Abstract
Background:
Bacillus cereus (B. cereus) biofilm is grown not only on medical devices but also on different substrata and is considered a potential hazard in the food industry. Quorum sensing plays a serious role in the synthesis of biofilm with its surrounding extracellular matrix enabling irreversible connection of the bacteria.
Aim:
The goal of the current investigation was to ascertain the prevalence, patterns of antimicrobial resistance, and capacity for B. cereus biofilm formation in meat and meat products in Egypt.
Methods:
In all, 150 meat and meat product samples were used in this study. For additional bacteriological analysis, the samples were moved to the Bacteriology Laboratory. Thereafter, the antimicrobial, antiquorum sensing, and antibiofilm potential of apple cider vinegar (ACV) on B. cereus were evaluated.
Results:
Out of 150 samples, 34 (22.67%) tested positive for B. cereus. According to tests for antimicrobial susceptibility, every B. cereus isolates tested positive for colistin and ampicillin but negative for ciprofloxacin and imipenem. The ability to form biofilms was present in all 12 multidrug-resistant B. cereus isolates (n = 12); of these, 6 (50%), 3 (25%), and 3 (25%) isolates were weak, moderate, and strong biofilm producers, respectively. It is noteworthy that the ACV demonstrated significant inhibitory effects on B. cereus isolates, with minimum inhibitory concentrations varying between 2 and 8 μg/ml. Furthermore, after exposing biofilm-producing B. cereus isolates to the minimum biofilm inhibitory concentrations 50 of 4 μg/ml, it demonstrated good antibiofilm activity (>50% reduction of biofilm formation). Strong biofilm producers had down-regulated biofilm genes (tasA and sipW) and their regulator (plcR) compared to the control group, according to reverse transcriptase quantitative polymerase chain reaction analysis.
Conclusion:
Our study is the first report, that spotlights the ACV activity against B. cereus biofilm and its consequence as a strong antibacterial and antibiofilm agent in the food industry and human health risk.
Keywords: Apple cider vinegar, B. cereus, Antimicrobial resistance, Biofilm
Introduction
High biological value proteins, vitamins particularly B and certain minerals all essential for human development and well-being can be found in meat products. One of the most underappreciated foodborne illnesses worldwide is contaminated meat products containing toxic Bacillus cereus (B. cereus) (Ceuppens et al., 2013; Ayako Kobashi et al., 2023). Bacterial biofilms that form in the food matrix or on tools can cause foodborne infections (Adame-Gómez et al., 2020). According to Boonyayatra et al. (2016), biofilm formation plays a significant role in the pathophysiology of numerous diseases in both humans and animals. Biofilm-forming bacteria have the ability to endure in unfavorable environments and, once inside an organism, to withstand the host immune system while developing resistance to the effects of antibiotics and disinfectants (Felipe et al., 2017). Food production is thought to pose a potential health risk due to the formation of biofilms by B. cereus (Lindsay et al., 2000). It is one of the leading causes of bacteria developing multidrug resistance (MDR) (Tewari et al., 2012).
The current understanding of biofilm cell differentiation in species belonging to the B. cereus group is lacking. Nonetheless, a number of studies showed that the primary regulatory pathways supporting the formation of biofilms in species belonging to the B. cereus group are conserved. Through the peptide PapR, the phospholipase C regulator (PlcR) is in charge of detecting external signals such as population density and nutrition. Its regulon contains some virulent factors that initiate the necrotrophic factor, neutral protease regulator (NprR), which promotes the expression of kurstakin and initiates biofilm formation (Dubois et al., 2012). According to Caro-Astorga et al. (2015), TasA has two paralogs: calY, which is located next to sipW-tasA, and tasA, which is a part of the sipW-tasA operon. Electron microscopy reveals that TasA and CalY are both involved in the production of fibers, and biofilm defects result from the eradication of their genes or sipW (Caro-Astorga et al., 2015).
Antibiotics are recognized as one of the most important weapons in the fight against illness (Thomas et al., 2015). Biofilm-forming bacteria are extremely adaptable and resistant to disinfectants and antibiotics. When treating both acute and chronic biofilm infections, high antibiotic resistance can be a barrier (Li and Lee, 2017). Many studies rely on mechanisms to disrupt bacterial quorum sensing by interfering with cell-cell communication to hinder the ability of B. cereus group strains, particularly B. cereus sensu stricto, to cause human infections. This process is known as quorum quenching (Waters and Bassler, 2005; Yehuda et al., 2018). The prevention of bacterial growth through the use of antimicrobial agents is the most effective strategy for inhibiting the formation of biofilm, a topic that has received significant attention recently (Roy et al., 2018). Consequently, a lot of research focused on nontraditional approaches such as biological products or herbal medicines as anti-biofilm agents (Schönborn et al., 2017).
Apple cider vinegar (ACV) is a naturally occurring product of apple fermentation made of apple, sugar, and yeast. On Gram-positive bacteria, it exhibits antibacterial activity (Watson et al., 2018). Its antioxidant and antibacterial properties against numerous pathogenic agents are attributed to a wide range of constituents, including vitamins, minerals, organic acids, polyphenols, and flavonoids (Xia et al., 2020; Budak et al., 2021). ACV had an antimicrobial effect on tested microorganisms such as Vibrio cholerae, Candida tropicalis, C. albicans, Echerchia coli O157:H7, and Salmonella typhi. Generally, a study has inspected the acetic acid effects, which is rich in vinegar, on the formation of biofilm and revealed that it reduced the biofilm formed by Staphylococcus aureus (Pedroso et al., 2018). Therefore, it needs to discover recent antibiofilm and antiquorum agents preferable to the traditional treatment to eradicate the biofilm that affects the control of B. cereus infection. Herein, we investigated the antimicrobial susceptibilities and biofilm-producing abilities of B. cereus recovered from meat products. Thereafter, the in vitro antibiofilm and antiquorum sensing activities of ACV were assessed against MDR B. cereus isolates followed by evaluating the efficacy of ACV on the expression profile of biofilm-associated genes via reverse transcriptase quantitative polymerase chain reaction (RT-qPCR).
Materials and Methods
Sampling
In all, 150 samples of meat and meat products including minced meat (40), shawarma (30), beef burger (25), beef kofta (25), beef luncheon (15), and sausage (15) were randomly collected from various supermarkets in the Sharkia Governorate of Egypt. As soon as possible, the samples were moved to the Bacteriology Laboratory, Department of Microbiology, Faculty of Veterinary Medicine, Zagazig University, for additional bacteriological analysis. They were aseptically placed into sterile containers, and stored in an icebox.
Isolation and identification of B. cereus group
The B. cereus group was isolated in compliance with ISO 21871 (2006). In summary, 90 ml of 0.1% buffered peptone water (Oxoid, UK) was used to suspend 20 g of each sample for a duration of 50 minutes at room temperature. To achieve a final dilution of 10−1, an additional 90 ml of 0.1% peptone water was added. Homogenization was done for 30 seconds using the Stomacher 400 (Seward Pharma, Mfrs, and DistribUAC house, UK) for processing. Bacillus cereus selective agar base (Oxoid, UK) was used for selective plating, and it was then incubated for 24 hours at 37°C. New colonies from every pure culture were inspected for biotyping purposes catalase, gelatin liquefaction, citrate utilization, starch hydrolysis, motility, and hemolysis tests (Quinn et al., 2002). The detection of para sporal protein toxin crystal and rhizoid growth was also carried out (Tallent et al., 2012). Genotypic identification of the B. cereus group was applied using the genus (16S rRNA) and group-specific (plcR) primers in conventional PCR (cPCR) at the Animal Health Research Institute’s Biotechnology Unite in Zagazig, Egypt. The used primer sequences, amplicon sizes, and thermo-cycling conditions are shown in Table 1.
Table 1. Target genes, oligonucleotide primer sequences, amplicons, and cycling conditions used for PCR.
| Target gene | Specificity | Primer sequence (5’–3’) | Amplified product size (bp) | PCR cycling condition | Reference |
|---|---|---|---|---|---|
| 16S rRNA | Housekeeping gene | F: ACTGGGACTGAGACACGG R: GATAACGCTTGCCACCTA |
242 | First, denaturation for 5 minutes at 94°C was followed by 35 cycles of 30 seconds at 94°C, 1 minute at 52°C, and 30 seconds at 55°C. Finally, elongation took place for 7 minutes at 72°C. | Huang et al., 2021 |
| plcR | Pleiotropic regulator | F: ACCCGACATTAAAATCGTTTG R: TAGTATGCCTTGCGCAGTTG |
200 | First, denaturation for 2 minutes at 94°C was followed by 30 cycles of 30 seconds at 94°C, 1 minute at 52°C, and 30 seconds at 72°C. Finally, elongation took place for 10 minutes at 72°C. | Oltuszak-Walczak, et al., 2013 |
| tasA | Amyloid like fiber | F: AGCAGCTTTAGTTGG TGG AG R: GTA ACT TAT CGC CTT GGA ATTG |
488 | First, denaturation for 5 minutes at 94°C was followed by 40 cycles of 30 seconds at 94°C, 45 seconds at 59°C, and 45 seconds at 72°C. Finally, elongation took place for 5 minutes at 72°C. | Caro-Astorga et al., 2015 |
| sipW | Signal peptidase | F: AGA TAA TTA GCA ACG CGA TCTC R: AGA AAT AGC GGA ATA ACC AAGC |
488 | First, denaturation for 5 minutes at 94°C was followed by 40 cycles of 30 seconds at 94°C, 45 seconds at 54°C, and 45 seconds at 72°C. Finally, elongation took place for 5 minutes at 72°C. |
Antimicrobial susceptibility testing of B. cereus isolates
Using the Kirby-Bauer disc diffusion method, the susceptibility of B. cereus isolates to different antimicrobials was investigated. Mueller Hinton agar media (MHA, Oxoid, UK) and eighteen standard antimicrobial discs (Oxoid, UK) representing fifteen antimicrobial groups were used in the experiment. The antimicrobial discs contained the following: ampicillin (AMP, 10 μg), cephalothin (kF, 30 μg), cefotaxime (CTX, 30 µg), imipenem (IPM, 10 μg), ciprofloxacin (CIP, 5 μg), ofloxacin (OX, 1 μg), trimethoprim-sulfamethoxazole (SXT, 1.25 μg/23.75 μg), doxycycline (DO, 30 μg), erythromycin (E, 15 μg), clindamycin (DA, 2 μg), vancomycin (VA, 30 μg), colistin (CT, 10 μg), tigecycline (TGC, 15 μg), fosfomycin (FF, 50 μg), and rifampicin (RA, 30 μg) (Bauer et al., 1966). To determine the minimum inhibitory concentrations (MICs) of vancomycin and colistin (Sigma-Aldrich, Seelze, Germany), the broth microdilution assay (Rankin, 2005) was used. Due to the absence of B. cereus interpretative criteria in pertinent CLSI documents, the results of antimicrobial susceptibilities were interpreted in accordance with the S. aureus guidelines of the Clinical and Laboratory Standards Institute (Yu et al., 2019, CLSI, 2010). As previously reported (Tambekar et al., 2006), the multiple antimicrobial resistance (MAR) indices for every antibiotic and every isolate were computed. The strain of B. cereus ATCC®14579TM was employed as a quality control.
Apple cider vinegar
The ACV (5% acetic acid) used in this study was purchased commercially and kept chilled at 4°C until needed. It was provided by Gardens Company, Egypt. It has no fat or protein and is made up of 94% water, 5% acetic acid, and 1% carbohydrates.
Antimicrobial activity of ACV against B. cereus isolates
The antimicrobial activities of different ACV concentrations against MDR B. cereus isolates were determined using the agar well diffusion assay (Valgas et al., 2007). The suspension turbidity of pure bacterial cell culture was adjusted to McFarland standard No. 0.5. (1–1.5 × 108 colony forming units/ml) using 0.85% physiological saline (Oxoid, UK). A bacterial suspension containing 100 μl was grown on MHA (Oxoid, UK) plates. Using a cork borer (7 mm in diameter), wells were made in the agar plate and then filled with 100 μl of prepared ACV at concentrations of 100%, 80%, 60%, 40%, and 20%. Controls were included, both positive (IPM, 10 μg) and negative (sterile distilled water). For 24 hours, the plates were incubated at 35°C. Millimeters were used to measure the diameters of the growth inhibition zones surrounding the wells. According to Choi et al. (2016), the isolates were categorized as resistant (0) for diameters less than 8 mm, moderately sensitive (+) for diameters between 8 and 20 mm, sensitive (++) for diameters between 20 and 30 mm, and very sensitive (+++) for diameters larger than 30 mm.
With the use of the broth microdilution assay, the MIC of ACV was ascertained (Rankin, 2005). Bacillus cereus inoculum size of approximately 5 × 105 CFU/ml was prepared in the appropriate broth culture media. Twofold serial dilutions of ACV were prepared in sterile distilled water from the stock solution (1,024 μg/ml). Aliquots (100 μl) of each ACV dilution were dispensed in sterile polystyrene, U-shaped bottom, 96-well culture plates (Techno Plastic Products, Switzerland). Each well received 100 µl of each bacterial suspension, which was then incubated for 24 hours at 37°C. After determining the ACV›s MIC and minimum bactericidal concentration (MBC) (Kwiecinski et al., 2009), an orderly array method was used to calculate the MIC50 and MIC90 (Hamilton-Miller, 1991). The examined antibacterial agent has bacteriostatic activity when a ratio of MBC to MIC ≥ 4 where MBC is more than two dilutions of the MIC (Pankey and Sabath, 2004). Meanwhile, it is considered bacteriocidal if MBC/MIC = ≤4 where MBC is within two dilutions of the MIC.
Phenotypic detection of B. cereus biofilm
As previously mentioned, the Congo red agar method was used to qualitatively detect B. cereus biofilm (Reid, 1999). Biofilm production was shown by black colonies with a dry crystalline consistency. In addition, the microtiter plate method was used to quantitatively detect the formation of biofilms using flat-bottom microtiter plates (Techno Plastic Products, Switzerland) (Adame-Gómez et al., 2020). In summary, isolates of B. cereus were cultured in tryptic soy broth (TSB; Oxoid, UK) at 37 °C. Following a 10-minute pelleting process at 6,000 × g, the bacterial cells were dissolved in 5 ml of fresh medium. Using an ELISA reader (stat fax 2100, USA), the optical densities (ODs) of the bacterial suspensions were measured and normalized to an absorbance of 1.00 at 600 nm. 200 μl of 0.1% aqueous crystal violet solution (Al-Gomhorya Company, Egypt) was added to each well, and the plates were left to stand for 15 minutes to quantify the biofilm. After that, the excess crystal violet was removed from the wells by washing them three times with sterile phosphate buffer saline (PBS). 200 μl of an 80:20 (v/v) mixture of ethyl alcohol and acetone (Al-Gomhorya Company, Egypt) was used to extract the crystal violet bound to the biofilm. The absorbance of the extracted crystal violet was then measured at 545 nm using an ELISA reader. As negative and positive controls, respectively, wells containing a noninoculated medium and a biofilm-producing bacterium (B. cereus ATCC®14579TM) were employed. Every biofilm assay was run in triplicate. The biofilm production was interpreted using the standards outlined by Stepanovic et al. (2007). These parameters define the OD cut-off value (ODc) as the negative control’s average OD plus three times its standard deviation (SD). The following standards were used to categorize the B. cereus isolate’s capacity to form biofilm: ODc < OD ≤ 2× ODc = Weak biofilm producer, 2× ODc < OD ≤ 4× ODc = Moderate biofilm producer, and 4× ODc < OD = Strong biofilm producer. OD ≤ ODc = Not a biofilm producer.
Antibiofilm activities of ACV
The antibiofilm activities of different concentrations of ACV were applied using the crystal violet staining assay as a trial to control B. cereus biofilm formation and to prevent bacterial colonization. In brief, a 96-well polystyrene microtiter plate with a flat bottom was selected, and 100 μl of MHB was added to each well. Following a double-fold serial dilution of 100 μl of ACV, 100 μl of B. cereus suspension (106 cells/ml) was added. The plates were allowed to form a biofilm by being incubated for 24 hours at 37°C. The wells’ contents were removed using an aspirator and three times cleaned in sterile PBS. The negative control (ACV well without B. cereus suspension) and the positive controls (wells containing B. cereus without ACV) were included. Three duplicates of each experiment were run. Biofilm inhibition % = (Control OD545 nm − Test OD545 nm)/(Control OD545 nm) × 100 was the formula used to determine the biofilm inhibition percentage (Raja et al., 2011). In addition, the minimal antimicrobial concentration was defined as the minimal biofilm inhibitory concentration (MBIC), which makes inhibition of the biofilm formation. Moreover, MBIC50 and MBIC90 of the antibacterial agents which are the lower concentrations of the antibacterial agent showing 50% or 90% reduction in biofilm formation, respectively, were also calculated (Raja et al., 2011).
The relative expression of biofilm biosynthesis genes and their regulator using SYPR Green RT-qPCR
Strong biofilm producers after being exposed to ACV’s MBIC, B. cereus isolates were incubated for 12 hours at 37°C. Biofilm producers of B. cereus that are not treated are used as controls. After being collected, the biofilms were gently cleaned with PBS to remove any nonadherent cells. Following the manufacturer’s instructions, total RNAs were extracted from the biofilms of both treated and untreated isolates using a QIAamp RNeasy Mini kit (Qiagen, Germany). By employing oligonucleotide primer sets and cycling conditions as specified in Table 1, the relative expression levels of the genes responsible for biofilm biosynthesis (tasA and sipW) and their regulator (plcR) were ascertained. Following the manufacturer’s instructions, RT-qPCR was performed in triplicate in the real-time PCR thermal cycler (MX3005P, Stratagene, La Jolla, CA) using the QuantiTect SYBR Green Kit (Qiagen, Germany). Melting curves were created to confirm the reaction’s specificity. The constitutive expression of the 16sRNA housekeeping gene served as a reference for normalizing the relative expression levels of the investigated genes. The comparative 2−ΔΔCT method (Livak Schmittgen, 2003) was used to determine the transcript levels fold changes of tested genes in treated B. cereus biofilm producers in relation to their levels in the untreated ones.
Statistical analysis
The GraphPad Prism 8 software (GraphPad Software, San Diego, CA) was used to analyze the data. The statistical differences in the number of positive isolates and antimicrobial resistance in B. cereus isolates recovered from meat and meat products were assessed using Pearson’s chi-square and Kruskal–Wallis tests. Analysis was conducted using the unpaired t-test for comparison of the fold change of gene expression between plcR, tasA, and sipW genes (in the presence of ACV) and the control ones (without ACV) (Yuan et al., 2006). The numerical data are shown as means ± standard errors (SEs) (Duncan, 1955). At p-values < 0.05, significant differences were taken into account (Yuan et al., 2006).
Results
Isolation and identification of B. cereus
Microbiological examination revealed 34 B. cereus isolates out of 150 (22.67%) examined meats and their derivatives. The higher recovery rate of B. cereus was reported in sausage (33.3%) followed by beef kofta (24%), and minced meat (22.5%) (Table 2). Nonsignificant differences were found in the amount of B. cereus recovered from different meat products by statistical analysis (p ˃ 0.05).
Table 2. Frequency of B. cereus in meat and meat products.
| Sample type (no.) | No. of B. cereus isolates (%) * | p-value |
|---|---|---|
| Minced meat (40) | 9 (22.5) | 0.936 |
| Shawarma (30) | 6 (20) | |
| Beef burger (25) | 5 (20) | |
| Beef kofta (25) | 6 (24) | |
| Beef luncheon (15) | 3 (20) | |
| Sausage (15) | 5 (33.3) | |
| Total (150) | 34 (22.67) |
* Number (%) of positive isolates relative to each source. p-values > 0.05 are statistically nonsignificant.
Bacillus cereus isolates were identified on B. cereus selective agar media by their crenated colonial morphology. The isolates have a typical peacock-blue colour enclosed by strong precipitation of the egg yolk lecithin giving a hazy turquoise zone with no mannitol fermentation (positive Nagler’s reaction). All the recovered isolates were hemolytic, could not grow at 6°C for 28 days, and did not show the rhizoid colonial appearance of Bacillus mycoides and Bacillus pseudomycoide as long hair-like colonies on nutrient agar plates. Biochemical identification revealed that isolates of B. cereus were motile and positive for citrate utilization, catalase, starch hydrolysis, and gelatin liquefaction. The isolates were free from protein crystals of B. thuringiensis after carbol fuchsin Zi ehl–Neelsen staining. Within the B. cereus group, there is currently only one isolated and recognized species, B. cereus. All isolates of B. cereus with phenotypically suspicion were subsequently subjected to cPCR analysis, which relied on the identification of specific species (plcR) and genus (16S rRNA) primers that produced amplicons at 242 and 200 bp, respectively.
Antibiogram of B. cereus isolates
Thirty-four B. cereus isolates were tested for in vitro antimicrobial susceptibility to 18 different antimicrobial agents. The results showed that all of the isolates were sensitive to ciprofloxacin and imipenem (100%, each) followed by clindamycin (26.47%), tobramycin (20.59%), vancomycin (20.59%), rifampicin (17.60%), and gentamicin (14.70%). However, all of the B. cereus isolates were resistant to the following drugs: ampicillin and colistin (100%, each), cefotaxime (94.12%), sulfamethoxazole-trimethoprim (91.20%), cephalothin and fosfomycin (91.18% each), chloramphenicol, erythromycin and tigecycline (88.23% each), ofloxacin (85.29%) and doxycycline (82.35%). It is interesting to note that every B. cereus isolate had an MDR (resistant to at least three antimicrobial classes) MAR index, ranging from 0.55 to 0.77, which was significantly higher than 0.3 (Table 3). Statistical analysis exposed a significant difference (p < 0.05) in the resistance of B. cereus isolates to tested antimicrobials. The frequency of B. cereus susceptibility to selected antimicrobial agents and their resistance pattern is shown in Table 4.
Table 3. Antibiogram of the B. cereus isolates recovered from meat and meat products.
| Isolate No. | Source | Antimicrobial resistance pattern | MAR index |
|---|---|---|---|
| 1 | Shawarma | SXT, DO, AMP, VA, CT, E, OX, KF, C, CN, TCG, FF | 0.667 |
| 2 | Minced meat | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, CN, TCG, FF | 0.778 |
| 3 | Beef kofta | SXT, DO, AMP, CT, DA, E, CTX, KF, C, CN, TCG, FF, TOB, RA | 0.778 |
| 4 | Sausage | SXT, DO, AMP, VA, CT, CTX, OX, KF, C, CN, TCG, FF | 0.667 |
| 5 | Shawarma | AMP, VA, CT, DA, E, CTX, OX, KF, C, TCG, FF, TOB, RA | 0.722 |
| 6 | Beef kofta | SXT, AMP, CT, DA, E, CTX, KF, C, TCG, FF, TOB | 0.611 |
| 7 | Minced meat | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, TCG, FF, RA | 0.778 |
| 8 | Beef burger | SXT, DO, AMP, VA, CT, DA, E, OX, KF, C, TCG, FF | 0.667 |
| 9 | Minced meat | SXT, DO, AMP, CT, DA, E, CTX, OX, KF, C, TCG, FF | 0.667 |
| 10 | Shawarma | SXT, DO, AMP, VA, CT, E, CTX, KF, C, TCG, FF, RA | 0.667 |
| 11 | Beef burger | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, TCG, FF, RA | 0.778 |
| 12 | Minced meat | DO, AMP, CT, DA, E, CTX, OX, KF, C, TCG, FF, TOB | 0.611 |
| 13 | Sausage | SXT, DO, AMP, VA, CT, E, CTX, KF, C, TCG, FF | 0.667 |
| 14 | Sausage | SXT, DO, AMP, VA, CT, DA, CTX, OX, KF, C, TCG, FF | 0.611 |
| 15 | Beef kofta | SXT, DO, AMP, VA, CT, E, CTX, OX, KF, C, TCG | 0.611 |
| 16 | Shawarma | SXT, DO, AMP, CT, DA, E, CTX, OX, KF, C | 0.556 |
| 17 | Beef luncheon | SXT, AMP, VA, CT, DA, E, CTX, OX, KF, C, RA | 0.611 |
| 18 | Minced meat | DO, AMP, VA, CT, E, CTX, OX, KF, C, FF, RA | 0.611 |
| 19 | Sausage | SXT, AMP, VA, CT, DA, E, CTX, OX, KF, C, TCG, FF, RA | 0.722 |
| 20 | Beef burger | AMP, VA, CT, E, CTX, OX, KF, C, TCG, FF | 0.556 |
| 21 | Minced meat | SXT, DO, AMP, CT, DA, E, CTX, OX, KF, C, TCG, FF | 0.667 |
| 22 | Beef kofta | SXT, DO, AMP, VA, CT, CTX, OX, KF, C, TCG, FF | 0.611 |
| 23 | Beef kofta | SXT, AMP, CT, DA, E, CTX, OX, KF, C, FF | 0.556 |
| 24 | Beef burger | SXT, DO, AMP, VA, CT, E, CTX, OX, KF, C, TCG, FF | 0.667 |
| 25 | Minced meat | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, TCG, FF | 0.722 |
| 26 | Beef luncheon | SXT, DO, AMP, VA, CT, E, CTX, OX, KF, CN, TCG, FF | 0.667 |
| 27 | Minced meat | SXT, DO, AMP, VA, CT, E, CTX, OX, KF, CN, TCG, FF | 0.667 |
| 28 | Beef burger | SXT, DO, AMP, CT, E, CTX, OX, KF, CN, TCG, FF | 0.611 |
| 29 | Shawarma | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, CN, TCG, FF | 0.778 |
| 30 | Beef luncheon | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, KF, C, CN, TCG, FF | 0.778 |
| 31 | Shawarma | SXT, DO, AMP, CT, E, CTX, OX, C, TCG, FF | 0.556 |
| 32 | Beef kofta | SXT, DO, AMP, VA, CT, DA, CTX, OX, KF, CN, TCG, FF | 0.667 |
| 33 | Sausage | SXT, DO, AMP, VA, CT, DA, E, CTX, OX, C, CN, TCG, FF | 0.722 |
| 34 | Minced meat | SXT, DO, AMP, VA, CT, E, CTX, OX, C, TCG, FF | 0.611 |
SXT, Trimethoprim/sulfamethaxole; DO, Doxycycline; AMP, ampicillin; VA, Vancomycin; CT, Colistin; DA, Clindamycin; E, Erythromycin; CTX, Cefotaxime; OX, Ofloxacin; KF, Cephalothin; C, Chloramphenicol; CN, Gentamicin; TGC, Tigecycline; FF, Fosfomycin; TOB, Tobramycin and RA, Rifampicin. MAR, Multiple antibiotic resistance.
Table 4. Frequency of antimicrobial resistance in B. cereus isolates (n = 34) recovered from meat and meat products.
| Antimicrobial group | Antimicrobial agent | No. (%) of resistant B. cereus | MAR index | p-value |
|---|---|---|---|---|
| Penicillin | AMP | 34 (100.00) | 0.056 | 0.001 |
| Cephalosporin | KF CTX |
31 (91.18) 32 (94.12) |
0.051 0.052 |
|
| Carbapenem | IMP | 0 (00.00) | 0.00 | |
| Fluoroquinolones | CIP OX |
0 (00.00) 29 (85.29) |
0.00 0.047 |
|
| Sulphonamides | SXT | 31 (91.18) | 0.051 | |
| Tetracyclines | DO | 28 (82.35) | 0.046 | |
| Macrolides | E | 30 (88.24) | 0.049 | |
| Lincosamides | DA | 20 (58.82) | 0.033 | |
| Aminoglycosides | CN TOB |
11 (32.35) 4 (11.76) |
0.018 0.007 |
|
| Glycopeptides | VA | 25 (73.53) | 0.041 | |
| Phenicoles | C | 30 (88.24) | 0.049 | |
| Polymyxins | CT | 34 (100.00) | 0.056 | |
| Glycylcyclines | TGC | 30 (88.24) | 0.049 | |
| Phosphonics | FF | 31 (91.18) | 0.051 | |
| Ansamycins | RA | 8 (23.53) | 0.013 |
AMP, ampicillin; KF, Cephalothin; CTX, Cefotaxime; IPM, Imipenem; CIP, Ciprofloxacin; OX, Ofloxacin; SXT, Trimethoprim/sulfamethaxole; DO, Doxycycline; E, Erythromycin; DA, Clindamycin; CN, Gentamicin; TOB, Tobramycin; VA, Vancomycin; C, Chloramphenicol; CT, Colistin; TGC, Tigecycline; FF, Fosfomycin and RA, Rifampicin; MAR, Multiple antibiotic resistance. p-values < 0.05 are statistically significant.
Antimicrobial activity of ACV against B. cereus
As presented in Table 5, all ACV concentrations (100%, 80%, 60%, 40%, and 20%) exhibited marked inhibitory activities against B. cereus isolates while applying the agar well diffusion assay. The inhibitory zone diameters ranged from 44–48 mm/100%, 42–46 mm/80%, 38–44 mm/60%, 37–42 mm/40%, and 36–39 mm 20% concentrations. To determine precisely the antimicrobial properties of ACV, MICs, and MBCs were necessarily performed on 12 MDR B. cereus isolates. The MIC results showed that ACV exhibited strong antimicrobial activities against examined isolates with MIC ranging from 2 to 8 μg/ml. MIC50 and MIC90 of ACV against the examined isolates are 4 and 8 μg/ml, respectively. Some of the examined isolates showed MBC value at the same MIC value, considering the AVC as a bacteriocidal agent.
Table 5. Inhibitory zone diameters, MIC, and MBC of ACV against MDR B. cereus isolates (n = 12) recovered from meat and meat products.
| Isolate no. | Source | Zone diameter (mm) against various ACV concentrations (%) | MIC (µg/ml) | MBC (µg/ml) | ||||
|---|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | 20 | ||||
| 1 | Mm 3 | 48 | 42 | 38 | 37 | 36 | 8 | 8 |
| 2 | Bk 4 | 45 | 43 | 41 | 39 | 37 | 2 | 8 |
| 3 | Sh 6 | 46 | 45 | 40 | 39 | 38 | 4 | 8 |
| 4 | Mm 8 | 47 | 46 | 44 | 38 | 36 | 4 | 8 |
| 5 | Bb 12 | 47 | 43 | 42 | 40 | 37 | 2 | 4 |
| 6 | S 20 | 48 | 45 | 43 | 40 | 38 | 2 | 4 |
| 7 | Mm 26 | 48 | 46 | 44 | 39 | 37 | 4 | 8 |
| 8 | BL 27 | 45 | 43 | 41 | 38 | 36 | 4 | 4 |
| 9 | Sh 30 | 44 | 43 | 42 | 40 | 38 | 2 | 4 |
| 10 | Bl 31 | 47 | 45 | 41 | 39 | 36 | 2 | 4 |
| 11 | Bk 33 | 47 | 46 | 43 | 42 | 37 | 4 | 8 |
| 12 | S 34 | 48 | 45 | 44 | 42 | 39 | 8 | 8 |
Mm: Minced meat, Sh: Shawarma, Bb: Beef burger, Bk: Beef kofta, Bl: Beef Luncheon, S: Sausage. MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; ACV, apple cider vinegar.
Biofilm production by B. cereus isolates
The qualitative recognition of biofilm on Congo red agar indicated that B. cereus biofilm producers (8/12; 66.67%) could alter transforming the media’s red hue into black because of sucrose consumption. On trypticase soya broth, 12 B. cereus isolates were cultured for quantitative detection of biofilm production. Three B. cereus isolates (25%) were strong biofilm producers, 3 (25%) were moderate, and 6 (50%) produced weak biofilm (Table 6). The results indicated that the quantitative detection of the biofilm is more accurate than the qualitative method using the Congo red agar.
Table 6. Biofilm production by B. cereus isolates.
| Isolate No. | Code | Qualitative detection | Biofilm production (OD) | Negative control (ODC) | Average reading |
|---|---|---|---|---|---|
| 1 | Mm 3 | Black | 2.784 | 0.691 | +++ |
| 2 | Bk 4 | Black | 0.925 | 0.462 | ++ |
| 3 | Sh 6 | Red | 0.709 | 0.589 | + |
| 4 | Mm 8 | Black | 0.642 | 0.421 | + |
| 5 | Bb 12 | Black | 1.301 | 0.649 | ++ |
| 6 | S 20 | Red | 0.587 | 0.344 | + |
| 7 | Mm 26 | Black | 1.054 | 0.524 | ++ |
| 8 | BL 27 | Red | 0.455 | 0.396 | + |
| 9 | Sh 30 | Black | 1.736 | 0.409 | +++ |
| 10 | Bl 31 | Red | 0.786 | 0.599 | + |
| 11 | Bk 33 | Black | 0.629 | 0.437 | + |
| 12 | S 34 | Black | 1.864 | 0.441 | +++ |
Mm: Minced meat, Sh: Shawarma, Bb: Beef burger, Bk: Beef kofta, Bl: Beef Luncheon, S: Sausage. OD, optical density; ODC, Cut-off optical density (OD of negative control + 3×SD of negative control); +, weak; ++, moderate; +++, strong. The data represents ELISA reading at OD at 545 nm. Black indicates biofilm production, while red indicates no biofilm production.
Antibiofilm activities of ACV against MDR B. cereus isolates
The ability of ACV to stop the development of biofilms in isolated B. cereus was investigated and the findings were compared with the untreated (positive control) B. cereus biofilm producer in addition to the negative control (Table 7). The inhibition of biofilm development in every isolate was expressed as antibiofilm activity and reported as inhibition or reduction %. Increasing the concentrations of the tested agent resulted in a significant (p ˂ 0.05) decrease in biofilm formation in all cases. The antibiofilm effect of ACV against biofilms produced by B. cereus isolates was found to be good, with over 50% inhibition of biofilm development. The most common minimum biofilm inhibitory concentration (MBIC50) was 4 μg/ml (Table7).
Table 7. Antibiofilm activity of ACV against B. cereus isolates.
| Isolate No. | Isolate code | Control +ve (OD) | OD of B. cereus biofilm ( inhibition %) against MICs of ACV (0.125–256 µg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 256 | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | p-value | |||
| 1 | Mm 3 | 2.784 | 0.006 (99.8) aA |
0.0272 (90.22) bB | 0.406 (85.41) cB |
0.579 (79.2) cC |
0.832 (70.1) aE |
0.909 (67.35) aF |
1.333 (52.1) bG |
1.506 (45.9%)dH |
1.0581 (43.21)cI |
1.658 (40.44) eK |
1.692 (39.22) bL |
1.714 (38.43)aM |
0.0001 |
| 2 | Bk 4 | 0.925 | 0.007 (99.21) aA |
0.097 (89.45) cB |
0.124 (86.56) bB |
0.227 (75.43) aD |
0.275 (70.21) aE |
0.356 (61.46) bF |
0.416 (54.96) aG |
0.447 (51.62) cG |
0.478 (48.31) cH |
0.518 (43.96) bK |
0.552 (40.32)aL |
0.569 (38.48) aM |
0.0001 |
| 3 | Sh 6 | 0.709 | 0.015 (97.83) bA |
0.0678 (90.44) bB |
0.131 (81. 56) aC |
0.208 (70.66) cD |
0.245 (65.42) cE |
0.288 (59.37) cF |
0.346 (51.24) cG |
0.359 (49.33) aG |
0.388 (45.27) bI |
0.408 (42.45) dK |
0.435 (38.64) cL |
0.459 (35.26) dM |
0.0001 |
| 4 | Mm 8 | 0.642 | 0.011 (98.23) bA |
0.073 (88.62) dB |
0.133 (79. 28) cC |
0.158 (75.38) aD |
0.194 (69.78) abE |
0.251 (60.90) bcF |
0.296 (53.89) abG |
0.317 (50.62) aH |
0.334 (47.97) cH |
0.362 (43.61) bK |
0.384 (40.19) aL |
0.395 (38.47) aM |
0.0001 |
| 5 | Bb 12 | 1.301 | 0.003 (99.80) aA |
0.096 (92.62) aA |
0.248 (80. 94) bC |
0.389 (70.10) cD |
0.513 (60.57) dE |
0.557 (57.19) dF |
0.633 (51.35) cG |
0.662 (49.12) bH |
0.699 (46.27) aI |
0.731 (43.81) bK |
0.788 (39.43) bL |
0.813 (37.51) bM |
0.0001 |
| 6 | S 20 | 0.587 | 0.005 (99.10) aA |
0.038 (93.52) aA |
0.109 (81. 43) aC |
0.144 (75.47) aD |
0.203 (65.42) bE |
0.233 (60.31) bcF |
0.279 (52.64) bG |
0.303 (48.38) bcH |
0.319 (45.66) bI |
0.333 (43.27) bK |
0.345 (41.13) aL |
0.357 (39.18) cL |
0.0001 |
| 7 | Mm 26 | 1.054 | 0.015 (98.58%) aA |
0.098 (90.70%) bB |
0.211 (79. 98) cC |
0.303 (71.25) bD |
0.399 (62.14) eE |
0.433 (58.92) dF |
0.508 (51.80) cG |
0.577 (47.15) cH |
0.591 (43.93) cI |
0.623 (40.89) eK |
0.647 (38.61) cL |
0.667 (36.72) cM |
0.0001 |
| 8 | Bl 27 | 0.455 | 0.009 (97.96%) bA |
0.037 (91.87) abB |
0.088 (80. 66) bC |
0.139 (69.45) dD |
0.174 (61.76) eE |
0.202 (55.60) eF |
0.227 (50.11) dG |
0.244 (46.37) cdH |
0.256 (43.73) cI |
0.266 (41.53) dK |
0.276 (39.34) bL |
0.285 (37.36) bM |
0.0001 |
| 9 | Sh 30 | 1.736 | 0.002 (99.88) aA |
0.169 (90.26) bB |
0.361 (79. 21) cC |
0.515 (70.33) cD |
0.628 (63.82) eE |
0.705 (59.39) cF |
0.803 (53.74) abG |
0.879 (49.37) bH |
0.918 (47.12) aI |
0.949 (45.33) cI |
0.990 (42.97) eK |
1.040 (40.10) bL |
0.0001 |
| 10 | Bl 31 | 0.786 | 0.004 (99.49) aA |
0.082 (89.57) cB |
0.167 (78. 75) dC |
0.241 (69.34) dD |
0.293 (62.72) dF |
0.329 (58.14) cdF |
0.358 (54.45) aG |
0.392 (50.13) bcG |
0.407 (48.22) cH |
0.432 (45.04) cI |
0.451 (42.62) eK |
0.476 (39.44) cL |
0.0001 |
| 11 | Bk 33 | 0.629 | 0.004 (99.36) aA |
0.071 (88.71) dB |
0.128 (79. 49) cC |
0.197 (68.68) dD |
0.262 (58.34) cF |
0.280 (55.48) eF |
0.311 (50.56) dG |
0.331 (47.38) cH |
0.345 (45.15)bI |
0.362 (42.45) cK |
0.375 (40.38) aL |
0.385 (38.79) aM |
0.0001 |
| 12 | S 34 | 1.864 | 0.010 (99.46) aA |
0.174 (90.65) bB |
0.344 (81. 52) aC |
0.540 (70.98) cD |
0.696 (62.60) eE |
0.759 (59.22)cdF |
0.862 (53.67) aG |
0.962 (48.30) cdH |
0.998 (46.37) abI |
1.030 (44.65) aK |
1.077 (42.12) eK |
1.177 (39.98) cL |
0.0001 |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |||
Mm: Minced meat, Sh: Shawarma, Bb: Beef burger, Bk: Beef kofta, Bl: Beef Luncheon, S: Sausage. OD: optical density. The data in the upper row of each isolate represent ELISA reading at OD 545 nm. The inhibition of biofilm formation of each isolate was stated as antibiofilm action (reduction or inhibition %) and was calculated as follows: (OD control-OD sample / OD control) *100; the inhibition % was indicated in the lower row of each isolate. The underlined OD numbers indicate nearly about 50% reduction in biofilm under MBIC50 of the antibacterial agents, which was defined as the lower concentration of the agent (µg/ml) which shows a 50% reduction in biofilm formation. Values in the same row carrying different capital superscripts or those that were defined in the same column carrying different small superscripts are significantly different (p < 0.05). **p-value < 0.05 is considered statistically different.
Transcriptional analysis of biofilm genes using RT-qPCR
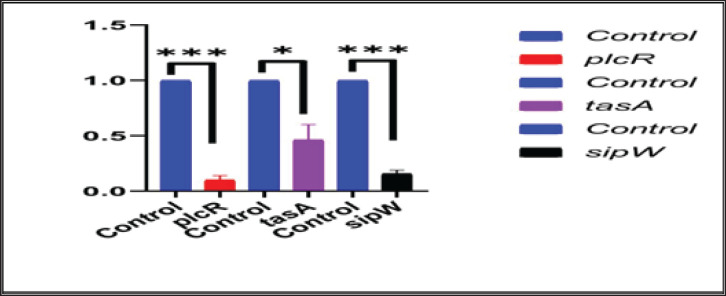
The relative expression of the pleiotropic regulator (plcR), amyloid such as fiber (tasA) and signal peptidase (sipW) genes were evaluated in MDR strong biofilm-producing B. cereus isolates by RT-qPCR. The relative expressions (fold-change of the expression levels) of the characteristic genes in MDR and adherent isolates after the addition of ACV as antibiofilm agent were matched with those in the control MDR and adherent isolates without the exposure to ACV. The 16S rRNA housekeeping gene was used for RT-qPCR normalization (Fig. 1). The results revealed down-regulation of biofilm genes in comparison to those of the control; this was highly significant (***) for plcR and sipW genes ( p < 0.0001), while was significant (*) with tasA gene (p < 0.05) (Table 8).
Fig. 1. Effect of ACV on the expression levels of B. cereus plcR, tasA, and sipW biofilm genes using RT-qPCR.
Table 8. Transcriptional analysis of biofilm genes using RT-qPCR after treatment with ACV.
| Gene | Control | Fold change | p-value |
|---|---|---|---|
| plcR | 1.00 ± 0.000 b | 0.1029 ± 0.03685 a*** | <0.0001 |
| tasA | 1.00 ± 0.000 b | 0.4642 ± 0.1368 a* | 0.0173 |
| sipW | 1.00 ± 0.000 b | 0.1596 ± 0.03004 a*** | <0.0001 |
Values bearing dissimilar superscripts (a,b) in the same row varied significantly (p < 0.05). Results were expressed as means ± SE.
Discussion
Biofilms can be produced by the B. cereus group of the genus Bacillus in a variety of beverage and food industry settings. Concerned about a significant source of post-process and recurrent cross-contamination in prepared foods that could lead to food poisoning or product degradation, biofilms are a major source of concern for the food trade. Vasudevan et al. (2003) state that biofilms are frequently to blame for recurrent infections. According to Wijman et al. (2007), control over biofilm formation is therefore dependent on providing mechanistic insight into the behavior of B. cereus in biofilms. Therefore, the main objectives of this study were to isolate, identify, and test for antibacterial susceptibility B. cereus that was isolated from samples of meat and meat products. The recovered B. cereus isolates were then used to detect biofilm formation both qualitatively and quantitatively. Furthermore, evaluation of ACV for a better understanding of its effect on the gene expression that is responsible for biofilm formation and its regulator, using RT-qPCR to reduce the drawbacks of antibiotic resistance.
The traditional identification methods were exposed as “presumptive B. cereus” because these methods could not distinguish B. cereus from other Bacillus group isolates (Vidic et al., 2020). Therefore, many laboratories’ stages should be performed to distinguish B. cereus from other Bacillus group species (Ramarao et al., 2020). The selective media and biochemical tests that these methods were designed to use are labor-intensive, time-consuming, and require specialized personnel (Zhu et al., 2016). However, molecular methods are more accurate for definitive identification. Herein, B. cereus was isolated from 34 out of 150 meat and meat product samples with an overall prevalence of 22.66%. Our results were consistent with those stated by Guven et al. (2006) (22.4%) and Amin and Tawfick (2021) (24%). On the other hand, higher percentages of B. cereus recovered from meat product samples were detected by Rather et al. (2011) (40%), Abd El Tawab et al. (2015) (38.33%), Yu et al. (2020) (35%), Tewari et al. (2015) (30.9%), Bashir et al. (2017) (29.33%), and Schlegelova et al. (2003) (28%). However, a lower prevalence rate was recorded by Konuma (1988) and Gharib et al. (2020) with percentages of 18.3% and 17.5%, respectively.
Bacillus cereus may be present in raw meat products because of unsanitary conditions during meat delivery, processing, or transportation. According to Floristean et al. (2007), improper storage temperatures of raw meat could potentially encourage the growth of bacteria. The processing of minced meat may be the reason for the high frequency of B. cereus isolation from edible meat products such as luncheons or the addition of expired additives, which can induce vegetation of Bacillus spores under insufficient heat treatment (Shawish and Tarabees, 2017).
Knowledge of antibiotic resistance patterns is of great alarm, as the major bacterial isolates may be highly resistant to frequently used antibacterial agents. Therefore, antimicrobial sensitivity testing is important to suggest suitable antibacterial agents for the treatment and prevention of resistance (Fiedler et al., 2019).
In this study, the B. cereus isolates exhibited 100% resistance to both ampicillin and colistin, next cefotaxime (94.12%), sulfamethoxazole-trimethoprim (91.20%), cephalothin and fosfomycin (91.18%, each), chloramphenicol, erythromycin and tigecycline (88.23%, each), ofloxacin (85.29%), and doxycycline (82.35%). On the other side, the highest sensitivity rates of B. cereus were reported for ciprofloxacin and imipenem (100%, each). Similar antimicrobial susceptibility results against B. cereus isolates were previously documented (El-Sayed, 2019; Solanki et al., 2019).
On the contrary, Fiedler et al. (2019) estimated the resistance of B. cereus to antibiotics and showed that it is highly resistant to penicillin G and cefotaxime (100%), ampicillin and amoxicillin/clavulanic acid combination (99.3%). However, these isolates were susceptible to ciprofloxacin, chloramphenicol, amikacin, imipenem, erythromycin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole by 99.3%, 98.6%, 98.0%, 93.9%, 91.8%, 88.4%, 76.2%, and 52.4%, respectively. In addition, Merzougui et al. (2014) indicated that the isolates B. cereus were resistant to penicillin, oxacillin, and cefepime (100%, each), ampicillin (98.4%), and tetracycline (90.6%), but they were susceptible to gentamicin, erythromycin, and chloramphenicol by 100%, 84.4%, and 67.2%, respectively.
According to Shalini and Rameshwar (2005), food can be thought of as the primary means of antibiotic-resistant bacteria spreading from humans to animals. One of the main causes of antibacterial resistance is excessive antibiotic exposure. The overuse of antibiotics in hospitals, agriculture, animal husbandry, and the general public, along with the freedom to buy antibiotics without a prescription and use them carelessly, may be the cause of the rise in antibiotic resistance. Serious and setting are most likely the main contributing factors to the widespread spread of nosocomial infections that are resistant to antibiotics and are difficult to treat in the health service (Fiedler et al., 2019).
The formation of biofilm is followed by significant alterations in the bacteria’s physiology and genetic makeup that reduce their susceptibility to practically all antibiotic classes (Melchior et al., 2006). Microorganisms are shielded from external aggression by it, and certain bacteria that form biofilms are resistant to antibiotics. Thus, the high antibiotic resistance of bacteria that form biofilms is intended to encourage the use of antimicrobial medications (Takaine et al., 2014) such as the use of substitute medicinal plants to treat diseases. Herein, 66.67% of B. cereus isolates (n = 12) were biofilm producers with black colonies on Congo red agar. Quantitatively, Six (50%), three (25%), and three (25%) produced weak, moderate, and strong biofilms, respectively, on tryptic soya broth in a microtitre plate. These findings came in parallel to those of several documents by JeeHoon and Beuchat (2005), Wijman et al. (2007), and Ozdemir and Arslan (2019) who reported B. cereus capability to form biofilms when examined either qualitatively or quantitatively.
A significant issue for food safety and human health is the formation of bacterial biofilms, which are produced by a variety of virulent bacteria. The most efficient way to prevent the formation of biofilm and stop bacterial growth when employing antimicrobial agents is to obstruct the formation of biofilm (Roy et al., 2018). Therefore, finding novel agents that can serve as an unconventional and alternate means of treating infections brought on by bacteria resistant to conventional treatments is crucial.
In the present work, vinegar solution (5% acetic acid) has a double effect, where it prevented the progress and formation of biofilm of B. cereus at MIC range 2–8 μg/ml. The ability of vinegar to reduce biofilm formation is positively related to its acetic acid antibacterial activity in this study and the use of marketable ACV reduced the viability of B. cereus and its biofilm-forming ability. Our result corresponded with those of Pedroso et al. (2018) who reported that ACV 70% reduced S. aureus biofilm formation; they added that acetic acid, which is abundant when the vinegar ferments and is included in 3%–5% concentration, influenced the biofilm formation. In addition, Halstead et al. (2015) revealed that 0.31% acetic acid inhibited the formation of biofilm by Pseudomonas aeruginosa. According to Tsang et al. (2018), biofilm-associated methicillin-sensitive S. aureus was eliminated by 5% and 3% acetic acid, respectively.
The inhibition zone diameter by the agar well diffusion assay revealed that ACV exhibited marked inhibitory activities against B. cereus at different concentrations with zone diameters ranging from 36 to 48 mm. These findings matched with other previous studies by Yagnik et al. (2021), Gaber et al. (2020), and Baldas and Altuner (2018). In addition, ACV had an antimicrobial effect on tested microorganisms such as V. cholerae, C. tropicalis, C. albicans, E. coli O157:H7, and S. typhi.
Our relative expression data of plcR, tasA, and sipW genes by RT-qPCR in strong biofilm-producing MDR B. cereus isolates in the presence of ACV compared to those the control without ACV where the used ACV was considered as an antibiofilm agent. The results revealed the down-regulation of biofilm genes in comparison to those of the control. Caro-Astorga et al. (2015) have documented the involvement of various genes in the formation of biofilms, including the sipW and tasA genes. The production of tasA has been linked to the formation of amyloid-like fibrils, which in turn cause foliar biofilms in Bacillus species. When it comes to sipW, it codes for a protease that helps process tasA. Without a doubt, B. cereus’s persistence in food trade equipment is largely due to its presence in biofilms as the biofilm protects vegetative cells and spores from sanitizer’s inactivation (Wijman et al., 2007). This is the first report locally and internationally, where no studies were conducted on the ACV effect on biofilm gene expressions (plcR, tasA, and sipW), and limited studies only highlighted its effect on bacterial biofilm formation.
Conclusion
The high isolation rate of MDR B. cereus from meat and meat products in Egypt was highlighted by this study. Moreover, using ACV as an antibiofilm agent might offer a valuable method to lower the hazard of B. cereus infection and its persistence in the food industry either in public spaces or at home.
Author contributions
Rana M. Mahmoud, Ahlam A. Gharib, Norhan K Abd El-Aziz, and Ahmed M. Ammar designed the research. The microbiological techniques were performed by Rana M. Mahmoud. The molecular analyses and data analysis were performed by Rana M. Mahmoud, Ahlam A. Gharib, and Norhan K. Abd El-Aziz. The study was conceived and designed by Ahmed M. Ammar, El-Shaimaa Mesallam Ali, Aml Mokhtar, and Ghada A. Ibrahim. Norhan K. Abd El-Aziz, Ahlam A. Gharib, and Rana M. Mahmoud wrote the manuscript’s first draft. The final manuscript was approved by all authors.
Funding
There was no special grant or funding for this research.
Data availability
The manuscript contains the data that support the study’s conclusions. Upon a reasonable request, the corresponding author will provide any additional data.
References
- Abd El Tawab A.A, Maarouf A.A, El-Hofy F.I, El-Said A.A. Bacteriological studies on some foodborne bacteria isolated from chicken meat and meat products in Kaliobia Governorate. BVMJ. 2015;29(2):47–59. [Google Scholar]
- Adame-Gómez R, Itzel-Maralhi C.F, Lilia-Lizette G.D, Yesenia R.S, Abigail P.V, Carlos O.P, Maria-Cristina S.D, Arturo R.P. Biofilm production by enterotoxigenic strains of Bacillus cereus in different materials and under different environmental conditions. Microorganisms. 2020;8(7):1071. doi: 10.3390/microorganisms8071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin H.M, Tawfick M.M. High risk of potential diarrheagenic Bacillus cereus in diverse food products in Egypt. J. Food Prot. 2021;84(6):1033–1039. doi: 10.4315/JFP-20-384. [DOI] [PubMed] [Google Scholar]
- Ayako K, Hideaki H, Tomoe S, Satowa S. Baseline and seasonal trends of Bacillus cereus and Bacillus subtilis from clinical samples in Japan. Infect. Prev. Pract. 2023;5(2):100272. doi: 10.1016/j.infpip.2023.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldas B, Altuner E.M. The antimicrobial activity of apple cider vinegar and grape vinegar, which are used as a traditional surface disinfectant for fruits and vegetables. Commun. Fac. Sci. Univ. Ankara Ser. C Biol. 2018;27:110. [Google Scholar]
- Bashir M, Malik M.A, Moien J.M, Badroo G.A, Bhat Altaf M, Singh M. Prevalence and characterization of Bacillus cereus in meat and meat products in and around Jammu region of Jammu and Kashmir, India. Int. J. Curr. Microbiol. App. Sci. 2017;6(12):1094–1106. [Google Scholar]
- Bauer A.W, Kirby W.M, Sherris J.C, Turk M. Antibiotic susceptibility testing by the standard single disc method. A. M. J. Clin. Pathol. 1966;45:493–446. [PubMed] [Google Scholar]
- Boonyayatra S, Pata P, Nakharuthai P, Chaisri W. Antimicrobial resistance of biofilm-forming Streptococcus agalactiae isolated from bovine mastitis. J. Vet. Sci. Technol. 2016;7:5. [Google Scholar]
- Budak H.N. Alteration of antioxidant activity and total phenolic content during the eight-week fermentation of apple cider vinegar. HortiS. 2021;38(1):39–45. [Google Scholar]
- Caro-Astorga J, Pérez-García A, De Vicente A, Romero D. Agenomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2015;5:745. doi: 10.3389/fmicb.2014.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens S, Uyttendaele M, Drieskens K, Heyndrickx M, Rajkovic A, Boon N, Van de Wiele T. Survival and germination of Bacillus cereus spores without outgrowth or enterotoxin production during in vitro simulation of gastrointestinal transit. Appl. Environ. Microbiol. 2012;78:7698–7705. doi: 10.1128/AEM.02142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O, Choi S.K, Kim J, Park C.G. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria. Asian Pac. J. Trop. Biomed. 2016;4:308–314. [Google Scholar]
- CLSI. 12th. Wayne, PA: M100-M60; 2010. Performance standards for antimicrobial susceptibility testing. Available via www.clsi.org . [Google Scholar]
- Dubois T, Faegri K, Perchat S, Lemy C, Buisson C, Nielsen-LeRoux C, Gohar M, Jacques P, Ramarao N, Kolstø A.B. Necrotrophism is a quorum-sensing-regulated lifestyle in Bacillus thuringiensis. PLoS Pathog. 2012;8:e1002629. doi: 10.1371/journal.ppat.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D.B. Multiple range and multiple F-test. Biometrics. 1955;11:124. [Google Scholar]
- El-Sayed A.M.A. 2019. Bacteriological and molecular studies on antimicrobial resistant bacteria isolated from meat and meat products. Master’s thesis, Faculty of Veterinary Medicine, Benha Univeristy, Benha, Egypt. [Google Scholar]
- Felipe V, Morgante C.A, Somale P.S, Varroni F, Zingaretti M.L, Bachetti R.A. Evaluation of the biofilm forming ability and its associated genes in Staphylococcus species isolates from bovine mastitis. Microb Pathog. 2017;104:278–286. doi: 10.1016/j.micpath.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Fiedler G, Schneider C, Igbinosa E, Kabisch J, Brinks E, Becker B, Stoll D, Cho G, Huch M, Franz C. Antibiotics resistance and toxin profiles of Bacillus cereus group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019;19(1):1–13. doi: 10.1186/s12866-019-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floristean V, Cretu C, Carp-Carare M. Bacteriological characteristics of Bacillus cereus isolates from poultry. Bull. USAMVCN. 2007;64(1–2):425–430. [Google Scholar]
- Gaber S.N, Bassyouni R.H, Masoud M, Ahmed F.A. Promising anti-microbial effect of apple vinegar as a natural decolonizing agent in healthcare workers. Alexandria J. Med. 2020;56(1):73–80. [Google Scholar]
- Gharib A.A, Abd El-Hamid M.A, Ayob E. Physiological and molecular studies on thermotolerance of Bacillus cereus isolated from some dairy products and fast foods. Zag. Vet. J. 2020;48(4):354–365. [Google Scholar]
- Goerke C, Bayer M.G, Wolz C. “Quantification of bacterial transcripts during infection using competitive reverse transcription-PCR (RT-PCR) and Light Cycler RTPCR. Clin. Diagn. Lab. Immunol. 2001;8(2):279–282. doi: 10.1128/CDLI.8.2.279-282.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güven K, Mutlu M.B, Avci Ö. Incidence and characterization of Bacillus cereus in meat and meat products consumed in Turkey. J. Food Safety. 2006;26(1):30–40. [Google Scholar]
- Halstead F.D, Rauf M, Moiemen N.S, Bamford A, Wearn C.M, Fraise A.P, Lund P.A, Oppenheim B.A, Webber M.A. The antibacterial activity of acetic acid against biofilm producin pathogens of relevance to burns patients. PLoS One. 2015;10(9):e0136190. doi: 10.1371/journal.pone.0136190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J.M.T. Calculating MIC50. J. Antimicrob. Chemother. 1991;27(6):863–875. doi: 10.1093/jac/27.6.863. [DOI] [PubMed] [Google Scholar]
- Huang X.D, Lu D.M, Ricciuto P.J, Hanson A.D, Richardson X, Lu E, Weng S, Nie L, Jiang E, Hou I.F, Steinmacher, Luo Y. A model-independent data assimilation (MIDA) module and its applications in ecology. Geosci. Model Dev. 2021;14(8):5217–5238. [Google Scholar]
- ISO. 21871. Geneva, Switzerland: International Organization for Standardization; 2006. Standard Microbiology of food and feeding stuffs. Horizontal method for the determination of low numbers of presumptive Bacillus cereus; p. 14. ISO—Bacillus cereus. [Google Scholar]
- Jee-Hoon R, Beuchat L. Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid–based Sanitizer. J. Food Protection. 2005;68:2614–2622. doi: 10.4315/0362-028x-68.12.2614. [DOI] [PubMed] [Google Scholar]
- Konuma H, Shinagawa K, Tokumar M, Onoue Y, Konno S, Fujino N, Shigehisa T, Kurata H, Kuwabara Y, Lopes C.A.M. Occurrence of B. cereus in meat products, raw meat and meat product additives. J. Food. Prot. 1988;51(4):324–326. doi: 10.4315/0362-028X-51.4.324. [DOI] [PubMed] [Google Scholar]
- Kwiecinski J, Eick S, Wojcik K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents. 2009;33(4):343–347. doi: 10.1016/j.ijantimicag.2008.08.028. [DOI] [PubMed] [Google Scholar]
- Li X.H, Lee J.H. Antibiofilm agents: a new perspective for antimicrobial strategy. J. Microbiol. 2017;55:753–766. doi: 10.1007/s12275-017-7274-x. [DOI] [PubMed] [Google Scholar]
- Lindsay D, Brozel V.S, Mostert J.F, von Holy A. Physiology of dairy associated Bacillus spp. over a wide pH range. Int. J. Food Microbia. 2000;54:49–62. doi: 10.1016/s0168-1605(99)00178-6. [DOI] [PubMed] [Google Scholar]
- Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−delta delta C (T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Melchior M.B, Vaarkamp H, Fink-Gremmels J. Biofilms: arole in recurrent mastitis infections? Vet. J. 2006;171:398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Merzougui S, Lkhider M, Grosset N, Gautier M, Cohen N. Prevalence, PFGE typing, and antibiotic resistance of Bacillus cereus group isolated from food in Morocco. Food borne Pathog. Dis. 2014;11(2):145–149. doi: 10.1089/fpd.2013.1615. [DOI] [PubMed] [Google Scholar]
- Oltuszak-Walczak E, Walczak P. PCR detection of cytK gene in Bacillus cereus group strains isolated from food samples. J. Microbiol. Methods. 2013;95:295–301. doi: 10.1016/j.mimet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Özdemir F, Arslan S. Biofilm production and antimicrobial susceptibility profiles of Bacillus spp. from meats. Sakarya Univ. J. Sci. 2019;22(6):1674–1682. [Google Scholar]
- Pankey G.A, Sabath L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram positive bacterial infections. Clin. Infect. Dis. 2004;38(6):864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- Pedroso J.D.F, Sangalli J, Brighenti F.L, Tanaka M.H, KogaIto C.Y. Control of bacterial biofilms formed on pacifiers byantimicrobial solutions in spray. Int. J. Paed. Dent. 2018;28(6):578–586. doi: 10.1111/ipd.12413. [DOI] [PubMed] [Google Scholar]
- Quinn P.J, Markey B.K, Carter M.E, Donnelly W.J, Leonard F.C, Meguire D. 2nd. Ames, Iowa, USA: Blackwell Science, Iowa State University Press; 2002. Veterinary microbiology and microbial disease; pp. 84–96. [Google Scholar]
- Raja A, Ali F, Khan I. Anti staphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011;11:54–62. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao N, Tran S.L, Marin M, Vidic J. Advanced methods for detection of Bacillus cereus and its pathogenic factors. Sensors. 2020;20(9):1–23. doi: 10.3390/s20092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin D.I. In: Manual of antimicrobial susceptibility testing american society for microbiology. 1st. USA: California Plaza Omaha; 2005. Test methods: MIC testing; pp. 53–62. [Google Scholar]
- Rather A.M, Aulakh R.S, Gill J.P.S, Rao S.T, Hassan N.M. Direct detection of Bacillus cereus and its enterotoxigenic genes in meat and meat products by polymerase chain reaction. J. Adv. Vet. Res. 2011;1:99–104. [Google Scholar]
- Reid G. Biofilms in infectious disease and on medical devices. Int. J. Antimicrob. Agents. 1999;11:223–226. doi: 10.1016/s0924-8579(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönborn S, Wente N, Paduch J.H. In vitro ability of mastitis causing pathogens to form biofilms. J. Dairy Res. 2017;84:198–201. doi: 10.1017/S0022029917000218. [DOI] [PubMed] [Google Scholar]
- Schlegelova J, Brychta J, Klimova E, Napravnikova E, Babak V. Prevalence and resistance to antimicrobial agents of Bacillus cereus isolates from foodstuffs. Vet. Med. Czech. 2003;48(11):331–338. [Google Scholar]
- Shalini M, Rameshwar S. Antibiotic resistance in food lactic acid bacteria—a review. Int. J. Food Microbiol. 2005;105:281–295. doi: 10.1016/j.ijfoodmicro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Shawish R, Tarabees R. Prevalence and antimicrobial resistance of Bacillus cereus isolated from beef products in Egypt. Open Vet. J. 2017;7(4):337–341. doi: 10.4314/ovj.v7i4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki K.S, Parmar B.C, Brahmbhatt M.N, Nayak J.B, Begadiya H.B. Cultural and biochemical characterization of B. cereus isolates and multidrug resistant detection of B. cereus isolates collected from various chicken shops of market in and around Anand, Gujarat, India. Int. J. Curr. Microbiol. App. Sci. 2019;8(3):910–915. [Google Scholar]
- Stepanovic S, Vukovi D, Hola V. Quantification of bio-film in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. Acta. Pathol. Microbiol. Immunol. Scand. 2007;115(8):891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- Tallent S.M, Kotewicz K.M, Strain E.A, Bennett R.W. Efficient isolation and identification of Bacillus cereus group. J. AOAC Int. 2012;95(2):446–451. doi: 10.5740/jaoacint.11-251. [DOI] [PubMed] [Google Scholar]
- Takaine M, Imada K, Numata O, Nakamura T, Nakano K. The meiosis-specific nuclear passenger protein is required for proper assembly of fore spore membrane in fission yeast. J. Cell Sci. 2014;127:4429–4442. doi: 10.1242/jcs.151738. [DOI] [PubMed] [Google Scholar]
- Tambekar D, Dhanorkar D, Gulhane S, Khandelwal V, Dudhane M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006;5:1562–1565. [Google Scholar]
- Tewari A, Singh P.S, Singh R. Prevalence of multidrug Resistant Bacillus cereus in foods and human stool samples in and around Pantnagar, Uttrakhand. J. Adv. Vet. Res. 2012;2:252–255. [Google Scholar]
- Tewari A, Singh S.P, Singh R. Incidence and enterotoxigenic profile of Bacillus cereus in meat and meat products of Uttarakhand, India. J. Food Sci. Technol. 2015;52:1796–1801. doi: 10.1007/s13197-013-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S.T.J, Gwynne P.J, Gallagher M.P, Simpson A.H.R. The biofilm eradication activity of acetic acid in the management of periprosthetic joint infection. Bone Joint Res. 2018;7(8):517–523. doi: 10.1302/2046-3758.78.BJR-2018-0045.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Jong A, Moyaert H, Simjee S, Garch F.E, Morrissey I. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: Vet Path result. Int. J. Antimicrob. Agents. 2015;46:13–20. doi: 10.1016/j.ijantimicag.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Valgas C, Machado de Souza S, Smânia E.F.A, Smânia A.J.R. Screening methods to determine antibacterial activity of natural products. Brazilian J. Microbiol. 2007;38(2):369–380. [Google Scholar]
- Vasudevan P, Nair M.K, Annamalai T. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003;92:179–185. doi: 10.1016/s0378-1135(02)00360-7. [DOI] [PubMed] [Google Scholar]
- Vidic J, Chaix C, Manzano M, Heyndrickx M. Food sensing: detection of Bacillus cereus spores in dairy products. Biosensors. 2020;10(3):1–16. doi: 10.3390/bios10030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C.M, Bassler B.L. Quorum sensing: cell-to-cell communication in bacteria. Ann. Rev. Cell Develop. Biol. 2005;21(1):319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Watson R.R, Preedy V.R, Zibadi S. 2nd. London, UK: Academic Press; 2018. Polyphenols: Mechanisms of action in human health and disease. [Google Scholar]
- Wijman J.G, de Leeuw P.P, Moezelaar R, Zwietering M.H, Abee T. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation, and dispersion. Appl. Environ. Microbio. 2007;73:1481–1488. doi: 10.1128/AEM.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Zhang B, Duan W, Zhang J, Wang M. Nutrients and bioactive components from vinegar: a fermented and functional food. J. Funct. Foods. 2020;64:103681. [Google Scholar]
- Yagnik D, Ward M, Shah A.J. Antibacterial apple cider vinegar eradicates methicillin resistant Staphylococcus aureus and resistant Escherichia coli. Sci. Rep. 2021;11(1):17. doi: 10.1038/s41598-020-78407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda A, Slamti R, Bochnik-Tamir L, Malach E, Lereclus D, Hayouka Z. Turning off Bacillus cereus quorum sensing system with peptidic analogs. Chem. Commun. 2018;54:9777–9780. doi: 10.1039/c8cc05496g. [DOI] [PubMed] [Google Scholar]
- Yu P, Yu S, Wang J, Guo H, Zhang Y, Liao X, Zhang J, Wu S, Gu Q, Xue L, Zeng H, Pang R, Lei T, Zhang J, Wu Q, Ding Y. Bacillus cereus isolated from vegetables in China: incidence, genetic diversity, virulence genes, and antimicrobial resistance. Front. Microbiol. 2019;10:948. doi: 10.3389/fmicb.2019.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.S, Reed A, Chen F, Stewart C.N. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, He J, Cao X, Huang K, Luo Y, Xu W. Development of a double-antibody sandwich ELISA for rapid detection of Bacillus cereus in food. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript contains the data that support the study’s conclusions. Upon a reasonable request, the corresponding author will provide any additional data.