Abstract
Background and study aims The long-term course of untreated asymptomatic esophageal eosinophilia (aEE) and minimally symptomatic eosinophilic esophagitis (mEoE) are not well understood. This study aimed to clarify this course.
Patients and methods A total of 36 patients with EE who were endoscopically followed up for more than 5 years, and who underwent more than one endoscopy evaluation after the first diagnosis, were investigated. These patients were divided into two groups according to the presence or absence of the continuous treatment: no treatment group (NT group, n=22) and proton pump inhibitor/potassium competitive acid blocker group (Tx group, n=14). Symptoms and endoscopic and histological findings were retrospectively reviewed according to endoscopic phenotypes. Endoscopic assessment was performed using the EoE endoscopic reference score (EREFS).
Results The median follow-up period was 84.5 months in the Tx group and 92 months in the NT group. During the follow-up period, about half of the patients in the Tx-diffuse group persisted EREFS >3, while the remaining half had EREFS ≤2. The total EREFS in the NT-diffuse group remained almost unchanged (median: 2–4) without apparent exacerbation. In contrast, EREFS in the NT-localized group exhibited an unchanged or gradually decreasing trend, with statistical significance from the first diagnosis to 72 to 83 months after.
Conclusions Untreated aEE and mEoE are not likely to worsen even without treatment at least for a median follow-up of 7 years. Instead, the localized type may spontaneously improve, implying a different pathogenesis in the presence of the diffuse type. Further studies should clarify the long-term prognosis.
Keywords: Endoscopy Upper GI Tract, Eosinophilic esophagitis, GI Pathology, Epidemiology
Introduction
Eosinophilic esophagitis (EoE) is a Th2 cell immune-mediated inflammatory disease characterized by dysphagia, food impaction, and intense esophageal eosinophilia (EE) 1 . Accumulating evidence suggests that EoE is a progressive disease that may cause fibro-stenotic changes, such as fixed rings and strictures, with an increased risk of food impaction over a long period of time 2 . Emergency Department visits due to EoE-associated food impaction have been continuously increasing in the United States over the past 15 years, imposing a burden on healthcare resources 3 . In contrast, dysphagia/food impaction and severe rings/strictures are much less common in Japan than in Western countries 4 . A recent comprehensive review of 886 Japanese adults with EE revealed that dysphagia/food impaction, heartburn, chest pain, and no symptoms were present in 52.9%, 25.3%, 6.7%, and 18.8% of participants, respectively 5 .
Asymptomatic EE (aEE) or minimally symptomatic EoE (mEoE) is occasionally diagnosed on endoscopic examinations during health check-up programs or gastric cancer screening programs in Japan, with a relatively high frequency ranging from 0.2 to 0.4% 6 7 8 . A population-based study from Sweden reported a prevalence of 0.4% for histologically diagnosed EoE in patients without troublesome symptoms 9 . Intriguingly, a population-based study in China detected EE in 0.4% of the studied patients, most of whom were asymptomatic 10 . Thus, while endoscopists occasionally encounter aEE or mEoE in daily endoscopic practice, EE is not diagnosed with EoE if there are no symptoms according to the current clinical guidelines 11 .
Endoscopic and histological findings do not differ substantially between aEE and symptomatic EoE 12 13 14 . Esophageal wall thickness and intraepithelial mast cells have been reported to be associated with the perception of esophageal symptoms 15 16 17 . In clinical practice, aEE and mEoE are likely to be followed up without active treatment; nonetheless, their natural course is not well-known 7 14 18 19 . Given the pathophysiology of EoE, which can potentially progress to esophageal stricture over time 20 21 22 , understanding the natural course of untreated aEE or mEoE is important for appropriate management. Therefore, the present study aimed to investigate the long-term clinical course of untreated aEE and mEoE and compare it with that of continuously treated EoE.
Patients and methods
Patients
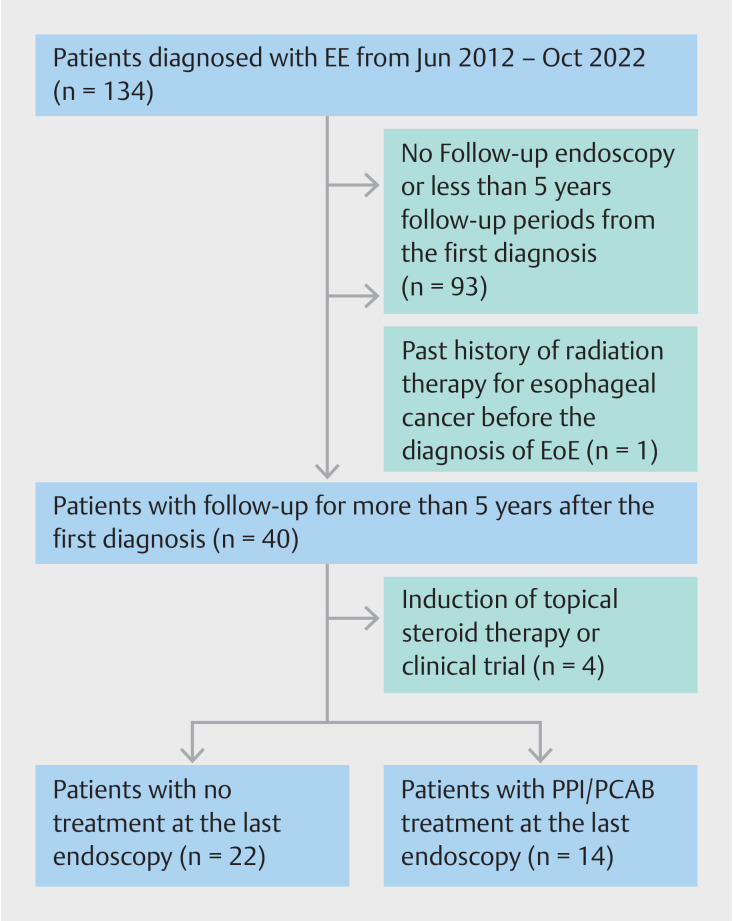
Fig. 1 shows a flow diagram illustrating the patients included in this retrospective study. All patients diagnosed with EE at the Yamagata University Hospital, JR Sendai Hospital, and Shinoda General Hospital from June 2012 to October 2022 were identified using medical charts and endoscopic databases. A total of 134 patients were diagnosed with EE at these three hospitals; however, the following were excluded: 1) 93 patients who did not undergo follow-up endoscopy or were followed up for <5 years from the first diagnosis; 2) one patient who had a history of radiation therapy for esophageal cancer prior to EE diagnosis; and 3) four patients who were treated with topical steroid therapy or enrolled in a clinical trial during the follow-up period. Ultimately, 36 patients were included in this study and divided into two groups according to treatment at the last follow-up endoscopy: the no treatment group (NT group, n=22) and proton pump inhibitor (PPI)/potassium competitive acid blocker (PCAB) group (Tx group, n=14).
Fig. 1.
Flow diagram showing patient enrollment in this retrospective study.
Definition of EoE, endoscopic assessment, and follow-up
EE was histologically defined as a peak of ≥15 eosinophils (eos) per high-power field (HPF), irrespective of esophageal symptoms. Possible secondary EE, including non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, drug-induced esophagitis, post-radiotherapy for esophageal cancer, or post-sublingual immunotherapy, was excluded. aEE was defined as absence of symptoms in patients based on medical chart review, whereas mEoE was defined as presence of minimal symptoms not requiring active medication in patients. Date of first diagnosis was defined as that of index endoscopy leading to diagnosis of EE. Two endoscopists certified by the Japanese Gastrointestinal Endoscopic Association (Y.A. and Y.S.) reviewed all endoscopic images from first diagnosis to last follow-up.
The EoE endoscopic reference score (EREFS) was utilized to evaluate edema (0–1), rings (0–3), exudates (0–2), furrows (0–1), and strictures (0–1), with the total score being mainly used for analysis 23 . The sum of scores for edema, exudates, and furrows was defined as the inflammatory score, whereas the sum of scores for rings and strictures was defined as the fibrostenotic score. EE was endoscopically subclassified into the diffuse type, localized type of the lower esophagus, and patchy type based on a previous report 18 ; the latter two types were regarded as the localized type. Intraepithelial eosinophil count (IEC) was recorded based on pathological reports at first diagnosis and at each follow-up endoscopy with esophageal biopsy. Presence or absence of endoscopic chronic gastritis and a history of endoscopic/surgical therapies for gastric lesions were also tabulated. The follow-up period was divided into 12 months from first diagnosis as 0 to 11 months, 12 to 23 months, 24 to 35 months, and so on. Data about all follow-up endoscopies were extracted until the last follow-up endoscopy procedure. If multiple endoscopies were performed during the same period, the earlier data were used for analysis.
This study was conducted in accordance with the principles embodied in the Declaration of Helsinki and was approved by the ethics committees of Yamagata University Hospital (approval no. 252), JR Sendai Hospital (approval no. 801), and Shinoda General Hospital (approval date May 27, 2021). Written informed consent was obtained from all patients prior to their participation in the study.
Treatment
EoE treatment was reviewed using medical charts from first diagnosis to last follow-up endoscopy. The attending physician arbitrarily decided whether to treat the patients on the basis of the severity of symptoms and/or endoscopic activity. In this study, patients who received second-line therapy, including swallowed topical corticosteroids, owing to non-response to the initial PPI/PCAB treatment were excluded. Patients were advised to undergo follow-up endoscopy at least once a year, irrespective of developing or worsening symptoms.
Statistical analyses
Data are expressed as medians with interquartile ranges (IQR) or as numbers with percentages. Continuous and categorical variables were analyzed using Wilcoxon’s rank-sum/signed-rank test and Fisher’s exact probability test, respectively. All statistical analyses were performed using JMP software version 14.1.0 (SAS Institute, Cary, North Carolina, United States), with statistical significance set at P <0.05.
Results
Clinical characteristics of the studied patients
Table 1 summarizes clinical characteristics of patients in the Tx group (n=14) and NT group (n=22) at first diagnosis. Median age at first diagnosis was not significantly different between the two groups (Tx group, 52.5 years old; NT group, 45.5 years old). Median follow-up period and number of follow-up endoscopies did not differ significantly between the two groups, even when divided according to endoscopic phenotypes. In the NT group, 4 (diffuse type, n=2; localized type, n=2) out of 22 patients received PPI treatment shortly after first diagnosis to evaluate any latent symptoms, although they showed almost no or minimal subjective symptoms. Consequently, all of them achieved histological remission (defined as ≤15 eos/HPF) for approximately 2 months; however, PPI treatment was discontinued until the last follow-up endoscopy because their symptoms did not change significantly before or after treatment. These patients were expediently assigned to the NT group. The remaining 18 patients in the NT group were consistently untreated since their first diagnosis. In the Tx group, one of 14 patients was on PPI due to new-onset dysphagia and heartburn from 36 months after first diagnosis to last follow-up endoscopy (60 months); this patient was expediently assigned to the Tx group. The remaining 13 patients in the Tx group, including one who was first diagnosed with EoE during PPI use, were continuously treated with PPI or PCAB. Most patients in both groups were diagnosed through medical check-ups.
Table 1 Demographic and clinical characteristics of the studied patients at time of first diagnosis.
| Tx group | NT group | P value | |
| *Close examination for gastrointestinal symptoms and other was unified and compared with medical check-up. †Excluding after ESD and after DG, chronic gastritis and no abnormal findings was compared. Tx, PPI/PCAB treatment; NT, no treatment; IQR, interquartile range; EGD, esophago-gastro-duodenoscopy; PPI, proton pump inhibitor; PCAB, potassium competitive acid blocker; EoE, eosinophilic esophagitis; EREFS, EoE endoscopic reference score; IEC, Intraepithelial eosinophil count; ESD, endoscopic submucosal dissection; DG, distal gastrectomy. | |||
| N | 14 | 22 | – |
| Male/female | 12/2 | 19/3 | 1 |
| Age at the first diagnosis, years, median (IQR) | 52.5 (46.3–63.8) | 45.5 (40.6–55) | 0.26 |
| Follow-up period, median, months (IQR) | 84.5 (65.3–114.3) | 92 (69.8–100) | 0.99 |
| Diffuse type/localized type | 92.5 (60–114.3)/80.5 (72–116.8) | 94 (84–98)/90 (69–100) | |
| Number of follow-up EGDs, median (IQR) | 7 (6–9.3) | 7 (5–9) | 0.67 |
| Diffuse type/localized type | 7 (5.5–9.3) / 7.5 (6.3–10.3) | 8 (7–9) / 6 (5–9) | |
| Initial PPI treatment (%) | – | 4 (18.2) | |
| Opportunity for EE diagnosis | |||
|
11 (78.6) | 19 (86.4) | 0.6582 * |
|
2 (14.3) | 0 | |
|
1 (7.1) | 3 (13.6) | |
| Allergic condition (%) | 6 (42.9) | 9 (40.9) | 1 |
|
5 (35.7) | 8 (36.4) | 1 |
|
1 (7.1) | 1 (4.6) | 1 |
|
0 | 1 (4.6) | 1 |
|
0 | 0 | – |
| Esophageal symptom (%) | 7 (50) | 3 (13.6) | 0.0262 |
| Dysphagia | 4 (28.6) | 1 (4.6) | 0.0637 |
| Food impaction | 1 (7.1) | 1 (4.6) | 1 |
| Heartburn | 5 (35.7) | 2 (9.1) | 0.0842 |
| EREFS score at first diagnosis, median (IQR) | |||
|
3 (2–5) | 3 (3–4) | 0.8243 |
|
3 (2–3) | 3 (2.75–3) | 0.5101 |
|
1 (0–1) | 0 (0–1) | 0.2503 |
| Endoscopic phenotype (%) | |||
|
10 (71.4) | 7 (31.8) | 0.0388 |
|
4 (3/1) (28.6) | 15 (13/2) (68.2) | |
| IEC at first diagnosis (/HFP, median (IQR)) | 56 (30–79.3) | 52.5 (27–70.5) | 0.745 |
| Background gastric mucosa (%) | |||
|
3 (21.4) | 10 (45.5) | 0.2673 † |
|
9 (64.3) | 10 (45.5) | |
|
0 (0)/2 (14.3) | 2 (9)/(0) | – |
| Successful H. pylori eradication after EoE diagnosis (%) | 1 (8.3) | 6 (30) | 0.2117 |
Esophageal symptoms were significantly less common in the NT group than in the Tx group (13.6% vs. 50%, P =0.0262). Total EREFS and IEC at the first diagnosis did not differ between the two groups; however, endoscopic phenotypes significantly varied between the two groups ( P =0.0388). In particular, the Tx group was dominated by the diffuse type (diffuse type, 71.4%; localized type, 28.6%), whereas the NT group was dominated by the localized type (diffuse type, 31.8%; localized type, 68.2%). In addition, chronic gastritis at first diagnosis was more common in the NT group, leading to an increase in subsequent Helicobacter pylori eradication.
Chronological change in EREFS
At first diagnosis, 60 to 71 months, and last follow-up
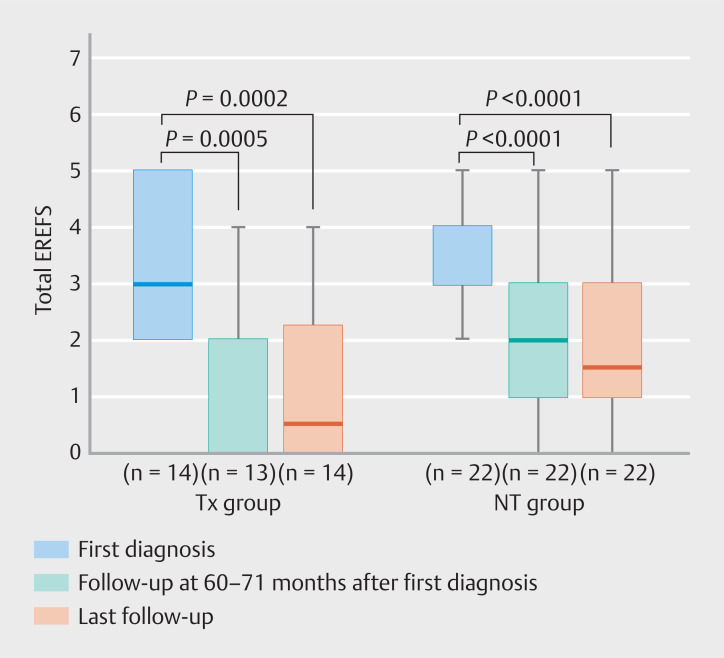
All patients except for one in the Tx group underwent follow-up endoscopy at 60 to 71 months; hence, we first compared the endoscopic activity at the first diagnosis, at 60 to 71 months, and at last follow-up. Total EREFS (median [IQR]) was significantly lower at 60 to 71 months and last follow-up than at first diagnosis in the Tx group (first diagnosis: 3 [2–5], 60 to 71 months: 0 [0–4], last follow-up: 1 [0–2.25]; first diagnosis vs. 60 to 71 months: P =0.0005; first diagnosis vs. last follow-up: P =0.0002) and NT group (first diagnosis: 3 [3–4], 60 to 71 months: 2 [1–3], last follow-up: 1.5 [0.75–3]; first diagnosis vs. 60 to 71 months: P <0.0001; first diagnosis vs. last follow-up: P <0.0001) ( Fig. 2 , Supplementary Table 1 ).
Fig. 2.
Total EREFS at first diagnosis, 60 to 71 months, and last follow-up is shown separately for the Tx and NT groups. The timing of the last follow-up varied from patient to patient. EREFS, EoE endoscopic reference score; Tx, PPI/PCAB treatment; NT, no treatment.
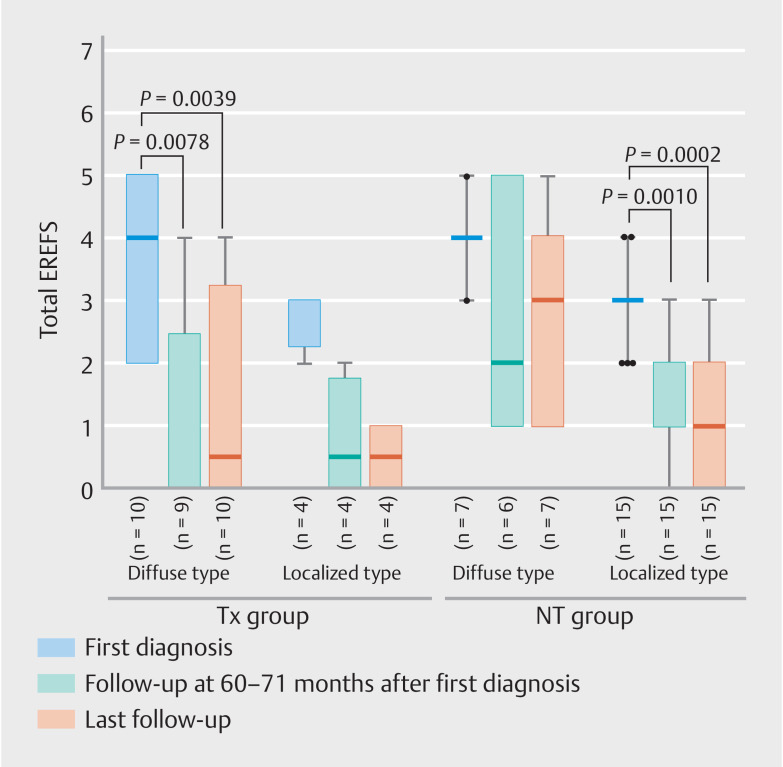
When the data were examined further, separately according to endoscopic phenotypes, decrease in total EREFS (median [IQR]) was statistically significant in the Tx-diffuse group (first diagnosis: 4 [2–5], 60 to 71 months: 0 [0–2.5], last follow-up: 1 [0–3.25]; first diagnosis vs. 60 to 71 months: P = 0.0078; first diagnosis vs. last follow-up: P = 0.0039) and NT-localized group (first diagnosis: 3 [3–3], 60 to 71 months: 1 [1–2], last follow-up: 1 [0–3]; first diagnosis vs. 60 to 71 months: P =0.0010; first diagnosis vs. last follow-up: P =0.0002) ( Fig. 3 , Supplementary Table 1 ). In the Tx-localized group, total EREFS at 60 to 71 months and last follow-up considerably decreased, compared with that at first diagnosis; nevertheless, this reduction did not reach statistical significance, probably because of the small number of patients. In the NT-diffuse group, total EREFS showed no significant changes in the three phases.
Fig. 3.
Total EREFS at first diagnosis, 60 to 71 months, and last follow-up is shown separately for the Tx and NT groups and for the endoscopic phenotypes. The timing of the last follow-up varies from patient to patient. Tx, PPI/PCAB treatment; NT, no treatment.
Annual chronological change in EREFS
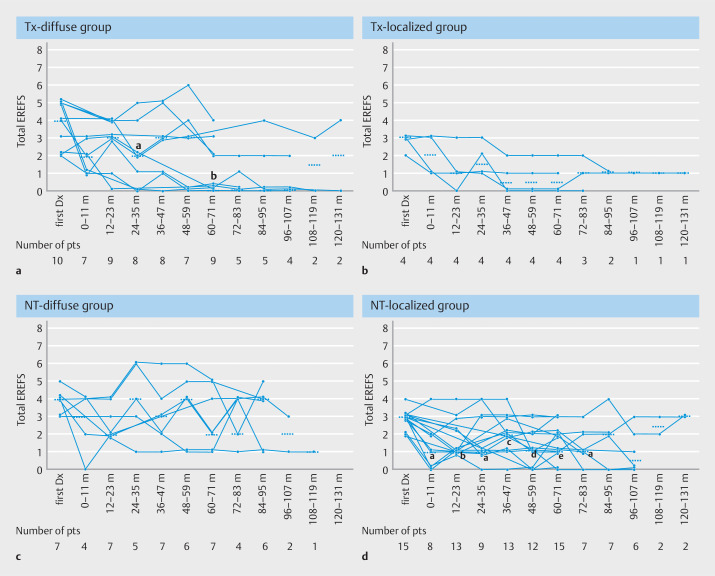
Fig. 4a , Fig. 4 b , Fig. 4 c , and Fig. 4 d show the annual chronological change in total EREFS from first diagnosis until last follow-up separately for the Tx and NT groups and according to endoscopic phenotypes. In the Tx-diffuse group, total EREFS was significantly lower at 24 to 35 months ( P =0.0313) and 60 to 71 months ( P =0.0078) than at the first diagnosis ( Fig. 4 a ). However, a total EREFS of ≥3 persisted in some patients with no obvious increase. In the Tx-localized group, which consisted of only four patients, total EREFS shortly decreased to ≤2 and was sustained in most patients ( Fig. 4 b ). In the NT-diffuse group, total EREFS remained unchanged, with a median of 2 to 4 during the follow-up period without an apparent increase ( Fig. 4 c ). Total EREFS in the NT-localized group showed an unchanged or gradually decreasing trend, in which statistical significance was found between the first diagnosis and all follow-up periods except for after 84 to 95 months ( Fig. 4 d ). Analysis of the number of patients exhibiting endoscopic remission (total EREFS ≤2) and non-remission (total EREFS >3) at each follow-up period revealed that approximately half or more of the patients in the NT-diffuse group continued to have an endoscopic non-remission, whereas the majority of the NT-localized group achieved endoscopic remission from an earlier phase of the follow-up period ( Supplementary Table 2 ) 24 .
Fig. 4.
Line graph showing the annual chronological change in the total EREFS from the first diagnosis to the last follow-up. The number of patients who underwent endoscopy at each follow-up period is shown at the bottom of the figure. The horizontal short dotted line represents the median value of the total EREFS at each follow-up period. EREFS, EoE endoscopic reference score; m, month; pts, patients; Dx, diagnosis; PPI, proton pump inhibitor; PCAB, potassium competitive acid blocker; aEE, asymptomatic esophageal eosinophilia; mEoE, minimally symptomatic EoE. a Tx-diffuse group, which includes patients with diffuse-type EoE who continuously received PPI/PCAB. a, P = 0.0313; b, P = 0.0078 (vs. at the first diagnosis). b Tx-localized group, which consists of patients with localized-type EoE who continuously received PPI/PCAB. c NT-diffuse group, which consists of patients with diffuse-type aEE or mEoE who were continuously untreated. d NT-localized group, which consisted of patients with localized-type aEE or mEoE who are continuously untreated. a, P =0.0313; b, P =0.0034; c, P =0.0137; d, P =0.0039; e, P =0.0010 (vs. at the first diagnosis).
Chronological change in IEC
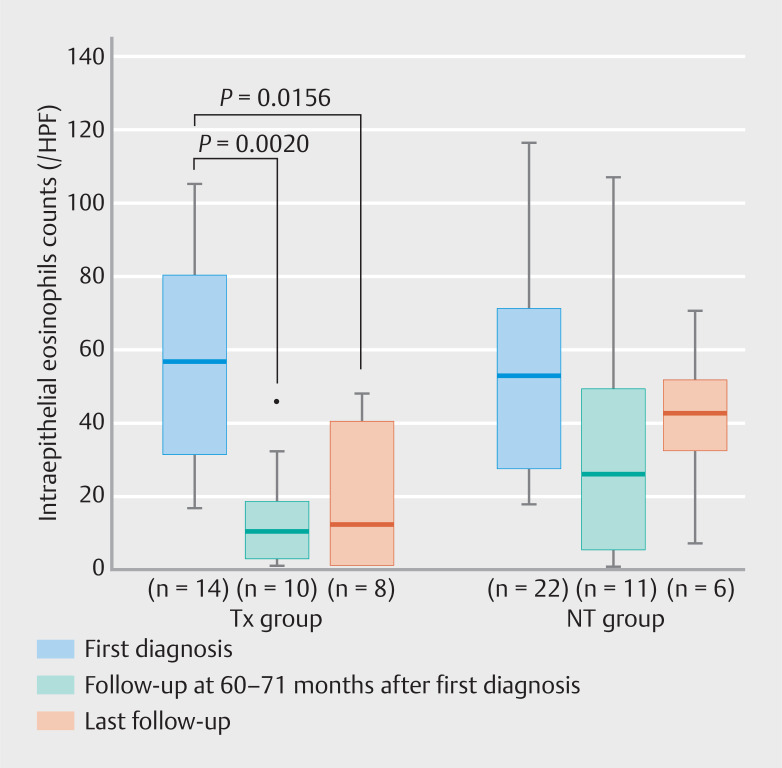
Esophageal biopsy was not performed during any of the follow-up endoscopies and was less frequently performed at the last follow-up. Analysis of the IEC at 60 to 71 months and last follow-up indicated that it was significantly lower in the Tx group at 60 to 71 months and last follow-up than at the first diagnosis (first diagnosis: 56 [30–79.3] eos/HPF, 60 to 71 months: 10 [1.5–17.8] eos/HPF, last follow-up: 11.5 [0–39.5] eos/HPF). In contrast, the IEC in the NT group did not differ among these three phases (first diagnosis: 52.5 [27–70.5] eos/HPF, 60 to 71 months: 25 [4–48] eos/HPF, last follow-up: 42 [31.5–51.3] eos/HPF) ( Fig. 5 ). This trend was similar when the data were examined separately according to endoscopic phenotypes ( Supplementary Fig. 1 ).
Fig. 5.
The IEC at first diagnosis, 60 to 71 months, and last follow-up separately shown by the Tx group and the NT group. Timing of the last follow-up varied from patient to patient. Tx, PPI/PCAB treatment; NT, no treatment; IEC, intraepithelial eosinophil count.
Discussion
The present study investigated the long-term (>5 years) course of untreated aEE and mEoE and compared it with that of EoE continuously treated with PPI/PCAB. We found that untreated EE did not exhibit remarkable endoscopic exacerbations, even without treatment, during a median follow-up period of 80–90 months (7–8 years) in our limited study population, most of whom were diagnosed through medical check-ups. The patients investigated in this study were approximately 10 to 15 years older than the patients commonly diagnosed with EoE in Western countries, who have a peak age of 30 to 40 years 2 . According to a recent review by Fujiwara et al, the mean age of patients with EoE in Japan was 49.8 years, most of whom (61.1%) belonged to the middle age group (40 to 69 years old). This difference is presumably due to the larger number of aEE or mEoE incidentally diagnosed through screening endoscopy for medical check-ups in Japan, including gastric cancer screening, which is generally undertaken at 40 to 50 years of age.
Because the extent of the inflamed esophageal mucosa has been recognized to be important in assessing disease activity and treatment response in EoE 25 26 , we conducted our analysis separately according to two endoscopic phenotypes – namely, the diffuse type and localized type. In the diffuse type with NT, endoscopic abnormalities persisted but were not exacerbated in most patients who did not develop symptoms that required active treatment. In contrast, in the localized type with NT, endoscopic activity remained unchanged or rather improved significantly, with a predominance of patients showing endoscopic remission (defined as total EREFS ≤2) in an earlier phase during follow-up 24 . We previously reported that endoscopic abnormalities remained unchanged in the diffuse type but were significantly improved in the localized type of aEE with NT for >3 years (median: 50 to 60 months) 18 . Findings from the present study reinforced our previous results by extending the follow-up period, increasing the number of patients, and uniformly assessing the endoscopic activity using the EREFS.
EoE is a chronic progressive disease that may cause esophageal strictures, the risk of which increases along with the length of diagnostic delay (usually with no active treatment) from symptom onset to definitive diagnosis 20 21 22 . Subjective symptoms can be masked by altering eating behavior, such as eating more slowly, chewing more, or eating while drinking water to avoid dysphagia or food impaction, which leads to diagnostic delay 27 28 . In contrast, Straumann et al. prospectively followed 30 adult patients with EoE who did not receive treatment, except for dilatation, for a mean of 7.2 years 29 . Dysphagia was increased in intensity in 23.3%, remained persistent in 36.7%, decreased in 36.7%, and completely disappeared in 3.3%. Endoscopic abnormalities persisted in most patients but did not differ between baseline and last follow-up, with histological improvement in infiltrated eosinophils or basal cell hyperplasia. Some recent reports described that most patients with EoE did not exhibit symptomatic and/or endoscopic exacerbations or have an improvement in their natural course for up to 10 years after diagnosis 7 30 31 . A recent nationwide survey from Japan reported that 66% of adult patients with definite EoE had the continuous type, while 14% had the intermittent type, 14% the single-flare form, and 5% the unclassifiable type 32 . Thus, EoE might be a composite entity encompassing different phenotypes ranging from progressive to regressive; nonetheless, little is known about the long-term course of EoE.
Several reports from Japan described the natural course of aEE or mEoE 7 14 18 19 . Ishibashi et al. showed that aEE remained endoscopically and symptomatically unchanged in 40% of patients, endoscopically worsened but was symptomatically unchanged in 40%, and 20% had newly developed symptoms (progression to EoE) with endoscopic exacerbation during a mean follow-up period of 40 months 19 . However, endoscopic exacerbation was mild because edema was subsequently complicated by linear furrows in most cases, and EREFS was not used in Ishibashi et al’s study to assess endoscopic findings. Three other studies also reported that endoscopic findings assessed with EREFS were unchanged or modestly exacerbated, with an increase of approximately 1 point in the total score during a mean follow-up period of about 2 to 6 years, with development of symptoms in a very small number of patients 7 14 18 . All of these reports, including the present study, involved a limited number of patients and had short follow-up periods and retrospective designs, and therefore, no definitive conclusions could be drawn. Therefore, there is no doubt that untreated aEE or mEoE should preferably be endoscopically monitored while considering the possibility of disease progression because of the difficulty in predicting endoscopic severity from subjective symptoms 33 . Our results also suggest that endoscopic surveillance for untreated EoE at least once every 5 years or less, especially for the localized type, may be acceptable for monitoring disease activity if symptoms do not worsen.
In this study, we also presented the long-term course of patients continuously treated with PPI/PCAB as a counterpart for untreated aEE and mEoE. Depending on the attending physician, treatment with PPI/PCAB was continued if symptomatic relief was achieved, regardless of endoscopic or histological findings. The chronological change in EREFS in the diffuse type showed a dichotomy between patients sustaining an EREFS > 3 and patients with an improved EREFS ≤2, indicating a mixture of PPI/PCAB responders and non-responders. In contrast, the localized type showed a much greater response to PPI/PCAB than the diffuse type, which is consistent with the results of a previous report 26 . Some studies reported that 20% to 30% of PPI responders could relapse during follow-up for at least 1 year 34 35 ; nevertheless, long-term data are lacking. In this study, no PPI/PCAB treatment responders had an apparent relapse of both the diffuse and localized types.
This study had several limitations. The biggest limitation was the small number of patients enrolled, attributable to the exclusion of 93 patients of a total 134 patients due to a short-term follow-up period of less than 5 years. Patients with newly developed or worsening symptoms might have sought care at a different hospital, which might have resulted in more patients with unchanged or improved disease activity in this study population. Medication was arbitrarily determined by the attending physician based on symptoms, as well as endoscopic and histological findings. Thus, due to the presence of selection bias and the small number of patients investigated, it would be inappropriate to draw any definitive conclusions about the long-term prognosis of aEE and mEoE based on findings from the present study alone. However, we believe that this study makes a significant contribution because aEE and mEoE are difficult to follow periodically over the long term because of the lack of symptoms. The endoscopic images had different conditions and qualities. To minimize variation in image evaluation, two board-certified endoscopists assessed the endoscopic findings using EREFS. The number and interval of follow-up endoscopies and the length of the follow-up period varied among the patients. Esophageal biopsy was not always performed during the follow-up period. Further studies with a larger number of patients should be conducted to elucidate the natural history of untreated aEE and mEoE.
Conclusions
In conclusion, this study showed that disease activity from aEE or mEoE would persist but not worsen even without treatment at least for up to 7 to 8 years. The localized type may improve and disappear over the long term, implying a different pathogenesis from that of the diffuse type. Further studies should be conducted to elucidate the natural history of untreated aEE and mEoE and to establish appropriate management strategies.
Acknowledgement
The authors thank Tetsurou Itou at the Shinoda General Hospital for providing patient care and managing the medical check-up program.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Lucendo AJ, Molina-Infante J, Arias Á et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154:319–332000. doi: 10.1053/j.gastro.2017.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam AY, Lee JK, Coward S et al. Epidemiologic burden and projections for eosinophilic esophagitis-associated emergency department visits in the United States: 2009–2030. Clin Gastroenterol Hepatol. 2023;21:3041–3.05E6. doi: 10.1016/j.cgh.2023.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Ishimura N, Okimoto E, Shibagaki K et al. Similarity and difference in the characteristics of eosinophilic esophagitis between Western countries and Japan. Dig Endosc. 2021;33:708–719. doi: 10.1111/den.13786. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara Y. Symptom-based diagnostic approach for eosinophilic esophagitis. J Gastroenterol. 2020;55:833–845. doi: 10.1007/s00535-020-01701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi K, Mishiro T, Tanaka S et al. Suitable biopsy site for detection of esophageal eosinophilia in eosinophilic esophagitis suspected cases. Dig Endosc. 2016;28:139–144. doi: 10.1111/den.12555. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Honma T, Nozawa Y et al. Eosinophilic esophagitis in Japanese patients: A mild and slow-progressing disorder. PLoS One. 2018;13:e0206621. doi: 10.1371/journal.pone.0206621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka F, Fukumoto S, Morisaki T et al. Obesity and hiatal hernia may be non-allergic risk factors for esophageal eosinophilia in Japanese adults. Esophagus. 2019;16:309–315. doi: 10.1007/s10388-019-00662-3. [DOI] [PubMed] [Google Scholar]
- 9.Ronkainen J, Talley NJ, Aro P et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: The population-based Kalixanda study. Gut. 2007;56:615–620. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Xu Q, Zheng Y et al. Prevalence of esophageal eosinophilia and eosinophilic esophagitis in adults: A population-based endoscopic study in Shanghai, China. Dig Dis Sci. 2015;60:1716–1723. doi: 10.1007/s10620-014-3512-9. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Liacouras CA, Molina-Infante J et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018;155:1022–1.033E13. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishimura N, Sumi S, Okada M et al. Is asymptomatic esophageal eosinophilia the same disease entity as eosinophilic esophagitis? Clin Gastroenterol Hepatol. 2019;17:1405–1407. doi: 10.1016/j.cgh.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura H, Tanaka F, Nadatani Y et al. Eosinophilic esophagitis and asymptomatic esophageal eosinophilia display similar immunohistological profiles. J Clin Biochem Nutr. 2021;68:246–252. doi: 10.3164/jcbn.20-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y, Iizuka T, Hosoi A et al. Clinicopathological differences between eosinophilic esophagitis and asymptomatic esophageal eosinophilia. Intern Med. 2022;61:1319–1327. doi: 10.2169/internalmedicine.8241-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muroi K, Kakushima N, Furukawa K et al. Subjective symptoms in patients with eosinophilic esophagitis are related to esophageal wall thickness and esophageal body pressure. Dig Dis Sci. 2021;66:2291–2300. doi: 10.1007/s10620-020-06527-5. [DOI] [PubMed] [Google Scholar]
- 16.Kanamori A, Tanaka F, Takashima S et al. Esophageal mast cells may be associated with the perception of symptoms in patients with eosinophilic esophagitis. Esophagus. 2023;20:333–341. doi: 10.1007/s10388-022-00967-w. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Ochiai Y, Hosoi A et al. Mucosal and submucosal thickening of esophageal wall is a promising factor in the development of symptoms in eosinophilic esophagitis. Gut Liver. 2024;18:50–59. doi: 10.5009/gnl220490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kon T, Abe Y, Sasaki Y et al. Clinical features of esophageal eosinophilia according to endoscopic phenotypes. Intern Med. 2020;59:2971–2979. doi: 10.2169/internalmedicine.4447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibashi F, Fukushima K, Onizuka R et al. Risk of progression to eosinophilic esophagitis in patients with asymptomatic esophageal eosinophilia: A retrospective pilot study. JGH Open. 2020;4:422–428. doi: 10.1002/jgh3.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–60. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Kim HP, Sperry SLW et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–850000. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol. 2016;50:134–140. doi: 10.1097/MCG.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 23.Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut. 2013;62:489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 24.Cotton CC, Woosley JT, Moist SE et al. Determination of a treatment response threshold for the Eosinophilic esophagitis Endoscopic Reference Score. Endoscopy. 2022;54:635–643. doi: 10.1055/a-1675-7860. [DOI] [PubMed] [Google Scholar]
- 25.Dellon ES, Khoury P, Muir AB et al. A clinical severity index for eosinophilic esophagitis: Development, consensus, and future directions. Gastroenterology. 2022;163:59–76. doi: 10.1053/j.gastro.2022.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawada A, Hashimoto A, Uemura R et al. Association between endoscopic findings of eosinophilic esophagitis and responsiveness to proton pump inhibitors. Endosc Int Open. 2019;7:E433–E439. doi: 10.1055/a-0859-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safroneeva E, Straumann A, Coslovsky M et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology. 2016;150:581–5.9E6. doi: 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander R, Alexander JA, Ravi K et al. Measurement of observed eating behaviors in patients with active and inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019;17:2371–2373. doi: 10.1016/j.cgh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Straumann A, Spichtin HP, Grize L et al. Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for UP to 11.5 years. Gastroenterology. 2003;125:1660–1669. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Bohm M, Jacobs JW, Gupta A et al. Most children with eosinophilic esophagitis have a favorable outcome as young adults. Dis Esophagus. 2017;30:1–6. doi: 10.1111/dote.12454. [DOI] [PubMed] [Google Scholar]
- 31.Podboy AJ, Lavey C, Mara K et al. Eosinophilic esophagitis is rarely continually symptomatic 10 years after an initial treatment course in adults. Dig Dis Sci. 2019;64:3568–3578. doi: 10.1007/s10620-019-05636-0. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Nagashima S, Yamada Y et al. Comparison of nonesophageal eosinophilic gastrointestinal disorders with eosinophilic esophagitis: A nationwide survey. J Allergy Clin Immunol Pract. 2021;9:3339–3349. doi: 10.1016/j.jaip.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Schreiner P, Biedermann L, Greuter T et al. How to approach adult patients with asymptomatic esophageal eosinophilia. Dis Esophagus. 2021;34:1–7. doi: 10.1093/dote/doaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okimoto E, Ishimura N, Ishihara S. Clinical characteristics and treatment outcomes of patients with eosinophilic esophagitis and eosinophilic gastroenteritis. Digestion. 2021;102:33–40. doi: 10.1159/000511588. [DOI] [PubMed] [Google Scholar]
- 35.Lucendo AJ, Molina-Infante J. Current treatment options and long-term outcomes in patients with eosinophilic esophagitis. Expert Rev Clin Immunol. 2022;18:859–872. doi: 10.1080/1744666X.2022.2096591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.