Abstract
All retroviral genomes contain a nucleotide sequence designated as the primer binding site (PBS) which is complementary to the tRNA used for initiation of reverse transcription. For human immunodeficiency virus type 1 (HIV-1), all naturally occurring genomes have a PBS complementary to tRNA3Lys. However, within HIV-1 virions, there are approximately equal amounts of tRNA1Lys, tRNA2Lys, and tRNA3Lys. We have used an endogenous reverse transcription-PCR technique specific for the tRNA species within isolated HIV-1 virions to demonstrate that in addition to tRNA3Lys, tRNA1Lys and tRNA2Lys could be used for initiation of HIV-1 reverse transcription. Using a single-round infection assay which employed an HIV-1 genome with a gpt gene encoding xanthine-guanine phosphoribosyl transferase in place of the env gene, we generated cell lines resistant to mycophenolic acid. Analysis of the U5-PBS from single-cell clones revealed PBS complementary to tRNA3Lys, not tRNA1Lys or tRNA2Lys. A mutant HIV-1 genome was then created which would favor the completion of reverse transcription with tRNA1,2Lys. Using this provirus in the complementation system, we again found only genomes with a PBS complementary to tRNA3Lys from proviral DNA isolated from gpt-resistant single-cell colonies. Finally, infection of cells with a mutant HIV genome with a PBS complementary to tRNA1,2Lys resulted in gpt- resistant cell colonies which contained integrated provirions with a PBS complementary to tRNA1,2Lys. The results of these studies suggest that the selection of tRNA3Lys for initiation of HIV-1 reverse transcription occurs both at the initiation and at a postinitiation step in reverse transcription prior to integration of the proviral DNA.
A distinguishing feature of retrovirus replication is the process by which the RNA genome is converted into a DNA form prior to integration into the host cell chromosome. The reverse transcription of the retroviral genome is accomplished by a virally encoded enzyme, reverse transcriptase (RT) (1, 25). The initiation of reverse transcription occurs at a region in the viral RNA genome designated as the primer binding site (PBS). The PBS is complementary to the 3′-terminal nucleotides of the cellular tRNA molecule used for initiation. The RT extends the 3′ OH of the cellular tRNA molecule bound at the PBS. Following inter- or intramolecular copying of the viral RNA genome, the DNA copy of the viral genome, termed the provirus, is integrated into the host cell chromosome (6, 18). During the process of reverse transcription, the RT copies the attached tRNA molecule used for initiation of reverse transcription. Thus, the sequence of the PBS from the integrated provirus reflects the tRNA primer used for initiation of reverse transcription.
The tRNA primer used for initiation of reverse transcription varies between different retroviruses (4, 7, 19). For human immunodeficiency virus type 1 (HIV-1), tRNA3Lys is exclusively used for initiation of reverse transcription (20, 21). Previous studies have found tRNA3Lys is present within HIV-1 virions. The viral polyprotein precursor, Gag-Pol, is probably responsible for the enrichment of tRNA3Lys in HIV-1 virions (16). Previous studies, though, have demonstrated that similar amounts of tRNA1Lys and tRNA2Lys to that of tRNA3Lys were also present in the HIV-1 virions (9, 30). The sequence homology between tRNA1,2Lys and tRNA3Lys is approximately 80% (there are only 2-nucleotide [nt] differences between tRNA1Lys and tRNA2Lys) (24). How the virus distinguishes between these three isoacceptors for the exclusive use of tRNA3Lys to initiate reverse transcription is not clear. Previous studies from this laboratory and others have demonstrated that HIV-1 has the capacity to utilize several different tRNA primers, including tRNA1,2Lys for the initiation of reverse transcription (5, 10, 13, 28, 29). However, upon extended in vitro culture, all of these viruses reverted back to utilizing tRNA3Lys for the initiation of reverse transcription.
Recently, chemical and enzymatic methodologies have been used to delineate additional regions of interaction between the HIV-1 RNA genome and tRNA3Lys (8). In particular, an A-rich region was identified which was found to interact with the anticodon loop of tRNA3Lys. This A-rich region is present at the end of a stem-loop RNA structure (hence A-loop) and has been shown in previous studies to be important for the efficient initiation of reverse transcription. Different A-loop–PBS combinations corresponding to nucleotide sequences complementary to the anticodon and 3′-terminal sequences of tRNAs other than tRNA3Lys have resulted in the production of HIV-1 which stably utilizes these alternative tRNAs for initiation of reverse transcription (11, 12, 26, 30).
In this study, we have addressed the specificity of the wild-type HIV-1 for tRNA3Lys for the initiation of reverse transcription. Using a sensitive endogenous RT-PCR, we demonstrate that either tRNA1Lys, tRNA2Lys, or tRNA3Lys can be used for the initiation of reverse transcription. Analysis of integrated proviral DNA sequences isolated from cell clones derived from a single infection found only proviral genomes with a PBS complementary to tRNA3Lys. Using a mutant virus which would be predicted to promote the selection of those genomes initiated with tRNA1,2Lys, we were still unable to recover any clones which were initiated with tRNA1Lys or tRNA2Lys. In contrast, drug-resistant clones with a PBS complementary to tRNA1,2Lys were only recovered if the starting virus contained a PBS complementary to tRNA1,2Lys. The results of these studies demonstrate that in addition to the preference for tRNA3Lys at the initiation of reverse transcription, there exists a second process during HIV-1 reverse transcription which favors the integration of proviral DNA initiated with tRNA3Lys.
MATERIALS AND METHODS
Cells and media.
293T and HeLa H1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% antibiotics at 37°C and 5% CO2. SupT1 cells were grown in RPMI 1640 medium containing 15% FCS and 1% antibiotics.
Constructs of mutant HIV-1.
The construction of the plasmid which contains the gpt gene of Escherichia coli encoding the enzyme xanthine-guanine phosphoribosyl transferase in place of the HIV-1 env gene has been previously described (17). A SalI-to-XbaI restriction fragment was excised from this plasmid and cloned into the infectious proviral clone pHXB2, resulting in pHXB2-gpt used for this study. To construct mutant plasmid pHXB2-G17-gpt, an A-to-G mutation was introduced by PCR at nt 17 in wild-type PBS complementary to tRNA3Lys (see Fig. 4C). The sense primer used in this PCR is the −48 reverse primer (New England BioLabs). The antisense primer is PBS-G17: (5′GTGCGCGCTTCAGCAAGCCGAGTCCTGCGTCGAGAGAGCTCCTCTGGTTTCCCTTTCGCTTTCAAGCCCCTGTTCG3′) (20). The template used in this PCR is M13mp18PBS (22). The PCR-generated fragment was digested with HpaI and BssHII and then directly cloned into pHXB2-gpt.
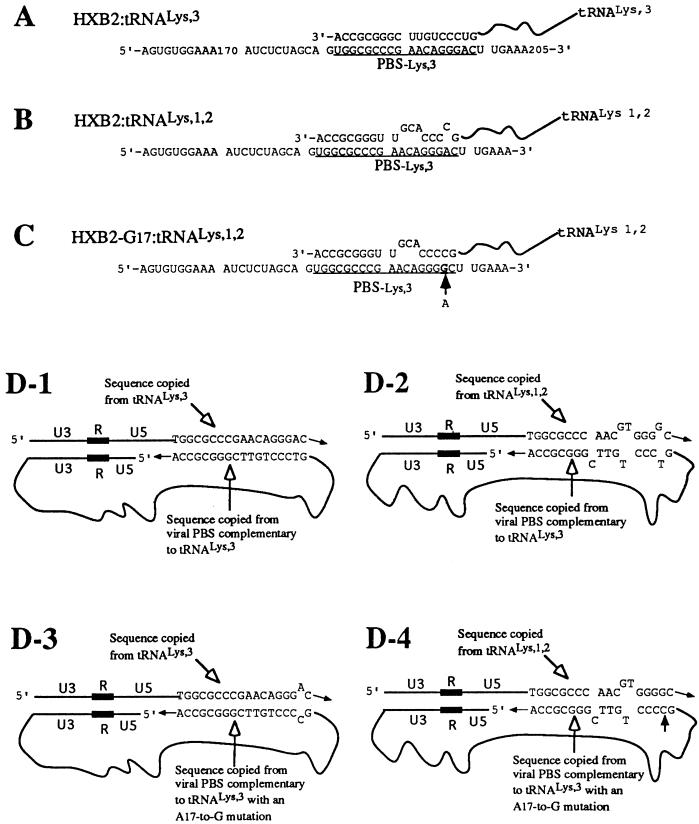
FIG. 4.
Construction of mutant HIV-1 provirus to optimize the use of tRNA1Lys or tRNA2Lys for reverse transcription. An A-to-G mutation was created at nt 17 in wild-type PBS complementary to tRNA3Lys by PCR. While this mutation would not be predicted to affect the interaction between the PBS and the 3′-end 18 nt of tRNA3Lys (A), it will increase complementarity between the PBS and the 3′-end 18 nt of tRNA1,2Lys (B and C), as well as that between the sequence copied from the viral PBS (in minus-strand DNA) and the sequence copied from the 3′-end 18 nt of tRNA1,2Lys (in plus-strand DNA) (D-2 and D-4). (A) Postulated interaction between the wild-type PBS and the 3′-end 18 nt of tRNA3Lys. (B) Postulated interaction between the wild-type PBS and the 3′-end 18 nt of tRNA1,2Lys. (C) Postulated interaction between the viral PBS that contains an A17-to-G mutation and the 3′-end 18 nt of tRNA1,2Lys. (D-1) Postulated interaction between the sequence copied from the viral PBS and the sequence copied from the 3′-end 18 nt of tRNA3Lys after second-strand translocation during reverse transcription. (D-2) Potential interaction between the sequence copied from the viral PBS and the sequence copied from the 3′-end 18 nt of tRNA1,2Lys after second-strand translocation. (D-3) Potential interaction between the sequence copied from the viral PBS that contains an A17-to-G mutation and the sequence copied from the 3′-end 18 nt of tRNA3Lys. (D-4) Potential interaction between the sequence copied from the viral PBS that contains an A-to-G mutation at nucleotide 17 and the sequence copied from the 3′-end 18 nt of tRNA1,2Lys.
To construct an HIV-1 clone, pHXB2-Lys1,2-gpt, which contains a gpt gene and a PBS sequence complementary to the 3′-end 18 nt of tRNA1,2Lys, primer 1 (5′-TAGACCAGATCTG AGCCTGGGAGCTC-3′: nt 13 to 48) and the PBS mutagenesis primer (5′-CCCTTTCGC TTTCAAGCCCCACGTTGGGCGCCACTGC-3′; partially complementary to nt 214 to 178) were used in the first round of PCR. The template DNA for this PCR was the wild-type pHXB2 plasmid DNA. PCR was performed for 35 cycles of amplification with Taq DNA polymerase (Gibco-BRL), each consisting of a denaturing step at 94°C for 1 min, an annealing step at 50°C for 1 min, and an extension step at 72°C for 1 min. A DNA fragment generated from the first-round PCR was agarose gel purified and served as a megaprimer in the second-round PCR. The other primer for this PCR was primer 2 (5′-CTCCTTCTAGCCTCCGCTAGTC-3′; complementary to nt 331 to 310). Vent DNA polymerase (New England BioLabs) was used in this round of PCR, which was performed for 35 cycles of amplification, each consisting of a denaturing step at 94°C for 1.5 min, an annealing step at 55°C for 2 min, and an extension step at 72°C for 1 min. The fragments obtained from the second-round PCR were digested with BglII and BssHII and cloned into a transfer vector, pUC119 PBS, which contains a wild-type HpaI-to-PstI DNA fragment from pHXB2 encompassing the original cellular sequence, a 5′ long terminal repeat sequence (U3-R-U5), PBS, and a partial gag coding sequence. The cloned DNA fragment was sequenced to obtain a clone that contained a PBS complementary to tRNA1,2Lys. The correct DNA fragment was reconstructed into recombinant provirus pHXB2-gpt through HpaI to BssHII.
The construction of the vesicular stomatitis virus G glycoprotein (VSV-G) expression plasmid, pLGRNL, has been previously described (3).
Endogenous reverse transcription-PCR.
Plasmid DNA containing wild-type HIV-1 proviral clone HXB2 was transfected into 293T cells by the CaPO4 method (Stratagene). To isolate the virus from culture medium of transfected cells, the culture medium was first subjected to a low-speed centrifugation (1,000 × g for 10 min) to pellet the cellular debris. The supernatant was then treated with RNase-free DNase I (Boehringer Mannheim) for 1 h at 37°C at a final concentration of 20 U/ml in the presence of 10 mM MgCl2. DNase-treated supernatant was overlaid onto 5-ml cushions containing 20% diatrizoate–80% TEN buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 100 mM NaCl) and centrifuged at 25,000 rpm (82,700 × g) for 3 h at 4°C in an SW28 rotor. The pelleted virus was resuspended in 50 μl of ice-cold TEN buffer, and the amount of virus was determined by p24 enzyme-linked immunosorbent assay (Coulter Laboratories). Virus preparation containing 1 ng of p24 antigen was added to 50 μl of endogenous reverse transcription reaction mixture (0.01% Triton X-100, 50 mM NaCl, 50 mM Tris-HCl [pH 8.0], 10 mM dithiothreitol, 5 mM MgCl2, 250 μM [each] dGTP, dATP, dTTP, and dCTP). The endogenous reaction was carried out at 37°C for 1 h and terminated by the addition of 50 μl of 2× stopping buffer (100 μg of proteinase K per ml, 3 mM EDTA [pH 8.0]). After incubation at 60°C for 1 h, the reaction mixture was boiled for 10 min.
An endogenous reverse transcription reaction was also carried out with virus produced from infected SupT1 cells. Virus produced from transfected 293T cells was used to infect SupT1 cells, which express CD4 receptor and support high-level HIV-1 replication. After 120 days of in vitro replication of the virus in SupT1 cells, culture medium containing 1 ng of p24 of HIV-1 antigen was used in an endogenous reverse transcription similar to that described for virus isolated from transfected 293T cells.
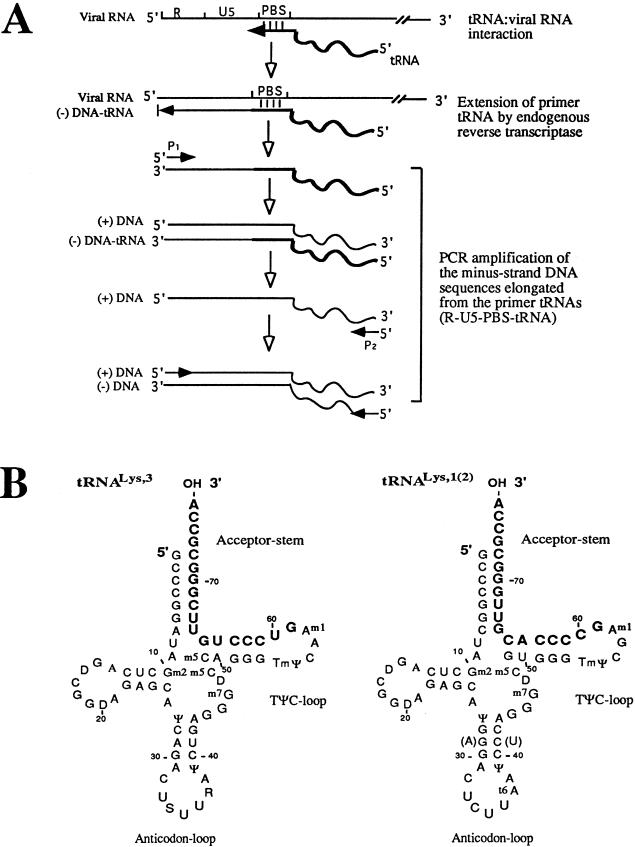
For PCR, 2.5 μl of endogenous reaction product containing 25 pg of p24 HIV-1 antigen was used in a 50-μl PCR mixture. The plus-sense primer is primer 1 (5′-TAGACCAGATCTGGCCTGGGAGCTC-3′; nt 13 to 48 of HXB2 RNA; nucleotide numbers correspond to those of Ratner et al. [21]), and the minus-sense primer is the Lys 1,2,3 primer (5′-TAGCTCAGTCGGTAGAGCA-3′; corresponding to nt 8 to 26 of tRNA1Lys, tRNA2Lys, and tRNA3Lys [24]). Taq DNA polymerase was chosen for this PCR, since a previous study showed that this enzyme possesses RT activity (15). Since the sequence of the minus-sense primer used in this PCR is shared by tRNA1Lys, tRNA2Lys, and tRNA3Lys, this PCR will simultaneously amplify the minus-strand strong-stop DNA products extended from the three tRNAs in endogenous reverse transcription (Fig. 1A). The PCR was performed for 35 cycles of amplification, each consisting of a denaturing step at 94°C for 1 min, an annealing step at 60°C for 1 min, and an extension step at 72°C for 1 min. PCR products were resolved in an agarose gel. A single DNA band corresponding to the expected size of 238 bp was isolated from the gel and ligated into pGEM-T-Easy vector (Promega). The ligation mixture was transformed into E. coli (DH5α). Plasmid DNAs from individual E. coli colonies were prepared and sequenced with primer 1. The identities of tRNA1Lys, tRNA2Lys, and tRNA3Lys were determined by their nucleotide sequences. (Note that there are only 2 nt that are different between tRNA1Lys and tRNA2Lys [Fig. 1B].)
FIG. 1.
Endogenous reverse transcription-PCR designed to specifically amplify the minus-strand strong-stop DNA elongated from tRNA1Lys, tRNA2Lys, and tRNA3Lys. (A) Endogenous reverse transcription reactions were carried out with HIV-1 produced from transfected 293T cells or infected SupT1 cells. PCR was performed with primer P1 (plus-strand sense DNA primer located in the R region) and primer P2 (Lys1,2,3 primer, corresponding to the common sequence [nt 8 to 26] shared by tRNA1Lys, tRNA2Lys, and tRNA3Lys). Note that, at the beginning of the PCR, P1 primer can only bind to the minus-strand strong-stop DNA that extended from endogenous tRNA primers. Taq DNA polymerase extends the P1 primer by copying the sequences of the minus-strand DNA and the still-attached tRNA. P2 primer binds to the DNA sequence copied from the 5′ ends of the attached tRNA1Lys or tRNA2Lys and tRNA3Lys. (B) Secondary structures of tRNA3Lys and tRNA1,2Lys. These three tRNAs share an overall 80% primary sequence homology (24). Note that there are only 2-nt differences between tRNA1Lys and tRNA2Lys. The 3′-terminal 18 nt in these tRNAs are highlighted in boldface.
Cloning and sequencing of proviral DNA.
Cellular chromosomal DNA was isolated from HIV-1-infected SupT1 cells at days 20, 45, 60, and 90 postinfection with the Wizard genomic DNA purification kit according to the manufacturer’s instructions (Promega). About 1.0 μg of isolated DNA was used to amplify the proviral DNA sequences encompassing the U5 and PBS regions by PCR with primer 1 (see above) and primer 2 (5′-CTCC TTCTAGCCTCCGCTAGTC-3′ [nt 333 to 311]). The PCR-amplified DNA fragment was gel purified and ligated into the pGEM-T-Easy vector (Promega). The ligation mixture was transformed into E. coli (DH5α); at each time point, plasmid DNAs from 10 to 15 individual E. coli colonies were prepared and sequenced with primer 1.
Single-round infection and cell cloning.
293T cells were cotransfected with pLGRNL (1 μg) and pHXB2-gpt (5 μg) or pHXB2-G17-gpt (5 μg) and pHXB2-Lys1,2-gpt (5 μg) in 35-mm-diameter dishes by the CaPO4 method (Stratagene). At 12 to 16 h after transfection, the medium was replaced with fresh medium. Culture supernatants were harvested from transfected 293T cells at 60 h posttransfection, filtered through a 0.45-μm-pore-size membrane, and quantitated for virus by a standard HIV-1 p24 antigen-capture enzyme-linked immunosorbent assay (Coulter Laboratories). 293T culture supernatant containing 20 pg of HIV-1 p24 was used to infect HeLa H1 cells in a 35-mm-diameter dish. At 12 h after infection, the medium was replaced with DMEM containing 10% FCS, 1% antibiotics, 250 μg of xanthine per ml, 50 μg of mycophenolic acid per ml, and 20 mM HEPES (pH 7.5). This medium was changed every 2 days until the foci of drug- resistant cells had formed (12 to 15 days). Dishes with drug-resistant foci were carefully washed once with phosphate-buffered saline (pH 7.0). Individual cell colonies were isolated with a cloning cylinder (8 by 8 mm) (Specialty Media, Lavallette, N.J.) and replated into a 35-mm-diameter culture plate. Drug-resistant colonies were further cultured in selection medium for an additional 10 days to allow sufficient growth for isolation of chromosomal DNA. Chromosomal DNAs were isolated from 7 to 15 individual colonies derived from different pseudovirus-infected cells by using a Wizard DNA isolation kit (Promega). The integrated HIV-1 proviral DNA sequences encompassing the U5-PBS region in these isolated DNAs were amplified by PCR with primer 1 and primer 2. The PCR-amplified fragments were each gel purified and ligated with pGEM-T-Easy vector. The ligation mixtures were transformed into E. coli (DH5α). Plasmid DNAs from four randomly selected E. coli colonies for each ligation transformation (originally from a single drug-resistant colony) were prepared and sequenced with primer 1.
RESULTS
Analysis of tRNALys primers used for the initiation of HIV-1 reverse transcription.
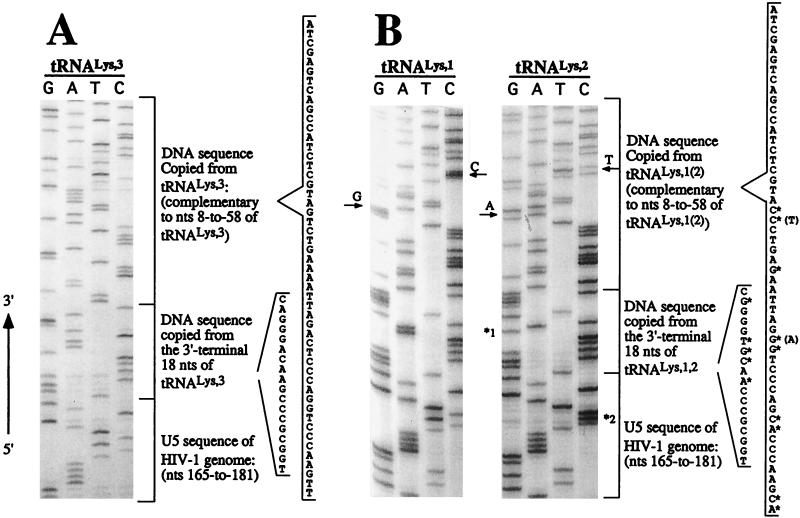
Previous studies have found that HIV-1 virions contain tRNA1Lys and tRNA2Lys as well as the cognate primer, tRNA3Lys (9, 30). To determine if tRNA1,2Lys could be used for initiation of reverse transcription, the endogenous reverse transcription-PCR method was used with PCR primers that could preferentially amplify the strong-stop DNA products that extended from all three tRNALys isoacceptors (Fig. 1A). For these studies, the wild-type HIV-1 proviral clone (pHXB2) was transfected into 293T cells; virus particles in the supernatant from transfected cells were isolated and used in an endogenous reverse transcription reaction. The products from endogenous reverse transcription reaction were then used as templates in a PCR. To absolutely confirm the identity of the PCR products, they were cloned and the DNA sequence was determined (Fig. 2). Among the 16 PCR clones analyzed, we found that 5 clones contained DNA sequence extended from tRNA1Lys, 4 contained DNA sequence extended from tRNA2Lys, and 7 contained DNA sequence extended from the cognate primer tRNA3Lys (Fig. 3). Thus, the virus produced from transfected 293T cells used either tRNA1Lys, tRNA2Lys, or tRNA3Lys as the primer for the initiation of HIV-1 reverse transcription. When a sense primer whose sequence is located in the U3 region and the tRNALys isoacceptor-specific primer (Lys 1,2,3 primer) were used in the endogenous RT-PCR, we also found that the virus used either tRNA1Lys, tRNA2Lys, or tRNA3Lys, suggesting that the minus-strand strong-stop DNA extended from tRNA1,2Lys can be elongated beyond the U3 region after the first strand translocation (data not shown).
FIG. 2.
Analysis of tRNALys isoacceptors used to initiate the reverse transcription in HIV-1. Viruses produced from transfected 293T or infected SupT1 cells were used in an endogenous reverse transcription reaction. The products of this reaction were amplified by a PCR with P1 primer and Lys1,2,3 primer (Fig. 1). PCR products were cloned, and the DNA sequence was determined. The identities of tRNAs used as primers in the initiation of reverse transcription were determined by their DNA sequence and comparison to known sequences (24). (A) DNA sequence (in genomic RNA sense) copied from the 3′ end of tRNA3Lys and the first strong-stop DNA product (only partial U5 sequence is shown). (B) DNA sequence from the 3′ ends of tRNA1Lys and tRNA2Lys and extended strong-stop DNA products. The individual nucleotides with an asterisk shown in the sequence indicate the nucleotide differences between tRNA3Lys and tRNA1,2Lys. The 2-nt differences between tRNA1Lys and tRNA2Lys are indicated by arrows. *1 indicates an A-to-G mutation in tRNA2Lys-extended DNA; *2 indicates the T-to-C mutation. Either mutation was probably created by HIV-1 RT or Taq DNA polymerase during the endogenous RT-PCR.
FIG. 3.
Frequencies with which tRNA1Lys, tRNA2Lys, and tRNA3Lys are used in the initiation of reverse transcription of HIV-1 isolated from transfected 293T cells and infected SupT1 cells. (a) PBS and surrounding sequence in wild-type proviral DNA of HIV-1 (HXB2 isolate) used for transfection of 293T cells. (b) DNA sequences recovered from endogenous reverse transcription-PCR conducted with HIV-1 produced from transfected 293T cells. The identities of tRNAs used as primers in the initiation of reverse transcription were determined by their complementary DNA sequence. The 18 nt (located in the PBS region) that were copied from the 3′ 18 nt of tRNA3Lys or tRNA1Lys and tRNA2Lys are underlined. Nucleotides in the sequences copied from tRNA1Lys and tRNA2Lys that are different from those in the sequence copied from tRNA3Lys are in boldface. The 2 nt that differ between tRNA1Lys and tRNA2Lys are also underlined. Identical nucleotides in the recovered DNA sequences to that in the input proviral DNA are represented by asterisks. (c) DNA sequences recovered from endogenous reverse transcription-PCR on HIV-1 produced from infected SupT1 cells. Frequencies (7/16, 5/16, etc.) of DNA sequences extended from each of tRNA1Lys, tRNA2Lys, and tRNA3Lys in PCR-amplified DNA clones are given to the right.
It is possible that the use of the different tRNALys isoacceptors during the endogenous reverse transcription reaction was due to the source of the cells (293T) which were used for transfection to produce the virus. To further substantiate our results, we carried out an endogenous reverse transcription reaction with HIV-1 which had continuously been grown in SupT1 cells for 3 months. Analysis of the PCR products revealed that among the 11 PCR clones analyzed, 2 clones contained DNA sequence extended from tRNA1Lys, 2 contained DNA sequence extended from tRNA2Lys, and 7 contained DNA sequence extended from tRNA3Lys (Fig. 3). Thus, HIV-1 produced from infected SupT1 cells also used tRNA1Lys and tRNA2Lys to initiate the reverse transcription.
Analysis of PBS obtained from proviruses after extended in vitro culture.
If the tRNA1Lys or tRNA2Lys can be used to initiate reverse transcription, we expect that analysis of proviruses after extended culture should reveal the presence of a PBS complementary to tRNA1,2Lys. To determine if this is the case, we analyzed the proviral DNA sequence in the U5-PBS region of HIV-1 that has been cultured in the SupT1 cells for 20, 45, 60, and 90 days. Chromosomal DNA was isolated, and the proviral DNA sequences encompassing the U5-PBS region were amplified by PCR. DNAs from individual PCR clones were sequenced. We found that all of the PBS sequences recovered from the integrated proviral DNA were complementary to the 3′-terminal 18-nt sequence of tRNA3Lys (data not shown). No PBS sequences complementary to the 3′-terminal sequences of tRNA1Lys and tRNA2Lys were found from our analysis at any of the times examined.
Analysis of PBS obtained from proviral DNA isolated from pseudovirus-infected cells.
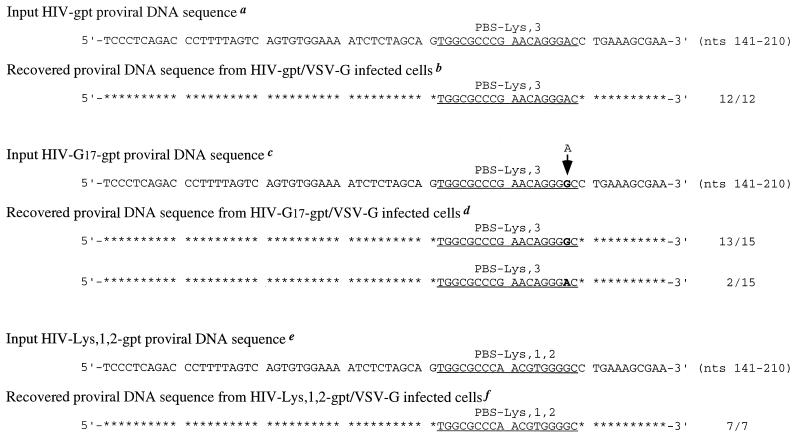
One possible reason for the lack of a PBS complementary to tRNA1Lys or tRNA2Lys in the integrated proviral DNA is that the viral sequence copied from tRNA1Lys or tRNA2Lys by the RT during plus-strand synthesis does not facilitate the transfer of the plus-strand DNA to the minus-strand template because of insufficient sequence complementarity (Fig. 4D-2). To investigate this possibility, we constructed a mutant HIV-1 with an A-to-G change at nt 17 in the wild-type PBS complementary to tRNA3Lys (Fig. 4C). This mutation will increase the complementarity between the PBS and the 3′-end sequence of tRNA1,2Lys (Fig. 4B and C), as well as the sequence copied from the viral RNA PBS (in the minus-strand DNA) and the sequence copied from the 3′-end sequence of tRNA1Lys or tRNA2Lys (in the plus-strand DNA) (Fig. 4D-2 and D-4). If the viral RT can copy the 3′-end 18-nt sequence of tRNA1Lys or tRNA2Lys into plus-strand DNA, this A-to-G mutation should facilitate the completion of the synthesis of proviral DNA. For this analysis, we conducted a single-round infection assay using the genome containing this mutation and a gpt gene in place of env (HXB2-G17-gpt). Cotransfection of this provirus with the plasmid encoding VSV-G (pLGRNL) resulted in a pseudotyped virus which can only undergo a single round of replication. The use of this system will avoid reversion of the PBS to the wild type (fully complementary to tRNA3Lys), which we have observed after multiple rounds of replication of viruses containing mutations in the PBS. As a control, virus was also produced from cotransfection of pHXB2-gpt that contains a wild-type PBS and a gpt gene in place of env and pLGRNL. Pseudoviruses were used to infect HeLa cells which were then grown in DMEM containing xanthine and mycophenolic acid to select cells that harbor the gpt gene (17). Chromosomal DNAs were isolated from individual drug-resistant colonies, and the integrated proviral DNA sequences in the U5-PBS region were amplified by PCR. The PCR-amplified products were independently cloned, and the DNA sequences of the U5- PBS were determined. When 12 drug-resistant foci grown up from HeLa cells infected with HXB2-gpt/VSV-G pseudovirus were analyzed, all of the recovered proviral DNAs were found to contain a wild-type PBS complementary to tRNA3Lys (Fig. 5). Thus, the proviral DNA synthesis initiated from tRNA1Lys or tRNA2Lys is not carried out through the second jump in reverse transcription. When DNAs isolated from 15 drug-resistant foci derived from HeLa cells infected with HIV-G17-gpt/VSV-G pseudovirus were analyzed, 2 were found to contain proviral DNA with a wild-type PBS, while 13 contained the proviral DNA with an input PBS (containing a G residue at position 17) (Fig. 5). Most importantly, we did not recover any U5- PBS DNA which contained a PBS sequence complementary to tRNA1Lys or tRNA2Lys. Thus, even with a mutation to promote complementarity between the minus-strand PBS DNA and the sequence copied from the 3′ end of tRNA1Lys or tRNA2Lys, the reverse transcription initiated from tRNA1,2Lys was not completed through plus-strand synthesis. Since plasmid DNAs from four bacterial colonies which represent four PCR fragments derived from each drug-resistant colony gave identical sequences, it is possible that these results reflect an unequal repair of the two strands of DNA when tRNA3Lys was used to initiate the reverse transcription on the mutant HXB2-G17-gpt genome. Additional experiments will be required to confirm this possibility.
FIG. 5.
Proviral DNA sequences recovered from mycophenolic acid-resistant HeLa cell colonies derived from single-round infection with HIV-1 pseudoviruses. (a) U5-PBS region sequence in proviral DNA of pHIV-gpt used for transfection of 293T cells. (b) DNA sequences recovered from 12 individual mycophenolic acid-resistant foci grown up from HIV-gpt/VSV-G pseudovirus-infected HeLa H1 cells. Identical nucleotides in the recovered DNA sequences to those in the input proviral DNA are represented by asterisks. The PBS sequence complementary to tRNA3Lys is underlined. (c) U5-PBS region sequence in mutant proviral DNA of pHIV-G17-gpt used for transfection of 293T cells. (d) DNA sequences recovered from 15 individual mycophenolic acid-resistant foci grown up from HIV-G17-gpt/VSV-G pseudovirus-infected HeLa H1 cells. (e) U5-PBS region sequence in proviral DNA of pHIV-Lys1,2-gpt used for transfection of 293T cells. (f) DNA sequences recovered from seven individual mycophenolic acid-resistant foci grown up from HIV-Lys1,2-gpt/VSV-G pseudovirus-infected HeLa H1 cells. Frequencies (12/12, 13/15, etc.) of DNA sequences in the analyzed drug-resistant foci that harbor the input or reverted proviral DNAs are given to the right.
To substantiate that the HIV-1 DNA synthesis initiated by the tRNA1,2Lys was blocked at the second strand transfer step, we constructed another mutant virus, pHXB2-Lys1,2-gpt, which contains a PBS complementary to the 3′-end 18 nt of tRNA1,2Lys. We reasoned that if the complementarity between the minus-strand PBS and the plus-strand PBS was the limiting factor for the completion of DNA synthesis initiated by tRNA1,2Lys, this mutant viral genome should allow tRNA1,2Lys-initiated DNA to go to completion and the integrated DNA that resulted from this mutant virus should contain a PBS complementary to tRNA1,2Lys. Indeed, analysis of the DNA from seven drug-resistant colonies derived from HeLa cells infected with HXB2-Lys1,2-gpt/VSV-G pseudovirus demonstrated that each of the recovered proviral DNAs contained a PBS complementary to tRNA1,2Lys (Fig. 5). Thus, the inability of the wild-type virus to use tRNA1,2Lys to complete reverse transcription was not due to an inherent feature of the virus.
DISCUSSION
In this report, we have provided genetic evidence to show that the tRNA1Lys and tRNA2Lys found in HIV-1 virions can be used to initiate reverse transcription. The reverse transcription initiated from the tRNA1Lys or tRNA2Lys, though, is probably not completed, as evidenced by the lack of integrated proviruses which contain a PBS complementary to these tRNAs.
The results of our studies provide us with important new insights into the mechanism by which HIV-1 selects tRNA3Lys to initiate reverse transcription and maintains a PBS complementary to tRNA3Lys. The major determinant for tRNA3Lys to be selected to initiate reverse transcription is probably its complementary to the PBS. Even though additional tRNAs are present within the virions of HIV-1, these tRNAs are not selected for reverse transcription because of insufficient complementarity to the PBS complementary to tRNA3Lys. However, sufficient complementarity exists between tRNA1Lys or tRNA2Lys and the PBS complementary to tRNA3Lys such that these tRNAs can interact with the wild-type PBS and be used for the initiation of reverse transcription. In fact, from cloning and sequencing of our endogenous RT-PCR products, we found that approximately 30% of the RT-PCR clones were from minus-strand DNA that extended from tRNA1Lys or tRNA2Lys. It was surprising then that we did not detect proviral genomes with a PBS complementary to tRNA1Lys or tRNA2Lys following analysis of cell clones obtained after single-round infection. We recovered only proviral genomes which contained a PBS complementary to tRNA3Lys. There are several possibilities to explain our results. First, the endogenous reverse transcription-PCR method which we used in this study might not reflect true initiation of minus-strand strong-stop DNA synthesis. This seems unlikely, because the endogenous reaction mixture contains all of the necessary viral proteins as well as genome requirements for the initiation of reverse transcription. Previous studies from our laboratory have also demonstrated that viruses which utilize alternative tRNAs to initiate reverse transcription also use the same tRNAs in the endogenous RT-PCR (14). Recently, we have genetically modified HIV-1 to use exclusively tRNA1,2Lys rather than tRNA3Lys to initiate reverse transcription (12). Using the reverse transcription-PCR method, we did not recover PCR clones with a PBS complementary to tRNA3Lys. Since HIV-1 virions with PBS complementary to alternative tRNAs still contain tRNA1,2Lys and tRNA3Lys as the major tRNA species (30), we believe the results further substantiate the fact that the clones of the PCR products represent minus-strand strong-stop DNA initiated with tRNA1,2Lys.
A second, more likely, possibility is that although tRNA1Lys or tRNA2Lys can be used for initiation of reverse transcription in the wild-type virus, the generation of a provirus from reverse transcription initiated by tRNA1Lys or tRNA2Lys was blocked. We speculate that the step at which the generation of the provirus might be blocked is at the completion of reverse transcription. The PBS is involved in both the initiation of reverse transcription and plus-strand synthesis required for the completion of the proviruses. In the latter process, a plus-strand copy of the PBS is generated when the 3′-terminal 18 nt of the tRNA molecule are copied by the reverse transcriptase. The complementarity between the plus- and minus-strand PBSs facilitates the translocation of the donor plus-strand DNA to the minus-strand template to generate the double-stranded proviral genome. Initiation of reverse transcription with a tRNA1,2Lys from a wild-type PBS (complementary to tRNA3Lys) and copying the 3′-terminal 18 nt of tRNA1,2Lys would be predicted to result in 3 mismatched bp between the donor plus- and minus-strand DNAs (Fig. 4D-2). Previous studies from our laboratory have shown that completion of proviral synthesis is affected by mutations which reduce the complementarity between the donor plus- and minus-strand DNAs (27). Thus, we predict that for the wild-type genome, reverse transcription initiated with tRNA1,2Lys would be much less efficient at the second-strand transfer because of incompatibility between the donor plus strand (generated from tRNA1,2Lys) and the minus strand (generated from copying of the PBS complementary to tRNA3Lys). If this were the only constraint to restrict the use of tRNA1,2Lys in reverse transcription, though, we would expect that infection with virus (HXB2-G17-gpt) with a PBS containing mutations to facilitate plus- and minus-strand DNA interaction would have resulted in proviruses with a PBS complementary to tRNA1,2Lys (Fig. 4D-4). However, this was not the case, as evidenced by the fact that analysis of recovered proviral genomes from cells infected with this mutant virus found only PBS complementary to tRNA3Lys. The results of this study suggest that the additional mismatched base pairs between the plus- and minus-strand PBSs might have reduced the efficiency of completion of reverse transcription (Fig. 4D-4). Support for this idea comes from the fact that we were able to recover integrated proviruses with a PBS complementary to tRNA1,2Lys from viruses derived from HXB2-Lys1,2-gpt. Thus, consistent with our previous studies, there is not a restriction at the level of viral proteins for use of tRNAs other than tRNA3Lys for reverse transcription (30).
The mechanism of how mismatched base pairs could result in an abortive completion of reverse transcription is not clear. Previous studies have suggested that the copying of the tRNA primer to generate a plus-strand DNA PBS used as a donor for completion of proviral synthesis might be influenced by the tRNA-PBS complex. Using an in vitro system, Ben-Artzi et al. found that the secondary structure of the tRNA-PBS complex could affect the copying of the tRNA primer and cause the RT to terminate before reaching the first modified Am at position 19 of the tRNA molecule (2). Prior to this study, it was generally believed that the RT copied the tRNA until it reached this modified base (23). The secondary structure formed by PBS-tRNA1Lys (or tRNA2Lys) might be different from that formed with tRNA3Lys due to the mismatched base pairs (Fig. 4). As a consequence of this altered structure, the plus-strand DNA synthesis carried by the HIV-1 RT might prematurely terminate, giving rise to an aborted plus-strand DNA product, which fails to facilitate the interaction between the plus- and minus-strand DNAs. Alternatively, the efficiency of completion with tRNA1,2Lys might be so low that to recover proviral genomes with a PBS complementary to tRNA1,2Lys, a greater number of single-cell clones would need to be analyzed. In either case, the efficiency for the completion of HIV-1 proviruses with a PBS complementary to tRNA1,2Lys would be much lower than that for viral genomes using tRNA3Lys so as to preclude the isolation of a naturally occurring HIV-1 with a PBS complementary to tRNA1,2Lys. Taken together, the results of our studies suggest that the selection of tRNA3Lys for reverse transcription occurs at both the initiation and later steps in the generation of the proviral genomes. The mechanism for selection probably involves complex interactions between the tRNA and PBS. Studies are ongoing to delineate these RNA-RNA interactions required for both initiation and completion of the proviral DNA.
ACKNOWLEDGMENTS
We thank members of the Morrow laboratory for helpful comments and Dee Martin for preparation of the manuscript. C.D.M. acknowledges the help of M.A.R. The virus growth was carried out at the UAB CFAR Virology Core (AI-27767).
This research was supported by a grant from the NIH (AI-34749) to C.D.M.
REFERENCES
- 1.Baltimore D. Viral RNA-dependent DNA polymerase. Nature (London) 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Artzi H, Shemesh J, Zeelon E, Amit B, Kleiman L, Gorecki M, Panet A. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996;35:10549–10557. doi: 10.1021/bi960439x. [DOI] [PubMed] [Google Scholar]
- 3.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and non mammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colicelli J, Goff S P. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1987;57:37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das A T, Klaver B, Berkhout B. Reduced replication of human immunodeficiency virus type 1 mutants that use reverse transcription primers other than the natural tRNA3Lys. J Virol. 1995;69:3090–3097. doi: 10.1128/jvi.69.5.3090-3097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilboa E, Mitra S W, Goff S, Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 7.Harada F, Sawyer R, Dahlberg J. A primer ribonucleic acid for initiation of in vitro Rous sarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975;250:3487–3497. [PubMed] [Google Scholar]
- 8.Isel C, Ehresmann C, Keith G, Ehresmann B, Marquet R. Initiation of reverse transcription of HIV-1: secondary structure of the HIV- 1 RNA/tRNALys,3 (template/primer) complex. J Mol Biol. 1995;247:236–250. doi: 10.1006/jmbi.1994.0136. [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, Mak J, Ladha A, Cohen E, Klein M, Rovinski B, Kleiman L. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol. 1993;67:3246–3253. doi: 10.1128/jvi.67.6.3246-3253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S-M, Wakefield J K, Morrow C D. Mutations in both the U5 region and primer binding site influence the selection of the tRNA used for the initiation of HIV-1 reverse transcription. Virology. 1996;222:401–414. doi: 10.1006/viro.1996.0437. [DOI] [PubMed] [Google Scholar]
- 11.Kang S-M, Zhang Z, Morrow C D. Identification of a sequence within U5 required for human immunodeficiency virus type 1 to stably maintain a primer binding site complementary to tRNAMet. J Virol. 1997;71:207–217. doi: 10.1128/jvi.71.1.207-217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang, S.-M., Z. Zhang, and C. D. Morrow. Critical role of human immunodeficiency virus type 1 in the discrimination between tRNALys1,2, and tRNALys,3 for initiation of reverse transcription. Submitted for publication.
- 13.Li X, Mak J, Arts E J, Gu Z, Kleiman L, Wainberg M A, Parniak M A. Effects of alterations of primer-binding site sequences on human immunodeficiency virus type 1 replication. J Virol. 1994;68:6198–6206. doi: 10.1128/jvi.68.10.6198-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang Z, Kang S-M, Buescher J L, Morrow C D. Insights into the interaction between tRNA and primer binding site from characterization of a unique HIV-1 virus which stably maintains dual PBS complementary to tRNAGly and tRNAHis. Virology. 1997;238:273–282. doi: 10.1006/viro.1997.8837. [DOI] [PubMed] [Google Scholar]
- 15.Lund A H, Duch M, Lovmand J, Jørgensen P, Pedersen F S. Complementation of a primer binding site-impaired murine leukemia virus-derived retroviral vector by a genetically engineered tRNA-like primer. J Virol. 1997;71:1191–1195. doi: 10.1128/jvi.71.2.1191-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak J, Jiang M, Wainberg M A, Hammarskjöld M-L, Rekosh D, Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J Virol. 1994;68:2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters G, Glover C. tRNAs and primer of RNA-directed DNA synthesis in mouse mammary tumor virus. J Virol. 1980;35:31–40. doi: 10.1128/jvi.35.1.31-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters G, Harada F, Dahlberg J E, Panet A, Haseltine W A, Baltimore D. Low-molecular-weight RNAs of moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977;21:1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratner L, Fisher A, Linda L, Agodzinski J, Mitsuya H, Liou R-S, Gallo R C, Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 21.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Ivanoff L, Petteway J S R, Pearson M L, Lautenberge J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature (London) 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 22.Rhim H, Park J, Morrow C D. Deletions in the tRNALys primer-binding site of human immunodeficiency virus type 1 identify essential regions for reverse transcription. J Virol. 1991;65:4555–4564. doi: 10.1128/jvi.65.9.4555-4564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth M J, Schwartzberg P L, Goff S P. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989;58:47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- 24.Sprintzl, M., T. Hartmann, J. Weber, J. Blank, and R. Zeidler. 1989. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 17(Suppl.):r1–r172. [DOI] [PMC free article] [PubMed]
- 25.Temin H M, Mizutani S. RNA-directed DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- 26.Wakefield J K, Kang S-M, Morrow C D. Construction of a type 1 human immunodeficiency virus that maintains a primer binding site complementary to tRNAHis. J Virol. 1996;70:966–975. doi: 10.1128/jvi.70.2.966-975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakefield J K, Morrow C D. Mutations within the primer binding site of the human immunodeficiency virus type 1 define sequence requirements essential for reverse transcription. Virology. 1996;220:290–298. doi: 10.1006/viro.1996.0317. [DOI] [PubMed] [Google Scholar]
- 28.Wakefield J K, Wolf A G, Morrow C D. Human immunodeficiency virus type 1 can use different tRNAs as primers for reverse transcription but selectively maintains a primer binding site complementary to tRNA3Lys. J Virol. 1995;69:6021–6029. doi: 10.1128/jvi.69.10.6021-6029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitcomb J M, Ortiz-Conde B A, Hughes S H. Replication of avian leukosis viruses with mutations at the primer binding site: use of alternative tRNAs as primers. J Virol. 1995;69:6228–6238. doi: 10.1128/jvi.69.10.6228-6238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Kang S-M, LeBlanc A, Hajduk S L, Morrow C D. Nucleotide sequences within the U5 region of the viral RNA genome are the major determinants for a human immunodeficiency virus type 1 to maintain a primer binding site complementary to tRNAHis. Virology. 1996;226:306–317. doi: 10.1006/viro.1996.0658. [DOI] [PubMed] [Google Scholar]