–15152. doi: 10.1074/jbc.270.25.15148. [DOI] [PubMed] [Google Scholar]
- 32.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostavasili I, Sahu A, Friedman H M, Eisenberg R J, Cohen G H, Lambris J D. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J Immunol. 1997;158:1763–1771. [PubMed] [Google Scholar]
- 34.Lapin L. Statistics, meaning and method. New York, N.Y: Harcourt Brace Jovanovich, Inc.; 1975. [Google Scholar]
- 35.McFadden G. Viroceptors, virokines and related immune modulators encoded by DNA viruses. R. G. Austin, Tex: Landes Co.; 1995. [Google Scholar]
- 36.McNearney T A, Odell C, Holers V M, Spear P G, Atkinson J P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987;166:1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merriam L T, Webster C, Joehl R J. Complement component C5 deficiency reduces edema formation in murine ligation-induced acute pancreatitis. J Surg Res. 1997;67:40–45. doi: 10.1006/jsre.1996.4916. [DOI] [PubMed] [Google Scholar]
- 38.Mold C, Bradt B M, Nemerow G R, Cooper N R. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med. 1988;168:949–969. doi: 10.1084/jem.168.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naganshumugam T, Lubinski J, Wang L, Goldstein L T, Weeks B S, Sundaresan P, Kang E H, Dubin G, Friedman H M. In vivo immune evasion mediated by herpes simplex virus type 1 immunoglobulin G Fc receptor. J Virol. 1998;72:5351–5359. doi: 10.1128/jvi.72.7.5351-5359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rother R P, Rollins S A, Fodor W L, Albrecht J-C, Setter E, Fleckenstein B, Squinto S P. Inhibition of complement-mediated cytolysis by the terminal complement inhibitor of herpesvirus saimiri. J Virol. 1994;68:730–737. doi: 10.1128/jvi.68.2.730-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai Y, Minigawa H, Ishibashi T, Inomata H, Mori R. Stromal keratitis induced by a unique clinical isolate of herpes simplex virus type 1. Ophthalmologica. 1994;208:157–160. doi: 10.1159/000310474. [DOI] [PubMed] [Google Scholar]
- 42.Sears A E, McGuire B S, Roizman B. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc Natl Acad Sci USA. 1991;88:5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons A, Nash A A. Zosteriform spread of herpes simplex as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanberry L R, Kern E R, Richards J T, Abbot T M, Overall J C. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of the recurrent disease. J Infect Dis. 1982;146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 45.Sunstrum J C, Chrisp C E, Levine M, Glorioso J C. Pathogenicity of glycoprotein C negative mutants of herpes simplex virus type 1 for the mouse central nervous system. Virus Res. 1988;11:17–32. doi: 10.1016/0168-1702(88)90064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tal-Singer R, Peng C, de Leon M P, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tal-Singer R, Seidel-Dugan C, Fries L, Huemer H P, Eisenberg R J, Cohen G H, Friedman H M. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J Infect Dis. 1991;164:750–753. doi: 10.1093/infdis/164.4.750. [DOI] [PubMed] [Google Scholar]
- 48.Wetzel, R. Unpublished data.
- 49.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]